7
Health Services Research In Cancer Care
What is Health Services Research?
Health services research is a multidisciplinary field that investigates the structure, processes, and effects of health care services (Box 7.1). Such research informs critical decisions by government officials, corporate leaders, clinicians, health plan managers, and consumers making choices about health care or health insurance. The National Cancer Policy Board (NCPB), in an effort to understand how resources for research are applied to questions regarding the quality of cancer care, undertook a review of the status of cancer-related health services research. This chapter first describes publication trends in cancer-related health services research and then summarizes support for health services research within the following organizations:
Federally Sponsored Research
- Department of Health and Human Services National Institutes of Health (National Cancer Institute) Agency for Health Care Policy and Research Health Care Financing Administration Centers for Disease Control and Prevention
- Department of Defense
- Department of Veterans Affairs
Privately Sponsored Research
- American Cancer Society
- Foundations (e.g., Robert Wood Johnson Foundation)
Although these organizations are not the only sponsors of cancer-related health services research, they represent the major funding sources for such research. Excluded from this review is health services research supported by health plans, insurers, pharmaceutical companies, and other private organizations. Much of the research in these settings is proprietary.
|
BOX 7.1 What Is Health Services Research? The Institute of Medicine defines health services research as: a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility, delivery, organization, financing, and outcomes of health care services to increase knowledge and understand the structure, processes, and effects of health services for individuals and populations. Several features of this definition are worth noting. First, health services research is a multidisciplinary field that draws from many academic and clinical disciplines such as economics, epidemiology, biostatistics, nursing, and medicine. Its boundaries are imprecise, particularly as they relate to policy and management studies and clinical research. A clinical trial, for example, could be categorized as health services research if the effectiveness of a health care technology or intervention was assessed in a ''real-world'' rather than in an ideal or highly controlled setting. Second, the reference to basic and applied research underscores the fact that health services research involves both questions about fundamental individual, organizational, and system behaviors and questions of direct practical interest to public and private decision makers. Third, by referring to both knowledge and understanding, the definition stretches the boundaries of the field to include work of a theoretical or conceptual nature. Finally, the definition includes research that can have either a group-or an individual-level focus. SOURCE: IOM, 1995. |
Health services research can be defined broadly to include behavioral and psychological research (e.g., assessments of individuals' preferences in health care), evaluations of programs that may fall outside the purview of the traditional health care system (e.g., school-based health programs), and randomized controlled clinical trials (e.g., studies of the effectiveness of health care technologies in situations representative of community practice). The National Cancer Policy Board accepted a broad definition of health services research and for this review applied the rubric used by the National Library of Medicine to select projects for inclusion in its health services research database (i.e., HSRProj) (Box 7.2).
Status of Cancer-Related Health Services Research
Publications
Evaluating trends in research publications is one way to assess the level of activity within a discipline. A resource for tracking such studies is the National Library of Medicine (NLM) Medline bibliographic database, which stores information about individual citations including index terms used to characterize each article (articles are indexed according to a dictionary of medical subject headings called MESH terms).
The volume of cancer-related health services research articles appears to have been relatively stable during the 1980s, but increased sharply in the 1990s according to Medline searches from 1980 to 1997. In 1997, there were more than 1,200 articles indexed that addressed health services research issues related to cancer (Figure 7.1). Although the number of health services research citations increased during this period, by 1997 they represented less than 3 percent of all cancer-related citations indexed in the medical literature (Figure 7.2). These trends reflect publications in English, but not necessarily articles written by U.S. investigators. Much of the literature reviewed in Chapter 5 was conducted in the United Kingdom and is represented here. Figures 7.1 and 7.2 therefore reflect trends in the general medical literature, not necessarily trends in the United States. These trends must be interpreted with caution because they may reflect changes in the way MESH headings are applied to index the literature rather than real increases in cancer-related health services research.
|
BOX 7.2 Topics in Health Services Research Health services research may address, or be conducted for the purposes of understanding or improving, areas such as the following:
SOURCE: HSRProj, 1998. |
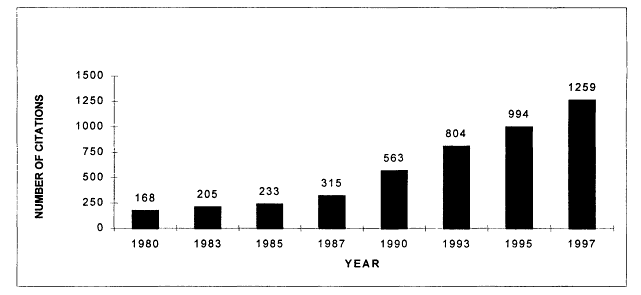
Figure 7.1
Medline citations for cancer-related health services research, 1980-1997. Research citations were identified in the NLM's Medline database using the MESH heading "neoplasms" (for cancer) and any one of the following major MESH headings "health services research," ''quality of health care," and "quality assurance." The last terms encompass cancer citations addressing guideline adherence, outcomes and process assessment, accreditation, and health planning. Only articles published in English are counted.
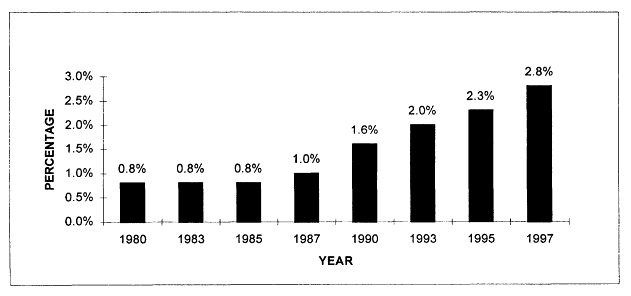
Figure 7.2
Medline citations for cancer-related health services research as a percentage of all cancer citations, 1980-1997. Percentages were calculated as the number of cancer-related health services research citations (as described in Figure 7.1) divided by the total number of citations identified using the "neoplasms" MESH term alone, for each given year. Only articles published in English are counted.
Research Support
A more direct way to assess the status of U.S.-based cancer-related health services research is to describe topics of investigation and levels of research spending. There is no one comprehensive source of information on health services research support, and as part of its review, the National Cancer Policy Board relied on the following sources:
- information catalogued in the HSRProj database (Health Services Research Project database) maintained by the National Library of Medicine—this database includes brief descriptions of ongoing extramural research sponsored by federal and state agencies, foundations, and other organizations;
- listings of research projects provided by some organizations (e.g., National Cancer Institute, Agency for Health Care Policy and Research, American Cancer Society);
- review of annual reports of research arms of certain agencies (e.g., the Department of Veterans Affairs);
- review of agency web sites (e.g., Department of Defense);
- informal contacts with agency representatives known to be involved in health services research (e.g., Health Care Financing Administration, Centers for Disease Control and Prevention); and
- meetings with senior agency representatives (i.e., Agency for Health Care Policy and Research, National Cancer Institute).
Despite the best efforts of the Board, the description of the nation's cancer-related health services research portfolio that follows may under-or over-estimate the actual level of research. Organizations varied in how they defined health services research and consequently, there is likely some inconsistency in what was included (or excluded) as a health services research activity. Furthermore, some health services research activities may have been missed because of limitations of research tracking systems. The review is limited to currently active research projects for most organizations.
Federally Sponsored Research
Department of Health and Human Services
The Department of Health and Human Services (DHHS) includes the Public Health Service (PHS), which in turn oversees several sites that house cancer research: the National Institutes of Health (NIH), the Agency for Health Care Policy and Research (AHCPR), and the Centers for Disease Control and Prevention (CDC). Within DHHS, the Health Care Financing Administration (HCFA), which is organizationally parallel to the PHS, also supports applied cancer research. DHHS reports to Congress each year about the amount it spends on a number of health-related areas, including cancer (McGeary, 1999).
à The National Cancer Institute (NCI), one of the National Institutes of Health is the largest single provider of funds for cancer research ($2.4 billion in FY 1997).
- AHCPR estimated it spent $3.9 million for research on health costs, quality, and outcomes related to cancer (out of a research budget of $95 million).
- HCFA spends enormous amounts on cancer—$16.7 billion in FY 1997—mostly on medical services and care, but it has a small program of research (see below)·
- CDC estimated that it spent $185 million on cancer-related programs in FY 1997. The categories included breast and cervical cancer ($139.7 million), cancer registries ($22.3 million), other chronic diseases ($8.1 million), infectious diseases ($450,000), environmental health ($1.7 million), and occupational safety and health ($12.7 million). All categories do not represent research programs For example, the breast and cervical cancer program is an early detection program aimed at underserved populations. Deleting this program and assuming the rest of the activities are research would leave $45.3 million.
National Institutes of Health
National Cancer Institute. Cancer Surveillance Research Program. Many of NCI's health services research activities are housed in the Division of Cancer Control and Population Sciences, Cancer Surveillance Research Program (CSRP). CSRP develops information systems and methods needed to conduct cancer surveillance research and makes these resources available to investigators throughout the research community. The linked Medicare-SEER (Surveillance, Epidemiology, and End Results) database, for example, is now widely used to answer cancer-related health services research questions (www.dccps.ims.nci.nih.gov/ARB/SEERMedicare). CSRP-sponsored research evaluates trends in cancer related to risk factors, health behaviors, and health services and assesses the influence of these factors on cancer burden (e.g., cancer incidence, morbidity, mortality, survival)· The division sponsors research related to patterns of care, diffusion of new technologies, cost of cancer care, and methodology and modeling.
Examples of health services research supported by the division include the following (Edwards, 1998a, b):
- Patterns of Care studies: SEER data are used to describe the dissemination of state-of-the-art cancer treatment and explanatory factors for variation in patterns of care. First conducted in 1987, samples of cases from SEER were obtained in 1988, 1989, 1990, 1991, 1995, and 1996. Currently, data are being collected on cases diagnosed in 1997 with cancer of the head and neck, cervix, childhood brain stem, and ductal carcinoma in situ of the breast. In previous years, cancer sites assessed have included: in situ and early-stage breast, colorectal, ovarian, urinary bladder, melanoma, non-small-cell lung, and childhood cancers. Annual budgets for the past three funding years ranged from $575,000 to $690,000 per year.
- Prostate cancer outcomes study: A longitudinal survey of 3,500 men with prostate cancer is underway regarding quality of life measured at 6 and 12 months and at 5 years following diagnosis (data collection to be completed in 1999). Practice patterns are also being assessed.
- Breast Cancer Surveillance Consortium (a national mammography screening and outcomes database): The performance of mammography screenings (i.e., its sensitivity, specific-
- ity, predictive value) in a community setting is being evaluated in eight sites across the country as part of a congressionally mandated study under the Mammography Quality Standards Act of 1992. The major objectives of the consortium are to enhance understanding of the accuracy, cost, and quality of breast cancer screening; to foster collaborative research among consortium participants; to assess factors associated with variations in mammography practice, accuracy, and subsequent diagnostic evaluation; and to provide a foundation for the conduct of clinical and basic science research that can improve understanding of breast cancer etiology and prognosis. By the year 2000, the consortium will have data on nearly 3.2 million screening mammographic examinations and more than 24,000 breast cancer cases. Through 1999, total funding for the consortium is $17.2 million (NCI provides 85-90 percent of the total funding, with the remainder coming from CDC and the DoD). An additional $31 million for the period FY 2000 through FY 2004 will support the extramural research effort.
- The SEER-Medicare database: This is a collaborative effort of the NCI, the SEER registries, and HCFA to create a large population-based source of information for cancer-related epidemiologic and health services research. The database links cases in SEER cancer registries to claims records in Medicare's administrative database. The currently available linked file includes all Medicare data through 1994 for persons diagnosed with cancer through 1993. An update of the linkage, which will incorporate SEER cancer cases diagnosed in 1994-1996, will be completed in 1999.
The SEER-Medicare data offer an opportunity to examine patterns of care prior to the diagnosis of cancer, during the period of initial diagnosis, and during long-term follow-up. Topics that can be addressed with the linked database include patterns of care for specific cancers, the use of health services, and the costs of treatment. There is also the potential for longitudinal surveillance of the health care of persons with cancer. These data can be used to assess health care directed toward the prevention of disease or disability, as well as the restoration or maintenance of health (Edwards, 1997). Active projects using the linked SEER-Medicare database include analyses of
- trends in treatment of in situ breast cancer;
- total lifetime payments for elderly cancer patients;
- differences in patterns of care and cancer survival between health maintenance organizations (HMOs) and fee-for-service (FFS) providers;
- breast cancer treatment patterns and trends;
- prostate cancer detection practices; and
- trends and variations in initial treatment for early-stage prostate cancer.
HMO Cancer Research Network. The purpose of the Cancer Research Network (CRN) is to encourage the expansion of collaborative cancer research among health care provider organizations that are oriented to community care; have access to large, stable, and diverse patient populations; and are able to take advantage of existing integrated databases that can provide patient-level information relevant to research studies on cancer control and to cancer-related population studies. Beginning in 1999, NCI will fund the first CRN project—a consortium of 10 large, not-for-profit, research-oriented HMOs. The CRN will conduct four main projects (Martin Brown, Head, Health Services and Economics Section, Applied Research Branch, Cancer Surveillance Re-
search Program, Division of Cancer Control and Population Sciences, NCI, personal communication, December 16, 1998):
- The development of an administrative infrastructure to support research collaboration, data quality, and integrity and to develop methods and organizational approaches to increase the participation of managed care patients in NCI-approved clinical trials. The infrastructure will include a data-coordinating center and expert teams to provide organized scientific input in the areas of biostatistics, health economics, survey measures, pharmacoepidemiology, genetics, clinical trials management, and survivorship.
- A study of the efficacy, reach, adherence to, and quality of delivery of smoking cessation programs in HMO practice settings.
- A study of late-stage breast and invasive cervical cancer cases to elucidate the patient, provider, and system factors that contribute to preventing advanced disease.
- A study of the effectiveness of the commonly used strategies of frequent mammography or prophylactic mastectomy, to prevent fatal breast cancer among women at increased risk for breast cancer.
Funding for this four-year extramural grant is approximately $4 million per year, with a total award of approximately $16 million.
The division has established an Outcomes Research Section to examine outcomes measures used in clinical trials and to monitor the national burden of cancer. The section will support research in the areas of measurement of quality of life, cost, and quality of care. Aspects of clinical trial organization and financing will also be addressed (e.g., integrating trials into routine care) (Martin Brown, Head, Health Services and Economics Section, Applied Research Branch, Cancer Surveillance Research Program, Division of Cancer Control and Population Sciences, NCI, personal communication, December 16, 1998).
Office of Cancer Survivorship. In 1996, NCI established the Office of Cancer Survivorship to develop and support a research agenda that explores the long-and short-term physical and psychological effects of cancer and its treatment. The office has provided $4 million to supplement existing cooperative agreements, grants, and contracts. An additional $700,000 was committed by the Susan Komen Foundation. Investigator-initiated research will be funded with an additional $3 million per year for five years. Most of the research funded to date has focused on treatment complications (e.g., effects of cancer treatment on gonadal function and reproductive health) and quality-of-life issues (e.g., quality of life for adult survivors of childhood leukemia), but a few awards have addressed health services research issues (e.g., medical care costs of cancer). Box 7.3 shows prioritized areas of research for the Office of Cancer Survivorship.
Health services research supported by the NCI is shown for breast cancer in Box 7.4, other cancer sites in Box 7.5, and other general research (i.e., not cancer-site specific) in Box 7.6.
Several other NIH institutes have supported extramural health services research (Table 7.1).
|
BOX 7.3 Prioritized Areas of Research for the Office of Cancer Survivorship (OCS) OCS has, to date, focused its research agenda on the issues of survivors who are at least two years post-treatment, because research data has been lacking for this group of individuals. Little information has been available about long-term cancer survivors (5-, 10-, and 15-year survivors) and the types of problems they face. OCS also aims to increase awareness of survivor issues among medical professionals and the general public. Prioritized areas of research include:
A number of survivor groups have been overlooked in studies to date, these include patients with certain diagnoses, survivors representing various ethnic and socioeconomic groups, and the elderly. In addition, longitudinal survivorship studies have been lacking and instrumentation has been inadequate to measure quality of life over time. SOURCE: Meadows A., presentation at the President's Cancer Panel, June 2, 1998. |
|
BOX 7.4 Current Extramural Health Services Research Projects on Breast Cancer Supported by NCI Prevention and Screening:
Treatment:
SOURCES: Brenda Edwards, Associate Director, CSRP, DCCPS, National Cancer Institute, personal communication to Maria Hewitt, November 1998; http://www.nih.gov/grants/guide/pa; Martin Brown, Head, Health Services and Economics Section, Applied Research Branch, CSRP, DCCPS, NCI, personal communication to Maria Hewitt, December 1998; HSRProj, 1998. |
|
BOX 7.5 Current Extramural Health Services Research Projects on Cancers Other than Breast Cancer Supported by NCI Prevention and Screening:
Treatment:
SOURCES: Brenda K. Edwards, Division of Cancer Control and Population Sciences, National Cancer Institute, personal communication to Maria Hewitt, November 1998; http://www.nih.gov/grants/ guide/pa; Martin Brown, Head, Health Services and Economics Section, Applied Research Branch, CSRP, DCCPS, NCI, personal communication to Maria Hewitt, December 1998; HSRProj, 1998. |
|
BOX 7.6 Current Extramural General Health Services Research Projects Supported by NCI Prevention and Cancer Control (Including Early Detection):
|
Palliative Care:
Rural Cancer Care:
Special Populations--Racial or Ethnic Minority Groups:
Cost Studies:
Education:
Clinical Trials:
|
|
Methodology
Provider Issues:
Other:
SOURCES: Brenda Edwards, DCCPS, National Cancer Institute, personal communication to Maria Hewitt, November 1998; http://www.nih.gov/grants/ guide/pa; Martin Brown, Head, Health Services and Economics Section, Applied Research Branch, CSRP, DCCPS, NCI, personal communication to Maria Hewitt, December 1998; HSRProj, 1998. |
TABLE 7.1
Current Extramural Health Services Research Projects Supported Elsewhere at NIH and Listed on HSRProj
|
Institute |
Project |
|
National Institute on Aging |
|
|
National Institute of Dental Research |
|
|
National Eye Institute |
|
|
National Library of Medicine |
|
|
SOURCE: HSRProj, 1998. |
|
Agency for Health Care Policy and Research
AHCPR is the lead agency within DHHS charged with supporting research on health care quality, outcomes, cost, utilization, and access. The agency supports intramural research as well as an extramural grants program with a budget of $171 million (1999 appropriation) (www.ahcpr.gov). Support for intramural and extramural cancer-related health services research projects active since FY 1995 totals $20 million and represents an estimated 6 percent of the research budget during this period (Wendy Perry, AHCPR, personal communication to Maria Hewitt, December 1998).
Ongoing extramural research projects supported by AHCPR since FY 1995 are listed in Box 7.7.
|
BOX 7.7 Extramural Cancer-Related Health Services Research Projects Active Since FY 1995, AHCPR Prevention, Screening, and Early Detection
Treatment
|
SOURCE: Wendy Perry, AHCPR, personal communication to Maria Hewitt, December 1998. |
Evidence-Based Practice Program. The Evidence-Based Practice Program supports literature syntheses on clinical effectiveness, a resource for developing clinical practice guidelines or performance measures. Two cancer-related topics were among the first 12 topics assigned to the evidence-based practice centers: testosterone suppression treatment for advanced prostate cancer and evaluation of cervical cytology. In addition, two assessments will be completed in 1999: (1) an assessment of the management of cancer pain and (2) an analysis of the use of erythropoietin in hematology and oncology.
CONQUEST. CONQUEST (Computerized Needs-Oriented QUality Measurement Evaluation System) is a database of clinical performance measures, a resource for quality monitoring activities. Included are measures related to the management of several cancers (i.e., colorectal, lung, prostate, breast), the use of screening tests (i.e., mammography, Pap smear), and cigarette use.
Patient Outcomes Research Teams (PORTs). PORTs conduct research and disseminate findings related to common disorders. Two of the 14 conditions funded to date relate to cancer, prostate disease, and local breast cancer.
Clinical Trials. AHCPR's budget is insufficient to permit sole funding of major randomized clinical trials, although it has on a few occasions collaborated with other agencies (e.g., the Department
of Veterans Affairs [VA] and several institutes within NIH) to take part in a larger comparative effectiveness study (U.S. Congress, 1994). AHCPR is, for example, supporting the Prostate Cancer Intervention Versus Observation Trial (PIVOT) in collaboration with NCI and VA. This randomized trial compares radical prostatectomy and palliative expectant management for the treatment of clinically localized prostate cancer. Information is being collected on patient outcomes such as functional status and quality of life. Costs and cost-effectiveness of alternative treatments are being assessed. Less than 5 percent of the total funding for this trial is from AHCPR.
Other Intraagency Agreements. In addition to the PIVOT trial, AHCPR sometimes transfers money to other agencies to support health services research. The Health Resources and Services Administration (HRSA), for example, received $100,000 to evaluate a multimedia education program on cervical cancer that was developed at NCI and the NLM for physicians, nurses, and other health care professionals. Funds were transferred to NCI to support an evaluation of minimal access surgery in cancer treatment (i.e., a comparative study of laparascopic versus open colectomy for the treatment of colon cancer). The assessment includes an analysis of cost-effectiveness. (AHCPR, personal communication to Maria Hewitt, December 1998).
Clinical Practice Guidelines. AHCPR no longer develops treatment guidelines, but it has recently issued guidelines on smoking cessation (1996) and the quality determinants of mammography (1994). In 1994, AHCPR published a practice guideline on cancer pain that will be updated with information forthcoming from an evidence practice center recently funded to review this topic. It has also recently issued a technical review of colorectal cancer screening (1998) (www.ahcpr.gov). AHCPR, in collaboration with the American Medical Association and the American Association of Health Plans, has developed a National Guideline Clearinghouse accessible by the Internet (www.guideline.gov). The website contains information on available guidelines, permits comparisons of guidelines recommendations, and facilitates communication among those involved in guideline development and dissemination (Stephenson, 1997). Of the first 414 guidelines accepted for inclusion, 45 relate to cancer (AHCPR, personal communication to Maria Hewitt, December 1998).
U.S. Preventive Services Task Force (USPSTF) and Put Prevention into Practice (PPIP). Since the 1980s, the USPSTF has evaluated scientific evidence for the effectiveness of clinical preventive services (e.g., screening tests, counseling, immunization, chemoprophylaxis) and produced age-and risk factor-specific recommendations for the services that should be included in a periodic health examination. PPIP is designed to help implement recommendations of the USPSTF. Roughly 20 percent of the services considered by USPSTF and PPIP relate to cancer detection or prevention.
Intramural Research Projects. Some of the research conducted by AHCPR staff concerns the quality of cancer care services (e.g., ''Drive-through mastectomy: How common and who's driving?'').
Basic Health Services Research. AHCPR supports research aimed at expanding the available array of quality measures. Some of these are not specific to cancer but could be relevant to cancer patients (e.g., quality measures for home and subacute care, health outcomes and quality-of-life meas-
ures for adolescents, aspects of clinician-patient interactions that improve patient satisfaction and outcomes) (Elaine Power, AHCPR, personal communication to Maria Hewitt, May 1998).
Research on Managed Care. To evaluate the effect of particular managed care policies on the quality of care provided to patients with chronic diseases (e.g., protocols governing the referral of patients to medical specialists, arrangements for paying physicians), AHCPR, in collaboration with HRSA (which is also part of DHHS) and the American Association of Health Plans Foundation, has recently funded a three-year research program. None of the conditions under study are cancers, but findings may be generalizable to cancer (www.ahcpr.gov/news/press/aahppr.htm).
Technology Assessment. AHCPR staff have evaluated the effectiveness of several cancer-related technologies including autologous peripheral stem cell transplantation, hematopoietic stem cell transplantation in multiple myeloma, and cryosurgery for recurrent prostate cancer following radiation therapy.
Health Care Financing Administration
Intramural Research—Office of Strategic Planning. Patterns and Outcomes of Cancer Care in the Medicare Population. HCFA analysts are attempting to answer the following questions with the linked SEER-Medicare database:
- What are overall Medicare costs, by type and stage of cancer?
- What Medicare costs are specifically related to cancer care?
- What comorbidities are associated with cancer and how do they influence Medicare use and cost?
- What is the mix of care (on a per-person basis) among community hospitals, teaching hospitals, and cancer centers?
- What institutional factors influence the type of inpatient hospital care received by cancer patients?
Breast Cancer Treatment Patterns Among Medicare Enrollees in HMOs and FFS. The linked SEER-Medicare database has recently been analyzed to examine the use of breast conserving surgery (BCS) versus mastectomy for early-stage breast cancer cases in HMOs and FFS. The study also compared the distributions of stage at diagnosis between HMO and FFS enrollees and examined the use of adjuvant radiation therapy among BCS patients. The study included all early-stage breast cancer cases diagnosed in 1988-1993 among elderly women entitled to Medicare residing in SEER reporting areas. (USDHHS, 1998; Riley et al, 1999).
Mammography Utilization Initiative. Rates of mammography use have been published by age and race, at the state and county levels, for 1993. The mammography use data book will be updated with 1994 and 1995 Medicare data and disseminated to public health and cancer organizations to help target outreach activities to areas with particularly low utilization. The data will be placed on HCFA's Internet home page (USDHHS, 1998).
Hospice Use. The linked SEER-Medicare database will be analyzed to examine the sociodemographic (e.g., age, sex, race or ethnicity, income, education) and health care (e.g., HMO status) determinants of hospice use among beneficiaries with colorectal and lung cancer (diagnosed in 1992 and 1993). The hospice benefit was originally designed as an alternative to aggressive care for beneficiaries with terminal illnesses. Few studies, however, have examined the utilization and cost of health care services among hospice patients. This study will assess the level and type of services both prior to and during hospice care (R. Mentnech, Health Care Financing Administration, personal communication to Maria Hewitt, November 1998.).
Intramural Research Center for Health Plans and Providers
Research Related to Payment Issues. Patterns of care and volume stability at the physician organization level will be studied using per capita measures of utilization for selected oncology services and for all Medicare services. The effect of the principal provider organization's characteristics, size, and case mix of oncology practice, as well as geographic location, on per capita costs will also be examined. These analyses will support the development of alternative service bundles and carve-out payments for the care of Medicare cancer patients. The linked SEER-Medicare database will be the principal source of data. The project is in the early development phase (USDHHS, 1998).
Research Training and Development. In an effort to promote research using its databases, HCFA has created the Research Data Assistance Center (ResDAC) to assist new researchers and promote familiarity with and use of its databases for research on Medicare and Medicaid issues. The initial ResDAC contract is with the University of Minnesota, which has formed a consortium with Boston University, Dartmouth College, and Georgetown University. ResDAC will facilitate and expedite the use of HCFA data for research on Medicare and Medicaid by providing education and training, improving researcher access to HCFA data, and providing expert consultation for researchers (USDHHS, 1998).
Extramural Research. According to the HSRProj database, HCFA has sponsored one extramural health services research project, "Estimating Mammography Utilization by Elderly Medicare Women."
Centers for Disease Control and Prevention
Most of the cancer-related health services research supported by CDC is funded through the National Center for Chronic Disease Prevention and Health, Division of Cancer Prevention and Control. The division plans and conducts epidemiologic studies and evaluations to identify the feasibility and effectiveness of cancer prevention and control strategies. Other activities include providing technical assistance to states, local public health agencies, and other health care provider organizations. Ongoing extramural research supported by the Division of Cancer Prevention and Control is shown in Box 7.8.
|
BOX 7.8 Current Extramural Health Services Research Projects Funded by CDC's National Center for Chronic Disease Prevention and Health, Division of Cancer Prevention and Control General
Breast and Cervical Cancer
Prostate Cancer
|
Colorectal Cancer
SOURCE: Kevin Brady, Acting Deputy Director, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health, CDC, personal communication to Maria Hewitt, March, 1999. |
Other cancer-related health services research is conducted within other areas of CDC. Two projects were identified within the Epidemiology Program Office: Mammography utilization in an HMO, and treatment issues related to breast and cervical cancer. Within the National Center for Health Statistics, researchers are evaluating the role of social class and race/ethnicity on the incidence of cancer (HSRProj, 1998).
Department of Defense
Beginning in FY 1992, the U.S. Congress directed the Department of Defense (DoD) to manage several appropriations for an extramural grant program directed toward specific research initiatives. The United States Army Medical Research and Materiel Command (USAMRMC) constituted the office of the Congressionally Directed Medical Research Programs (CDMRP) to administer these funds. To date, between FY 1992 and 1999, $1.1 billion has been targeted by Congress for research on breast cancer, prostate cancer, ovarian cancer, neurofibromatosis, defense women's health, and osteoporosis. The CDMRP strives to identify gaps in funding and provide award opportunities that will enhance program research objectives without duplicating existing funding opportunities.
The three DoD programs targeted at cancer research include the following:
- Breast cancer: Between FY 1992 and 1999, $883.8 million has been appropriated to the Breast Cancer Research Program (BCRP). Proposals are sought across all areas of laboratory, clinical, behavioral, and epidemiological research including all disciplines within the basic, clini-
- cal, psychosocial, behavioral, sociocultural, and environmental sciences: nursing, occupational health, alternative therapies, public health and policy, and economics. In FY 1993-1994 the program applied the recommendations of the Institute of Medicine and focused on traditional research and infrastructure (IOM, 1997). In FY 1996, the program deemphasized traditional awards to fund innovative "idea awards" and clinical translational research. The BCRP also offers training support, such as predoctoral, postdoctoral, sabbatical, and career development awards.
- Prostate cancer: Between FY 1997 and 1999, $135 million has been appropriated to the Prostate Cancer Research Program for basic and clinical research. The goals of the program are to pursue breakthrough ideas and approaches, prepare new scientists, encourage established investigators, and promote prostate cancer public awareness and education among the public. The program encourages innovative, multi-institutional, and multidisciplinary research and has funded new investigator awards, idea development awards, and minority population-focused training awards.
- Ovarian cancer: Between FY 1997 and 1999, $27.5 million has been appropriated to the Ovarian Cancer Research Program. To date, comprehensive, multidisciplinary, preventive center grants have been funded to foster the development of a sustained national ovarian cancer research enterprise.
Extramural research identified in HSRProj is shown in Box 7.9.
|
BOX 7.9 Current Extramural Health Services Research Projects Funded by DoD General
Prostate Cancer
|
Breast Cancer
SOURCES: HSRProj 1998; S. Young-McCaughan, Deputy Director for Research Programs, CDMRP, personal communication to Maria Hewitt, December 1998. |
Department of Veterans Affairs
The Department of Veterans Affairs (VA) Office of Research and Development supports intramural biomedical, rehabilitation, and health services research. Health services research focuses on conditions that are common among veterans, including cancer (especially prostate and lung). Recent initiatives have addressed factors affecting the delivery of health care such as managed care; the implementation of clinical practice guidelines; ethnic, cultural, and gender issues; continuity of care, and patient-centered care. VA support for cancer-related health services research totaled $9.5 million in fiscal years 1997 and 1998.
Eleven Centers of Excellence have been established to link health services research to patient and administrative needs and to provide technical expertise in certain areas, for example, the measurement of chronic disease outcomes, health economics, and tobacco use and cessation. Studies of cancer treatments, chronic pain, and end-of-life care are being conducted as part of the VA's quality improvement program, the Quality Enhancement Research Initiative (QUERI).
The VA is supporting two clinical trials with health services research components:
- PIVOT, a randomized trial comparing radical prostatectomy versus palliative expectant management for the treatment of clinically localized prostate cancer (other sponsors include NCI and AHCPR); and
- A VA cooperative trial to assess whether 18-F-Fluorideoxyglucose Positron Emission Tomography (PET) imaging can be used to accurately determine if solitary pulmonary nodules are malignant. The trial will assess the costs and benefits of this technology (e.g., avoidance of unnecessary procedures).
In 1998, the VA's Office of Research and Development began collaborating with the Department of Defense for studies on prostate diseases including prostate cancer.
Other VA intramural health services research is shown in Box 7.10.
|
BOX 7.10 Intramural Health Services Research Projects, Department of Veterans Affairs General
Prostate Cancer
|
Breast Cancer
Other Cancers
SOURCE: Department of Veterans Affairs, Office of Research and Development, Mary Jones, personal communication to Maria Hewitt, January 7, 1999. |
Private Organizations Funding Research
American Cancer Society
The American Cancer Society (ACS) is the largest non-government funder of cancer research in the United States ($93.4 million in 1996) (McGeary, 1999).
Intramural Research Programs
Department of Epidemiology and Surveillance Research. Assessment of the Quality of Treatment Data. The ACS in collaboration with the American College of Surgeons and three state cancer registries (Illinois, Kentucky, Louisiana) is evaluating the completeness and quality of treatment data for patients with colon cancer. Different approaches to collecting data from both hospital and outpatient settings will be assessed with the aim of estimating the proportion of colon cancer patients who receive optimal treatment, given the stage of their disease at diagnosis. Data acquired in a more timely fashion could be used by clinicians, individual hospitals, and state health department officials as benchmarks to gauge the quality of care provided. Success in this feasibility study could lead to the study of other cancer sites in additional states. Funding for this feasibility study is less than $100,000 (P. Wingo, Department of Epidemiology and Surveillance Research, American Cancer Society, personal communication to Maria Hewitt, October 1998).
Patterns of Care Study. The ACS is analyzing the National Hospital Discharge Surveys from 1988 to 1995 to describe patterns of use of inpatient surgical procedures for treating cancers of the lung, colorectum, prostate, and female breast, by age, race, gender, and geographic region. This is an intramural research activity of the Department of Epidemiology and Surveillance Research (P. Wingo, Department of Epidemiology and Surveillance Research, American Cancer Society, personal communication to Maria Hewitt, October 1998).
Behavioral Research Center. Although not designed as health services research initiatives per se, several activities within ACS's Behavioral Research Center could have applications to health services research (ACS, 1998).
Population-Based Surveys of Cancer Survivors. The Behavioral Research Center is conducting two large population-based surveys of cancer survivors at a cost of $2 million for the pilot phases (Baker, VP Behavioral Research, ACS, personal communication to Maria Hewitt, October 1997). The first is the "Study of Cancer Survivors—Incidence." This survey of up to 100,000 cancer survivors is underway in a pilot phase and is designed as a 10-year prospective study of survivors enrolled within the first year after diagnosis of any one of the ten most common cancers (i.e., prostate, female breast, lung, colorectal, urinary bladder, non-Hodgkin's lymphoma, skin melanoma, uterine, kidney, ovarian). A population-based sample is being selected from area cancer registries in sufficient numbers to provide state-level estimates. The major aim of the survey is to examine the behavioral, psychosocial, treatment, and support factors that influence quality of life and survival of cancer patients. The survey is being fielded on a pilot basis in four states (Iowa, Minnesota, Wisconsin, Georgia), and plans are to extend the study to other states that have adequate cancer registration and an interest in participating. The survey includes a number of scales that have been validated (e.g., problems in daily living, physical and mental health functioning, problems with work) along with basic information about the cancer (type of cancer), treatment, health insurance, and site of health care. It should be possible, therefore, to examine quality-of-life issues by insurance or site of care, controlling for type of cancer (although it is unclear what information on comorbidity will be available).
The second survey is the "Study of Long-Term Cancer Survivors—Prevalence." This survey is a cross-sectional study of 6,000 long-term survivors (i.e., those who are 5, 10, and 15 years beyond diagnosis) of six cancers (prostate, breast, colorectal, bladder, melanoma, uterine). There will be 1,000 respondents for each type of cancer. Twenty-seven states have registries that were established in 1983 or earlier, and four SEER metro area registries also meet this requirement, which is necessary to identify 15-year survivors. Only 12 state registries and all four SEER registries have complete data (85 percent complete) for 1983, 1988, and 1993.
Complementary Therapies. Surveys of complementary therapies (e.g., acupuncture, visualization, yoga) have been conducted to determine the extent to which people with cancer are using these unconventional treatments and what their impact is on quality of life. In addition, surveys of oncology physicians, nurses, and social workers have been completed regarding the extent to which providers are aware of commonly used complementary therapies and whether they are supportive of cancer patients' use of these therapies.
Barriers to Care. Plans are underway to conduct research on factors that inhibit or are barriers to the participation of minorities and other special populations in prevention programs, screening, clinical trials, and effective treatment.
Extramural Research Program
Very little ACS extramural research is devoted to health services research, even when broadly defined to include behavioral, psychosocial, and quality-of-life research. Information from the ACS on institutional, research, and training grants in effect as of September 1, 1998, indicates that less than 5 percent of the total $171,336,000 grant program is allocated to health services research (Box 7.11). The brief descriptions of research projects included in the grant summary list do not always provide sufficient detail to allow one to distinguish between health services and other kinds of research. Consequently, this list may under-or overestimate the level of health services research support.
|
BOX 7.11 ACS Extramural Grant Program Health Services Research Support (as of September 1998) Breast Cancer
Colon Cancer
Prostate Cancer
|
Other Cancers
Other
SOURCE: ACS, 1998. |
Several other private organizations support cancer-related health services research to a limited extent (Table 7.2).
TABLE 7.2
Current Extramural Health Services Research Projects Supported by Foundations or Private Organizations and Listed on HSRProj
|
Organization |
Project |
|
Robert Wood Johnson Foundation |
Smoking and cancer screening: chronic disease prevention for older women |
|
|
Supporting quality improvement and Joint Commission on Accreditation of Healthcare Organizations standard setting for pain management in hospitals |
|
|
Research on cancer screening among Hispanic women |
|
United Hospital Fund |
Improving clinical care for early-stage breast cancer patients: changing physician practices |
|
Aetna, Inc. |
Preparing African-American men for decision making about prostate cancer and early detection |
|
|
Using performance measures to motivate process improvement: a randomized trial |
|
SOURCE: HSRProj 1998. |
|
The Cochrane Collaboration
Although not strictly health services research, the Cochrane Collaboration is a not-for-profit international organization that ''aims to help people make well-informed decisions about healthcare by preparing, maintaining, and promoting the accessibility of systematic reviews of the effects of healthcare interventions'' (Box 7.12). Evidence reviewed comes from a number of sources, with an emphasis on published and unpublished randomized clinical trials.
Since 1997, the Cochrane Cancer Network (http://www.canet.demon.co.uk) has coordinated the work of site-specific groups and plans to develop a database of all past and present controlled or randomized trials and systematic reviews in cancer. To date, the network has registered nearly 15,000 reports of controlled and randomized trials in cancer (www.canet.demon.co.uk). Cancer or cancer-related collaborative review groups that are registered or that are developing include:
- breast cancer,
- colorectal cancer,
- ear, nose, and throat disorders,
- eye cancer,
- gynecological cancer,
- head and neck cancer,
- hematological malignancies,
- liver cancer,
- lung cancer,
- oral cancer,
- pain, palliative, and supportive care,
- prostatic and urological cancers,
- skin cancer,
- stomach and pancreatic cancer, and
- tobacco addiction.
The Cochrane Cancer Network is developing a specialized database for cancer, called the Cancer Library in Europe, which will serve as a comprehensive source of information about cancer for consumer groups and other members of the cancer community (http://www.canet.demon.co.uk).
Key Findings
- Many public and private organizations are funding a diverse set of health services research topics, but with the information currently available, one cannot estimate total spending on cancer-related health services research. The best estimates suggest that it represents a very small share of total cancer research funding.
- Currently funded health services research is addressing important issues related to the quality of cancer care, for example, the impact of managed care, patterns of care, outcomes studies, issues in cancer survivorship, cancer control in minority communities, barriers to access to cancer care, cancer surveillance, pain management, and end-of-life care.
- NCI appears to be the primary sponsor of cancer-related health services research. The health services research budget is not known, but quality-related research appears to represent a very small fraction of the overall NCI research budget ($2.4 billion in FY 1997).
- AHCPR has a small budget ($171 million in 1999) and a limited but balanced (by cancer site) portfolio of extramurally funded cancer health services research. An estimated 6 percent of the research budget is spent on cancer-related health services research. AHCPR is developing an infrastructure for evidence synthesis and is disseminating information about clinical practice guidelines, both or which have relevance to cancer care.
- Analysts at NCI and HCFA are conducting important cancer-related health services research with the linked SEER-Medicare claims file. NCI and HCFA are attempting to assist new researchers and to promote familiarity with, and use of, this database.
- DoD is supporting breast, prostate, and ovarian cancer research through its Congressionally Directed Medical Research Programs, but very few of the awards are for health services research.
- Very few private organizations (e.g., foundations) appear to be funding cancer-related health services research. The American Cancer Society has an extramural research budget of $171 million of which 5 percent of support is devoted to health services research. The ACS is internally funding the development of two very large surveys of cancer survivors, which will provide valuable information about the experience of cancer, especially quality-of-life issues.
- The Cochrane Collaboration, an international organization devoted to synthesizing the results of clinical trials, has begun to organize groups based on cancer site.
References
American Cancer Society. 1998a. American Cancer Society Extramural Grants Programs in Effect September 1, 1998. Atlanta, GA: American Cancer Society.
American Cancer Society. 1998b. Behavioral Research Center: Program Description and Progress Report. Atlanta, GA: American Cancer Society.
Brown M. 1998. Head, Health Services and Economics Section, Applied Research Branch, Cancer Surveillance Research Program, personal communication to Maria Hewitt, December 16, 1998.
The Cochrane Collaboration. 1999. http://hiru.mcmaster.ca/cochrane/default.htm.
Edwards BK. 1997a. Associate Director, Cancer Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. Presentation to National Cancer Policy Board. Washington, D.C.
Edwards BK. 1997b Briefing Book prepared for National Cancer Policy Board. Updated by personal communication.
Edwards, BK. 1998. Associate Director, Cancer Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, personal communication to Maria Hewitt, November 2, 1998.
Eisenberg JM. 1998. AHCPR focuses on information for health care decision makers. Health Services Research 33 (4): 767-785.
HSRProj Database (Health Services Research Project Database). 1998. http://www.ahsr.org.
IOM (Institute of Medicine). 1995. Health Services Research.' Work Force and Educational Issues. Field MJ, Tranquada RE, Feasley JC, eds. Washington, D.C.: National Academy Press.
IOM. 1997. A Review of the Department of Defense's Program for Breast Cancer Research. Committee to Review the Department of Defense's Breast Cancer Research Program. Washington, D.C.: National Academy Press.
McGeary, M. 1999. Cancer Research Funding in the United States. Draft presented to National Cancer Policy Board, Washington, D.C.
Riley GF, Potosky AL, Klabunde CN, et al. 1999. Stage at diagnosis and treatment patterns among older women with breast cancer: An HMO and fee-for-service comparison. Journal of the American Medical Association 281 (8):720-726.
Stephenson J. 1997. Revitalized AHCPR pursues research on quality. Journal of the American Medical Association 278(19): 1557.
U.S. Congress, Office of Technology Assessment. 1994. Identifying Health Technologies That Work: Searching for Evidence. OTA-H-608. Washington, D.C.: U.S. Government Printing Office.
U.S. Department of Defense. 1998. Congressionally Directed Medical Research Programs: Prostate Cancer Research Program. http://cdmrp.army.mil/prostate.
U.S. Department of Health and Human Services, Health Care Financing Administration. 1998. Active Projects Report: Research and Demonstration in Health Care Financing. Washington, D.C.
U.S. Department of Veteran Affairs, Office of Research and Development. 1997. Refining Research Priorities: New Initiatives Meeting Veteran Needs. Washington, D.C.