3
Ensuring Access to Cancer Care
The link between poor access to care and poor health outcomes is well established (Hoffman, 1998; IOM, 1994), but the reasons for inadequate access are not well understood. Some of the connections are intuitive and obvious: women without health insurance have breast cancer detected at later stages and have poorer survival rates than women with insurance (Ayanian et al., 1993). One in seven Americans lacks health insurance, which creates a general barrier to getting medical care of any kind. Some other barriers to receiving appropriate medical care are less obvious. Nonfinancial barriers that may prevent people from ''getting to the door" of a health care provider include geography, language, fear and distrust of health care providers, and difficulties getting through appointment or "gatekeeper" systems. Once "in the door," other barriers to access may surface when attempting to navigate the system: for example, getting from a primary care provider to a specialist. Within the system, providers may lack current information on treatment, have difficulty communicating with patients, or have insufficient staff to coordinate care and provide all the services patients need. The cancer care system is complex; consequently, various barriers that serve to limit access may surface during each phase of care.
Access, as defined by the Institute of Medicine (IOM, 1994), is the timely use of personal health services to achieve the best possible health outcomes. This definition of access incorporates both the use of health services and the quality of such services to assess the degree of access that has been achieved. The test of equity of access involves first determining whether there are systematic differences in use and outcomes among groups in U.S. society and, if there are, the reasons for these differences (IOM, 1994).
This report brings together the best evidence from the published literature about the barriers to health care for cancer patients and the roots of these barriers. The body of available literature clearly documents differences in access to particular phases of care for particular cancers, at specific points in time and place. In no case, however, does it provide an overall picture of access to cancer care across society in the late 1990s, and in some cases conflicting evidence is pre-
sented on the same topic. Some discrepancies may be due to different research methods, but many of them probably reflect the actual situation—that access has varied across time and place and that the variation has multidimensional causes and effects. Nonetheless, the material presented here is a useful guide to general patterns of differential access and to some of the interventions that have been successful in improving access.
Evidence of Access Problems
Individuals who are poor, have low educational attainment, or are members of racial or ethnic minority groups tend to have poorer cancer outcomes than members of other groups. This is supported by findings from the literature relating to different aspects of cancer care:
- Survival from cancer is associated with social class (characterized by income and education): lower social classes tend to have poorer survival (Gordon et al., 1992; Greenwald et al., 1996; Kogevinas and Porta, 1997; Savage et al., 1984).
- Overall cancer mortality is higher in the lower social classes, even after risk factors such as smoking are taken into account (Lantz et al., 1998).
- Death rates among African-American hospital patients with colorectal cancer are higher than rates for white patients, even when differences in patient characteristics, insurance status, clinical factors, and providers are accounted for (Ball and Elixhauser, 1996; Cooper et al., 1996).
- Hispanic cancer patients have lower colorectal survival rates than non-Hispanics (Goodwin et al., 1996).
- Five-year survival rates for Native American compared to white, non-Hispanic individuals, ascertained in 1978-1981, were substantially lower for colorectal cancer (37 versus 51 percent), lung and bronchial cancer (5 versus 12 percent), and female breast cancer (53 versus 75 percent) (Miller 1996).
- Individuals with cancer who are elderly, women, and members of racial/ethnic minority groups are more likely to have poor pain relief than others (Bernabei et al., 1998; Cleeland et al., 1994, 1997).
Why Do These Differences Exist?
Some of the factors that have been investigated as possibly affecting access to optimal cancer care are
- health insurance coverage and type of coverage;
- cost, including health insurance and out-of-pocket costs;
- attributes of the health care delivery system (e.g., geographic distribution of cancer care facilities, lack of service coordination);
- attributes of individuals (e.g., lack of knowledge or misperceptions about cancer prevention and treatment, linguistic or cultural attributes); and
- attributes of health care providers (e.g., lack of knowledge about cancer prevention and treatment, communication styles).
These factors and others may come into play in different ways at one or more of the steps along the path from cancer detection and treatment to care at the end of life, and all can potentially contribute to differences in outcomes. In this chapter, the role of financial barriers in the context of cancer care is reviewed, in particular, problems related to health insurance coverage and out-of-pocket costs. Then the literature exploring the sources of the mortality differentials among sociodemographic groups is summarized by the following phases of care (For a more in-depth review of this literature and a conceptual framework regarding issues of access to cancer care, see the NCPB commissioned paper by Mandelblatt and colleagues [Mandelblatt et al., 1998, available on line at: www.nas.edu/cancerbd], upon which this review is based):
- Phase 1: Early detection,
- Phase 2: Evaluation of abnormal screening results,
- Phase 3: Cancer treatment,
- Phase 4: Posttreatment surveillance and recurrence care, and
- Phase 5: End-of-life care.
Financial Barriers To Access To Cancer Care
Health Insurance and Type of Coverage
Individuals with cancer are very likely to be insured, because the large majority is over age 65 and covered by Medicare. Nevertheless, of the 1.3 million new cases of cancer diagnosed in 1997, an estimated 86,000 individuals, or 7 percent, would be expected to be uninsured (estimate based on age-specific cancer incidence rates and the age distribution of the uninsured). Nationally, 16 percent of the population was uninsured in 1997 (U.S. Bureau of the Census, 1998). In addition, many individuals with health insurance experience lapses in coverage (an estimated 12 million in 1992).
The diagnosis of cancer can, in itself, lead to a loss of health insurance coverage or to higher insurance premiums. In 1992, 7 percent of cancer survivors who were insured prior to their diagnosis reported that their health insurance changed following their cancer diagnosis (e.g., 5 percent said that their insurance costs increased) (Hewitt, 1998). Congress tried to remedy this problem in 1996, enacting the Health Insurance Portability and Accountability Act (Kennedy-Kassebaum Act) to improve the portability and continuity of health insurance coverage in private insurance markets and among employer-sponsored group health plans. The act limits the ability of insurers to deny or discontinue coverage because of preexisting conditions such as cancer. The increased cost of premiums for portable insurance products and difficulties in implementing the law, however, have limited the value of these new protections for consumers (U.S. General Accounting Office, 1997).
If individuals are uninsured, medical expenses related to cancer may force them to "spend down" to become eligible for Medicaid—that is, to deplete their assets until they meet eligibility criteria. Alternatively, individuals who are disabled by cancer for a period of two years may become eligible for Medicaid coverage through the Supplemental Security Income (SSI) program. Some hospitals are obligated to provide some charity care to the uninsured (i.e., under the Hill-
Burton Act of 1946); some state and federal programs provide free cancer screening and sometimes treatment for the uninsured (e.g., the Centers for Disease Control and Prevention [CDC] National Breast and Cervical Cancer Early Detection Program; the Maryland state program that pays for treatment for uninsured women with breast cancer); at least 50 pharmaceutical companies have patient assistance programs to help defray the costs of expensive chemotherapy drugs for those who are poor and uninsured (or underinsured);1 and some charitable organizations provide free services or financial assistance to individuals with cancer. These programs and services cannot substitute for adequate insurance coverage for cancer treatment, but they can ease the financial burden for those who receive them.
- The American Cancer Society has a volunteer-based program called Road to Recovery that provides transportation for cancer patients to and from medical appointments and treatments (Anne Marie Oria, Texas American Cancer Society, personal communication to Elizabeth Kidd, October 1998).
- Cancer Care, a nonprofit, voluntary agency serving primarily the New York City area provides, on a limited basis, financial assistance for treatment-related expenses (e.g., transportation, child care, home care, pain medication) (Sherry Fremont, personal communication to Elizabeth Kidd, October 1998).
- St. Jude Children's Research Hospital provides free medical care, transportation, and other supportive services for children with cancer and other conditions (Jerry Chipman, personal communication to Elizabeth Kidd, September 1998).
- The Organ Transplant Fund provides health care support services, financial assistance, and advocacy programs to transplant candidates and their families (Organ Transplant Fund, 1998).
At least 27 states sell comprehensive health insurance to state residents with serious medical conditions who cannot find a company to insure them or who cannot afford the high cost of coverage. The insurance provided through these so-called state risk pools (also known as Guaranteed Access Programs) generally costs more than regular insurance, and in some states, there are waiting periods for coverage of preexisting conditions and lifetime caps on benefits (e.g., sometimes as low as $250,000) (Matt Hayes, Patient Advocate Foundation, personal communication to Elizabeth Kidd, September 1998).
Cost, Including Health Insurance and Out-of-Pocket Costs
Health insurance coverage may not adequately protect individuals from the high costs associated with cancer treatment. Some policies have high deductibles (e.g., catastrophic policies typically contain a deductible of $15,000 or more), and copayments or coinsurance over the course of cancer treatment can be substantial (HIAA, 1998). Furthermore, many insurers, including Medicare, do not cover all of the drugs and treatments used by cancer patients (see discussion of prescription drug coverage below).
Relatively few studies specific to cancer exist regarding the magnitude of the financial burden associated with out-of-pocket costs, but available evidence suggests that it is substantial. In a study conducted in 1986, Medicare was found to cover an estimated 83 percent of typical total charges for lung cancer and 65 percent of typical charges for breast cancer (Sofaer et al., 1990). For these two cancers, investigators assessed the extent to which supplemental Medigap plans reduced out-of-pocket costs and found that plans varied widely in the financial protection offered. Out-of-pocket expenses ranged from less than $100 under some health maintenance organization (HMO) plans to nearly $4,000 under some private Medigap plans (1986 dollars) (Sofaer et al., 1990). Unlike the great majority of employer-provided insurance plans, Medicare does not cap beneficiaries' total payments for cost sharing (AARP, 1997). Medicare HMOs typically have lower cost sharing than the traditional Medicare program and may offer additional benefits, such as outpatient prescription drug coverage (AARP, 1997).
Prescription Drug Coverage. Insurance policies often lack comprehensive coverage for prescription drugs, a benefit needed by most individuals with cancer. Medicare, for example, does not cover the costs of most outpatient prescription drugs, which can include pain medications and other drugs to treat the effects of cancer and its treatment. Many Medicare beneficiaries are subject to these costs because only one-third of them have insurance policies that cover prescription drugs (e.g., Medicaid, employer-provided, or privately purchased policies) (Gluck, 1999). Most chemotherapy is administered in outpatient settings and is covered by Medicare. Even when insurance does offer prescription drug coverage, out-of-pocket expenses can be high because of limits to coverage.2 An estimated 7 percent of the elderly with chronic illnesses spend at least 10 percent of their household income on prescription drugs (Rogowski et al., 1997).
Review of the Literature, By Phase of Care
Phase 1: Early Detection
Early detection tests for breast, cervical, and colorectal cancers are effective in reducing mortality. For women age 50 to 69, for example, mammography screening reduces the death rate from breast cancer by about one-third (USDHHS, 1991). Although effective, these tests are underutilized, for example,
- 56 percent of women age 50 and older in 1994 had had a mammogram to detect breast cancer within the past 2 years,
- 77 percent of women age 18 and older in 1994 had had a Pap smear to detect cervical cancer within the past 3 years,
- 30 percent of people age 50 and older in 1992 had had a fecal occult blood test (FOBT) to detect colorectal cancer within the past 2 years, and
- 33 percent of people age 50 and older in 1992 had ever had a proctosigmoidoscopy to detect colorectal cancer (NCHS, 1997).
For those cancers for which effective screening tests exist, diagnosis at advanced stages among those eligible for screening suggests that tests are underused. Overall, 6 percent of breast cancers, 8 percent of cervical cancers, and 21 percent of colorectal cancers are diagnosed late (i.e., advanced) (Ries et al., 1997), but late stage at diagnosis is more common among some sociodemographic groups than others:
- People living in areas with high rates of poverty and unemployment are more likely to have their colorectal cancer diagnosed at a late stage than those living in other areas (Mandelblatt et al., 1996).
- Women living in poorer neighborhoods are more likely than women living in wealthier areas to have invasive, rather than localized, cervical cancer at diagnosis (Breen and Figueroa, 1996).
- African-American and other minority group members diagnosed with cancer are more likely to be diagnosed at advanced stages of disease than are whites (Farley and Flannery, 1989; Mandelblatt et al., 1991, 1996; Wells et al., 1992). For cervical cancer, this racial gap has increased over time despite greater use of Pap tests among African-American, compared to white women (Mitchell and McCormack, 1997).
- African-American and Hispanic women are more likely to have breast cancer diagnosed at late stages than white women, when setting of care, income, and education are controlled for (Mandelblatt et al., 1991).
- Women are more likely than men to have late-stage colorectal cancer at diagnosis (Mandelblatt et al., 1996).
Financial Barriers to Cancer Screening
Lack of health insurance is clearly linked to lower rates of cancer screening (Ayanian, 1993; Hedegaard et al., 1996; Katz and Hofer, 1994; Mickey et al., 1997) and to diagnosis at more advanced stages of disease (Figure 3.1). However, even in countries where health care coverage is universal, screening rates are not uniformly high. In Canada, for example, individuals with high compared to low household incomes are more likely to be screened for cancer (Katz and Hofer, 1994). Conversely, despite not having health insurance, 40 to 50 percent of uninsured women in the United States report that they have been screened for cervical and breast cancer (Hoffman, 1998).
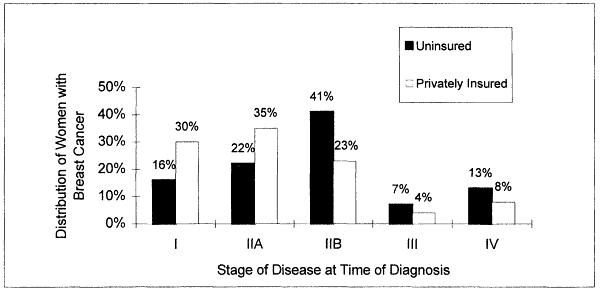
Figure 3.1
Distribution of women with breast cancer by disease stage at time of diagnosis.
Source: Hoffman, 1998.
Insurance policies vary in the extent of coverage they offer for cancer screening. People covered by plans with no or low levels of cost sharing are more likely to be screened for cancer than those in plans with higher out-of-pocket costs (Lurie et al., 1987). Among Medicare beneficiaries, those with private supplemental insurance are more likely to be screened for cancer than beneficiaries with Medicare supplemented by Medicaid or with Medicare alone (Blustein, 1995; Potosky et al., 1998). Cancer screening tests were a covered benefit for Medicare beneficiaries, but until 1998 a copayment was required for these tests.
Health Care Delivery and Cancer Screening
The way health care is delivered also affects the use of cancer screening tests. Individuals covered by managed care plans have higher rates of cancer screening than those covered by fee-for-service plans (Burack and Gimotty, 1997; Potosky et al., 1998). This is true also of Medicare beneficiaries in HMOs, whose cancer screening rates are the highest of the elderly population (Potosky et al., 1998).
Even for those with insurance, screening tests may be inaccessible because there are no facilities within a reasonable distance. Perhaps for this reason, residents of rural areas use cancer screening tests less often than their urban counterparts (Hayward et al., 1988b; Katz and Hofer, 1994). Some specific findings include the following:
- Women living in areas with no or few mammogram facilities are less likely to have mammograms than those living in areas with more facilities (Mandelblatt, 1995).
- Women living in areas with primary care shortages are less likely to have regular mammograms than women living elsewhere (Phillips et al, 1998).
Role of Patient Beliefs, Knowledge, and Racial Or Socioeconomic Characteristics in Screening
A lack of awareness of the benefits of cancer screening can pose as significant a barrier as lack of insurance or distance from screening facilities. Many individuals know little about cancer, and do not know that it can be successfully treated or when and why screening tests are useful (Grady et al., 1992; Myers et al., 1991). Low level of education (which usually occurs in conjunction with low household income) is associated with lower cancer screening (and re-screening) use (Lannin et al., 1998; Mickey, 1997; Rutledge et al., 1988).
Concerns about inconvenience, discomfort, trouble, embarrassment, fear of radiation, and pain involved in screening are among the reasons people forgo cancer screening tests (Davis et al., 1996; Glanz et al., 1996; Myers et al., 1991; Stein et al., 1990). Other attitudes—fatalism, a feeling that one's health cannot be affected by traditional medicine, and religious or cultural beliefs—may also preclude cancer screening (Kagawa-Singer, 1997; Lannin et al., 1998; Mo, 1992).
In one breast cancer study, culturally based attitudes and beliefs were more predictive of advanced stage at diagnosis (suggesting low screening rates) than were social class and race (Lannin et al., 1998). In this study, African-American women had three times the odds of white women (i.e., an "odds ratio" of 3) of being diagnosed with late-stage disease (Stages III and IV). When social class was taken into account in the analysis, the odds ratio decreased to 1.8. When measures of cultural beliefs (e.g., the devil can cause you to get cancer, air causes cancer) were also controlled for in the analysis, African-American women no longer had increased odds of late-stage disease.
Use of mammography appears to account for much of the variation of stage at diagnosis of breast cancer that can be attributed to race (Breen and Figueroa, 1996; Mandelblatt et al., 1995). Studies have found the following:
- There were no differences in stage at diagnosis among women who were regular mammography users according to comparisons of mammography histories of elderly African-American and white women. However, among women who had not participated in screening mammography, the odds of being diagnosed with late-stage breast cancer were 2.5 times greater for African-American than for white women (McCarthy et al., 1998).
- Women who receive medical care through the Department of Defense, and who should therefore all have the same access to care, demonstrate no difference in stage among Caucasian, African-American, and Hispanic women diagnosed with breast cancer (Zaloznik, 1995, 1997).
- Among elderly women, African-Americans as compared to whites use mammography less often. More frequent use of mammography is associated with more visits to a primary care physician in both groups, but the deficit for African-American women persists at each income level, even after primary care use is considered. Primary care visits are less likely to "boost" mammography use for African-American women than for white women (Burns et al., 1996).
Some evidence suggests that certain racial and ethnic groups that appear to have adequate access to care are not getting appropriate screening services. In one study, women living in Appalachia and Hispanic women living in urban Texas had relatively low cancer screening rates despite having access to care (NCI, 1995b). Hispanics and some Asian groups (e.g., Chinese, Vietnamese) tend to have lower screening rates than whites or African Americans, which may be attributable, at least in part, to language and cultural barriers on the part of patients and providers (Hiatt and Pasik, 1996). Studies have found that
- physicians discussed mammography less often with Hispanic than with non-Hispanic patients (Fox and Stein, 1991),
- African-American women enrolled in managed care plans were less likely than white women to have had a doctor advise them to get a mammogram. Nevertheless, African-American and white women had similar self-report mammography use (Glanz et al., 1996); and
- African-American patients were less likely to report receiving advice about cancer screening or receiving screening tests than white patients seeing the same physicians (Gemson et al., 1988).
The older people are, the less likely they are to be screened for breast and cervical cancers (Fox et al., 1994; Hedegaard et al., 1996; NCHS, 1997; NCI, 1995). The elderly may hold beliefs that inhibit testing, but they are likely to comply with physicians' recommendations to be screened (Fox et al., 1994; Mandelblatt et al., 1991). Some physicians may also mistakenly believe that routine cancer screening is unimportant in elderly patients (Weisman et al., 1989).
Many people who are screened for cancer once do not have the tests repeated at recommended intervals. Rates of adherence to regular or ''interval" screening are significantly lower than for the initial screening procedure (Burack and Gimotty, 1997; De Waard et al., 1984). Adherence to lifetime cancer screening is measured as the number of cancer screens received per number recommended. For example, if five screens were recommended for a 55-year-old woman and she had received only four of the five, she would be considered 80 percent adherent. The effect of age on adherence to interval screening appears to vary by cancer type, with higher screening rates observed for the elderly with colorectal cancer (Brown et al., 1990; Mandelblatt et al., 1996), but lower rates for cervical cancer (De Waard et al., 1984; Fink et al., 1972; Mandelblatt et al., 1998). One study indicates that adherence to lifetime breast cancer screening is higher among women who are younger, are members of a higher social class, and have access to care, especially membership in an HMO (the study compared women of similar age, race, education, and income) (Philips et al., 1998).
Role of the Physician in Cancer Screening Access
With or without insurance, lacking a regular source of care also leads to lower rates of cancer screening (Bindman et al., 1996; Fox et al., 1994; Gordon et al., 1998; Zapka, 1994; Zapka et al., 1992). In one study, women who did not have a regular doctor were 3.5 times more likely to be diagnosed with late-stage breast cancer than women who had seen their regular doctor within the past year (Lannin et al., 1998). In a study of multiethnic black and Hispanic women in New York,
breast and cervical cancer screening rates increased when women had a usual source of care and when they had a regular clinician at their usual source (O'Malley et al., 1997). One of the strongest predictors of whether a person will be screened for cancer is whether the physician recommends testing (Fox and Stein, 1991; Grady et al., 1992; Mickey et al., 1997; Zapka et al., 1991). Overall, physicians order fewer cancer screening tests than are recommended in preventive health care guidelines (Fox et al., 1988; Schwartz et al., 1991). Some researchers have looked into the reasons physicians may not recommend screening tests and have found the following:
- Physicians are generally aware of guidelines, but they may not perceive screening tests to be beneficial in the absence of symptoms (Schapira et al., 1993).
- Screening recommendations change, and some providers may not keep up with current standards, whereas others may be confused by conflicting guidelines.
- Many individuals seek health care only when they have an acute illness and providers may miss opportunities to provide screening if they focus only on the presenting illness.
- A lack of reimbursement for counseling about screening, time pressures, and health system infrastructure limitations (e.g., a lack of tracking or reminder systems) may also contribute to providers' underuse of cancer screening tests.
Screening practices also vary by physician specialty. Obstetrician-gynecologists are more likely than family practitioners to order cancer screening tests for women. Internists generally recommend screening at lower rates than other primary care providers, and subspecialists providing primary care tend to screen at the same, or lower rates than primary care providers (Albanes et al., 1988; Bassett, 1985; Bergner et al., 1990; Mann et al., 1987; Schwartz et al., 1991; Weinberger et al., 1991; Weisman et al., 1989; Zapka et al., 1992). Some studies suggest that women cared for by female physicians are more likely to be screened for cancer than women cared for by male physicians (Lurie et al., 1997).
The manner in which screening is presented by health care providers can affect whether a person actually has the test. A higher level of enthusiasm for the recommendation can influence the likelihood of screening (Mickey et al., 1997). Women who say that they participated in the initial decision to be screened for breast cancer were also more likely to adhere to the recommended follow-up mammography regimen than those who felt the doctor had made the decision for them (Phillips et al., 1998).
Interventions to Improve Screening Rates
A number of interventions have been demonstrated to increase cancer screening rates. Telephone and mailed reminders from providers, multimedia educational interventions, financial incentives, and peer counseling can all increase women's use of mammography (Clementz et al., 1990; Davis et al., 1997; Irwig et al., 1990; Janz et al., 1997; Kendall, 1993; Kiefe et al., 1994; King et al., 1994; Landis et al., 1992; Lantz et al., 1995; Mickey et al., 1997; Mohler, 1995; Taplin et al., 1994). Among women already screened for cancer, reminders to return for screening increase interval testing (Mayer et al., 1994; Schapira et al., 1992). Providing general information about cancer screening alone to those who are eligible, however, has not been effective in increasing test use (Champion, 1994b; Nattinger et al., 1989; Skinner et al., 1994).
Interventions aimed at providers can also improve screening use. Physician reminder systems, chart audit with feedback, and physician education about appropriate screening practices contribute to higher screening test use (Becker et al., 1989; Burack et al., 1997; Chambers et al., 1989; Cheney and Ramsdell, 1987; Cowan et al., 1992; Landis et al., 1992; McPhee et al., 1989, 1991; Nattinger et al., 1989; Ornstein et al., 1991; Tierney et al., 1986; Yarnall et al., 1993). However, in one study, written feedback and financial incentives were ineffective in improving physician compliance to cancer screening guidelines in primary care sites serving women age 50 and older cared for in a Medicaid HMO (Hillman et al., 1998).
Phase 2: Evaluation of Abnormal Screening Results
Follow-Up of Abnormal Results
Screening tests alone do not provide a diagnosis of cancer; this can be made only with further testing. In fact, most people with abnormal results from a single cancer screening test will not be found to have cancer, so definitive testing is essential if the benefits of screening are to be realized (Mandelblatt et al., 1997). Nonetheless, many individuals fail to receive timely, or any, follow-up of an abnormal screening test, with large variations in the rates of nonresolution across settings and populations. Different studies have reported that 20-99 percent of women who have abnormal mammograms receive appropriate diagnostic follow-up (Kerlikewske, 1996; Manelblatt et al., 1993b), and 20-74 percent of women with abnormal Pap smears receive appropriate follow-up (Lacey et al., 1993; Marcus et al., 1992; Mandelblatt et al., 1997; Michielutte et al., 1985).
In one study of the reasons for delays between the time of the initial medical consultation and the establishment of a diagnosis among women with breast cancer, providers and the health care systems were found to be responsible for 45 percent of cases with significant delays. Delays were attributed to difficulties in scheduling or physician inaction. In about 25 percent of the cases, the delay was attributed to patients, and the most common reason for inaction provided by women was that the problem was not perceived as important. In another 17 percent of cases, both the patient and system were determined to be responsible. For the balance of cases, no reason for the delay was ascertained (Caplan et al., 1996). Several patient characteristics are associated with inadequate follow-up of abnormal cancer screening results: rural residence (Fox et al., 1997), relatively less education (Michielutte et al., 1985), low income (McCarthy et al., 1996a), and being a member of a racial or ethnic minority group (Chang et al., 1996; Kerlikowske, 1996; Mandelblatt et al., 1996; Rojas et al., 1996), but these factors are not all independent predictors of follow-up. Some of the observed racial differences may, in part, be attributable to socioeconomic status, age, marital status, and history of previous mammogram. For instance, when these factors were controlled for in an analysis of the effect of race on screening follow-up, the effect of race diminished substantially (McCarthy et al., 1996a). It is unclear whether certain patients fail to heed advice about follow-up; whether personal characteristics predict the likelihood that a physician will make follow-up recommendations; whether certain institutions lack tracking systems; or whether certain patients have difficulties navigating the system.
Studies have shown that out-of-pocket costs and the type of health care delivery system may influence follow-up rates. Women in HMOs who made copayments waited an average of 1.25 months longer between initial suspicion of cancer and obtaining a definitive diagnosis than women without copayments (Greenwald, 1987). In another study, colorectal cancer patients treated in HMOs had delays in diagnosis or treatment relative to patients in fee-for-service settings (Francis et al., 1984).
Some inadequate follow-up may be traced to poor provider-patient communication. In one study, more than half of the women with abnormal mammograms who had not sought follow-up care indicated that they thought their mammograms were normal (McCarthy et al., 1996b).
Among those who are informed of an abnormal screening test, some may not seek follow-up care for a variety of reasons:
- concern about cost,
- fear of learning that something is wrong,
- anxiety about painful diagnostic procedures (Rojas et al., 1996), or
- concern that they are too old for treatment (Mandelblatt, 1993a).
Interventions To Improve Follow-Up Rates
Several techniques have been tested to improve follow-up rates. Findings from key studies include the following:
- Telephone reminders are more effective than letters in increasing the follow-up of screening tests among women with abnormal Pap smears (Lerman et al., 1992; Marcus et al., 1992; Miller et al., 1997; Paskett, 1990).
- Computerized tracking systems can improve follow-up of abnormal screening results, but their success depends on adequate system staffing and support (Monticciolo and Sickles, 1990; Mandelblatt et al., 1998).
- A comprehensive review of interventions to increase colorectal cancer screening adherence found that the most intensive strategies delivered to eligible persons rarely increased adherence to FOBT above 50 percent. These intensive strategies included the use of a letter signed by one's own physician and including FOBT kits in the mailout to intensive follow-up with instructional telephone calls (Vernon, 1997).
- A review of the literature on strategies to increase adherence to breast and cervical cancer screening among underserved women determined that management systems directed to both patients and providers were consistently effective for most underserved women. Community-based outreach and integration of preventive services at the primary health care site are effective strategies for both African-American and Hispanic women. Use of mass media has been successful when targeted toward Hispanic women, but not when targeted toward African-American women. Mobile units and integration of preventive services at primary health care sites are effective strategies for elderly women (Vellozzi et al., 1996).
- One hospital serving a low-income population instituted a ''patient navigator" system that was successful in improving follow-up of abnormal screening or cancer diagnostic tests. Patient navigators were employed to ensure that individuals with abnormal tests were brought into care. Navigators made intensive efforts to contact women, including home visits, and facilitated follow-up appointments once women had been contacted (e.g., arranged child care, transportation) (Freeman et al., 1995).
Access to Definitive Cancer Staging
After a cancer diagnosis, additional tests are used to further classify and stage the disease. These staging tests provide critical information for selecting among treatment options and also provide prognostic information (e.g., likelihood of survival). Cancer patients who have a complete set of staging tests have better survival compared to those who do not (although the reason for this is not obvious) (Lee-Feldstein et al., 1994; Mandelblatt et al., 1998).
There is significant variation in oncologists' and surgeons' use of tests for diagnosis and staging for cancer (Plawker et al., 1997), and standard diagnostic workup and staging are not performed consistently in all population groups. There is evidence suggesting that appropriate staging is completed more frequently for
- younger women with breast cancer (Hillner et al., 1996; Kosary et al., 1995; Lash and Silliman, 1998; Silliman et al., 1989);
- men compared to women (e.g., for colorectal cancers, lung cancers) (Kosary et al., 1995);
- Medicare beneficiaries in HMOs compared to those in fee-for-service (e.g., for breast, cervical, colon, and prostate cancers) (Riley et al., 1994);
- whites compared to African Americans (e.g., for bladder, breast, colorectal, lung or bronchus, uterine, cervical, renal, and prostate cancers) (Ball and Elixhauser, 1996; Harris et al., 1997; Kosary, 1995; Liff et al., 1991); and
- urban compared to rural residents (Liff et al., 1991).
Phase 3: Cancer Treatment
Physicians use information from the diagnostic workup and staging process to formulate treatment recommendations. The treatment that patients actually receive depends on a number of factors, however, including the availability of health care resources, insurance coverage, physicians' awareness of treatment options, and patients' treatment preferences. These variations often show up as differences in the geographic distribution of cancer treatments (Ballard-Barbash et al., 1996; Farrow et al., 1992, 1996; Harlan et al., 1995; Nattinger et al., 1992; Samet et al., 1990). For example, use of breast conserving therapy ranged from 48 percent in Minnesota to 74 percent in Massachusetts (Guadagnoli et al., 1998). Rates of use of systemic chemotherapy also show wide geographic variation (Osteen and Karnell, 1994).
The availability of health care resources explains some geographic variation in cancer treatment:
- Women with breast cancer were more likely to get breast conserving surgery (BCS) than other types of surgery if they resided in counties with a cancer center or in a large city (Samet et al., 1994).
- Women were more likely to receive BCS when they were treated in hospitals with a high volume of breast cancer cases, a medical school affiliation, radiation facilities, and geriatric services (Nattinger et al., 1992).
- In two studies, women with early breast cancer, who were cared for in teaching hospitals, were more likely to receive BCS than those seen in nonteaching settings (Lee-Feldstein et al., 1994; Studnicki et al., 1993).
Having health insurance and the type of coverage one has are also associated with differential treatment patterns:
- Among individuals with non-small-cell lung cancer, patients without private insurance receive surgery less often than those with it (Greenberg et al., 1988).
- Rates of bone marrow transplantation for leukemia or lymphoma have been from one-third to one-half lower among self-pay and Medicaid patients than among privately insured patients (Mitchell et al., 1997).
Physicians may not recommend expensive chemotherapy for uninsured or underinsured patients for financial reasons or because they believe that such groups are less likely to comply with the treatment regimen (Begg and Carbone, 1983).
In one study, one-third or more patients undergoing treatment for cancer in Texas reported out-of-pocket costs exceeding $100 per visit for chemotherapy or radiotherapy. Hispanics were more likely than whites or blacks to have out-of-pocket costs higher than $200 per visit, probably because they were uninsured or underinsured (Guidry et al., 1998).
These same investigators found that black and Hispanic cancer patients being treated in Texas with chemotherapy or radiotherapy consistently reported that barriers such as distance, access to an automobile, and availability of someone to drive them to the treatment center were major problems (Guidry et al., 1997).
The major barriers influencing whether or not patients with cancer seek or continue treatment identified in a recent review of the literature include (Guidry et al., 1996):
- communication problems between patients and providers,
- lack of information about side effects,
- cost of treatment,
- difficulties in obtaining and maintaining insurance coverage, and
- absence of social support networks.
Access barriers generally were found to be greater for older women, members of minority groups, and patients of lower socioeconomic status.
Variation in Cancer Treatment By Age
In many cases (though not all), older people are less likely to get effective cancer treatments than are younger people, despite evidence that the elderly can tolerate and benefit from them (Begg and Carbone, 1983). The underuse of aggressive treatment among older people is often assumed to be related to the presence of coexisting conditions, but even among those without potentially complicating conditions, treatment differences exist by age (Newschaffer et al., 1996). Physicians may underuse some cancer treatment for elderly patients because they do not know that the elderly can tolerate aggressive therapy, they make mistaken assumptions about patient preferences, or they underestimate life expectancy. Some evidence suggests that the elderly are as likely as younger patients to prefer aggressive, lifesaving treatment (McQuellon et al., 1995; Yellen et al., 1994). Several research studies have documented the underuse of cancer treatments among older patients:
- Older patients with localized or regional non-small-cell lung cancer were less likely than younger patients to receive any therapy, and among those who did receive therapy, older patients were more likely to receive radiotherapy than the more aggressive surgical treatment (Smith et al., 1995).
- The elderly are less likely to receive bone marrow transplantation for leukemia or lymphoma (Mitchell et al., 1997).
- Among women with breast cancer, older women are less likely to receive BCS instead of mastectomy (Chu et al., 1987; Farrow et al., 1992; Mor et al., 1985; Newschaffer et al., 1996; Satariano, 1992), and among those getting BCS, older women have lower rates of adjuvant radiotherapy (Greenfield et al., 1987).
- Use of adjuvant chemotherapy declines with age among women with localized breast cancer (Hillner et al., 1996).
- Physicians deviate from recommended chemotherapy regimens more frequently when treating older patients with cancer (Schleifer et al., 1991).
- In New Mexico between 1984 and 1986, 43 percent of women age 85 and older, 84 percent of women age 75-84, and 92 percent of women age 65-74 received definitive treatment for localized breast cancer (defined as lumpectomy or excisional biopsy followed by radiation therapy or mastectomy). Age remained significant when access to transportation, physical activity levels, income, social support, ability to perform activities of daily living, mental status, and the presence of other medical illnesses were taken into account (Goodwin et al., 1993).
- In a comparable population of women in Virginia in 1985-1989, 66 percent of women age 65-69 and 7 percent of women age 85 and older received the appropriate radiation therapy after BCS. In addition, 44 percent of patients with positive lymph nodes received any adjuvant therapy, and 33 percent received hormone therapy (even though adjuvant therapy is recommended for all patients with node-positive disease) (Hillner et al., 1996).
- Based on Surveillance, Epidemiology, and End Results Program (SEER) data, 76 percent of women age 65-69, 68 percent age 70-74, 56 percent age 75-79, and 24 percent age 80 years or older received radiation therapy after breast conserving surgery for Stage I or II cancer. Controlling for differences in comorbidity narrowed, but did not eliminate, the difference associated with age (Ballard-Barbash et al., 1996).
Similar differences in treatment by age have been reported from other studies (Farrow et al., 1992; Greenfield et al., 1987; Lazovich et al., 1991).
Not all the evidence points to underuse of treatment in older people, however. One of the more thorough studies, of postmenopausal women with early breast cancer treated in 1993 in Minnesota, found that most (92 percent) women with node-positive breast cancer received some form of adjuvant therapy (Guadagnoli et al., 1997). The likelihood of treatment with adjuvant therapy did decline slightly with age, but the decline was not statistically significant. The use of adjuvant therapy was less frequent in women with node-negative breast cancer and did decline with age, but the age-associated differences were not significant after adjusting for various demographic and disease-associated factors.
Variations in Cancer Treatment By Race, Social Characteristics, and Gender
Differences in treatment by race have been well documented: African-American patients are less likely than white patients to undergo surgical resection for colorectal cancer (Cooper et al., 1996), to receive bone marrow transplantation for leukemia or lymphoma (Mitchell et al., 1997), to receive radical prostatectomy and radiation for localized prostate cancer (Harlan et al., 1995), or to have breast conserving surgery (BCS) for breast cancer or receive radiation therapy following BCS (Farrow et al., 1992; Muss et al., 1992; Nattinger et al., 1992). However, it appears that these effects may actually be more closely related to social class than to race.
In one study, elderly residents of areas characterized by low compared to high educational attainment were more likely to have received no treatment for non-small-cell lung cancer and, when treated, to receive radiation instead of surgical therapy, despite having similar clinical profiles (Smith et al., 1995). In another study, low educational attainment and a high percentage of the population with poverty-level incomes were associated with lower rates of BCS for women with breast cancer (Samet et al., 1994).
There are few large differences in survival among cancer patients by gender and studies of patterns of treatment by gender for bladder or colorectal cancer, leukemia, and lymphoma do not suggest any differences in care for men and women (Harris et al., 1997; Mitchell et al., 1997).
Physician-Associated Variation in Cancer Treatment
Physicians' treatment recommendations are influenced by a number of factors, including physician age (Liberati et al., 1987), gender (GIVIO, 1988), specialty (Deber and Thompson, 1987), and belief in efficacy of care (Liberati et al., 1987). The content of physician communication also varies according to patient characteristics, including age, income, education, race or ethnicity, and expected prognosis (Waitzkin, 1985).
Variations in the use of breast conserving surgery (BCS) instead of mastectomy for patients with early breast cancer may, in part, be explained by physician specialty, training, and experience. When asked about treatment preferences, medical oncologists were more likely than surgeons to prefer BCS (Deber and Thompson, 1987), surgeons were more likely than primary care physicians to prefer BCS, and among surgeons, those with postgraduate specialty training in
surgical oncology were more likely than surgeons with general board certification to recommend BCS to elderly patients (Mandelblatt et al., 1998).
Delays in adoption or lack of compliance with practice guidelines can limit access to recommended cancer care. In 1985, for example, a consensus statement of expert opinion was published accepting the use of BCS and radiation therapy in selected patients with early-stage breast cancer (Harris, et al., 1985). By 1990, the rates of BCS among women 65 to 79 in the Medicare program ranged from only 8 to 26 percent across regions of the United States and rates of BCS use had not increased appreciably nationally (i.e., from 14 percent in 1986 to 15 percent in 1990) (Nattinger et al., 1996). Physicians have also not adhered to recommended chemotherapy regimens. In one study, more than half of patients had their chemotherapy modified by their physicians in ways that were considered inappropriate (Schleifer et al., 1991).
The quality of physician-patient communication can affect clinical outcomes (Greenfield et al., 1988; Kaplan et al., 1989), adjustment to cancer (Roberts et al., 1994), patient quality of life, and satisfaction with care (Greenfield et al., 1988; Kaplan et al., 1989). Evidence of communication gaps is therefore worrisome. Patients and their physicians report the content of their interactions differently (Mackillop et al., 1988; Mosconi et al., 1991; Siminoff et al., 1989), assess the role of the patient in the decision-making process differently (Strull et al., 1984), and have different expectations of treatment benefits (Mackillop et al., 1988; Mosconi et al., 1991; Siminoff et al., 1989). In one study, relatively few women with breast cancer (15 to 27 percent) who had had a mastectomy reported that their physicians discussed BCS as a treatment option (Guadagnoli et al., 1998). Physicians who believe that patients should participate in treatment decisions are more likely to recommend BCS (Liberati et a1., 1991).
Cancer Treatment in Clinical Trials
Clinical trials are the mechanism through which new technologies, pharmaceuticals, or therapeutic strategies are evaluated against current standards of care. For patients with cancer, clinical trials can provide access to the best available and most promising new treatments.
There are striking variations in age-specific rates of participation in cancer clinical trials: more than 70 percent of children with cancer participate, but fewer than two percent of individuals age 50 and older with cancer participate in cooperative group clinical trials sponsored by the National Cancer Institute (Tejeda et al., 1996). Pediatric cancers are relatively rare and care within the context of a clinical trial has become standard practice (Simone and Lyons, in press). The elderly have historically been excluded from cancer clinical trials because of concerns about treatment complications or side effects, comorbid conditions, and interactions with current medications (although this has been changing).
Studies conducted to identify inequalities in access to clinical trials among racial or ethnic groups suggest that African Americans are represented in treatment trials, but underrepresented in screening and chemoprevention trials, in proportion to their age-specific rates of cancer (Bleyer et al., 1997a, b; Chlebowshi et al., 1993; Tejeda et al., 1996; Thompson et al., 1995). Negative attitudes toward medicine and research may inhibit African-American participation in clinical trials (Mouton et al., 1997; Robinson, 1996). The National Institutes of Health (NIH) Revitalization Act of 1993 specified that women, minorities, and other subpopulations must be
included in all government-sponsored Phase III clinical trials (which are usually large, randomized trials), or justification for their exclusion must be documented (NIH, 1994).
Physicians underrefer patients to clinical trials because of concerns about patient age, frailty, inadequate health insurance coverage, ability to travel to the clinical trial center, and other aspects of participation that might be considered a burden to the patient (Foley and Moertel, 1991). Physicians also report being concerned about the amount of time associated with participation in a trial (patient and physician), being uncomfortable with discussions of the uncertainty of trial treatment, and being concerned about changes in the physician's role as a result of trial participation (Farrar, 1991; Kaluzny et al., 1993; Taylor et al., 1984).
Access to clinical trials can be limited by insurance policies. Most insurers do not cover the cost of participation in clinical trials as a matter of policy (e.g., Medicare, most state Medicaid programs, most managed care organizations).
Phase 4: Posttreatment Surveillance and Recurrence Care
Patients are monitored for recurrent cancer and psychosocial distress for the first several years following their primary and adjuvant treatment when the probability of recurrence and adjustment difficulties is greatest (Schiffer et al., 1997). For most cancers, however, little evidence exists from which to develop guidelines for "appropriate" follow-up procedures, so variations in treatment cannot necessarily be interpreted as better or worse care. Not surprisingly, there is relatively little research on access to care during this phase of disease management, although there are indications that the intensity of follow-up care does vary, at least for some cancers (e.g., lung and colorectal cancers) (Johnson et al., 1996a, b; Virgo et al., 1995). Some of this variation is explained by physician experience and training (Johnson et al., 1996c). One study of the effect of race on the follow-up care of men with prostate cancer in the U.S. military health care system suggests that when health care is uniformly available, follow-up care is similar for whites and African Americans (Moul et al., 1996).
Phase 5: End-Of-Life Care
Care at the end of life for patients dying from cancer may include heroic attempts at cure, pain management, treatment for psychological problems, or combinations of these. Caregivers may be the same as those involved in earlier phases of treatment but are likely to include others, among them, hospice caregivers from various disciplines. For most patients, palliation eventually becomes the focus of care. Barriers to the best end-of-life care may stem from financial constraints, and from provider and patient attitudes and knowledge.
Financial Factors
The uninsured and inadequately insured may not be able to afford important components of end-of-life care (e.g., pain medication, nutritional supplements, outpatient nursing services)
(Underwood, 1995). Even among the well insured, the costs of end-of-life care can contribute to financial hardship. In one study, more than half of the families involved in the care of a seriously ill family member reported at least one severe burden ranging from loss of family savings, or loss of income, to changes in future educational plans or employment status (Covinsky et al., 1994). One commonly cited financial burden relates to Medicare reimbursement policy. Medicare will reimburse for pain management at an inpatient facility, but not for outpatient oral analgesics. This is a major barrier to adequate pain management for terminal cancer patients who choose to die at home.
An additional barrier to end-of-life care is the absence of a primary care provider. The poor and uninsured may be less likely to have a regular clinician with whom they are comfortable discussing end-of-life care issues.
Patient Factors
Patients dying of cancer often suffer avoidable pain and distress (Cleeland et al., 1994; Passik et al., 1998). Certain patient attitudes or beliefs can act as barriers to good end-of-life care. Stoicism can lead to underreporting of pain, nausea, or depression; concerns about becoming addicted to pain medication or a belief in the inevitability of pain with cancer can contribute to the underuse of pain medication (Ward et al., 1993). Some patients are reluctant to communicate symptoms to their providers for fear of diverting attention from the pursuit of a cure (Ward et al., 1993). Some evidence suggests that these attitudinal barriers may be more prevalent among certain sociodemographic groups (e.g., those with low educational attainment) and certain racial or ethnic minority groups (Cleeland et al., 1997; Rimer et al., 1987; Ward et al., 1993).
Provider Factors
Aside from financial barriers, most impediments to adequate end-of-life care are associated with health care providers. For example, although there are effective pharmacological strategies to manage pain, providers consistently undertreat pain (Levin et al., 1998; Levy, 1996; McCaffery and Ferrell, 1995). In one study, 42 percent of patients with recurrent or metastatic cancer were inadequately managed for their pain (Cleeland et al., 1994). The elderly, women, and members of racial or ethnic minority groups are more likely than others to have poor pain relief (Bernabei et al., 1998; Cleeland et al., 1994, 1997). Physicians report concerns about management of side effects, patient tolerance of analgesia, and regulatory scrutiny when prescribing narcotics as barriers to effective pain management (Van Roenn et al., 1993). Simple measures, such as attaching a patient-completed pain assessment sheet to the front of the medical chart, can increase effective pain management (Trowbridge et al., 1997).
Physicians also do not adequately identify signs of depression among patients with cancer. In one study, only 13 percent of patients with evidence of moderate to severe depression were identified by their physicians (Passik et al., 1998). Providers may not have clear guidance on how to manage terminal cancer care. Guidelines are available to assist in the management of cancer-associated pain (e.g., from the Agency for Health Care Policy and Research), but there are
no standards of care for other common symptoms of cancer such as anxiety, anorexia, or the wasting often associated with cancer.
Communication among various members of the end-of-life care team and with patients may be less than optimal. In one study of terminally ill patients, communication between physicians and patients was poor—only 41 percent of patients in the study reported talking to their physician about prognosis or about their wishes regarding resuscitation (SUPPORT, 1995; Lo, 1995). Physicians infrequently discuss advance directives, and when these are discussed, there is considerable disagreement on the outcome of such discussions. Patients' requests for end-of-life care (e.g., withholding of CPR) are frequently not documented in the medical chart (Haidet et al., 1998; SUPPORT, 1995).
A recent study reports that the vast majority of people with terminal cancer overestimate their chances of surviving their illness (Smith et al., 1998; Weeks et al., 1998). Those who thought they were going to live for six months were more than two times as likely to choose aggressive anticancer therapy instead of palliative or hospice care, which is designed to relieve symptoms. The overoptimistic patients did indeed live longer than those who had more realistic expectations. Patients who overestimated their survival and received aggressive therapy, however, had exactly the same median survival as those who received palliative care and were more likely to have a hospital readmission, undergo attempted resuscitation, or die while receiving ventilatory support. Some patients choose aggressive chemotherapy, even when there is little chance of benefit (Slevin et al., 1990). To achieve the goals of supporting patient values and minimizing the prospect of utilizing therapies that will not increase survival, physicians must make sure that patients understand their prognosis by initiating a dialogue, asking what patients want to know, providing estimates of survival duration, explaining the poor efficacy and debilitating side effects of therapy common at this stage, and discussing all treatment options, including palliative care alone (Smith et al., 1998).
About half of cancer patient deaths involve hospice care. ''Hospice" is a philosophy of care that emphasizes the coordinated delivery of many services for terminally ill patients and their families including: nursing care; physician services; homemakers and home health aides; physical, occupational, and speech therapy; and psychological counseling and social services. Medicare provides a hospice benefit that has criteria for patient admissions and use of therapies. Eligibility for hospice services under Medicare requires an expected survival of less than six months, but most hospice patients live for less than two months following their admission. Physicians appear to delay referring patients for hospice care, in part because it is difficult to predict accurately the expected survival of the terminally ill. Delays in referral may also be due to a physician's lack of knowledge of hospice services, poor communication with the palliative care team, or reluctance to engage in uncomfortable discussions or feelings about end-of-life care (Christakis and Escarce, 1996). Hospice benefits for non-Medicare patients are variable and Medicaid coverage of hospice care varies from state to state.
The National Cancer Policy Board sought additional information about barriers to effective end-of-life care for cancer patients by commissioning interviews with 19 expert physicians, nurses, social workers, and health services researchers. The findings are summarized in the paper, Issues in End of Life Care for People with Cancer: Interviews with Selected Providers and Researchers (Gelband et al., 1999). This approach was taken to complement the 1997 report, Approaching Death: Improving Care at the End of Life, completed by IOM's Committee on Care at the End of Life (IOM, 1997). The 1997 report is a thorough review of end-of-life issues, ending
with a series of recommendations that were accepted by the NCPB for cancer patients (Box 3.1). For more detail and context, the reader is referred to the complete IOM report.
A recent review examined outcome measures that have been used, or proposed for use in the clinical audit of palliative care of patients with advanced cancer. Identified measures met some, but not all of the objectives of measurement in palliative care, and fulfilled some, but not all of the criteria established for validity, reliability, responsiveness, and appropriateness (Hearn et al., 1997).
Key Findings
A persistent, vexing problem for many Americans is lack of health insurance or insufficient coverage to help defray the expense of a costly illness such as cancer. Although most individuals diagnosed with cancer are elderly and have Medicare coverage, an estimated 7 percent of those facing a new diagnosis of cancer lack health insurance. Health insurance offers some, but often incomplete, protection against the high costs of cancer care. High deductibles, copayments or coinsurance, and limits on coverage can all contribute to high out-of-pocket costs. Medicare was, for example, estimated to cover only 83 percent of typical total charges for lung cancer and 65 percent of typical charges for breast cancer in 1986. Some individuals have additional protection through other insurers (e.g., Medigap policies, Medicaid), but even then, the financial burden of cancer can be substantial. A particular problem for many with cancer involves limitations on prescription drug coverage, an expensive and widely used benefit.
Individuals who are poor, have low educational attainment, or are members of racial or ethnic minority groups tend to have less favorable outcomes with cancer than other groups. Limited access to primary care or cancer screening contributes to having cancer diagnosed at later stages when the prognosis is worse. Having health insurance coverage improves access, but does not guarantee that cancer screening tests are used. Other factors that can impede access to screening are culturally based attitudes and beliefs, not having services available in the local community, and health care providers not able to speak the language of the people they serve.
It is often health care providers who can be held accountable for the underuse of cancer screening tests. One of the strongest predictors of whether a person will be screened for cancer is whether the physician recommends testing, and evidence suggests that physicians order fewer screening tests than they should. The use of screening tests is improved in managed care plans, compared to fee-for-service plans.
Even when screening is used, many individuals fail to receive timely, or any, follow-up of abnormal screening results. Both screening and follow-up rates can be improved with interventions aimed at those eligible for screening (e.g., telephone and mailed reminders from providers, educational interventions) and health care providers (e.g., reminder systems).
Differences in treatment by race have been well documented. However, it appears that the effect may actually be related more closely to social class than to race. Another group that appears to be vulnerable in the cancer care system is the elderly. Older people are often less likely to get effective cancer treatments than are younger people, despite evidence that the elderly can tolerate and benefit from them. Some undertreatment is explained by provider attitudes toward treating the elderly, who are perceived as less willing or able to tolerate aggressive treatment.
There is evidence of widespread quality problems in end-of-life care, especially in the area of pain management. The elderly, women, and members of racial or ethnic minority groups are more likely than others to have poor pain relief, which appears to be due to a combination of factors: poor palliative care practices on the part of providers, attitudes of patients (e.g., stoicism), and miscommunication between patients and health care providers.
|
BOX 3.1 "Recommendations and Future Directions" from Approaching Death: Improving Care at the End of Life
SOURCE: IOM, 1997. |
References
Albanes D, Weinberg GB, Boss L, Taylor PR. 1988. A survey of physicians' breast cancer early detection practices. Preventive Medicine 17(5):643-652.
American Association of Retired Persons Public Policy Instiute and the Lewin Group. 1997. Out-of-Pocket Health Spending by Medicare Beneficiaries Age 65 and Older: 1997 Projections.
Ayanian JZ, Kohler BA, Abe T, Epstein AM. 1993. The relation between health insurance coverage and clinical outcomes among women with breast cancer. New England Journal of Medicine 329:326-331.
Ball JK, Elixhauser A. 1996. Treatment differences between blacks and whites with colorectal cancer. Medical Care 34:970-984.
Ballard-Barbash R, Potosky AL, Harlan LC, et al. 1996. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. Journal of the National Cancer Institute 88(11):716-726.
Bassett AA. 1985. Occult breast cancer and changing patterns of surgical practice. Canadian Journal of Surgery 28(4):297-298.
Becker DM, Gomez EB, Kaiser DL, et al. 1989. Improving preventive care at a medical clinic: How can the patient help? American Journal of Preventive Medicine 5(6):353-359.
Begg CB, Carbone PP. 1983. Clinical trials and drug toxicity in the elderly: The experience of the Eastern Cooperate Oncology Group. Cancer 52:1986-1992.
Bergner M, Allison CJ, Diehr P, et al. 1990. Early detection and control of cancer in clinical practice. Archives of Internal Medicine 150(2):431-436.
Bernabei R, Gambassi G, Lapane K, et al. 1998. Management of pain in elderly patients with cancer. Journal of the American Medical Association 279(23):1877-1882.
Bindman AB, Grumbach K, Osmond D, et al. 1996. Primary care and receipt of preventive services. Journal of General Internal Medicine 11(5):269-276.
Bleyer WA, Tejeda HA, Murphy SB, et al. 1997a. Equal participation of minority patients in U.S. national pediatric cancer clinical trials. Journal of Pediatric Hematology/Oncology 19(5):423-427.
Bleyer WA, Tejeda H, Murphy SB, et al. 1997b. National cancer clinical trials: Children have equal access; adolescents do not. Journal of Adolescent Health 21:366-373.
Blustein J. 1995. Medicare coverage, supplemental insurance, and the use of mammography by older women. New England Journal of Medicine 332:1138-1143.
Breen N, Figueroa JB. 1996. Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: A prevention policy perspective. American Journal of Preventive Medicine 12:319-326.
Brown ML, Potosky AL, Thompson GB, Kessler LG. 1990. The knowledge and use of screening tests for colorectal and prostate cancer: Data from the 1987 national health interview survey. Preventive Medicine 19(5):562-574.
Burack RC, Gimotty PA. 1997. Promoting screening mammography in inner-city setting. The sustained effectiveness of computerized reminders in a randomized controlled trial. Medical Care 35:921-931.
Burns RB, McCarthy EP, Freund KM, et al. 1996. Black women receive less mammography even with similar use of primary care. Annals of Internal Medicine 125(3):173-182.
Caplan LS, Helzlsouer KJ, Shapiro S, et al., 1996. Reasons for delay in breast cancer diagnosis. Preventive Medicine 25(2):218-224.
Chambers CV, Balaban DJ, Carlson BL, et al. 1989. Microcomputer-generated reminders. Improving the compliance of primary care physicians with screening guidelines . Journal of Family Practice 29(3):273-280.
Champion VL. 1994a. Beliefs about breast cancer and mammography by behavioral stage. Cancer 72: 1100-1112.
Champion VL. 1994b. Strategies to increase mammography utilization. Medical Care 32(2):118-129.
Champion VL, Huster G. 1994. Effect of interventions on stage of mammography adoption. Journal of Behavioral Medicine 18(2):169-187.
Chang SW, Kerlikowske K, Napoles-Springer A, et al. 1996. Racial differences in timeliness of follow-up after abnormal screening mammography. Cancer 78:1395-1402.
Cheney C, Ramsdell JW. 1987. Effect of medical records' checklists on implementation of periodic health measures. American Journal of Medicine 83:129-136.
Chlebowski RT, Bulter J, Nelson A, et al. 1993. Breast cancer chemoprevention. Tamoxifen: Current issues and future prospective. Cancer 2(3 Suppl.):1032-1037.
Christakis NA, Escarce JJ. 1996. Survival of Medicare patients after enrollment in hospice programs. New England Journal of Medicine 335(3):172-178.
Chu J, Diehr P, Feigle P, et al. 1987. The effect of age on the care of women with breast cancer in community hospitals. Journal of Gerontology 42(2):185-190.
Cleeland CS, Gonin R, Baez L, et al. 1997. Pain and pain treatment in minority outpatients with metastatic cancer: The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Annals of Internal Medicine 127:813-816.
Cleeland CS, Gonin R, Hatfield AK, et al. 1994. Pain and its treatment in outpatients with metastatic cancer. New England Journal of Medicine 330:592-596.
Clementz GL, Aldag JC, Gladfelter TT, et al. 1990. A randomized study of cancer screening in a family practice setting using a recall model. Journal of Family Practice 30:537-541.
Cooper GS, Yuan Z, Landefeld CS, Rimm AA. 1996. Surgery for colorectal cancer: Race-related differences in rates and survival among Medicare beneficiaries . American Journal of Public Health 86(4):582-586.
Covinsky KE, Goldman L, Cook EF, et al. 1994. The impact of serious illness on patients' families. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Journal of the American Medical Association 272(23):1839-1844.
Cowan JA, Heckerline PS, Parker JB. 1992. Effect of a fact sheet reminder on performance of the periodic health examination: A randomized controlled trial. American Journal of Preventive Medicine 8:104-109.
Davis NA, Nash E, Bailey C, et al. 1997. Evaluation of three methods for improving mammography rates in a managed care plan. American Journal of Preventive Medicine 13(4):298-302.
Davis TC, Arnold C, Berkel HJ, et al. 1996. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer 78:1912-1920.
Deber RB, Thompson GG. 1987. Who still prefers aggressive surgery for breast cancer? Implications for the clinical applications of clinical trials. Archives of Internal Medicine 147:1543-1547.
De Waard F, Collette HJA, Rombach JJ, et al. 1984. The DOM project for the early detection of breast cancer, Utrecht, The Netherlands. Journal of Chronic Diseases 37:1-44.
Elixhauser A, Ball JK. 1994. Black/white differences in colorectal tumor location in a national sample of hospitals. Journal of the National Medical Association (United States) 85:449-458.
Farley TA, Flannery JT. 1989. Late-stage diagnosis of breast cancer in women of lower socioeconomic status: Public health implications. American Journal of Public Health 79(11):1508-1512.
Farrar WB. 1991. Clinical trials: Access and reimbursement. Cancer 67(6 Suppl.):1779S-1782S.
Farrow DC, Hunt WC, Samet JM. 1992. Geographic variation in the treatment of localized breast cancer. New England Journal of Medicine 326:1097-1101.
Farrow DC, Samet JM, Hunt WC. 1996. Regional variation in survival following the diagnosis of cancer. Journal of Clinical Epidemiology 49(8):843-847.
Fink R, Shapiro S, Roester R. 1972. Impact of efforts to increase participation in repetitive screenings for early breast cancer detection. American Journal of Public Health 62:328-336.
Foley JF, Moertel CG. 1991. Improving accrual into cancer clinical trials. Journal of Cancer Education 6:165-173.
Fox E. 1997. Predominance of the curative model of medical care: A residual problem. Journal of the American Medical Association 278(9):761-764.
Fox P, Amsberger P, Zhang X. 1997. An examination of differential follow-up rates in cervical cancer screening. Journal of Community Health 22:199-209.
Fox SA, Stein JA. 1991. The effect of physician-patient communication on mammography utilization by different ethnic groups. Medical Care 29(11):1065-1082.
Fox SA, Klos DS, Tsou CV. 1988. Underuse of screening mammography by family physicians. Radiology 166:431-433.
Fox SA, Roetzheim RG, Kington RS. 1997. Barriers to cancer prevention in the older person. Clinics in Geriatric Medicine (United States) 13(1):79-95.
Fox SA, Siu AL, Stein JA. 1994. The importance of physician communication on breast cancer screening of older women. Archives of Internal Medicine 154:2058-2068.
Fox SA, Tsou CV, Klos DS. 1985. An intervention to increase mammography screening by residents in family practice. Journal of Family Practice 20(5):467-471.
Francis AM, Polissar L, Lorenz AB. 1984. Care of patients with colorectal cancer. A comparison of a health maintenance organization and fee-for-service practices. Medical Care 22(5):418-429.
Freeman HP, Muth BJ, Kerner JF. 1995. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Practice 3(1):19-30.
Gelband H, Greene A, Kidd E. 1999. Issues in End of Life Care for People with Cancer: Interviews with Selected Providers and Researchers . Washington, D.C.: National Academy Press.
Gemson DH, Elinson J, Messeri P. 1988. Differences in physician prevention practice patterns for white and minority patients. Journal of Community Health 13:53-64.
GIVIO. 1988. Survey of treatment of primary breast cancer in Italy. British Journal of Cancer 57:130-134.
Glanz K, Resch N, Lerman C, Rimer BK. 1996. Black-white differences in factors influencing mammography use among employed female health maintenance organization members. Ethnicity and Health 1(3):207-220.
Gluck ME. 1999. A Medicare Prescription Drug Benefit. National Academy of Social Insurance, Medicare Brief April 1999 (1):1-13.
Goodwin JS, Hunt WC, Samet JM. 1993. Determinants of cancer therapy in elderly patients. Cancer 72(2):594-601.
Goodwin JS, Samet JM, Hunt WC. 1996. Determinants of survival in older cancer patients. Journal of the National Cancer Institute (United States) 88(15):1031-1038.
Gordon NH, Crowe JP, Brumberg DJ, Berger NA. 1992. Socioeconomic factors and race in breast cancer recurrence and survival. American Journal of Epidemiology 135:609-618.
Gordon NP, Rundall TG, Parker L. 1998. Type of health care coverage and the likelihood of being screened for cancer. Medical Care 36(5):636-645.
Grady KE, Lemkau JP, McVay JM, Reisine ST. 1992. The importance of physician encouragement in breast cancer screening of older women. Preventive Medicine 21:766-780.
Greenberg ER, Chute CG, Stukel T, et al. 1988. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. New England Journal of Medicine 318:612-617.
Greenfield S, Blanco DM, Slashoff RM, Ganz PA. 1987. Patterns of care related to age of breast cancer patients. Journal of the American Medical Association 257:2766-2770.
Greenfield S, Kaplan SH, Ware JE, et al. 1988. Patient's participation in medical care: Effects of blood sugar control and quality of life in diabetes. Journal of General Internal Medicine 3:448-457.
Greenwald HP. 1987. HMO membership, copayment, and initiation of care for cancer: A study of working adults. American Journal of Public Health 77(4):461-466.
Greenwald HP, Borgatta EF, McCorkle R, Polissar N. 1996. Explaining reduced cancer survival among the disadvantaged. Milbank Quarterly 74(2):215-239.
Guadagnoli E, Shapiro C, Gurwitz JH, et al. 1997. Age-related patterns of care: Evidence against ageism in the treatment of early stage breast cancer. Journal of Clinical Oncology 15:2338-2344.
Guadagnoli E, Weeks JC, Shapiro CL, et al. 1998. Use of breast-conserving survey for treatment of Stage I and Stage II breast cancer. Journal of Clinical Oncology 16(1):101-106.
Guidry JJ, Aday LA, Zhang D, Winn RJ. 1997. Transportation as a barrier to cancer treatment. Cancer Practice 5(6):361-366.
Guidry JJ, Aday LA, et al. 1998. Cost considerations as potential barriers to cancer treatment. Cancer Practice 6(3):182-187.
Guidry JJ, Greisinger A, Aday LA, et al. 1996. Barriers to cancer treatment: A review of published research. Oncology Nursing Forum 23(9):1393-1398.
Haidet P, Hamel MB, Davis RB, et al. 1998. Outcomes, preferences for resuscitation, and physician-patient communication among patients with metastatic colorectal cancer. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. American Journal of Medicine 105(3):222-229.
Harlan L, Brawley O, Pommerenke F, et al. 1995. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. Journal of Clinical Oncology (United States) 13:93-100.
Harris DR, Andrews R, Elixhauser A. 1997. Racial and gender differences in use of procedures for black and white hospitalized adults. Ethnicity and Disease 7:91-105.
Harris JR, Hellman S, Kinne DW. 1985. Limited surgery and radiotherapy for early breast cancer. New England Journal of Medicine 313:1365.
Hayward RA, Shapiro MF, Freeman HE, Corey CR. 1988a. Inequities in health services among insured Americans. Do working-age adults have less access to medical care than the elderly? New England Journal of Medicine 318:1507-1512.
Hayward RA, Shapiro MF, Freeman HE, Corey CR. 1988b. Who gets screened for cervical and breast cancer? Archives of Internal Medicine 148:1177-1181.
Health Insurance Association of America. 1998. http://www.hiaa.org.
Hearn J, Higginson IJ. 1997. Outcome measures in palliative care for advanced cancer patients: A review. Journal of Public Health Medicine 19(2)193-199.
Hedegaard HB, Davidson AJ, Wright R. 1996. Factors associated with screening mammography in low-income women. American Journal of Preventive Medicine 12(1):51-56.
Hewitt M. 1998. Special tabulations, 1992 National Health Interview Survey, Cancer Survivorship Section.
Hiatt RA, Pasick RJ. 1996. Unsolved problems in early breast cancer detection: Focus on the underserved. Breast Cancer Research and Treatment 40:37-51.
Hillman AL, Ripley K, Goldfarb N, et al. 1998. Physician financial incentives and feedback: Failure to increase cancer screening in Medicaid managed care. American Journal of Public Health 88(11):1699-1701.
Hillner BE, Penberthy L, Desch CE, et al. 1996. Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Research and Treatment 40:75-86.
Hoffman C. 1998. Uninsured in America: A Chart Book. The Kaiser Commission on Medicaid and the Uninsured.
IOM (Institute of Medicine). 1994. Access to Health Care in America . Washington, D.C.: National Academy Press.
IOM. 1997. Approaching Death: Improving Care at the End of Life. MJ Field, CK Cassel, eds. Washington, D.C.: National Academy Press.
Irwig L, Turnbull D, McMurchie M. 1990. A randomized trial of general practitioner-written invitations to encourage attendance at screening mammography. Community Health Studies 14:357-364.
Janz NK, Schottenfeld D, Doerr KM, et al. 1997. A two-step intervention to increase mammography among women aged 65 and older. American Journal of Public Health 87:1683-1686.
Johnson FE, McKirgan LW, Coplin MA, et al. 1996a. Geographic variation in patient surveillance after colon cancer surgery. Journal of Clinical Oncology 14:183-187.
Johnson FE, Naunheim KS, Coplin MA, Virgo KS. 1996b. Geographic variation in the conduct of patient surveillance after lung cancer surgery. Journal of Clinical Oncology 14:2940-2949.
Johnson FE, Novell LA, Coplin MA, et al. 1996c. How practice patterns in colon cancer patient follow-up are affected by surgeon age. Surgical Oncology 5:127-131.
Kagawa-Singer M. 1997. Addressing issues for early detection and screening in ethnic populations. Oncology Nursing Forum 24(10):1705-1711.
Kaluzny A, Brawley O, Garson-Angert D, et al. 1993. Assuring access to state-of-the art care for U.S. minority populations: The first 2 years of the minority-based community clinical oncology program. Journal of the National Cancer Institute 85(23):1945-1950.
Kaplan SH, Greenfield S, Ware JE. 1989. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Medical Care 27:S110-127.
Katz SJ, Hofer TP. 1994. Socioeconomic disparities in preventive care persist despite universal coverage. Breast and cervical cancer screening in Ontario and the United States. Journal of the American Medical Association 272(7):530-534.
Kendall C, Hailey BJ. 1993. The relative effectiveness of three reminder letters on making and keeping mammogram appointments. Behavioral Medicine 19(1):29-34.
Kerlikowske K. 1996. Timeliness of follow-up after abnormal screening mammography. Breast Cancer Research and Treatment 40:53-64.
Kiefe CI, McKay SV, Halevy A, Brody BA. 1994. Is cost a barrier to screening mammography for low-income women receiving Medicare benefits? A randomized trial. Archives of Internal Medicine 154:1217-1224.
King ES, Rimer BK, Seay J, et al. 1994. Promoting mammography use through progressive interventions: Is it effective? American Journal of Public Health 84:104-106.
Kogevinas M, Porta M. 1997. Socioeconomic differences in cancer survival: A review of the evidence. IARC Scientific Publication 138:177-206.
Kosary CL, Ries LAG, Miller BA, et al. 1995. SEER Cancer Statistics Review, 1973-1992: Tables and Graphs. Bethesda, MD: National Cancer Institute, National Institutes of Health.
Lacey L, Whitfield J, DeWhite W, et al. 1993. Referral adherence in an inner city breast and cervical cancer screening program. Cancer 72:950-955.
Landis SE, Hulkower SD, Pierson S. 1992. Enhancing adherence with mammography through patient letters and physician prompts: A pilot study. North Carolina Medical Journal 53:575-578.
Lannin DR, Mathews HF, Mitchell J, et al. 1998. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. Journal of the American Medical Association 279:1801-1807.
Lantz PM, House JS, Lepkowski JM, et al. 1998. Socioeconomic factors, health behaviors, and mortality. Results from a nationally representative prospective study of U.S. adults. Journal of the American Medical Association 279:1703-1708.
Lantz PM, Stencil D, Lippert MT, et al. 1995. Breast and cervical cancer screening in a low-income managed care sample: The efficacy of physician letters and phone calls. American Journal of Public Health 85:834-836.
Lash TL, Silliman RA. 1998. Re: Prevalence of cancer. Journal of the National Cancer Institute 90(5):399-400.
Lazovich D, White F, Thomas DB, Moe RE. 1991. Under-utilization of breast-conserving surgery and radiation therapy among women with Stage I or II breast cancer. Journal of the American Medical Association 266:3433-3438.
Lee-Feldstein A, Anton-Culver H, Feldstein PJ. 1994. Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. Journal of the American Medical Association 271(15):1163-1168.
Lerman C, Caputo C, Milller S, et al. 1992. Telephone counseling improves adherence to colposcopy among lower-income minority women . Journal of Clinical Oncology 10:330-333.
Levin ML, Berry JI, Leiter J. 1998. Management of terminally ill patients: Physician reports of knowledge, attitudes, and behavior. Journal of Pain and Symptom Management 15:27-40.
Levy MH. 1996. Drug therapy: Pharmacologic treatment of cancer pain. New England Journal of Medicine 335:1124-1132.
Liberati A, Apolone G, Nicolucci A, et al. 1991. The role of attitudes, beliefs, and personal characteristics of Italian physicians in the surgical treatment of early breast cancer. American Journal of Public Health 81:38-42.
Liberati A, Patterson WB, Biener L, McNeil BJ. 1987. Determinants of physicians' preference for alternative treatments in women with early breast cancer. Tumori 73:601-609.
Liff JM, Chow WH, Greenberg RS. 1991. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer 67:1454-1459.
Lo B. 1995. Improving care near the end of life: Why is it so hard? Journal of the American Medical Association 274(20):1634-1636.
Lurie N, Manning WG, Peterson C, et al. 1987. Preventive care: Do we practice what we preach? American Journal of Public Health 77(7):801-804.
Lurie N, Margolis KL, McGovern PG, et al. 1997. Why do patients of female physicians have higher rates of breast cancer and cervical screening? Journal of General Internal Medicine 12:34-43.
Mackillop WJ, Stewart WE, Ginsburg AD, et al. 1988. Cancer patients' perception of their disease and treatment. British Journal of Cancer 58:355-358.
Mandel JS, Bond JH, Church TR, et al. 1993. Reducing mortality from colorectal cancer by screening for fecal occult blood. New England Journal of Medicine 328:1365-1371.
Mandelblatt J, Kanetsky PA. 1995. Effectiveness of interventions to enhance physician screening for breast cancer. Journal of Family Practice 40:162-170.
Mandelblatt J, Andrews H, Kao R, et al. 1995. Impact of access and social context on breast cancer stage at diagnosis. Journal of Health Care for the Poor and Underserved 6(3):342-351.
Mandelblatt J, Andrews H, Kao R, et al. 1996. The late-stage diagnosis of colorectal cancer: Demographic and socioeconomic factors. American Journal of Public Health 86:1794-1797.
Mandelblatt J, Andrews H, Kerner J, et al. 1991. Determinants of late stage of diagnosis of breast and cervical cancer: The impact of age, race, social class, and hospital type. American Journal of Public Health 81:646-649.
Mandelblatt J, Freeman H, Winczewski D, et al. 1997. The costs and effects of cervical cancer screening in a public hospital emergency room. American Journal of Public Health 87(7):1182-1189.
Mandelblatt JS, Ganz PA, Kahn KL. 1998. A proposed agenda for the measurement of breast cancer quality of care outcomes in oncology practice. Submitted to Journal of Oncology.
Mandelblatt J, Richart R, Thomas L, et al. 1992a. Is human papillomavirus associated with cervical neoplasia in the elderly? Gynecologic Oncology 46:6-12.
Mandelblatt J, Traxler M, Lakin P, et al. 1992b. Mammography and Papanicolaou smear use by elderly poor black women. The Harlem Study Team. Journal of the American Geriatrics Society 40(10):1001-1007.
Mandelblatt JS, Wheat ME, Monane M, et al. 1992c. Breast cancer screening for elderly women with and without comorbid conditions. A decision analysis model. Annals of Internal Medicine 116:722-730.
Mandelblatt J, Traxler M, Lakin P, et al. 1993a. Breast and cervical cancer screening of poor, elderly, black women: Clinical results and implications. American Journal of Preventive Medicine 9(3):133-138.
Mandelblatt JS, Traxler M, Lakin P, et al. 1993b. A nurse practitioner intervention to increase breast and cervical cancer screening for poor, elderly black women. Journal of General Internal Medicine 8:173-178.
Mandelblatt J, Traxler M, Lakin P, et al. 1993c. Targeting breast cancer and cervical cancer screening to elderly poor black women: Who will participate? Preventive Medicine 22:20-33.
Mandelblatt J, Yabroff KR, Kerner J. 1998. Access to quality cancer care: Evaluating and ensuring equitable services, quality of life and survival. National Cancer Policy Board commissioned paper.
Mann LC, Hawes DR, Ghods M, et al. 1987. Utilization of screening mammography: Comparison of different physician specialties. Radiology 164:121-122.
Marcus AC, Crane LA, Kaplan CP, et al. 1992. Improving adherence to screening follow-up among women with abnormal Pap smears. Results from a large clinic-based trial of three intervention strategies. Medical Care 30:216-230.
Mayer JA, Clapp EJ, Bartholomew S, Elder J. 1994. Facility-based inreach strategies to promote annual mammograms. American Journal of Preventive Medicine 10(6):353-356.
McCaffery M, Ferrell BR. 1995. Nurses' knowledge about cancer pain: A survey of five countries. Journal of Pain and Symptom Management 10:356-369.
McCarthy BD, Yood MU, Janz NK, et al. 1996a. Evaluation of factors potentially associated with inadequate follow-up of mammographic abnormalities. Cancer 77:2070-2076.
McCarthy BD, Yood MU, Boohaker EA, et al. 1996b. Inadequate follow-up of abnormal mammograms. American Journal of Preventive Medicine 12:282-288.
McCarthy EP, Burns RB, Coughlin SS, et al. 1998. Mammography use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Annals of Internal Medicine 128(9):729-736.
McPhee SJ, Bird JA, Fordham D, et al. 1991. Promoting cancer prevention activities by primary care physicians. Results of a randomized, controlled trial. Journal of the American Medical Association 266(4):538-544.
McPhee SJ, Bird JA, Jenkins CNH, Fordham D. 1989. Promoting cancer screening. A randomized, controlled trial of three interventions. Archives of Internal Medicine 149:1866-1872.
McQuellon RP, Muss HB, Hoffman SL, et al. 1995. Patient preferences for treatment of metastatic breast cancer: A study of women with early-stage breast cancer. Journal of Clinical Oncology 13:858-868.
Michielutte R, Diseker RA, Young LD, May WJ. 1985. Noncompliance in screening follow-up among family planning clinic patients with cervical dysplasia. Preventive Medicine 14(2):248-258.
Mickey RM, Vezina JL, Worden JK, Warner SL. 1997. Breast screening behavior and interactions with health care providers among lower income women. Medical Care 35(12):1204-1211.
Miller BA, Kolonel LN, Bernstein L, et al., (eds.). 1996. Racial/Ethnic Patterns of Cancer in the United States, 1988-1992. Bethesda MD: National Cancer Institute.
Miller SM, Siejak KK, Schroeder CM, et al. 1997. Enhancing adherence following abnormal Pap smears among low-income minority women: A preventive telephone counseling strategy. Journal of the National Cancer Institute 89(10):703-708.
Mitchell JB, McCormack LA. 1997. Time trends in late-stage diagnosis of cervical cancer: Differences by race/ethnicity and income. Medical Care 35(12):1220-1224.
Mitchell JM, Meehan KR, Kong J, Schulman KA. 1997. Access to bone marrow transplantation for leukemia and lymphoma: The role of sociodemographic factors. Journal of Clinical Oncology (United States) 15:2644-2651.
Mo B. 1992. Modesty, sexuality, and breast health in Chinese-American women. Western Journal of Medicine 157:260-264.
Mohler PJ. 1995. Enhancing compliance with screening mammography recommendations: A clinical trial in a primary care office. Family Medicine 27:117-121.
Monticciolo DL, Sickles EA. 1990. Computerized follow-up of abnormalities detected at mammography screening. American Journal of Roentgenology 155:751-753.
Mor V, Masterson-Allen S, Goldberg RJ, et al. 1985. Relationship between age at diagnosis and treatments received by cancer patients. Journal of the American Geriatric Society 33:585-589.
Mosconi P, Meyerowitz BE, Liberati, et al. 1991. Disclosure of breast cancer diagnosis: Patient and physician reports. Annals of Oncology 2:273-280.
Moul JW, Douglas TH, McCarthy WF, McLeod DG. 1996. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. Journal of Urology 155:1667-1673.
Mouton CP, Harris S, Rovi S, et al. 1997. Barriers to black women's participation in cancer clinical trials. Journal of the National Medical Association (United States) 89:721-727.
Muss HB, Hunter CP, Welsey M, et al. 1992. Treatment plans for black and white women with Stage II node-positive breast cancer—The National Cancer Institute Black/White Cancer Survival Study. Cancer 70:2460-2467.
Myers RE, Ross EA, Wolf TA, et al. 1991. Behavioral interventions to increase adherence in colorectal cancer screening. Medical Care 29:1039-1050.
National Cancer Institute. 1995a. Breast and cervical cancer screening among underserved women. Archives of Family Medicine 4:617-624.
National Cancer Institute. 1995b. Cancer Screening Consortium for Underserved Women. Bethesda, MD: National Institutes of Health.
National Center for Health Statistics. 1997. Healthy People 2000 Review. Hyattsville, MD: Public Health Service.
National Institutes of Health. 1994. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Federal Register 59:14508-14513.
Nattinger AB, Gottlieb MS, Hoffman RG, et al., 1996. Minimal increase in use of breast-conserving surgery from 1986 to 1990. Medical Care 34(5)479-489.
Nattinger AB, Gottlieb MS, Veum J, et al. 1992. Geographic variation in the use of breast-conserving treatment for breast cancer. New England Journal of Medicine 326:1102-1127.
Nattinger AB, Panzer RJ, Janus J. 1989. Improving the utilization of screening mammography in primary care practices. Archives of Internal Medicine 149:2087-2092.
Newschaffer CJ, Penberthy L, Desch CE, et al. 1996. The effect of age and comorbidity in the treatment of elderly women with nonmetastatic breast cancer. Archives of Internal Medicine 156:85-90.
O'Malley AS, Mandelblatt J, Gold K, et al. 1997. Continuity of care and the use of breast and cervical cancer screening services in a multiethnic community. Archives of Internal Medicine 157(13): 1462-1470.
Organ Transplant Fund, Inc. 1998. http//:www.otf.org.
Ornstein SM, Garr DR, Jenkins RG, et al. 1991. Computer-generated physician and patient reminders. Tools to improve population adherence to selected preventive services. Journal of Family Practice 32:82-90.
Osteen RT, Karnell LH. 1994. The National Cancer Data Base report on breast cancer. Cancer 73:1994-2000.
Paskett ED, White E, Carter WB, Chu J. 1990. Improving follow-up after an abnormal Pap smear: A randomized controlled trial. Preventive Medicine 19(6):630-641.
Passik SD, Dugan W, McDonald MV, et al. 1998. Oncologists' recognition of depression in their patients with cancer. Journal of Clinical Oncology 16:1594-1600.
Phillips KA, Kerlikowske K, Baker LC, et al. 1998. Factors associated with women's adherence to mammography screening guidelines. Health Services Research 33(1):29-53.
Plawker MW, Fleisher JM, Vapnek EM, Macchia RJ. 1997. Current trends in prostate cancer diagnosis and staging among United States urologists. Journal of Urology 158(5):1853-1858.
Potosky AL, Breen N, Graubard BI, Parsons PE. 1998. The association between health care coverage and the use of cancer screening tests. Results from the 1992 National Health Interview Survey. Medical Care 36:257-270.
Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK, eds. 1997. SEER Cancer Statistics Review, 1973-1994. NIH Pub. No. 97-2789. Bethesda, MD: National Cancer Institute, National Institutes of Health.
Riley GF, Potosky AL, Lubitz JD, Brown ML. 1994. Stage of cancer at diagnosis for Medicare HMO and fee-for-service enrollees. American Journal of Public Health 84:1598-1604.
Rimer B, Levy M, Kleintz MK, et al. 1987. Improving cancer patients' pain control through education. Progress in Clinical and Biological Research 248:123-127.
Roberts CS, Cox CE, Reintgen DS, et al. 1994. Influence of physician communication on newly diagnosed breast patients' psychologic adjustment and decision making. Cancer 74(1 Suppl.):336-341.
Robinson SB, Ashley M, Haynes MA. 1996. Attitudes of African-Americans regarding prostate cancer clinical trials. Journal of Community Health 21(2):77-87.
Rogowski J, Lillard L, Kington R. 1997. The financial burden of prescription drug use among elderly persons. Gerontologist 37(4):475-482.
Rojas M, Mandelblatt J, Cagney K, et al. 1996. Barriers to follow-up of abnormal screening mammograms among low-income minority women. Ethnicity and Health 1(3):221-228.
Rutledge DN, Hartmann WH, Kinman PO, Winfield AC. 1988. Exploration of factors affecting mammography behaviors. Preventive Medicine 17:412-422.
Samet JM, Hunt WC, Farrow DC. 1994. Determinants of receiving breast-conserving surgery. Cancer 73:2344-2351.
Samet JM, Hunt WC, Goodwin JS. 1990. Determinants of cancer stage. A population-based study of elderly New Mexicans. Cancer 66:1302-1307.
Satariano W. 1992. Cormorbidity and functional status in older women with breast cancer: Implications for screening, treatment, and prognosis. Journal of Gerontolology 47:24-31.
Savage D, Lindenbaum J, Van Ryzin J, et al. 1984. Race, poverty, and survival in multiple myeloma. Cancer 54(12):3085-3094.
Schapira DV, Kumar NG, Clark RA, Yag C. 1992. Mammography screening credit card and compliance. Cancer 70:509-512.
Schapira DV, Panies RJ, Kumar NB, et al. 1993. Cancer screening. Knowledge, recommendations, and practices of physicians. Cancer 71:839-843.
Schiffer CA, Dodge R, Larson RA. 1997. Long-term follow-up of cancer and leukemia group B studies in acute myeloid leukemia. Cancer 80(11):2210-2214.
Schleifer SJ, Bhardwaj S, Lebovits A, et al. 1991. Predictors of physician nonadherence to chemotherapy regimens. Cancer 67:945-951.
Schwartz JS, Lewis CE, Clancy C, et al. 1991. Internists' practices in health promotion and disease prevention. A survey. Annals of Internal Medicine (United States) 114:46-53.
Selby JV, Friedman GD, Quesenberry CP Jr., Weiss N. 1992. A case-control study of sigmoidoscopy and mortality from colorectal cancer. New England Journal of Medicine 326:653-657.
Shapiro S, Venet W, Strax P, et al. 1982. Ten-to-fourteen-year effect of screening on breast cancer mortality. Journal of the National Cancer Institute 69:349.
Silliman RA, Guadagnoli F, Weitberg AB, Mor V. 1989. Age as a predictor of diagnostic and initial treatment intensity in newly diagnosed breast cancer patients. Journal of Gerontology 44:M46-50.
Siminoff LA, Fetting JH, Abeloff MD. 1989. Doctor-patient communication about breast cancer adjuvant therapy. Journal of Clinical Oncology 7:1192-1200.
Simone J, Lyons J. In press. Superior cancer survival in children compared to adults: A superior system of cancer care? Salt Lake City: Huntsman Cancer Institute, University of Utah.
Skinner CS, Strecher VJ, Hospers H. 1994. Physician's recommendations for mammography: Do tailored messages make a difference? American Journal of Public Health 84:43-49.
Slevin ML, Stubbs L, Plant HJ, et al. 1990. Attitudes to chemotherapy: Comparing views of patients with cancer with those of doctors, nurses, and general public. British Medical Journal 300(6737): 1458-1460.
Smith TJ, Penberthy L, Desch CE, et al. 1995. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer 13:235-252.
Smith TJ, Swisher K. 1998. Telling the truth about terminal cancer. Journal of the American Medical Association 279(21) 1746-1748.
Sofaer S, Davidson B, Goodman R, et al. 1990. Helping Medicare beneficiaries choose health insurance: The illness episode approach. Gerontologist 30(3):308-315.
Stein JA, Fox SA, Murata PJ. 1990. The influence of ethnicity, socioeconomic status, and psychological barriers on use of mammography. Journal of Health and Social Behavior 32:101-113.
Strull WM, Lo B, Charles G. 1984. Do patients want to participate in decision making? Journal of the American Medical Association 252(21):2990-2994.
Studnicki J, Schapira DV, Bradham DD, et al. 1993. Response to the National Cancer Institute alert—The effect of practice guidelines on two hospitals in the same medical community. Cancer 72: 2986-2992.
SUPPORT Principal Investigators. 1995. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). Journal of the American Medical Association 274(20):1591-1598.
Taplin SH, Anderman C, Grothaus L, et al. 1994. Using physician correspondence and postcard reminders to promote mammography use. American Journal of Public Health 84:571-574.
Taylor KM, Margolese RG, Soskolne CL. 1984. Physicians' reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancer. New England Journal of Medicine 310:1363-1367.
Tejeda HA, Green SB, Trimble EL, et al. 1996. Representation of African Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. Journal of the National Cancer Institute 19(88):812-816.
Thompson IM, Colman CA, Brawley OW, et al. 1995. Chemoprevention of prostate cancer. Seminars in Urology 13:122-129.
Tierney WM, Hui SL, McDonald CJ. 1986. Delayed feedback of physician performance versus immediate reminders to perform preventive care. Medical Care 24:659-666.
Trowbridge R, Dugan W, Jay SJ, et al. 1997. Determining the effectiveness of a clinical practice intervention in improving the control of pain in outpatients with cancer. Academic Medicine 72:798-800.
Underwood SM. 1995. Enhancing the delivery of care to the disadvantaged: The challenge to providers. Cancer Practice 3:31-36.
U.S. Bureau of the Census. 1998. Health Insurance Coverage:1997. www.census.gov/hhes/hlthins/hlthin97/hi97t2.html.
U.S. Department of Health and Human Services. 1991. Healthy People 2000. National Health Promotion and Disease Prevention Objectives . Publication No. (PHS)91-50213
. Washington, D.C.U.S. Department of Health and Human Services. 1996. Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. Second Edition. Washington, D.C.
U.S. General Accounting Office. 1997. The Health Insurance Portability and Accountability Act of 1996: Early Implementation Concerns. GAO-HEHS-97-200R. Washington, D.C.
U.S. General Accounting Office. 1998. Many HMOs Experience High Rates of Beneficiary Disenrollment. GAO-HEHS-98-142. Washington, D.C.
Van Roenn JH, Cleeland CS, Gonin R, et al. 1993. Physician attitudes and practice in cancer pain management: A survey from the Eastern Cooperative Oncology Group. Annals of Internal Medicine 119:121-126.
Vellozzi CJ, Romans M, Rothenberg RB. 1996. Delivering breast and cervical cancer screening services to underserved women: Part I. Literature review and telephone survey. Women's Health Issues 6(2):65-73.
Vernon SW. 1997. Participation in colorectal cancer screening: A review. Journal of the National Cancer Institute 89(19)1406-1422.
Virgo KS, McKirgan LW, Caputo MC, et al. 1995. Post-treatment management options for patients with lung cancer. Annals of Surgery 222(6):700-710.
Waitzkin H. 1985. Information giving in medical care. Journal of Health and Social Behavior 26(2):81-101.
Ward SE, Goldberg N, Miller-McCauley V, et al. 1993. Patient-related barriers to management of cancer pain. Pain 52:319-324.
Weeks JC, Cook EF, O'Day SJ, et al. 1998. Relationship between cancer patients' prediction of prognosis and their treatment preferences. Journal of the American Medical Association 279(21)1709-1714, June 3.
Weinberger MW, Saunders AF, Samsa GP, et al. 1991. Breast cancer screening in older women: Practices and barriers reported by primary care physicians. Journal of the American Geriatric Society 39:22.
Weisman C, Celentano D, Teitelbaum M, Klassen A. 1989. Cancer screening services for the elderly. Public Health Reports 104:209-214.
Wells BL, Horm JW. 1992. Stage at diagnosis in breast cancer: Race and socioeconomic factors. American Journal of Public Health 82(10):1383-1385.
Yarnall KS, Michener JL, Broadhead WE, Tse CK. 1993. Increasing compliance with mammography recommendations: Health assessment forms. Journal of Family Practice 36:59-64.
Yellen SB, Cella DF, Leslie WT. 1994. Age and clinical decision making in oncology patients. Journal of the National Cancer Institute 86(23):1766-1770.
Zaloznik AJ. 1995. Breast cancer stage at diagnosis: Caucasians versus Afro-Americans. Breast Cancer Research and Treatment 34(3):195-198.
Zaloznik AJ. 1997. Breast cancer stage at diagnosis: Caucasians versus Hispanics. Breast Cancer Research and Treatment 42(2):121-124.
Zapka JG. 1994. Promoting participation in breast cancer screening. American Journal of Public Health 84(1):12-13.
Zapka JG, Berkowitz E. 1992. A qualitative study about breast cancer screening in older women: Implications for research. Journal of Gerontology 47:93-100.
Zapka JG, Hosmer D, Costanza ME, et al. 1992. Changes in mammography use: Economic, need and service factors. American Journal of Public Health 82:1345-1351.
Zapka JG, Stoddard A, Maul L, Costanza ME. 1991. Interval adherence to mammography screening guidelines. Medical Care 29:697-707.

































