A
Overview of the Immune System and Other Host Defense Mechanisms
William R. Beisel1
Immunity, if defined broadly, encompasses all mechanisms and responses used by the body to defend itself against foreign substances, microorganisms, toxins, and noncompatible living cells. Such immunity may be conferred by the immune system itself, or by the protective role of other generalized host defensive mechanisms. Every aspect of immunity and host defense is dependent upon a proper supply and balance of nutrients (Chandra, 1988; Cunningham-Rundles, 1993; Forse, 1994; Gershwin et al., 1985; Watson, 1984).
The generalized primary forms of host defense are termed "innate," "inborn," or ''nonspecific" immunity (Abbas et al., 1995; Brostoff et al., 1991). These initial defensive mechanisms guard the body by contributing protective responses that are effective against a diverse variety of threats. Nonspecific defensive mechanisms may be active or passive in nature (Beisel, 1991). Although nonspecific immunity does not require participation of the immune system per se, it may trigger secondary immune system actions.
The second, or subsequent, form of host defense is termed "adaptive," "acquired," or "antigen-specific" immunity. This form of protection is delivered by the immune system itself, with its complex and highly interactive network of lymphocyte species and their products. The immune system is characterized by antigen specificity and antigen-related memory. Beginning at birth and continuing throughout life, the immune system's immunological repertoire expands as a myriad of new and different antigens are encountered.
Generalized (Nonspecific) Host Defenses
Nonspecific host defenses are provided by both passive and active mechanisms. These mechanisms are involved in defining host susceptibility or resistance to infection, trauma, or other disease threats, and they may be drastically impaired by diverse forms of malnutrition.
Passive Defensive Measures
Passive defenses include anatomical barriers and pathways (skin and mucous membranes, fascial planes, body spaces, tubular structures); exogenous body secretions (mucin, saliva, bronchial fluids, gastric HCI, properdin, opsonins, lysozyme, etc.); physicochemical environments within normal tissues; normal ciliary activity; normal physiological factors (age, sex, race, circadian rhythms); normal microbiological flora in various locations; and even occupational and environmental factors.
Passive defenses can be compromised by malnutrition, injury or trauma, fatigue, specific illnesses (for example, diabetes, leukemia, Hodgkin's disease, alcoholism), prescribed or addictive drugs (for example, corticosteroids, antimetabolites, antimicrobials, hallucinogens, crack cocaine), and medically implanted foreign bodies (for example, vascular prostheses, catheters, drains).
Active Nonspecific Defenses
Nonspecific active defensive measures include a diverse variety of physiological responses (for example, elevated body temperatures tachycardia, vomiting and diarrhea, pituitary-adrenal activation), phagocytic cell activation, creation of inflammatory reactions, formation of nitric oxide from arginine, and a stereotyped pattern of acute-phase reactions (including fever, myalgias, arthralgias, headache and somnolence, anorexia, and a markedly altered pattern of protein synthesis and breakdown in liver and muscle, respectively). In contrast to the synthesis of antigen-specific antibodies by the immune system, nonspecific active humoral defense mechanisms include the production of cytokines, hormones, acute-phase plasma proteins, and sometimes the activation of protein components of the complement, kinin, and coagulation systems.
However, it is important to point out that not all of these components may come into play in any one inflammatory or nonantigen-specific response.
Cytokines
Cytokines are small peptides that function as intercellular signals and mediators. Cytokines are produced by many different types of cells throughout the body. Most cytokines have a diverse variety of actions, depending on the cells they stimulate. Cytokines involved in immune function include interleukins, interferons, colony stimulating factors, and a variety of other closely related mediators. In addition to their multiplicity of actions, cytokines tend to have great redundancy, with overlapping actions being common. Moreover, certain cytokines can stimulate the synthesis and release of other cytokines.
In their role as active participants in nonspecific immunity, a group of "proinflammatory cytokines" (that is, interleukin-1 [IL-1], IL-6, IL-8, tumor necrosis factor [TNF], and interferon-gamma [INF-γ]) initiate acute-phase reactions, launch immune system activities, trigger central nervous system (CNS) responses, and stimulate the release (or suppression) of hormones. Proinflammatory cytokines also participate in inflammatory reactions. Interferons have both antiviral and immunological properties. INF-γ (produced by lymphocytes) also participates in inflammatory and acute-phase processes. Yet another category of cytokines, colony stimulating factors, whose main functions are as hematopoietic factors, may also play a role in inflammation in select responses (Aggarwal and Puri, 1995).
Although new information about the cytokines is still being generated, the fundamental importance of their diverse functions is now fully recognized. As an example of cytokine biology, when macrophages or monocytes are activated (by microorganisms, antigen-antibody complexes, toxins, chemicals, etc.), they quickly respond by producing IL-1, TNF, and other cytokines. These cytokines travel via the blood or interstitial fluid between cells to interact with specific receptor proteins located on the walls of many different target cells throughout the body. The union of a cytokine with its specific cellular receptor leads to the activation of phospholipase enzymes within the target cell wall and the subsequent release into the cell of arachidonic acid (from n-6 polyunsaturated fatty acids [PUFAs] in the plasma membrane), or eicosapentaenoic acid (from n-3 PUFA).
Then, depending on the enzymes (cyclooxygenases or lipoxygenases) contained within a target cell, the arachidonic and eicosapentaenoic acids (EPAs) are converted into eicosanoids (for example, prostaglandins, leukotrienes, thromboxanes, lipoxins) of different potencies. These eicosanoid messenger-effector molecules, in turn, initiate target cell-specific responses (some of which can be blocked by glucocorticoids, aspirin, or ibuprofen) (Beisel, 1995).
Although these cytokine-induced responses are generally protective in nature, an excess production and/or activity of cytokines can be harmful. In fact, an excess of proinflammatory cytokines can lead to hypotensive shock, multiorgan failure, and death. The body possesses an elaborate system of checks and balances to control the production and activity of individual cytokines, a system far more complicated than those that regulate endocrine functions.
The effects of cytokines can be inhibited by a number of different mechanisms. Cytokines with inhibitory actions can block the synthesis and release of other cytokines. In addition, target cells release cytokine receptors into the plasma, and these soluble free receptors can intercept and inactivate the cytokine before it reaches the target cell. Blocking proteins can obstruct cell wall receptors so that cytokines cannot exert their cellular effects. Finally, hormones such as the glucocorticoids can inhibit the intracellular effects of certain cytokines.
Every aspect of cytokine biology requires proper nutrition. A full range of essential nutrients is required to (1) permit the replication of cytokine-producing cells; (2) allow the activation of these cells and the subsequent synthesis and release of cytokines into plasma; (3) allow target cells to synthesize receptor proteins and cytokine-related enzymes; (4) provide a full spectrum of cell wall PUFAs and permit their multistep conversion into eicosanoids; (5) enable target cells to respond appropriately to specific eicosanoid stimuli; and (6) allow the simultaneous development of mechanisms used for controlling excess cytokine activity (Beisel, 1995; Cunningham-Rundles, 1993; Jeng et al., 1995; see Jeffrey Rossio, Chapter 8).
Acute-Phase Reactions
Acute-phase reactions constitute an interrelated group of physiologic and metabolic changes that occur in response to generalized acute infectious illnesses, trauma, severe inflammatory processes, tissue injury, and other medical and surgical diseases (Beisel, 1995; Cunningham-Rundles, 1993; Forse, 1994). Acute-phase reactions are initiated by proinflammatory cytokines and generally have an acute onset.
Acute-phase reactions include fever, generalized malaise, somnolence, anorexia, arthralgia or myalgia, skeletal muscle proteolysis, endocrine system participation, water and salt retention, and cachexia accompanied by negative body balances of nitrogen, phosphate, magnesium, and zinc (Beisel, 1991). Acute-phase reactions are accompanied by a stimulated production of white blood cells and by immune system activation, and are characterized metabolically by a transient intolerance to glucose, hypertriglyceridemia, the sequestration of iron and zinc, and a diminished production of hemoglobin. Numerous other metabolic responses include a massive reprioritization of hepatocyte functions that involves the synthesis of acute-phase reactant
glycoproteins, hepatic enzymes, and metallothioneins, concomitant with a depressed production of plasma albumin (Beisel, 1991).
Acute-phase proteins are synthesized within hours; they include C-reactive protein (CRP), haptoglobin, ceruloplasmin, orosomucoid, α1-antitrypsin, serum amyloid A protein, fibrinogen, and others.
Proinflammatory cytokines, especially IL-1 and TNF, trigger all of these physiological and cellular events. Cytokine actions within the CNS trigger the onset of fever (by causing the release of prostaglandins within the temperature-regulating center), anorexia, somnolence, and the release (or suppression) of hormones produced in the CNS and pituitary gland.
Inflammatory Reactions
Localized inflammatory reactions serve to control and confine infectious microorganisms, to attract cells and their products to localized areas of injury, and to initiate the healing process. Inflammatory reactions involve many pathophysiological processes and cell types.
Inflammatory reactions are characterized by heat and redness, swelling, and pain. Despite these noxious symptoms, inflammatory reactions serve to localize a disease process and prevent it from becoming generalized.
Inflammatory reactions initially include dilation of local blood vessels, vascular congestion, and the binding of white blood cells to the endothelium. At the same time, immunoglobulin G (IgG) molecules become attached to mast cells and basophils, activating them to release histamine and other inflammation-producing complement activation aids in this process. Polymorphonuclear (PMN) leukocytes, monocytes, and other cells penetrate blood vessel endothelium to enter the area of inflammation; this penetration is abetted by the release of chemoattractants.
These initial inflammatory reactions are accompanied by extensive cellular activation that may include the following: the release of prostaglandins, defensins, cathepsins, and thromboxanes from phagocytes; release of lysozyme from macrophages or monocytes; release of histamine from basophils; release of cationic and basic proteins by eosinophils; deposition of fibrin; binding of iron to PMN-synthesized lactoferrin; microbicidal killing; and destruction of many participating cells with localized release of their contents, including enzymes and free oxygen radicals.
The healing process involves the proliferation of fibroblasts, the synthesis of collagen and glucosaminoglycans, phagocytic removal of inflammatory debris, and eventual disappearance of vascular congestion and edema.
The Complement System
As one of the principal mediators of the inflammatory reaction, the complement system participates in both adaptive and cell-mediated immunological responses. It assists in phagocytosis, chemotaxis, cellular activation, the respiratory burst of phagocytes, anaphylaxis, increased capillary permeability, and damage to microorganism surfaces (Abbas et al., 1994; Brostoff et al., 1991) (see Figure A-1). It is a highly adaptive system of profound importance, but its activity can be curtailed by malnutrition.
The complement system consists of a group of 17 plasma proteins that, when the system is activated, are cleaved and/or linked in a sequential manner (termed the complement cascade). Induction of the cascade by antibodies produces the ''classic activation pathway," and initiation by endotoxins or microbial antigens produces the "alternate activation pathway."
In the classic complement activation pathway, aggregated antigen—antibody complexes bind to C1 (first complement component), converting it to a protease enzyme that activates C4 and C2, in turn, to produce fragments C4b and C2a. These fragments then form a complex that activates C3.
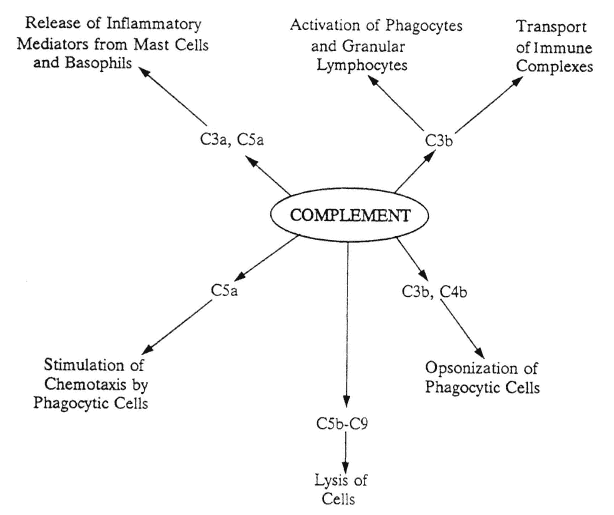
FIGURE A-1
Actions of complement fragments. After complement component C3 is activated via either the classic or the alternate pathway, C3 cleavage fragments have a variety of actions, as shown. C3b is also the starting point for the lytic pathway, which involves complement components C5-C9, to initiate cellular lysis. Lytic pathway fragments also help stimulate chemotaxis.
In the alternate pathway, the presence of microbial cell wall lipopolysaccharides activates plasma proteins, including properdin, to trigger the cleavage of C3 to C3b.
Each complement pathway leads to the activation of C3, which in turn is cleaved into the biologically active molecules C3a (anaphylatoxin) and C3b. The effector molecule C3b is the starting point of the lytic pathway, which proceeds via the participation of C5, C6, C7, C8, and C9. Lytic components can stimulate chemotaxis or produce lesions in cell membranes.
Antigen-Specific Host Defenses
Assisting the nonspecific defenses of the host, the immune system provides additional defensive measures by focusing on and reacting to the highly specific molecular structures of antigens (unique molecular components of microorganisms, food, tissues, inert substances, chemicals, etc.).
The immune system is divided into two major branches that provide (1) antigen-specific cell-mediated immunity (CMI) and (2) humoral immunity (see Figure A-2). CMI is controlled by the thymus gland and provided by T-lymphocytes and natural killer (NK) cells. Humoral immunity is generated by B-lymphocytes. When stimulated appropriately by an antigen plus IL-2 and other cytokines, B-cells undergo clonal expansion and/or are converted into plasma cells, the cellular factories that produce antigen-specific antibodies. The ability of the immune system to recognize and respond to foreign antigens has five cardinal features (Abbas et al., 1995):
- specificity, the ability of lymphocytes to recognize determinant configurations, known as epitopes, on antigenic molecules;
- diversity, the huge number (> 109) of distinct antigenic determinants recognizable in the human lymphocyte repertoire;
- memory, the primary and secondary recognition of a specific antigen by lymphocytes: after the first lymphocytic identification of a new foreign antigen, clonal proliferation occurs, memory cells eventually survive as programmed lymphocytes that will respond to that same antigenic determinant for the indefinite future;
- self-limitation, the waning of an immune response after initial interactions with (and generally, elimination of) a foreign antigen; and
- discrimination of self from nonself, the ability of lymphocytes to distinguish foreign antigens from the self-antigens contained in body tissues. Abnormalities in the maintenance of "self-tolerance" lead to autoimmune diseases.
-
Immune responses occur in three distinct phases: cognitive, activation , and effector (Abbas et al., 1995). (1) In the initial cognitive phase, foreign antigens
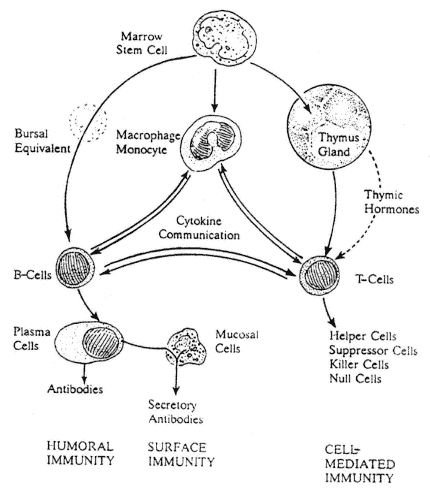
FIGURE A-2
Overview of the immune system. Lymphocytes, derived initially from marrow stem cells, comprise the humoral or cell-mediated arms of the immune system. Humoral immunity is provided by B-cells, which are influenced in mammals by an unidentified equivalent of the bursa of chickens. When stimulated by an appropriate antigen, B-cells undergo clonal expansion and transformation into antibody-producing plasma cells. IgA molecules are joined by J and "secretory" pieces produced by mucosal and epithelial cells, and the resultant IgA dimers are then secreted to generate surface immunity. Cell-mediated immunity is provided by T-cells that originate in the thymus gland and remain thereafter under the influence of circulating thymic hormones. Band T-cells also are stimulated (or inhibited) by a complex network of cytokines (released by macrophages/monocytes and a variety of other body cells, including the lymphocytes themselves), which provide cell-to-cell communications. T-cells also assist antigen-presenting cells in the cell-to-cell delivery of processed antigen to B-cells.
become bound to specific receptors on the cell wall of mature lymphocytes. B-lymphocytes express specific antibodies on their surfaces that can bind soluble foreign proteins, polysaccharides, or lipids, while T-lymphocytes express receptors that recognize only short peptide sequences on foreign antigens (including those located on the surfaces of other cells). (2) In the activation phase, lymphocytes that have recognized a foreign antigen proliferate, leading to the creation of lymphocyte clones. This involves both of the major arms, or branches, of the immune system. In the humoral arm (see Figure A-2), humoral immunity is generated by B-cells, which, when stimulated by an antigen plus IL-2 and other cytokines, differentiate into antibody-secreting plasma cells. Simultaneously, in the CMI arm of the immune system,
antigen-stimulated T-cells (Figure A-2) differentiate from null cells to helper or suppressor cells or into killer cells. The CMI arm is controlled by the thymus gland and its zinc-containing hormones. The CMI serves to support the humoral arm of the immune system, recruit other defensive cells, and kill any cell recognized as foreign. Responding T- and B-lymphocytes eventually migrate to sites of antigen administration or antigen penetration into body tissues. (3) In the third, effector phase, newly secreted antibodies serve to eliminate the foreign antigen and also to activate the complement cascade, stimulate the degranulation of mast cells, and initiate the release of mediators from other cells. Activated T-cells secrete cytokines that enhance the functions of B-cells and phagocytes and stimulate nonspecific inflammatory responses.
The immune system performs its unique functions in two anatomically distinct but interrelated loci. A systemic component generates both CMI and humoral responses whenever it is penetrated by a foreign antigen. A second and separate (but cofunctioning) surface component of the immune system recognizes foreign antigens on body surfaces (including the respiratory and intestinal mucosa) and produces antibodies for secretion in tears and in mucosal, dermal, and intestinal fluids.
Other functioning aspects of the immune system can generate inappropriate or exaggerated tissue-damaging responses. Hypersensitivity or allergic reactions, or autoimmunity, result in damaging long-term immune responses as the body makes antibodies against its own tissues.
Cell-Mediated Immunity
CMI protects the specific, genetically determined tissue type of the body (host) from anything foreign. Through CMI, the body recognizes and defends against infusions of incompatible blood cells or transplanted tissues. Constant surveillance by NK-cells is maintained to detect any body cells that may undergo malignant mutations and, if possible, destroy them (Abbas et al., 1995; Bostoff et al., 1991). NK-lymphocytes arise from "previously uncommitted or null" cells and can, with the help of IL-2, lyse individual bloodborne tumor stem cells without prior sensitization or major histocompatibility complex (MHC). NK-cells also function in graft-versus-host reactions and have been implicated in antibacterial and antiviral defense mechanisms.
Antibody responses to T-cell-dependent antigens require direct physical contact (regulated by genetically determined MHC proteins and adhesion molecules on cell surfaces) between T-helper cells, antigen-presenting cells, and B-cells, and the activation of B-cells to produce appropriate antibodies. CMI includes the lymphocytic secretion of and response to a variety of cytokines. CMI also is involved in eliciting delayed hypersensitivity reactions, that is, reactions that result 24–48 h after contact with an antigen to which the body has been exposed previously.
CMI, particularly T-cell number and function, is a major target of malnutrition. Generalized protein-energy malnutrition (PEM) may cause severe atrophy of lymphoid tissues, especially in their T-cell areas. Deficiencies of other nutrients, including vitamins A and B6 and the minerals iron, zinc, copper, and selenium, also can produce CMI dysfunction. Zinc is of special concern because, in addition to its role in nucleic acid synthesis and the activity of many metalloenzymes, it is a component of the thymic hormones and is essential for their functions. Severe generalized malnutrition also causes the disappearance of clinical allergies and hypersensitivities (Chandra, 1988; Cunningham-Rundles, 1993; Forse, 1994; Gershwin et al., 1985; Watson, 1984).
T-Cells
T-cells are long-lived lymphocytes that circulate continually throughout the body, periodically returning (homing) to the site of their individual origins. At all locations, T-cell activities are influenced by the thymus gland through the effects of its thymic hormones.
T-cells have diverse functions and activities, many of which are triggered when a native T-cell is first stimulated by a new foreign antigen. These functional responses develop in three distinct phases: (1) the cognitive phase, (2) the activation phase, and (3) the effector phase (see Figure A-3).
Many different subsets of T-cells exist. Each T-cell subset has unique (but sometimes overlapping) actions and can be identified by specific cluster of differentiation (CD) antigens (glycoproteins localized on their exterior cell membranes). Subsets include CD4+ T-helper cells, CD8+ T-suppressor cells, and killer cells. T-cells may recognize both specific antigens presenting on membranes of body cells and antigen fragments associated with MHC proteins on these membranes.
CD4+ T-cells recognize antigen fragments associated with Class II MHC molecules in antigen-presenting cells (APCs). APCs engulf (endocytose) and process antigens and link recognizable antigenic fragments to the Class II MHC molecules on their surfaces, which stimulates initiating or helping activities.
When CD4+ cells recognize complexes of antigen and Class II (MHC) molecules on the surface of a macrophage, they activate the macrophage, stimulating it to destroy engulfed organisms. However, if the complexes are on the surface of a B-lymphocyte, the CD4+ cells release cytokines, including IL-2, that lead to B-cell activation, clonal expansion, and antibody production.
CD8+ T-cells recognize antigen fragments associated with Class I MHC molecules on cell surfaces. Cells infected by a virus may exhibit viral peptide antigens linked to surface Class I molecules. CD8+ T-cells thus can recognize and destroy virally infected cells.
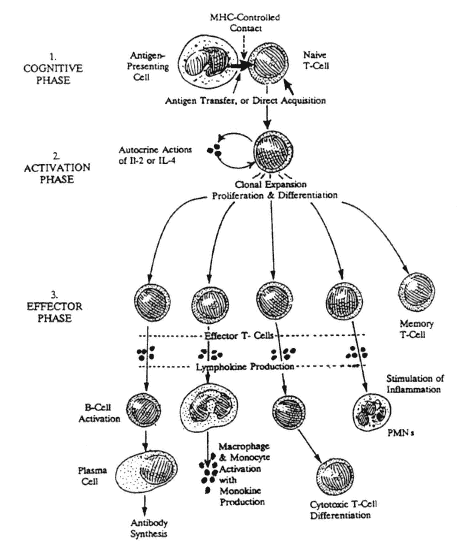
FIGURE A-3
Initial contact of a new foreign antigen with a naive T-cell initiates the cognitive phase. This is immediately followed by the release of lymphokines (IL-2 and/or IL-4) that function as auto-crines to stimulate growth, proliferation, and differentiation of the newly stimulated T-cell during the activation phase to produce clones of effector and memory T-cells. Effector T-cells have many functions, including the triggering of antibody production by B-cells, activation of monocytes and macrophages, the stimulation of inflammatory reactions, and further differentiation into cytotoxic T-cells.
Humoral Immunity
Humoral immunity involves the production of antibodies against specific antigens. Like CMI, humoral immunity has both a systemic component (which produces serum antibodies IgG, IgM, and IgA) and a surface component (which produces secretory IgA).
B-Cells
B-cells are primarily responsible for humoral immunity. They recognize both intact, ''whole," extracellular antigens and processed antigens delivered by antigen-presenting cells. B-cells receive help from CD4+ T-helper cells and stimulatory cytokines. During B-cell interactions with antigen-presenting cells, the role of MHC molecules is essential. There are many genetically determined
MHC haplotypes that vary in their efficiency, resulting in differences in the quantity and quality of subsequent immune responses.
Following the presentation of an antigen to a naive B-cell, the B-cell undergoes clonal expansion to produce daughter cells that will respond to the same antigen whenever it is encountered in the future. Antigen-activated B-cells, stimulated further by IL-2 and other cytokines, mature into plasma cells, the major antibody-producing cells.
Antibodies
Antibodies are bifunctional (immunoglobulin) molecules created to interact with specific antigens. The primary molecular structure of antibodies consists of four peptides (chains) connected by sulfhydryl bonds and arranged in the shape of a Y. Two heavy chains form the Y, and two light chains are attached to the arms of the Y. These arms are termed the Fab (fragment-antibody) regions, and they serve to interact with (bind to) specific intact antigens, either free or on the surface of a microorganism or cell. The stem of the Y is termed the Fc region, and it interacts with receptors on lymphocytes and other cells, or directly with complement.
There are several types of antibodies (immunoglobulins): IgG, IgM, IgA, secretory IgA, IgD, and IgE. A brief description of these immunoglobulins is provided in the glossary (Appendix B).
After the first exposure of a B-cell to a new foreign antigen, systemic antibody production focuses predominantly on IgM, with relatively little IgG produced. Subsequent exposures to the same antigen result in predominant production of IgG (Figure A-4). In contrast, the formation of a secretory antibody by the mucosal immune system involves the initial production of IgA molecules, the joining of two IgAs by a small protein J (joiner) peptide, and actual secretion of the IgA dimer by epithelial or mucosal cells, which add another secretory fragment or peptide to the process.
Antibodies have a variety of distinct functions such as the direct neutralization of circulating antigens and the formation of immune complexes with circulating antigens. After creation of an immune complex in plasma, the Fc regions of the antibody can act as adapters that cross-link the antigen–antibody complex to Fc receptors on the surface of host phagocytic cells (that is, macrophages and PMN leukocytes) that express Fc receptors. This linkage then can lead to the cellular uptake and destruction of the antigen–antibody complex:
- Recognition and sensitization of "foreign" cell targets or parasites for attack by cytotoxic killer cells that possess Fc receptors. This is called antibody-dependent cell-mediated cytotoxicity and is used by eosinophils and large, granular lymphocytes.
- Participation in inflammatory reactions. IgG antibodies can bind to and sensitize mast cells and basophils via their Fc receptors. This binding activates
- the cells to release inflammation-producing mediators, such as histamine, that contribute to the local inflammatory reaction.
- Activation of complement. This is followed by the release of proinflammatory mediators.
The effects of malnutrition on humoral immunity are far less pronounced than on CMI. Atrophy of B-cell areas of lymphoid tissues is relatively small, and preexisting antibodies continue to be produced. In fact, a slightly paradoxical increase in plasma antibody concentrations is a common finding in children with severe PEM. Antibody responses to new antigens, such as those in vaccines, can generally be detected, although the titers and activities of such new antibodies may be reduced.
Hypersensitivity (or Allergic) Reactions
When immune responses are inappropriate or exaggerated and lead to tissue damage, the terms "hypersensitivity" or "allergy" are used. These responses occur only in certain individuals, as a result of second (or numerous)
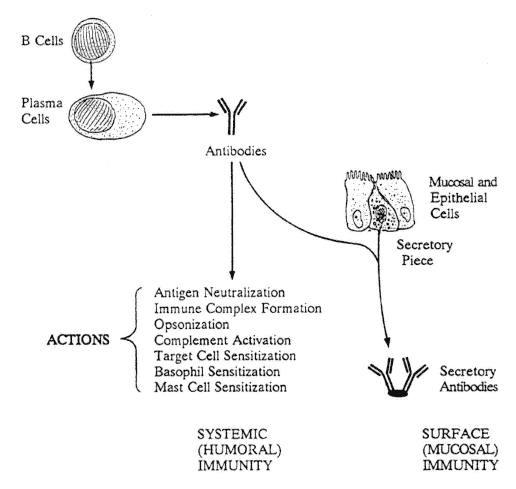
FIGURE A-4
Antibody actions. Plasma antibodies have a multiplicity of actions that provide the interior of the body with systemic humoral immunity. Plasma IgA molecules are joined by J- and "secretory" pieces produced by mucosal and epithelial cells, and the resultant IgA dimers are then secreted to generate surface (mucosal and epithelial) immunity.
contacts with a particular antigen (antigens that produce these reactions are generally termed allergens). Hypersensitivity responses can occur in a variety of distinct but sometimes overlapping types, as listed below.
- Type I reactions are immediate reactions that result from the interaction of an allergen with IgE-sensitized mast cells and basophils. Histamine and other mediators are released, causing acute responses in the skin (weal and flare reactions, urticaria), eyes, nose, bronchial tree, and so forth. These reactions are commonly called allergies. If of extreme, immediate, life-threatening severity, they are termed anaphylactic responses.
- Type II reactions are cytolytic or cytotoxic reactions triggered by the interaction of IgG or IgM with antigens on the surface of specific cells or tissues. These reactions lead to tissue destruction, as seen in autoimmune diseases; the rejection of transplanted foreign tissue; or skin diseases such as pemphigus.
- Type III reactions are immune complex reactions that are also triggered by IgG or IgM and are caused by the interaction of soluble antigen–antibody complexes with complement. If these complexes are deposited within certain tissues, they can cause localized damage. In the kidney, they induce glomerulonephritis, and in the skin, diseases such as erythema multiforme and erythema induratum. Interaction with fungal antigens causes farmer's lung disease. Skin testing with the inciting allergen produces Arthus reactions of 5- to 24-h duration.
- Type IV reactions are delayed-type dermal hypersensitivity reactions to the intracutaneous injection of tuberculin or other antigens. If positive, these modified inflammatory responses progress slowly and peak in 24 to 48 h. In deeper tissues, these reactions induce granulomatous responses that are characteristic of tuberculosis.
Severe malnutrition, by resulting in immunological dysfunction, can cause both hypersensitivity and allergic reactions to disappear.
References
Abbas, A.K., A.H. Lichtman, and J.S. Pober, eds. 1994. Cellular and Molecular Immunology, 2nd ed. Philadelphia: W.B. Saunders Co.
Abbas, A.K., V.L. Perez, L. Van Parijs, and R.C. Wong. 1995. Differentiation and tolerance of CD4+ T lymphocytes. Ciba Found. Symp. 195:7-13.
Aggarwal, B.B., and R.K. Puri, eds. 1995. Human Cytokines: Their Role in Disease and Therapy. Cambridge, MA: Blackwell Science.
Anderson, A.O. 1997. New technologies for producing systemic and mucosal immunity by oral immunization: Immunoprophylaxis in Meals, Ready-to-Eat. Pp. 451-500 in Emerging Technologies for Nutrition Research: Potential for Assessing Military Performance Capability, S.J. Carlson-Newberry and R.B. Costello, eds. Committee on Military Nutrition Research, Food and Nutrition Board, Institute of Medicine. Washington, D.C.: National Academy Press.
Beisel, W.B. 1991. Nutrition and infection. Pp. 507-542 in Nutritional Biochemistry and Metabolism, 2nd ed., M.C. Linder, ed. New York: Elsevier.
Beisel, W.R. 1995. Herman Award Lecture, 1995: Infection-induced malnutrition--From cholera to cytokines. Am. J. Clin. Nutr. 62:813-819.
Brostoff, J., G.K. Scadding, D. Male, and I.M. Roitt, eds. 1991. Clinical Immunology. London: Gower Medical Publishing.
Chandra, R.K., ed. 1988. Nutrition and Immunology. New York: Alan R. Liss, Inc.
Cunningham-Rundles, S., ed. 1993. Nutritional Modulation of the Immune Response. New York: Marcel Decker, Inc.
Forse, R.A., ed. 1994. Diet, Nutrition, and Immunity. Boca Raton, Fla.: CRC Press.
Gershwin, M.E., R.S. Beach, and L.S. Hurley, eds. 1985. Nutrition and Immunology. Orlando, Fla.: Academic Press.
Jeng, K-C.G., C-S. Yang, W-Y. Siu, Y-S. Tsai, W-J. Liao, and J-S. Kuo. 1996. Supplementation with vitamins C and E enhances cytokine production by peripheral blood mononuclear cells in healthy adults. Am. J. Clin. Nutr. 64:960-965.
Watson, R.R., ed. 1984. Nutrition, Disease Resistance, and Immune Function. New York: Marcel Decker, Inc.
| This page in the original is blank. |
















