Page 83
4
Health Effects of Arsenic
THIS chapter presents the subcommittee's review of the evidence of health effects in humans resulting from ingestion of inorganic arsenic. The source of exposure in the large majority of studies reviewed is drinking water contaminated with inorganic arsenic from natural sources. A few studies involve other sources of exposure, however, such as industrially contaminated drinking water, medicinal use of arsenic, and arsenical pesticides. The focus of the chapter is on causal inference, which in risk-assessment terminology is often referred to as hazard identification. The chapter first provides the evidence for cancer and then other effects.
Although evidence for dose-response relationships is presented as it relates to causal inference, the actual quantification of dose-response relationships has not been undertaken in this chapter. Statistical issues in dose-response quantification for risk-assessment purposes are presented in Chapter 10.
Cancer Effects
The carcinogenic role of arsenic compounds was first noted over 100 years ago in the Hutchinson (1887) observation that an unusual number of skin tumors develop in patients treated with arsenicals. In a 1980 review of arsenic, the International Agency for Research on Cancer (IARC 1980) determined that inorganic arsenic compounds are skin and lung (via inhalation) carcinogens in humans. Data suggesting an increased risk for cancer at other sites were noted to be inadequate for evaluation. Since 1980, several additional studies of cancer and exposure to arsenic in drinking water have been completed.
The epidemiological studies outlined in this chapter clearly show associa-
Page 84
tions of arsenic with several internal cancers at exposure concentrations of several hundred micrograms per liter of drinking water. However, they provide few data about the degree of association at exposure concentrations below a few hundred micrograms per liter.
An extensive literature describes cases of skin and internal cancers following medicinal treatment with potassium arsenite (Fowler's solution) for a variety of conditions (Sommers and McManus 1953; Rosset 1958; Robson and Jelliffe 1963; Jackson and Grainge 1975; Popper et al. 1978; Prystowsky et al. 1978; Reymann et al. 1978; Nagy et al. 1980; Falk et al. 1981; Roat et al. 1982; Robertson and Low-Beer 1983), exposure to arsenical pesticides (Sommers and McManus 1953; Kjeldsberg and Ward 1972; Popper et al. 1978), or consumption of industrially contaminated drinking water or pesticide-contaminated wine (Roth 1957). The case reports and case series do not provide the needed data for quantitative risk assessment. However, the occurrence of these tumors in high numbers after long-term ingestion of arsenic in relatively young patients, or at anatomic sites where cancer is an extremely rare occurrence (e.g., liver angiosarcoma), increases the likelihood that many of the documented cancers were induced by arsenic. The observations also assist in identifying major cancer end points. The most common types of malignancy described in the reports are skin cancer, lung cancer, angiosarcoma of the liver (probably noted because of its rarity), prostate cancer, and bladder cancer. Reports of other cancers also appear: leukemia; other hematopoietic cancers; and cancers of the breast, colon, stomach, parotid gland, nasopharynx, larynx, buccal cavity, kidney, and others. Additional case reports describe internal cancers after the appearance of Bowen's disease, a type of superficial intraepidermal carcinoma that has been linked with arsenic exposure (Graham and Helwig 1959; Epstein 1960; Peterka et al. 1961; Hugo and Conway 1967).
The second group of studies comprises epidemiological investigations. Most of them did not provide the informational quality necessary for interpretation of dose-response relationships. However, many of the studies included data that are valuable in establishing the level of risk of particular internal cancers associated with a range of likely arsenic exposures (see Table 4-1). The form of arsenic was not specified in the epidemiological studies cited except for Cuzick et al. (1992), who observed mortality in a cohort of patients medicinally treated with potassium arsenite. Ecological studies are considered first, followed by cohort studies. Studies are summarized in Tables 4-1 through 4-6. When evaluating the epidemiological evidence to help judge whether arsenic in drinking water is a likely cause of internal cancers or other diseases, the subcommittee used the evaluation criteria that have been discussed by Hill (1965) and others (Cox 1972; Susser 1973; Rothman 1986).
Page 85
The primary criteria that were used in attempting to distinguish causal from noncausal associations included (1) strength of the association (the magnitude of the risk ratio between exposed and nonexposed populations); (2) temporality (the disease must follow the exposure); (3) biological gradient (exposure to higher concentrations of arsenic or exposure for longer periods should result in a greater effect than low-concentration exposures or exposures of short duration); and (4) epidemiological coherence (are similar observations made in diverse populations?).
| TABLE 4-1 Summary of Cancer End Points Available for Quantitative Risk Assessment of Cancer and Ingested Arsenic Exposures | ||||||||
| Cancer Site | ||||||||
| Study | Skin | Bladder | Lung | Kidney | Nasal | Liver | Prostate | Other |
| Ecological studies | ||||||||
| Tseng et al. 1968 |
| |||||||
| Wu etal. 1989 |
|
|
|
|
|
|
| |
| Chen and Wang 1990 |
|
|
|
|
|
|
| |
| Guo et al. 1997 |
|
| ||||||
| Hopenhayn-Rich et al. |
|
|
|
| ||||
| 1996, 1998 | ||||||||
| Smith et al. 1998 |
|
|
|
|
| |||
| Cohort studies | ||||||||
| Cuzick et al. 1992 |
|
|
|
| ||||
| Tsuda et al. 1995 |
|
|
|
| ||||
| Chiou et al. 1995 |
|
| ||||||
| Case-control studies | ||||||||
| Bates et al. 1995 |
| |||||||
|
| ||||||||
Ecological Studies
Summary results of the ecological studies are shown in Tables 4-2, 4-3, and 4-4.
Page 86
| TABLE 4-2 Bladder-Cancer Mortality, Urinary-Cancer Incidence, and Arsenic Exposure in Ecological Studies | |||||||
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | ||
| Mortality studies | |||||||
| Chen and Wang 1990 | Taiwan | Data from 83,656 wells, national survey | b(SE) from regression: | Mortality, 1972-1983 in 314 precincts and townships; regression-coefficient (b) estimates increase in age-adjusted mortality per 100,000 per increase in arsenic at 100 mg/L of water | |||
| Male | Female | ||||||
| National data | 3.9 (0.5) | 4.2 (0.5) | |||||
| Rate: | |||||||
| Wu et al. 1989 | SW Taiwan | Average arsenic: <0.30 ppm | Male | Female | Male | Female | Mortality, 1973-1986 in 42 villages in Taiwan |
| 23 | 30 | 22.6 | 25.6 | ||||
| 36 | 36 | 61.0 | 57.0 | ||||
| 26 | 30 | 92.7 | 111.3 | ||||
| SMR: | Mortality, 1986-1991; national rates | ||||||
| Hopenhayn- Rich et al. 1996,1998 | Cordoba Province, Argentina | Few high concentration Scattered high concentration | Male | Female | Male | Female | for 1989 used as the standard for the SMR; SMR for COPD below the expected level, indicating low smoking rates; also no trend with stomach cancer SMR |
| 113 | 39 | 0.80 (0.7-1.0) | 1.21 (0.9-1.6) | ||||
| 93 | 24 | 1.42 (1.1-1.7) | 1.58 (1.0-2.4) | ||||
| 131 | 27 | 2.14 (1.8-2.5) | 1.82 (1.2-2.6) | ||||
| SMR: | |||||||
| Smith et al. 1998 | Region II, Northern Chile | 420 mg/L average; 5-yr average ranged from below100 mg/L after 1980 to 569 µg/L in1955-1959; by city and 5- yr period, range was 40-870 µg/L | Male | Female | Male | Female | Mortality, 1989-1993; national rates for 1991 used as the standard for the SMR; arsenic concentration is population-weighted average for major cities or towns in Region II, 1950-1974; information in paper adequate to calculate increase in risk per unit exposure |
Page 87
(Table continued from previous page)
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | |
| Incidence studies | ||||||
| Guo et al. 1997a | Taiwan | Data from 83,656 wells, national survey: | b(SE) from regression: | Incidence, 1980-1987; results shown are for transitional-cell carcinoma, the most common form of bladder cancer | ||
| <0.05 ppm | Mixed results for exposure | |||||
| 0.05-0.08 ppm | levels; at >0.64 ppm, the | |||||
| 0.09-0.16 ppm | b(SE) was | |||||
| 0.17-0.32 ppm | ||||||
| 0.33-0.64 ppm | Male | Female | ||||
| >0.64 ppm | National rates | 0.57 (0.07) | 0.33 (0.04) | |||
| Transitional-cell carcinoma only. | ||||||
| Abbreviations: b(SE), regression coefficient (standard error); SMR, standardized mortality ratio; COPD, chronic obstructive pulmonary disease | ||||||
Page 88
| TABLE 4-3 Kidney-Cancer Mortality, Urinary-Cancer Incidence, and Arsenic Exposure in Ecological Studies | |||||||||
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | ||||
| Mortality studies | |||||||||
| SMR: | |||||||||
| Chen et al. | SW | Blackfoot-disease | Male | Female | Female | Male | SMRs; age-specific Taiwan rates | ||
| 1985 | Taiwan | endemic area | 42 | 62 | 772 | 1,119 | as standard | ||
| Chen and | Taiwan | Data from 83,656 | b(SE) from regression: | Mortality, 1972-1983 in 314 | |||||
| Wang 1990 | wells; national | Male | Female | precincts and townships; | |||||
| survey | National data | 1.1(0.2) | 1.7 (0.2) | regression-coefficient (¬) estimates | |||||
| increase in age-adjusted mortality | |||||||||
| per 100,000 per increase in arsenic | |||||||||
| at 100 pg/L of water | |||||||||
| Rate: | |||||||||
| Wu et al. | SW | Average arsenic: | Male | Female | Male | Female | Mortality, 1973-1986 in 42 villages | ||
| 1989 | Taiwan | <0.30 ppm | 9 | 4 | 8.42 | 3.42 | in Taiwan | ||
| 0.30-0.59 ppm | 11 | 13 | 18.90 | 19.42 | |||||
| ³0.60 ppm | 6 | 16 | 25.26 | 57.98 | |||||
| SMR: | |||||||||
| Hopenhayn- | Cordoba | County group: | Male | Female | Male | Female | SMRs using national age-specific | ||
| Rich et al. | Province, | Low | 66 | 38 | 0.87 | 1.00 | rates as the standard | ||
| 1996,1998 | Agentina | Medium | 66 | 34 | 1.33 | 1.36 | |||
| High | 53 | 27 | 1.57 | 1.81 | |||||
(Table continued on next page)
Page 89
(Table continued from previous page)
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | ||
| Incidence studies | |||||||
| Guo et al. | Taiwan | Data from 83,656 | Incidence, 1980-1987; results | ||||
| 1997a | wells; national | shown are for renal-cell carcinoma | |||||
| survey: | b(SE) from regression: | only | |||||
| <0.05 ppm | Mixed results for exposure | ||||||
| 0.05-0.08 ppm | levels; at >0.64 ppm, the | ||||||
| 0.09-0.16 ppm | b(SE) was | ||||||
| 0.17-0.32 ppm | |||||||
| 0.33-0.64 ppm | Male | Female | |||||
| >0.64 ppm | National rates | 0.03 (0.02) | 0.142 (0.013) | ||||
| aTransitional-cell carcinoma only. | |||||||
| Abbreviations: b(SE), regression coefficient (standard error); SMR, standardized mortality ratio. | |||||||
Page 90
| TABLE 4-4 Lung-Cancer Mortality and Arsenic Exposure in Ecological Studies | |||||||||||||
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | ||||||||
| Chen and | Taiwan | 1974-1976 data from | b(SE) from regression: | Mortality, 1972-1983 in 314 | |||||||||
| Wang 1990 | 83,656 wells, national | Male | Female | precincts and townships; | |||||||||
| survey: average for | 5.3(0.9) | 5.3(0.7) | regression-coefficient (P) | ||||||||||
| National data | estimates increase in age- | ||||||||||||
| each of 314 precincts | adjusted mortality per | ||||||||||||
| or townships | 100,000 per increase in | ||||||||||||
| arsenic at 100 µg/L of water | |||||||||||||
| Rate: | |||||||||||||
| Wu et al. | SW | Average arsenic: | Male | Female | Male | Female | Mortality, 1973-1986 in 42 | ||||||
| 1989 | Taiwan | <0.30 ppm | 53 | 43 | 49.16 | 36.71 | villages in Taiwan | ||||||
| 0.30-0.59 ppm | 62 | 40 | 100.67 | 60.82 | |||||||||
| ¬0.60 ppm | 32 | 38 | 104.08 | 122.16 | |||||||||
| SMR: | Mortality, 1989-1993; | ||||||||||||
| Smith et al. | Region II, | 420 µg/L average; 5-yr | Male | Female | Male | Female | national rates for 1991 used | ||||||
| 1998 | Northern | average ranged from | 544 | 154 | 3.8(3.5-4.1) | 3.1(2.7-3.7) | as the standard for the SMR; | ||||||
| Chile | below 100 µg/L after | arsenic concentration is | |||||||||||
| 1980 to 569 µg/L in 1955- | population-weighted average | ||||||||||||
| 1959; by city and 5-yr | for major cities and towns in | ||||||||||||
| period, range was 40-870 | Region II, 1950-1974; | ||||||||||||
| µg/L | information in paper | ||||||||||||
| adequate to calculate | |||||||||||||
| increase in risk per unit | |||||||||||||
| exposure | |||||||||||||
Page 91
(Table continued from previous page)
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | ||
| SMR: | Mortality, 1986-1991; national | ||||||
| Hopenhayn- | Cordoba | County group: | Male | Female | Male | Female | rates for 1989 used as the |
| Rich et al. | Province, | Low | 826 | 194 | 0.92(0.85-0.98) | 1.24(1.06-1.42) | standard for the SMR; SMR for |
| 1998 | Argentina | Medium | 914 | 138 | 1.54(1.44-1.64) | 1.34(1.12-1.58) | COPD below the expected |
| High | 708 | 156 | 1.77(1.63-1.90) | 2.16(1.83-2.52) | level, indicating low smoking | ||
| rates; also no trend with | |||||||
| stomach cancer SMR | |||||||
| Abbreviations: b(SE), regression coefficient (standard error); SMR, standardized mortality ratio. | |||||||
Page 92
Villages in southwestern coastal Taiwan switched from surface to groundwater (artesian wells) for drinking in the 1920s, motivated by the need to improve the microbiological quality of drinking water. Unexpectedly, aquifers were contaminated with naturally occurring arsenic, and the shift resulted in widespread exposure to relatively high concentrations. Tseng et al. (1968) and Tseng (1977) conducted individual medical examinations, with an emphasis on skin lesions, of 40,421 inhabitants of 37 villages in that area of Taiwan by the end of 1965. The arsenic content of well water ranged from 0.01 to 1.82 parts per million (ppm); most wells had arsenic concentrations of 0.4-0.6 ppm. Prevalence of skin cancer, keratoses, and hyperpigmentation were calculated for villages in three exposure groups: 0.0-0.29 ppm, 0.30-0.59 ppm, and 0.60 ppm and over. Hyperpigmentation was the most common condition (183.5/1,000), followed by keratosis (71.0/1,000) and skin cancer (10.6/1,000). In both sexes in three broad age groups, the prevalence of skin lesions increased with exposure to arsenic. For example, among males 60 and over, the prevalence of skin cancer per 1,000 persons was 46.1 in the 0.00.29-ppm group, 163.4 in the 0.30-0.59-ppm group, and 255.3 in the 0.60 ppm-and-over group. Among females 60 years of age and over, skin-cancer prevalence was 9.1, 62.0, and 110.1 per 1,000 persons in the three exposure groups. Study results were used by EPA for a risk assessment of ingested arsenic (EPA 1988). The primary limitation of this study, beyond the problems common to ecological studies, is related to the lack of detail and specificity provided for exposure estimates. Those issues are discussed in Chapter 2 of this report.
Arsenic-related risks of internal and skin cancers were studied by Chen et al. (1985). This study reported standardized mortality ratios (SMRs) in 84 villages in four townships in southwestern Taiwan where blackfoot disease was prevalent and where earlier studies had detected increased skin-cancer rates. Mortality over the period 1968-1986 was compared with expected mortality based on nationwide age- and sex-specific rates. Significantly increased mortality was observed among males and females for bladder, kidney, skin, lung, liver, and colon cancers as follows: bladder-cancer SMRs = 11.0 (95% confidence interval (CI) = 9.3-12.7) for males (M) and 20.1 (95% CI = 17.0-23.2) for females (F); kidney-cancer SMRs = 7.7 (95% CI = 5.4-10.1) (M) andll.2 (95% CI = 8.4-14) (F); skin-cancer SMRs = 5.3 (95% CI = 3.8-6.9) (M) and 6.5 (95% CI = 4.7-8.4) (F); lung-cancer SMRs = 3.2 (95% CI = 2.9-3.5) (M) and 4.1 (95% CI = 3.6-4.7) (F); liver-cancer SMRs = 1.7 (95% CI = 1.5-1.9) (M) and 2.3 (95% CI = 1.9-2.7) (F); colon-cancer SMRs = 1.6 (95% CI = 1.2-2.0) (M) and 1.7 (95% CI = 1.32.1) (F). Other cancer sites (small intestine, esophagus, nasopharynx, rectum, stomach, and thyroid) did not show statistically meaningful associations. The
Page 93
SMR for leukemia was 1.4 (95% CI = 1.0-1.8) for men and 0.9 (95% CI = 0.5-1.3) for women. Concentrations of arsenic in the drinking water were not presented in the Chen et al. (1985) report. However, an exposure-response gradient for risk of bladder, kidney, skin, lung, and liver cancers was noted in evaluating the risk in areas with shallow wells (presumably low arsenic exposure), both shallow and artesian wells (intermediate exposure), and artesian wells only (highest exposure). In villages with artesian wells, SMRs were approximately 30.0, 9.0, 10.0, 5.0, and 2.0 (CIs not reported) for bladder, kidney, skin, lung, and liver cancers, respectively.
Wu et al. (1989) provided quantitative information on arsenic concentrations in the drinking water of 42 villages in southwestern Taiwan and calculated age-adjusted cancer mortality during the period 1973-1986 within three groups of villages stratified by exposure concentration (less than 0.30 mg/L, 0.30-0.59 mg/L, and 0.60 mg/L or more). Among males, mortality increased with increasing arsenic concentrations in water for cancers of all sites combined, and cancers of the bladder, kidney, skin, lung, liver, prostate, and leukemia when considered separately. Among females, increases in mortality were observed for all sites combined and cancers of the bladder, kidney, skin, lung, and liver. Nationwide mortality rates for those cancers were not provided by Wu et al. (1989). However, age-adjusted mortality for Taiwan was noted by Chen et al. (1985) for the years 1968-1982. Among males, the ratio of mortality in high-arsenic-exposure villages compared with national mortality, varied with increases of about 3-fold for liver cancer and 30-fold and over for bladder cancer. Among females, analogous mortality ratios increased more than 80-fold for bladder cancer.
Chen and Wang (1990) analyzed nationwide mortality data from Taiwan using water arsenic concentrations from 83,656 wells located in 314 precincts and townships from 1974 to 1976. Using a multiple regression approach, the authors compared age-adjusted mortality for 1972-1983 with the arsenic concentrations in those locations. A significant association with arsenic concentration was found for cancers of the liver, nasal cavity, lung, skin, bladder, and kidney in both sexes and for prostate cancer in males. Using multiple linear regression models, Chen and Wang calculated a regression coefficient indicating the change in age-adjusted mortality per 100,000 person-years for every 0.1-ppm increase in arsenic in well water, after adjusting for indices of industrialization and urbanization. The regression coefficients were 6.8, 0.7, 5.3, 0.9, 3.9, and 1.1 for men and 2.0, 0.4, 5.3, 1.0, 4.2, and 1.7 for women for cancers of the liver, nasal cavity, lung, skin, bladder, and kidney, respectively. The regression coefficient for prostate cancer was 0.5. Regression models included indices of urbanization and industrialization and were weighted by the square root of person-years at risk in each place. The
Page 94
number of wells per precinct or township ranged from 12 to 3,160. In more than 90% of precincts and towns, more than 50 wells were examined. The method for calculating average exposure for each geographic area was not presented. Chen and Wang (1990) stated that ''there was a significant intra-area homogeneity and interarea heterogeneity in arsenic concentration." Supporting data were not presented. High concentrations of arsenic were found in water in the northeast of Taiwan and in the coastal area in the southwest of Taiwan, where other studies have been conducted.
Guo et al. (1997) used tumor registry data along with the same exposure data from the 1974-1976 nationwide water-quality survey used previously by Chen and Wang (1990). The authors used arsenic concentrations in drinking water from 243 townships with about 11.4 million residents. The annual incidence of bladder and kidney cancers for townships in 1980-1987 and subcategories of those cancer diagnoses were regressed against a model that included six variables for the proportions of wells in each of six categories of arsenic concentration in each township. Sex-specific models were adjusted for age and included an urbanization index and the annual number of cigarettes sold per capita. Regression models were weighted by the total population of each township. A total of 1,962 bladder, 726 kidney, 170 ureter, and 57 urethral cancers were included. Guo et al. (1997) found associations of high arsenic concentrations (more than 0.64 ppm) in both sexes with transitional-cell carcinomas of the bladder, kidney, and ureter and all urethral cancers combined, but they did not present relative risk estimates, so the results cannot be compared directly with other studies. Associations of the township proportion of wells with arsenic at concentrations lower than 0.64 ppm were not significant, and some regression coefficients were negative. No association was found with cigarette sales, but a positive link was observed with urbanization. The overall crude annual bladder-cancer incidence rate (2.15 per 100,000 population) reported by Guo et al. (1997) is far below that of comparable Asian populations, suggesting under-ascertainment of newly diagnosed bladder cancer in the voluntary national cancer registry. Cancer reporting is likely to be better in urbanized areas than in rural areas, such as the high-arsenic regions of southwest and northeast Taiwan. Uncertainties in exposure estimates previously cited apply also to this study.
In two reports, Hopenhayn-Rich et al. (1996, 1998) examined SMRs for bladder, kidney, lung, liver, skin, and stomach cancers for 1986-1991 in the 26 counties of Cordoba Province, Argentina. The authors grouped counties into three strata according to the arsenic concentration in their drinking water. The low and intermediate exposure groups were defined qualitatively by the authors. In the highest exposure group, comprising two counties, arsenic exposure data were presented in tabular form by town. Arsenic concentra-
Page 95
tions ranged from 40 to 433 µg/L of drinking water in the towns of one county and 50 to 353 µg/L in those of the other. Separate average concentrations in each county were 181 and 174 µg/L. SMRs were calculated using sex- and age-specific rates for Argentina as the referent. Significant increases with exposure concentration were found for the following cancers: bladder-cancer SMRs = 0.80 (95% CI = 0.66-0.96), 1.42 (95% CI = 1.14-1.74), and 2.14 (95% CI = 1.78-2.53) (M) and 1.21 (95% CI = 0.85-1.64), 1.58 (95% CI = 1.01-2.35), and 1.82 (95% CI = 1.19-2.64) (F); lung-cancer SMRs = 0.92 (95% CI = 0.85-0.98), 1.54 (95% CI = 1.44-1.64), and 1.77 (95% CI = 1.63-1.90) (M) and 1.24 (95% CI = 1.06-1.42), 1.34 (95% CI = 1.12-1.58), and 2.16 (95% CI = 1.83-2.52) (F); and kidney-cancer SMRs = 0.87 (95% CI = 0.66-1.10), 1.33 (95% CI = 1.02-1.68), and 1.57 (95% CI = 1.17-2.05) (M) and 1.00 (95% CI = 0.71-1.37), 1.36 (95% CI = 0.941.89), and 1.81 (95 % CI = 1.19-2.64) (F). Skin-cancer (risk did not increase monotonically with exposure) SMRs = 2.04 (95% CI = 1.38-2.89), 1.49 (95% CI = 0.83-2.45), and 1.49 (95% CI = 0.71-2.73) (M) and 0.85 (95% CI = 0.42-1.51), 0.82 (95% CI = 0.32-1.68), and 2.78 (95% CI = 1.614.44) (F). Skin cancers due to arsenic are generally not fatal, and with the small numbers of deaths, the confidence intervals were broad. The information presented above is in terms of relative risk. When considered in absolute risk terms, the major component of risk was lung cancer. For example, for men in the high exposure counties, there were 708 lung cancer deaths observed, with 400.73 expected, from which the excess number of deaths can be calculated to be 307. The corresponding excess number of deaths for bladder cancer was 70, indicating that the number of excess lung cancer deaths was over 4 times that for bladder cancer. Among women, the number of excess lung cancer deaths was 84 in the high exposure counties, which is 7 times more than the corresponding number of 12 for bladder cancer. Deaths from chronic obstructive pulmonary disease (COPD) were not related to arsenic concentrations in drinking water, indicating that smoking is an unlikely confounder of the lung, bladder, and kidney cancer results. This study has limitations shared by most ecological investigations.
Smith et al. (1998) examined bladder and lung-cancer mortality (19891993) among persons 30 years of age and over in a region of northern Chile (Region II) with a population of about 400,000. Concentrations of arsenic in drinking water were well-documented and had been high in all major population centers of Region II, especially before 1975. The population-weighted average in the years 1950-1974 was 420 µg/L, the maximum being 870 µg/L in Antofagasta, the largest city, between 1955 and 1969. SMRs for Region II were increased for every 10-year age group for each sex, using national rates as the standard. For bladder cancer, the overall SMRs were 6.0 (95%
Page 96
CI = 4.8-7.4) for men and 8.2 (95% CI = 6.3-10.5) for women. Lung-cancer SMRs were 3.8 (95% CI = 3.5-4.1) for men and 3.1 (95% CI = 2.73.7) for women. As in Argentina, the main contribution to cancer risks involved lung cancer. The reported number for excess lung cancer deaths among men (400.8) was about 5 times that for bladder cancer (77.5). Among women, the number of excess lung cancer deaths (105) was about twice that for bladder cancer (56.2). Although having the limitations of an ecological design, this study has several strengths. Population exposures to arsenic were well defined compared with several other exposed populations, and mortality due to COPD was low, indicating low smoking rates in the population. The importance of the Hopenhayn-Rich studies in Argentina (1996, 1998) and the Smith study in Chile (1998) is that they confirmed earlier observations from Taiwan of increased risk for bladder and lung cancers after exposure to inorganic arsenic in drinking water.
Cohort Studies
The results of the cohort studies are shown in Tables 4-5 and 4-6.
Cuzick et al. (1992) observed excess bladder-cancer mortality among 478 patients medicinally treated with potassium arsenite in 1945-1969; 5 deaths occurred. Based on age-, sex-, and calendar-year-adjusted rates for England and Wales, 1.6 bladder-cancer deaths were expected (p < 0.05). Among the five cases, total arsenic doses were 224, 504, 963, 1,901, and 3,324 mg. The SMR was 5.00 (95 % CI = 2.0-15) for the four patients treated with more than 500 mg of arsenic. No excess of lung-cancer deaths was observed (14 observed, 14.0 expected). Relatively little opportunity existed for bias in this follow-up study. Cigarette-smoking habits among patients were not described, but it is unlikely that smoking could have accounted for the excess, even if all bladder-cancer patients had smoked. With circulatory diseases as indicators (SMR = 0.91; 95% CI = 0.74-1.1), smoking rates in the overall cohort do not appear to be unusual.
Tsuda et al. (1995) found excess mortality due to the following cancers among a cohort of 113 persons exposed to arsenic above 1.0 mg/L of industrially contaminated drinking water in villages of Niigata Prefecture, Japan: urinary-tract cancer (3 observed, 0.10 expected) SMR = 31.18 (95% CI = 8.62-91.75), lung cancer (8 observed, 0.51 expected) SMR = 15.69 (95% CI = 7.38-31.02), liver cancer (2 observed, 0.28 expected) SMR = 7.17 (95% CI = 1.28-26.05), and uterine cancer (2 observed, 0.15 expected) SMR = 13.47 (95% CI = 2.37-48.63). The observed-to-expected ratios were near or below expectation among persons exposed to arsenic at less than 0.05 mg/L.
Page 97
| TABLE 4-5 Lung-Cancer Mortality or Incidence and Arsenic Ingestion in Cohort Studies | |||||
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments |
| SMR: | 478 patients treated with Fowler's solution | ||||
| Cuzick et al. | United | 14 | 1.00 (0.5-1.7) | (potassium arsenite) | |
| 1992 | Kingdom | ||||
| Chiou et al. | SW Taiwan | Cumulative arsenic: | Relative risk: | Incidence among a cohort of 2,556 subjects | |
| 1995 | <0.1 mg/L/yr | 3 | 1.0 | ||
| 0.1-19.9 mg/L/yr | 7 | 3.1 (0.8-12.2) | (263 blackfoot-disease patients and 2,293 | ||
| ³20 mg/L/yr | 7 | 4.7 (1.2-18.9) | healthy individuals) followed for 7 yr | ||
| Average arsenic: | |||||
| <0.05 mg/L | 5 | 1.0 | |||
| 0.05-0.70 mg/L | 7 | 2.1 (0.7-6.8) | |||
| ³0.71 mg/L | 7 | 2.7 (0.7-10.2) | |||
| SMR: | |||||
| Tsuda et al. | Niigata | <0.05 µg/L | 0 | 0.0 (0-2.4) | Exposure among 113 persons who drank |
| 1995 | Prefecture, | 0.05-0.99 µg/L | 1 | 2.3 (0.1-13.4) | from contaminated wells for approximately 5 |
| Japan | ³1.0 mg/L | 8 | 15.7 (7.4-31.0) | yr (1955-1959), then followed for 33 yr | |
| Abbreviation: SMR, standardized mortality ratio. | |||||
Page 98
| TABLE 4-6 Bladder-Cancer Mortality or Incidence and Arsenic Ingestion in Cohort Studies | ||||||
| Study | Location | Exposure | Cases, No. | Study Outcome | Comments | |
| SMR: | ||||||
| Cuzick et al. | United | 224 mg | 1 | 1.20 (0.04-7.0) | 478 patients treated with Fowler's solution | |
| 1992 | Kingdom | 504 mg | 1 | (potassium arsenite); also, one death at age | ||
| 963 mg | 1 | 5.00 (2.0-15) | 85 and mention of bladder cancer as | |||
| 1,901 mg | 1 | (exposure ³500 mg) | contributing cause to another death | |||
| 3,324 mg | 1 | |||||
| Chiou et al. | SW Taiwan | Cumulative arsenic: | Relative risk: | Incidence among a cohort of 2,556 subjects | ||
| 1995 | <0.1 mg/L/yr | 4 | 1.0 | (263 blackfoot-disease patients and 2,293 | ||
| 0.1-19.9 mg/L/yr | 7 | 2.1 (0.6-7.2) | healthy individuals) followed for 7 yr | |||
| 20+mg/L/yr | 9 | 5.1 (1.5-17.3) | ||||
| Average arsenic: | ||||||
| <0.05 mg/L | 6 | 1.0 | ||||
| 0.05-0.70 mg/L | 7 | 1.8 (0.6-5.3) | ||||
| 0.71+ mg/L | 7 | 3.3 (1.0-11.1) | ||||
| SMR: | ||||||
| Tsuda et al. | Niigata | <0.05 µg/L | 0 | 0.0 (0-12.5) | Exposure among 113 persons who drank | |
| 1995 | Prefecture, | 0.05-0.99 µg/L | 0 | 0.0 (0-47.1) | from contaminated wells for approximately 5 | |
| Japan | ³1.0 µg/L | 3 | 31.2 (8.6-91.8) | yr (1955-1959), then followed for 33 yr | ||
| Abbreviation: SMR, standardized mortality ratio. | ||||||
Page 99
Results in this low-exposure group were statistically unstable. Expected deaths numbered less than two for each cancer cause of death. Expected numbers of deaths were based on sex-, age-, and cause-specific mortality in Niigata Prefecture from 1960 to 1989.
Chiou et al. (1995) followed 2,556 subjects in the blackfoot-disease area of southwestern Taiwan for periods ranging up to 7.7 years (an average of 4.97 years) from 1986 to 1993. In contrast to many other studies that evaluate mortality, incident cancer was the outcome of interest. Followup occurred many years after exposure to elevated arsenic concentrations in drinking water ended. Blackfoot-disease patients numbered 263, and healthy individuals numbered 2,293. Information was gathered in individual interviews. Several measures of exposure were evaluated, including average concentration of arsenic in artesian wells and cumulative arsenic exposure from drinking artesian well water. Relative risks were calculated using Cox's proportional hazards regression analysis (Cox 1972). For average arsenic concentrations of less than 0.05 mg/L, 0.05-0.70 mg/L, and 0.71 mg/L or more, relative risks were as follows: bladder cancer, 1.0 (referent), 1.8 (95% CI = 0.65.3), and 3.3 (95% CI = 1.0-11.1); lung cancer, 1.0 (referent), 2.1 (95% CI = 0.7-6.8), and 2.7 (95 % CI = 0.7-10.2). For cumulative arsenic exposures of 0, 0.1-19.9, and 20 mg/L or more times the number of years, relative risks were as follows: bladder cancer, 1.0 (referent), 2.1 (95% CI = 0.6-7.2), and 5.1 (95% CI = 1.5-17.3); lung cancer, 1.0 (referent), 3.1 (95% CI = 0.812.2); and 4.7 (95% CI = 1.2-18.9). Results were adjusted for cigarette-smoking habit and thus are likely to be more precise than those of other studies. A weakness of this study, shared with others from the same geographic area, is the small number of precise estimates of arsenic in drinking water.
Case-Control Studies
Bates et al. (1995) used information from 117 bladder-cancer cases and 266 population-based controls in the State of Utah to evaluate bladder-cancer risk after relatively low-level exposure to arsenic in drinking water. Subjects were interviewed in 1978 as part of the National Bladder Cancer Study sponsored by the National Cancer Institute. Individual exposures to arsenic in drinking water were estimated by linking residential-history information with water-sampling information from public-water supplies. Eighty-one of the 88 Utah study towns (92%) had arsenic at concentrations of less than 10 µg/L, and only one town had water with arsenic at more than 50 µg/L. Overall, no association was found between bladder-cancer risk and arsenic
Page 100
exposure. Using cumulative dose as the exposure metric, relative to a lifetime exposure of less than 19 mg, odds ratios were 1.56 (95% CI = 0.8-3.2), 0.95 (95% CI = 0.4-2.0), and 1.41 (95% CI = 0.7-2.9) for exposures of 19 to less than 33 mg, 33 to less than 53 mg, and 53 mg or more, respectively. However, among smokers, increased risk was suggested in time-window analyses of exposures occurring for years 20-29 (16 cases in the highest-exposure group) and 30-39 years (9 high-exposure cases) before the interview. The exposure index in this instance was expressed as the product of micrograms per liter and years of exposure.
Occupational Studies
Inhaled inorganic arsenic is a recognized cause of lung cancer. That effect is pertinent to arsenic ingestion and lung cancer because it supports the biological plausibility of a causal relationship between ingested inorganic arsenic and lung cancer. In addition, dose-response patterns observed between inhalation of inorganic arsenic and lung-cancer risk might be applicable to ingested arsenic and cancer risk and thus useful in assessing risk of ingested inorganic arsenic. At least five major studies of occupationally exposed individuals have reported excess risk of lung cancer from inhalation of arsenic (Ott et al. 1974; Lee-Feldstein 1986; Enterline et al. 1987; Jarup et al. 1989; Taylor et al. 1989). The question of the dose-response relationships found in those study populations was examined by most investigators. In one case, urinary arsenic levels were available to estimate exposures (Enterline et al. 1987). Enterline's first analysis of mortality data from a cohort of smelter workers (Enterline and Marsh 1982) indicated a supralinear dose-response relationship, based on estimates of airborne exposure. However, a reanalysis of the data using urinary arsenic levels was consistent with a linear dose-response association (Enterline et al. 1987). Data from the five studies were investigated by Hertz-Picciotto and Smith (1993). The data from each study were consistent with a supralinear dose-response relationship when exposures were estimated from air concentrations of inorganic arsenic in each workplace. The finding of linearity by Enterline et al. (1987) raises the possibility that the supralinear shape of the dose-response curve was due to misclassification of exposure based on air concentrations; such misclassification might have been due to workers avoiding exposure as much as possible (e.g., with the use of respirators) when air concentrations were high. In summary, the data from occupational studies suggest that the risk of lung cancer after inhalation of inorganic arsenic compounds is at least linear with exposure.
Page 101
This observation might have implications for dose-response relationships with ingested arsenic.
Noncancer Effects
Arsenic exposure interferes with the action of enzymes, essential cations, and transcriptional events in cells throughout the body, and a multitude of multisystemic noncancer effects might ensue. This discussion focuses on selected noncancer effects from chronic ingestion of arsenic in drinking water that are most relevant to the effect on health. Because experimental animals appear to be less sensitive than humans to the chronic effects of arsenic (Byron et al. 1967; Heywood and Sortwell 1979), data from human studies are emphasized. Potential reproductive and developmental effects associated with chronic ingestion of arsenic are discussed in a later section.
Cutaneous Effects
In contrast to many of the early nonspecific multisystemic signs and symptoms of chronic arsenic poisoning that are challenging to diagnose, the classic cutaneous manifestations are distinctive and characteristic. Their appearance usually follows a temporal progression, beginning with hyperpigmentation, then progressing to palmar-plantar hyperkeratoses. As discussed further below, patients might subsequently develop a variety of nonmelanoma skin cancers, occasionally, but not always, at pre-existing areas of hyperkeratosis. Although cutaneous manifestations have been most commonly reported following ingestion of arsenic containing groundwater (Yeh 1973; Zaldivar 1974; Cebrian et al. 1983; Chakraborty and Saha 1987) medicinals (Black 1967; Tay 1974), or contaminated grape beverages (Luchtrath 1983), cohorts primarily exposed via inhalation have also been affected (Perry et al. 1948; Hamada and Horiguchi 1976).
The hyperpigmentation of chronic arsenic poisoning commonly appears in a finely freckled, "raindrop" pattern that is particularly pronounced on the trunk and extremities but that might also involve mucous membranes such as the tongue or buccal mucosa (Black 1967; Yeh 1973; Tay 1974; Saha 1995). The raindrop appearance results from the presence of numerous rounded hypopigmented maculas (typically 2-4 mm in diameter) widely dispersed against a tan-to-brown hyperpigmented background (Tay 1974). Although less common, other patterns include diffuse hyperpigmentation (Tay 1974; Saha 1995); localized or patchy pigmentation, particularly affecting skinfolds (Tay 1974; Szuler et al. 1979; Luchtrath 1983); and so-called leukoderma or
Page 102
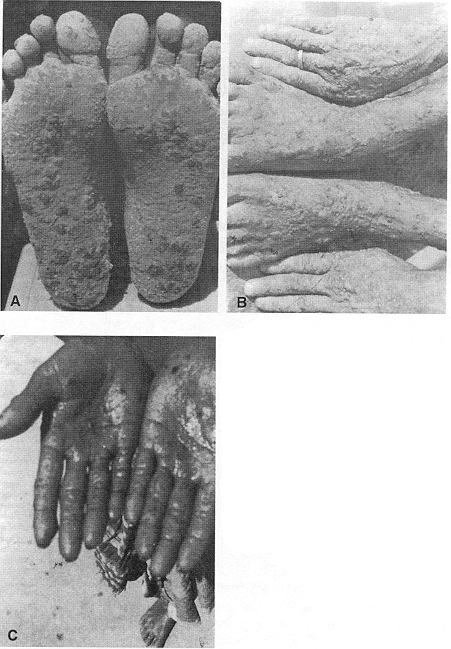
FIGURE 4-1
Photographs of West Bengal patients with arsenic-induced skin lesions. (A) Arsenic keratosis on
plantar aspect of the feet. (B) Arsenic keratosis on the dorsum of feet and hands. (C) Arsenic
keratosis on palm. Source: Subramanian and Kosnett (1998). Photos courtesy of Dr. D. Chakraborti,
School of Environmental Studies, Jadavpur University, Calcutta. Reprinted with permission from the
International Journal of Occupational and Environmental Health; copyright 1998,
Hanley & Belfus, Philadelphia.
Page 103
leukomelanosis, in which the hypopigmented maculas take on a spotty, white appearance. Mandal et al. (1996) reported that raindrop pigmentation might evolve to leukomelanosis several years after the subject is no longer exposed. Pigmentation is not histopathologically related to arsenical hyperkeratoses, nor is it a direct precursor to cancer.
Arsenical hyperkeratoses appear predominantly on the palms and the plantar aspects of the feet, although involvement of the dorsum of the extremities and the trunk has also been described. In early stages, the involved skin might have an indurated, gritlike character that can be best appreciated by palpation; however, the lesions usually advance to form raised, punctate, 2-4 mm wartlike keratoses that are readily visible (Tay 1974). Occasional lesions might be larger (approximately 1 cm) and have a nodular or horny appearance. Histological examination of the lesions typically reveals hyperkeratosis with or without parakeratosis, acanthosis, and enlargement of the rete ridges. In some cases, there might be evidence of cellular atypia, mitotic figures, or large vacuolated epidermal cells (Black 1967; Tay 1974; Ratnam et al. 1992; Alain et al. 1993). Yeh (1973) classified arsenical keratoses into two types: a benign type A, further subgrouped into those with no cell atypia and those with mild cellular atypia; and a malignant type B. Lesions of Bowen's disease (intraepithelial carcinoma, or carcinoma in situ), basal-cell carcinoma, or squamous-cell carcinoma might arise in the hyperkeratotic areas or might appear on nonkeratotic areas of the trunk, extremities, or head (Sommers and McManus 1953; Yeh 1973). The concurrent appearance of multiple skin cancers, particularly in non-sun-exposed areas, is particularly characteristic of arsenic causation (Tseng 1977; Zaldivar et al. 1981).
The magnitude of arsenic dose and the time frame of exposure necessary to induce the hyperpigmentation and hyperkeratoses characteristic of chronic arsenic intoxication have undergone limited investigation. Among the population exposed to arsenic in drinking water in the Antofagasta region of Chile, cases of cutaneous arsenicism, apparently including both hyperpigmentation and hyperkeratoses, have been described in children as young as 2 years of age (Rosenberg 1974; Zaldivar and Guillier 1977). The estimated mean arsenic dose in Antofagasta was estimated to be approximately 0.06 mg/kg per day for subgroups of children aged 3.13 ±3.33 years but was approximately 0.02 mg/kg per day for subgroups in their teens and twenties and 0.006 mg/kg per day for a subgroup in their sixties, indicating an inverse relationship between daily arsenic dose rate per kilogram of body weight and age (Zaldivar and Ghai 1980). In the cohort of 40,421 inhabitants of southeastern Taiwan investigated by Tseng and colleagues, the youngest subjects found to have hyperpigmentation and hyperkeratosis were reported to be ages 3 and 4, respectively, in an early report (Tseng et al. 1968) and 5 and 15 in a later
Page 104
account (Tseng 1977). The concentration of arsenic consumed by those children was not specified. Foy et al. (1992) reported palmar-plantar hyperkeratosis in a 4-year-old child residing in a region of southern Thailand with a mean well-water arsenic content of 0.82 mg/L. In a clinical evaluation conducted among 296 residents of Region Lagunera in northern Mexico where ingested groundwater contained a mean arsenic concentration of approximately 0.4 mg/L, the shortest time of exposure associated with ''hypopigmentation" was 8 years, increasing to 12 years for hyperpigmentation and palmar-plantar hyperkeratosis (Cebrian et al. 1983). Wagner et al. (1979) reported a patient who presented with anemia, peripheral neuropathy, and palmar-plantar hyperkeratoses 4 months after ingesting arsenic from well water at a dose estimated to be between 3.4 and 3.9 mg per day.
In a long-term follow-up of patients who had consumed medicinal arsenic in the form of Fowler's solution (potassium arsenite), the median total arsenic dose among 69 subjects with cutaneous signs of arsenicism (keratoses, hyperpigmentation, or skin cancer) was 672 mg, compared with a median arsenic dose of 448 mg in 73 subjects without skin signs (Cuzick et al. 1982). Those patients were drawn from a larger cohort that ingested arsenic for a mean duration of 8.92 months at a dose rate of approximately 8.3 mg per day. Subjects were evaluated at a mean follow-up time of 20.3 years, and the latency period for onset of cutaneous signs was not evaluated. In a retrospective study of 262 adults treated with Fowler's solution, Fierz (1965) reported the minimal latency period for hyperkeratoses to be 2.5 years, following ingestion of approximately 2.2 g of arsenite. Other case reports of adults ingesting medicinal arsenic have described doses and latencies associated with the onset of cutaneous manifestations. Rattner and Dorne (1943) reported the development of hyperpigmentation within 6-12 months of the start of treatment with arsenite at a dose of 4.75 mg day. Hyperkeratoses appeared at approximately 3 years. Pascher and Wolf (1952) described a 24-year-old asthmatic individual who was found to have hyperpigmentation and hyperkeratoses when examined 3 months after termination of a 15-month course of arsenite taken at a dose of 10 mg per day. Silver and Wainman (1952) reported a patient with the onset of "freckling of the skin and darkening of the nipples" 13 months after taking an asthma mixture delivering arsenic at a dose of 9 mg per day for 9 months, followed by 4.5 mg per day for 4 months. Ribard et al. (1986) reported a subject who presented with chronic arsenic intoxication, including hyperpigmentation, after 18 months of ingestion of an anti-asthma remedy at a dose of 2.8 mg of arsenic per day.
Limited evidence indicates that hyperpigmentation and keratoses due to arsenic exposure might serve as markers of susceptibility for other outcomes, including skin cancers and internal cancers. A small study in England of
Page 105
cancer among patients treated with medicinal arsenic noted that cancer deaths occurred only among those with prior skin manifestations due to arsenic (Cuzick et al. 1982, 1984). In a further follow-up of that cohort, a threefold increased risk of bladder-cancer mortality was found, and all five deaths occurred in patients with previous signs of arsenic poisoning (Cuzick et al. 1992). However, Tsuda et al. (1995) found an SMR for bladder cancer of 10.34 (95% CI = 2.82-30.37) among the subcohort of arsenic-exposed subjects without skin findings (see Tsuda et al. 1995, Table 5). In studies that have observed a positive relationship between arsenic ingestion and cancer, the doses of ingested arsenic were of a sufficient magnitude to cause cutaneous signs of arsenicism (hyperpigmentation or hyperkeratoses) in at least some members of the study population. At the present time, epidemiological data are insufficient to demonstrate an observed risk of cancer in populations exposed to ingested arsenic at doses too low to result in overt nonmalignant cutaneous effects.
Gastrointestinal Effects
With acute or subacute exposure (greater than several milligrams of inorganic arsenic per day), arsenic might induce overt gastrointestinal disturbances, ranging from mild abdominal cramping and diarrhea to severe life-threatening hemorrhagic gastroenteritis associated with shock. Mild-tomoderate hepatocellular necrosis, evidenced by increases in serum transaminase, might occur.
With chronic exposure to arsenic, overt gastrointestinal symptoms are often absent; however, chronic concentrations might nonetheless be associated with important organ-system pathology. Noncirrhotic portal hypertension is an uncommon but relatively specific gastrointestinal manifestation associated with chronic inorganic arsenic ingestion via medications or groundwater. Because hepatic function remains intact, the condition might not come to clinical attention until advanced stages when the patient might present with gastrointestinal hemorrhage secondary to esophageal varices (Morris et al. 1974; Szuler et al. 1979; Upshaw et al. 1979; Robertson and Low-Beer 1983; Nevens et al. 1990). Physical examination of affected patients might reveal hepatic or splenic enlargement (Guha Mazumder et al. 1997), but that finding is not invariable. Cutaneous signs of chronic arsenic poisoning are usually apparent. Liver-function tests are generally normal. Histopathological examination of the liver might find periportal fibrosis associated with slight-tomoderate enlargement of the portal tracts and mild or no inflammatory-cell infiltration. Obliterative intimal hypertrophy of intrahepatic venules have been reported which results in obstruction of portal venous flow, increased splenic
Page 106
pressures, and hypersplenism (Morris et al. 1974; Nevens et al. 1990). Hepatic arsenic content may also be markedly increased (Morris et al. 1974; Datta 1976; Guha Mazumder et al. 1997).
A few case reports noted the evolution of noncirrhotic portal hypertension to cirrhosis in patients exposed to arsenite-containing medicines (Franklin et al. 1950; Cowlishaw et al. 1979; Upshaw and Claiborne 1995). A high prevalence of cirrhosis has been reported in an autopsy series of former vineyard workers who ingested Haustrunk, a wine substitute made from an aqueous infusion of pressed grapes contaminated with arsenic insecticide residues (Luchtrath 1972, 1983). It is also noteworthy that two cohorts of arsenic-exposed copper-smelter workers have experienced increased mortality from cirrhosis of the liver (Axelson et al. 1978; Welch et al. 1982), albeit the number of cases was small and other smelter cohorts have not been similarly affected. Unfortunately, in most reports of arsenic-associated cirrhosis the authors have not been able to determine subjects' ethanol consumption, the most common cause of cirrhosis. Therefore, the etiological link with arsenic remains uncertain.
Cardiovascular Effects
Acute or subacute exposure to inorganic arsenic in the range of milligrams to grams per day have induced the rapid appearance of serious overt cardiovascular manifestations, including hypotension, congestive heart failure, and cardiac arrhythmias. The latter are often preceded by electrocardiographic prolongation of the Q-T interval, occasionally leading to polymorphic ventricular tachycardia (torsades de pointe) (Zettel 1943; Glazener et al. 1968). These acute or subacute effects are reversible.
Chronic ingestion of inorganic arsenic, most notably from drinking-water sources and arsenic-contaminated wine or wine substitutes, has been associated with the insidious development of peripheral vascular disease. The most prominent reports of arsenic-related peripheral vascular disease have originated from southwestern Taiwan, where the ingestion of arsenic-containing artesian well water from the early 1900s through the late 1950s to early 1960s was associated with the development of more than 1,000 cases of blackfoot disease. A severe manifestation of peripheral vascular insufficiency resulted in gangrene of the extremities, particularly the feet (Tseng et al. 1961; Tseng 1977). In the villages with the highest number of cases, the reported arsenic concentration of the well water ranged from 0.35 to 1.14 ppm, with a median value of 0.78 ppm (Chen et al. 1962). Clinically, the disease begins with patients' subjective complaints of coldness or numbness in the extremities
Page 107
(usually the feet) and intermittent claudication, progressing over the course of several years to ulceration, gangrene, and spontaneous amputation (Tseng et al. 1961). Histological examination of tissue from affected limbs revealed evidence of thromboangiitis obliterans and arteriosclerosis obliterans, particularly affecting small vessels (Yeh and How 1963; Yu et al. 1984). Blackfoot disease usually occurs in combination with the classic cutaneous manifestations of chronic arsenic poisoning: among 360 cases reported by Tseng et al. (1968), 280 (78 %) had hyperpigmentation and 135 (38 %) had hyperkeratoses. Although humic substances isolated from artesian well water in the blackfoot-disease endemic areas of Taiwan have been associated with thrombogenic actions in experimental models (Lu 1990; Yang et al. 1996), there is no epidemiological or human histopathological data linking humic substances to blackfoot disease (Chen 1990; Engel et al. 1994).
An increased prevalence of signs and symptoms of peripheral vascular disease has also been reported among the residents of Antofagasta, Chile, where the public drinking-water supply from the mid-to-late 1950s through 1970 contained inorganic arsenic at a mean concentration of approximately 0.6 ppm (Zaldivar 1974). Borgono et al. (1977) compared the prevalence of several manifestations of cardiovascular disease in 146 Antofagasta residents with abnormal skin pigmentation, and 36 with normally pigmented skin. Among those with abnormal pigmentation, there was an increased prevalence of Raynaud's syndrome (38.8% versus 9.3%), acrocyanosis (24.3% versus 12.5 %), and hyperkeratosis (43.7% versus 3.1%). In a clinical evaluation of 100 children with cutaneous manifestations of chronic arsenic poisoning, 19 had arterial spasms in the fingers and toes, one had gangrene of a finger, and four had ischemia of the tongue (Zaldivar and Guillier 1977). Autopsies performed on five children, aged 1 to 7 years, with cutaneous manifestations of chronic arsenic poisoning yielded findings that included mesenteric artery thrombosis, cerebrovascular disease, and coronary artery occlusions (Zaldivar 1974). An autopsy series on five Antofagasta children reported by Rosenberg (1974) described arterial intimal thickening comparable to that seen in the blackfoot-disease patients of Taiwan. Similarly, the postmortem findings on five children reported by Borgono and Greiber (1971) were noteworthy for extensive coronary or cerebrovascular occlusions, as well as ischemia of the tongue in a 2-year-old child, and Raynaud's syndrome and gangrene of the toe in a 4-year-old child.
In a recent extensive review of the vascular effects of arsenic, Engel et al. (1994) cited several German reports of overt peripheral vascular disease, including thromboangiitis obliterans and overt gangrene of the extremities, in German vintners with chronic arsenic poisoning from consumption of arseniccontaminated wine substitutes (Butzengeiger 1940, as cited in Engel et al.
Page 108
1994; Roth 1957; Grobe 1976, as cited in Engel et al. 1994). Hotta (1989) described a case study of 125 patients diagnosed with chronic arsenic poisoning as a result of occupational or environmental exposure to arsenic from smelter emissions contaminating the air, surface drinking water, and the residential environment of an arsenic mining and refining area in Toruku, Japan. The magnitude of exposure to arsenic and to other suspected pollutants, such as sulfur dioxide and other metals and particulates, was not quantified, but 93% of the subjects had melanosis, 92% had hyperkeratosis, and approximately 45 % had Bowen's disease. Hypertension was reported in 68 % of the subjects, Raynaud's syndrome in 26 %, and gangrene of the extremities in two male subjects, neither of whom had a history of diabetes. Tsuda et al. (1990) investigated the mortality experience of 141 certified arsenic-poisoning patients, with a mean age of 60.8 ± 10.4 years and followed for an average of 9.3 ± 4.0 years in Toruku from 1972 to 1990. The SMR for ischemic heart disease was 214 (95 % CI = 100-437), and the SMR for cerebrovascular disease was 77 (95% CI = 36-153).
Although extensive environmental exposure to arsenic in drinking water, accompanied by cutaneous manifestations, has also been reported in Region Lugunera in northern Mexico, the Cordova and Salta provinces of Argentina, and recently in West Bengal, India, investigators in those regions have reported little, if any, evidence of an excess prevalence of overt peripheral vascular disorders. A brief report by Cebrian (1987) refers to a 4% prevalence of "peripheral vascular alterations" and a 0.7% prevalence of blackfoot disease, apparently in reference to a study population of 296 residents of Region Lugunera exposed to a mean arsenic concentration in drinking water of 0.41 ppm (Cebrian et al. 1983; Engel et al. 1994). A recent angiographic study of 12 patients with cutaneous arsenic poisoning from the Argentina Salta Province (Torres Soruco et al. 1991, as cited in Engel et al. 1994) found evidence of microangiopathies in all patients, half of whom were under age 25, but overt peripheral vascular disease in that region has not been apparent.
Three other studies examined subclinical vascular effects of arsenic. Lagerkvist et al. (1986) detected a heightened vasospastic response to an experimental cooling of the fingers in 47 arsenic-exposed smelter workers compared with controls. Arsenic exposure of the subjects at the time of the study was estimated to be 300 µg or less per day, with a mean cumulative average uptake of 4 g. In a follow-up study of 21 of the affected workers, the vasospastic tendency displayed no change before and after 4-8 weeks of summer vacation, suggesting that cumulative rather than recent arsenic exposure was a determining factor (Lagerkvist et al. 1988). Tseng et al. (1995) used laser Doppler flowmetry to assess baseline and heat-induced cutaneous perfusion of the big toe in 45 long-term residents of the blackfoot-
Page 109
disease endemic area of Taiwan and 51 age-matched controls. All subjects were nonsmokers and were selected on the basis of the absence of any overt signs or symptoms or history of cardiac or peripheral vascular disease. The exposed subjects, with a mean age of 60 years, consumed artesian well water with high arsenic content for at least 30 years ending 20-30 years ago. The presence of cutaneous signs of chronic arsenic poisoning and the magnitude of past or current arsenic exposure were not reported. Both basal and heat-induced cutaneous perfusion was substantially lower in the formerly exposed subjects, suggesting that subclinical microcirculatory changes associated with chronic arsenic ingestion can persist long after exposure has ended.
Chen et al. (1995) recently examined the relationship between cumulative ingestion of arsenic in drinking water and the prevalence of hypertension among residents of the endemic area of blackfoot disease in Taiwan. Hypertension was defined as a systolic blood pressure equal to 160 mm Hg, a diastolic blood pressure equal to 95 mm Hg, or a history of treatment with antihypertensive drugs. Compared with several other reports of morbidity associated with chronic ingestion of arsenic in drinking water, this study was distinguished by an attempt to characterize each individual's cumulative arsenic exposure by use of detailed interviews and a geographical database of the arsenic content of the region's artesian wells. Data on 898 subjects over 30 years of age were analyzed, including 119 subjects who had never ingested artesian well water. Two analytical approaches found ingestion of arsenic to be associated with an increased risk of hypertension. In a multiple logistic regression model controlling for age, sex, diabetes, proteinuria, body-mass index, and fasting serum concentrations of triglycerides, cumulative arsenic exposure was associated with a significantly increased odds ratio for hypertension, peaking at 3.8 (95 % CI = 1.4-10.3) for subjects with arsenic exposure at 14.8-18.5 mg/L-yr compared with subjects with no exposure. Alcohol consumption, cigarette smoking, physical activity at work, and duration of dried-sweet-potato consumption as a staple food were not found to be significant risk factors. In a second analytical approach, subjects from the endemic blackfoot-disease area were found to have a 1.5-fold increase in age- and sex-adjusted prevalence of hypertension compared with residents from a nonendemic area that were studied in separate but comparable investigations. Chen et al. (1995) did not present data on the relationship between cutaneous manifestations of chronic arsenic poisoning and hypertension prevalence in their study subjects.
In 1982, the Michigan Department of Public Health conducted a pilot health study of individuals who consumed water from private wells within an area of Huron County found on a prior environmental survey to contain arsenic at a concentration of greater than 50 µg/L. Of 221 eligible residents,
Page 110
163 participated in the health study. Medical histories, limited physical examinations, and 24-hr urine collections were conducted on 53 males (mean age of 49.3 years) and 61 females (mean age of 46 years). Well-water arsenic concentrations ranged from 31 to 335 µg/L, with a median of 75 µg/L, for the 51 wells in the 60 households included in the study. The magnitude of typical daily arsenic consumption was calculated from questionnaire responses and well-water arsenic analysis, but duration of well-water use was not determined. Arsenic consumption amounts were found to be substantially higher for subjects (25 of the 114) who indicated having had "high blood pressure," but comparative exposure data, urine values, medication usage, and blood-pressure measurements were not reported. No relationship was reported between electrocardiogram findings and indices of arsenic exposure (Michigan Department of Public Health 1982).
Because hypertension and vascular occlusion are risk factors for death from ischemic heart disease or other cardiovascular illnesses, several investigators have examined the association between arsenic exposure and cardiovascular mortality. The experience in cohorts occupationally exposed to arsenic by inhalation has been inconsistent. Welch et al. (1982) found a risk ratio of 1.77 (95% CI = 1.32-2.31) for mortality from ischemic heart disease in a heavily exposed subgroup of smelter workers in Anaconda, Montana, and Wall (1980) reported a risk ratio of 1.32 (95 % CI = 1.20-1.44) for mortality from circulatory disease among smelter workers in Ronnskar, Sweden. However, no such increase has been observed in other cohort studies of arsenic-exposed smelter workers (Rencher et al. 1977; Enterline and Marsh 1982) or insecticide manufacturers (Mabuchi et al. 1980; Sobel et al. 1988). Assessing those studies and others on cardiovascular mortality in occupational cohorts exposed to arsenic by inhalation, Engel et al. (1994) noted that the risk ratio for mortality from circulatory diseases in most cases exceeds unity, a relationship that might in fact be underestimated because of the healthy-worker effect.
Cuzick et al. (1992) reported cause-specific mortality through 1990 in a cohort of 478 dermatological clinic patients who were treated with Fowler's solution, an oral medication containing 1 % potassium arsenite, between 1945 and 1969. Duration of treatment ranged from 2 weeks to 12 years. The magnitude of each subject's daily exposure was not stated but, on the basis of therapeutic practices in dermatology, can be estimated to have ranged from 5 to 10 mg of arsenite daily. The SMRs were less than 1.0 for all circulatory diseases (SMR = 0.91, 95% CI = 0.74-1.1), ischemic heart disease (SMR = 0.85, 95% CI = 0.6-1.1), and cerebrovascular diseases (SMR = 0.72, 95% CI = 0.4-1.1).
The relationship between arsenic ingestion in drinking water and
Page 111
cardiovascular-disease mortality has been examined in populations in Taiwan and the United States. Chen et al. (1988) examined the mortality experience of a cohort of 789 blackfoot-disease patients residing in four townships in southwestern Taiwan with high arsenic content in artesian wells. The diagnosis of blackfoot disease was based on a physician-confirmed finding of objective and subjective evidence of peripheral vascular insufficiency. The nonconcurrent cohort consisted of prevalent cases of blackfoot-disease patients alive on January 1, 1968, and all new cases registered after that date, followed through December 31, 1983. A total of 7,578 person-years was investigated. Death certificates and family interviews were used to determine cause of death, and SMRs for different end points were determined using age- and sex-specific mortality for all of Taiwan and, in a separate analysis, for the blackfoot-disease endemic area. In addition to finding increased SMRs for several malignancies, statistically significant SMRs of 1,243 (CIs not specified) for peripheral vascular disease and 209 (CIs not specified) for cardiovascular diseases were noted. Using the mortality of the blackfoot-disease endemic area as standard rates, the SMRs decreased to 351 for peripheral vascular disease and 160 for cardiovascular diseases. Causes of death from cardiovascular diseases in this study included endocarditis and pulmonary embolism, as well as end points more closely related to coronary atherosclerosis. SMRs for cerebrovascular disease were 118 (compared with all of Taiwan) and 107 (compared with the blackfoot-disease endemic area) and were statistically nonsignificant.
A large ecological study in the blackfoot endemic area of southwestern Taiwan (Wu et al. 1989) examined the relationship between arsenic well-water concentration and age-adjusted cause-specific mortality (as assessed from death certificates) from 1973 to 1986. A statistically increased trend was observed for increased mortality from peripheral vascular disease and cardiovascular disease with increasing median arsenic concentrations in well water in the decedent's community. The rates in the highest-arsenic-exposure areas (0.60 ppm) were approximately double those in the lowest-arsenic-exposure areas (less than 0.30 ppm). A smaller trend was observed in increased cerebrovascular accident mortality, a trend that was statistically nonsignificant.
Chen et al. (1996) reported the results of ischemic-heart-disease mortality in a prospective cohort study of 263 blackfoot-disease patients and 2,293 other residents of the blackfoot-disease endemic area. For each subject, lifetime residence histories, measurement data on arsenic in well water throughout Taiwan, and age-specific estimates of well-water consumption were used to generate exposure variables of average concentration of arsenic in drinking water and cumulative arsenic exposure from drinking water. In Cox's proportional-hazards regression analyses, the impact of arsenic exposure on
Page 112
ischemic-heart-disease mortality during an average follow-up period of 5 years (beginning in the late 1980s) was determined after adjusting for the covariates of age, sex, cigarette smoking, body-mass index, serum concentrations of cholesterol and triglycerides, hypertension, and diabetes. Relative risks were 2.46 (95% CI = 0.53-11.37), 3.97 (CI = 1.01-15.59), and 6.47 (CI = 1.8822.24) for subjects with a cumulative arsenic exposure of 0.1-9.9 mg/L-yr, 10.0-19.9 mg/L-yr, and 20.0 mg/L-yr or more, respectively, compared with those without arsenic exposure. Adjusting for cumulative arsenic consumption and other listed covariates, the relative risk associated with blackfoot disease was 2.48 (CI = 1.14-5.40).
The relationship between the prevalence of cerebrovascular disease and ingestion of inorganic arsenic in drinking water was investigated by Chiou et al. (1997) in a cross-sectional study of 8,102 men and women 40 years of age and over residing in the Layang Basin at the northeastern coast of Taiwan. Residents of this study area were reported to have commonly used shallow wells (less than 40 m deep) as a source of drinking water from the 1940s through the 1990s, when a tapwater system began to be used by some area residents. The well-water arsenic concentration in the region's villages ranged from "undetectable" to 3.59 mg/L (median values in these villages ranged from undetectable to 0.14 mg/L). Subjects were recruited from a registry of all adult residents within 18 villages, but the criteria for village selection, methods of recruitment, and percentage participation were not stated. Medical history, cerebrovascular risk factors, and detailed lifetime history of residential village-water consumption according to water source and duration were obtained by individual subject interviews. A well-water sample obtained from each household (3,901 in number) was analyzed for arsenic by hydride-generation flameless-atomic-absorption spectrophotometry. The sensitivity and precision of the laboratory analyses were not stated, a major concern given that the data analyses categorized some samples as containing more than, or less than 0.1 µg/L, a value that is usually below the limit of detection of this method (see further comments on analytical precision on page 8-5). The water analyses and lifetime water-consumption histories were used to calculate cumulative lifetime arsenic consumption from drinking water and average arsenic consumption from water for each subject. The investigators stated that cases of cerebrovascular disease identified by personal interview were verified by review of hospital medical records, according to World Health Organization (WHO) criteria. However, quantitative data were not reported on the subdivision of suspected cases into "definite stroke," "no stroke," or ''insufficient data" according to the WHO MONICA study criteria (Asplund et al. 1988), a key consideration in prevalence studies given that a recent investigation of hospital discharge summaries found a false-positive rate
Page 113
for stroke of 31.5% (Stegmayr and Asplund 1992). The basis for identifying a reported diagnostic subcategory of "cerebral infarction" in the Layang Basin cohort was not specified; that designation requires confirmation by computerized-tomography scan under the WHO MONICA criteria.
In a multivariate logistic regression model of cerebrovascular-disease prevalence, the adjusted odds ratio were 2.26 (95 % CI = 1.23-4.15) and 2.69 (95 % CI = 1.35-5.38) for cumulative arsenic exposures of 0.1-4.9 mg/L-yr and 5.0 mg/L-yr or more, respectively, compared with less than 0.1 mg/L-yr. Odds ratios were adjusted for age, sex, cigarette smoking, alcohol consumption, hypertension, and diabetes mellitus. The adjusted odds ratios were 2.53 (95% CI = 1.47-4.35), 2.78 (95% CI = 1.55-4.97), and 3.60 (95% CI = 1.83-7.11) for lifetime average drinking-water arsenic concentrations of 0.150 µg/L, 50.1-299.9 µg/L, and 300 µg/L or more, respectively, compared with less than 0.1 µg/L. Interestingly, the authors reported that although one-third of their study subjects were exposed to arsenic at an average concentration of 50 µg/L or more in well water, the age-adjusted prevalence rates of cerebrovascular disease in the entire study cohort (15.8/1,000 for males, 13.2/1,000 for females) were still similar to those of the general population in Taiwan.
Engel and Smith (1994) investigated the ecological relationship between cardiovascular mortality and arsenic in drinking water in 30 counties in the United States where the average arsenic concentration was greater than 5 µg/L. The mean arsenic concentration in the public-drinking-water-supply systems in those counties ranged from 5.4 to 91.5 µg/L. County-specific and national mortality figures from 1968 to 1984 were derived from data from the National Center for Health Statistics, and population demographics were obtained from records from the U.S. Census Bureau. In the five counties where the mean arsenic value exceeded 20 µg/L, SMRs for diseases of arteries, arterioles, and capillaries (DAAC) were 1.9 (90% CI = 1.7-2.1) for females and 1.6 (90% CI = 1.5-1.8) for males. However, mortality from ischemic heart disease for both sexes was decreased in those 5 counties and in 10 counties with mean arsenic values of 10-20 µg/L. SMRs were 0.8 (90% CI = 0.8-0.9) for females and 0.7 (90% CI = 0.7-0.7) for males. SMRs for cerebrovascular disease, all malignant neoplasms, and lung cancer were close to unity.
Hematological Effects
Acute and chronic arsenic poisoning might result in anemia, leukopenia, and thrombocytopenia. Effects on those cell lineages can be simultaneous and can appear within a week of high-dose acute arsenic poisoning. The anemia,
Page 114
a consequence of hemolysis or marrow suppression, might be normocytic or megaloblastic. Basophilic stippling of erythrocytes and erythrocyte karyorrhexis can be present on bone-marrow examination or peripheral blood smear (Kyle and Pease 1965; Lerman et al. 1980; Eichner 1984). Reticulocytosis is a common but not invariable finding. The marrow can also reveal erythroid hyperplasia, with megaloblastic features in both erythroid and myeloid maturation. Leukopenia can be characterized by neutropenia or lymphopenia, and relative eosinophilia is common (Kyle and Pease 1965; Westhoff et al. 1975; Feussner et al. 1979). In practically all cases, the hematological abnormalities are reversible; normalization of most cell lineages occurs within weeks of termination of exposure (Rezuke et al. 1991).
The hematological consequences of subacute and chronic arsenic poisoning can appear similar to that resulting from acute arsenic poisoning. Thus, Mizuta et al. (1956) detected frequent anemia and leukopenia in the peripheral blood of 32 subjects after 2-3 weeks of ingesting approximately 3 mg of arsenic per day in contaminated soy sauce. The hematological effects were characterized as "slight, " with red-blood-cell (RBC) counts of 3-4.5 x 106 and white-blood-cell (WBC) counts of 3-6 x 103. Accounts of anemia or leukopenia in patients treated with 1-10 mg of inorganic arsenic per day in the form of potassium arsenite (Fowler's solution) or arsenic sulfide have been reported (Tay and Seah 1975; Swanson and Cook 1977). Terada et al. (1962) described a characteristic pattern of anemia, leukopenia, and thrombocytopenia among 55 individuals exposed to arsenic in drinking water in the Niigata Perfecture of Japan. Underground disposal of arsenic trioxide and arsenic trisulfide from a nearby factory was the source of the exposure, which occurred for approximately 5 years (Tsuda et al. 1995). The magnitude of exposure to patients examined by Terada et al. (1962) was not specified, but the arsenic concentration of some wells in the area exceeded 1,000 µg/L, and approximately half the subjects had characteristic arsenic-related skin lesions.
Winski and Carter (1998) recently examined the effect of inorganic arsenate and arsenite on human erythrocyte morphology in an in vitro model. Slight hemolysis (approximately 1.2 %) occurred after 5 hr of incubation with 10 mM of sodium arsenate but was not detected after incubation with the same amount of sodium arsenite. Sodium arsenate induced dose-dependent echinocytic transformation beginning at a concentration of 0.1 mM, and irreversibly transformed sphero-echinocytes appeared at concentrations of 5 mM and 10 mM of arsenate. The morphological effects induced by sodium arsenite were less severe and were reported only after incubation at a concentration of 10 mM. The observed morphological changes were noted to be consistent with the effects of adenosine triphosphate (ATP) depletion, and spectrophotometric measurements confirmed that arsenate incubation induced a dose-dependent
Page 115
depletion in erythrocyte ATP at concentrations of 0.01 mM and higher. By comparison, arsenite incubation had little effect on erythrocyte ATP. Based on those measurements, the authors estimated that human erythrocytes are at least 1,000 times more sensitive to arsenate, or As(V), than to arsenite, or As(III). The results suggest that arsenic-induced changes in erythrocyte membrane integrity and deformability could contribute to microvascular occlusion and related peripheral vascular effects of chronic arsenic exposure (see Cardiovascular Effects above). However, as noted in Chapter 6, the blood arsenic concentration reported in human populations with chronic exposure to arsenic in drinking water has been on the order of 0.0001 to 0.001 mM, and therefore, the direct clinical relevance of the study is uncertain.
Rodent models of arsenic exposure have demonstrated disturbances in heme biosynthesis, characterized particularly by an increase in urinary uroporphyrin excretion (Woods and Fowler 1978; Martinez et al. 1983; Conner et al. 1995). In a study of the effect of arsenic on human urinary porphyrin excretion, Garcia-Vargas et al. (1994) compared urinary porphyrin profiles in 36 subjects consuming water with an arsenic concentration of 400 µg/L to profiles in 31 control subjects consuming water with an arsenic concentration of 20 µg/L. A linear relationship between total urinary porphyrin concentration and urinary arsenic was limited to subjects whose spot-urine arsenic concentration exceeded 1,000 µg/g of creatinine. Compared with controls, more subjects in the high-arsenic-exposure group had a coproporphyrin-to-uroporphyrin ratio of less than 1.0, as well as a decreased ratio of coproporphyrin III to coproporphyrin I. Cutaneous signs of arsenic poisoning were present in 15 of the 36 subjects from the town with high arsenic concentrations in water, but porphyrin profiles and clinical findings were not associated in the report.
Harrington et al. (1978) examined complete blood counts from 183 subjects drawn from 59 households in the Ester Dome area of Alaska where well water contained a mean arsenic concentration of 0.22 mg/L (range of 0.001 to 2.45). No associations were found between estimated daily arsenic ingestion and any hematological abnormality. Morse et al. (1979) found no hematological abnormalities, nor correlation of blood counts or white-cell differential with urinary arsenic concentrations (mean 47.5 µg/L) in 132 children (mean age 10.9 years) in the copper smelter town of Ajo, Arizona. The children were exposed to varying amounts of arsenic in drinking water and dust. Among 73 of the children consuming groundwater with a mean arsenic concentration of 0.09 mg/L, urinary arsenic concentrations measured 59.0 ± 41.9 µg/L. Dermatological and neurological examinations were negative. Southwick et al. (1983) (see Neurological Effects below) found no increased prevalence of anemia or significant difference in hematocrit in 137
Page 116
subjects from two arsenic-exposed towns in Utah (mean arsenic drinking-water concentrations of 0.180 mg/L and 0.270 mg/L) and in 100 subjects from a control town (mean arsenic drinking-water concentration of 0.019 mg/L). In the 1982 health survey of residents of Huron County, Michigan, where well-water arsenic concentrations ranged from 0.031 to 0.335 mg/L (median 0.075 mg/L) (Michigan Department of Health 1982), hemoglobin concentrations were significantly correlated with log urinary arsenic concentration (r = -0.22, p = 0.03, 103 samples), but not daily arsenic consumption (r = -0.05,p not specified, 86 samples) . Other measurements within the complete blood count (WBC, RBC, hematocrit, mean cell volume, mean cell hemoglobin, and mean cell hemoglobin concentration) were not significantly correlated with either mean well-water arsenic concentration, daily arsenic consumption, or log urinary arsenic excretion.
Pulmonary Effects
The possible role of chronic arsenic ingestion in the genesis of nonmalignant pulmonary disease has been suggested in a few case series describing medical problems among individuals chronically exposed to increased concentrations of arsenic in drinking water. Among a total study cohort of 180 residents of Antofagasta, Chile, exposed to drinking water containing arsenic at 0.8 mg/L, 38.8% of 144 subjects with "abnormal skin pigmentation" complained of chronic cough, compared with 3.1 % of 36 subjects with normal skin (Borgono et al. 1977). In autopsies of five children from the Antofagasta region with an antecedent history of cutaneous arsenicism and postmortem findings of extensive (nonpulmonary) vascular disease, two of the subjects were noted to have slight pulmonary fibrosis (Rosenberg 1974). In a study of arsenic in drinking water in West Bengal, India, Guha Mazumder et al. (1997) noted a complaint of cough in 89 (57 %) of 156 patients with arsenical hyperpigmentation. Lung-function tests performed on 17 of those patients showed features of restrictive lung disease in 9 (53%) and combined obstructive and restrictive disease in 7 (41%) (Guha Mazumder et al. 1997). The results of more-extensive investigation to characterize the nature of the pulmonary disease among patients with chronic arsenic poisoning in West Bengal have not been reported.
In an ecological investigation of the relationship of arsenic in drinking water to vascular-disease mortality in 30 U.S. counties (see above), standardized mortality ratios for both chronic airway obstruction and emphysema were significantly increased (in the range of 1.2 to 1.7) (Engel and Smith 1994). However, as noted by the authors, the lack of any significant increase in lung
Page 117
cancer or ischemic heart disease in the same counties suggests the possibility that the increased pulmonary disease mortality might not be attributable to smoking, the most common underlying cause of death from chronic obstructive pulmonary disease. In like manner, in a recent ecological investigation of mortality in a region in northern Chile, which formerly had increased concentrations of arsenic in drinking water, a striking increase was observed in chronic obstructive disease mortality that was limited to the age category of 30-39 years (Smith et al. 1998). From 1989 to 1993, four deaths occurred in men, compared with 0.8 expected deaths, and six deaths occurred in women, compared with 0.1 expected deaths. The authors noted that the decedents would have been young children during the period 1955-1970, when arsenic concentrations in the region's drinking water were at their peak (approximately 0.57 mg/L).
Immunological Effects
Immunomodulating and immunotoxic effects of arsenic have been demonstrated in several experimental models in vitro and in vivo. McCabe et al. (1983) measured the response of in vitro cultures of human and bovine peripheral lymphocytes to phytohemagglutinin (PHA) stimulation in the presence of several concentrations of sodium arsenite and sodium arsenate. At low doses of arsenite (2 x 10-6 M) and arsenate (5 x 106 M), PHA-induced stimulation of cultured human lymphocytes was increased 49% with arsenite and 19% with arsenate. Conversely, at high doses of arsenite (1.9 x 10-5 M) and arsenate (6 x 104 M), PHA-induced stimulation was completely inhibited.
Gonsebatt et al. (1992) studied the response to PHA stimulation of peripheral blood lymphocytes from healthy human volunteers incubated with arsenite and arsenate at concentrations of 10-9 M, 10-8 M, or 10-7 M, concentrations equivalent to those observed in the blood of arsenic-exposed human populations. Delays in cell-cycle kinetics, assessed by the proportion of PHA-treated cells in M1, M2, and M3 phases, were observed at all concentrations of arsenite and arsenate in a dose-dependent pattern. Arsenite and arsenate did not differ in their capacity to effect cell proliferation.
In a follow-up study, Gonsebatt et al. (1994) compared lymphocytere-plicating ability in 33 individuals consuming drinking water with a mean arsenic concentration of 412 µg/L and in 30 control subjects consuming water with a mean arsenic concentration of 37 µg/L. Arsenic concentrations from first-morning-void urine from the exposed and control groups yielded means of 758 µg/L and 37 µg/L, respectively. The peripheral blood lymphocyte
Page 118
count of the arsenic-exposed subjects was slightly increased relative to the controls (3.1 ± 1.0 x 106 versus 2.6 ± 1.1 x 106). The progression of lymphocytes from the S phase of the cell cycle to the M phase following PHA incubation was decreased in the exposed subjects, suggesting an impairment of immune response.
Sikorski et al. (1989) examined the effect of a single intratracheal dose of sodium arsenite at 10 mg/kg (the maximally tolerated dose with respect to mortality) on several immune responses in female mice. The immunoglobulin M (IgM) antibody response of splenocytes to T-dependent antigen sheep red blood cells was decreased 24 % but without a change in splenic weight or cell number. The proliferate response of splenocytes to mitogens and the mixed lymphocyte response to DBA/2 spleen cells were not affected. Arsnite diminished by 47 % the delayed hypersensitivity response to an intradermally injected antigen. The functional ability of peritoneal exudate cells to phagocytize chicken red blood cells was substantially increased. Host resistance to intraperitoneal injections of bacteria and tumor cells (B16F10 melanoma) was not decreased.
Gainer and Pry (1972) examined the effect of inorganic arsenic on host resistance to viral challenge in mice. Mice given drinking water containing 0.002 M arsenate for 2 weeks experienced a greater than 3-fold increase in mortality from a subsequent intraperitoneal challenge of pseudorabies virus. Impaired host resistance was also demonstrated in additional experiments using subcutaneous arsenic injections and intraperitoneal exposure to encephalomyocarditis virus and St. Louis encephalitis virus. Resistance to Western encephalitis virus increased when arsenic was administered at the time of viral inoculation (a possible direct antiviral effect of the arsenic) but decreased if arsenic exposure followed the inoculation by 1 day.
From the late eighteenth through the mid-twentieth century, oral preparations of inorganic arsenic were used therapeutically for a variety of health problems, particularly for such illnesses as asthma, rheumatic fever, and skin conditions (eczema and psoriasis) (Stockman 1902; Pope 1902) in which an immunosuppressant effect might have contributed to an improvement in symptoms. An immunosuppressant action might also have been responsible for a common side effect of medicinal arsenic, the development of herpes zoster (Farquharson 1880; Reynolds 1901; Costello 1942). Long-term daily ingestion of medicinal arsenite in those cases probably ranged from 5 to 10 mg (Stockman 1902; Pope 1902). An epidemic increase in the number of cases of herpes zoster led to the initial suspicion of arsenic as a cause of poisoning among several thousand beer drinkers in the Manchester beer incident of 1900 (Reynolds 1901), when arsenic ingestion might have been 1-5 mg per day for weeks or months. An increased incidence of herpes labialis has been reported
Page 119
in acute poisoning incidents from other arsenic contaminated foodstuffs, including a single acute exposure to 1-3 g of arsenic in cider (Lawson et al. 1925) or 2-3 weeks of exposure to 3 mg of arsenic per day in soy sauce (Mizuta et al. 1956).
In the 1982 Huron County, Michigan, health survey of residents exposed to arsenic in drinking water (see description in Cardiovascular Effects above), 37 of 114 adult participants (32.5%) related a history of "cold sores" (herpes labialis), making it the most common health problem reported in the study. The daily consumption of arsenic in drinking water and the log-transformed concentration of arsenic in urine were higher for the eight subjects who reported a history of shingles (herpes zoster) than for the other subjects (Michigan Department of Health 1982).
Neurological Effects
Acute inorganic arsenic intoxication that produces initial gastrointestinal or cardiovascular symptoms can be followed by the delayed onset of central or peripheral nervous-system involvement. The central-nervous-system effects, which generally occur within 1-5 days of acute poisoning, can range from headache and mild confusion to florid encephalopathy, seizures, and coma (O'Shaughnessy and Kraft 1976; Freeman and Couch 1978; Greenberg et al. 1979; Fincher and Koerker 1987; Levin-Scherz et al. 1987). Evidence of peripheral neuropathy, a more common but not invariable finding, emerges within 1-4 weeks (Heyman et al. 1956; Le Quesne and McLeod 1977; Donofrio et al. 1987). Initial neuropathic symptoms typically consist of sensory dysesthesias in a symmetric stocking-glove distribution, but can be accompanied by ascending weakness and flaccid paralysis in severe cases. Histopathological examination of the peripheral nerves is consistent with a sensorimotor axonopathy (Ota 1970; Le Quesne and McLeod 1977), although electrophysiological testing can sometimes suggest segmental demyelination (Murphy et al. 1981; Donofrio et al. 1987).
In subacute or chronic exposure, arsenic can occasionally result in subclinical or overt peripheral neuropathy in the absence of antecedent gastrointestinal or cardiovascular signs or symptoms (Buchanan 1901; Heyman et al. 1956; Feldman et al. 1979; Lagerkvist and Zetterlund 1994).
Prominent arsenical peripheral neuropathy has been reported following subacute ingestion of drinking water containing arsenic at a concentration of 10 mg/L or more. Ingestion of well water containing arsenic at concentrations of 11.8-21.0 mg/L, apparently from pesticide contamination, resulted in multisystemic illness, including neuropathy, in several subjects exposed over
Page 120
a 10-week period (Feinglass 1973). A 36-year-old male developed a slowly progressive sensorimotor peripheral axonopathy 4 months after daily consumption of well water containing arsenic of geological origin at a concentration of 25 mg/L (Kosnett and Becker 1988). Multisystemic illness, including neuropathy, occurred in a husband and wife consuming well water containing arsenic at 9.0-10.9 mg/L (Franzblau and Lilis 1989). Wagner et al. (1979) described a 41-year-old female who developed subacute multisystemic arsenical intoxication, including lower-extremity paresthesias, following 4 months of consumption of well water found to contain arsenic at a concentration of only 1.2 mg/L. However, a conservative estimate of her intake of coffee prepared from the well water was 12-14 cups per day.
The occurrence of peripheral neuropathy is inconsistent in individuals chronically exposed to arsenic in drinking water at concentrations of 0.1-1.0 mg/L. As noted and reviewed by Hotta (1989), peripheral neuropathy has not been identified as a common or significant finding in the cohorts affected by chronic arsenical dermatitis from well water in southeastern Taiwan; Cordoba, Argentina; or Antofagasta, Chile. Basu et al. (1996) recently described a sensory predominant distal polyneuropathy in eight patients with arsenical dermatoses in West Bengal, India, where there is chronic consumption of well water containing arsenic at concentrations of 0.2-2.0 mg/L. Hindmarsh et al. (1977) reported electromyographic findings in 32 of 110 members of a Canadian community consuming well water containing arsenic at a concentration of more than 0.05 mg/L (range of 0.06 to 1.40 mg/L) and in 12 control subjects who used wells with arsenic at less than 0.05 mg/L. Seven of 14 subjects exposed to well-water arsenic exceeding 0.10 mg/L had abnormal electromyograms, compared with 3 of 18 subjects exposed to well-water arsenic at 0.05-0.10 mg/L and none of the 12 control subjects.
Kreiss et al. (1983) conducted blinded clinical neurological examinations and nerve-conduction-velocity (NCV) measurements on 147 subjects from Ester Dome, Alaska, a community in which many residences use wells containing increased concentrations of arsenic (mean 0.347 mg/L; range of 0.001 to 4.78). Subjects had a mean age of 36.3 years (range of 17 to 57 years) and had lived an average of 74 months in their residence. An index of usual daily arsenic consumption in water for each subject ranged from 0.0 to 4,521 µg per day (one outlying value was 14,479 µg per day) and correlated closely with first-morning-void and spot-urine arsenic concentrations (Pearson correlation coefficient for log values = 0.730, p < 10-5). When daily water arsenic consumption was divided into three categories (0-100 µg, 101-1,000 kg, and 1,001-15,000 mg), no significant differences were found in the prevalence of subjects with an abnormal neurological examination or abnormal NCV. Neurological findings on physical examination and NCV results were
Page 121
poorly correlated. Kreiss et al. (1983) concluded that there was no evidence of arsenic-induced clinical or subclinical neuropathy in the study cohort.
Southwick et al. (1983) conducted a controlled cross-sectional study of the effects of chronic arsenic ingestion in drinking water among residents of Millard County, Utah. Participants included 145 residents of two exposed communities whose well-water arsenic concentration averaged 0.180 mg/L and 0.270 mg/L and 105 control subjects whose well water averaged 0.019 mg/L. On the basis of individual interviews, the annual arsenic ingestion in the exposed subjects was found to average 152.4 mg per year (0.42 mg per day), compared with 24.2 mg per year (0.07 mg per day) in the controls. Blinded dermatological examinations did not reveal any apparent evidence of arsenic-related skin manifestations. Among a subset of participants undergoing NCV measurements, 13 of 83 exposed subjects (15.7%) and 8 of 67 controls (11.9%) were found to have at least one nerve with abnormal conduction. Blinded neurological examinations showed that the mean NCV for each particular nerve was not significantly different in the exposed subjects and the control subjects. In a regression analysis, the annual arsenic dose was not significantly associated with NCV.
Endocrinological Effects
Inhibition of the pyruvate dehydrogenase enzyme complex and the consequent effects on carbohydrate metabolism and cellular respiration have long been recognized as major effect of acute arsenic intoxication (Stocken and Thompson 1949). Experimental studies of the effect of arsenicals on glucose metabolism have yielded considerable interspecies differences. For example, acute and subacute exposure of rats to relatively high doses of sodium arsenite (i.e., 10 mg/kg intraperitoneally for 1-7 days) has been associated with hyperglycemia and impaired glucose tolerance (Ghafghazi et al. 1980), whereas exposure of mice to a single dose of sodium arsenite (10 mg/kg intraperitoneally) induces hypoglycemia (Boquist et al. 1988). Similarly, the organic arsenical phenylarsine oxide inhibits insulin-stimulated glucose transport in the rat skeletal muscle, but increases the same process in human skeletal muscle in vitro (Carey et al. 1995). Neither hyperglycemia nor hypoglycemia has been described in human accounts of overt subacute arsenic intoxication in humans (Mizuta et al. 1956; Terada et al. 1960; Armstrong et al. 1984; Franzblau and Lilis 1989).
Lai et al. (1994) investigated the relationship between cumulative arsenic exposure from artesian well water and the prevalence of diabetes mellitus in 891 adults who were over 30 years of age and residing in the blackfoot-disease
Page 122
hyperendemic area of southeastern Taiwan in 1989. Most (78.3 %) of the subjects had begun residing in the area before 1960, when median well-water arsenic concentrations ranged from 0.70 to 0.93 mg/L. Cumulative lifetime ingestion of arsenic in drinking water (parts per million-years) could be estimated for 718 (80.6%) of the subjects. The presence of diabetes mellitus in all subjects was assessed by a fasting blood glucose test, an oral glucose tolerance test, or a history of insulin or sulfonylurea medication. In a multiple logistic regression analysis controlling for age, sex, body-mass index, and physical activity at work, the odds ratio (95% CI) for diabetes mellitus was 6.61 (0.86-51.0) and 10.05 (1.30-77.9) for cumulative arsenic exposures of 0.1-15.0 ppm-yr and more than 15.0 ppm-yr, respectively. The prevalence of diabetes mellitus in 108 unexposed subjects was only 0.9 %, considerably lower than the 5.1 % background prevalence cited by the authors as characteristic of the rural population of Taiwan at ages of 40 years or older. The study did not report a logistic model that included an apparent age-sex interaction or a continuous (rather than categorical) arsenic-exposure variable, and detailed recruitment information describing the relationship of the study subjects to the total population at risk was not supplied.
Prompted by the Taiwanese report, Swedish investigators (Rahman and Axelson 1995) examined the relationship between diabetes mellitus and arsenic exposure in two occupational cohorts. In a nested case-control study of a history of diabetes mellitus among 116 deceased male residents of a northern Swedish parish who had been employed at a copper smelter, the Mantel-Haenszel age-adjusted odds ratio associated with job-related arsenic exposure was 3.3 (95% CI = 0.5-30). Cases consisted of 12 decedents with diabetes mellitus noted in the death certificates or the employment medical records; the selected controls were 31 decedents who the authors considered to be free from diagnoses potentially related to arsenic ingestion (i.e., any cancer, cardiovascular, or cerebrovascular disease). The odds ratios associated with increasing arsenic exposure categories were (reference level, 1)2.0, 4.2, and 7.0. The 95 % CIs for each strata of exposure included unity; however, the test for trend was statistically significant (X2(1) = 4.68, p = 0.03). Rahman et al. (1996) conducted a case-control study of diabetes mellitus as an underlying or contributing cause of death between 1950 and 1982 of 5,498 decedents who resided in the glass-producing area of southeastern Sweden. Controls were decedents without any indication on their death certificates of cancer, cardiovascular disease, or diabetes. A history of employment as a glassblower, (glass) foundry worker, or unspecified glass worker (job categories with probable arsenic exposure) was associated with a Mantel-Haenszel odds ratio for diabetes mellitus of 1.4 (95 % CI = 0.92-2.2; 209 subjects). The unspecified glass workers, thought to include persons with high arsenic exposure,
Page 123
carried the highest risk (Mantel-Haenszl odds ratio 1.8, 95% CI = 1.1-2.8).
Rahman et al. (1998) conducted a cross-sectional study in western Bangladesh of the prevalence of diabetes mellitus among residents who had hyperkeratosis attributed to consumption of tube well water containing increased concentrations of arsenic. Arsenic-exposed subjects 30 years of age or older with keratoses (163 subjects) were recruited by a door-to-door survey in seven villages known to have arsenic contaminated drinking water, and unexposed subjects (854 subjects) were recruited by a similar door-to-door method in four suburbs of Dhaka, which has no arsenic contamination (i.e., arsenic in drinking water was less than 0.01 mg/L). The presence of diabetes mellitus was determined by a history of symptoms, previously diagnosed diabetes, glucosuria, and blood glucose after oral glucose challenge. There were 21 confirmed cases of diabetes mellitus in the 163 arsenic-exposed subjects with keratosis. The crude prevalence ratio for diabetes mellitus among the arsenic exposed subjects with keratosis was 4.4 (95 % CI = 2.5-7.7), which increased to 5.2 (95% CI = 2.5-10.5) after adjustment for age, sex, and body-mass index. When current well-water arsenic content and historical well-usage data were used to estimate the time-weighted-average drinking-water arsenic concentration, a dose-response pattern emerged. Using the control population's age- and sex-adjusted prevalence as a reference (1.0), the prevalence ratios for diabetes mellitus were calculated to be 2.6, 3.9, and 8.8 for time-weighted-average arsenic-exposure categories of less than 0.5 mg/L, 0.5-1.0 mg/L, and greater than 1.0 mg/L.
Reproductive and Developmental Effects
Epidemiological Associations
Very few studies have been conducted on arsenic and reproductive success in humans, and nothing conclusive can be stated from those studies. Nordstrom et al. (1978a,b) reported an increase in spontaneous abortions and low birth weight in populations living near a copper smelter, which emitted considerable arsenic. Tabacova et al. (1994) reported similar findings in Bulgaria, where pregnancy complications and perinatal deaths attributable to congenital malformations were higher in women living near a copper smelter than in those living in a less-exposed region (as cited in Zelikoff et al. 1995). However, copper smelters are also a source of other metals, including mercury, lead, zinc, and copper. Some reports have made associations between arsenic concentration in drinking water and adverse pregnancy outcomes and birth defects. Reported effects include coarctation of the aorta, but not other
Page 124
heart abnormalities (Zierler et al. 1988), and spontaneous abortion (Aschengrau et al. 1989). On the other hand, Swan et al. (1995) reported no association between occupational exposure to arsenic in the semiconductor industry and miscarriage.
Investigators in Hungary reported a correlation between increased concentrations of arsenic in well water (60-270 µg/mL) and increased incidence of spontaneous abortion and perinatal death without an increase in premature births from 1980 to 1987 (Dési 1992). The arsenic content of the drinking water in the affected region has subsequently been lowered. Another series of evaluations of demographic data from 1970 to 1987 indicated that frequency of stillbirths and spontaneous abortions was increased in a population drinking from wells with arsenic concentrations exceeding 100 µg/L (Börzsönyi et al. 1992; Rudnai et al. 1996).
Animal Data
Arsenic has been shown to be teratogenic and embryotoxic in several commonly used animal models, including rats, mice, hamsters, rabbits, and chicks. The syndrome of malformations includes neural tube, eye, skeletal, and urogenital abnormalities. This syndrome is reasonably consistent across species, although the incidence varies. The potency of the different forms of arsenic also varies across species. The inorganic forms, arsenite and arsenate, are more toxic than the methylated forms.
Arsenic teratogenicity was first reported in studies on chick embryos in which tail-bud abnormalities were observed after arsenic administration (Ancel and Lallemand 1941). The teratogenic potential of arsenic in mammals was investigated almost three decades later. Ferm and Carpenter (1968) demonstrated that intravenous administration of sodium arsenate to pregnant golden hamsters on gestation day 8 produced exencephaly (a neural-tube defect comparable to anencephaly in humans) in embryos. Exposure at 20 mg/kg produced exencephaly in 66% of the embryos. That concentration also produced a 35% incidence of embryo lethality; 40 mg/kg was lethal to 100% of the embryos. Sodium arsenate administered at 5 mg/kg did not produce malformations, although the resorption (embryonic death) incidence of 16% was somewhat higher than that in the control group. Definitive conclusions cannot be drawn from that experiment because only three litters were evaluated. A repetition of the experiment using 20 mg/kg intravenously on gestation day 8 confirmed the previous results; 49% of the embryos in the later experiment were malformed (Holmberg and Ferm 1969).
Ferm et al. (1971) extended those observations in hamsters by intravenous
Page 125
administration of sodium arsenate at 15-25 mg/kg in the morning, afternoon, or evening of gestation day 8. In addition to exencephaly and encephalocele (another defect of the developing central nervous system), renal agenesis and other urogenital abnormalities, rib malformations, cleft lip and palate, and anophthalmia were observed. Neural-tube defects and rib abnormalities decreased with administration later on day 8, and urogenital anomalies occurred at roughly the same rate irrespective of administration time. Constant infusion of arsenate beginning on one of gestation days 4-7 and continuing until at least day 9 produced comparable malformations (Ferm and Hanlon 1985). The malformations were correlated with dose but not with duration of exposure.
The developmental toxicity of arsenite has also been evaluated in hamsters. Arsenite was more embryotoxic than arsenate: an intravenous injection of sodium arsenite at 10 mg/kg on gestation day 8 killed 90% of the embryos; at 20 mg/kg, sodium arsenate killed 44% (Willhite 1981). Sodium arsenite exposure at 2 mg/kg resulted in only a 4% incidence of resorptions (not different from background) but still produced malformations. The syndrome of malformations was comparable to that produced by arsenate. Oral gavage of sodium arsenite at 25 mg/kg on gestation day 8, 11, or 12 increased the death rate in fetuses exposed on gestation day 8 or 12 and decreased the weights of the surviving fetuses exposed on day 12 (Hood and Harrison 1982).
Mice are also sensitive to the developmental effects of arsenic. Intraperitoneal injection of sodium arsenate at 45 mg/kg on one of gestation days 6-12 in Swiss-Webster mice caused terata, resorptions, and decreased fetal weight. Resorption incidence was highest with later exposure and was as high as 78 % on gestation day 12. Malformation incidence was highest in the litters of animals exposed on day 9-63 % of the live fetuses had malformations. The spectrum of malformations was dependent on gestational stage at exposure and included exencephaly, hydrocephalus, anophthalmia, micrognathia, cleft lip, micromelia, ectrodactyly, and various skeletal abnormalities (Hood and Bishop 1972). The authors reported that exposure at 25 mg/kg had no effect. The same laboratory compared the effects of oral gavage and intraperitoneal administration of sodium arsenate in mice. Arsenate was given intraperitoneally at a dose of 40 mg/kg or by mouth at a dose of 120 mg/kg to CD-1 mice on one of gestation days 7-15. Those doses produced comparable maternal toxicity, and preliminary results with lower oral doses indicated that up to 100 mg/kg was without developmental effect. The intraperitoneal administration produced results that were comparable to the earlier study. Oral exposure to arsenate significantly decreased fetal weight when administered on gestation days 10, 11, or 15 but not on other days. The only statistically significant increase in resorption incidence was in the group exposed on
Page 126
day 11. No significant increases in malformation rates occurred after oral exposure (Hood et al. 1978).
The developmental toxicity of arsenic acid (the acid form of arsenate) was evaluated in mice using the regulatory standard Segment II testing protocol, which involves dosing pregnant animals daily through the period of major organogenesis, occurring on days 6-15 in this species (Nemec et al. 1998). The compound was administered by gavage at doses of 7.5, 24, and 48 mg/kg per day. The highest concentration was maternally toxic and increased the rate of resorptions and decreased fetal weight. There was a 2.03 % malformation incidence in that group compared with a 0.98% incidence in controls. The malformations were exencephaly and thoracogastroschisis. No significant effects were observed at the two lowest concentrations, although one case of exencephaly and two of thoracogastroschisis occurred in those groups. Neither malformation was observed in the controls. The authors concluded that these malformations were not treatment-related and that the no-observed-adverse-effect level (NOAEL) for developmental effects for arsenic acid is 7.5 mg/kg per day (equivalent to arsenic at 3 mg/kg per day). In a separate review, Golub (1994) concluded that the malformations were attributable to arsenic and that the NOAEL was less than that value. Although these conflicting opinions cannot be resolved in this report, it is clear that the NOAEL for arsenic must be close to 3 mg/kg. The effects of arsenic acid were also evaluated in a two-generation study in mice in which it was added to the diet at concentrations of 20, 100, and 500 ppm (approximate daily doses of 0.5, 2.7, and 13.3 mg/kg) (Hazelton Laboratories 1990, as cited in Golub et al. 1998). The highest concentration was severely maternally toxic and had profound effects on litter size, offspring growth, and viability. The intermediate dose resulted in a mild (approximately 10%) growth retardation in offspring during the suckling period, and the lowest concentration was without effect. The fertility of adults appeared to be unaffected.
Recently completed studies reported in abstract form describe the results of oral and inhalation developmental toxicity tests using arsenic trioxide in rats. In the inhalation test, arsenic trioxide was administered at concentrations up to 10 mg/m3 for 6 hr per day, 7 days per week, beginning 14 days before mating and continuing throughout gestation. No effect on any developmental measurement was observed (Stump et al. 1998a). In the oral test, arsenic trioxide was again administered 14 days before mating and daily throughout gestation. A concentration of 10 mg/kg per day caused a decrease in fetal weight and an increase in two skeletal variants; that dose also produced some maternal toxicity. A concentration of 5 mg/kg per day was without observable developmental or maternal effect (Stump et al. 1998b). In a recent review Golub et al. (1998) confirmed that the primary effect of arsenic was increased prenatal mortality and decreased fetal weight.
Page 127
Arsenite was more potent than arsenate in the mouse. Intraperitoneal injection of sodium arsenite at 10-12 mg/kg to Swiss-Webster mice on one of gestation days 7-12 increased the resorption rate at all injection times, decreased fetal weight after injection on gestation days 7-11, and increased malformation incidence especially after injection on days 9 and 10. Malformations were similar to those seen after arsenate exposure: exencephaly, open eyes, and micrognathia; and rib, vertebral, and tail defects (Hood 1972). Sodium arsenite was given by gavage to CD-1 mice on one of days 8-15 of gestation at doses of 20, 40, or 45 mg/kg. The lowest dose was reported to be without effect. The two highest doses produced a 19% and 36% incidence of maternal deaths, respectively, and also decreased fetal weights and increased resorption incidence. There was also a small increase above the control rate in malformations (exencephaly and open eyes) after dosing on gestation days 8-10 (Baxley et al. 1981). In a multigeneration study in which arsenite at a concentration of 5 ppm was added to the diets of CD mice for three generations, fertility or generation time were not affected, the number of offspring per litter decreased slightly, abnormalities were not observed, and the sex ratio was skewed in favor of males in one of the three generations (Schroeder and Mitchener 1971). Because numerous other studies examining the developmental effects of arsenic have not noted anything similar to the last observation, it should be viewed as uncorroborated and unlikely.
Beaudoin (1974) confirmed the teratogenicity of intraperitoneal sodium arsenate in the rat. Doses of 20-50 mg/kg given to Wistar rats on one of gestation days 7-12 were evaluated. Embryo lethality was complete at 50 mg/kg. The lowest doses also increased the resorption rate and the malformation rate in surviving fetuses. The highest rate of malformation was after dosing on gestation day 9. Eye defects, exencephaly, renal agenesis, gonadal agenesis, and rib and vertebral abnormalities were the most commonly observed malformations.
A Segment II study was conducted in rabbits using gavaged arsenic acid at doses of 0.19, 0.75, and 3.0 mg/kg per day on gestation days 6-18 (Nemec et al. 1998). Seven of 18 animals in the highest-dose group died as a result of exposure. No effects on fetal weight were observed, and malformation rates were low in all groups. One case of renal agenesis was observed at the highest concentration, and three cases (all from the same litter) of fused ribs were observed at the intermediate concentration. Those effects are consistent with the effects of arsenic in other species but are also observed spontaneously in rabbits, particularly fused ribs, which occur at a mean rate of 0.3% of fetuses in 225 studies compiled from industry and contract laboratory records from 1989 to 1992 (MARTA 1996). Therefore, 1 mg/kg per day is a NOAEL for developmental toxicity in the rabbit study.
Page 128
The developmental effects of other forms of arsenic have been studied. Arsine gas had no developmental effects in rats or mice exposed at up to 2.5 ppm by inhalation for 6 hr per day on gestation days 6-15, even though evidence of hemolysis was observed in the dams exposed at the highest concentration (Morrissey et al. 1990). Dimethylarsinic acid (cacodylic acid) administered by gavage to rats and mice on gestation days 6-15 was developmentally toxic. All the doses administered to mice (200-600 mg/kg per day) decreased maternal weight gain, and the highest dose produced over 50% maternal mortality. Developmental effects including decreased fetal weight and increased incidence of cleft palate at 400 and 600 mg/kg per day. Maternal weight gain in rats decreased at 40 mg/kg per day and above, and lethality began at 50 mg/kg per day. Fetal weight decreased at 40 mg/kg per day, and variations in palate structure were noted at 15 mg/kg per day and above but not at 7.5 mg/kg per day (Rogers et al. 1981). Variations of palate structure are considered within the range of normal variability in response to a stressor and are typically classified as nonadverse effects. Neither monomethylarsonic acid (MMA) nor dimethylarsinic acid (DMA) produced developmental toxicity in hamsters (Willhite 1981).
In summary, the animal data indicate that arsenic in a variety of forms has the potential to cause developmental toxicity, including malformations, in a variety of species. The spectrum of malformations that has been produced is remarkably consistent across species. Arsenite has been repeatedly shown to be more potent than arsenate, and organic forms appear to be considerably less potent than the inorganic forms. Along with other manifestations of developmental toxicity, malformation rates are highest after parenteral administration but have been observed after oral administration. The test regimen in a two-generation study in mice in which arsenic was administered in the diet as arsenic acid probably represents the closest of any of the test regimens to expected chronic human exposure to arsenic in drinking water, although it should be noted that mice convert organic arsenic to DMA much more efficiently than do humans (see Chapter 5). In that study, arsenic at a concentration of 20 ppm (approximately 0.5 mg/kg per day) was clearly without effect, and 100 ppm (approximately 2.65 mg/kg per day) had a reversible effect on growth during early life. Fertility of adult animals appears to be unaffected, although assessment of adult reproductive health has not been comprehensive. In marmoset monkeys, which do not methylate inorganic arsenic (Vahter et al. 1982), and in mice and hamsters, which are efficient in arsenic methylation (Lindgren et al. 1982), arsenic accumulates in the epididymis. In contrast to the rodents, the marmoset accumulated arsenic in the testis as well. Thus, there is reason to investigate the effects of arsenic exposure on male reproductive health.
Page 129
Placental Transfer
Arsenic appears to move freely across the placenta, and substantial concentrations of arsenic have been measured in the embryo after oral or intraperitoneal administration of arsenate or arsenite in mice (Hood et al. 1987, 1988). This transfer occurs during all stages of gestation. During the embryonic period in the mouse and marmoset, accumulation of arsenic is especially great in the neuroepithelium, as assessed by autoradiography. During the fetal period, arsenate, but not arsenite, is distributed to developing bone, and both forms are distributed to the skin, liver, and gastrointestinal tract (Lindgren et al. 1984). The distribution of arsenic to those organs might be attributable to the in vivo reduction of arsenate to arsenite (see Chapter 5).
Recent studies found that arsenic readily crosses the human placenta, giving rise to arsenic concentrations that are about as high in cord blood as in maternal blood (Concha et al. 1998). However, more than 90% of the arsenic in plasma and urine was in the form of DMA, a percentage that was significantly higher in pregnant women than in nonpregnant women. That finding indicated an increased methylation of arsenic during pregnancy. Thus, the fetus seems to be exposed mainly to DMA, at least at term. As mentioned above, animal data indicate that less developmental toxicity is caused by the methylated metabolites of arsenic than by arsenite.
Pathogenesis and Possible Mechanisms of Teratogenesis
A few studies have evaluated the pathogenesis and potential mechanisms of action of arsenic-induced teratogenesis. Observations in hamster embryos several hours after parenteral administration of arsenate showed a delay in the elevation of the neural folds (Willhite 1981; Carpenter 1987), an event that precedes neural-tube closure. A paucity of cephalic mesoderm was also reported (Willhite 1981), along with a shortening and abnormal folding of the notochord (Marin-Padilla 1979), which is the structure that organizes and induces the neural plate, the precursor of the central nervous system. In addition to those effects on the mesoderm, the neuroepithelium was reported to thin in response to arsenate exposure (Marin-Padilla 1980). That effect was also observed in rat embryos, in which an increase in apoptosis of the neuroectoderm was observed several hours after maternal arsenate exposure (Takeuchi 1979).
Histological studies of the developing urogenital system in rat embryos after maternal arsenate exposure revealed that the first observable change is a retardation in the growth of the mesonephric duct. The retardation led to absence of the ureteric bud (which arises from the mesonephric duct) and
Page 130
resulted in the absence of the vas deferens, seminal vesicle, and part of the epididymis (Burk and Beaudoin 1977).
Neural-tube closure and mesonephric-duct elongation involve morphogenetic movements affected by cytoskeletal elements and cell-adhesion molecules. The function of these proteins is dependent in part on ionic and sulfhydryl interactions, both of which are known to be affected by arsenic. Therefore, it would be an interesting hypothesis to test for altered function of these molecules in arsenic-exposed embryos.
The adverse developmental effects of arsenic were frequently observed in conjunction with maternal toxicity. There are instances in which the maternal toxicity, and not the exogenous insult per se, is the causative factor in abnormal development (Daston 1994). One common mechanism by which that occurs is through the induction of metallothionein in the maternal liver, leading to a systemic redistribution of zinc and a transitory, but developmentally adverse, embryonic zinc deficiency. Arsenate has produced those effects in the pregnant rat (Taubeneck et al. 1994). However, explanted rodent embryos exposed to arsenic in the absence of the maternal system developed abnormally (Chaineau et al. 1990; Mirkes and Cornel 1992; Zelikoff et al. 1995), indicating that arsenic exposure has direct effects. In addition, Golub (1994) thoroughly analyzed the literature for maternal- and developmental-toxicity relationships and found the correlation to be less than perfect. Therefore, arsenic is likely to have direct embryotoxicity in vivo, but its effects might be exacerbated by maternal toxicity.
Summary And Conclusions
Ingestion of inorganic arsenic is an established cause of skin cancer. Recent studies strengthen the evidence that ingestion of arsenic can also cause cancers of the lung and the urinary bladder. Based on findings of increased risks of bladder- and lung-cancer mortality in three countries (Taiwan, Argentina, and Chile), the subcommittee believes that the evidence is now sufficient to include bladder and lung cancer among the cancers that can be caused by ingestion of inorganic arsenic. With minor exception, the epidemiological evidence for cancer comes from places where exposed populations were exposed to arsenic concentrations in drinking water of at least several hundred micrograms per liter. Few data address the degree of cancer risk at lower concentrations of ingested arsenic. Studies of lung cancer among workers exposed to airborne arsenicals indicate a linear dose-response relationship over a broad range of exposure.
Increased risks of other cancers, such as kidney and liver cancer, have
Page 131
Increased risks of other cancers, such as kidney and liver cancer, have also been reported. However, the strength of the association for these sites is not as strong as for lung and bladder cancers, and increased risk for some have not been noted in all studies. Thus, further confirmatory studies are needed to establish arsenic as a cause of the other cancers.
Data derived from population-based studies, clinical-case series, and case reports relating to the ingestion of inorganic arsenic in drinking water, medications, or contaminated food or beverages show the capacity of arsenate and arsenite to adversely affect multiple-organ systems. The clinical appearance of the noncancer manifestations of arsenic intoxication in humans is dependent on the magnitude of the dose and the time course of exposure. Although the toxicokinetic and toxicodynamic interaction between those two measures has not been well characterized, several general findings emerge from the available data. Diffuse or spotted hyperpigmentation, the initial nonmalignant cutaneous effect of chronic arsenic ingestion, can first appear within 6 months to 3 years of chronic ingestion at concentrations in excess of approximately 0.04 mg/kg per day. Lower exposure rates, on the order of 0.01 mg/kg per day or higher, can also result in hyperpigmentation after intervals as long as 5 to 15 years. Palmar-plantar hyperkeratoses, the other principal nonmalignant cutaneous manifestation of chronic arsenic exposure, usually follows the initial appearance of arsenical hyperpigmentation within a period of years.
Weeks to months of ongoing ingestion of inorganic arsenic at doses of approximately 0.04 mg/kg per day or higher can result in overt nonspecific gastrointestinal complaints, such as diarrhea and cramping, and hematological effects, including anemia and leukopenia. A sensory predominant axonal peripheral neuropathy can also occur after months to years of this level of exposure. Such gastrointestinal, hematological, and neurological effects generally improve or resolve following cessation of exposure. Irreversible noncirrhotic portal hypertension appears to have occurred after years of arsenic ingestion at concentrations of 0.01 to 0.02 mg/kg per day or higher. Recent studies from Mexico suggest that those low concentration rates can also perturb porphyrin metabolism; however, the impact of this disturbance on clinical function is not fully determined.
Peripheral vascular disease has been associated with chronic arsenic ingestion in epidemiological studies conducted in populations exposed via drinking water in Taiwan. Clinical-case series and case reports also linked arsenic ingestion to peripheral vascular disease in subjects exposed to arsenic in drinking water in Latin America and northern Mexico and to arsenic from multiple sources in Japan and Germany (Moselle vintners). The patients with peripheral vascular disease were drawn from populations in areas where arsenic exposure occurred over a period of years at concentrations sufficient
Page 132
to result in cutaneous manifestations. However, data on the latency and progression of arsenic-induced peripheral vascular disease remain sparse.
Recent epidemiological investigations in the arsenic-affected areas of southwestern Taiwan associated cumulative arsenic ingestion with a risk of hypertension and cardiovascular disease mortality. Although investigators estimated individual arsenic doses in several studies, the reports do not reveal the extent of the cardiovascular risk, in the absence of cutaneous effects, from exposure to low concentrations of arsenic. A small cross-sectional study conducted in Michigan suggested a possible link between chronic exposure to low concentrations of arsenic and hypertension. However, a cohort study of individuals exposed to higher concentrations of arsenic in medication revealed no evidence of increased cardiovascular mortality. Cohort studies of occupational arsenic exposure have suggested a small increase in cardiovascular-related mortality, but the relationship has not been found consistently. Recently, a study conducted in the Layang Basin of northeastern Taiwan reported that chronic ingestion of groundwater containing arsenic at a concentration as low as 0.1 to 50 µg/L was associated with an increased prevalence of cerebrovascular disease. However, uncertainties relating to that study's design and negative findings in other cohorts require that the relationship between arsenic exposure and cerebrovascular disease undergo further evaluation.
Recent studies in southwestern Taiwan and Bangladesh associated chronic arsenic ingestion in drinking water with an increased risk of diabetes mellitus. The study subjects were drawn from populations with overt cutaneous signs of arsenic intoxication; information is lacking on the magnitude of the potential risk associated with exposure to low concentrations of arsenic. Two small Swedish case-control studies suggested that arsenic exposure of smelter workers and art glass workers might also be associated with an increased risk of diabetes mortality.
Inorganic arsenic has been shown to have immunomodulating and immunotoxic effects in experimental models. Subacute or chronic arsenic exposure at high doses (more than 0.05 mg/kg per day) has been associated with a decline in peripheral leukocytes, and as discussed in Chapter 8, the capacity of arsenic to suppress aspects of the immune response might have formed the basis for its former use as a therapeutic agent. The potential effect of exposure to low concentrations of arsenic on immune function has not been adequately investigated in field research; however a small cross-sectional study from Michigan is consistent with an immunomodulating effect. The potential effect of ingested arsenic on respiratory function has been suggested by studies from Chile, West Bengal, and the United States, but the specific pathology of the effect has not been investigated.
Page 133
Arsenic administered parenterally has been shown to be teratogenic in a number of mammalian species, but there is little evidence to suggest teratogenicity by oral or inhalation routes. Although some studies show an association between arsenic exposure and adverse pregnancy outcomes, they are inadequate to draw firm conclusions. No effects on fertility were observed in a multigeneration study in mice; however, arsenic does accumulate in the epididymides of hamsters, mice, and marmosets and in the testes of marmosets, suggesting that potential reproductive effects of arsenic should be investigated further.
Recommendations
Epidemiological studies are needed to characterize the dose-response relationship for arsenic-associated cancer and noncancer end points, especially at low doses. Such studies are of critical importance for improving the scientific validity of risk assessment. With respect to cancer, studies are recommended to define the dose-response relationship between arsenic ingestion and cancer of the skin, bladder, and lung, and to investigate the effect of arsenic on cancer at other sites. With respect to noncancer effects, particular emphasis should be placed on epidemiological study of arsenic-associated cutaneous effects, cardiovascular and cerebrovascular disease, diabetes mellitus, and adverse reproductive outcomes. Data related to latency and the relationship between magnitude of dose and time course of exposure should be obtained. Other studies of less critical importance but nonetheless needed to fill important data gaps include the following:
—Detailed clinical studies and preliminary epidemiological studies to better characterize the effect of low-to-moderate chronic arsenic exposure (0.01 to 0.03 mg/kg per day) on immune function, porphyrin metabolism, and respiratory function.
—Studies investigating the possible role of certain nonmalignant arsenic-associated effects as biomarkers of susceptibility to arsenic-induced malignancies. Such effects could include specific cutaneous manifestations or elaboration of specific gene products.
References
Alain, G., J. Tousignant, and E. Rozenfarb. 1993. Chronic arsenic toxicity. Int. J. Dermatol. 32:899-901.
Ancel, P., and S. Lallemand. 1941. Sur l'arret de developpement du borgeon caudal obtenu experimentalement chez 1'embryon de poulet. Arch. Phys. Biol. 15:27-29.
Page 134
Armstrong, C.W., R.B. Stroube, T. Rubio, E.A. Siudyla, and G.B. Miller, Jr. 1984. Outbreak of fatal arsenic poisoning caused by contaminated drinking water. Arch. Environ. Health 39:276-279.
Aschengrau, A., S. Zierler, A. and Cohen. 1989. Quality of community drinking water and the occurrence of spontaneous abortion. Arch. Environ. Health 44:283-290.
Asplund, K., J. Tuomilehto, B. Stegmayr, P.O. Wester, and H. TunstallPedoe. 1988. Diagnostic criteria and quality control of the registration of stroke events in the MONICA project. Acta. Med. Scand. 728(Suppl.):26-39.
Axelson, O., E. Dahlgren, C.D. Jansson, and S.O. Rehnlund. 1978. Arsenic exposure and mortality: A case-referent study from a Swedish copper smelter. Br. J. Ind. Med. 35:8-15.
Basu, D., J. Dasgupta, A. Mukherjee and D.N. Guha Mazumder. 1996. Chronic neuropathy due to arsenic intoxication from geo-chemical source-A five-year follow-up. JANEI. 1:45-47.
Bates, M.N., A.H. Smith, and K.P. Cantor. 1995. Case-control study of bladder cancer and arsenic in drinking water. Am. J. Epidemiol. 141:523-530.
Baxley, M.N., R.D. Hood, G.C. Vedel, W.P. Harrison, and G.M. Szczech. 1981. Prenatal toxicity of orally administered sodium arsenite in mice. Bull. Eviron. Contam. Toxicol. 26:749-756.
Beaudoin, A.R. 1974. Teratogenicity of sodium arsenate in rats. Teratology 10:153-157.
Black, M.M. 1967. Prolonged ingestion of arsenic. Pharm. J. (Dec. 9):593597.
Boquist, L., S. Boquist, and I. Ericsson. 1988. Structural B-cell changes and transient hyperglycemia in mice treated with compounds inducing inhibited citric acid cycle enzyme activity. Diabetes 37:89-98.
Borgono, J.M., and R. Greiber. 1971. Epidemiological study of arsenicism in the city of Antofagasta [in Spanish]. Rev. Med. Chil. 99:702-707.
Borgono, J.M., P. Vicent, H. Venturino, and A. Infante. 1977. Arsenic in the drinking water of the city of Antofagasta: Epidemiological and clinical study before and after the installation of the treatment plant. Environ. Health Perspect. 19:103-105.
Börzsönyi, M., A. Berecsky, P. Rudnai, M. Csanady, and A. Horvath. 1992. Epidemiological studies on human subjects exposed to arsenic in drinking water in southeast Hungary. Arch. Toxicol. 66:77-78.
Buchanan, R.J.M. 1901. Cases of arsenical peripheral neuritis. Lancet (Jan. 19): 170-172.
Burk, D., and A.R. Beaudoin. 1977. Arsenate-induced renal agenesis in
Page 135
rats. Teratology 16:247-259.
Butzengeiger, K.H. 1940. Uber periphere Zirkulationsstorungen bei chronischer Arsenvergiftung. Klin Wochenschr. 22:523-527.
Byron, W.R., G.W. Bierbower, J.B. Brouwer, and W.H. Hansen. 1967. Pathologic changes in rats and dogs from two-year feeding of sodium arsenite or sodium arsenate. Toxicol. Appl. Pharmacol. 10:132-147.
Carey, J.O., J.L. Azevedo, P.G. Morris, W.J. Pories, and G.L. Dohm. 1995. Okadaic acid, vanadate, and phenylarsine oxide stimulate 2-deoxy-glucose transport in insulin-resistant human skeletal muscle. Diabetes 44:682-688.
Carpenter, S.J. 1987. Developmental analysis of cephalic axial dysraphic disorders in arsenic-treated hamster embryos. Anat. Embryol. 176:345365
Cebrian, M. 1987. Some Potential Problems in Assessing the Effects of Chronic Arsenic Exposure in North Mexico [preprint extended abstract]. Preprint of paper presented at the 194th National Meeting of the American Chemical Society, Aug. 30-Sept. 4, 1987, New Orleans, La.
Cebrian, M.E., A. Albores, M. Aguilar, and E. Blakely. 1983. Chronic arsenic poisoning in the north of Mexico. Hum. Toxicol. 2:121-133.
Chaineau, E., S. Binet, D. Pol, B. Chatellier, and V. Meininger. 1990. Embryotoxic effects of sodium arsenite and sodium arsenate on mouse embryos in culture. Teratology 41:105-112. Chakraborty, A.K., and K.C. Saha. 1987. Arsenical dermatosis from tubewell water in West Bengal. Indian J. Med. Res. 85:326-334.
Chen, C.J. 1990. Blackfoot disease [letter]. Lancet 336(8712):442.
Chen, C.J., and C.J. Wang. 1990. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 50:5470-5474.
Chen, C.J., Y.C. Chuang, T.M. Lin, and H.Y. Wu. 1985. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: High-arsenic artesian well water and cancers. Cancer Res. 45:5895-5899.
Chen, C.J., M.M. Wu, S.S. Lee, J.D. Wang, S.H. Cheng, and H.Y. Wu. 1988. Atherogenicity and carcinogenicity of high-arsenic artesian well water. Multiple risk factors and related malignant neoplasms of blackfoot disease. Arteriosclerosis 8:452-460.
Chen, C.J., Y.M. Hsueh, M.S. Lai, M.P. Shyu, S.Y. Chen, M.M. Wu, T.L. Kuo, and T.Y. Tai. 1995. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension 25:53-60.
Chen, G.Q., J. Zhu, X.G. Shi J.H. Ni, H.J. Zhong, G.Y. Si, X.L. Jin, W. Tang, X.S. Li, S.M. Xong, Z.X. Shen, G.L. Sun, J. Ma, P. Zhang, T.D. Zhang, C. Gazin, T. Naoe, S.J. Chen, Z.Y. Wang, and Z. Chen. 1996.
Page 136
In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RARa/PML proteins. Blood 88:1052-1061.
Chen, K.P., H.Y. Wu, and T.C. Wu. 1962. Epidemiologic studies on blackfoot disease in Taiwan. 3. Physicochemical characteristics of drinking water in endemic blackfoot disease areas. Mem. Coll. Med. Natl. Taiwan Univ. 8:115-129.
Chiou, H.Y., Y.M. Hsueh, K.F. Liaw, S.F. Horng, M.H. Chiang, Y.S. Pu, J.S. Lin, C.H. Huang, and C.J. Chen. 1995. Incidence of internal cancers and ingested inorganic arsenic: A seven-year follow-up study in Taiwan. Cancer Res. 55:1296-1300.
Chiou, H.Y., Y.M. Hsueh, L.L. Hsieh, L.I. Hsu, Y.H. Hsu, F.I. Hsieh, M.L. Wei, H.C. Chen, H.T. Yang, L.C. Leu, T.H. Chu, C. Chen-Wu, M.H. Yang, and C.J. Chen. 1997. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat. Res. 386:197207.
Concha, G., G. Vogler, D. Lezeano, B. Nermell, and M. Vahter. 1998. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 44:185-190.
Conner, E.A., H. Yamauchi, and B.A. Fowler. 1995. Alterations in the heme biosynthetic pathway from the III-V semiconductor metal, indium arsenide (InAs). Chem.-Biol. Interact. 93:273-285.
Costello, M.J. 1942. Herpes zoster following administration of mapharsen. Arch. Dermatol. Syphilol. 45:1188-89.
Cowlishaw, J.L., E.J. Pollard, A.E. Cowen, and L.W. Powell. 1979. Liver disease associated with chronic arsenic ingestion. Aust. NZ J. Med. 9:310-313.
Cox, D.R. 1972. Regression models and life-tables. J. R. Stat. Soc. Ser. B 34:187-220.
Cuzick, J., S. Evans, M. Gillman, and D.A. Price Evans. 1982. Medicinal arsenic and internal malignancies. Br. J. Cancer 45:904-911.
Cuzick, J., R. Harris, and P.S. Mortimer. 1984. Palmar keratoses and cancers of the bladder and lung. Lancet i(8376): 1530-1533.
Cuzick, J., P. Sasieni, and S. Evans. 1992. Ingested arsenic, keratoses, and bladder cancer. Am. J. Epidemiol. 136:417-421.
Daston, G.P. 1994. Relationships between maternal and developmental toxicity. Pp.189-212 in Developmental Toxicology, 2nd Ed., C.A. Kimmel, and J. Buelke-Sam, eds. New York: Raven.
Datta, D.V. 1976. Arsenic and non-cirrhotic portal hypertension [letter].
Page 137
Lancet i(7956):433.
Dési, I. 1992. Arsenic contamination of drinking water in south-east Hungary. Geogr. Med. 22:45-53.
Donofrio, P.D., A.J. Wilbourn, J.W. Albers, L. Rogers, V. Salanga and H.S. Greenberg. 1987. Acute arsenic intoxication presenting as GuillainBarre-like syndrome. Muscle Nerve 10:114-120.
Eichner, E.R. 1984. Erythroid karyorrhexis in the peripheral blood smear in severe arsenic poisoning: A comparison with lead poisoning. Am. J. Clin. Pathol. 81:533-537.
Engel, R.R., and A.H. Smith. 1994. Arsenic in drinking water and mortality from vascular disease: An ecological analysis in 30 counties in the United States. Arch. Environ. Health 49:418-427.
Engel, R.R., C. Hopenhayn-Rich, O. Receveur, and A.H. Smith. 1994. Vascular effects of chronic arsenic exposure: A review. Epidemiol. Rev. 16:184-209.
Enterline, P.E. and G.M. Marsh. 1982. Cancer among workers exposed to arsenic and other substances in a copper smelter. Am. J. Epidemiol. 116:895-911.
Enterline, P.E., V.L. Henderson, and G.M. Marsh. 1987. Exposure to arsenic and respiratory cancer: A reanalysis. Am. J. Epidemiol. 125:92938.
EPA (U.S. Environmental Protection Agency). 1988. Special Report on Inorganic Arsenic: Skin Cancer; Nutritional Essentiality. EPA 625/387/013. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, D.C.
Epstein, E. 1960. Association of Bowen's disease with visceral cancer. Arch. Dermatol. 82:349-351.
Falk, H., J. Herbert, S. Crowley, K.G. Ishak, L.B. Thomas, H. Popper, and G.G. Caldwell. 1981. Epidemiology of hepatic angiosarcoma in the United States: 1964-1974. Environ. Health Perspect. 41:107-113.
Farquharson, R. 1880. On the use of arsenic in skin-diseases. Br. Med. J. (May 29) 802-804.
Feinglass, E.J. 1973. Arsenic intoxication from well water in the United States. N. Engl. J. Med. 268:828-830.
Feldman, R.G., C.A. Niles, M. Kelly-Hayes, D.S. Sax, W.J. Dixon, D.J. Thompson, and E. Landau. 1979. Peripheral neuropathy in arsenic smelter workers. Neurology 29:939-944.
Ferm, V.H., and S.J. Carpenter. 1968. Malformations induced by sodium arsenate. J. Reprod. Fertil. 17:199-201.
Ferm, V.H., and D.P. Hanlon. 1985. Constant rate exposure of pregnant hamsters to arsenate during early gestation. Environ. Res. 37:425-432.
Page 138
Ferm, V.H., A. Saxon, and B.M. Smith. 1971. The teratogenic profile of sodium arsenate in the golden hamster. Arch. Environ. Health 22:557-560.
Feussner, J.R., J.D. Shelbume, S. Bredehoeft and H.J. Cohen. 1979. Arsenic-induced bone marrow toxicity: Ultrastructural and electron-probe analysis. Blood. 53:820-827.
Fierz, U. 1965. Catamnestic investigations of the side effects of therapy of skin diseases with inorganic arsenic [in German]. Dermatologica 131:4158.
Fincher, R.E., and R.M. Koerker. 1987. Long-term survival in acute arsenic encephalopathy: Follow-up using newer measures of electrophysiologic parameters. Am. J. Med. 82:549-552.
Foy, H.M., S. Tarmapai, P. Eamchan, and O. Metdilogkul. 1992. Chronic arsenic poisoning from well water in a mining area in Thailand. Asia Pacific. J. Public Health 6(3):150-152.
Franklin, M., W. Bean and R.C. Harden. 1950. Fowler's solution as an etiologic agent in cirrhosis. Am. J. Med. Sci. 219:589-596.
Franzblau, A., and R. Lilis. 1989. Acute arsenic intoxication from environmental arsenic exposure. Arch. Environ. Health. 44:385-390.
Freeman, J.W. and J.R. Couch. 1978. Prolonged encephalopathy with arsenic poisoning. Neurology 28:853-855.
Gainer, J.H., and T. W. Pry. 1972. Effects of arsenicals on viral infections in mice. Am. J. Vet. Res. 33:2299-2307.
Garcia-Vargas, G.G., L.M. Del Razo, M.E. Cebrian, A. Albores, P. Ostrosky-Wegman, R. Montero, M.E. Gonsbatt, C.K. Lim, and F. De Matteis. 1994. Altered urinary porphyrin excretion in a human population chronically exposed to arsenic in Mexico. Hum. Exp. Toxicol. 13:839847.
Ghafghazi, T., J.W. Ridlington, and B.A. Fowler. 1980. The effects of acute and subacute sodium arsenite administration on carbohydrate metabolism. Toxicol. Appl. Pharmacol. 55:126-130.
Glazener, F.S., J.G. Ellis, and P.K. Johnson. 1968. Electrocardiographic findings with arsenic poisoning. Calif. Med. 109:158-162.
Golub, M.S. 1994. Maternal toxicity and the identification of inorganic arsenic as a developmental toxicant. Reprod. Toxicol. 8:283-295.
Golub, M.S., M.S. Macintosh, and N. Baumrind. 1998. Developmental and reproductive toxicity of inorganic arsenic: Animal studies and human concerns. J. Toxicol. Environ. Health B Crit Rev. 1:199-241.
Gonsebatt, M.E., L. Vega, L.A. Herrera, R. Montero, E. Rojas, M.E. Cebrian, and P. Ostrosky-Wegman. 1992. Inorganic arsenic effects on human lymphocyte stimulation and proliferation. Mutat. Res. 283:91-95.
Gonsebatt, M.E., L. Vega, R. Montero, G. Garcia-Vargas, L.M. Del Razo,
Page 139
A. Albores, and M.E. Cebrian. 1994. Lymphocyte replicating ability in individuals exposed to arsenic via drinking water. Mutat. Res. 313:293299.
Graham, J.H., and E.B. Helwig. 1959. Bowen's disease and its relationship to systemic cancer. Arch. Dermatol. 80:133-159.
Greenberg, C., S. Davies, T. McGowan, A. Schorer, and C. Drage. 1979. Acute respiratory failure following severe arsenic poisoning. Chest 76:596-598.
Grobe, J.V. 1976. Peripheral circulatory disorders and acrocyanosis in arsenic exposed Moselle wine-growers. [ in German]. Berufsdermatosen 24(3):78-84.
Guha Mazumder, D.N., J. Das Gupta, A. Santra, A. Pal, A. Ghose, S. Sarkar, N. Chattopadhaya, and D. Chakraborti. 1997. Non-cancer effects of chronic arsenicosis with special reference to liver damage. Pp. 112-123 in Arsenic: Exposure and Health Effects, C.O. Abernathy, R.L. Calderon, and W.R. Chappell, eds. London: Chapman & Hall.
Guha Mazumder, D.N., R. Haque, N. Ghosh, B.K. De, A. Santra, D. Chakraborty, and A.H. Smith. 1998. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int. J. Epidemiol. 27:871-877.
Guo, H.R., H.S. Chiang, H. Hu, S.R. Lipsitz, and R.R. Monson. 1997. Arsenic in drinking water and incidence of urinary cancers. Epidemiology 8:545-550.
Hamada T., and S. Horiguchi. 1976. Occupational chronic arsenical poisoning: On the cutaneous manifestations. Jpn. J. Ind. Health 18(2): 103115.
Harrington, J.M., J.P. Middaugh, D.L. Morse, and J. Housworth. 1978. A survey of a population exposed to high concentrations of arsenic in well water in Fairbanks, Alaska. Am. J. Epidemiol. 108:377-385.
Hazelton Laboratories. 1990. Two-generation dietary reproductive study with arsenic acid in mice. Study No. HLA 6120-138. Hazleton Laboratories America, Vienna, Va.
Hertz-Picciotto, I., and A.H. Smith. 1993. Observations on the doseresponse curve for arsenic exposure and lung cancer. Scan. J. Work Environ. Health 19:217-226.
Heyman, A. J.B. Pfeiffer, R.W. Willett, and H.M. Taylor. 1956. Peripheral neuropathy caused by arsenical intoxication: A study of 41 cases with observations on the effects of BAL (2,3 dimercapto-propanol). N. Engl. J. Med. 254:401-409.
Heywood, R., and R.J.Sortwell. 1979. Arsenic intoxication in the rhesus monkey. Toxicol. Lett. 3:137-144.
Page 140
Hill, A.B. 1965. The environment and disease: Association or causation? Proc. R. Soc. Med. 58:295-300.
Hindmarsh, J.T., O.R. McLetchie, L.P. Heffernan, O.A. Hayne, H.A. Ellenberger, R.F. McCurdy, and H.J. Thiebaux. 1977. Electromyographic abnormalities in chronic environmental arsenicalism. J. Anal. Toxicol. 1:270-276.
Holmberg, R.E., and V.H. Ferm. 1969. Interrelationships of selenium, cadmium, and arsenic in mammalian teratogenesis. Arch. Environ. Health 18:873-877.
Hood, R.D. 1972. Effects of sodium arsenite on fetal development. Bull. Environ. Contam. Toxicol. 7:216-222.
Hood, R.D., and S.L. Bishop. 1972. Teratogenic effects of sodium arsenate in mice. Arch. Environ. Health 24:62-65.
Hood, R.D., and W.P. Harrison. 1982. Effects of prenatal arsenite exposure in the hamster. Bull. Environ. Contarn. Toxicol. 29:679-687.
Hood, R.D., G.T. Thacker, B.L. Patterson, and G.M. Szczech. 1978. Prenatal effects of oral versus intraperitoneal sodium arsenate in mice. J. Environ. Pathol. Toxicol. 1:671-678.
Hood, R.D., G.C. Vedel-Macrander, M.J. Zaworotko, F.M. Tatum, and R.G. Meeks. 1987. Distribution, metabolism and fetal uptake of pentavalent arsenic in pregnant mice following oral or intraperitoneal administration. Teratology 35:19-25.
Hood, R.D., G.C. Vedel, M.J. Zaworotko, and R.G. Meeks, 1988. Uptake, distribution, and metabolism of trivalent arsenic in the pregnant mouse. J. Toxicol. Environ. Health 25:423-434.
Hopenhayn-Rich, C., M.L. Biggs, A. Fuchs, R. Bergoglio, E.E. Tello, H. Nicolli, and A.H. Smith. 1996. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology 7:117-124.
Hopenhayn-Rich C., M.L. Biggs, and A.H. Smith. 1998. Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba, Argentina. Int. J. Epidemiol. 27:561-569.
Hotta, N. 1989. Clinical aspects of chronic arsenic poisoning due to environmental and occupational pollution in and around a small refining spot [in Japanese]. Nippon Taishitsugaku Zasshi [Jpn. J. Const. Med.] 53(1/2):49-70.
Hugo, N.E., and H. Conway. 1967. Bowen's disease: Its malignant potential and relationship to systemic cancer. Plast. Reconstr. Surg. 39:190-194.
Hutchinson, J. 1887. Arsenic cancer. Br. Med. J. 2:1280-1281.
IARC (International Agency for Research on Cancer). 1980. Some Metals and Metallic Compounds. IARC Monographs on the Evaluation of
Page 141
Carcinogenic Risks to Humans, Vol. 23. Lyon, France: International Agency for Research on Cancer.
Jackson, R. and J.S. Grainge. 1975. Arsenic and cancer. Can. Med. Assoc. J. 113:396-401.
Jarup, L., G. Pershagen, and S. Wall. 1989. Cumulative arsenic exposure and lung cancer in smelter workers: A dose-response study. Am. J. Ind. Med. 15:31-41.
Kjeldsberg, C.R. and H.P. Ward. 1972. Leukemia in arsenic poisoning. Ann. Intern. Med. 77:935-937.
Kosnett, M.J., and C.E. Becker. 1988. Dimercaptosuccinic acid: Utility in acute and chronic arsenic poisoning [abstract]. Vet. Hum. Toxicol. 30:369.
Kreiss, K., M.M. Zack, P.J. Landrigan, R.G. Feldman, C.A. Niles, J. Chirico-Post, D.S. Sax, M.H. Boyd, and D.H. Cox. 1983. Neurologic evaluation of a population exposed to arsenic in Alaskan well water. Arch. Environ. Health. 38:116-121.
Kyle, R.A., and G.L. Pease. 1965. Hematologic aspects of arsenic intoxication. N. Engl. J. Med. 273:18-23.
Lagerkvist, B.J., and B. Zetterlund. 1994. Assessment of exposure to arsenic among smelter workers: A five year follow-up. Am. J. Ind. Med. 25:477-488.
Lagerkvist, B., H. Linderholm, and G.F. Nordberg. 1986. Vasospastic tendency and Raynaud's phenomenon in smelter workers exposed to arsenic. Environ. Res. 39:465-474.
Lagerkvist, B.E., H. Linderholm, and G.F. Nordberg. 1988. Arsenic and Raynaud's phenomenon: Vasospastic tendency and excretion of arsenic in smelter workers before and after the summer vacation. Int. Arch. Occup. Environ. Health. 60:361-364.
Lai, M.S., Y.M. Hsueh, C.J. Chen, M.P. Shyu, S.Y. Chen, T.L. Kuo, M.M. Wu, and T.Y. Tai. 1994. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am. J. Epidemiol. 139:484-492.
Lawson, G.B., W.P. Jackson, and G.S. Cattanach. 1925. Arsenic poisoning: Report of twenty-eight cases. JAMA 35:24-26.
Lee-Feldstein, A. 1986. Cumulative exposure to arsenic and its relationship to respiratory cancer among copper smelter employees. J. Occup. Med. 28:296-302.
Le Quesne, P.M., and J.G. McLeod. 1977. Peripheral neuropathy following a single exposure to arsenic. Clinical course in four patients with electrophysiological and histological studies. J. Neurol. Sci. 32:437-451.
Lerman, B.B., N. Ali, and D. Green. 1980. Megaloblastic, dyserythropoietic anemia following arsenic ingestion. Ann. Clin. Lab. Sci. 10:515-517.
Page 142
Levin-Scherz, J.K., J.D. Patrick, F.H. Weber, and C. Garabedian, Jr. 1987. Acute arsenic ingestion. Ann. Emerg. Med. 16:702-704.
Lindgren, A., M. Vahter, and L. Dencker. 1982. Autoradiographic studies on the distribution of arsenic in mice and hamster administered 74Asarsenite or -arsenate. Acta Pharmacol. Toxicol. 51:253-365.
Lindgren, A., B.R. Danielsson, L. Dencker, and M. Vahter. 1984. Embryotoxicity of arsenite and arsenate: Distribution in pregnant mice and monkeys and effects on embryonic cells in vitro. Acta Pharmacol. Toxicol. 54:311-320.
Lu F.J. 1990. Blackfoot disease: arsenic or humic acid? Lancet 336(8707): 115-116.
Luchtrath, H. 1972. Cirrosis of the liver in chronic arsenical poisoning of vintners. Ger. Med. 2:127-128.
Luchtrath, H. 1983. The consequences of chronic arsenic poisoning among Moselle wine groweres. Pathoanatomical investigations of post-mortem examinations performed between 1960 and 1977. J. Cancer Res. Clin. Oncol. 105:173-182.
Mabuchi, K., A.M. Lilienfeld and L.M. Snell. 1980. Cancer and occupational exposure to arsenic: A study of pesticide worker. Prev. Med. 9:51-77.
Mandal, B.K., T.R. Chowdhury, G. Samanta, G.K. Basu, P.P. Chowdhury, C.R. Chanda, D. Lodh, N.K. Karan, R.K. Dhar, D.K. Tamili, D. Das, K.C. Saha, and D. Chakraborti. 1996. Arsenic in groundwater in seven districts of West Bengal, India-The biggest arsenic calamity in the world. Curr. Sci. (Bangalore) 70:976-986.
Marin-Padilla, M. 1979. Notochordal-basichondrocranium relationships: abnormalities in experimental axial skeletal (dysraphic) disorders. J. Embryol. Exp. Morphol. 53:15-38.
Marin-Padilla, M. 1980. Morphogenesis of experimental encephalocele (cranioschisis occulta). J. Neurol. Sci. 46:83-99.
MARTA (Middle Atlantic Reproduction and Teratology Society). 1996. Historical control data. Pp. 713-733 in Handbook of Developmental Toxicology, R.D. Hood, ed. Boca Raton, Fla.: CRC Press.
Martinez, G., M. Cebrian, G. Chamorro, and P. Jauge. 1983. Urinary uroporphyrin as an indicator of arsenic exposure in rats. Proc. West. Pharmacol. Soc. 26:171-174.
McCabe, M., D. Maguire, and M. Nowak. 1983. The effects of arsenic compounds on human and bovine lymphocyte mitogenesis in vitro. Environ. Res. 31:323-331.
Michigan Department of Public Health. 1982. Arsenic in Drinking Water-A Study of Exposure and a Clinical Survey. Division of Environmental Epidemiology, Bureau of Disease Control and Laboratory Services.
Page 143
Environmental Health Statistics Unit, Technical Services Section, Office of Vital and Health Statistics, Department of Public Health, Lansing, Mich.
Mirkes, P.E., and L. Cornel. 1992. A comparison of sodium arsenite- and hyperthermia-induced stress responses and abnormal development in cultured postimplantation rat embryos. Teratology 46:251-259
Mizuta, N., M. Mizuta, F. Ito, T. Ito, H. Uchida, Y. Watanabe, H. Akama, T. Murakami, F. Hayashi, K. Nakamura, T. Yamaguchi, W. Mizuia, S. Oishi and H. Matsumura. 1956. An outbleak of acute arsenic poisoning caused by arsenic-contaminated soy-sauce (shoyu): A clinical report of 220 cases. Bull. Yamaguchi Med. Sch. 4(2):131-149.
Morris, J.S., M. Schmid, S. Newman, P.J. Scheuer, M.R.C. Path and S. Sherlock. 1974. Arsenic and noncirrhotic portal hypertension. Gastroenterology 66:86-94.
Morrissey, R.E., B.A. Fowler, M.W. Harris, M.P. Moorman, C.W. Jameson, and B.A. Schwetz. 1990. Arsine: Absence of developmental toxicity in rats and mice. Fundam. Appl. Toxicol. 15:350-356.
Morse D.L., J.M.. Harrington, J. Housworth, P.J. Landrigan, A. Kelter. 1979. Arsenic exposure in multiple environmental media in children near a smelter. Clin Toxicol 14(4):389-399.
Murphy, M.J., L.W. Lyon, and J.W. Taylor. 1981. Subacute arsenic neuropathy: Clinical and electrophysiological observations. J. Neurol. Neurosurg. Psychiatry 44:896-900.
Nagy, G., A. Nemeth, F. Bodor, and E. Fiscor. 1980. Cases of bladder cancer caused by chronic arsenic poisoning [in Hungarian]. Orv. Hetil. 121:1009-1011.
Nemec, M.D., J.F. Holson, C.H. Farr, and R.D. Hood. 1998. Developmental toxicity assessment of arsenic acid in mice and rabbits. Reprod. Toxicol. 12:647-658.
Nevens, F., J. Fevery, W. Van Steenbergen, R. Sciot, V. Desmet, and J. De Groote. 1990. Arsenic and non-cirrhotic portal hypertension: A report of eight cases. J. Hepatol. 11:80-85.
Nordstrom, S., L. Beckman, and I. Nordenson. 1978a. Occupation and environmental risks in and around a smelter in northern Sweden. I. Variations in birth weight. Hereditas 88:43-46.
Nordstrom, S., L. Beckman, and I. Nordenson. 1978b. Occupation and environmental risks in and around a smelter in northern Sweden. III. Frequencies of spontaneous abortion. Hereditas 88:51-54
O'Shaughnessy, E., and G.H. Kraft. 1976. Arsenic poisoning: Long-term follow-up of a nonfatal case. Arch. Phys. Med. Rehabil. 57:403-406.
Ota, M. 1970. Ultrastructure of sural nerve in a case of arsenical
Page 144
neuropathy. Acta Neuropathol. 16:233-242.
Ott, M.G., B.B. Holder, and H.L. Gordon. 1974. Respiratory cancer and occupational exposure to arsenicals. Arch. Environ. Health 29:250-255.
Pascher, F., and J. Wolf. 1952. Cutaneous sequelae following treatment of bronchial asthma with inorganic arsenic: Report of two cases. JAMA 148:734-736.
Perry, K., R.G. Bowler, H.M. Buckell, H.A. Druett, and R.S.F. Schilling. 1948. Studies in the incidence of cancer in a factory handling inorganic compounds of arsenic: II. Clinical and environmental investigations. Br. J. Ind. Med. 5:6-15.
Peterka, E.S., F.W. Lynch, and R.W. Goltz. 1961. An association between Bowen's disease and internal cancer. Arch. Dermatol. 84:623-629.
Pope, F.M. 1902. Arsenic in the treatment of chorea. Br. Med. J. 2:12291230.
Popper, H., L.B. Thomas, N.C. Telles, H. Falk, and I.J. Selikoff. 1978. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Am. J. Pathol. 92:349-369.
Prystowsky, S.D., G.J. Elfenbein and S.I. Lamberg. 1978. Nasopharyngeal carcinoma associated with long-term arsenic ingestion. Arch. Dermatol. 114:602-603.
Rahman, M., and O. Axelson. 1995. Diabetes mellitus and arsenic exposure: a second look at case-control data from a Swedish copper smelter. Occup. Environ. Med. 52:773-774.
Rahman, M., G. Wingren, and O. Axelson. 1996. Diabetes mellitus among Swedish art glass workers-An effect of arsenic exposure? Scand. J. Work Environ. Health 22:146-149.
Rahman, M., M. Tondel, S.A. Ahmad, and O. Axelson. 1998. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am. J. Epidemiol. 148:198-203.
Ratnam, K.V., M.J. Espy, S.A. Muller, T.F. Smith, and W.P. Su. 1992. Clinicopathologic study of arsenic-induced skin lesions: No definite association with human papillomavirus. J. Am. Acad. Dermatol. 27:120122.
Rattner, H., and M. Dome. 1943. Arsenical pigmentation and keratoses. Arch Dermatol. Syphilol. 48:458-460.
Rencher, A.C., M.W. Carter, and D.W. McKee. 1977. A retrospective epidemiological study of mortality at a large western copper smelter. J. Occup. Med. 19:754-758.
Reymann, F., R. Moller, and A. Nielsen. 1978. Relationship between arsenic intake and internal malignant neoplasms. Arch. Dermatol. 114:378-381.
Page 145
Reynolds, E.S. 1901. An account of the epidemic outbreak of arsenical poisoning occurring in beer-drinkers in the north of England and the midland counties in 1900. Lancet (Jan. 19):166-170.
Rezuke, W.N., C. Anderson, W.T. Pastuszak, S.R. Conway and S.I. Firshein. 1991. Arsenic intoxication presenting as a myelodysplastic syndrome: A case report. Am. J. Hematol. 36:291-293.
Ribard, P., J.F. Bergman, V.G. Levy, and M. Thomas. 1986. Chronic selfpoisoning by arsenic [ in French] [letter]. Presse Med. 15(36):1833.
Roat, J.W., A. Wald, H. Mendelow, and K.I. Pataki. 1982. Hepatic angiosarcoma associated with short-term arsenic ingestion. Am. J. Med. 73:933-936.
Robertson, D.A.F., and T.S. Low-Beer. 1983. Long term consequences of arsenical treatment for multiple sclerosis. Br. Med. J. 286:605-606.
Robson, A.O., and A.M. Jelliffe. 1963. Medicinal arsenic poisoning and lung cancer. Br. Med. J. 5351:207-209.
Rogers, E.H., N. Chernoff, and B.J. Kavlock. 1981. The teratogenic potential of cacodylic acid in the rat and mouse. Drug Chem. Toxicol. 4:49-61.
Rosenberg, H.G. 1974. Systemic arterial disease and chronic arsenicism in infants. Arch. Pathol. 97(6):360-365.
Rosset, M. 1958. Arsenical keratoses associated with carcinomas of the internal organs. Can. Med. Assoc. J. 78:416-419.
Roth, F. 1957. The sequelae of chronic arsenic poisoning in Moselle vintners. Germ. Med. Monthly. 2:172-175.
Rothman, K.J. 1986. Modern Epidemiology. Boston: Little, Brown. 358 pp.
Rudnai, P., M. Csanády, E. Sárkány, V. Kiss, and G. Mucsi. 1996. Association between arsenic levels of drinking water in reproductive outcomes in Hungary [abstract]. International Seminar on Arsenic Exposure: Health Effects, Remediation Methods in Treatment Costs. Universidad de Chile, Oct. 8-10.
Saha, K.C. 1995. Chronic arsenical dermatoses from tube-well water in West Bengal during 1983-87. Ind. J. Dermatol. 40:1-12.
Schroeder H.A., and M. Mitchener. 1971. Toxic effects of trace elements on the reproduction of mice and rats. Arch. Environ. Health 23:102-106.
Sikorski, E.E., J.A. McCay, K.L. White, Jr., S.G. Bradley, and A.E. Munson. 1989. Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1 mice. Fundam. Appl. Toxicol. 13:843-858.
Silver, A.S., and P.L. Wainman. 1952. Chronic arsenic poisoning following use of an asthma remedy. JAMA 150:584-585.
Smith, A.H., M. Goycolea, R. Haque, and M.L. Biggs. 1998. Marked
Page 146
increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 147:660-669.
Sobel, W., G.G. Bond, C.L. Baldwin, and D.J. Ducommun. 1988. An update of respiratory cancer and occupational exposure to arsenicals. Am. J. Ind. Med. 13:263-270.
Sommers, S.C., and R.G. McManus. 1953. Multiple arsenical cancers of skin and internal organs. Cancer 6:347-359.
Southwick, J.W., A.E. Western, M.M. Beck, T. Whitley, R. Isaacs, J. Petajan and C.D. Hansen 1983. An epidemiological study of arsenic in drinking water in Millard County, Utah. Pp. 210-225 in Arsenic: Industrial, Biomedical, Environmental Perspectives, W.H. Lederer and R.J. Fensterheim, eds. New York: Van Nostrand Reinhold.
Stegmayr, B., and K. Asplund. 1992. Measuring stroke in the population: Quality of routine statistics in comparison with a population-based stroke registry. Neuroepidemiology. 11(4-6):204-213.
Stocken, L.A., and R.H.S. Thompson. 1949. Reactions of British antilewisite with arsenic and other metals in living systems. Physiol. Rev. 29:168-194.
Stockman, R. 1902. The therapeutic value of arsenic and the justification of its continued use in the light of recent observations concerning its toxic action. Br. Med. J. (Oct. 18):1227-1229.
Subramanian, K.S., and M.J. Kosnett. 1998. Human exposures to arsenic from consumption of well water in West Bengal, India. Int. J. Occup. Environ. Health 4:217-230.
Stump, D.G., C.E. Ulrich, J.F. Holson, and C.H. Farr. 1998a. An inhalation developmental toxicity study of arsenic trioxide in rats. Teratology 57(45):216.
Stump, D.G., K.J. Clevidence, J.F. Knapp, J.F. Holson, and C.H. Farr. 1998b. An oral developmental toxicity study of arsenic trioxide in rats. Teratology 57(4-5):216-217.
Susser, M. 1973. Causal Thinking in the Health Sciences: Concepts and Strategies of Epidemiology. New York: Oxford University Press. 181 PP.
Swan, S.H., J.J. Beaumont, S.K. Hammond, J. Von Behren, R.S. Green, M.F. Hallock, S.R. Woskie, C.J. Hines, and M.B. Schenker. 1995. Historical cohort study of spontaneous abortion among fabrication workers in the Semiconductor Health Study: Agent-level analysis. Am. J. Ind. Med. 28:751-769.
Swanson, M., and R. Cook. 1977. Drugs, Chemicals and Blood Dyscrasias: A summary of blood abnormalities associated with exposure to specific drugs and chemicals. Hamilton, Ill.: Drug Intelligence Publications.
Page 147
Szuler, I.M., C.N. Williams, J.T. Hindmarsh, and H. Park-Dinesoy. 1979. Massive variceal hemorrhage secondary to presinusoidal portal hypertension due to arsenic poisoning. Can. Med. Assoc. J. 120:168171.
Tabacova, S., D.D. Baird, L. Balabaeva, D. Lolova, and I. Petrov. 1994. Placental arsenic and cadmium in relation to lipid peroxides and glutathione levels in maternal-infant pairs from a copper smelter area. Placenta 15:873-881.
Takeuchi, I.K. 1979. Embryotoxicity of arsenic acid: Light microscopy and electron microscopy of its effect on neurulation-stage rat embryo. J. Toxicol. Sci. 4:405-416.
Taubeneck, M.W., G.P. Daston, J.M. Rogers, and C.L. Keen. 1994. Altered maternal zinc metabolism following exposure to diverse developmental toxicants. Reprod. Toxicol. 8:25-40.
Tay, C.H. 1974. Cutaneous manifestations of arsenic poisoning due to certain Chinese herbal medicine. Australas. J. Dermatol. 15(3):121-131.
Tay, C.H., and C.S. Seah. 1975. Arsenic poisoning from anti-asthmatic herbal preparations. Med. J. Aust. 2:424-428.
Taylor, P.R., Y.L. Qiao, A. Schatzkin, S.X. Yao, J. Lubin, B.L. Mao, J.Y. Rao, M. McAdams, X.Z. Xuan, and J.Y. Li. 1989. Relation of arsenic exposure to lung cancer among tin miners in Yunnan Province, China. Br. J. Ind. Med. 46:881-886.
Terada, H., K. Katsuta, T. Sasakawa, H. Saito, H. Shirota, K. Fukuchi, C. Skiya, Y. Yokoyama, S. Hirokawa, G. Watanabe, K. Hasegawa, T. Oshina, and C. Sekiguchi. 1960. Clinical observations of chronic toxicosis by arsenic [in Japanese]. Nihon Rinsho 118:2394-2403. Translation Doc. TR 74-106 by Leo Kanner Associates, Redwood City, Calif., for the U.S. Environmental Protection Agency.
Terada, H., T. Sasagawa, H. Saito, H. Shirata and T. Sekiya. 1962. Chronic arsenical poisoning and hematopoietic organs. Acta Med. Biol. 9(4):279-292.
Torres Soruco, C.A., R.E. Bagini, and M.A. Salvador. 1991. Arteriopatia en pacientes con hidroarsenicismo crónico. La Semana Med. 175:35-38.
Tseng, W.P. 1977. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ. Health Perspect. 19:109-119.
Tseng, W.P., W.Y. Chen, J.L. Sung, and J.S. Chen. 1961. A clinical study of blackfoot disease in Taiwan: An epidemic peripheral vascular disease. Mem. Coll. Med. Natl. Taiwan Univ. 7:1-18
Tseng, W.P., H.M. Chu, S.W. How, J.M. Fong, C.S. Lin, and S. Yeh. 1968. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 40:453-463.
Page 148
Tseng, C.H., C.K. Chong, C.J. Chen, B.J. Lin, and T.Y. Tai. 1995. Abnormal peripheral microcirculation in seemingly normal subjects living in blackfoot-disease-hyperendemic villages in Taiwan. Int. J. Microcirc. Clin. Exp. 15:21-27.
Tsuda, T., T. Nagira, M. Yamamoto, and Y. Kume. 1990. An epidemiological study on cancer in certified arsenic poisoning patients in Toroku. Ind. Health 28(2):53-62.
Tsuda, T., A. Babazono, E. Yamamoto, N. Kurumatani, Y. Mino, T. Ogawa, Y. Kishi, H. Aoyama. 1995. Ingested arsenic and internal cancer: A historical cohort study followed for 33 years. Am. J. Epidemiol. 141:198-209.
Upshaw, C.B., and T.S. Claiborne. 1995. Medicinal arsenic poisoning: 27 year follow-up. South. Med. J. 88:892-893.
Upshaw, C.B., M.F. Bryant, and T.S. Claiborne. 1979. Noncirrhotic portal hypertension after arsenic ingestion. South. Med. J. 72:1332-1334.
Vahter, M., E. Marafante, A. Lindgren, and L. Dencker. 1982. Tissue distribution and subcellular binding of arsenic in marmoset monkeys after injection of 74As-arsenite. Arch. Toxicol. 51:65-77.
Wagner, S.L, J.S. Maliner, W.E. Morton, and R.S. Braman. 1979. Skin cancer and arsenical intoxication from well water. Arch. Dermatol. 115:1205-1207.
Wall, S. 1980 Survival and mortality pattern among Swedish smelter workers. Int. J. Epidemiol. 9:73-87.
Welch, K., I. Higgins, M. Oh, C. Burchfiel. 1982. Arsenic exposure, smoking and respiratory cancer in copper smelter workers. Arch. Environ. Health 37:325-335.
Westhoff, D.D., R.J. Samaha, and A. Barnes. 1975. Arsenic intoxication as a cause of megaloblastic anemia. Blood 45:241-246.
Willhite, C.C. 1981. Arsenic-induced axial skeletal (dysraphic) disorders. Exp. Mol. Pathol. 34:145-158.
Winski, S.L., and D.E. Carter. 1998. Arsenate toxicity in human erythrocytes: characterization of morphologic changes and determination of the mechanism of damage. J. Toxicol. Environ. Health. 53:345-355.
Woods, J.S., and B.A. Fowler. 1978. Altered regulation of mammalian hepatic heme biosynthesis and urinary porphyrin excretion during prolonged exposure to sodiium arsenate. Toxicol. Appl. Pharmacol. 43:361-371.
Wu, M.M., T.L. Kuo, Y.H. Hwang, and C.J. Chen. 1989. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am. J. Epidemiol. 130:1123-1132.
Yang, H.L., H.C. Chiu, and F.J. Lu. 1996. Effects of humic acid on the
Page 149
viability and coagulant properties of human umbilical vein endothelial cells. Am. J. Hematol. j51:200-206.
Yeh, S. 1973. Skin cancer in chronic arsenicism. Hum. Pathol. 4:469-485.
Yeh, S., and S.W. How. 1963. Rep. Inst. Pathol. Natl. Taiwan Univ. 14:2573.
Yu, H.S., H.M. Sheu, S.S. Ko, L.C.Chiang, C.H. Chien, S.M. Lin, B.R. Tserng, C.S. Chen. 1984. Studies on blackfoot disease and chronic arsenism in southern Taiwan: with special reference to skin lesions and fluorescent substances. J. Dermatol. 11:361-370.
Zaldivar, R. 1974. Arsenic contamination of drinking water and food-stuffs causing endemic chronic poisoning. Beitr. Pathol. 151:384-400.
Zaldivar, R., and G.L. Ghai. 1980. Mathematical model of mean age, mean arsenic dietary dose and age-specific prevalence rate from endemic chronic arsenic poisoning: A human toxicology study. Zentralbl. Bakteriol. Abt. 1 Orig. B 170(5-6):402-408.
Zaldivar, R., and A. Guillier. 1977. Environmental and clinical investigations on endemic chronic arsenic poisoning in infants and children. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. Reihe B 165(2):226-234.
Zaldivar R, L. Prunes, and G. Ghai. 1981. Arsenic dose in patients with cutaneous carcinomata and hepatic hemangio-endothelioma after environmental and occupational exposure. Arch. Toxicol. 47:145-154.
Zelikoff, J.T., J.E. Bertin, T.M. Burbacher, E.S. Hunter, R.K. Miller, E.K. Silbergeld, S. Tabacova, and J.M. Rogers. 1995. Health risks associated with prenatal metal exposure. Fundam. Appl. Toxicol. 25:161-170.
Zettel, H. 1943. Der einfluss chronischer arsenschadigung auf herz und gefasse. Z. Klin. Med. 142:689-703.
Zierler S., M. Theodore, A. Cohen, and K.J. Rothman. 1988. Chemical quality of maternal drinking water and congenital heart disease. Int. J. Epidemiol. 17:589-594.



































































