assessment is the requirement to be explicit in all the evaluations and judgments that must be made to document conclusions.
Risk Assessment and Food Safety
Basic Concepts
Risk assessment is a scientific undertaking having as its objective a characterization of the nature and likelihood of harm resulting from human exposure to agents in the environment. The characterization of risk typically contains both qualitative and quantitative information and includes a discussion of the scientific uncertainties in that information. In the present context, the agents of interest are nutrients, and the environmental media are food, water, and nonfood sources such as nutrient supplements and pharmacologic preparations.
Performing a risk assessment results in a characterization of the relationships between exposure(s) to an agent and the likelihood that adverse health effects will occur in members of exposed populations. Scientific uncertainties are an inherent part of the risk assessment process and are discussed below. Deciding whether the magnitude of exposure is "acceptable" in specific circumstances is not a component of risk assessment; this activity falls within the domain of risk management. Risk management decisions depend on the results of risk assessments but may also involve the public health significance of the risk, the technical feasibility of achieving various degrees of risk control, and the economic and social costs of this control. Because there is no single, scientifically definable distinction between "safe" and "unsafe'' exposures, risk management necessarily incorporates components of sound, practical decision making that are not addressed by the risk assessment process (NRC, 1983, 1994).
A risk assessment requires that information be organized in rather specific ways but does not require any specific scientific evaluation methods. Rather, risk assessors must evaluate scientific information using what they judge to be appropriate methods; and they must make explicit the basis for their judgments, the uncertainties in risk estimates, and when appropriate, alternative interpretations of the available data that may be scientifically plausible (NRC, 1994; OTA, 1993).
Risk assessment is subject to two types of scientific uncertainties: (1) those related to data and (2) those associated with inferences that are required when directly applicable data are not available (NRC, 1994). Data uncertainties arise when evaluating information obtained from the epidemiologic and toxicologic studies of nutrient intake levels that are the basis for risk assessments. Examples of inferences include the use of data from experimental animals to estimate responses in humans and the selection of uncertainty factors to estimate inter and intraspecies variabilities in response to toxic substances. Uncertainties arise
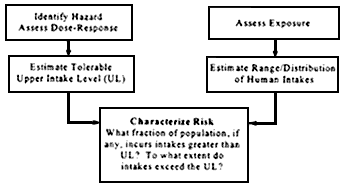
FIGURE 1.
Risk assessment model for nutrient toxicity.
whenever estimates of adverse health effects in humans are based on extrapolations of data obtained under dissimilar conditions (for example, from experimental animal studies). Options for dealing with uncertainties are discussed below and in detail in Appendix B.
Steps in the Risk Assessment Process
The organization of risk assessment is based on a model proposed by the NRC (1983, 1994); that model is widely used in public health and regulatory decision making. The steps of risk assessment as applied to nutrients are as follows (see also Figure 1):
- Step 1. Hazard identification involves the collection, organization, and evaluation of all information pertaining to the adverse effects of a given nutrient. It concludes with a summary of the evidence concerning the capacity of the nutrient to cause one or more types of toxicity in humans.
- Step 2. Dose-response assessment determines the relationship between nutrient intake (dose) and adverse effect (in terms of incidence and severity). This step concludes with an estimate of the UL—it identifies the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. Different ULs may be developed for various life stage groups.
- Step 3. Intake assessment evaluates the distribution of usual total daily nutrient intakes among members of the general population.
- Step 4. Risk characterization summarizes the conclusions from Steps 1 through 3 and evaluates the risk. Generally, the risk is expressed as the fraction
- of the exposed population, if any, having nutrient intakes (Step 3) in excess of the estimated UL (Steps 1 and 2). If possible, scientific characterization also covers the magnitude of any such excesses. Scientific uncertainties associated with both the UL and the intake estimates are described so that risk managers understand the degree of scientific confidence they can place in the risk assessment.
The risk assessment contains no discussion of recommendations for reducing risk; these are the focus of risk management.
Thresholds
A principal feature of the risk assessment process for noncarcinogens is the long-standing acceptance that no risk of adverse effects is expected unless a threshold dose (or intake) is exceeded. The adverse effects that may be caused by a nutrient or food component almost certainly occur only when the threshold dose is exceeded (NRC, 1994; WHO, 1996). The critical issues concern the methods used to identify the approximate threshold of toxicity for a large and diverse human population. Because most nutrients are not considered to be carcinogenic in humans, the approach to carcinogenic risk assessment (EPA, 1996) is not discussed here.
Thresholds vary among members of the general population (NRC, 1994). For any given adverse effect, if the distribution of thresholds in the population could be quantitatively identified, it would be possible to establish ULs by defining some point in the lower tail of the distribution of thresholds that would be protective for some specified fraction of the population. However, data are not sufficient to allow identification of the distribution of thresholds for all but a few, well-studied nutrients and compounds found in food (for example, acute toxic effects or for chemicals such as lead, where the human database is very large). The method described here for identifying thresholds for a general population is designed to ensure that almost all members of the population will be protected, but it is not based on an analysis of the theoretical (but practically unattainable) distribution of thresholds. By using the model to derive the threshold, however, there is considerable confidence that the threshold, which becomes the UL for nutrients or food components, lies very near the low end of the theoretical distribution, and is the end representing the most sensitive members of the population. For some nutrients, there may be subpopulations that are not included in the general distribution because of extreme or distinct vulnerabilities to toxicity. Such distinct groups, whose conditions warrant medical supervision, may not be protected by the UL.
The Joint FAO/WHO Expert Commission on Food Additives and various national regulatory bodies have identified factors (called uncertainty factors [UFs]) that account for interspecies and intraspecies differences in response to



