9
Methodological Issues in Assessment of Human Immune Function
Susanna Cunningham-Rundles1
Introduction
Assessment of immune function in the context of studies presented in this volume is based on the underlying premise that in vitro or ex vivo2 measurements of immune response during the stress of training reflect the innate status of the immune system and, furthermore, may be interpreted to predict future response. However, the fundamental relationships that are valid in other well-studied settings may be different for two main reasons. First, stress associated with military training conditions does not have an exact parallel with conditions associated with military deployment. Second, a greater or more
severe stress reaction does not necessarily predict poorer recovery, and recovery may be the critical parameter for assessing potential response to future stress and long-term health of the immune system.
Factors influencing immune response of the individual to potential environmental pathogens at any single point in time include such interacting host factors as genetic predisposition, general fitness or state of health, previous history of exposure, and nutrient status. Although immunization can be protective against known pathogens (that is, where exposure can be predicted), nutrients may support general improvement of immune function or act as specific cofactors, which suggests that repletion or supplementation could ultimately provide an approach for optimizing immune response.
Evidence is increasing that nutrients are involved specifically with the development of an immune response (Cunningham-Rundles, 1993). Further, current studies suggest that key elements in the diet, for example some trace elements, have a profound influence on immune response even within a period of days (Prasad et al., 1988). Conversely, nutrient deprivation may have long-term consequences, especially when these deprivations are unrecognized and uncorrected (Ohshima et al., 1991).
Development of a rational experimental design for the measurement of general immune function and how this may relate to future immunity is important if the operation of these nutrition-immune function interactions is to be clarified in essentially healthy persons under stress. Quantitative measures of response to a test stimulus may indicate but may not predict response to an unforeseen pathogen. In contrast, the relationship among immunization, subsequent development of serum antibody titer, and protection against encounter with the immunizing pathogen can be more easily quantified and predicted.
The subsequent discussion presents some key elements in the evaluation of immune response. Some of the key questions that must be considered are:
- How is immune function defined?
- What is the setting?
- How is immune function measured?
- Is there change over time?
- Do measures of immune response in vitro or ex vivo correlate with immune response in vivo?
- Can measures of immune response predict future response?
Rationale for Immune Assessment
The rationale for undertaking immune assessment is an important consideration in selecting the tests. Immune tests have been developed from several areas of primary research, including susceptibility to and recovery from specific infections, development of vaccines, study of congenitally impaired
host defense, investigations into the basis of graft rejection, and the science of blood transfusion (Paxton et al., 1995).
Although the impact of chronic protein calorie deprivation on susceptibility to infection has been well documented, less is known about the impact of acute deprivation. The fundamental premise that nutritional status has a major impact on immune function is soundly based on a wide range of studies indicating that nutritional deprivation, especially chronic deprivation, produces a measurable impairment of immune response that reflects both duration and degree of nutrient deficit (Cunningham-Rundles and Cervia, 1996; Shronts, 1993). For most human studies, the circumstances of nutritional status impairment have been either in the context of (1) pathological disease states associated with altered nutrient intake, altered absorption, or changes in utilization, or (2) increased and unmet special nutrient requirements such as those that occur during childhood development, during aging, or under conditions of inadequate nutrient intake and food scarcity. Although it is generally thought to be relatively rare in this country, malnutrition is frequently associated with immune deficiency and causes significant vulnerability to infections (Chandra, 1993). Taken together, these studies indicate that nutrients are critical cofactors for immune response and suggest that (1) there is a potential benefit from repletion where deficits are found and (2) better long-term nutrient status is associated with better immune response, lowered incidence of infections, and possibly cancer prevention.
The critical questions about immune status in Special Forces troops, however, are how acute nutrient deprivation of the fundamentally healthy person undergoing highly stressful training in preparation for possible defense activities may influence long-term host defense and whether temporary nutritional deficits incurred during training or in combat conditions may produce long-term vulnerability. The studies of nutrient immune interaction in the settings noted above do not provide an adequate blueprint for answering these questions.
The central question of how the stress of military training influences immune response must be posed in the context of relevance to recovery. The immune system responds to challenge via a vast array of interrelated processes, and the healthy person may be made essentially stronger by these encounters if recovery can be supported properly.
The generalized lack of response to immune signals, called anergy, may indicate failure of immune response, as is commonly encountered in primary immune deficiency, granulomatous diseases, acute infection, trauma, and cancer. However, simple malnutrition, for example that associated with aging or other conditions where nutrient intake is inadequate, can produce anergy, as measured by loss of the secondary response to recall (memory) antigens in the delayed-type hypersensitivity skin test in vivo and in vitro (Blot et al., 1993; Peretz et al., 1991; Smythe et al., 1971). Thus, conditions resulting in acquired anergy may be improved with appropriate intervention.
The Immune System
The immune system is an organ system consisting of primary tissues (bone marrow, fetal liver, and thymus) and secondary lymphoid tissues (spleen and lymph nodes). The work of two main immune cell types (Haynes et al., 1990; Paul, 1993) is often used to define immune activity. T-lymphocytes (T-cells) are defined by expression of (1) the T-cell receptor that binds to antigen, and (2) CD3, a surface determinant associated with the T-cell receptor that is essential for activation. T-cells have an array of clonally variable receptors for a large range of antigens, require thymic maturation for normal function, and mediate cellular immunity. B-lymphocytes (B-cells) are identified by surface immunoglobulin (detected by monoclonal antibodies such as CD19, CD20) and, upon appropriate activation, develop into plasma cells secreting specific antibody and thus mediate humoral immunity.
The normal functions of these cells have been defined in situations of pathological absence or loss. Loss of the normal thymus has been found to compromise T-cell function and affect T-dependent B-cell activation. Failure at the bone marrow level has been observed to affect both T-cell and B-cell immune response.
The distinction between specific and nonspecific immune response is an intrinsic feature of immunity that permits differentiation between self and nonself at the cellular level. In general, this is accomplished by the integration of the molecular complex called the major histocompatibility complex (MHC) "self"-antigen system into the antigen recognition phase. Antigen must be processed and presented in the context of self-MHC to be recognized and to lead to the development of immune response. T-cell activation is a highly controlled event. Antigens are recognized by T-cells only after the antigen has been processed and presented on the surface of an antigen-presenting cell, APC, which may be a monocyte, dendritic cell, or B-cell. The antigen is presented as a MHC/peptide combination. The antigen-processing function is carried out by antigen-presenting cells, the best studied of which is the monocyte. This response triggers lymphocyte activation and proliferation and leads to the production of effector cells (natural killer cells, phagocytes, or cytotoxic T-cells) and triggering of B-cells to produce antibody. This kind of immunity, often termed adaptive immunity, is retained as "memory" and is typically elicited following immunization or natural infection (Owen and Jenkinson, 1993).
A second fundamental type of immunity can be described as innate immunity, as it is not stored as a memory function and is not improved by repeated contact. This immunity is mediated by phagocytic cells, some of which, like the monocyte, can also process and present antigen. Innate immunity is mediated by certain cytokines such as interferons (Cohen, 1994; Germain and Margulies, 1993), which confer nonspecific protection. Natural killer (NK) cells are an integral component of the nonadaptive, innate immune system. Unlike
phagocytic cells, NK cells are not functionally developed at birth (Cunningham-Rundles et al., 1993), probably because the key cytokine, interferon-gamma (INF-γ), which is needed for development and maturation of this system, is also downregulated at birth.
This third arm, represented by the NK cell, can be defined as neither B-cell nor exactly T-cell-like, in having neither surface immunoglobulin nor a rearranged T-cell receptor. This cell, once called the "K" cell, "null" cell, or ''third population," has eluded conventional classification by cell lineage analysis. Currently, CD56 is considered the most definitive marker of the NK cell (Trinchieri, 1992). However, NK cells are best known as cells that can kill nonspecifically (naturally) viral-infected cells and bacteria and prevent tumor cell metastasis. If activated by a key cytokine, interleukin (IL)-2, NK cells will differentiate into lymphokine-activated killer (LAK) cells (Ortaldo and Longo, 1988). These cells can kill many tumor cell types. The NK cell system is constitutively active and does not have to be primed by antigen to kill. When armed with specific antibody, however, these cells can kill specifically. Functional evaluation of this cell population can be readily achieved using a short-term chromium release assay (known as NK cell cytotoxicity assay and using NK cell-sensitive tumor cell lines as targets) and is a valuable tool in assessing immune response.
Although assessment of all aspects of immunity is not required to implement immune testing, examination of the range of possible changes in a pilot study is advisable so that possible critical interactions are not overlooked.
Selection and Development of the Test System
Measurement of "immunity" has often focused on measurement of humoral immunity to detect the presence of potentially protective antibodies to an infectious agent introduced by natural infection or by immunization. Cellular immune function is fundamentally more complex and less easy to measure. Basic humoral immune tests are usually carried out to measure the specific antibody product of a response formed in the past in vivo to a specific virus or microbe. An outline of tests for humoral immunity appears in Figure 9-1.
In contrast, cellular immune assays measure current response in vitro , and sometimes in vivo, by elicitation of a functional response at the time of the test. An outline of cellular immune tests appears in Figure 9-2.
Human studies have been based on observation of peripheral blood immune cells because the peripheral compartment is most accessible and readily measured, but this approach may not reflect regional events (Cunningham-Rundles, 1994). Knowledge of the differences between systemic and mucosal immune response may ultimately explain many current paradoxes arising when immune response measured in vitro or ex vivo is compared with host defense in vivo.
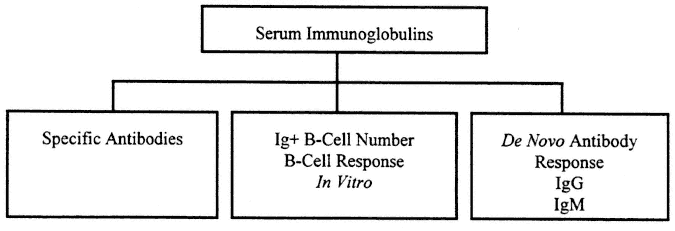
FIGURE 9-1
Evaluation of humoral immune response includes assessment of the general level of serum immunoglobulins and specific antibodies. This reflects previous immunization and serves to assess intrinsic integrity of the B-cell system. In addition, studies may include examination of B-cell response in vitro and response to de novo immunization that may show differences associated with current, perhaps transient, immune impairment.
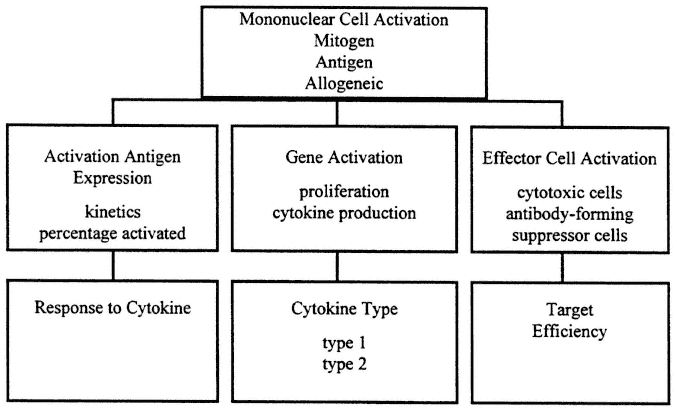
FIGURE 9-2
Evaluation of cellular immune response is characteristically conducted as a three-step process. First, the level of activation is studied using the three basic types of activators: mitogen, antigen, and allogeneic activation. Then the activation pathway is examined for integrity of the process in general terms. Finally, the effector phase is examined for specificity of cytokine production, and response capacity towards key intermediates, the cytokines, and strength of the effector process. All aspects of this process may reflect current immune deficits since response is elicited in vitro.
Systemic cellular immune function appears to be regulated through functionally distinct T-helper type cytokine patterns, such that when T-helper type 1 (Th1) cytokines, IL-2, and INF-γ are produced, cellular immune host defense is favored, and when T-helper type 2 (Th2) cytokines IL-4, IL-5, Il-6, and IL-10 are produced, B-cell response is induced (Barnes et al., 1993; Yamamura et al., 1991).
In contrast to systemic immunity, the primary activity of mucosal immune response is to protect the mucosa by blocking microbial, toxin, and antigen entry; this protection is mediated by secretion and transport of IgA to the lumen of the gut, a process mediated by a special type of memory T-cells with reduced proliferative capacity and capability to promote B-cell activation (Kagnoff, 1993; Marsh and Cummins, 1993).
Recent studies suggest that normal mucosa may downregulate mucosal T-cell reactivity (Qiao et al., 1993). Triggering of these cells, however, can produce an inflammatory response. Although there are few noninvasive methods to evaluate the gastrointestinal immune response, events in this compartment of the immune system may be crucial during response to stress.
In Vivo Assessment of Immune Response
In vivo skin testing and examination of humoral immune response to previously encountered vaccines can be useful in establishing both a baseline response and a response due to the impact of military training.
An issue that frequently must be confronted in immune studies is how to evaluate the potential significance of impaired immune response detected in vitro as a reliable reflection of susceptibility to future infection in vivo. Studies of even relatively well-defined disorders have shown that there may be very significant differences in the clinical impact of immune deficiency. One way to test this is to undertake a planned immunization, which can be accomplished, for example, by assessing response to a flu vaccine. If proliferative response is measured in vitro to the immunizing antigen, both T-and B-cell response can be assessed.
The use of a skin test panel can be an important way to measure delayed-type hypersensitivity in vivo (Kniker et al., 1979). Experience with the delayed-type hypersensitivity skin test during previous decades has shown good overall correlation between lack of reactivity, or anergy, and immune deficiency in a variety of settings. The skin test is not very quantifiable. The use of the purified protein derivative (PPD) skin test to assess possible presence of Mycobacterium tuberculosis is an exception, although anergic individuals do not respond. In addition, there are false positives with persons who have been vaccinated with BCG (Bacillus Calmette-Guerin3).
Some studies have been based on a de novo immunization skin test using dinitrofluorobenzene (DNFB). Although this approach was once used rather extensively, it is no longer considered useful because of ambiguities in the underlying mechanism of reaction, which produces some contact sensitivity, and because of lingering alteration in skin. The introduction of the "skin window"4 (Black et al., 1988) test may ultimately provide a more quantitative and informative measure of in vivo immune response because the reaction can be used to test autologous response, and the types of cells entering the region can be studied.
Despite some reservations (including issues with variable level of response with this test and difficulties in interpretation of negative results), the importance of the skin test as a convincing demonstration that immune defects noted in vitro may have prognostic significance in vivo should not be underestimated (Kniker et al., 1979). The use of test systems that provide a broad range of test antigens as well as positive and negative controls are particularly recommended.
In Vitro Assessment of Immune Response
Functional studies need to be carried out on fresh anticoagulated blood whenever possible (or blood stored at room temperature in the dark for under 24 hours) before mononuclear cells are isolated. When blood is being sent by air or transported to a distant lab, it is advisable to include a control specimen drawn in parallel from control persons (those not subjected to the military training conditions) to serve as an internal standard for the shipping process.
The question of when the blood should be drawn is important. In general, most data have been obtained with blood drawn in the morning as there are circadian effects that may influence results. When this schedule cannot be followed, it is helpful to continue to maintain a uniformity of drawing time for individual subjects.
Proliferative Response
Since peripheral blood lymphocytes are resting cells (not cycling), the cellular immune reaction must be generated de novo in the test system with the introduction of the stimulant; this stimulant must be potentially capable of triggering the response. In addition, the culture system must be capable of supporting the reaction by providing all needed elements, and there must be a measurable end point.
All of these tests require experience and are best used in the context of defined questions. The establishment of laboratory normal ranges and maintenance of reagent quality control, especially for certain variable elements such as serum, is essential for accuracy and sensitivity of these tests.
The use of whole blood to assess immune response is a valuable approach because it approximates in vivo conditions and, further, is often more practical in field conditions. However, use of this system to pinpoint critical interactions appears to require further development.
The choice of stimulant is very important. If the stimulant is "nonspecific" (for example, mitogens that directly trigger response or allogeneic cells that express MHC class II antigens recognized as "foreign"), a relatively large fraction of isolated mononuclear cells from all healthy persons will be capable of reacting in an appropriately designed system. If the test signal is an antigen previously encountered in vivo, then a smaller proportion of the cells from the donor will react. This latter type of reaction, the classic delayed-type hypersensitivity type IV reaction (Kirkpatrick, 1988), is a secondary response comparable to a humoral immune response to a "booster" immunization.
Development of Effector Cell Response
The development of an effector response (appearance or activity of effector cells such as NK cells or cytotoxic cells) may be affected or altered by the kinetics of the proliferative response. This may occur because of delayed secondary recruitment during the amplification phase of the response or because of changes in cytokine production. Cytokine patterns are critical for the development of effector activity. Effector functions may be missing or impaired during a stress response. Evaluation of this may require a detailed approach, however, since alterations in subpopulations and kinetics must be considered. Attempts to restore response by cytokine addition may be useful as a means of identifying the lesion.
When suppressor cell mechanisms (actions of specific cell types or cytokines that serve to inhibit the immune response) are suspected, removal of cells by magnetic or flow cytometric approaches may be useful. Monitoring population characteristics in these cases is also critical. Studying the response of subpopulations will also require careful evaluation of normal cells in parallel since, to a large degree, immune response is under negative control.
Assessment of Cell Types by Flow Cytometry
The development of monoclonal antibodies directed against human immune cell surface determinants has provided a more accurate and ultimately quantifiable way to define lymphocyte subpopulations (Lanier et al., 1986; Reinherz and Schlossman, 1980).
Flow cytometry also has the potential to become a major approach to the study of immune function. Certain key cellular events occurring along the T-cell activation-proliferation pathway can be measured directly in a flow cytometer.
As shown in Figure 9-3, several different approaches can be combined in the development of test systems. Although early events, such as the rapid increase in intracellular free calcium that leads to change in pH and in membrane potential, can be measured cytometrically, lack of standardization is a limitation at present. However, increased expression of cell surface molecules, which occurs following lymphocyte activation, is relatively easily measured. For example, after T-cell activation, the first measurable surface marker induced is CD69, which is not expressed on resting T-cells. The kinetics of appearance and decline have been well defined (Lopez-Cabrera et al., 1993). Other cell surface markers appearing on activated T-cells following activation include CD25 (the alpha chain of the IL-2 receptor), CD71, and HLA-DR.5 Use of these markers as a means of probing critical events in lymphocyte activation provides a new approach to the development of methods that can measure subtle but clinically significant events (Cunningham-Rundles et al., 1990).
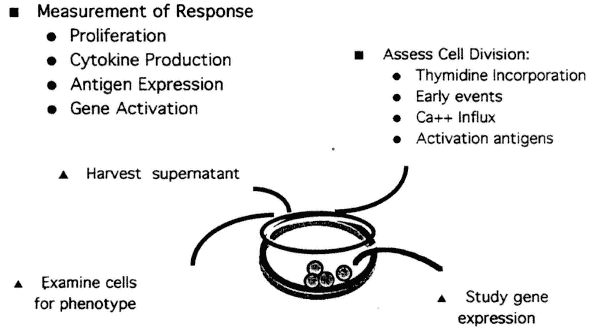
FIGURE 9-3
Measurement of immune response can be performed in microtier tissue culture plates, typically in triplicate. The figure depicts how parallel studies may be performed under the same culture conditions using the same activator, shown here at the level of the single well, and then be used to follow the consequences of activation through the ensuing stages: (1) early events such as gene activation, (2) activated antigen expression, (3) cytokine production, (4) proliferation (cell division), and (5) effector cell activity.
Author's Conclusions
The central question of how the stress of military training influences immune response must be posed in the context of significance for recovery. The immune system provides response to challenge at several levels. The fundamentally healthy person may become essentially stronger by these encounters if recovery can be supported properly.
The key question involving the immune status of Special Forces troops is how acute nutrient deprivation during training may influence long-term host defense and whether temporary nutritional and immune deficits incurred during training might produce long-term vulnerability. Studies of nutrient-immune interaction in most previous settings are not necessarily relevant to this question. Undertaking a properly organized and implemented study for the purpose of establishing the exact range and nature of changes in immune response that occur during the stress of training is an important first step. As discussed here, it is critical to standardize and quantify an approach that includes several levels of immune response.
Further research is needed to determine how specific immune deficits emerging during emotional and physical stress are influenced by other imposed stressors such as caloric and micronutrient deprivation. Knowledge is also needed about how these stressors may affect the kinetics and the degree of recovery of immune response leading to the support of host defense.
References
Barnes, P.F., L. Shuzhuang, J.S. Abrams, E. Wang, M. Yamamura, and R.L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61(8):3482-3489.
Black, M.M., R.E. Zachrau, and R.H. Ashikari. 1988. Skin window reactivity to autologous breast cancer: An index of prognostically significant cell-mediated immunity. Cancer 62:72-83.
Blot, W.J., J.Y. Li, P.R. Taylor, W. Guo, S. Dawsey, G.Q. Wang, C.S. Yang , S.F. Zheng, M. Gail, G.Y. Li, Y. Yu, B. Liu, J. Tangrea, Y. Sun, F. Liu, J.F. Fraumeni Jr., Y.H. Zhang, and B. Li. 1993. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease specific mortality in the general population. J. Natl. Cancer Inst. 85(18):1483-1491.
Chandra, R.K. 1993. Nutrition and the immune system. Proc. Nutr. Soc. (England) 52(1):77-84.
Cohen, J.J. 1994. Cells involved in host defense: The new immunology. J. Nutr. Immunol. 2(4):73-86.
Cunningham-Rundles, S., ed. 1993. Nutrient Modulation of Immune Response. New York: Marcel Dekker.
Cunningham-Rundles, S. 1994. Malnutrition and gut immune function. Curr. Opin. Gastroenterol. 10: 664-670.
Cunningham-Rundles, S., and J.S. Cervia. 1996. ''Malnutrition and host defense" in nutrition. Pp. 295-307 in Pediatrics: Basic Science and Clinical Application, 2d ed., W.A. Walker and J.B. Watkins, eds. Basel, Switzerland: Marcel Dekker.
Cunningham-Rundles, C., C. Chen, J.B. Bussel, C. Blankenship, M.B. Veber, D. Sanders-Laufer, T. Hinds, J.S. Cervia, and P. Edelson. 1993. Human immune development: Implications for congenital HIV infections. Ann. N.Y. Acad. Sci. 693:20-34.
Cunningham-Rundles, S., R.R. Yeger-Arbitman, R. Nachman, S.A. Kaul, and M. Fotino. 1990b. New variant of MHC class II deficiency with interleukin-2 abnormality. Clin. Immunol. Immunopathol. 56:116-123.
Germain, R.N., and D.H. Margulies. 1993. The biochemistry and cell biology of antigen processing and presentation. Ann. Rev. Immunol. 11:403-450.
Haynes, B.F., S.M. Denning, P.T. Le, and K.H. Singer. 1990. Human intrathymic T-cell differentiation. Semin. Immunol. 2:67-77.
Kagnoff, M.F. 1993. Immunology of the intestinal tract. Gastroenterology 105(5):1275-1280.
Kirkpatrick, C.H. 1988. Delayed hypersensitivity. Pp. 261-277 in Immunological Diseases, M. Sampter, D.W. Talmage, M.M. Frank, K.F. Austem, and H.N. Claman, eds. Boston and Toronto: Little, Brown.
Kniker, W.T., C.T. Anderson, and M. Roumiantzeff. 1979. The MULTITESTR system: A standardized approach to evaluation of delayed-type hypersensitivity and cell-mediated immunity. Ann. Allergy 43:73-79.
Lanier, L., J.H. Phillips, J. Hackett Jr., M. Tutt, and V. Kumar. 1986. Natural killer cells: Definition of a cell type rather than a function. J. Immunol. 137:2735-2739.
Lopez-Cabrera, M., A. G. Santis, E. Fernandez-Ruis, R. Blacher, F. Esch, P. Sanchez-Mateos, and F. Sanchez-Madrid. 1993. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal transmitting receptors. J. Exp. Med. 178:537-547.
Marsh, M.N., and A.G. Cummins. 1993. The interactive role of mucosal T-lymphocytes in intestinal growth, development, and enteropathy. J. Gastroenterol. Hepatol. 8:270-278.
Ohshima, T., T. Nakaya, K. Saito, H. Maeda, and T. Nagano. 1991. Child neglect followed by marked thymic involution and fatal systemic pseudomonas infection. Int. J. Legal Med. 104(3):167-171.
Ortaldo, J.R., and D.L. Longo. 1988. Human natural lymphocyte effector cells: Definition, analysis of activity, and clinical effectiveness. J. Natl. Cancer Inst. 80:999-1010.
Owen, M.J., and E. Jenkinson. 1993. Ontogeny of the immune response. Pp. 3-12 in Clinical Aspects of Immunology, P.J. Lachman, D.K. Peters, F.S. Rosen, and M.J. Walport, eds. London: Blackwell Scientific.
Paul, W.E. 1993. Fundamental Immunology, 3d ed. New York: Bauer Press.
Paxton, H., S. Cunningham-Rundles, and M.R.G. O'Gorman. 1995. Laboratory evaluation of the cellular immune system. Pp. 887-912 in Clinical diagnosis and Management by Laboratory Methods, J.B. Henry, ed. Philadelphia: W.B. Saunders and Company.
Peretz, A., J. Neve, J. Duchateau, V. Siderova, K. Huygen, J.P. Famaey, and Y.A. Carpentier. 1991. Effects of selenium supplementation on immune parameters in gut failure patients on home parenteral nutrition. Nutrition 7(3):215-221.
Prasad, A.S., S. Meftah, J. Adballah, J. Kaplan, G.J. Brewer, J.F. Bach, and M. Dardenne. 1988. Serum thymulin in human zinc deficiency. J. Clin. Invest. 82:1202-1210.
Qiao, L., G. Schurmann, F. Autschbach, R. Wallich, and S.C. Meuer. 1993. Human intestinal mucosa alters T-cell reactivities. Gastroenterology 105:814-819.
Reinherz, E.L., and S.F. Schlossman. 1980. The differentiation and function of human T-lymphocytes. Cell 19:821-827.
Shronts, E.P. 1993. Basic concepts of immunology and its application to clinical nutrition. Nutr. Clin. Pract. 8(4):177-183.
Smythe, P.M., M. Schonland, G.G. Brereton-Stiles, H.M. Coovadia, H.J. Grace, W.E.K. Loening, A. Mafoyne, M.A. Parent, and G.H. Vos. 1971. Thymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet 2:939-943.
Trinchieri, G. 1992. The hematopoietic system and hematopoiesis. Pp. 1-25 in Neoplastic Hematopathology, D.M. Knowles, eds. Baltimore: Williams and Wilkins.
Yamamura, M.K., R.J. Uyemura, K. Deans, T.H. Weinberg, B. Rea, B. Bloom, and R.L. Modlin. 1991. Defining protective responses to pathogens: Cytokine profiles in leprosy lesions. Science 254:277-279.
Discussion
JOHANNA DWYER: I was wondering, in the slides you presented of the dietary fat and PGE2 response, what specific fatty acids were in the fat?
SUSANNA CUNNINGHAM-RUNDLES: We do not have that information yet. It has all been done by food recall. But basically what we are going to do in the future in that particular study is provide people with a standardized diet. This was actually done with free-living people. The results were helped by taking out all of the noncompliant people.
JOHANNA DWYER: This must have reduced your sample size.
SUSANNA CUNNINGHAM-RUNDLES: With human subjects, it does not always work out.
ARTHUR ANDERSON: Regarding giving several oxygenase inhibitors to mimic the effect of high-stress training, would that have an effect similar to the dietary change?
SUSANNA CUNNINGHAM-RUNDLES: I think it well might. It would be very interesting to know that. Prostaglandin secretion is not normally activated. Prostaglandin level would be undetected unless we stimulated cells in vitro. Clearly, this was a person who probably did have a genuine risk for tumor development. The level of prostaglandin that was documented was very interesting. You do not see that commonly. But, in terms of responsiveness to PHA and the development of prostaglandin, yes, in that setting [of a high-stress training environment] you probably would see a suppression of that. I think it would be interesting to do the study and find out what the effects would be.
STEVE GAFFIN: While the subject is mainly the problem of malnutrition and immunosuppression, among those tests that you have just described, are there any populations of humans in whom overstimulation of the immune system is observed, in whom there is immuno-activation, and through whom we could then search for dietary components to that, apart from genetic factors?
SUSANNA CUNNINGHAM-RUNDLES: This is not easy to do, but I would suggest using a model of chronic viral infections. We do have chronic activation of the immune system in those settings, in the development of cytokines that then eventually produce the loss of lean body mass. There are some good studies. I would very much approve of what Dr. Chandra said, for example, about parallels between HIV-associated malnutrition and protein calorie malnutrition. The problem is that HIV is such a hellish infection that you hesitate to extrapolate from that. But you could look at other chronic viral infections with that kind of evidence. You could look at autoimmune disease. But I personally think that autoimmune disease is a very unusual setting, and you would be on very shaky ground when you tried to apply it because you would have deregulation of the immune mechanisms. I am not sure that that would really apply to a healthy person under stress.
STEVE GAFFIN: I meant healthy people in different populations around the world. Are any of them relatively more resistant to certain infections, which might relate to that?
SUSANNA CUNNINGHAM-RUNDLES: That is true. Generally, however, those things appear to be somewhat more related to genetics. This actually is quite an interesting area to talk about. It is not easily addressed. But different MHC genes do affect response to particular stimulants, basically, most of the stimulants that we encounter. Personally, as a microbiologist, I believe it is all microbes. So I think that our response to microbes is extraordinarily fundamental. I do not know of anything that would exactly fit what you are looking for, though.
MELINDA BECK: We know that a lot of response to infection occurs locally and in the blood. What is your feeling about looking at, say, nasal lavage to study a respiratory infection?
SUSANNA CUNNINGHAM-RUNDLES: I really think that is very excellent and very much on target.














