1
A Review of the Role of Nutrition in Immune Function
The infectious disease threats facing soldiers are multiple and vary with geography. In fact, during major wars, infectious diseases usually have accounted for more noneffective days than combat wounds or nonbattle injuries. Combined stressors may reduce the normal ability of soldiers to resist pathogens, may increase their susceptibility to biological agents employed against them, and may reduce the effectiveness of vaccines intended to protect them. Military studies in multistressor environments have demonstrated that higher energy intakes will better sustain the indices of immune status. Troops must be supplied with foods of high biological quality that will enable them to sustain performance and that will counter an array of immunological impairments caused by a myriad of unknown stressors.
The Committee's Task
As part of its responsibility to the Military Nutrition Division (currently the Military Nutrition and Biochemical Division) at the U.S. Army Research Institute of Environmental Medicine (USARIEM), the Committee on Military Nutrition Research (CMNR) has, on many occasions, evaluated both research plans and ongoing research efforts funded by U.S. Department of Defense appropriations. Examples include a 1996 review of research activities at the Louisiana State University's Pennington Biomedical Research Center, a 1995 review of issues related to the iron status of women enrolled in U.S. Army Basic Combat Training, and a review of the results of a nutrition intervention project conducted during a 1992 U.S. Army Ranger training class.
On May 20–21, 1996, the CMNR convened a workshop in response to a request from Army representatives to provide information on the impact of nutritional status on immune function (see Appendix E for agenda). The purpose of the workshop was to assess the current state of knowledge about how military stresses (including food deprivation) could unfavorably influence immune function and to evaluate ongoing research efforts by USARIEM scientists to study immune status in Special Forces troops. Army representatives asked the CMNR to include in its response the answers to the following five questions:
- What methods for assessment of immune function are most appropriate in military nutrition laboratory research, and what methods are most appropriate for field research?
- What are the significant military hazards or operational settings most likely to compromise immune function in soldiers?
- The proinflammatory cytokines have been proposed to decrease lean body mass, mediate thermoregulatory mechanisms, and increase resistance to infectious disease by reducing metabolic activity in a way that is similar to the reduction seen in malnutrition and other catabolic conditions. Interventions to sustain immune function can alter the actions, nutritional costs, and potential changes in the levels of proinflammatory cytokines. What are the benefits and risks to soldiers of such interventions?
- What are the important safety and regulatory considerations in the testing and use of nutrients or dietary supplements to sustain immune function under field conditions?
- Are there areas of investigation for the military nutrition research program that are likely to be fruitful in the sustainment of immune function in stressful conditions? Specifically, is there likely to be enough value added to justify adding to operational rations or including an additional component?
To assist the CMNR in responding to these questions, the workshop included presentations from individuals with expertise in immune function. As a background to these presentations, an Army representative provided an overview of why the Army is interested in nutrition and immune function. In preparing their presentations, the invited speakers were asked to address the questions posed by the Army. The speakers discussed their presentations with committee members at the workshop and submitted written reports based on their verbal presentations. The committee met after the workshop on May 22, 1996, to discuss the proceedings and the information provided. Later, the CMNR reviewed the workshop presentations and drew on its collective expertise and the scientific literature to summarize the information pertinent to nutrition and immune function, and to evaluate the potential contribution of military nutrition research to the maintenance or enhancement of the ability of the immune system to protect soldiers engaged in military operations. The committee's responses to the five questions posed by the Army appear in Chapter 2; while its conclusions and recommendations are contained in Chapter 3. The final report was reviewed and approved by the entire committee.
Stage Setting: The Military Situation
The opportunities to study the effects of multiple stressors on the immune system occur infrequently, intermittently, and largely in uncontrolled environments. As described by LTC Karl E. Friedl in Chapter 4, studies in multistressor environments, such as basic training and the Special Forces' Assessment and Selection Course (SFAS), demonstrated that higher energy intakes were better able to sustain the indices of immune status. As nutritional studies conducted with Ranger trainees (Ranger I: Moore et al., 1992; Ranger II: Shippee et al., 1994) demonstrated, troops must be supplied with high-quality foods that will enable them to sustain performance and counter an array of immunological impairments caused by a myriad of unknown stressors.
Studies of immunocompetence conducted under field conditions, where inadequate energy intake, strenuous exercise, adverse environmental and physical conditions, and psychological stress interact to create an extremely complex stimulus, are markedly different from those conducted in the laboratory. Under laboratory conditions, confounding variables can be controlled and only the variables of interest investigated. In Chapters 5 and 6 respectively, LTC Ronald L. Shippee and Pål Wiik present data describing the effect on the immune system of various training regimens imposed on elite military troops.
The Army's Interest in Nutrition and Immune Function
The primary goal of the Army Operational Medicine Program is to develop physiological strategies (including nutritional, pharmacological, and diagnostic strategies) to protect and sustain deployed soldiers, thereby enhancing readiness by maintaining their ability to accomplish assigned missions. One program with a critical need to enhance and maintain readiness is the U.S. Army Special Operations Training Program. Both the U.S. Army Ranger Training and the SFAS are physically and psychologically demanding programs used by the U.S. Army to screen male officers and enlisted soldiers for entry into Special Operations units. Since the summer of 1992, a number of studies have been conducted with these Special Operations schools through collaborative research between the U.S. Army Medical Research and Materiel Command (USAMRMC), the Soldier System Command (SSCOM), the U.S. Department of Agriculture, and industry. Currently, U.S. Ranger training consists of three 3-week phases conducted at widely varying sites with differing physical demands: military base training, mountain training, and swamp training. A desert phase originally was included in Ranger training but recently was omitted from the training program. The last 10 d of each phase were conducted entirely in stressful field situations. An overview of studies conducted by the Army Operational Medicine Program with Special Operations training courses and clinical assessment procedures employed during these studies is presented in Table 1-1.
Ranger I, the first joint USAMRMC–SSCOM study (Moore et al., 1992), was conducted from July 1991 through October 1991. Baseline assessments were performed on 190 soldiers, and this group was followed throughout the course, although attrition from the course left a final sample size of 55.
Ranger II (a nutritional intervention study; Shippee et al., 1994) was conducted from August through October 1992, and provided for increased rations to mitigate the weight loss and immune dysfunctions that occurred in Ranger I. The primary objective of the Ranger II study was to test the effect of increasing the daily caloric intake by approximately 15 percent (above that documented in the Ranger I study) on body weight loss and on the status indicators for body composition and immune function. It was hoped that the number of infections documented during previous U.S. Ranger studies would be decreased by nutritional intervention. In Ranger II, there was an overall decrease in the incidence of infections (for example, cellulitis, conjunctivitis, acute gastroenteritis, otitis, upper respiratory tract infection, and sore throat) documented in the medical records. Reports of abrasions, injuries, and knee problems also were more common during the earlier training classes. Baseline and periodic assessments were performed on 175 soldiers. Attrition during Ranger II left a final sample size of 53. The Ranger II training course was
composed of four phases that included training exercises in different environmental conditions at four geographically diverse locations. In the summer of 1993, the first nutritional and immune study of SFAS entrants was conducted using a research design and methodology similar to that in the Ranger studies. The SFAS is a physically and psychologically demanding 21-day course. In this course, unlike the Ranger studies, food deprivation was not used as an overt training stressor.
Research conducted at the U.S. Army Medical Research Institute of Infectious Diseases by William R. Beisel demonstrated that febrile infections induce a state of hypermetabolism with subsequent losses of protein, minerals, and vitamins, leading to a wasting of muscle mass (Beisel, 1977; Beisel and Sobocinski, 1980; Beisel et al., 1967). These effects were later found to be mediated through the cytokines. Current military research focuses on the effects of nutrition on the sustainment and enhancement of immune status in healthy individuals, rather than on the nutritional consequences of infection and their causal relationship to cytokine responses.
During World War II, diseases such as the diarrheal disease encountered by Rommel's troops in North Africa and the malaria suffered by units such as Merrill's Marauders in the China—Burma theater were of major concern to the military. Today, these diseases are still part of the military's high-priority research because of their common and widespread occurrence and the Army's limited ability to provide specific protections. In Chapter 4, LTC Karl E. Friedl hypothesizes that the soldier's defense against biological threat agents may depend on physiological enhancement of the immune system, possibly through various nutritional strategies.
Knowledge arising from civilian (that is, academic, governmental, private, and industrial) medical research may not be applicable to troops who are exposed to the stress of a variety of combat—work conditions. This lack of relationship between military immunological data and data from civilian hospital records exists because of the differences in the two populations. Members of the military are initially normal, nutritionally intact, physically fit, and relatively young. In contrast, nonmilitary medical patients are generally older, often demonstrate altered nutritional status (obesity, undernutrition), are rarely physically fit, and often have chronic confounding disease processes (for example, diabetes, atherosclerosis, and cancer) or undesirable life-style habits (for example, drugs, alcohol, and tobacco) that greatly modify their immunological baseline and limit their ability to respond to subsequent stimuli. The stressors faced by military personnel include altered environments (heat or cold, varying altitudes and terrains), excessive work loads, alterations in nutrient intake, and possible exposure to new pathogens and/or chemical toxins. The stress experienced by the military patient may include an emergency or elective
TABLE 1-1 Overview of Army Operational Medicine Program Studies with Special Forces Training Courses: Demographics and Clinical Assessments
|
|
Ranger I Class 91-11 |
Ranger II Class 92-11 |
Fort Jackson BCT |
SFAS-1 Class 07-93 |
|
Dates |
7/91–10/91 |
8/92–10/92 |
5/93–6/93 |
6/93 |
|
Days in training |
63 |
63 |
63 |
21 |
|
No. of subjects at start |
190 (male) |
175 (male) |
174 (female) |
100 (male) |
|
No. of subjects to finish |
55 |
50 |
158 |
37 |
|
Procedures |
|
|
|
|
|
Dietary assessment |
|
|
|
|
|
Energy intakes |
X (estimation of food provided) |
X (estimation of food provided) |
X (visual estimation) |
X (diet logs, visual estimation) |
|
Energy expenditure (DLW) |
X |
X |
– |
X |
|
Survey of food knowledge and attitudes |
– |
– |
X |
– |
|
Activity monitor |
|
|
|
|
|
TDEE |
– |
X |
– |
X |
|
Body composition |
|
|
|
|
|
Circumference and skinfolds |
X |
X |
X |
X |
|
Body fat (DXA) |
X |
X (and BIA) |
X (and BIA) |
X |
|
Bone mineral |
X |
X |
X |
– |
|
|
Ranger I Class 91-11 |
Ranger II Class 92-11 |
Fort Jackson BCT |
SFAS-1 Class 07-93 |
|
Strength testing |
X |
X |
X |
X |
|
Biochemistry |
|
|
|
|
|
Clinical |
X |
X |
X |
X |
|
Nutritional* |
X |
X |
X (no copper, zinc) |
X |
|
Hormones |
X† |
X |
X‡ |
– |
|
Immune assessment (N) |
49 |
41 |
48 |
37 |
|
Leukocytes§ |
X |
X |
X |
X |
|
Lymphocyte proliferation studies |
X |
X |
X |
X |
|
Cytockines|| |
|
|
|
|
|
IL-2 cellular production |
X |
X |
X |
X |
|
IL-2 receptor |
X |
X |
X |
X |
|
IL-6 cellular production and plasma |
X |
X |
X |
X |
|
DTH |
X |
– |
– |
– |
|
CD |
– |
3,4,8,19,4/8 |
– |
– |
|
Throat and nasal cultures |
X |
– |
– |
– |
operation, severe infection, or possibly acute hemorrhage. Thus, it may be difficult to compare the immune defects described in civilian patients with those observed in military personnel under combat conditions.
The ability to carry out research on the impact of these operational stressors presents many challenges. Ethical considerations limit the ability to design experiments that incorporate many of these stressors and employ human experimental models. Therefore, many experiments have evolved as opportunistic field studies in which investigators do not impose the stressors but are able to study the consequences. From these field studies, recommendations often are made to correct the problem associated with the stressors evaluated, which in turn limits the ability to study this problem further through the field model. For example, the increase in food intake recommended to Ranger II program participants appeared to reduce the high incidence of infections that had been observed in Ranger I (Shippee et al., 1994). The Ranger training studies have provided a unique opportunity to evaluate some of these stressors because of the rigors of the program and the duration of the course, permitting the investigator an evaluation period beyond what may be the manifestation of an initial acute-phase response. A number of investigations have grown out of the use of the Ranger training model. These studies established a relationship between energy deficit (as rate of weight loss) and the suppression of lymphocyte response (see Table 1-2, which is taken from Friedl, Chapter 4).
The most important feature of Table 1-2 is the relationship it shows between increasing energy deficit (as a rate of weight loss) and suppression of the lymphocyte response. The lowest levels of interleukin-6 (IL-6) were found in soldiers with the highest stress conditions. Additionally, the very lowest level of IL-6 was demonstrated in the individual soldier with the greatest relative weight loss.
Since soldiers do not maintain adequate energy intakes while participating in various simulated combat programs, the intake of protein, vitamins, minerals, and trace elements may be reduced proportionally, thus limiting the supply of cofactors necessary for optimal host defense. Moreover, stress could hypothetically increase the need for antioxidant vitamins and minerals that also serve as cofactors to enhance immunological functions. If this is the case, immunological responses could be attenuated by the lack of adequate nutrients. The potential of specific dietary supplements (pills, foods, or liquid formulas given, in addition to meals, to increase nutrient intakes above the RDA [Recommended Dietary Allowance] to sustain immune function, or even offer superimmunity (a state of enhanced function of the immune system), has not been evaluated fully. There are currently few military studies evaluating the potential for nutritional supplements or whole foods to provide sustained benefits. One such pilot study (Kramer et al., 1997) did report a markedly enhanced mitogen-stimulated lymphocyte proliferation response (a measure of
TABLE 1-2 Energy Deficit and Its Relation to Stress Indices and PHA-Stimulated T-Lymphocyte Proliferation
T-cell activity) in unstressed individuals given a whole-food supplement that consisted of kale, sweet potato, and tomato juice and thus was high in antioxidants.
There is a concern with the inappropriate use of dietary supplements by individual soldiers, particularly in elite units. These soldiers are susceptible to the claims of many manufacturers regarding enhanced performance following use of such products. Since these products are readily available in post exchanges and commissaries, there is, in the view of many, an implied military endorsement of their use. However, the use of these supplements may carry some risk (Herbert, 1997; Rock et al., 1996) and may impair rather than enhance readiness, because enhanced responsiveness of the immune system may not always be desirable. An example of a large-scale epidemic of severe inflammatory illness and mortality associated with a food supplement was the 1-tryptophan eosinophilia myalgia syndrome epidemic of 1989 (Crofford et al., 1990; Silver et al., 1990). At that time, 1-tryptophan was freely available over the counter and was taken for insomnia, muscle building, depression, and premenstrual syndrome. Although the major etiologic factor in the cause of the epidemic was impure 1-tryptophan manufactured by a Japanese petrochemical
company (Showa Denko K.K.), further testing in animals showed that pure 1-tryptophan in doses comparable to the large doses consumed by the patients was associated with related deleterious risks, such as pancreatic acinar hyperplasia (Love et al., 1993). Thus, without adequate research, it cannot be assumed that ''natural products'' are safe, even if manufactured according to Good Manufacturing Practices. Clearly there is a need for studies to evaluate the benefits, risks, and safety limits, if any, associated with intakes of dietary supplements.
U.S. Army Training Courses
In Chapter 5, Shippee describes four years of studies with the U.S. Army Ranger Training Brigade and SFAS. These studies were designed to test proposed guidelines for sleep deprivation, food restriction, and environmental exposure, with immune response as one of the variables of concern. Shippee points out that in modern warfare, nonbattle injury and infection account for more casualties than actual military action.
Currently, U.S. Ranger training consists of three 3-week phases conducted at widely varying sites with differing physical demands: military base field training, mountain training, and swamp training. The last 10 d of each 21-day phase involve a field training period. Changes to the training course have occurred over the years in response to infections, accidents, and death (Consolazio et al., 1966; Johnson et al., 1976), as well as in response to findings from independent research studies (IOM, 1993; Moore et al., 1992; Shippee et al., 1994) that evaluated the effects of training and possible strategies for improved outcome. Typically, data collection occurred pre-course and at the end of each phase before refeeding, sleep, or hygiene (except for dual-energy x-ray absorptiometry determinations, measurement of muscle strength, and particular anthropometric assessments, samples were collected in a fasted state [IOM, 1992]). Blood samples were obtained 3–6 h after individuals returned to the training camp site.
Energy was provided initially in these studies by one Meal, Ready-to-Eat (MRE) per day, and in Ranger I, a negative energy balance was reported (-1,203 kcal/d [Moore et al., 1992]). Consequently, mean weight loss over the 62 d of training was significant (15.9 percent), with a large proportion of this loss resulting from the depletion of fat stores, from 14.6 to 5.8 percent. Changes in endocrine function (especially decreases in blood testosterone and triiodothyronine [T3] concentrations) compromised the individual's ability to adapt to environmental stress. Assessment of immune function using an in vitro T-lymphocyte proliferation assay showed that the immune system was significantly suppressed by training. These results explain, in part, the high
infection rates observed among the troops (8, 25, and 24 percent at the end of the desert, jungle, and swamp phases [Moore et al., 1992] respectively) during the latter phases of Ranger training.
The CMNR (IOM, 1993) recommended increasing food intake by use of a Long-Life Ration Packet (LLRP) in place of the MRE in Ranger II (Shippee et al., 1994). The resultant energy balance was less negative (-847 kcal), with a weight loss of only 12 percent (a decrease from 14.7 to 8.4 percent body fat) (Shippee et al., 1994). Under these circumstances, immune function was somewhat improved (infection rates were reduced in Ranger II to 8, 12, and 2 percent during the mountain, desert, and swamp phases, respectively [Shippee et al., 1994]); however, responses to mitogens that stimulate lymphocyte proliferation were still significantly depressed in Ranger II subjects (see Table 1-3). The persistent problem of inadequate energy and nutrient intake by soldiers in the field was the focus of a 1995 CMNR report entitled Not Eating Enough (IOM, 1995a). As discussed in that report and subsequently in the report of the Subcommittee on Body Composition, Nutrition, and Health of Military Women, Assessing Readiness in Military Women (IOM, 1998), the Army Natick Research Laboratory, in conjunction with USARIEM, continually reevaluates and modifies the MRE and other operational rations to improve palatability, acceptability, portability, nutrient delivery, and nutritional labeling.
Additional studies have been performed with the SFAS to address the efficacy of various supplement in improving immune function. Evaluation of the effect of a carbohydrate—electrolyte beverage on performance showed no significant effect on lymphocyte proliferation; however, the study involved more-than-adequate energy intakes (4,890–7,846 kcal/d) and was probably not directly applicable to the field situation (Montain et al., 1995). A study employing a drink that supplied 15 g of glutamine and 200 kcal/d, showed no effect on in vitro lymphocyte proliferation but did improve delayed-type hypersensitivity (DTH) responses to tetanus toxoid compared to a control group (see Shippee, Chapter 5). In comparison, three MREs per day contain between 4.2 and 10.8 g of glutamine. Another study, involving a beverage supplying 200 kcal, as well as the antioxidant vitamins A (as β–carotene, 30 mg), E (400 IU RRR-α-tocopherol), and C (1 g); and selenium (200 μg), produced only a slight decrease in lymphocyte proliferation in the treated group and an improved booster response to tetanus toxoid (see Shippee, Chapter 5).
An additional stressor during the Ranger I and II studies was the lack of restful and uninterrupted sleep. The average sleep period for both studies was identical at 3.6 h/d. The longest sleep periods (averaging approximately 4.25 h/d) were experienced in Ranger II during the swamp phase, and this sleep was of poor quality as measured by indices of movement during sleep and of sleep fragmentation. Similarly, sleep periods during the desert phase of Ranger II averaged approximately 3.4 h/d and were of poor quality (Shippee et al., 1994).
In summary, it has been shown that the multistressor components of rigorous military training induce weight loss, alterations in immunological indices and, at times, increases in the incidence of infectious illnesses. In contrast, higher energy intakes appear to preserve immune functions. Based on the results of Ranger I, the focus of Ranger II in terms of immune function was to determine the effect of increased energy intake on T-lymphocyte function and IL-6 levels. Data indicated that there was slightly less suppression of T-lymphocyte function during Ranger II compared to Ranger I; however, plasma IL-6 continued to be reduced. At an earlier presentation to the CMNR (Moldawer, 1997), the urinary excretion of IL-6 was suggested as a diagnostic measure of an acute-phase response. However, care must be taken in interpretating lymphocyte proliferation tests to evaluate immune function because they yield measures that are relatively insensitive and nonspecific. They are less responsive to stress hormones than are cytokine response patterns, which may explain, in part, the apparently improved immune function and lower infection rates demonstrated in Ranger II. Results of micronutrient intervention studies may offer potential benefits of military significance.
Norwegian Ranger Training
In Chapter 6, Wiik describes the Norwegian Ranger Training program undertaken by cadets in the Norwegian Military Academy. This training program was designed to stress individuals to their limits, both physiologically and psychologically, over a short period of seven days. During this time, cadets were engaged in continuous military activities of average work intensity (32 percent Vo2 max for 24 h/d). Using heart rate monitoring as an indicator of hydration status, total energy expenditure was estimated in one study to be 8,500 kcal/d (although Wiik admits that this value may have been an overestimate). Energy intake in this training regimen was very low. In one study, energy intake averaged 420 kcal/d, with no food intake at all during 4 out of 7 days. Intakes in another study averaged 1,620 kcal/d when a supplement of 1,200 kcal/d was provided with the daily ration. Additionally, cadets averaged 3 hours of sleep per day (Wiik et al., 1996).
No significant increase in infectious disease was observed in cadets participating in these studies. Changes in specific immune parameters documented during these training periods show a general decline in immune function, similar to that seen in response to strenuous exercise (see Table 1-3). In these troops, an increase in energy intake of 1,200 kcal/d appeared to have little effect on modulating the overall immune response to exercise.
TABLE 1-3 Combined Data on Immunological Status During Military Training Exercises
|
|
U.S. Ranger I (N = 49) |
U.S. Ranger II (N = 41) |
Norwegian Ranger (N = 20) |
BCT (N = 48) |
SFAS (N = 37) |
|||||
|
Immunological Parameter |
Early (4 weeks) |
Late (8 weeks) |
Early (4 weeks) |
Late (8 weeks) |
Days 1–3 |
Days 4–7 |
4 Weeks |
8 Weeks |
3 Weeks |
|
|
White blood cell studies |
||||||||||
|
Granulocyte number* |
|
– |
|
|
|
|
|
|
|
|
|
Granulocyte function† |
|
|
|
|
|
|
|
|
|
|
|
Monocyte number* |
|
|
|
|
|
|
|
|
|
|
|
Monocyte function† |
|
|
|
|
|
|
|
|
|
|
|
Eosinophils |
|
|
|
|
|
|
|
|
|
|
|
Lymphocyte count |
|
– |
|
|
|
|
|
|
|
|
|
CD4+ helper lymphocytes |
|
|
|
|
|
|
|
|
|
|
|
CD8+ suppressor |
|
|
|
|
|
|
|
|
|
|
|
CD4+:CD8+ |
|
|
|
|
|
|
|
|
|
|
|
NK-cells |
|
|
|
|
|
|
|
|
|
|
|
B-lymphocyte count |
|
|
|
|
|
|
|
|
|
|
|
Lymphocyte response to mitogens |
|
|
|
|
|
|
|
|
|
|
|
T-cell |
|
|
|
|
|
|
|
|
|
|
|
B-cell |
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Ranger I (n = 49) |
U.S. Ranger II (n = 41) |
Norwegian Ranger (n = 20) |
BCT (n = 48) |
SFAS (n = 37) |
||||
|
Immunological Parameter |
Early (4 weeks) |
Late (8 weeks) |
Early (4 weeks) |
Late (8 weeks) |
Days 1–3 |
Days 4–7 |
4 weeks |
8 weeks |
3 weeks |
|
Immunoglobulin measurements |
|||||||||
|
IgG |
|
|
|
|
|
|
|
|
|
|
IgA |
|
|
|
|
|
|
|
|
|
|
IgM |
|
|
|
|
|
|
|
|
|
|
Cytokine measurements |
|||||||||
|
TNF* |
|
|
|
|
– |
– |
|
|
|
|
IL-1* |
|
|
|
|
– |
– |
|
|
|
|
IL-2 cellular |
|
|
|
|
– |
– |
|
|
|
|
IL-4 |
|
|
|
|
– |
– |
|
|
|
|
IL-6* cellular and plasma |
– |
|
|
|
|
|
|
|
|
|
– |
|
|
|
|
|
|
|
|
|
|
GMCSF |
|
|
|
|
|
|
|
|
|
|
NOTE: * Participates in acute-phase response. † Z As detected by chemiluminescence methodology. |
|||||||||
In an attempt to provide a plausible explanation for the training-induced changes in immune parameters, Wiik presents data on blood concentrations of the hormone vasoactive intestinal polypeptide (VIP). VIP and other neuropeptides play a role in immune regulation at the local level, for example in the spleen, lymph nodes, and Peyer's patches in the gut which are innervated by the autonomic nervous system and at local sites of inflammation. VIP is known to inhibit lymphocyte mitogenic responses and the monocyte production of oxygen free radicals. Additionally, VIP stimulates resident central nervous system (CNS) glial cell production of a wide range of cytokines. Within the CNS, such cytokine production plays a role in neuronal cell death and survival. Wiik reports that blood concentrations of VIP increased during the Norwegian Ranger training, and VIP-receptor activity was upregulated, resulting in the observed inhibition of monocyte activity. In contrast, catecholamine receptor activity was found to be downregulated in Norwegian Ranger trainees. It is important to note that all of these neuropeptides and neurotransmitter systems are components of stress-responsive neuronal systems that contribute to immune changes in stress situations. Changes in any neuropeptide or altered neuroendocrine autonomic responses are important when considering immune changes in stress situations (Sternberg, 1997b).
Additionally, studies of the combined effects of glucocorticoids and fasting on immune function in rats support the hypothesis that the interaction of these two factors may result in greater diminution of immune activity than either alone (Wiik, 1995).
The immune response seen in U.S. Ranger studies is similar in most respects to that observed in the Norwegian training. The exception is that cytokine-mediated immunity, as evaluated by plasma or cellular levels of IL-1, IL-12, and IL-4, did not change in the Norwegian study, whereas in the U.S. Ranger studies, the response increased or decreased depending on the cytokine evaluated (IL-2 or IL-4). Differences in immune response among participants in the two programs may be explained by (1) the more intense nature of the Norwegian program, (2) differences in the duration of training, and especially, (3) differences in assessment methodologies (cell-adjusted in Norwegian versus. whole-blood preparations in U.S. studies, as described by Tim R. Kramer in Chapter 10. It has also been reported that relative increases or decreases of different cytokines are important because glucocorticoids (released during stress) differentially suppress some cytokines and stimulate others (DeRijk et al., 1997).
Clearly, the various training courses reviewed, differ in many respects, and interpretation of these findings is difficult. Nevertheless, several conclusions can be drawn from these studies:
- Longer-term strenuous training is more debilitating in general (increase in incidence of upper respiratory infection [URI], cellulitis, and pneumococcal pneumonia as seen in U.S. Ranger trainees) than short, intense training situations.
- Partial restoration of energy balance over the short run may not have a significant impact on immune function (any potential improvement in terms of positive gains may be overridden by physical and psychological stresses).
- Restoration of energy requirements over the long run can improve the composition of weight loss dramatically, influencing immune function less severely.
- Nonspecific granulocyte function seems to be stimulated during the first days of training and then suppressed, which is consistent with the effects of glucocorticoids.
- Stressful military training of up to 1-week duration may be well tolerated in healthy individuals; however, training of longer duration may be associated with a decline in immune and other physiological functions.
Introduction to Immune Function
In Chapter 7, Ranjit K. Chandra reviews what is known about the stress exerted by compromised nutrition on the immune system and elucidates several additional factors that come into play in the military setting, thus providing an immunological background for this report. Stephen S. Morse (see Appendix D) provides insights into the impact of emerging infections and the movement of soldiers around the globe.
As William R. Beisel mentions in the overview in Appendix A, knowledge about the cytokines is now of key importance in the practice of medicine and surgery and in understanding many aspects of disease progression. Jeffrey L. Rossio (see Chapter 8) describes the roles of many families of cytokines, including the interleukins, interferons, various hematopoietic growth and differentiation factors, and cytotoxic–cytostatic inducers and effectors. Many actions of the immune system are initiated and controlled by cytokines. Although immune system cells often interact by direct, histocompatibility-requiring contact, they also employ cytokines as their principal agents for humoral communication and cellular proliferation, activation, and maturation to effector cell function. Many fundamental texts containing a more detailed review of immunology are available (for example, Abbas et al., 1991; Kuby, 1997) to the reader, as well as reviews of the immune system and its dysfunction in immunodeficiency disorders (Keusch, 1994; Myrvik, 1994; Roitt and Brostoff, 1991).
Current State of Knowledge of the Field
Chandra (see Chapter 7) summarizes much of the state of current knowledge regarding the interaction of nutrition and the immune response. This area of investigation is relatively new, with much of the available information having emerged in the past 25 years. The 1968 World Health Organization (WHO) monograph Interactions of Nutrition and Infection (Scrimshaw et al., 1968) suggested for the first time that the relationship between infection and malnutrition is a synergistic one. Three factors have provided the impetus to study the immune system in states of compromised nutrition: (1) the compilation of an epidemiologic database; (2) the development of new disease concepts and assessment techniques in immunology; and (3) the emergence of dramatic human interest cases, emanating largely from studies of protein-energy malnutrition (PEM) in young children, which stimulated the interest of researchers and the concerned public.
Severe PEM can cause significant alterations in the immune response. Research using laboratory animals and work with human subjects extended these observations to the recognition that nutritional deficiencies are associated with a large number of alterations in cell-mediated immunity, decreased lymphocyte stimulation response to mitogens and antigens, altered production of cytokines, lower secretory immunoglobulin A (IgA) antibody response on mucosal surfaces, decreased antibody affinity, and phagocyte dysfunction. Deficiencies of many individual nutrients, including protein, essential fatty acids, vitamin A, pyridoxine, folic acid, zinc, iron, copper, and selenium, have been associated with altered immune function (Figure 1-1).
Based on the scientific literature (Beisel, 1982; Chandra 1988; Cunningham-Rundles, 1993; Forse, 1994; Gershwin et al., 1985; Keusch and Farthing, 1986; Watson and Retro, 1984) highlighting the integral relationship between nutrition and immunity, Chandra proposes the following general principles, which are developed throughout this chapter:
- PEM and deficiencies of individual nutrients, even subclinical deficits, may be associated with a catabolic response, an impaired immune response, and an altered risk of infection (Beisel, 1982; Keusch and Farthing, 1986).
- Excessive intakes of some nutrients may result in reduced immune responses.
- Dose–response curves should form the basis of recommendations for optimal nutrient intake.
- Immune responses provide sensitive and functional indices of nutritional status and can aid in assessing prognosis in medical and surgical patients.
- Many factors other than nutrition can modulate immune competence.
- Basic knowledge of nutrition–immune interactions can be utilized to formulate nutritional recommendations and interventions that may be expected to reduce illness and improve chances for survival.
As mentioned by Chandra and discussed later in this chapter, many nonnutritional factors may also influence the immune response; these include genetics, physical and thermal trauma, environmental and body temperature, infection, emotional stress, and physical activity. For example, the reduced immunologic response in elderly subjects pre- and postbereavement is an example of emotional stress that may interact with nutritional or other factors to reduce immune responses. Moderate, graded exercise in elderly subjects has been shown to enhance immune responses and decrease the incidence of infection. However, strenuous exercise, both severe and prolonged, reduces immunity and increases the incidence of infection in the short term (see Chandra, Chapter 7). A J curve of risk has been proposed (see Chapter 17 by David C. Nieman), which states that at moderate activity, the risk to the individual of developing a URI is lower than in a sedentary or strenuously active situation.
Emerging Infections, Nutritional Status, and Immunity
Emerging infections, discussed by Morse (see Appendix D), are those that suddenly appear in the population, or in some new geographic range, or that rapidly increase in prevalence. The Ebola outbreak that occurred in Africa and the Ebola–Reston virus are two recent emerging infections that have been in the news (Breman et al., 1997). Another of the more surprising infections to emerge in recent years has been the human immunodeficiency virus (HIV) infection that leads to AIDS. Finally, one of the most common of the emerging infections throughout the world is dengue hemorrhagic fever (Gubler and Trent, 1993), a severe manifestation of dengue infection in children.
The military has a long-standing interest in the threat of emerging infections. Soldiers serve as efficient sentinels for these infections, intentionally or unintentionally, as observed during the Korean War in 1951 when thousands of U.S. and Un troops began to develop Korean hemorrhagic fever, a severe infection previously unknown to U.S. physicians, although similar illnesses had been recognized (but not widely reported) in Manchuria, Northern Russia, and Scandinavia. Morse describes in detail how a common field mouse that lives in the rice fields throughout Korea (and now China) introduced the Hantavirus responsible for Korean hemorrhagic fever. A similar situation is occurring in North America, but the vector is a deer mouse, and the virus causes Hantavirus pulmonary syndrome.
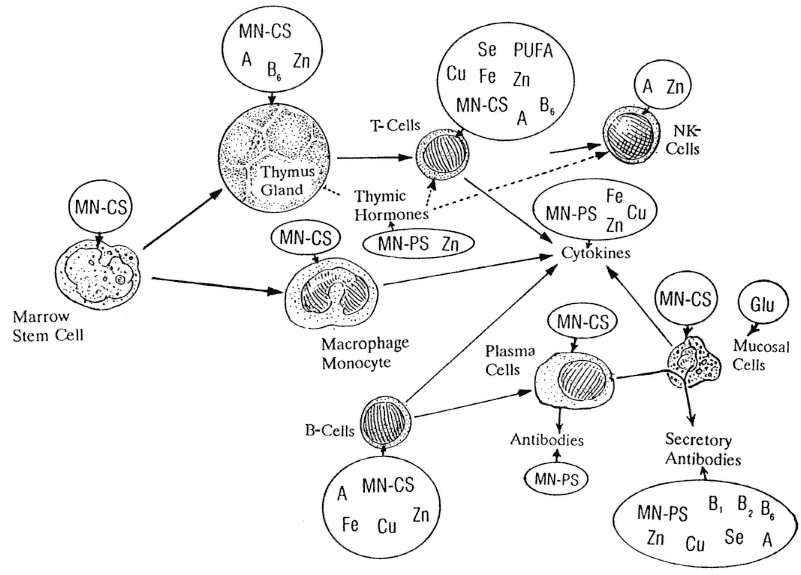
FIGURE 1-1
Nutrients known to be involved with immune system function. A, vitamin A; B1, vitamin B1; B2, vitamin B2; B6, vitamin B6; Cu, copper; Fe, iron; Glu, glutamine; MN-CS, multiple nutrients for cellular synthesis; MN-PS, multiple nutrients for protein synthesis; NK, natural killer; PUFA, polyunsaturated fatty acid; Se, selenium; Zn, zinc. SOURCE: Beisel (1982); Chandra (1988).
The process of infectious disease emergence occurs in two steps: introduction and dissemination. Many infections that are introduced into humans are not disseminated successfully. Factors that affect the introduction of an infectious disease include ecological changes, land-use changes, human demographics, and behavior. Human behavior, including the high rates of international travel and commerce that move people and goods across continents, also plays a very important role in the second step of dissemination as experienced with HIV.
There is concern not only about the movement of people, but also about biological products and foodstuffs, which may harbor pathogens (for example, a toxic and deadly form of Escherichia coli [0157H7] that can produce hemolytic uremic syndrome [Griffin and Tauxe, 1991]; cyclospora, a parasite that causes diarrheal disease [Pieniazek and Herwaldt, 1997]; or the agent responsible for bovine spongiform encephalopathy [Dealler and Lacey, 1990; Almond and Pattison, 1997]). Also important is the highly adaptive nature of microbes, as evidenced by the rise of new antibiotic-resistant organisms. Reemerging diseases such as diphtheria are often present and a continual threat to military troops, and outbreaks of salmonellosis are not uncommon.
Both macro- and micronutrient status must be considered among relevant host factors. Nutrition and nutritional practices can be additive or synergistic with the biologic factors noted above and can have indirect effects. Many important infectious diseases are food- or waterborne. Relatively little is known about the role of nutrition or malnutrition in affecting cytokine balance or the many factors involved in producing resistance to viral infections in humans.
The Cytokine System
Rossio (see Chapter 8) enumerates the most significant characteristics of cytokines: multiple biologic activities (pleiotropy; for example, IL-1 exhibits more than 50 unique and separate functions), redundancy of actions (for example, IL-1, IL-6, and tumor necrosis factor [TNF] can all induce fever), and synergistic activity.
Like hormone activities, cytokine activities are controlled partially by biological checks and balances; these control measures are much more complex for the cytokines than those that regulate endocrine functions. Target cells can shed their cytokine receptors into the plasma to inactivate circulating cytokines; receptor-blocking proteins can be produced; hormones can blunt the actions of proinflammatory cytokines; and certain cytokines can inhibit the production of others.
Although concentrations of individual plasma cytokines can now be measured, interpretation of these measurements is confounded by the need also
to know the interacting effects of circulating receptors, receptor antagonists, and inhibitory cytokines. Interpretation of plasma cytokine values also is complicated by their sporadic production and by the fact that accumulations of large amounts of cytokines in specific tissues or in pathologic lesions may not be reflected by increases in their plasma values.
As noted earlier, cytokines have multiple and overlapping roles and can function as two-edged swords. Cytokines that control the growth and differentiation of T- and B-lymphocytes initiate many ''beneficial'' immunological responses, such as cell killing and antibody production. On the other hand, excess production of the proinflammatory cytokines (IL-1, IL-6, IL-8, IL-17, γ-interferon, and TNF) during sepsis can initiate lethal hypotensive shock (Moldawer, 1997). Stress and glucocorticoid responses during stress are important regulators of cytokine production, tending to shift immune responses from T-helper 1 (Th1) to Th2-type responses (cellular to humoral), and are immunosuppressive overall (DeRijk et al., 1997; Sternberg, 1997a). Fever and other hypermetabolic components of acute-phase reactions can deplete the body of essential nutrients. Losses include muscle protein and amino acids, vitamins, minerals, and essential trace elements. In Chapter 8. Rossio points out that immunological dysfunctions observed during various forms of malnutrition may include a reduced ability of the body to synthesize cytokines, as broadly depicted in Figure 1-2.
Cytokine-induced malnutrition can become extremely severe, as seen clinically in many victims of trauma, infections, or other wasting illnesses. The pathogenesis of cytokine-induced malnutrition differs drastically from malnutrition due to starvation alone (see Table 1-4) (Beisel, 1995), but because cytokine-induced acute-phase reactions often include (and induce) severe anorexia, these two forms of malnutrition can coexist in a synergistic relationship during generalized febrile infections or other severe medical and surgical illnesses.
The importance of the cytokines for intercellular communications within the immune system and for the multiple host responses (immunological, physiological, and nutritional, as depicted in Figure 1-3) during inflammatory and infectious stress is emphasized by numerous references to cytokine functions by authors throughout this report. Additional viewpoints are provided in Chapter 19 by Leonard P. Kapcala and are discussed later in this chapter.
Assessment of Immune Function
A review of techniques for the assessment of immune function, as well as a discussion of assessment in the context of nutritional deprivation and
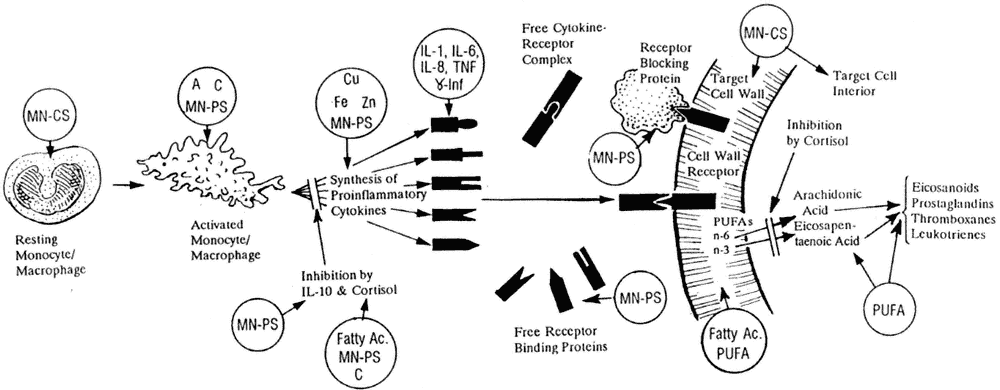
FIGURE 1-2
Macrophage/monocyte production of cytokines is representative of all body cells that produce proinflammatory cytokines. Nutrients needed for the synthesis, action, and control of proinflammatory cytokines (IL–1, IL–6, IL–8, TNF, and γ-interferon [γ-Inf]): A, vitamin A; C, vitamin C; Cu, copper; Fatty Ac., fatty acid; Fe, iron; MN-CS, multiple nutrients for cellular synthesis; MN-PS, multiple nutrients for protein synthesis; PUFA, polyunsaturated fatty acid; Zn, zinc.
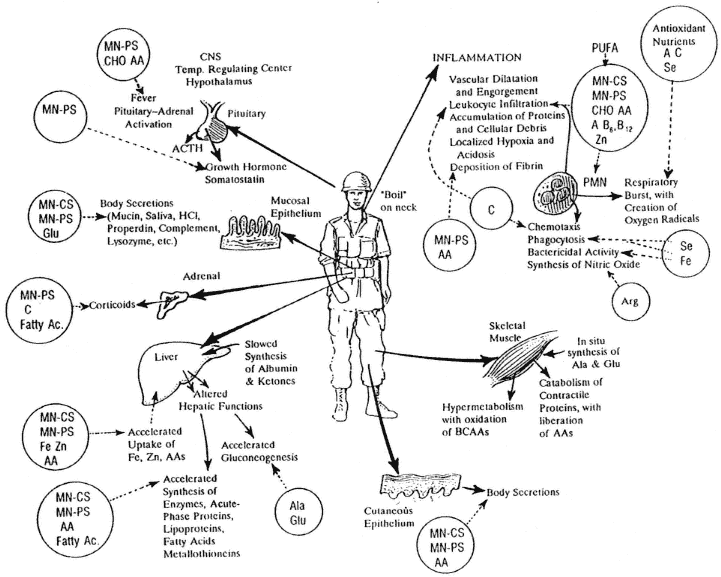
FIGURE 1-3
Nutrients needed for maintenance and function of other neuroendocrine or other endogenous mechanisms of host defense: A, vitamin A; AA, amino acid; Ala, alanine; Arg, arginine; B6, vitamin B6; B12, vitamin B12; BCAA, branch chain amino acid; C, vitamin C; CHO, carbohydrate; CNS, central nervous system; Cu, copper; Fatty Ac, fatty acid; Fe, iron; Glu, glutamine; HC1, hydrochloric acid; MN-CS, multiple nutrients for cellular synthesis; MN-PS, multiple nutrients for protein synthesis; PMN, polymorphonuclear (leukocyte); PUFA, polyunsaturated fatty acid; Se, selenium.
TABLE 1-4 Differences in the Pathogenesis of Malnutrition
military field operations, is presented by Susanna Cunningham-Rundles in Chapter 9; in Chapter 10, Tim R. Kramer presents a description of the use of one particular technique for the assessment of several parameters of immune function in cultures of whole blood.
Assessment Techniques
There is no criterion test for immune deficiency. Many existing indicators such as lymphocyte proliferation assays tend to be poor predictors of disease attributable to immune deficiency (Straight et al., 1994). However, assays of whole-blood cytokine production (De Rijk et al., 1996, 1997) may provide a more sensitive method than was available previously to measure immune changes associated with exercise and stress. The choice of techniques will depend on the investigator's resources and the availability and accessibility of tissue, blood, or biological fluid to be assayed.
A number of examples exist of test panels that are in use or have been recommended by government agencies and private-sector scientists involved in immunologic field assessment (Beisel and Talbot, 1985; Taylor, 1993). One such group, the Agency for Toxic Substances and Disease Registry (ATSDR) of the Department of Health and Human Services convened a workshop in 1992 to
refine panels of standardized immune tests previously recommended by the Centers for Disease Control and Prevention for use in environmental health field studies (Straight et al., 1994). The outcomes of this workshop included recommendations for a panel of tests for assessment of immune deficiency as well as factors that must be considered in the design of field studies. The test panel recommended by the ATSDR is two tiered: the first tier consists of basic tests of immune status; the second tier consists of more focused tests to be used for individuals with suspected immune deficiency. To provide an example, the tests comprising this panel are described in Appendix C.
Considerations in Study Design
The desire to measure the influence of any stimulus on immune function, in this case the stresses of training and field operations, is based on two underlying assumptions presented by Cunningham-Rundles (see Chapter 9):
- Measurement of immune function, either in vitro or ex vivo (following removal of cells from the body and placement in culture), reflects the internal status of the immune system;
- These measures may be interpreted to predict future immune system response.
-
Among the problems cited are that relationships observed under various (artificial) conditions (for example, in previous experiments on nutritional status and immune function conducted in a clinical or laboratory setting) may not parallel those seen in training or deployment situations, particularly when clinical subjects were exposed to only one type (Cunningham-Rundles, Chapter 9). For example, the experimental conditions may involve the use of endocrine (cortisol and/or epinephrine) or bacterial endotoxin injections, for which the metabolic and immunologic responses in normal volunteers have been studied. Many, but not all, of these observed responses are similar to those described in injured or infected patients (Bessey et al., 1984; Michie et al., 1988; Watters et al., 1986).
An additional problem is that recovery (defined by Cunningham-Rundles as the ability to recover from a threat to the immune system) may be the most critical parameter for assessing the likely response to future immune exposure but may not be predictable based on the magnitude of the immune response in a test situation.
Other problems include the fact that the response of an individual to a pathogen at any given time is influenced by a multitude of independent as well as interdependent factors. In addition to nutritional status, these factors include
general health status, genetic predisposition, pharmacologic effects, presence of immunosuppressive diseases, neuroendocrine stress, and prior exposure. Therefore, it may be impossible to isolate the effect of a single nutrient or even nutritional status as a whole. In addition, the nutritional effects may be those of a single nutrient as well as the effects of nutrient interactions, and each may manifest with a complicated time course (which influences whether immune function assessments performed at isolated times will detect any effects). Cunningham-Rundles' recommendations include the need to document the type and range of alterations in immune function expected under typical training conditions, followed by careful design of studies allowing assessment at several levels of immune response.
One of the primary challenges of performing immune function assessments in field settings is that the necessity for the subjects to continue to perform physically challenging work significantly limits the amount of blood that can be sampled. Kramer (see Chapter 10) describes the ongoing efforts of his laboratory to modify techniques for the assessment of immune indices in samples of whole blood. According to Kramer, assaying whole blood samples, rather than isolating peripheral blood mononuclear cells (PBMCs) as is customarily done, has several advantages. First, additional indices of immune function can be assayed using smaller samples. Second, more samples can be processed in less time (using less equipment and fewer technical staff) because the requirement to purify cells has been eliminated. Kramer shows that the coefficients of variation for measurement of mitogen-stimulated proliferation of cells compared favorably with those in PBMCs in a study of zinc-deficient women in Thailand (Kramer et al., 1990). Shephard and coworkers (1994b), in reviewing studies of the impact of exercise on the immune system, describe the finding of Shinkai et al. (1993) that washing of PBMCs tends to lead to incomplete recovery but if care is taken, the results can agree well with those from whole blood. In contrast, Shephard et al. (1994b) report a study (Radomski et al., 1980) showing that whole-blood responses may be modified by soluble factors found in the blood. A study by Bocchieri and coworkers (1995), comparing mitogen-induced proliferation in both whole-blood cultures and PBMCs to actual disease state and T-cell phenotype in HIV-seropositive individuals and controls, found stronger correlations between proliferation in whole-blood cultures and other disease indicators than between the same indicators and proliferation in PBMCs. According to Cunningham-Rundles (Chapter 9), the whole-blood method has the advantage of reflecting the number of cells actually circulating as well as all plasma proteins and nutrients present in vivo. Whole blood is also a better medium for measurement of cytokine stimulation and glucocorticoid sensitivity ex vivo (DeRijk et al., 1996, 1997). If an observed defect must be shown to be intrinsic to cell function, then isolation and study in a standardized test is needed. However, whole blood is a
physiological medium that is well suited to testing under field conditions. Whole blood also is currently preferred for phenotypic analysis of cellular subsets; it is advantageous for field studies to use both functional and phenotypic analysis.
In summary, the design of an immune function assessment study is a significant challenge. According to Cunningham-Rundles and others, population research must move in the direction of identifying correlations among changes in individual parameters of immune function and patterns of immune responses, imposition of specific stressors, and disease outcome. Table 1-5 provides an outline of assessment methods for immune function.
Nutrition
Most nondeployed military personnel live at home or in nonbarracked conditions, consuming a diet ad libitum. Depending on food preferences and the use of vitamin or mineral supplements, there may be great variations in the quantities of nutrients ingested. As these individuals are deployed and move from their self-selected diets to a constant ration, there may be profound alterations in nutrient intake, and these changes may result in altered host defense.
Part V of this report (see Douglas W. Wilmore, Chapter 11; Richard D. Semba, Chapter 12; Laura C. Rall and Simin Nikbin Meydani, Chapter 13; Darshan S. Kelley, Chapter 14; Gerald T. Keusch, Chapter 15; and Melinda A. Beck, Chapter 16) discusses essential individual nutrients known to be important for sustaining immune system functions. However, it must not be forgotten that, worldwide, the most significant adverse impact of nutritional status on the immune system results from PEM. Its adverse effects on the immune system may be magnified by the deficiencies of essential single nutrients that almost always accompany PEM. These poorly defined nutritional interrelationships were undoubtedly of importance during the short-term effects of PEM associated with the sizable losses of weight and muscle mass experienced during Ranger I training and may have been major contributing factors in the immunological dysfunctions detected in these Rangers (Beisel, 1991).
Given the evidence that deficits in energy, protein, and certain fatty acids adversely affect immune function, it is also important to consider other nutrients such as vitamins and minerals more explicitly. Finally, it must be emphasized that in many cases, an "overdose" of a nutrient, as well as a deficiency, can lead to negative consequences.
Nutrients with Roles Implicated in Immune Function
Evidence demonstrates that severe protein or calorie malnutrition in humans results in impairment of both humoral and cell-mediated immune functions (Bistrian et al., 1975; McMurray et al., 1981; Neumann et al., 1975; Watson and Retro, 1984). There is also evidence that moderate energy restriction, such as that experienced by overweight individuals on weight loss diets, interferes with normal immune function (Kelley et al., 1994; Nieman et al., 1996; Stallone et al., 1994).
Protein and Amino Acids
Protein deficiency is consistently observed to interfere with maintaining resistance to infection because most immune mechanisms are dependent on cell replication or the production of active protein compounds. As might be expected, deficiencies of essential amino acids can result in an altered humoral response, whereas deficiencies of single nonessential amino acids may have little effect on the immune system, although there are some exceptions.
Certain amino acids have been shown to play a direct role in immunity. Glutamine and arginine are two amino acids that have been shown to have immunoregulatory functions (Kirk and Barbul, 1992; Reynolds et al., 1988).
Glutamine. Glutamine is an abundantly available, nonessential (or "conditionally essential") amino acid that functions in the regulation of both energy and nitrogen balance. Most glutamine in the body is synthesized in skeletal muscle, from which it is released into the circulation and supplied to the visceral organs (Souba et al., 1985). Glutamine appears to have numerous important functions within the body: in the liver, it plays an important role in gluconeogenesis, amino acid synthesis, and the production of urea and glutathione; in the kidneys, it functions to promote ammonia excretion and thus neutralize acid loads; and in cells such as intestinal enterocytes, colonocytes, lymphocytes, and macrophages, it functions as a major source of carbohydrate skeletons for fuel and promotes cell proliferation.
In catabolic states such as stress, surgery, and disease, both synthesis and release of muscle glutamine increase (Muhlbacher et al., 1984) simultaneously with demand. The competition for glutamine increases among the visceral organs, the major consumers being the liver, gastrointestinal mucosa, kidney, and immunological tissues. Under such conditions, glutamine may become a conditionally essential amino acid, because the supply can no longer keep up with the demand. In such cases, the diet may become an important source, although at present the extent to which dietary glutamine might supply the additional glutamine required is unknown.
TABLE 1-5 Assessment of Immune Function
|
Method |
Advantages |
Disadvantages |
Cost (1–4, 4 = most expensive) |
Applicability (F, field; E, experimental) |
|
Clinical epidemiology |
Determines the "true" incidence of infection |
|
2–3 |
F |
|
Common lab procedures |
None |
1 |
F,E |
|
|
Assay of acute-phase reactants |
Helps define course of illness |
Special reagents required |
1–2 |
F,E |
|
Measures of humoral immunity |
||||
|
Serum immunoglobulins and antibody levels |
|
Individuals with normal levels may be immunosuppressed |
1 |
F,E |
|
In vitro humoral immune responses |
None |
Consistent differences cannot be detected with present methods |
3–4 |
E |
|
Measures of cell-mediated immunity |
||||
|
Skin testing |
Involves all phases of classic immune response, predictions of outcome |
48 h needed Difficult to quantitate |
2 |
F,E |
|
Method |
Advantages |
Disadvantages |
Cost (1–4, 4 = most expensive) |
Applicability (F, field; E, experimental) |
|
Determination of cell number, populations, and subpopulations |
Semiautomated techniques |
Some cell preparation necessary for blood sample Special handling required* |
2–3 |
E,F |
|
Assay of circulating cytokines and soluble receptors (whole-blood cytokine production assays) |
|
|
1–2 |
F,E |
|
Assessment of in vitro lymphocyte function |
Evaluate DNA synthesis or IL-2 release following a mitogenic stimulus |
|
3–4 |
E |
|
Functional assays of specific cells |
Only method used to determine NK-cell activity and cytotoxicity |
|
3–4 |
E |
|
Phagocytic cell assays |
Only method used to note phagocytosis or respiratory burst |
|
3–4 |
E |
|
NOTE: CRP, C-reactive protein; ELISA, enzyme-linked immunosorbent assay; ESR, erythrocyte sedimentation rate; IL-2, Interleukin-2; NK, natural killer. * Lab tests may require special sample transport, handling, preparation, and storage, plus skilled technicians, and expensive equipment and/or reagents. Sample size requirements may be limiting. |
||||
Wilmore (see Chapter 11) focuses his discussion of glutamine on its role in immune function. Over the past decade, glutamine has been studied for its ability to promote immune cell proliferation and enhance immune function. Glutamine appears to promote lymphocyte proliferation and macrophage phagocytosis (Parry-Billings et al., 1990), the bactericidal activity of neutrophils (Ogle et al., 1994), the generation of lymphokine-activated killer cells (Juretic et al., 1994), and monocyte surveillance (Roth et al., 1982). Because of these effects, the amino acid has been studied for its potential use in reducing infection in patients undergoing surgical, chemotherapeutic, and cancer treatment procedures, where endogenous glutamine availability may become insufficient to supply increased metabolic needs. In several trials, some evidence has been obtained that glutamine administration can reduce infection and promote recovery (see MacBurney et al., 1994; Ziegler et al., 1992). Castell et al. (1996) found that the provision of glutamine in a sports drink decreased the incidence of infections (self-reported cold, cough, sore throat, or influenza) in marathon runners during the week following participation in various types of exhaustive, prolonged exercise. This effect is not uniformly positive, suggesting that glutamine may be efficacious in some, but not all, infectious conditions.
The gastrointestinal tract also has been the focus of study relating to the use of glutamine to improve host defenses and immune function. Under conditions of stress, infection, and injury, the permeability of the bowel to pathogens and toxic molecules increases, making the body more susceptible to disease. Glutamine is known to enhance intestinal mucosal growth and integrity and has been found to improve intestinal function in surgical patients (Van der Hulst et al., 1993) and patients with diseases of the large intestine (Zoli et al., 1995). Such findings suggest that glutamine ultimately may be found useful in promoting the recovery of patients from surgical treatments and disease states in which immune function may be compromised. In a controlled clinical trial, the administration of glutamine to previously immune-suppressed bone marrow transplant patients was found to reduce the length of hospital stay significantly, primarily due to reductions in clinical infections (Ziegler et al., 1992). A reduction in mortality was observed in patients with intra-abdominal sepsis who received glutamine (Griffiths et al., 1997). Unfortunately, studies that have reported beneficial effects of glutamine have used a parenteral or enteral (gastric tube) route of administration. This factor may explain the inability of Shippee and coworkers (see Chapter 5) to observe any effect of oral glutamine supplements.
In summary, the administration of exogenous glutamine appears to improve immune functions in patients, whose glutamine demands may not be met by endogenous production or dietary supply. Such effects may derive from one or more of the amino acid's many metabolic and cell proliferative effects in the body. If additional work continues to show a positive effect of glutamine in
reducing infection and disease, the amino acid ultimately may prove to be of value prophylactically in reducing sickness and speeding the recovery of soldiers from illness, although the adequacy of normal dietary glutamine will have to be assessed as well.
Arginine. The nonessential amino acid arginine, in addition to glutamine, should also be considered as a possible immune-enhancing nutrient. Arginine has multiple biological effects that are beneficial in a variety of situations, such as trauma, tumors, infections, and depressed immunity. l-Arginine supplementation in healthy human subjects has been shown to increase blood lymphocyte proliferation and suppressor T-cell numbers (Barbul et al., 1981) and to enhance phagocytic activity of alveolar macrophages in rats bearing tumor transplants (Tachibana et al., 1985). Arginine is the sole precursor of nitric oxide, a newly recognized but important microbicidal molecule (Koshland, 1992) that appears to be involved in macrophage killer function and in regulating interactions between macrophages and lymphocyte adhesion and activation (Denham and Rowland, 1992; Kirk et al., 1992; Kubes et al., 1991; Liew et al., 1990).
Vitamins
Vitamin A and Carotenoids. The effects of vitamin A deficiency on immune function are significant, and there is convincing evidence for a role of vitamin A in resistance to infection, although the mechanism is not known (Kjolhede and Beisel, 1995). Epidemiologic studies, clinical trials, and experimental studies in animal models have firmly established that vitamin A deficiency is a nutritionally acquired immunodeficiency disorder that is characterized by widespread immune alterations and increased infectious disease and mortality (Semba, 1994). Infections are known to accelerate the metabolic degradation of vitamin A and to increase its urinary excretion. Evidence has accrued from many types of laboratory studies and some clinical studies (reviewed in Ross and Hämmerling, 1994) that vitamin A deficiency affects immunocompetence through several processes. A hallmark of vitamin A deficiency is depressed antibody responses to T-cell-dependent and independent antigens, which may be mediated by alterations in the production of some cytokines. However, some viral infections do not reduce and may increase immunoglobulin G response, which may reduce or otherwise alter other immune responses (Ross and Stephenson, 1996). It should be noted, however, that most of the data available are from children because vitamin A deficiency is relatively rare in adults (especially in the United States) and quite difficult to induce experimentally. Trauma, or sterile inflammation, may cause a significant decrease in some plasma nutrients by reducing the biosynthesis of their transport proteins in liver,
including retinol-binding protein and prealbumin, the transport proteins for retinol (Aldred and Schreiber, 1993). A reduction in plasma retinol, into the range considered marginally vitamin A-deficient, has been produced in well-nourished rats following induction of acute inflammation (Rosales et al., 1996), and low plasma retinol has been reported during infections in children and adults (reviewed in Ross and Stephensen, 1996).
Reviews of randomized, controlled epidemiological studies as well as clinical trials have led to the conclusion that although the incidence of infectious disease does not appear to be greatly increased by vitamin A deficiency, vitamin A supplementation (to correct a deficiency) can reduce the severity of some infections, including diarrheal diseases (Beaton, 1996; Kirkwood, 1996). Because excess vitamin A can be toxic and is a suspected teratogen, no case can be made for supplementation of nondeficient individuals with amounts of preformed vitamin A significantly beyond the RDA level. Provitamin A carotenoids (vitamin A precursors from plant sources), however, are more limited in their toxicity and may have some effects on the immune system that are not seen with preformed vitamin A. Semba (see Chapter 12) discusses the varying bioavailability of carotenoids from dietary fruits and vegetables.
Vitamin E. The predominant physiologic function of vitamin E is in its role as an antioxidant required for the protection of cellular as well as membrane polyunsaturated fatty acids. Vitamin E also protects membrane-bound nucleic acids and thiol-rich proteins from oxidative damage. It is also known that antioxidants such as vitamin E are important for controlling signal transduction and genetic expression of the various cytokines and ultimately proliferation of the cells that synthesize them. This is particularly important for cells of the immune system because their membranes contain a high level of PUFAs that are exposed to high concentrations of free-radical products. So, not only are the levels of antioxidants reduced during normal cellular processes, but they also can be reduced by the presence of a pathogen itself. Because of the rare occurrence of symptomatic vitamin E deficiency in humans, most studies related to immunity have been conducted in either nondeficient subjects or laboratory animals, and in fact, animal studies have demonstrated improvements in immune function expressed through changes in cell proliferation (Meydani and Hayek, 1992).
Rall and Meydani (see Chapter 13) describe a number of controlled clinical trials performed in Meydani's laboratory demonstrating that vitamin E supplementation of elderly subjects as well as healthy young subjects may enhance immune function (Meydani et al., 1990, 1994). Meydani found that supplementation with 400 mg RRR-α-tocopherol (natural form) or placebo for 6 months resulted in an increased DTH response in both young and elderly subjects.
Rall and Meydani also present some data interrelating the role of vitamin E to prostaglandins and immune function. Prostaglandins have a regulatory role in maintaining the function of T-cells as well as inhibiting lymphocyte proliferation, NK cell cytotoxicity, antibodies, and certain cytokines (Meydani et al., 1990). For example, prostaglandin E2 (PGE2) can downregulate the function of Th1 cells and also upregulate the function of Th2 cells. Meydani hypothesized and later demonstrated in aged mice that vitamin E, acting as an antioxidant, would inhibit PGE2 production (by altering the cyclooxygenase pathway) and would thus be effective in enhancing the immune response as monitored by T-cell-mediated functions (Kramer et al., 1991; Meydani et al., 1986). Meydani and coworkers (1990) have also demonstrated enhanced cell-mediated immune indices of DTH, lymphocyte proliferation to concanavalin A (ConA), and IL-2 production in a group of healthy elderly subjects receiving 800 IU of vitamin E daily for 30 days. The authors therefore believe that vitamin E supplements act to improve the immune response by decreasing the production of PGE2, which in turn moderates cyclooxygenase activity. Thus, benefits may be conferred on the elderly in the face of PGE2 levels and oxidative tissue damage that tend to rise with age.
Vitamin C. Despite widespread coverage by the popular press of the influence of vitamin C on the common cold, its role in modulating immune function remains controversial. Based on epidemiological studies of individuals consuming diets deficient in vitamin C and on administration of vitamin C supplements to injured and surgical patients, several mechanisms have been proposed for the apparent immunomodulatory effect, but none have been confirmed definitively.
Vitamin C is known to function as an antioxidant and in this capacity may serve to protect the integrity of plasma, other extracellular fluids, plasma membranes, and intracellular spaces. Neutrophil activity (but not number) is attributed to the high levels of vitamin C in these cells (Khaw et al., 1995; Myrvik, 1994). A recent study in rats has shown that the antioxidant effect of vitamin C also serves to protect the level of cell energetics in burned tissues (Lalonde and Boetz-Marquard, 1997).
Other proposed mechanisms of vitamin C's role in immune function include stimulation of lymphocyte blastogenesis and synthesis of other immune modulators such as prostaglandins, prostacyclins, histamine (Myrvik, 1994), and IL-1. Vitamin C is also essential for the locomotion of neutrophils and other phagocytic cells (Beisel, 1982). Because the decrease in vitamin C status noted with smoking is associated with increased plasma values of IL-6 and TNF soluble receptors (Borelli et al., 1996), and vitamin C status is also inversely associated with levels of C-reactive protein (CRP) and other acute-phase proteins (Khaw et al., 1995), the effects of vitamin C on immune function have
been proposed to be mediated through these changes. However, in the latter studies, cause and effect were not determined.
Attempts to show a relationship among dietary vitamin C intake or vitamin C supplementation, vitamin C status, and incidence of infection or cancer have provided controversial and often contradictory results, raising questions in the minds of some scientists regarding how requirements for the vitamin should be determined (Levine et al., 1996). Several types of surgical procedures as well as burn injuries, physical overtraining, and smoking are associated with decreased vitamin C status (Ballmer and Staehlin, 1994; Lalonde and Boetz-Marquard, 1997; Peters et al., 1993; Tappia et al., 1995), which has been proposed to be responsible for the increased incidence of infection in these individuals. However, attempts to show that normalization of vitamin C status by dietary supplementation decreases the incidence of infection have resulted in contradictory observations. Although one study observed enhanced resistance to URIs in supplemented (versus unsupplemented) marathon runners (Peters et al., 1993), a meta-analysis of the largest supplementation studies involving humans concluded that vitamin C supplementation influences susceptibility to URIs (colds) only in those individuals with the lowest initial dietary intakes of vitamin C (Hemila, 1997).
A significant amount of attention has recently focused on whether antioxidant nutrients, particularly vitamins E and C, may help to reduce oxidative stress and damage during exercise (Cannon et al., 1991; Kanter et al., 1993; Meydani et al., 1993; Witt et al., 1992). Although antioxidant supplementation may attenuate oxidative stress following prolonged and strenous exertion, the effect of this attenuation on the exercise-induced immune response is uncertain (see David C. Nieman, Chapter 17).
In summary, the effects of vitamin A deficiency on immune function are significant, and infections can accelerate its loss of vitamin A. Vitamin A supplements have been shown to reduce the severity of some infections if a deficiency is present; however, an excess can be toxic. The carotenoids are less toxic and may have some effects on the immune system that are not seen with preformed vitamin A. Vitamins C and E are both powerful antioxidants, have been shown to enhance the immune response, and are relatively nontoxic. There is considerable evidence regarding the possible benefits of vitamin E and C supplementation in terms of reducing oxidative stress and/or damage during exercise. However, data remain incomplete, particularly in terms of the optimal amount of supplementation that should be recommended to achieve these benefits.
B-Vitamins. The B vitamins are involved in a broad spectrum of cellular metabolic reactions and, as a group, have been shown to have an effect on cellular disease resistance and the immune response (Bendich and Chandra, 1990). Vitamins B6, B12, and folate are particularly important for cell-mediated
immunity (CMI) functions, and thiamine, is necessary for the synthesis of antibodies or the expression of humoral immunity (Beisel, 1991, 1992). Experimental vitamin B6 deficiency in humans results in only slight impairment of antibody formation in response to a challenge by tetanus toxoid or typhoid (Hodges et al., 1962). Vitamin B6 deficiency is not uncommon in humans, although when present, it is usually found in combination with PEM and a deficiency of other B vitamins such as riboflavin. Lymphocyte differentiation and maturation are altered by a deficiency of vitamin B6, DTH responses are reduced, and antibody production may be directly impaired (Chandra, 1991; Rall and Meydani, 1993). Although repletion of vitamin B6 restores these functions, megadoses do not produce benefits beyond those observed with moderate supplementation (Rall and Meydani, 1993). Folate deficiency can lead to decreased responses of T-cells to phytohemagglutinin (PHA) as well as to decreased cytotoxic T-cell function (Gross and Newberne, 1976; Hollingsworth and Carr, 1973). CMI is depressed in individuals with anemia due to folate deficiency (Gross et al., 1975). Folate and vitamin B12 are both essential to cellular replication, and experimental deficiencies interfere with antibody formation and replication of stimulated leukocytes. In humans, neither phagocytosis nor the bactericidal capacity of neutrophils toward Staphylococcus aureus is altered by folate deficiency (Gershwin et al., 1985), but responses are reduced by vitamin B12 deficiency.
Fatty Acids
Among the fatty acids present in the diets of humans, the PUFAs are immunologically the most important. The PUFAs are substrates for the synthesis of two families of immunologically important products: the eicosanoids (i.e., prostaglandins, prostacyclins, and thomboxanes) through the cyclooxygenase pathway, and the leukotrienes and lipoxins through the lipoxygenase pathway. These compounds affect many physiological functions (including immunity and inflammation) to varying degrees, depending on structure, amounts, and ratios. Fatty acids affect immune function not only by the total amount of fat present, but also by the amounts of and ratio between the n-6 and n-3 types of PUFA that act primarily through prostaglandins and leukotriene production and activity, as depicted in Figure 1-4. For example, linoleic acid (18:2n-6) occurs in particularly high concentration in sunflower, safflower, corn, and soybean oils. On the other hand, soybean, linseed, and canola oils have a high concentration of linolenic acid (18:3n-3). Animal tissues are unable to synthesize linoleic and α-linolenic acids, so these ''essential'' fatty acids must be consumed in the diet. Typical North American diets provide 7 percent of energy as linoleic acid, much more than is needed to prevent deficiency (Lands, 1991). The n-6 and n-3
FIGURE 1-4
Metabolic pathways for conversion of dietary PUFAs to eicosanoids. Depending on their concentration and type, prostaglandins and leukotrienes stimulate or inhibit the activity of immune cells. Other 20-carbon fatty acids compete with arachidonic acid for the lipoxygenase and cyclooxygenase enzymes and reduce the products formed from arachidonic acid, including eicosanoids of the 2-series and leukotriene B4, which are potent modulators of the immune cell: PGE1, PGI2, PGI3, and PGE2, prostaglandins E1, I2, I3, and E2, respectively. PMN, polymorphonuclear (leukocyte).
NOTE: For a more detailed review of dietary fatty acids and the Immune system, the reader is referred to Calder (1998).
SOURCE: Pathway adapted from Palombo et al. (1997); 1Meydani (1993); 2Hughes (1996); 3Wasserman et al. (1987); 4Meydani (1990); 5Dinarello (1983); 6Chouarh and Fradeliz (1982).
families of PUFAs are not metabolically interconvertible in mammals. High intakes of 18:2n-6 with low intakes of 18:3n-3 (because of hydrogenation of polyunsaturated oils) and relatively low intakes of other oils (fish oils) could result in competitive pressure against n-3 fatty acids. As noted earlier in this chapter, increasing amounts of PUFAs in the diet increase the vitamin E requirement because of the propensity of PUFAs to undergo lipid peroxidation. The richest sources of vitamin E in the U.S. diet are vegetable oils. Clinical and experimental studies have demonstrated that the structural and functional properties of immune cells can be modified by dietary supplementation with n-3 PUFAs from fish oil or linolenic acid, and in vivo tests are the most appropriate approach for determining the effect of different dietary fatty acids on immune function and inflammation, but few studies have been reported in humans (Calder, 1998).
Amount of Fatty Acid Intake
The effect on immune function of reducing total fat intake has been studied in healthy men (Kelley et al., 1989). In this study, the baseline diet supplied PUFA at 6 percent of energy, and the experimental diets supplied PUFA at either 3.5 percent (four subjects) or 12.9 percent (four subjects) of energy. In both groups, immune function improved as measured by such indices as the numbers of circulating T- and B-lymphocytes and their proliferation in response to specific mitogens. Neutrophils and serum complement C3 decreased, and leukocyte counts and plasma concentrations of all major classes of immunoglobulins remained unchanged.
In another study cited, Kelley and colleagues (1992a) measured the immune status of seven healthy women fed diets reduced in total fat with higher or lower levels of PUFA than the stabilization diet containing 5.2 percent of energy as PUFA and 40 percent of energy as total fat. The experimental diets contained either 3.2 percent of energy as PUFA and 26.1 percent as total fat, or 9.1 percent of energy as PUFA and 31.1 percent as total fat. In this study, a number of parameters reflecting immune status improved on the lower-fat diets. In contrast, no differences in these indices were observed between individuals consuming diets containing 9.1 percent of energy as PUFA or 3.2 percent as PUFA. The authors suggested but did not conclude that immune function may have improved in response to the reduction of total fat intake.
Amount of n-6 PUFA
In the two studies cited above, changing the level of linoleic acid (LA) from 3 to 13 percent of energy but reducing total fat intake did not adversely affect
the indices of immune function. In another study cited (Barone et al., 1989), results were more equivocal, with adverse effects sometimes being observed when dietary n-6 PUFA increased along with an increase in total fat. Inconsistencies apparent among studies measuring the effect of PUFA on immune function may be due to differences in total fat intake, antioxidants, duration of feeding, and the immune indices measured. Another obvious factor is the ratio of n-6 to n-3 PUFA in the diets. To evaluate this more fully, Kelley et al. (1996) used a crossover study design in which healthy men were fed a baseline diet containing 30 percent of energy as fat (with equal amounts of saturated fatty acids, monounsaturated fatty acids, and PUFAs) and containing 200 mg of arachidonic acid, which was subsequently increased to 1.5 g. This moderate level of arachidonic acid (that is, the intake of additional PUFA) had no adverse effect on various indices of immune function, including DTH response, NK-cell activity, lymphocyte proliferation in response to the mitogens ConA and PHA, and in vitro secretion of IL-1, IL-2, and TNF. It appears that n-6 PUFAs have little effect, if any, on immune function, independent of fat and n-3 PUFA intake.
Amount of n-3 PUFA
A number of studies have been carried out to evaluate the effect of n-3 PUFAs derived from plant sources (Bjerve et al., 1989; Caughey et al., 1996; Kelley et al., 1991), as well as those derived from marine oils (Caughey et al., 1996; Endres et al., 1989, 1993; Kelley et al., 1992b; Kramer et al., 1991; Lee et al., 1985; Madden et al., 1991; Molvig et al., 1991; Payan et al., 1986; Virella et al., 1989), on immune function. These studies have suggested that consumption of n-3 PUFA reduces a number of indices of immune function and, because of this, has been reported to be beneficial in the management of autoimmune diseases such as arthritis in humans.
Kelley et al. (1991) added flaxseed oil (to provide 6 percent of energy as α-linolenic acid [ALA]) to a baseline diet containing 23 percent of energy as total fat, and fed this diet to healthy, young, adult male volunteers for 8 weeks. The addition of flaxseed oil inhibited the proliferation of PBMCs in response to T- and B-cell-specific mitogens because of either the added n-3 PUFA or the higher fat level (a control group receiving 30 percent of energy as total fat was not included).
In a study by Meydani et al. (1993) utilizing fish oil supplements, 22 healthy adults were fed a baseline diet for 6 weeks, followed by a 24-week test diet. The low-fish-oil test diet, low in marine n-3 PUFA (0.13 percent EPA + DHA), increased the response to T-cell mitogens and had no effect on DTH, IL-6, granulocyte macrophage colony stimulating factor (GMCSF), or PGE2
production. In contrast, the high-fish-oil diet, high in marine n-3 PUFA (0.54 percent EPA + DHA), decreased the percentage of CD4+ cells and increased the percentage of CD8+ cells. In addition, this diet decreased responses to T-cell mitogens, the hypersensitivity skin response, and the production of cytokines IL-1β, TNF, and IL-6 by mononuclear cells. In another study, Kelley et al. (1992b) observed that the immune response of healthy young men (25–40 years old) was not inhibited by consumption of 500 g/d of salmon that supplied 2.1 percent of energy as marine oil-derived n-3 PUFA. In a recent study not previously cited, Hughes et al. (1996) reported that fish oil supplementation inhibited the expression of major histocompatibility complex Class II molecules and adhesion molecules on human monocytes. Since these surface molecules are involved in the immune response to presenting antigens, the authors suggested that this is a potential mechanism by which n-3 PUFA may suppress the immune response.
A series of studies has examined the effects of fish oil medium-chain triglycerides (so-called structured lipids) on the incidence of postoperative infection and other indicators of renal, hepatic, and immune function. Bistrian and coworkers (Swails et al., 1997) report significant decreases in infection and improved clinical indices in patients fed enteral diets containing these structured lipids in the immediate postoperative period following surgery for gastrointestinal malignancies.
Mixtures of fish oils and arginine, with or without nucleic acids, have also been administered in the immediate postoperative period with mixed results. Two large clinical trials, one in the United States and the other in Germany, have demonstrated significant decreases in postoperative infection and length of hospitalization following immediate postsurgical feeding with an enteral supplement (Trade name: Impact, manufactured by Sandoz) (Bower et al., 1995; Senkal et al., 1997). In a more recent study, patients given a mixture of n-3-fatty acids (contained in fish oils) and arginine in the immediate postoperative period experienced no beneficial effects on immune function.
Calder (1997) has suggested that diets enriched in fish oil n-3 PUFAs may be of use in the therapy of acute and chronic inflammation and disorders involving inappropriately active immune responses (such as autoimmune disorders). High-fat diets also have the potential to affect immune function adversely as evidenced by the fact that such diets were once used to delay or prevent transplant rejection (Beisel, 1992).
In summary, limited data suggest that moderate reductions in total fat calories (i.e., 26 percent versus 30 percent) may have some beneficial effects in enhancing immune function. To interpret data in this area, a number of caveats are important. Many variables (including total fat, n-6 PUFA, n-3 PUFA, and antioxidants among others) were changed simultaneously in all of these studies. These variables significantly interact with each other, and in human studies, the
possible number of subjects does not allow for the kind of statistical analysis necessary to sort through all variables and interactions. The most important caveat may be that in all of these studies, surrogates of immune function were measured, rather than immune function itself (that is, immunity to disease and resistance to infection). Finally, it should be noted that although increasing the consumption of marine oils that supply eicosapentaenoic acid (EPA) and DHA may reduce the risk of heart disease and be useful in treating autoimmune disease and inflammation, such diets may well reduce immune function.
Minerals
Iron
In Chapter 15, Keusch provides an overview of iron metabolism and the role of iron in both host defense and the virulence of the invading pathogen. As with many other aspects of the immune system, iron has both positive and negative effects, promoting host defense or microbial virulence under differing circumstances. Iron is highly reactive, with considerable ability to generate free radicals that are toxic to both host and microbial cells. The host and the invading organism both require the biological mechanisms to acquire and detoxify iron. The battle between host and pathogen is partly a battle of binding affinity in which the chelator protein that binds iron with greater affinity is able to strip it from the protein that binds it with less affinity.
In the mammalian host, iron is bound primarily to protein complexes, including iron transport (transferrin or lactoferrin) and storage proteins (ferritin), enzymes (cytochrome c), and oxygen transport systems (heme) (Griffiths, 1987). The mammalian iron acquisition system is very efficient, having the ability to compete for ferric iron even from insoluble ferric hydroxide. Because most iron is present in the bound form, the free iron pool is very small. Free radicals that are formed are destroyed by iron-containing enzymes such as catalase. Synthesis of the transferrin receptor and synthesis of ferritin (for iron transport and storage) are regulated reciprocally by iron concentration via a posttranscriptional mechanism (Klausner et al., 1993). Iron influences immune functions via cytokines and nitric oxide (Weiss et al., 1995), and iron-containing enzymes play a key role in the bactericidal activities of phagocytic cells. Morikawa et al. (1995) reported that ferritin directly suppresses the differentiation and maturation of human B-lymphocytes into antibody-producing cells. Lactoferrin, the milk protein, has a direct transcriptional role that may help explain the direct transmission of passive immunity from mother to child (Fleet, 1995).
Iron also is required for microbial growth; hence, microorganisms compete with the host for iron by using analogous systems of acquisition, transport, and
detoxification. Many microbes make siderophores (iron-binding chelators), which are high-affinity binding molecules for the ferric ion that have the ability to remove iron from host iron-binding proteins including ferritin (Neilands, 1995). In response to low iron availability, E. coli, for example, depresses transcription of genes involving iron acquisition and transport, including iron chelators, outer membrane siderophores, and inner membrane transport proteins. Under anaerobic conditions, ferrous iron is more available because of its increased solubility. In addition, a ferrous iron transport gene has been demonstrated in E. coli. It is clear that pathogenic microorganisms adapt to low iron availability through the regulation of gene transcription and translation by iron. In Keusch's view, this raises doubts about enhancing immunity by withholding iron.
Iron Deficiency. Iron is needed by both pathogens and their hosts and is required for host immune function. Transferrin iron is required for the clonal expansion of lymphocytes via ribonucleotide reductase, and iron uptake also must precede DNA synthesis (Kay and Benzie, 1986; Phillips and Azari, 1975). In humans with iron deficiency anemia, decreases in CD3+ and CD4+ B-lymphocytes, and killer-cell activity have been reported (Santos and Falcao, 1990). In addition, iron deficiency is associated with a decrease in delayed-type skin test reactivity to antigens and with impaired mitogen-stimulated lymphocyte proliferation in vitro (Krantman et al., 1982). Data from animal studies suggest that antibody production and CMI are likely to be impaired by iron deficiency because of the role of iron metalloenzymes in DNA synthesis and cell proliferation. Whether iron deficiency affects the host–pathogen relationship at the clinical level remains to be demonstrated conclusively. However, as discussed below, it is clear that iron deficiency does not and will not enhance immune responses.
Iron Excess. Because invading microorganisms require iron and compete for it, it has been hypothesized that withholding therapeutic iron during infection will protect the host, and excess iron will enhance infection (Weinberg, 1984). It has been reported that iron overload states such as β-thalassemia, sickle cell anemia with multiple transfusions, or idiopathic hemochromatoses result in iron-saturated transferrin. Any excess iron then can form loose complexes with albumin, thereby increasing the availability of iron to microorganisms (Hershko and Peto, 1987). Increased infections and fatal outcomes have been associated with these conditions (Barrett-Conner, 1971; Buchanan, 1971) and also have been observed in animal models of hemochromatosis. However, it is difficult to attribute these adverse effects completely to free iron-related microbial growth and infection rather than to damage occurring to the reticuloendothelial system and disrupted cellular function resulting from iron-mediated oxidation or peroxidation effects (Hershko et al., 1988). For example, although 20 percent of β-thalassemia
deaths result from infection, nearly all occur in splenectomized patients. Nevertheless, free iron does increase oxidative damage to cells, and iron excess is, in fact, likely to impair immune function. A number of defects in immune mechanisms have been demonstrated in thalassemia patients. The addition of increasing amounts of iron to T-lymphocytes diminishes clearing efficiency and reduces the proliferative response to mitogens (Good et al., 1988; Munn, 1981). Reductions in the number and function of CD4+ cells and decreases in NK-cell activity have been reported in iron-overload patients (Akbar et al., 1986).
Clinical Studies. To clarify the conditions under which iron deficiency or iron excess is harmful, data obtained from well-controlled clinical studies are essential. In reviewing published clinical studies dealing with the effects of iron deficiency or overload on susceptibility to infection, Keusch (1994) concludes that most studies are flawed in design for one reason or another. The most frequently cited studies showing a benefit of iron fortification are those that involve a comparison of infant formula with and without added iron (Andelman and Sered, 1966). Unfortunately, in these studies, the morbidity data involving respiratory and intestinal disease were obtained by maternal recall rather than observation by trained personnel. Thus, the clinical consequences of iron deficiency with respect to immune function remain uncertain.
Several clinical studies, however, have reported that iron overload can sometimes increase the severity of infections. These typically occur under conditions where the host does not compete well with the invading pathogen for protein-bound iron; thus the pathogen benefits from the increase in free iron that results from iron overload. For example, life-threatening infections from low-virulence strains such as Yersinia enterocolitica have occurred in patients on iron chelation therapy or with iron overload following massive ingestion of iron (Carniel et al., 1987, 1989). High plasma iron values (following nutritional therapy) have also induced lethal cerebral malaria in asymptomatic, parasitemic, malnourished African children (Murray et al., 1978). Clearly iron in relation to infection has both positive and negative effects.
Iron Status of Military Personnel. In December 1995, the CMNR responded to a request by USARIEM and the Commander, USAMRMC to examine data pertaining to the iron status of women in basic combat training (BCT) and to make recommendations on the extent of the problem, how to treat it, and any additional research necessary. According to data presented (Friedl et al., 1990; Klicka et al., 1993; Westphal et al., 1995), when the criterion for iron deficiency was defined as a serum ferritin concentration of less than 12 μg/L and the criteria for iron deficiency anemia were defined as a combination of low serum ferritin and a hemoglobin concentration of less than 120 g/L, 17 percent of new female recruits who were tested at entry to BCT fit the criteria for iron deficiency while 8 percent could be classified as having iron deficiency anemia. A survey of a similar (but not the same) population of women at the end of BCT
showed that by the end of training, 33 percent were iron deficient and 26 percent were anemic. When iron deficiency is defined as a serum ferritin concentration of less than 12 µg/L, the sensitivity for detecting iron deficiency is 61 percent and the specificity is 100 percent (Hallberg et al., 1993).
Iron deficiency anemia can be expected to have adverse effects on military performance of both men and women depending in part on its severity. Performance deficits in both men and women due to compromised iron status have been demonstrated most clearly during exercise of prolonged duration such as long-distance running (Newhouse and Clement, 1988). Iron deficiency anemia may also have an adverse impact on recovery from trauma, especially trauma involving significant blood loss. However, data to support deficits in physical performance in iron-compromised individuals have not been systematically collected by the military. Some preliminary evidence suggests that iron supplementation of nonanemic women can improve aerobic capacity (J. Haas, Cornell University, personal communication, 1997).
After reviewing the data, the CMNR recommended to the Army that personnel with iron deficiency or iron deficiency anemia should receive appropriate medical treatment and monitoring until laboratory results show a return to normal values; a delay in deployment was also recommended for personnel with iron deficiency anemia. Current guidelines for treatment of nonpregnant women of childbearing age recommend an oral dose of 60–120 mg/d of iron with nutritional counseling. If, after 4 weeks, the anemia does not respond to iron treatment despite compliance with supplementation and the absence of illness, further evaluation is warranted using other laboratory tests (CDC, 1998).
In summary, mechanistic data exist to suggest strongly that both iron deficiency and iron excess can increase susceptibility to infection, albeit by different mechanisms. It is important to note, however, that there is a fairly large range of iron intakes over which the immune system can function normally. Although the evidence for iron excess may be compelling, it is likely that for the military, the potential reduction in immune function due to iron deficiency is of more immediate and consequential importance than iron overload. The negative consequences of low iron status are especially dire for women in the military.
Zinc
Zinc is clearly the most important trace element with respect to immune function. Many animal studies have shown that zinc deficiency leads to decreased T-cell function, impaired antibody response, reduced thymus size, and depletion of macrophages and lymphocytes in the spleen (Beisel, 1982). The inherited defect in intestinal zinc absorption, acrodermatitis enteropathica,
causes a severe (but treatable) zinc deficiency state in afflicted infants, resulting in similar widespread immunological dysfunctions. There also is evidence that zinc deficiency in elderly persons can result in heightened susceptibility to infectious disease (Chandra, 1988). Conversely, excess intakes of zinc also have been reported to be immunosuppressive so that both excess and deficient zinc status can have adverse effects on immune function (Chandra, 1982). Given that zinc is a vital cofactor for many different enzymes, regulates some immune-related genes, influences cytokine effects in some situations, and may play a key role as a component of thymic hormones that help regulate all T-cell functions, it is not surprising that deficiency of this essential trace element results in numerous immunological impairments, but its precise role in proper immune system functioning has yet to be clarified fully.
Clinically, zinc deficiency is almost impossible to prove in individual patients, in contrast to groups, by any current, clinically available, diagnostic methods. However, zinc deficiency almost always coexists with severe PEM, and it is difficult to separate the overlapping effects of these two states on immune system function. Animal data show that unifactorial, experimental zinc deficiency leads to a greatly heightened susceptibility to infection and immune system dysfunctions similar to those seen in PEM (Fraker, 1993). Zinc deficiency also impairs the body's ability to mobilize its stores of vitamin A (Udomkesmalee et al., 1992).
In contrast, excess intakes of zinc can have an adverse impact on the immune system (Chandra, 1982). Long-term clinical studies (Tang et al., 1996) showed that HIV patients with zinc intakes greater than 20.2 mg/d had decreased survival rates beginning at about 1,000 d and worsening through a 2,500-d period of observation, compared to HIV patients whose zinc intake was less than 14.2 mg/d. Furthermore, excess zinc intake can interfere with intestinal absorption of copper (Kramer et al., 1993). Imbalances between zinc and copper may occur because of either deficient or excessive copper intake, or excessive zinc intake relative to copper. There is some evidence to suggest that the interactive effects of zinc and copper on the immune response may involve differential cytokine stimulation (Scuderi, 1990). In studies with children, zinc has been shown to reduce the morbidity associated with secretory diarrhea (Rosado et al., 1997; Sazawal et al., 1995). This effect of supplemental zinc is of unknown mechanistic basis but could be related to enhanced immune function of the intestine. An effect on diarrheal disease in the field is possible.
Copper
Copper, like zinc, is a necessary constituent of numerous metalloenzymes, and deficiency of this essential trace element results in an increased
susceptibility of animals to a wide range of infectious agents. Copper deficiency in a variety of animal models has caused a decreased antibody response to a number of antigens, decreased T-cell proliferative response, decreased NK-cell activity, and thymic atrophy (Bala, 1991; Blakley and Hamilton, 1987; Prohaska et al., 1983; Lukasewycz and Prohaska, 1990). Copper deficiency is rare in human adults and, if present, is characterized by leukopenia and anemia (Prasad et al., 1978). Moreover, children suffering from Menkes disease, an inborn error that results in failure to absorb copper, normally die of infectious bronchopneumonia. A well-controlled human metabolic feeding study showed that lymphoproliferative responses to mitogens were markedly impaired in healthy adult men fed a low-copper diet for 66 d (Hopkins and Failla, 1997). Despite compelling evidence that copper is required for a normal immune system, more research is needed to clarify its role in proper immune function.
Selenium
Selenium is now known to be a part of several mammalian enzymes including four glutathione peroxidases (Burk, 1997), three deiodinases (Arthur and Beckett, 1994; Berry and Larsen, 1992; Croteau et al., 1995), and thioredoxin reductase, as well as a variety of microbial enzymes (Chaudiere et al., 1984). Selenium also is found in a number of selenoproteins (selenoprotein P, selenoprotein W) whose enzymatic activities have yet to be determined. Many of selenium's effects can be explained most simply on the basis that it is a required constituent of the glutathione peroxidase family of enzymes. Selenium deficiency has been associated with an increased susceptibility to certain infectious pathogens, perhaps because of decreased antibody production and impaired lymphoproliferative responses in the deficient state (Kiremidjian-Schumacher and Stotzky, 1987). In China, selenium deficiency also has been associated with an endemic juvenile cardiomyopathy (known as Keshan disease). This association has led to the discovery that the selenium status of the host may have an effect on the genetic makeup of a pathogen. Because of pronounced seasonal and annual variations in the incidence of Keshan disease, an infectious component may be involved in its etiology. In fact, a number of enteroviruses were isolated from Keshan disease patients, and one of these, a coxsackievirus B4, was tested by the Chinese for its cardiotoxic effects in mice fed diets of varying selenium content (Bai et al., 1980).
Beck (see Chapter 16) and Orville A. Levander confirmed the Chinese results by showing that feeding mice a selenium-deficient diet increased damage to the heart caused by a myocarditic viral strain, coxsackievirus B3/20 (CVB3/20). Subsequently, they showed that a normally amyocarditic strain,
coxsackievirus B3/0 (CVB3/0) (that is, a strain that normally does not cause heart damage) became myocarditic when inoculated into selenium-deficient mice. Similar changes in Coxsackievirus virulence (i.e., the myocarditic strain became more so and the amyocarditic strain became myocarditic) have been demonstrated in vitamin E-deficient, fish oil-fed mice. All of these dietary effects (selenium deficiency, vitamin E deficiency, fish oil supplementation) can be explained most readily on the basis of an increased oxidative stress imposed on the host by the diet.
The biochemical mechanisms responsible for these dietary effects on viral virulence in mice are not known, but defects in certain host immune functions were observed (impaired mitogen- and antigen-stimulated T-cell proliferation). Other immune parameters (antibody production, NK-cell function) were not affected by selenium or vitamin E deficiency.
Isolation of the CVB3/0 that exhibited virulence in selenium-deficient mice (now called CVB3/0Se), followed by passage through cell culture and reinoculation into a normal mouse, revealed that this virus had undergone a genomic alteration that was stable for at least one such passage. This observation appears to be the first report that the nutritional status of the host might affect the genetic makeup of a pathogen.
If these results are generally applicable to other viruses (or perhaps even other microbial pathogens) and/or other nutritional deficiencies, the implications for public health in general and military health in particular could be profound. As pointed out by Morse (see Appendix D ), soldiers often must perform in crowded and unsanitary environments, which increases their vulnerability to infection by a variety of pathogenic agents. If, for any reason, the nutritional well-being of military personnel is compromised, they could become unwitting incubators of novel viruses with unpredictable properties.
In summary, dietary deficiencies of a variety of nutritionally essential trace elements (zinc, copper, selenium) have all been shown to have an adverse impact on a number of immune functions in laboratory animals. Moreover, deficiencies of zinc and/or copper have resulted in increased susceptibility of humans to certain infections. However, excessive intake of some trace elements (for example, zinc) may lead to immunosuppressive effects; so there clearly exists an optimal range of nutritional status for proper immune function.
Other Factors
Alcohol
Excessive alcohol consumption is a major health problem in the United States, and high rates of excessive consumption have been observed among military personnel (Bray et al., 1995). Although data were not presented at the
workshop, it is important to discuss the various mechanisms by which alcohol interferes with the complex mechanisms of nutritional immunomodulation. Alcohol acts directly on mechanical barriers in the gastrointestinal tract and increases the permeability of the intestinal wall, which results in a greater absorption of immunogenic material in the intestine. Alcohol further affects granulocytopoiesis and suppresses various immune functions (Watzl and Watson, 1992).
Indirect effects on immune response also can be caused by alcohol-induced malnutrition. Heavy alcohol intake (abuse) is associated with a high percentage of total energy intake being contributed by alcohol and sometimes with an inadequate intake of protein, vitamins, and minerals. In addition, alcohol abuse impairs absorption, utilization, storage, and excretion of nutrients, which in combination with inadequate nutrient intake results in nutritional immunosuppression. The nutrients most likely at risk of becoming depleted are folate, thiamine, vitamins A and B6, and zinc.
Alcohol in vivo causes abnormalities in the function and/or structure of a broad array of cells involved in humoral and cellular immunity—including lymphocytes, Kupffer cells (mononuclear phagocytes found on the luminal surface of liver), and other macrophages—and cytokines, namely, TNF, IL-1, and IL-6 (Martinez et al., 1992).
Health and Stress
In Chapter 17, David C. Nieman discusses how the immune system responds to chronic exercise of varying intensity and duration, both in health and during periods of compromised health. In Chapters 18 and 19, respectively, Seymour Reichlin and Leonard P. Kapcala review the role of the neuroendocrine system in moderating immune function. Reichlin describes the hypothalamic–pituitary–adrenal, thyroid, and gonadal system interrelationships and responses to inflammatory disease conditions, whereas Kapcala discusses the involvement of individual cytokines in stimulating the hypothalamic–pituitary–adrenal axis. Superimposed on the demands of exercise and neurohormonal or neuroimmunological activation, an individual's innate biologic rhythms also may affect changes in the body's immune response, as described by Erhard Haus in Chapter 20.
Exercise, Infection, and Immunity
The interaction of exercise and immunity has two facets: the first is the effect of exercise on various elements of the immune system; the second is the
effect of infection on exercise performance and the ability to recover. In Chapter 17, Nieman addresses both of these issues.
The effect of exercise on the immune system depends on a variety of factors, the most important of which seem to be the intensity and duration of exercise and the immune component being discussed. The acute response (within 1–2.5 h) to strenuous exercise (> 65 percent Vo2 max) includes neutrophilia and lymphocytopenia. This response is probably due to an exercise-induced increase in stress hormones (especially cortisol) and increased cytokine concentrations. Increased levels of cortisol are associated with neutrophilia, eosinophilia, lymphocytopenia, and a suppression of NK- and T-cell activity (Cupps and Fauci, 1982). Mitogen-induced lymphocyte proliferation (Eskola et al., 1978; Nieman et al., 1995b), upper airway neutrophil phagocytosis, and neutrophil oxidative burst (Macha et al., 1990; Müns, 1993) have been shown to be suppressed for several hours after strenuous activity, leading to the idea that exercise creates a window of opportunity for infection (see Nieman, Chapter 17). Other stresses such as chronic mental stress and anxiety have been associated with similar suppression of immune function, probably through the same hormonal mechanism.
Highly trained endurance athletes, both young and old, appear to have enhanced NK-cell activity and decreased neutrophil function in comparison to age- and sex-matched sedentary controls (Nieman et al., 1993b, 1995; Pedersen et al., 1989; Tvede et al., 1991). However, measurements taken after 8–15 weeks of moderate exercise show that this exercise does not seem to affect either parameter. Mitogen-induced lymphocyte proliferative response (a measure of T-cell activity) does not appear to be changed by moderate physical activity, although strenuous activity may negatively affect the immune system in the young. Swedish studies indicated that moderate physical training stimulates the immune system, whereas exhaustive and long-lasting exercise is followed by a temporary immunodeficiency and an increased susceptibility to respiratory tract infections (Friman et al., 1997).
In the elderly, intense physical training is associated with an increase in NK- and T-cell activity (Shinkai et al., 1995) and PHA-induced lymphocyte proliferation (Nieman et al., 1993b), compared to individuals having a sedentary life-style. However, moderate activity does not seem to have the same effect, and training may have to be sufficient to induce changes in body weight and fitness to be effective in stimulating the immune system in the elderly as well as the young.
A J curve of risk has been proposed by Nieman, which means that at moderate activity, the risk to the individual of developing a URI is lower than in a sedentary or strenuously active situation (Nieman, 1994). In addition, the symptoms associated with any URI are diminished in those who are moderately active regardless of age; however, he points out the dearth of well-controlled
studies of this phenomenon. Infection does decrease various measures of performance. Nieman suggests that mild exercise during infection with localized symptoms is not contraindicated, but exercise should be curtailed for 2 to 4 weeks after severe infections with systemic involvement.
The military relevance of the interaction between nutrition and physical activity and the effect of this interaction on the immune system are pointed out by Nieman (see Chapter 17). Low energy intake, weight reduction, and increased levels of circulating glucocorticoids often associated with field maneuvers (see Shippee and Wiik, Chapters 5 and 6, respectively) may suppress the immune system independently, and superimposing physical activity may increase vulnerability. The use of flu shots to minimize risk in exercising troops is recommended by Nieman. In addition, the use of immunomodulator drugs, such as indomethacin, aspirin, and ibuprofen, could be considered; however, studies on these compounds are ongoing, and no clear conclusions are available at present. Studies of glutamine (see Wilmore, Chapter 11), vitamin C (see Rall and Meydani, Chapter 13), n-acetylcysteine, and carbohydrate-containing beverages (by way of their ability to decrease cortisol levels) suggest that these may be candidates for prophylactic agents.
Two final conclusions regarding exercise and immunity can be drawn:
- Low- to moderate-intensity exercise (< 60 percent Vo2 max, such as that performed by most troops in daily life) of reasonable duration (< 60 min) may exert less stress on the immune system than more strenuous or longer exercise, with little change in immune function being documented under conditions of moderate exercise.
- Repeated bouts of strenuous activity may put individuals at risk for infection (especially URIs) because of suppression of neutrophil activity. Risk of illness is particularly high during the first 2 weeks following a bout of prolonged strenuous exercise.
-
Hormonal Responses to Stress
The anterior pituitary gland secretes a number of hormones vital for normal physiological processes, including those that are involved in the regulation of other endocrine glands. Secretion of hormones from the anterior pituitary is regulated by neurohormones produced in the hypothalamus and delivered to the pituitary gland through a portal circulation system that directly connects the hypothalamus to the anterior pituitary (Rang et al., 1995).
The Stress Response Model
The body responds to bouts of moderate–severe exercise, changes in environmental exposure (heat, cold), or hypovolemia (due to excess sweating, decreased intake, or diarrhea) in a rather stereotypic manner by elaborating ''stress hormones.'' All of the hormones that regulate carbohydrate metabolism participate in host responses to infection. Among these are the glucocorticoids from the adrenal cortex, the catecholamines from the adrenal medulla and sympathetic nervous system, and glucagon, a pancreatic hormone. These three families of stress hormones have been infused into normal volunteers, and the metabolic and immunologic responses monitored. Many, if not all, of the changes observed are similar to those described in injured and infected humans (Bessey et al., 1984; Watters et al., 1986). The glucocorticoid cortisol, when infused into healthy volunteers, has major immunological effects; lymphocyte counts increase, while the proportions of T3-, T4-, and T11-lymphocytes decrease (Barber et al., 1993; Calvano et al., 1987). When epinephrine was added to the infusion, the marginating pool of circulating neutrophils was mobilized, but chemotaxis measured after 6 h of infusion was reduced (Davis et al., 1991). When cortisol alone was administered for 6 h before an endotoxin challenge, the immunological response was greatly reduced compared to the response in volunteers receiving endotoxin alone (Barber et al., 1993). When the three stress hormones were administered together to volunteers, NK-cell activity was suppressed (Blazar et al., 1986). Different cytokines show differential sensitivity to suppression after administration of glucocorticoids in vivo and ex vivo, with IL-6 relatively resistant to suppression by glucocorticoids (DeRijk et al., 1997).
Neuroendocrine Consequences of Systemic Inflammation
In Chapter 18, Reichlin focuses on the manner in which infection and inflammatory disease alter the secretion of hypothalamic and pituitary hormones and the subsequent functioning of their target tissues.
Hypothalamic–Pituitary–Adrenal System
The hypothalamic–pituitary–adrenal system is altered significantly by inflammatory disease. Infections or injections of bacterial toxins stimulate the release of a number of cytokines including IL-1, IL-2, IL-6, and TNF. Within the hypothalamus, these cytokines directly stimulate the synthesis and secretion of corticotropin-releasing hormone (CRH) and vasopressin (VP) (Kapcala, 1997; Reichlin, 1993). Acting in concert, these hormones increase the release of
adrenocorticotrophic hormone (ACTH) from the anterior pituitary, which in turn augments the secretion of cortisol from the adrenal gland. Additionally, in the hypothalamus, CRH activates the peripheral autonomic nervous system, enhancing the release of epinephrine from the adrenal medulla. Epinephrine acts synergistically with CRH and VP to stimulate the secretion of ACTH, thereby regulating the circulating concentrations of cortisol. Since almost all components of the immune response are inhibited by cortisol and other glucocorticoids, pituitary–adrenal activation is accompanied by a reduction in the intensity of the immune response (Kapcala, 1997; Reichlin 1993) (Figure 1-5).
The pituitary–adrenal response to stress, which is characterized by intense mobilization of cytokines and related inflammatory mediators that can compromise healing, has been proposed to have a suppressive effect on excessive inflammatory reactions (Munck et al., 1984), and this has been supported by several animal models (Sternberg et al., 1992).
Humans with adrenal insufficiency are affected more detrimentally by sepsis than are individuals with normal adrenal function. However, clinical studies indicate that the treatment of sepsis with glucocorticoids does not improve survival in patients with normal adrenal function (Reichlin, 1993).
Hypothalamic–Pituitary–Thyroid System
A variety of acute and chronic illnesses can lead to abnormalities in the hypothalamic–pituitary–thyroid system, characterized by low circulating levels of triiodothyronine, low or normal blood levels of thyroxine (T4), and depressed thyroid-binding proteins (Wartofsky and Burman, 1982). Additionally, the depressed plasma concentrations of thyroid hormones fail to stimulate the secretion of thyroid-stimulating hormone (TSH), thus indicating an impairment in the pituitary–thyroid feedback system (Beisel, 1991, 1992; Reichlin, 1993; Shambaugh-Beisel, 1966). These abnormalities are the result of cytokine-induced alterations at all levels of this system (Reichlin, 1993, 1994). Within the hypothalamus, cytokines suppress the synthesis and secretion of thyrotropin-releasing hormone (TRH) (Kakuscska et al., 1994) and increase the secretion of somatostatin. Within the pituitary, cytokines inhibit secretion and reduce the biological potency of TRH. Finally, responsiveness to TRH is reduced in the thyroid, and circulating concentrations of thyroid hormones are low (Reichlin, 1993). Starvation can exacerbate the effects of inflammation on thyroid functioning, as noted by decreased T4 to T3 conversion. Whether these inflammation-induced changes in the hypothalamic–pituitary–thyroid are beneficial, remains open to question.
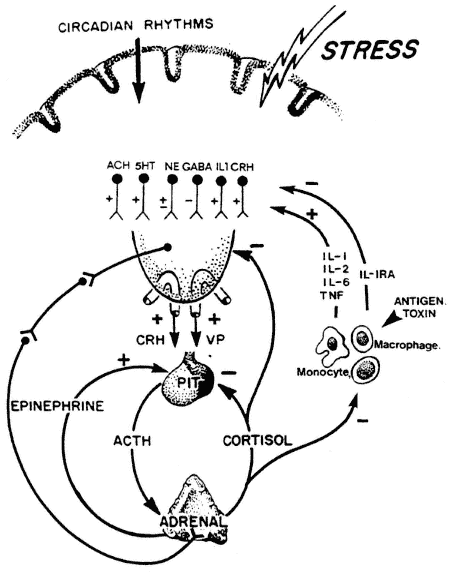
FIGURE 1-5
Schematic outline of neuroendocrine factors that regulate the secretion of the adrenal cortex. ACTH release from the pituitary is stimulated by corticotropin-releasing hormone (CRH) and vasopressin (VP) acting synergistically. Circulating epinephrine (the response of the activated sympathetic nervous system) is also synergistic for ACTH release. Secretion of ACTH is inhibited at the pituitary level by circulating glucocorticoids, and at the hypothalamic level, CRH and VP are also under negative feedback control by glucocorticoids. VP, CRH neurons are in turn subject to a wide range of influences—circulating cytokines, prostaglandins, and many neurotransmitters. Some, such as acetylcholine (ACH), are stimulatory; others, such as gama-aminobutyric acid (GABA), are inhibitory. Interacting with the hypothalamic–pituitary–adrenal axis is the peripheral immune system (as well as endothelia and other structures), which releases cytokines into the blood that then can activate the adrenals. Each of the major inflammatory cytokines, IL-1 (α or β), IL-2, IL-6 and TNF-α, is capable of activating ACTH release. The peripheral immune system provides a chemosensory system by which the presence of foreign molecules can be communicated to the brain and induce an appropriate response, a model of "bidirectional communication between the brain and the immune system" (Blalock, 1989).
SOURCE: Blalock, 1989.
NOTE: 5HT, 5-hydroxy tryptamine; NE, norephrine.
Pituitary–Gonadal System
Gonadal function also is reduced in severe inflammatory disease. Burns, sepsis, and severe trauma are associated with a reduction in plasma concentrations of sex steroids in both males and females. The reductions in steroid values are related in part to the direct actions of cytokines on the testes and ovaries. Normally, low circulating levels of estrogens or testosterone stimulate the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus. However, in inflammatory disease, IL-1 inhibits the pulsatile release of GnRH from the hypothalamus, which in turn inhibits the release of gonadotropins from the pituitary and thus contributes to the low plasma concentrations of circulating gonadal steroids (Reichlin, 1993). Although a decreased availability of testosterone might be hypothesized to be associated with muscle wasting, the functional role of gonadal hormone suppression in inflammatory disease is unknown at present.
Growth Hormone
Inflammatory disease often is accompanied by increases in plasma growth hormone (GH) concentrations (Beisel et al., 1968; Reichlin, 1993). However, circulating levels of GH may not be representative of the effects of this hormone on the tissues themselves. GH-directed protein metabolism is mediated by insulin-like growth factor-I (IGF-I, somatomedin C), a protein derived from the liver whose secretion is regulated by GH and the availability of metabolic substrate. In sepsis and severe burns, plasma GH concentrations are elevated, while IGF-I values are low, suggesting that in these conditions, there may be resistance to GH at the tissue level. Because administration of GH to individuals with severe burns can improve graft healing and survival (Knox et al., 1995), the burn-induced increase in GH may be positive but suboptimal. Reichlin suggests that administration of IGF-I to sepsis or burn patients might thus be preferable to administration of GH. IGF-I infusion has been shown to lower protein oxidation and to stimulate glucose uptake in a small study of burn patients (Cioffi et al., 1994), and IGF-I enhances the immune response in rats (Hinton et al., 1995).
In summary, a variety of changes in the hypothalamic–pituitary system occur via activation of inflammatory responses (and cytokine release) through products of cell injury or toxins affecting pituitary–endocrine end organ functioning. Some of these changes in neuroendocrine function are valuable because they help to promote healing and survival. However, others may potentiate the detrimental consequences of inflammation. Reichlin concludes that interactions between cytokine-mediated changes in neuroendocrine activity
and poor nutrition could detrimentally affect immunological activity and resistance to stress in military personnel.
Inflammatory Stress and the Immune System
In Chapter 19, Kapcala deals principally with how cytokines act through the brain to induce glucocorticoid and epinephrine secretion. As previously mentioned by Reichlin, the cytokines most involved in the stimulation of the hypothalamic–pituitary–adrenal axis (HPAA) are IL-1 and IL-6, which are the most potent, and TNF (Chrousos, 1995). Kapcala reiterates the effect of IL-1 on the CRH–ACTH–cortisol axis and discusses data indicating that cytokines might act at all levels of the HPAA to induce glucocorticoid release, but he concludes that the dominant effect is mediated through the brain and that the CRH neurons are probably the most important site. Since peptides, including IL-1, normally do not cross the blood–brain barrier in appreciable amounts (Coceani et al., 1988), one or more specialized mechanisms must exist for giving circulating cytokines access to brain if they are to influence central CRH neurons.
Under conditions of infection and/or inflammation, and even in the absence of infection or inflammation, cytokines may permeate the brain through specialized regions termed circumventricular organs (CVOs) to influence CRH (see Kapcala, Chapter 19). This communication also may occur via circulating cytokines that can activate peripheral sensory neurons, relaying input to the brain, which ultimately stimulates CRH neurons (Wan et al., 1994; Watkins et al., 1995). Also, cytokines may signal the brain through binding to receptors on cerebral blood vessel endothelial cells and activating synthesis of second messengers, prostaglandins, and nitric oxide (Sternberg, 1997b). Regardless of the mechanism, stimulation of central IL-1 receptors by peripheral and central IL-1 is thought to promote CRH release and ultimately ACTH and glucocorticoid secretion (Chrousos, 1995).
The importance of glucocorticoid release in limiting the toxic effects of cytokine overexpression also is of concern. The lethality of an infectious agent in animals with a compromised glucocorticoid response has been documented (Bertini et al., 1988; Fan et al., 1994; Kapcala et al., 1995; Nakano et al., 1987), and the administration of glucocorticoid protects against these effects (Bertini et al., 1988; Butler et al., 1989; Kapcala et al., 1995; MacPhee et al., 1989; Nakano et al., 1987). Moreover, compromised adrenal function is associated with a prolonged elevation in circulating concentrations of IL-1 and TNF, providing further evidence that the adrenal responses normally limit widespread cytokine effects (Butler et al., 1989; Zuckerman et al., 1989). One such mechanism by which increased glucocorticoid secretion would counterbalance the toxic effects of excessive cytokine actions in the body involves inhibition by glucocorticoids
of the production of a variety of cytokines and other mediators (for example, prostaglandins and leukotrienes) of the inflammatory response (Munck et al., 1984; Williams and Yarwood, 1990).
CRH neurons in the brain also influence sympathetic output, and their activation by cytokines (IL-1) leads to a clear rise in sympathetic firing and immune suppression. This could be tied pharmacologically to intact sympathetic functioning (Sundar et al., 1990).
In summary, infection leads to a host response that includes the release of cytokines, which promote and amplify cytotoxic actions to kill the infectious agent. Many such cytotoxic actions are not specific to the pathogen and, left unchecked, can damage the host. The additional ability of several cytokines to activate the HPAA and thus to raise levels of circulating glucocorticoids (which have immune-suppressive functions) via direct and indirect actions on central neurons is seen as one mechanism by which the initial immune reaction to infection is contained and toxic effects on the host are limited.
Biologic Rhythms in the Immune System and Nutrition
In Chapter 20 of this volume, Haus reviews the effects of biological rhythms on the functioning of the immune system. Alterations in the immune system are observed across the 24-hour day (circadian rhythm), 7-day week (circaseptan rhythm), and year (circannual rhythm).
Circadian rhythms are observed at all points in the development of the immune response, including the generation of immunoreactive molecules, immunoglobulin synthesis, and proliferation of immunocompetent cells. These rhythms result in differences in the immune response across the circadian cycle and are important for the response to both a primary antigenic stimulation and some extraneous challenge to the immunized subject. Alterations of the normal circadian (temporal) pattern of functioning of the cells in the immune system can have detrimental consequences.
Circaseptan rhythms also have been observed in immune responses. For example, the response of the body after kidney damage or exposure to an antigen (DeVecchi et al., 1981) or treatment with immunosuppressive compounds (Hrushesky and Marz, 1994) varies across an approximately 7-day period.
Immune responses vary across the year. These circannual rhythms may reflect variations in environmental variables, such as daily light levels, temperature, and differences in exposure to antigens, and may be mediated by seasonal changes in the function of the pineal or thyroid gland (Arendt, 1994; Nicolau and Haus, 1994). Circannual rhythms also have been observed in
animals kept for generations under controlled environmental conditions and thus in some cases may be endogenous and genetically fixed.
Biologic Rhythms in the Number and Function of White Blood Cells
The number of white blood cells (WBCs) found in the circulation varies across the 24-h day. Several factors, including influx from storage sites, proliferation of cells, release of newly formed cells into the circulation, and cell destruction and removal, may contribute to the circadian variation in leukocyte number. Although circadian alterations in WBC number are relatively consistent within an individual, there is substantial interindividual variation in circadian patterns. Determinations of WBC number in 150 healthy individuals revealed that peaks or troughs in the values could occur at any hour of the day (Haus et al., 1983). Additionally, different cell types (such as lymphocytes, neutrophil leukocytes, monocytes, and eosinophils) display different rhythmic patterns. In most individuals, the number of lymphocytes peaks during the night, with the highest values between midnight and 1:00 a.m.
Lymphocyte function also varies across the day. Circadian variations in responses to external stimuli are reflected in changes in the number of cells in the peripheral blood and bone marrow. For example, the T-cell response to PHA and B-cell activation with pokeweed mitogen has been found to vary across the 24-h cycle (Haus et al., 1983). As another example, NK-cell activity in the blood of healthy adults is highest in the early morning and reaches a nadir during the night (Gatti et al., 1988).
Haus (see Chapter 20) discusses a number of factors, including alterations in the sleep–wake cycle, lymphoid tumors, and infection with lymphotrophic human HIV that can alter the circadian rhythms of circulating lymphocytes. For example, in HIV-infected patients, circadian rhythm disturbances in WBC numbers occur as an early consequence of the disease (Haus, 1996). Cytokine ratios also have been shown to vary in relation to circadian changes in glucocorticoid levels (DeRijk et al., 1997).
Biologic Rhythms in Cytokines and Their Inhibitors
Circadian variations in cytokines and their soluble receptors (for example, IL-1, IL-2, and IL-6) are found in the serum of healthy individuals. For example, serum concentrations of IL-6, which plays an important role in host defense mechanisms, inflammation, and immune responses, are highest during the night and lowest at midmorning (10:00 a.m.) (Sothern et al., 1995). Circadian rhythms in soluble IL-2 receptors have been found in healthy subjects, and rhythms in IL-6 (Arvidson et al., 1994; Suteanu et al., 1995) and
neopterin (Suteanu et al., 1995) have been observed in patients with rheumatoid arthritis. Monocyte IL-1β and IL-1rα secretion and urinary IL-1rα excretion are significantly increased during the follicular phase of the menstrual cycle in premenopausal women relative to the luteal phase and significantly greater than those of men at all times (Lynch et al., 1994). The time at which cytokines reach the hypothalamus may in part determine the response to cytokines within the CNS and the subsequent activity of the endocrine system.
Biologic Rhythmicity of the Humoral Immune Response
In both animals and humans, there are circadian variations in the response of immunocompetent cells to an antigen and in the response of the immunized organism to secondary challenge following reintroduction of the antigen. For example, in mice injected with sheep red blood cells (commonly used in rodent studies), the fewest plaque-forming cells were found when the animals were injected at the beginning of the dark portion of the daily cycle (Fernandes et al., 1977). In humans, the concentrations of immunoglobulins and other components of serum proteins critical for immune function vary in a circadian manner. In healthy subjects, maximal concentrations of the three major types of immunoglobulins are detected in the early to late afternoon. Alterations in the circadian rhythms of these immunoglobulins have been noted in clinical conditions such as allergic asthma and allergic rhinitis.
Other rhythmic variations in humoral immune responses have been reported. Haus notes that circaseptan rhythms in antibody formation occur in a large number of animal species. Moreover, circannual rhythms in serum immunoglobulins, with peak concentrations during mid-summer to mid-autumn, have been reported in healthy human subjects.
Potential Clinical Relevance of Biologic Rhythms in Immune Function
Human immune responses to inoculation may vary as a function of the time of day at which an antigen is introduced. However, this variation may occur only with some antigens (for example, hepatitis B antigen) and may depend on other factors such as seasonal variations. Available data indicate that although all subjects achieve some level of immunity irrespective of the time of vaccination, higher antibody titers result when inoculations are given at midday (Pöllman and Pöllman, 1988). However, circadian variations in antibody responses to inoculations may be particularly relevant for the success of vaccinations in subjects with poor antibody formation.
Human immune responses to common antigens also vary in a circadian manner. For example, the greatest sensitivity to antigens of ragweed, house dust,
and grass pollen is observed approximately 6 h preceding the middle of the patient's sleep cycle (see, for example, Lee et al., 1977). Similar rhythms have been reported for the hyperreactivity of the bronchial tree to histamine. The times of greatest cutaneous sensitivity to antigens and greatest susceptibility to asthmatic attacks correspond to the low point in the 24-h cycle of adrenocorticosteroid secretion and plasma catecholamine concentration. Haus suggests that these alterations in adrenocorticosteroids and catecholamines could contribute to the differences in day–night sensitivity to allergens and frequency of asthma attacks.
Factors That Interact with Circadian Rhythms in Immune Function
A number of factors, including patterns of sleep, the menstrual cycle, exercise, and the timing of food intake, can alter circadian rhythms associated with the functioning of the immune system. For example, sleep deprivation leads to alterations in biological rhythms and decrements in immune function and, if severe enough, can result in death (Everson, 1993). Impairments in immune function also have been noted in shift workers and individuals suffering from "jet lag." However, because shift work and jet lag are frequently associated with some degree of sleep deprivation, it is not known whether the alterations in immune function observed in these situations are primarily a consequence of sleep deprivation or of circadian desynchronization.
Circadian rhythms in immune function also are influenced by levels of physical activity and patterns of food intake. The effects of exercise on the immune system depend on the intensity of the activity, as described by Nieman in Chapter 17. Moderate exercise appears to improve certain immune functions, whereas extreme or exhaustive activities serve as immunosuppressants. Restricting food intake to a certain time of day (for example, all food eaten in the morning or at night) is associated with alterations in the circadian rhythms in digestive and metabolic functions, including endocrine and exocrine secretions from the pancreas and the secretion of liver enzymes (Fuller and Snoddy, 1968). These alterations could affect the timing of certain immune system responses, although critical evidence for such a relationship is not yet available.
Chronopharmacology and Chronotherapeutics
Knowledge of rhythmic changes in the immune system is important for treating of immune-related disorders and reducing the toxic effects of chemotherapeutic agents (see Haus, Chapter 20). Haus suggests that the timing of treatment relative to the critical periods of a cycle may permit optimal therapeutic effects with a minimal drug dose. Additionally, in the case of
chemotherapy for cancer, understanding both circadian and seasonal rhythms may make it possible to obtain the desired treatment benefits with minimal toxicity (Levi, 1994).
In summary, these rhythms are important in mediating the body's responses to external stimuli such as pain, bacterial and viral infections, toxins, other antigens, and pharmacological agents. Many of these rhythms are inherited; however, others are adjusted in response to environmental variables such as light–dark cycles, environmental temperature, social situation, exercise, and food intake. Because military personnel may encounter infectious agents during field operations and can be subjected to extremes of environmental factors (for example, extreme heat or cold, intense physical exercise, sleep deprivation, and limited times for food consumption), it is important to consider biological rhythms in immune function. Additionally, knowledge of these time-related variations in immune function could be useful in determining the optimum timing of treatment for immune-related disorders.
Effects of Other Stressors on Immune Function
In addition to nutritional deficiency, physical exertion, and alterations in circadian rhythms, a number of other factors have been examined with respect to their influence on immune function or susceptibility to infection. These include temperature extremes, high altitude, sleep deprivation, emotional stress, smoking, and exposure to other environmental pollutants.
Extremes of Temperature
Attempts to examine the effects of cold temperature on immune function of laboratory animals have shown immune suppression in cold-exposed animals; however, the exposure of animals to cold temperatures is an established method of inducing stress. In addition, cold exposure influences sleep patterns for a period of time prior to acclimation (Pozos, 1996). Thus, it is not possible to conclude from this literature whether cold temperature exposures have effects on the immune system that are independent from those of a generalized stress response or sleep disturbance. Hot environments have been hypothesized to stimulate an increase in oxidative stress that could indirectly influence immune function (Young, 1990). As discussed by Morse in his presentation in Appendix D and others (Russell, 1998), attempts to study the effects of climate change on human health and immune function are also confounded by the altered pattern of infectious agents present in various climates.
Exposure to High Altitude
The influence of high-altitude exposure on immune function has been examined by several groups of investigators. Simon-Schnass (IOM, 1996) suggests that the increase in oxidative stress that appears to be induced at high altitude may be due to hypoxia, UV exposure, and other effects on the tricarboxylic acid cycle. However it is difficult to separate these effects from those of the anorexia that is induced by high altitude, the generally increased levels of physical exertion of people in field exercises at high altitude, and the extremes of temperature experienced at high altitude. Japanese investigators studying the mechanism of high-altitude pulmonary edema (HAPE) report significant elevations in many parameters of immune function, including IL-1β, IL-6 and 8, TNF-α, IL-1 receptor agonist, total WBC count, neutrophils, macrophages, lymphocytes, and a number of proteins (Kubo et al., 1998). These changes are believed to be associated with the early stage of HAPE and possibly with pulmonary hypertension; however it is not clear how the ability of the immune system to respond to infectious agents is affected.
Sleep Deprivation
The influence of sleep deprivation on immune function was mentioned earlier as a factor in the decreased immune response observed in the Ranger studies. Dinges and coworkers (1995) have reviewed studies of the influence of sleep deprivation on immune function. No consistent alterations in immune parameters have been reported across studies, suggesting that sleep has no consistently immunosuppresive effects that can be separated from the effects of other (simultaneously imposed) stressors. For example, the work of Haus (see Chapter 20) suggests that the effects of sleep deprivation would not be separable from those of changes in diurnal rhythms.
Emotional Stress
Deployed soldiers experience high levels of both chronic and acute emotional stress. As mentioned by Chandra (see Chapter 7), the stress of grieving for a bereaved spouse has been associated with depressed immune function in elderly individuals. Other studies by the same group have revealed that mucosal wound healing is slowed by the stress induced by final exams (Marucha et al., 1998), that immune response to vaccination and to HSV-1 exposure is inhibited by chronic stress (Glaser et al., 1998; Glaser and Kiecolt-Glaser, 1997), and that response to acute stress is influenced by the presence of chronic stress (Cacioppo et al., 1998). These researchers have proposed a model
(the reactivity hypothesis) in which stress-related changes in HPA A activation are responsible for decreased immune response (Cacioppo et al., 1998).
Smoking and Environmental Pollutants
The effects of smoking on immune function are well known and are reviewed briefly in the discussion of vitamin C and immune function. Because the proportion of active-duty military personnel who smoke is higher than that of the general population, the effects of smoking on immune function are often superimposed on other stressors. Similarly, deployed troops may be exposed to a number of environmental pollutants (such as oil smoke) whose effects on the immune system must be determined. An upcoming CMNR workshop will examine these effects and their ability to be remedied by antioxidant administration.
Effects of Combined Stressors
It should be clear from the foregoing discussion that in the field, exposure to single stressors is not typical and the sources of stress may not always be immediately identifiable. Only well-controlled studies in clinical laboratory settings can assess the effects of single and combined stressors on immune function.
Summary
The Ranger studies as well as other Army Operational Training Program studies have provided an opportunity to evaluate the effects of multiple physical, psychosocial, and nutritional stressors on immune system function. In this report, information is provided regarding the assessment of immune status under field conditions; the impact of compromised nutrition status on immune function; the interaction of health, exercise, and stress (both physical and psychological) in immune function; and the role of nutritional supplements and newer biotechnology methods reported to enhance immune function. The advantages and disadvantages of vitamin and mineral supplementation have been considered not only for their effects on immune system function, but for overall health in general. Finally, the physiologic and immune responses to alterations in neuroendocrine function are examined in depth. Included also is a thorough review of the interrelationship of biologic rhythms and immune system function.
During the workshop, a number of additional questions arose regarding the role of nutrition in immune function, and these are discussed at the conclusion of each author's chapter. These questions focused on assessment methodologies and individual stressors that could possibly alter nutrient requirements, which might in turn affect performance and immune function.
The CMNR's summary of the workshop and selected review of the nutrition and immunology literature set the stage for responding to the questions posed by the Army. The committee's responses, as well as its conclusions and recommendations, are presented in the next two chapters.
References
Abbas, A.K., A.H. Lichtman, and J.S. Prober. 1991. Cellular and Molecular Immunology. Philadelphia, Pa.: W.B. Saunders Co.
Akbar, A.N., P.A. Fitzgerald-Bacarsly, M. DeSousa, P.J. Giardina, M.W. Hilgartner, and R.W. Grady. 1986. Decreased natural killer activity in thalassemia major: A possible consequence of iron overload. J. Immunol. 136:1635-1640.
Aldred, A.R., and G. Schreiber. 1993. The negative acute phase proteins. Pp. 21-37 in Acute Phase Proteins: Molecular Biology, Biochemistry, and Clinical Applications, A. Mackiewicz, I. Kushner, and H. Bauman, eds. Boca Raton, Fla.: CRC Press.
Almond, J., and J. Pattison. 1997. Human BSE. Nature 389:437-438.
Andelman, M.B., and B.R. Sered. 1966. Utilization of dietary iron by term infants: A study of 1,048 infants from a low socioeconomic population. Am. J. Dis. Child. 111:45-55.
Arendt, J. 1994. The pineal. Pp. 348-362. In Biologic Rhythms in Clinical and Laboratory Medicine, Y. Touitou and E. Haus, editors. 2nd ed. Heidelberg: Springer-Verlag.
Arthur, J.R., and G.J. Beckett. 1994. Roles of selenium in type I iodothyronine 5'-deiodinase and in thyroid hormone and iodine metabolism. Pp. 93-115 in Selenium in Biology and Human Health, R.F. Burk, ed. New York: Springer-Verlag.
Arvidson, N.G., B. Gudbjornson, L. Elfman, and A.C. Ryden. 1994. Circadian rhythm of serum interleukin 6 in rheumatoid arthritis. Ann. Rheumat. Dis. 53(8):521-524.
Bai, J., S. Wu, K. Ge, X. Deng, and C. Su. 1980. The combined effect of selenium deficiency and viral infection on the myocardium of mice. Acta Acad. Med. Sci. Sin. 2:29-31.
Bala, S., M.L. Failla and J.K. Lunney. 1991. Alterations in splenic lymphoid cell subsets and activation antigens in copper-deficient rats. J. Nutr. 121(5):745-753.
Ballmer, P.E., and H.B. Staehlin. 1994. Beta carotene, vitamin E, and lung cancer. N. Engl. J. Med. 330(15):1029-1035.
Barber, A.E., S.M. Coyle, M.A. Marano, E. Fischer, S.E. Calvano, Y. Fong, L.L. Moldawer, and S.F. Lowry. 1993. Glucorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 150:1999-2006.
Barbul, A., D.A. Sisto, H.L. Wasserkrug, and G. Efron. 1981. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery 90(2):244-251.
Barone, J., J.R. Herbert, and M.M. Reddy. 1989. Dietary fat and natural-killer-cell activity. Am. J. Clin. Nutr. 50(4):861-867.
Barret-Connor, E. 1971. Bacterial infection and sickle cell anaemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine 50:97-112.
Beaton, G.H. 1996. Basic biology and biology of interventions: A synthesis view. In Beyond Nutritional Recommendations: Implementing Science for Healthier Populations, C. Garza, J.D. Haas, J-P. Habicht, and D.L. Pelletier, eds. Proceedings from the 14th Annual Bristol-Myers Squibb/Mead Johnson Nutrition Research Symposium, June 5-7, 1995. Ithaca, N.Y.: Cornell University.
Beisel, W.R. 1977. Symposium on impact of infection on nutritional status of the host. Am. J. Clin. Nutr. 30:1203-1371, 1439-1566.
Beisel, W.R. 1982. Single nutrients and immunity. Am. J. Clin. Nutr. 35(suppl.):415-468.
Beisel, W.R. 1991. Nutrition and infection. Pp. 507-542 in Nutritional Biochemistry and Metabolism, 2nd ed., M.C. Linder, ed. New York: Elsevier.
Beisel, W.R. 1992 Metabolic responses of the host to infections. Pp. 1-13 in Textbook of Pediatric Infectious Diseases, 3d ed., R.D. Feigin and J.D. Cherry, eds. Philadelphia: W.B. Saunders Co.
Beisel, W.R. 1995. Herman Award Lecture, 1995: Infection-induced malnutrition--From cholera to cytokines. Am. J. Clin. Nutr. 62:813-819.
Beisel, W.R., and P.Z. Sobocinski. 1980. Metabolic Aspects of Fever. Pp. 39-48 in Fever, J.M. Lipton, ed. New York: Raven Press.
Beisel, W.R., and J.M. Talbot, eds. 1985. Research Opportunities on Immunocompetence in Space. Report of the Life Science Research Office, FASEB. Prepared for the Life Science Division, Office of Space Science and Applications, NASA, Washington, D.C. Bethesda, Md.: Life Science Research Office, FASEB.
Beisel, W.R., W.D. Sawyer, E.D. Ryll, and D. Crozier. 1967. Metabolic effects of intracellular infections in man. Ann. Int. Med. 67:744-779.
Beisel, W.R., K.A. Woeber, P.J. Bartelloni, and S.H. Ingbar. 1968. Growth hormone response during sandfly fever. J. Clin. Endocrinol. Metab. 28(8):1220-1223.
Bendich, A., and R.K. Chandra, ed. 1990. Micronutrients and immune functions. New York, N.Y.: New York Academy of Sciences.
Berry, M.J., and P.R. Larsen. 1992. The role of selenium in thyroid hormone action. Endocr. Rev. 13:207-219.
Bertini, R., M. Bianchi, and P. Ghezzi. 1988. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J. Exp. Med. 167:1708-1712.
Bessey, P.Q., J.M. Watters, T.T. Aoki, and D.W. Wilmore. 1984. Combined hormonal infusion simulates the metabolic response to injury. Ann. Surg. 200:264-281.
Bistrian, B.R., G.L. Blackburn, N.S. Scrimshaw, J.P. Flatt. 1975. Cellular immunity in semistarved states in hospitalized adults. Am. J. Clin. Nutr. 28:1148-1155.
Bjerve, K.S., S. Fischer, F. Wammer, and T. England. 1989. α-linolenic acid and longchain omega-3 fatty acid supplementation in three patients with omega-3 fatty acid deficiency. Am. J. Clin. Nutr. 49:290-300.
Blakley, B.R., and D.L. Hamilton. 1987. The effect of copper deficiency on the immune response in mice. Drug Nutr. Interact. 5:103-111.
Blalock, J.E. 1989. A molecular basis for bidirectional communication between the immune and neuroendcorine systems. Physiol. Rev. 69:1-32.
Blazar, B.A., M.L. Rodrick, J.B. O'Mahoney, J.J. Wood, P.Q. Bessey, D.W. Wilmore, and J.A. Mannick. 1986. Suppression of natural killer-cell function in humans following thermal and traumatic injury. J. Clin. Immunol. 6:26-36.
Bocchieri, M.H., M.A. Talle, L.M. Maltese, I.R. Ragucci, C.C. Hwang, and G. Goldstein. 1995. Whole blood culture for measuring mitogen induced T cell proliferation provides superior correlations with disease state and T cell phenotype in asymptomatic HIV-infected subjects. J. Immunol. Meth. 181:233-243.
Borelli, M.I., F.E Estivariz, and J.J. Gagliardino. 1996. Evidence for the paracrine action of islet-derived corticotropin-like peptides on the regulation of insulin release. Metabolism 45(5):565-570.
Bower, R.H., F.B. Cerra, B. Bershadksy, J.J. Licari, D.B. Hoyt, G.L. Jensen, C.T. Van Buren, M.M. Rothkopf, J.M. Daly and B.R. Adelsberg. 1995. Early enteral administration of a formula (Impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: results of a multicenter, prospective, randomized, clinical trial. Crit. Care Med. 23(3):436-449.
Bray, R.M., L.A. Kroutil, S.C. Wheeless, M.E. Marsden, S.L. Bailey, J.A. Fairbank, and T.C. Harford. 1995. Health behavior and health promotion. Department of Defense Survey of Health-Related Behaviors among Military Personnel. Report No. RTI/6019/06-FR. Research Triangle Park, N.C.: Research Triangle Institute.
Breman, J.G., G. van der Groen, C.J. Peters, and D.L. Heymann. 1997. International colloquium on Ebola virus research: summary report. J. Infect. Dis. 176(4):1058-1063.
Buchanan, W.M. 1971. Shock in bantu siderosis. Am. J. Clin. Pathol. 55:401-406.
Burk, R.F. 1997. Selenium-dependent gluthathione peroxidases. In Comprehensive Toxicology, vol. 3, Biotransformation, F.P. Guengerich, ed. Oxford: Pergamon.
Butler, L.D., N.K. Layman, P.E. Riedl, R.L. Cain, and J. Shellhaas. 1989. Neuroendocrine regulation of in vivo cytokine production and effects: I. In vivo regulatory networks involving the neuroendocrine system, interleukin-1 and tumor necrosis factor-α. J. Neuroimmunol. 24:143-153.
Cacioppo, J.T., G.G. Berntson, W.B. Malarkey, J.K. Kiecolt-Glaser, J.F. Sheridan, K.M. Poehlmann, M.H. Burleson, J.M. Ernst, L.C. Hawkley, and R. Glaser. 1998. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann. NY Acad. Sci. 840:664-673.
Calder, P.C. 1997. n-3 polyunsaturated fatty acids and cytokine production in health and disease. Ann. Nutr. Metab. 41:203-34.
Calder, P.C. 1998. Dietary fatty acids and the immune system. Nutr. Rev. 56:S70-83.
Calvano, S.E., J.D. Albert, A. Legaspi, B.C. Organ, K.J. Tracey, S.F. Lowry, G.T. Shires, and A.C. Antonacci. 1987. Comparison of numerical and phenotypic leukocyte changes during constant hydrocortisone infusion in normal humans with those in thermally injured patients. Surg. Gynecol. Obstet. 164:509-520.
Cannon, J.G., S.N. Meydani, R.A. Fielding, M.A. Fiatarone, M. Meydani, M. Farhangmehr, S.F. Orencole, J.B. Blumberg, and W.J. Evans. 1991. The acute-phase response in exercise. II. Associations between vitamin E, cytokines and muscle proteolysis. Am. J. Physiol. 260:R1235-R1240.
Carniel, E., D. Mazigh, and H.H. Mollaret. 1987. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect. Immun. 55:277-280.
Carniel, E., O. Mercereau-Puijalon, and S. Bonnefoy. 1989. The gene coding for the 190,000-dalton iron-regulated protein of Yersinia species is present only in the highly pathogenic strains. Infect. Immun. 57:1211-1217.
Castell, L.M., J.R. Poortmans, and E.A. Newsholme. 1996. Does glutamine have a role in reducing infections in athletes? Eur. J. Appl. Physiol. 73:488-490.
Caughey, G.E., E. Montzioris, R.A. Gibson, L.G. Cleland, and M.J. James. 1996. The effect on human tumor necrosis factor α and interleukin 1β production of diets enriched in n-3 fatty acids from vegetable oil or from fish oil. Am. J. Clin. Nutr. 63:116-122.
CDC (Centers for Disease Control and Prevention). 1998. Recommendations to Prevent and Control Iron Deficiency in the United States. MMWR 47(RR-3):1-36.
Chandra, R.K. 1982. Excessive intake of zinc impairs immune response. J. Am. Med. Assoc. 252:1443-1446.
Chandra, R.K., ed. 1988. Nutrition and Immunology. New York: Alan R. Liss, Inc.
Chandra, R.K. 1991. 1990 McCollum award lecture. Nutrition and immunity: Lessons from the past and new insights into the future. 53:1087-1101.
Chaudiere, J., E.C. Wilhelmsen, and A.L. Tappel. 1984. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocarboxylic acids and other mercaptans. J. Biol. Chem. 259:1043-1050.
Chrousos, G.P. 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. New Engl. J. Med. 332:1351-1362.
Cioffi, W.G., D.C. Gore, L.W. Rue III, G. Carrougher, H.P. Guler, W.F. McManus, and B.A. Pruitt Jr. 1994. Insulin-like growth factor-1 lowers protein oxidation in patients with thermal injury. Ann. Surg. 220:310-316.
Coceani, F., J. Lees, and C.A. Dinarello. 1988. Occurrence of interleukin-1 in cerebrospinal fluid of the conscious cat. Brain Res. 446:245-250.
Consolazio, C.F., L.O. Matoush, R.A. Nelson, R.S. Harding, and J.E. Canham. 1966. Nutritional survey: Ranger Department, Fort Benning, Georgia. Laboratory Report No. 291. Denver, Colo.: U.S. Army Medical Research and Nutrition Laboratory, Fitzimons General Hospital.
Crofford, L.J., J.I. Rader, M.C. Dalakas, R.H. Hill Jr., S.W. Page, L.L. Needham, L.S. Brady, M.P. Heyes, R.L. Wilder, P.W. Gold, et al. 1990. 1-tryptophan implicated in human eosinophilia-myalgia syndrome causes fascitis and perimyositis in the Lewis rat. J. Clin. Invest. 86:1757-1763.
Croteau, W., S.L. Whittemore, M.J. Schneider, and D.L. St. Germain. 1995. Cloning and expression of a cDNA for a mammalian type III iodothyronine deiodinase. J. Biol. Chem. 270:16569-16575.
Cunningham-Rundles, S., ed. 1993. Nutritional Modulation of the Immune Response. New York: Marcel Decker, Inc.
Cupps, T.R., and A.S. Fauci. 1982. Corticosteroid-mediated immunoregulation in man. Immunol. Rev. 65:133-155.
Davis, J.M., J.D. Albert, K.J. Tracy, S.E. Calvano, S.F. Lowry, G.T. Shires, and R.W. Yurt. 1991. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J. Trauma 31:725-731.
Dealler, S., and R.W. Lacey. 1990. Beef and bovine spongiform encephalopathy: the risk persists. Nutr. Health 7(3)117-133.
Denham, S., and I.J. Rowland. 1992. Inhibition of the reactive proliferation of lymphocytes by activated macrophages: The role of nitric oxide. Clin. Exp. Immunol. 87:157-162.
DeRijk, R., D. Michelson, B. Karp, J. Petrides, E. Galliven, P. Deuster, G. Paciotti, P.W. Gold, and E.M. Sternberg. 1997. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 β (IL-1 β), Il-6, and tumor necrosis factor-α (TNF α) production in humans: High sensitivity of TNF Α and resistance of IL-6. J. Clin. Endocrinol. Metab. 82(7):2182-2191.
DeRijk, R.H., J. Petrides, P. Deuster, P.W. Gold, and E.M. Sternberg. 1996. Changes in corticosteroid sensitivity of peripheral blood lymphocytes after strenuous exercise in humans. J. Clin. Endocrinol. Metab. 81(1):228-235.
DeVecchi, A., F. Halberg, R.B. Sothern, A. Cantaluppi, and C. Ponticelli. 1981. Circaseptan rhythmic aspects of rejection in treated patients with kidney transplant. Pp. 339-353 in Chronopharmacology and Chemotherapeutics, C.A. Walker, C.M. Winget, and K.F.A. Soliman, eds. Tallahasse, FL: A and M University Foundation.
Dinges, D.F., S.D. Douglas, S. Hamarman, L. Zaugg, and S. Kapoor. 1995. Sleep deprivation and human immune function. Adv. Neuroimmunol. 5:97-110.
Endres, S., R. Ghorhani, V.E. Kelley, K. Georgilis, G. Lonnemann, J.W.M. van der Meer, J.G. Cannon, T.S. Rogers, M.S. Klempner, P.C. Weber, E.J. Schaefer, S.M. Wolff, and C.A. Dinarello. 1989. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Eng. J. Med. 320:265-271.
Endres, S., S.N. Madden, R. Ghorbani, R. Schindler, and C.A. Dinarello. 1993. Dietary supplementation with n-3 fatty acids suppresses interleukin-2 production and mononuclear cell proliferation. J. Leukocyte Biol. 54:599-603.
Eskola J., O. Ruuskanen, E. Soppi, M.K. Viljanen, M. Järvinen, H. Toivonen, and K. Kouvalainen. 1978. Effect of sport stress on lymphocyte transformation and antibody formation. Clin. Exp. Immunol. 32:339-345.
Everson, C.A. 1993. Sustained sleep deprivation impairs host defense. Am. J. Physiol. 265:R1148-R1154.
Fairbrother, B., E.W. Askew, M.Z. Mays, R. Shippee, K.E. Friedl, R.W. Hoyt, M. Kramer, T.R. Kramer, and K. Popp. 1993. Nutritional assessment of soldiers during the special forces assessment and selection course. USARIEM Study Protocol, May 1993. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Fan, J., X. Gong, J. Wu, Y. Zhang, and R. Xu. 1994. Effect of glucocorticoid receptor (GR) blockade on endotoxemia in rats. Circul. Shock 42:76-82.
Fernandes, G., E.J. Yunis, and F. Halberg. 1977. Circadian aspect of immune response in the mouse. Pp. 233-249 in Chronobiology in Allergy and Immunology, J.P. McGovern, M.H. Smolensky, and A. Reinberg, eds. Chicago, Ill.: Charles C Thomas.
Fleet, J.C. 1995. A new role for lactoferrin: DNA binding and transcription activation. Nutr. Rev. 53:226-227.
Forse, R.A., ed. 1994. Diet, Nutrition, and Immunity. Boca Raton, Fla.: CRC Press.
Fraker, P.J., L.E. King, B.A. Garry, and C.A. Medina. 1993. The immunopathology of zinc deficiency in humans and rodents. Pp. 267-283 in Human Nutrition--A Comprehensive Treatise, D.M. Klurfeld, ed., vol. 8: Nutrition and Immunology. New York: Plenum Press.
Friedl, K.E., L.J. Marchitelli, D.E. Sherman, and R. Tulle. 1990. Nutritional assessment of cadets at the U.S. Military Academy: Part 1. Anthropometric and biochemical measures. Technical Report No. T4-91. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Friman, G., E. Larsson, and C. Rolf. 1997. Interaction between infection and exercise with special reference to myocarditis and the increased frequency of sudden deaths among young Swedish orienteers 1979-1992. Scand. J. Infect. Dis. Supp. 104:41-49.
Fuller, R., and H. Snoddy. 1968. Feeding schedule alteration of daily rhythm in tyrosine Α-ketoglutaride transaminase in rat liver. Science 159:738
Gatti, G., R. Cavallo, M.L. Sartori, R. Carignola, R. Masera, D. Delponte, A. Salvadori, and A. Angeli. 1988. Circadian variations of interferon-induced enhancement of human natural killer (NK) cell activity. Cancer Detec. 12:431-438.
Gershwin, M.E., R.S. Beach, and L.S. Hurley, eds. 1985. Nutrition and Immunology. Orlando, Fla.: Academic Press.
Glaser, R., and J.K. Kiecolt-Glaser, 1997. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann. Behav. Med. 19:78-82.
Glaser, R., J.K. Kiecolt-Glaser, W.B. Malarkey, and J.F. Sheridan. 1998. The influence of psychological stress on the immune response to vaccines. Ann. NY Acad. Sci. 840:649-655.
Good, M.F., L.W. Powell, and J.W. Halliday. 1988. Iron status and cellular immune competence. Blood Rev. 2:43-49.
Griffin, P.M., and R.V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli , and the associated hemolytic uremic syndrome. Epidemiol. Rev.
Griffiths, E. 1987. Iron in biological systems. Pp. 1-25 in Iron and Infection , J.J. Bullen and E. Griffiths, eds. London: John Wiley & Sons.
Griffiths, R.D., C. Jones, and T.E. Palmer. 1997. Six month outcome of critically ill patients given glutamine-supplemental parenteral nutrition. Nutrition 13:295-302.
Gross, R.L., and P.M. Newberne. 1976. Malnutrition, the thymolymphatic system and immunocompetence. Adv. Exp. Med. Biol. 73:179-187.
Gross, R.L., J.V.O. Reid, P.M. Newberne, B. Burgess, R. Marston, and W. Hift. 1975. Depressed cell-mediated immunity in megaloblastic anemia due to folic acid deficiency. Am. J. Clin. Nutr. 28:225-232.
Gubler, D.J., and D.W. Trent. 1993. Emergence of epidemic dengue/dengue hemorrhagic fever as a public health problem in the Americas. Infect. Agents Dis. 2:383-393.
Hallberg, L., C. Bengtsson, L. Lapidus, G. Lindstedt, P.A. Lundberg and L. Hulten. 1993. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women . Br. J. Haematol. 85(4):787-798.
Haus, E. 1996. Biologic rhythms in hematology. Path. Biol. 44(6):618-630.
Haus, E., D.J. Lakatua, J. Swoyer, and L. Sackett-Lundeen. 1983. Chronobiology in hematology and immunology. Am. J. of Anatomy 168:467-517.
Hemila, H. 1997. Vitamin C intake and susceptibility to the common cold. Br. J. Nutr. 77(1):59-72.
Herbert, V. 1997. Destroying immune homeostasis in normal adults with antioxidant supplements. Am. J. Clin. Nutr. 65(6):1901-1903.
Hershko, C., and T.E.A. Peto. 1987. Non-transferrin plasma iron. Brit. J. Haematology 66:149-151.
Hershko, C., T.E.A. Peto, and D.J. Weatherall. 1988. Iron and infection. Br. Med. J. 296:660-664.
Hinton, P.S., C.A. Peterson, H.C. Lo, H. Yang, D. McCarthy, and D.M. Ney. 1995. Insulinlike growth factor-I enhances immune response in dexamethasone-treated or surgically stressed rats maintained with total parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 19:444-452.
Hodges, R.E., W.B. Bean, M.A. Ohlson, and R.E. Bleiler. 1962. Factors affecting human antibody response. IV. Pyridoxine deficiency. Am. J. Clin. Nutr. 11:180-186.
Hollingsworth, J.W., and J. Carr. 1973. 3H-uridine incorporation as a T lymphocyte indicator in the rat. Cell Immunol. 8(2):270-279.
Hopkins, R.G., and M.L. Failla. 1997. Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J. Nutr. 127(2):257-262.
Hrushesky, W.J.M., and W.J. Marz. 1994. Chronochemotherapy of malignant tumors: Temporal aspects of antineoplastic drug toxicity. Pp. 611-634 in Biologic Rhythms in Clinical and Laboratory Medicine, 2nd ed., Y. Touitou and E. Haus, eds. Heidelberg: Springer-Verlag.
Hughes D.A., A.C. Pinder, Z. Piper, I.T. Johnson, and E.K. Lund. 1996. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am. J. Clin. Nutr. 63:267-272.
IOM (Institute of Medicine). 1992. A Nutritional Assessment of U.S. Army Ranger Training Class 11/91. A brief report of the Committee on Military Nutrition Research, Food and Nutrition Board. March 23. Washington, D.C.: National Academy Press.
IOM (Institute of Medicine). 1993b. Review of the Results of Nutritional Intervention, Ranger Training Class 11/92 (Ranger II), B.M. Marriott, ed. A report of the Committee on Military Nutrition Research, Food and Nutrition Board. Washington, D.C.: National Academy Press.
IOM (Institute of Medicine). 1995a. Not Eating Enough, Overcoming Underconsumption of Military Operational Rations, B.M. Marriott, ed. A report of the Committee on Military Nutrition Research, Food and Nutrition Board. Washington, D.C.: National Academy Press.
Johnson, H.L., H.J. Krzywicki, J.E. Canham, J.H. Skala, T.A. Daws, R.A. Nelson, C.F. Consolazio, and P.P. Waring. 1976. Evaluation of calorie requirements for Ranger training at Fort Benning, Georgia. Technical Report No. 34. Presidio of San Francisco, Calif.: Letterman Army Institute of Research.
Juretic, A., G.C. Spagnoli, H. Hörig, R. Babst, K. von Bremen, F. Harder , and M. Heberer. 1994. Glutamine requirements in the generation of lymphokine-activated killer cells. Clin. Nutr. 13:42-49.
Kakuscska, I., L.I. Romero, B.D. Clark, J.M. Rondeel, Y. Qi, S. Alex, C.H. Emerson, and R.M. Lechan. 1994. Suppression of thyrotropin-releasing hormone gene expression by interleukin-1β in the rat: Implications for nonthyroidal illness. Neuroendocrinology 59(2):129-137.
Kanter, M.M., L.A. Nolte, and J.O. Holloszy. 1993. Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J. Appl. Physiol. 74:965-969.
Kapcala, L.P., T. Chautard, and R.L. Eskay. 1995. The protective role of the hypothalamic-pituitary-adrenal axis against lethality produced by immune, infectious, and inflammatory stress. Ann. N.Y. Acad. Sci. 771:419-437.
Kay, J.E., and C.R. Benzie. 1986. The role of the transferrin receptor in lymphocyte activation. Immunol. Lett. 12:55-58.
Kelley, D.S., D.B. Branch, and J.M. Iacono. 1989. Nutritional modulation of human status. Nutr. Res. 9:965-975.
Kelley, D.S., L.B. Branch, J.E. Love, P.C. Taylor, Y.M. Rivera, and J.M. Iacono. 1991. Dietary α-linolenic acid and immunocompetence in humans. Am. J. Clin. Nutr. 53:40-46.
Kelley, D.S., P.A. Daudu, L.B. Branch, H.L. Johnson, P.C. Taylor, and B. Mackey. 1994. Energy restriction decreases number of circulating natural killer cells and serum levels of immunoglobulins in overweight women. Eur. J. Clin. Nutr. 48(1):9-18.
Kelley, D.S., R.M. Dougherthy, L.B. Branch, P.C. Taylor, and J.M. Iacono. 1992a. Concentration of dietary n-6 polyunsaturated fatty acids and human status. Clin. Immunol. Immunopath. 62:240-244.
Kelley, D.S., G.J. Nelson, L.B. Branch, P.C. Taylor, U.M. Rivera, and P.C. Schmidt. 1992b. Salmon diet and human immune status. Eur. J. Clin. Nutr. 46:397-404.
Kelley, D.S., P.C. Taylor, G.J. Nelson, P.C. Schmidt, and B.E. Mackey. 1996. Effects of dietary arachidonic acid on human response. FASEB J. 10:A557.
Keusch, G.T. 1994. Nutrition and infection. Pp. 1241-1258 in Modern Nutrition in Health and Disease, 8th ed., M.E. Shils, J.A. Olson, and M. Shike, eds. Malvern, Pa.: Lea and Febiger.
Keusch, G.T., and M.J.G. Farthing. 1986. Nutrition and infection. Annu. Rev. Nutr. 6:131-154.
Khaw, K.T., P. Woodhouse. 1995. Interrelation of vitamin C, infection, haemostatic factors, and cardiovascular disease. BMJ 310(6994):1559-1563.
Kiremidjian-Schumacher, L.O., and G. Stotzky. 1987. Selenium and immune responses. Environ. Res. 42:277-303.
Kirk, S.J., and A. Barbul. 1992. Arginine and immunity. Pp. 160-161 in Encyclopedia of Immunology, Book I, I.M. Roitt and P.J. Delves, eds. London: Academic Press.
Kirk, S.J., M.C. Regan, H.L. Wasserkrug, M Sodeyama, and A. Barbul. 1992. Arginine enhances T-cell responses in athymic nude mice. J. Parenter. Enter. Nutr. 16:429-432.
Kirkwood, B.J. 1996. Epidemiology of interventions to improve vitamin A status in order to reduce child mortality and morbidity. In Beyond Nutritional Recommendations: Implementing Science for Healthier Populations, C. Garza, J.D. Haas, J-P. Habicht, and D.L. Pelletier, eds. Proceedings from the 14th Annual Bristol-Myers Squibb/Mead Johnson Nutrition Research Symposium, June 5-7, 1995. Ithaca, N.Y.: Cornell University.
Kjolhede, C., and W.R. Beisel. 1995. Vitamin A and the immune function: A symposium. J. Nutr. Immunol. 4:xv-143.
Klausner, R. D., T.A. Roualt, and J.B. Harford. 1993. Regulating the fate of mRNA: The control of cellular iron metabolism. Cell 72:19-28.
Klicka, M.V., D.E. Sherman, N. King, K.E. Friedl, and E.W. Askew. 1993. Nutritional assessment of cadets at the U.S. Military Academy: Part 2. Assessment of nutritional intake. Technical Report No. T94-1. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Knox, J.R. Demling, D. Wilmore, P. Sarraf, and A. Santos. 1995. Increased survival after major thermal injury: The effect of growth hormone therapy in adults. J. Trauma 39:526-530.
Koshland Jr., D.E. 1992. Science's 1992 molecule of the year [editorial]. Science 258:1861.
Kramer, T.R., R.J. Moore, R.L. Shippee, K.E. Friedl, L. Martinez-Lopez, M.M. Chan, and E.W. Askew. 1997. Effects of food restriction in military training on T-lymphocyte responses. Int. J. Sports Med. 18:S84-S90.
Kramer, T.R., K. Praputpittaya, Y. Yuttabootr, R. Singkamani, and M. Trakultivakorn. 1990. The relationship between plasma zinc and cellular immunity to Candida albicans in young females of northern Thailand. Ann. N.Y. Acad. Sci. 587:300-302.
Kramer, T.R., N. Schoene, L.W. Douglass, J.T. Judd, R. Ballard-Barbash, P.R. Taylor, H.N. Bhagavan, and P.P. Nair. 1991. Increased vitamin E intake restores fish-oil-induced suppressed blastogenesis of mitogen-simulated T-lymphocytes. Am. J. Clin. Nutr. 54:896-902.
Kramer, T.R., E. Udomkesmalee, S. Dhanamitta, S. Sirisinha, S. Charoenkiatkul, S. Tuntipopipat, O. Banjong, N. Rojroongwasinkul, and J.C. Smith Jr. 1993.
Lymphocyte responsiveness of children supplemented with vitamin A and zinc. Am. J. Clin. Nutr. 58:566-570.
Krantman, H.J., S.R. Young, B.J. Ank, C.M. O'Donnell, G.S. Rachelefsky, and G.S.Stiehm. 1982. Immune function in pure iron deficiency. Am. J. Dis. Child. 136:840-844.
Kubes, P., M. Suzuki, and D.N. Granger. 1991. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 88:4651-4655.
Kubo, K., M. Hanaoka, T. Hayano, T. Miyahara, T. Hachiya, M. Hayasaka, T. Koizumi, K. Fujimoto, T. Kobayashi, and T. Honda. 1998. Inflammatory cytokines in BAL fluid and pulmonary hemodynamics in high-altitude pulmonary edema. Respir. Physiol. 111:301-310.
Kuby, J. 1997. Immunology, 3d ed. New York: W.H. Freeman and Company.
Lalonde, R., and T. Boetz-Marquard. 1997. The neurobiological basis of movement initiation. Rev. Neurosci. 8(1):35-54.
Lands, W.E. 1991. Biosynthesis of prostaglandins. Annu. Rev. Nutr. 11:41-60.
Lee, R.E., M.H. Smolensky, C.S. Leach, and J.P. McGovern. 1977. Circadian rhythms in the cutaneous reactivity to histamine and selected antigens, including phase relationship to urinary cortisol excretion. Ann. Allergy 38(4):231-236.
Lee, T.H., R.L. Hoover, J.D. Williams, R.I. Sperling, J. Ravalese, B.W. Spur, D.R. Robinson, E.J. Corey, R.A. Lewis, and K.F. Austen. 1985. Effect of dietary enrichment with eicosapentaenoic acid and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Engl. J. Med. 312:1217-1224.
Levi, F.A., R. Zidani, J.M. Vannetzel, B. Perpoint, C. Focan, R. Faggiuolo, P. Chollet, C. Garufi, M. Itzhaki, and L. Dogliotti. 1994. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J. Natl. Cancer Inst. 86(21):1608-1617.
Levine, M., C. Conry-Cantilena, Y. Wang, R.W. Welch, P.W. Washko, K.R. Dhariwal, J.B. Park, A. Lazarev, J.F. Graumlich, J. King, and L.R. Cantilena. 1996. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. 93(8):3704-3709.
Liew, F.Y., S. Millott, C. Parkinson et al. 1990. Macrophage killing of Leishmania parasite in vivo is medicated by nitric oxide from L-arginine. J. Immunol. 144:4794-4797.
Love, L.A., J.L. Rader, L.J. Crofford, R.B. Raybourne, M.A. Principato, S.W. Page, M.W. Trucksess, M.J. Smith, E.M. Dugan, and M.L. Turner. 1993. Pathological and immunological effects of ingesting L-tryptophan and 1,1'-ethylidenebis (L-tryptophan) in Lewis rats. J. Clin. Invest. 91:804-811.
Lukasewycz, O.A., and J.R. Prohaska. 1990. Immune response in copper deficiency. Ann. N.Y. Acad. Sci. 587:147-159.
Lynch, E.A., C.A. Dinarello, and J.G. Cannon. 1994. Gender differences in IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist secretion from mononuclear cells and urinary excretion. J. Immunol. 153(1):300-306.
MacBurney, M., L.S. Young, T.R. Ziegler, and D.W. Wilmore. 1994. A cost-evaluation of glutamine-supplemented parenteral nutrition in adult bone marrow transplant patients. J. Am. Diet. Assoc. 94:1263-1266.
Macha, M., M. Shlafer, and M.J. Kluger. 1990. Human neutrophil hydrogen peroxide generation following physical exercise. J. Sports Med. Phys. Fit. 30:412-419.
MacPhee, I.A., F.A. Antoni, and D.W. Mason. 1989. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J. Exp. Med. 169:431-445.
Madden, S.N., S. Endres, M.N. Woods, B.R. Goldin, C. Soo, A. Morrill-Labrode, C.A. Dinarello, and S.L. Gorbach. 1991. Oral n-3 fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: Comparison between young and old women. J. Nutr. 121:547-555.
Martinez, F, E.R. Abril, D.L. Earnest, and R. R. Watson. 1992. Alcohol and cytokine secretion. Alcohol 9(6):455-458.
Marucha, P.T., J.K. Kiecolt-Glaser, and M. Favagehi. 1998. Mucosal wound healing is impaired by examination stress. Psychosom. Med. 60:362-365.
McMurray, D.N., S.A. Loomis, L.J. Casazza, H. Rey, and R. Miranda. 1981. Development of impaired cell-mediated immunity in mild and moderate malnutrition . Am. J. Clin. Nutr. 34(1):68-77.
Meydani, S.N., and M. Hayek. 1992. Vitamin E and immune response. Pp. 105-128 in Nutrition and Immunology, R.K. Chandra, ed. St. John's, Newfoundland: ARTS Biomedical.
Meydani, S.N., M.P. Barklund, S. Liu, M. Meydani, R.A. Miller, J.G. Cannon, F.D. Morrow, R. Rocklin, and J.B. Blumberg. 1990. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am. J. Clin. Nutr. 52(3):557-563.
Meydani, S.N., A.H. Lichtenstein, S. Cornwall, M. Meydani, B.R. Goldin, H. Rasmussen, C.A. Dinarello, and E.J. Schaefer. 1993b. Immunologic effects of national cholesterol education panel step-2 diets with and without fish-derived n-3 fatty acid enrichment. J. Clin. Invest. 92:105-113.
Meydani, S.N., M. Meydani, L.C. Rall, F. Morrow, and J.B. Blumberg. 1994. Assessment of the safety of high-dose, short-term supplementation with vitamin E in healthy older adults . Am. J. Clin. Nutr. 60(5):704-709.
Meydani, S.N., M. Meydani, C.P. Verdon, A.C. Shapiro, J.B. Blumberg, and K.C. Hayes. 1986. Vitamin E supplementation suppresses prostaglandin E2 synthesis and enhances the immune response of aged mice. Mech. Aging Dev. 34:191-201.
Michie, H.R., K.R. Manogue, D.R. Spriggs, A. Revhaug, S.T. O'Dwyer, C.A. Dinarello, A. Cerami, S.M. Wolff, and D.W. Wilmore. 1988. Detection of circulating tumor necrosis factor after endotoxin administration. N. Engl. J. Med. 318:1481-1486.
Moldawer, L.L. 1997. The validity of blood and urinary cytokine measurements for detecting the presence of inflammation. In Emerging Technologies for Nutrition Research: Potential for Assessing Military Performance Capability, S.J. Carlson-Newberry and R.B. Costello, eds. Committee on Military Nutrition Research, Food and Nutrition Board. Washington, D.C.: National Academy Press.
Molvig, J., F. Pociot., H. Worssaae, L.D. Wogensen, L. Baek, P. Christensen, T. Mandrup-Poulsen, K. Anderson, P. Madsen, J. Dyerberg, and J. Nerup. 1991. Dietary supplementation with omega-3-polyunsaturated fatty acids decreases mononuclear cell proliferation and interleukin-1β content but not monokine secretion in healthy and insulin-dependent diabetic individuals. Scan. J. Immunol. 34:399-410.
Montain, S.J., R.L. Shippee, W.J. Tharion, and T.R. Kramer. 1995. Carbohydrate-electrolyte solution during military training: Effects of physical performance, mood state and immune function. Technical Report No. T95-13, AD-A297 258. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Moore, R.J., K.E. Friedl, T.R. Kramer, L.E. Martinez-Lopez, R.W. Hoyt, R.E. Tulley, J.P. DeLany, E.W. Askew, and J.A. Vogel. 1992. Changes in soldier nutritional status
and immune function during the Ranger training course. Technical Report No. T13-92 AD-A257 437. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Morikawa, K., F. Oseko, and S. Morikawa. 1995. A role for ferritin in hematopoiesis and the immune system [review]. Leuk. Lymphoma Res. 18:429-433.
Muhlbacher, F., C.R. Kapadia, M.F. Colpoys, R.J. Smith, and D.W. Wilmore . 1984. Effects of glucocorticoids on glutamine metabolism in skeletal muscle. Am. J. Physiol. 247:E75-E83.
Munck, A. P.M. Guyre, and N.J. Holbrook. 1984. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol. Rev. 5:25-44.
Munn, C.G., A.L. Markenson, A. Kapadia, and M. deSousa. 1981. Impaired T cell mitogen responses in some patients with thalassemia intermedia. Thymus 3:119-128.
Müns, G. 1993. Effect of long-distance running on polymorphonuclear neutrophil phagocytic function of the upper airways. Int. J. Sports Med. 15:96-99.
Murray, M.J., A.B. Murray, N.J. Murray, and M.B. Murray. 1978. Diet and cerebral malaria: The effects of famine and refeeding. Am. J. Clin. Nutr. 31:57-61.
Myrvik, Q.N. 1994. Immunology and nutrition. Pp. 623-662 in Modern Nutrition in Health and Disease, 8th ed., M.E. Shils, J.A. Olson, and M. Shike, eds. Malvern, Pa.: Lea and Febiger.
Nakano, K., S. Suzuki, and C. Oh. 1987. Significance of increased secretion of glucocorticoids in mice and rats injected with bacterial endotoxin. Brain Behav. Immun. 1:159-172.
Neilands, J.B. 1995. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726.
Neumann, C.G., G.J. Lawlor Jr., E.R. Stiehm, M.E. Swenseid, C. Newton, J. Herbert, A.J. Ammann, and M. Jacob. 1975. Immunologic responses in malnourished children. Am. J. Clin. Nutr. 28:89-104.
Nicolau, G.Y. and E. Haus. 1994. Chronobiology of the hypothalamic-pituitary-thyroid axis. Pp. 330-347 in Biologic Rhythms in Clinical and Laboratory medicine, 2nd ed., Y. Touitou and E. Haus, ed. Heidelberg: Springer-Verlag.
Nieman, D.C. 1994. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports Exerc. 26:128-139.
Nieman, D.C., K.S. Buckley, D.A. Henson, B.J. Warren, J. Suttles, J.C. Ahle, S. Simandle, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1995c. Immune function in marathon runners versus sedentary controls. Med. Sci. Sports Exerc. 27:986-992.
Nieman, D.C., D.A. Henson, G. Gusewitch, B.J. Warren, R.C. Dotson, D.E. Butterworth, and S.L. Nehlsen-Cannarella. 1993a. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 25:823-831.
Nieman, D.C., A.R. Miller, D.A. Henson, B.J. Warren, G. Gusewitch, R.L. Johnson, J.M. Davis, D.E. Butterworth, J.L. Herring, and S.L. Nehlsen-Cannarella. 1994. Effects of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int. J. Sports Med. 15:199-206.
Nieman, D.C., A.R. Miller, D.A. Henson, B.J. Warren, G. Gusewitch, R.L. Johnson, J.M. Davis, D.E. Butterworth, and S.L. Nehlsen-Cannarella. 1993b. The effects of high-versus moderate-intensity exercise on natural killer cell cytotoxic activity. Med. Sci. Sports Exerc. 25:1126-1134.
Nieman, D.C., S.I. Nelson-Cannarella, D.A. Henson, D.E. Butterworth, O.R. Fagoaga, B.J. Warren, and M.K. Rainwater. 1996. Immune response to obesity and moderate weight loss. Int. J. Obes. Relat. Metab. Disord. 20(4):353-360.
Ogle, C.K., J.D. Ogle, J-X. Mao, J. Simon, J.G. Noel, B-G. Li, and J.W. Alexander. 1994. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN 18:128-133.
Palombo, J.D., S.J. DeMichele, E. Lydon, and B.R. Bistrian. 1997. Cyclic vs continuous enteral feeding with omega-3 and gamma-linolenic fatty acids: effects on modulation of phospholipid fatty acids in rat lung and liver immune cells. JPEN J. Parenter. Enteral Nutr. 21(3):123-132.
Parry-Billings, M., J. Evans, P.C. Calder, and E.A. Newsholme. 1990. Does glutamine contribute to immunosuppression after major burns? Lancet 336:523-525.
Payan, D.G., M.Y.S. Wong, T. Chernov-Rogan, F.H. Valone, W.C. Pickett, V.A. Blake, and W.M. Gold. 1986. Alteration in human leukocyte function induced by ingestion of eicosapentaenoic acid. J. Clin. Immunol. 6:402-410.
Pedersen, B.K., N. Tvede, L.D. Christensen, K. Klarlund, S. Kragbak, and J. Halkjaer-Kristensen. 1989. Natural killer cell activity in peripheral blood of highly trained and untrained persons. Int. J. Sports Med. 10:129-131.
Peters, E.M, J.M. Goetzsche, B. Grobbelaar, and T.D. Noakes. 1993. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am. J. Clin. Nutr. 57(2):170-174.
Philips, J.L., and P. Azari. 1975. Effect of iron transferrin on nucleic acid synthesis in phytohaemagglutinin-stimulated human lymphocytes. Cell Immunol. 15:94-99.
Pieniazek, N.J. and B.L. Herwaldt. 1997. Reevaluating the molecular taxonomy: is human-associated Cyclospora a mammalian Eimeria species? Emerg. Infect. Dis. 3(3):381-383.
Pöllman, L., and B. Pöllman. 1988. Variations of the efficiency of hepatitis B vaccination. Annual Rev. Chronopharmacol. 5:45-48.
Pozos, R.S., D.E. Roberts, A.C. Hackney, and S.J. Feith. 1996. Military Schedules vs. Biological Clocks. Pp. 149-160 in Nutritional Needs in Cold and in High-Altitude Environments, Applications for Military Personnel in Field Operations, B.M. Marriott and S.J. Carlson, eds. A report of the Committee on Military Nutrition Research, Food and Nutrition Board, Institute of Medicine. Washington, D.C.: National Academy Press.
Prasad, A.S., G.J. Brewer, E.B. Schoomaker, and P. Rabbani. 1978. Hypocupremia induced by zinc therapy in adults. J. Am. Med. Assoc. 240:2166-2168.
Prohaska, J.R., S.W. Downing, O.A. Lukasewycz. 1983. Chronic dietary copper deficiency alters biochemical and morphological properties of mouse lymphoid tissues. J. Nutr. 113(8):1583-1590.
Radomski, M.W., B.H. Sabiston, and P. Isoard. 1980. Development of ''sport anemia'' in physically fit men after daily sustained submaximal exercise. Aviat. Space Environ. Med. 51(1):41-45.
Rall, L.C., and S.N. Meydani. 1993. Vitamin B6 and immune competence. Nutr. Rev. 51:217-225.
Rang, H.P., M.M. Dale, J.M. Ritter, and P. Gardner. 1995. Pharmacology. New York: Churchill Livingstone.
Reichlin, S. 1993. Neuroendocrine-immune interactions [review article]. New Engl. J. Med. 329(17):1246-1253.
Reichlin, S. 1994. Neuroendocrine consequences of systemic inflammation. Pp. 83-96 in Advances in Endocrinology and Metabolism, E.L. Mazzaferri, R.S. Bar, and R.A. Kreisberg, eds. St. Louis: Mosby.
Reynolds, J.V., J.M. Daly, S. Zhang, E. Evantash, J. Shou, R. Sigal, and M.M. Ziegler. 1988. Immunomodulatory mechanisms of arginine. Surgery 104(2):142-151.
Rock, C.L., R.A. Jacob, and P.E. Bowen. 1996. Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E, and the carotenoids . J. Am. Diet. Assoc. 96(7):693-702.
Roitt, I.M., and J. Brostoff. 1991. Immunology. London: Gower.
Rosado, J.L., P. Lopez, E. Munoz, H. Martinez, and L.H. Allen. 1997. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am. J. Clin. Nutr. 1997. 65:13-29.
Rosales, F.J., S.J. Ritter, R. Zolfaghari, J.E. Smith, and A.C. Ross. 1996. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J. Lipid Res. 37:962-971.
Ross, A.C., and U.G. Hammerling. 1994. Retinoids and the immune system. Pp. 521-543 in The Retinoids: Biology, Chemistry, and Medicine, 2nd ed., M.B. Sporn, A.B. Roberts, and D.S. Goodman, eds. New York: Raven Press.
Ross, A.C., and C.B. Stephensen. 1996. Vitamin A and retinoids in antiviral responses. FASEB J. 10:979-985.
Roth, E., J. Funovics, F. Muhlbacher, P. Sporn, W. Mauritz, and A. Fritsch. 1982. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle. Clin. Nutr. 1:25.
Russell, R.C. 1998. Mosquito-borne arboviruses in Australia: the current scene and implications of climate change for human health. Int. J. Parasitol. 28:955-169.
Santos, P.C., and R.P. Falcao. 1990. Decreased lymphocyte subsets and K-cell activity in iron deficiency anemia. Acta Haemtologica 84:118-121.
Sazawal, S., R. E. Black, M.K. Bhan, N. Bhandari, A. Sinha, and S. Jalla. 1995. Zinc supplementation in young children with acute diarrhea in India. N. Engl. J. Med. 333:839-844.
Scrimshaw, N.S., C.E. Taylor, and J.E. Gordon. 1968. Interactions of nutrition and infection. Monograph. Geneva: World Health Organization.
Scuderi, P. 1990. Differential effects of copper and zinc on human peripheral blood monocyte cytokine secretion. Cell Immunol. 126:391-405.
Semba, R.D. 1994. Vitamin A, immunity, and infection. Clin. Infect. Dis. 19:489-499.
Senkal, M., A. Mumme, U. Eickhoff, B. Geier, G. Spath, D. Wulfert, U. Joosten, A. Frei, M. Kemen. 1997. Early postoperative enteral immunonutrition: clinical outcome and cost-comparison analysis in surgical patients. Crit. Care Med. 25:1489-96.
Shambaugh, G.E., and W.R. Beisel. 1966. Alterations in thyroid physiology during pneumococcal septicemia in the rat. Endocrinology. 79(3):511-523.
Shephard, D.S., J.A. Walsh, E. Kleinau, S. Stansfield, and S. Bhalotra. 1995a. Setting priorities for the Children's Vaccine Initiative: a cost-effectiveness approach. Vaccine 13(8):707-714.
Shephard, R.J., S. Rhind, and P.N. Shek. 1994b. Exercise and training: Influences on cytotoxicity, interleukin-1, interleukin-2 and receptor structures. Int. J. Sports Med. 15(suppl. 3):S154-S166.
Shinkai, S., H. Kohno, K. Kimura, T. Komura, H. Asai, R. Inai, K. Oka, Y. Kurokawa, and R.J. Shephard. 1995. Physical activity and immunosenescence in elderly men. Med. Sci. Sports Exerc. 27:1516-1526.
Shippee, R., K. Friedl, T. Kramer, M. Mays, K. Popp, E.W. Askew, B. Fairbrother, R. Hoyt, J. Vogel, L. Marchitelli, P. Frykman, L. Martinez-Lopez, E. Bernton, M. Kramer, R. Tulley, J. Rood, J. DeLany, D. Jezior, and J. Arsenault. 1994. Nutritional and
immunological assessment of Ranger students with increased caloric intake. Report No. T95-5. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Silver, R.M., M.P. Heyes, J.C. Maize, B. Quearry, M. Vionnet-Fuasset, and E.M. Sternberg. 1990. Scleroderma, fascitis, and eosinophilia associated with the ingestion of tryptophan [see comments]. N. Engl. J. Med. 322(13):874-881.
Simon-Schnass, I. 1996. Oxidative Stress at High Altitudes and Effects of Vitamin E. Pp. 393-418 in Nutritional Needs in Cold and in High-Altitude Environments, Applications for Military Personnel in Field Operations, B.M. Marriott and S.J. Carlson, eds. A report of the Committee on Military Nutrition Research, Food and Nutrition Board, Institute of Medicine. Washington, D.C.: National Academy Press.
Sothern, R.B., B. Roitman-Johnson, E.L. Kanabrocki, J.G. Yager, M.M. Roodell, J.A. Weatherbee, M.R. Young, B.M. Nemchausky, and L.E. Scheving. 1995. Circadian characteristics of circulating interleukin-6 in men. J. Allergy Clin. Immunol. 95:1029-1035.
Souba, W.W., R.J. Smith, and D.W. Wilmore. 1985. Glutamine metabolism by the intestinal tract. J. Parenter. Enteral Nutr. 9:608-617.
Stallone, D.D., A.J. Stunkard, B. Zweiman, T.A. Wadden, and G.D. Foster. 1994. Decline in delayed-type hypersensitivity response in obese women following weight reduction. Clin. Diagn. Lab. Immunol. 1(2):202-205.
Sternberg, E.M. 1997a. Emotions and disease: From balance of humors to balance of molecules. Nat. Med. 3(3):264-267.
Sternberg, E.M. 1997b. Perspectives Series: Cytokines and the brain: Neural-Immune interactions in health and diseases. J. Clin. Invest. 100(11):107.
Sternberg, E.M., G.P. Chrousos, R.L. Wilder, and P.W. Gold. 1992. The stress response and the regulation of inflammatory disease. Ann. Intern. Med. 11:854-866.
Straight, J.M., H.M. Kipen, R.F. Vogt, and R.W. Amler. 1994. Immune Function Test Batteries for Use in Environmental Health Studies. U.S. Department of Health and Human Services, Public Health Service. Publication Number: PB94-204328.
Sundar, S.K., M.A. Cierpial, C. Kilts, J.C. Ritchie, and J.M. Weiss. 1990. Brain IL-1-induced immunosuppression occurs through activation of both pituitary-adrenal axis and sympathetic nervous system by corticotropin-releasing factor. J. Neurosci. 10:3701-3706.
Suteanu, S., E. Haus, L. Dumitriu, G.Y. Nicolau, E. Petrescu, H. Berg, L. Sackett-Lundeen, I. Ionescu, R. Reilly. 1995. Circadian rhythm of pro- and anti-inflammatory factors in patients with rheumatoid arthritis- No. 226 [abstract]. Biological Rhythm Research 26(4):446.
Swails, W.S., A.S. Kenler, D.F. Driscoll, S.J. DeMichele, T.J. Babineau, T. Utsunamiya, S. Chavali, R.A. Forse, and B.R. Bistrian. 1997. Effect of a fish oil structured lipid-based diet on prostaglandin release from mononuclear cells in cancer patients after surgery. J. Parenter. Enteral. Nutr. 21:266-274.
Tachibana, K., K. Mukai, I. Haraoka, S. Moriguchi, S. Takama, and Y. Kishino. 1985. Evaluation of the effects of arginine-enriched amino acid solution on tumor growth. J. Parenter. Enter. Nutr. 9:428-434.
Tang, A.M., N.M.H. Graham, and P. Saah. 1996. Effects of micronutrient intake on survival in human immunodeficiency virus 1 infection. Am. J. Epidemiol. 143:1244-1256.
Tappia, P.S., K.L. Troughton, S.C. Langley-Evans, and R.F. Grimble. 1995. Cigarette smoking influences cytokine production and antioxidant defences. Clin Sci (Colch) 88(4):485-489.
Taylor, G.R. 1993. Overview of spaceflight immunology studies. J. Leukocyte Biol. 54:179-188.
Tvede, N., J. Steensberg, B. Baslund, J. Halkjaer-Kristensen, and B.K. Pedersen. 1991. Cellular immunity in highly-trained elite racing cyclists and controls during periods of training with high and low intensity. Scand. J. Sports Med. 1:163-166.
Udomkesmalee, E., S. Dhanamitta, S. Sirisinha, S. Charoenkiatkul, S. Tantipopipat, O. Banjong, N. Rojroongwasinkul, T.R. Kramer, and J.C. Smith Jr. 1992. Effect of vitamin A and zinc supplementation on the nutriture of children in Northeast Thailand. Am. J. Clin. Nutr. 56:50-57.
Van der Hulst, R.R., B.K. van Kreel, M.F. von Meyenfeldt, R.J. Brummer, J.W. Arends, N.E. Deutz, and P.B. Soeters. 1993. Glutamine and the preservation of gut integrity. Lancet 334:1363-1365.
Virella, G., J.M. Kilpatrick, M.R. Rugeles, B. Hayman, and R. Russell. 1989. Depression of humoral responses and phagocytic functions in vivo and in vitro by fish oil and eicosapentaenoic acid. Clin. Immunol. Immunopathol. 52:257-270.
Wan, W., L. Wetmore, C.M. Sorensen, A.H. Greenberg, and D.M. Nance. 1994. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 34:7-14.
Wartofsky, L., and K.D. Burman. 1982. Alterations in thyroid function in patients with systemic illness: the "euthyroid sick syndrome." Endocr. Rev. 3(2):164-217.
Watkins, L.R., L.E. Goehler, J.K. Relton, N. Tartaglia, L. Silbert, D. Martin, and S.F. Maier. 1995a. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: Evidence for vagal mediation of immune-brain communication. Neuroscience Lett. 183:27-31.
Watson, R.R., and T.M. Retro. 1984. Resistance to bacterial and parasitic infections in the nutritionally compromised host. CRC Crit. Rev. Microbiol. 10:297-315.
Watters, J.M., P.Q. Bessey, C.A. Dinarello, S.M. Wolff, and D.W. Wilmore. 1986. Both inflammatory and endocrine mediators stimulate host responses to sepsis. Arch. Surg. 121:179-190.
Watzl, B., and R.R. Watson. 1992. Role of alcohol abuse in nutritional immunosuppression. J. Nutr. 122 (3 suppl.):733-737.
Weinberg, E.D. 1984. Iron withholding: A defense against infection and neoplasia. Physiol. Rev. 64:65-102.
Weiss, G., H. Wachter, and D. Fuchs. 1995. Linkage of cell-mediated immunity to iron metabolism [review]. Immunol. Today 16:495-500.
Westphal, K.A., K.E. Friedl, M.A. Sharp, N. King, T.R. Kramer, K.L. Reynolds, and L.J. Marchitelli. 1995a. Health, performance, and nutritional status of U.S. Army women during basic combat training. Technical Report No. T96-2. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Wiik P., P.K. Opstad and A. Bøyum. 1996. Granulocyte chemiluminescence response to serum opsonized particles ex vivo during long-term strenuous exercise, energy, and sleep deprivation in humans. Eur. J. Appl. Physiol. 73:251-258.
Wiik, P., K.K. Skrede, S. Knardahl, A.H. Haugen, C.E. Ærø, P.K. Opstad, A. Bøyum. 1995. Effect of in vivo corticosterone and acute food deprivation on rat resident peritoneal cell chemiluminescence after activation ex vivo. Acta Physiol Scand 154(3):407-416.
Williams, T.J., and H. Yarwood. 1990. Effect of glucocorticosteroids on microvascular permeability. Am. Rev. Resp. Dis. 141:S39-S43.
Witt, E.H., A.Z. Reznick, C.a. Viguie, P. Starke-Reed, and L. Packer. 1992. Exercise, oxidative damage, and effects of antioxidant manipulation. J. Nutr. 122 (suppl. 3):766-773.
Ziegler, T.R., L.S. Young, K. Benfell, M. Scheltinga, K. Hortos, R. Bye, F.D. Morrow, D.O. Jacobs, R.J. Smith, J.H. Antin, and D.W. Wilmore. 1992. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann. Intern. Med. 116:821-828.
Zoli, M., M. Carè, C. Falco, R. Spanò, G. Bernardi, and I. Gasbarrini. 1995. Effect of oral glutamine on intestinal permeability and nutritional status in Crohn's disease. Gastroenterology 108:A766.
Zuckerman, S.H., J. Shellhaas, and L.D. Butler. 1989. Differential regulation of lipopolysaccharide-induced interleukin-1 and tumor necrosis factor synthesis: Effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur. J Immunol. 19:301-305.
| This page in the original is blank. |
















































































