4
Research Opportunities and the Elements of Materials Science and Engineering
In Chapter 3, research opportunities in materials science and engineering were described in terms of the functional roles of materials. Such a breakdown has the advantage of making explicit the link between fundamental research and its applications. Inevitably, however, a presentation organized in that way omits some important areas of research. Often in basic research, the focus is structure, properties, phenomena, or behavior of materials; the functional utility of a new material may not be appreciated until its properties have been adequately characterized. One example is quasi-crystals, a class of materials neither exactly crystalline nor amorphous but with some attributes of both. These materials were discovered only in this decade. Properties of this class of materials are still being measured, and it is not clear which functional property will result in an application. Other research topics may be linked to applications but may still not be adequately described by a breakdown of research opportunities according to materials function, because such topics may relate broadly to several, or all, of the functional classes of materials. An example is rapid solidification processing, which has already had important applications in metals for both structural and magnetic applications, and in ceramics for structural electronic applications.
Science is a process of collecting and organizing knowledge about nature. The motivation of the scientist may vary widely. In some cases, it is to understand a phenomenon at its most fundamental level. In others, the motivation is to understand and gain knowledge in a particular area that is believed to be useful in developing materials for a particular application. The latter motivation is easy to justify because it aims at immediate results. What is less easy to explain is the pursuit of science when application is not
the primary motivation. In physics and chemistry, the value of seeking knowledge for its own sake tends to be taken for granted. In the field of materials science and engineering, the role of fundamental science is just as essential and forms the basis for many of the great developments that have already changed our society and will continue to transform it.
For example, quantum mechanics is the basis of our understanding of solids. The use of quantum mechanical principles in building transistors or future quantum-well devices is a case in point. Without the framework provided by this fundamental knowledge, the power of materials science and engineering, which led to the integrated circuit, would never have revolutionized information technology. The importance of quantum mechanics appeared again in the invention of the first and of all subsequent lasers. It is now playing a central role in the development of high-Tc superconductors. The discovery of high-speed superconducting Josephson junctions was not motivated by applications. But their development into a high-speed switch (to which materials science and engineering made a contribution) was so motivated. The new high-temperature superconductors were not discovered during a search for applications. But their development as useful materials will require scientific research directed toward applications.
The point is that fundamental scientific advances are essential components of materials science and engineering, even though the motivation for them may, at the outset, have no obvious connection with applications. Once a major scientific breakthrough occurs, the full power of materials science and engineering is needed to make something useful of it.
The methodology for developing materials for applications, which provides an underlying coherence to this diverse field, is the framework for this chapter’s examination of research opportunities in materials science and engineering. Assessing research opportunities in terms of the four basic elements of the field (see Figure 1.10) allows study of their relative significance regardless of materials class, functional applications, or position in the spectrum from basic research to engineering. It also provides another perspective from which to discern and monitor broad trends in materials science and engineering. In this chapter, emphasis is given to synthesis and processing as the element of the field that especially requires the concentrated efforts of U.S. researchers.
In the past, many viewed materials science and engineering as focusing primarily on structure-property-performance relationships in materials. This view of the field has had its counterpart in the activities viewed as important by materials scientists and engineers. Materials scientists and engineers have studied the structure and composition of materials on scales ranging from the electronic and atomic through the microscopic to the macroscopic. They have measured materials properties of all kinds, such as mechanical strength, optical reflectivity, and electrical conductivity. They have predicted and
evaluated the performance of materials as structural or functional elements in engineering systems.
In conducting all these studies, materials scientists and engineers have recognized that properties and performance depend on structure and composition, which in turn are the result of the synthesis or processing of a material. Only recently, however, have synthesis and processing come to be widely viewed as an essential and integral element of materials science and engineering. As discussed in Chapter 3, synthesis of new materials by unusual chemical routes and by various physical and chemical means has led to an era in which atom-by-atom fabrication can be achieved. Coincidentally, processing has received renewed attention, partly in response to challenges from international competitors who have reaped the benefits of improved quality and uniformity of traditional materials, and partly in response to the demands for process control to achieve the promise of advanced materials.
Increasingly, work in materials science and engineering involves interactions among groups working in all elements of the field. One example of this trend is the development of new high-temperature intermetallic composites to achieve a complex array of performance-driven properties for applications such as the proposed national aerospace plane. If materials science and engineering is to remain healthy and productive, research on all elements of the field—and on their relationships—is vital. Nonetheless, the committee has emphasized synthesis and processing as the aspect of the field representing the greatest national weakness and also the ripest opportunities. In addition, concluding sections of this chapter discuss instrumentation and modeling, which are the areas of research critical to synthesis and processing as well as to the other elements of the field.
More detailed discussions of these significant aspects of materials science and engineering are presented in Appendixes A, B, C, D, and E, which describe important research opportunities in synthesis, processing, performance, instrumentation, and analysis and modeling, respectively. This information should be useful to the practitioners of materials science and engineering as well as to the federal agencies seeking specific advice on technical areas of research.
PROPERTIES AND PERFORMANCE
Properties are the descriptors that define the functional attributes and utility of materials. The brilliance and transparency of diamond, for example, give rise to its use as gemstones as well as sophisticated optical coatings, while its great hardness and thermal conductivity permit quite different applications such as cutting tools and media. A micrograph of a diamond thin film is shown in Figure 4.1. Metals are ductile, a property that facilitates their being processed into wires for electrical conduction or for mechanical retention.
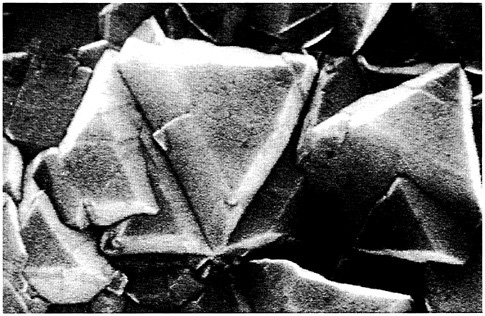
FIGURE 4.1 Polycrystalline diamond film deposited on silicon wafer by the hot filament chemical vapor deposition method. The largest triangular face is approximately 2 µm on a side. (Courtesy National Institute of Standards and Technology.)
Ceramics have high melting points, great strength, and chemical inertness that promote their use as liners or protective coatings in advanced heat engines; however, their lack of plasticity is a detrimental property that currently limits the widespread application of ceramics. Unique physical properties of polymers make possible diverse products such as sonar devices, liquid-crystal displays, electronic package encapsulation, and automobile interiors, but, conversely, the transport properties of polymers’ constituents accelerate degradation, as illustrated by the “new car” smell present in newly assembled vehicles.
In the broadest sense, materials properties represent the collective responses of materials to external stimuli; for instance, electrical or thermal conductivity are the measured result of the application of an electric field or temperature gradient. Similarly, magnetic susceptibility, superconducting transition temperature, optical absorption, mechanical strength, elastic constants, and the like are responses to other stress fields. Collectively, properties are the quantitative measures of the electrical, magnetic, optical, thermal, and mechanical character of materials and result from the structure and composition of the synthesized or processed substance, be it in solid, liquid, or gas form or in the microscopic or macroscopic size regime.
Property measurements and their analysis are the domains of theoretical
and experimental physicists, physical chemists, metallurgists, ceramists, polymer chemists, and engineers of all fields. Characterizing of some properties on an atomic scale is a research area at the forefront of theoretical physics—the theories of phase transitions in magnetic materials and in ferroelectrics are cases in point. Similarly, elucidation of the fracture mechanics of brittle ceramic and metallic materials is a focus of research by metallurgists, ceramists, and solid-state physicists alike. The understanding of properties and structure in tandem has enabled synthetic chemists to make materials with “better,” or at least predictably different, properties. This interrelationship affects the ultimate utility of materials—their performance. Two of the three Nobel Prizes cited in Chapter 1—for discovery of the quantum Hall effect and for work on high-Tc superconductivity—were awarded for research based on innovative property determinations. The work on high-Tc superconductivity, of course, was also a triumph of synthesis and analysis.
With increased understanding of the origin of properties in the structure and composition of materials has come the opportunity to design desired combinations of properties. For example, in years past, engineers were limited by materials that were either strong or tough, but rarely both. Today these properties can be achieved in appropriate balance in a variety of materials, from high-strength steels to polymer composites to toughened ceramics. These new materials are often slowly introduced into applications due to the need for engineering design data that have been evaluated for accuracy and are available in convenient computer format. The need for such materials property data for design is universally recognized but is rarely given adequate attention. Collaboration among universities, government laboratories, and, most importantly, industries (in which most of the data are first generated) is critical to the development of such evaluated data bases.
Performance is the element in which the inherent properties of a material link up with product design, engineering capabilities, and human needs. The properties of a material are put to use to achieve desired performance in a device, component, or machine. Examples of measures of performance include lifetime, speed (of a device or vehicle), energy efficiency (of a machine or current carrier), safety, and life cycle costs.
Materials researchers who are concerned with performance seek to develop models that relate device performance to the fundamental properties of the component materials. They also seek to understand how the materials properties are affected in service and how to predict and improve these changes in properties. The working environment is usually highly complex, involving multiple, often synergistic stimuli and forces, such as heat and mechanical stress cycling, moisture and oxidation exposure, and irradiation. For structural materials, for example, it is necessary to know how the materials respond to stresses caused by service loading, mechanical contacts, or temperature variation; how they react to corrosive or otherwise hostile environments; and
how they undergo internal degradation. The crucial issues are reliability, durability, life prediction, and life extension at minimum cost. Understanding of failure modes and development of rational simulative test procedures are crucial to the development of improved materials, designs, and processes. Such issues are relevant not only for materials used in large structures or machines, but also for those that form structural and other elements in electronic, magnetic, or optical devices.
Behavior in service generally refers to the behavior of a material in some end use—as in a turbine blade, piston head, containment vessel, energy-absorbing bumper, airplane wing, concrete roadbed, artificial heart valve, or component in an integrated circuit. In general, the study of materials performance strongly overlaps with design. Although synthesis and processing are often thought of in serial fashion (i.e., the equipment or product designer specifies needed properties, and materials personnel then choose or develop materials that have those properties), it is becoming increasingly common to consider the synthesis and processing and the behavior of materials as an integral part of the device or equipment design process. In all real applications, many materials properties play roles in the design of a system. For example, in a relatively simple system such as an automobile hood, relevant properties include not only density, corrosion resistance, strength, stiffness, and forming and welding parameters, but also electrical conductivity (because of the possibility of radio frequency interference between engine devices and the antenna) and magnetic properties (which come into play in the separation of iron for recycling after use). More complex combinations of materials and properties interact in subtle ways in most machines, devices, and components, from engine components to electronic packaging elements.
Effective research programs bearing on performance require broad interactions of materials science and engineering researchers with participants from manufacturing and other engineering disciplines. Except in some industrial and federal laboratories, such interactions do not occur effectively at present. Indeed, performance-oriented research is not adequately pursued today in academic materials science departments in the United States—a shortcoming, given its importance to materials science and engineering. This lack of attention by academia seems to revolve around the perception that performance-oriented research is “too macroscopic” or “not fundamental enough.” But performance-oriented research involves many intellectually challenging problems, ranging from understanding the microstructural response of interfaces between complex solids to predicting lifetimes for structural materials subject to stress or corrosion. Progress in these areas depends on tools and perspectives drawn from all of traditional materials science and engineering plus related aspects of chemistry, physics, mathematics, and engineering.
Performance is similar to synthesis and processing in that it has been underemphasized in the United States, particularly in federally funded research in areas relevant to commercial issues. Additional efforts to evaluate and predict the performance of materials in the context of their use have the potential to contribute substantially to materials problems of economic importance. Design of materials for improved performance relies on improved experimental techniques and improved theoretical understanding of such performance-related properties of materials as susceptibility to mechanical fatigue, an example of which is shown in Figure 4.2, which illustrates cracking. Performance research will be carried out best at facilities where creative approaches to synthesis and processing, characterization, design, and performance are found and where cooperative mechanisms exist to draw these elements of materials science and engineering together.
The interaction between materials synthesis, materials performance, and component or equipment design is becoming increasingly sophisticated and complex. It is further complicated by the fact that current and future products increasingly call for intimate combinations of novel materials. Optimum trade-offs have to be found in design versus performance. To find them by older empirical methods would generally be too time-consuming and costly. Instead, increasing use must be made of analysis and modeling, which often call for intensive computing capabilities. Likewise, there are growing demands for improved instrumentation, particularly for monitoring or deducing the performance of materials in their design environment.
STRUCTURE AND COMPOSITION
A given material contains a hierarchy of structural levels, from the atomic and electronic to the macrostructural level. At all of these structural levels, chemical composition and distribution may vary spatially. These structures and compositions are the result of the synthesis and processing that have been applied to make a given material. In turn, the nearly infinite variety of possible structures gives rise to the similarly complex arrays of properties exhibited by materials. Because of the fundamental role of structure and composition, understanding them at all levels is an essential aspect of materials science and engineering.
Critical to the rapid advances in materials science and engineering over recent decades has been the development of continually more powerful tools for probing structure and composition. Fifty years ago, the major tools used were the light microscope, x-ray diffraction, and infrared and ultraviolet spectroscopy. Today, researchers have access to an enormous range of new instruments and technologies. The scanning tunneling microscope enables
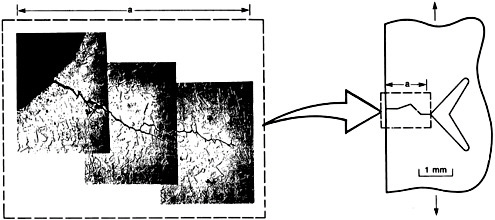
FIGURE 4.2 Left: Micrograph of a typical surface flaw in stainless steel. Right: Schematic of the plastic zone at the tip of a flaw. The nature and extent of the plastic zone determine a material’s fracture resistance and consequently its performance in service. (Reprinted from National Materials Advisory Board, The Impact of Supercomputing Capabilities on U.S. Materials Science and Technology, National Academy Press, Washington, D.C., 1987.)
determination of atomic arrangement and electronic structure at and near the surface of materials. Solid-state nuclear magnetic resonance allows determination of chemical makeup in complex polymer systems. Electron microscopy can show atomic arrangements and chemical compositions at near-atomic resolution; at lower magnifications, it allows the determination of maps of chemical inhomogeneity on larger scales. A host of spectroscopies enable the chemical characterization of surfaces. High-intensity neutron beams from reactors and photon beams from synchrotron sources have made possible a vast array of techniques for chemical and structural characterization.
This new instrumentation has increased understanding but brings with it major concerns for the field of materials science and engineering. The high cost of characterization has become an issue demanding care in the balance of allocations by funding agencies and has, in many instances, become a limiting factor in the progress of research. Furthermore, the availability of large characterization facilities in only a few geographic locations has led to changes in the way research is carried out for many materials scientists and engineers. Rather than do these experiments in their own laboratories, they may now travel thousands of miles to do their research at a major national facility in the midst of the exciting intellectual ferment present at such facilities.
Concurrent with the development of techniques for characterizing the structure and composition of materials has been the development of analytical and modeling techniques to explain the origins of these observations, for example, quantum calculations to describe electronic structure and crystal structure stability; equilibrium and nonequilibrium thermodynamics to describe multiphase materials; and hydrodynamics and instability analysis to explain the development of microstructures in crystalizing metals and polymers.
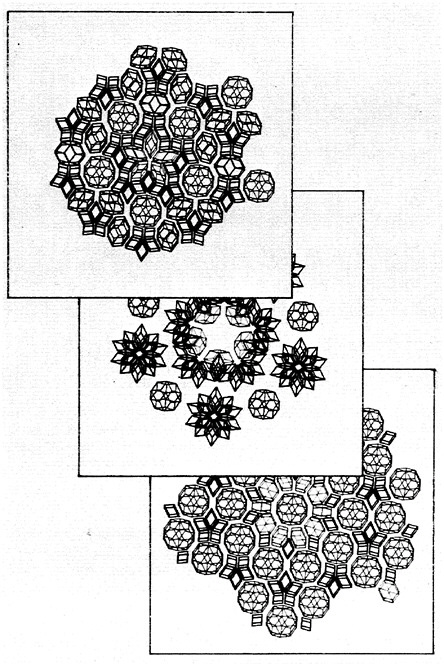
Historically, the development of materials has involved many key discoveries made at the macroscopic level (such as continuum behavior or mechanical properties). In recent decades, there has also been increased emphasis on the microscopic or atomic level, both in research and in education. New generations of electrical engineers, ceramists, metallurgists, polymer chemists, and condensed-matter physicists, who have been trained in the basic interactions of atoms and molecules, understand the fundamental concepts underlying and unifying previously disparate classes of materials. Opportunities to increase fundamental understanding in this area continue to occur. Three years ago, for example, the apparently well-defined science of geometric crystallography was jolted by the discovery of icosahedral symmetry in solids. These so-called quasi-crystals possess orientational order without translational periodicity. Study of these quasi-crystals promises to lead to a deeper understanding of the conditions leading to different atomic arrangements in solids. Figure 4.3, a picture of icosahedral Al6Li3Cu, shows the triacontahedral faceting that occurs upon slow quench of the phase. Figure 4.4
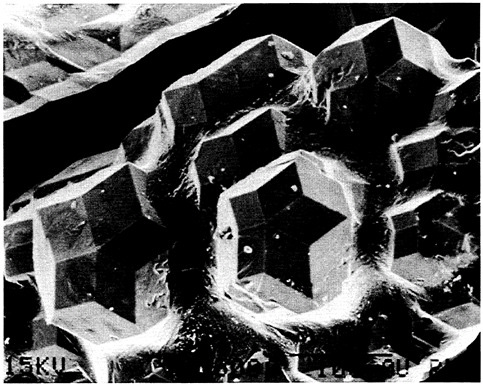
FIGURE 4.3 Icosahedral Al6Li3Cu. (Reprinted, by permission, from P.A.Heiney, P.A.Bancel, P.M.Horn, J.L.Jordan, S.LaPlaca, J.Angilello, and F.W.Gayle, 1987, Disorder in Al-Li-Cu and Al-Mn-Si Icosahedral Alloys, Science 238:660–663. Copyright © by The American Association for the Advancement of Science.)
shows three slices of a three-dimensional icosahedral quasi-crystal; pieces include a rhombic triacontahedron.
A new emphasis in materials science and engineering centers on the nanometer size regime, which is intermediate between the well-studied macroscopic and atomic levels. This regime is pivotal in understanding the magnetic, electronic, and optical properties of materials. Knowledge of structural and compositional features in the nanometer size range is also important for interfaces between dissimilar materials such as those that occur in composite structural materials or in the complex multimaterial devices that make up integrated electronic circuitry. Studies of synthesis and processing have focused increasingly on the nanometer size regime, as is demonstrated by development of block copolymers, ultrafine ceramic powders, metallic microstructures, and superlattice electronic devices. The increased understanding of materials at this level has shifted the fundamentals of process control
away from purely macroscopic phenomena. Moreover, numerous new synthetic techniques have made it possible to fabricate nanometer-scale structures that exhibit new physical phenomena, which in turn can form the basis for new technologies. Increasingly, the properties and performance of materials are determined by the nanostructure of the materials, and society is using more of these materials each year. Development of such materials represents a scientific frontier with technological importance to many industries.
SYNTHESIS AND PROCESSING
Synthesis and processing are terms that refer to the building of new arrangements of atoms, molecules, and molecular aggregates; the control of structure at all levels from the atomic to the macroscopic; and the development of processes to produce materials and components effectively and competitively. Synthesis is often used alone to refer to the physical and chemical means by which atoms and molecules are assembled. Processing may be used in a similar way, for example, in the phrase electronic materials processing. Processing may also imply changes on a larger scale, including materials manufacturing. It is often applied to such macroscopic manipulations as ingot solidification, mechanical modification, sintering, and joining. These macroscopic manipulations, of course, also cause important structural changes at the levels of atoms and grains.
In materials science and engineering, the distinctions between synthesis and processing have become increasingly blurred in recent years. The fabrication of artificially structured materials, which involves synthesis of materials on the atomic scale, is typically referred to as processing. The preparation of ceramics, which in the past generally involved sintering of mixtures of mineral-derived oxides, now involves considerable synthetic chemistry in some instances. Broadly, it may be stated that synthesis and processing form a continuous range of activities in which assemblages of atoms, molecules, and molecular aggregates are transformed into useful products.
Synthesis and processing research is evolving to the point that, in some cases, new materials can be tailored, atom by atom, to achieve a desired set of properties or to obtain new and sometimes unexpected phenomena. Synthesis and processing encompass a comprehensive array of techniques and technologies as diverse as rolling of sheet steel, pressing and sintering of ceramic powders, ion implantation of silicon, creation of artificially structured materials, ladle-refining of steel, sol-gel production of fine ceramic powders, pouring of polymer-modified concrete, shaping by machining or chip processes, thermomechanical processing of alloys, preparation of polymers by chemical reactions, coating of turbine blades for corrosion resistance, zone refining of silicon, growth of gallium arsenide crystals, and laying-up of composite materials. Some of these technologies are quite new and may,
in time, lead to major technological advances and industrial growth. Others are well embedded in established industries but require continual improvement if the U.S. industries that rely on them are to remain competitive with foreign industries.
Synthesis and processing are key to production of high-quality, low-cost products throughout a broad spectrum of manufacturing. They are central to translation of new research and new designs into useful devices, systems, and products. They are essential to efficient introduction of advanced materials or materials combinations into the marketplace. A case in point are the ceramic components used in catalytic converters. A key element in many catalytic converters is the monolithic substrate (Figure 4.5). The substrate consists of several ceramic layers, the final one added to assure a high surface area. The catalyst, composed of precious metals such as platinum, palladium, and rhodium, is applied as a thin coat about 30 to 50 µm thick, and the assembly is incorporated as the active component into the catalytic converter.
Synthesis and processing also represent a large area of basic research in materials science and engineering. From this basic research come wholly new materials, for example, new conductive polymers, new compositions
FIGURE 4.5 Monolithic substrate configurations developed as active components in catalytic converters. The circular and oval-shaped versions are currently preferred shapes. (Reprinted, by permission, from Ford Motor Company. Copyright © 1989 by Ford Motor Company.)
of ceramic superconductors, dislocation-free single crystals, and artificially structured materials. Another important thrust of basic processing research is to develop a fundamental understanding of kinetic phenomena involved in materials processing, to serve as the foundation for changes and improvements in processing. Examples of such phenomena are rheological behavior in die filling, atomistic mechanisms of crystal growth, atomistic mechanisms of removal of materials in machining, and mass transport mechanisms in consolidation processes.
The United States suffers from a serious weakness in synthesis and processing with respect to new materials, manufacturing technology, and education in materials science and engineering. In many areas, the synthesis and processing of materials have been emphasized less in government, industrial, and university laboratories in the United States than in laboratories of other countries. Not only does this sharply limit the techniques that can be brought to bear on problems in this area, but it also curbs opportunities for unexpected discoveries.
An important but often overlooked aspect of synthesis and processing advances is the continued development of new machinery and equipment for synthesis and processing. As discussed below in the section “Instrumentation,” research devoted to equipment development receives only limited support in the United States, with a commensurate loss of equipment markets to foreign competition. Notable examples in process technology include the markets for machine tools and semiconductor processing equipment. To ameliorate the flow of resources for manufacturing equipment to foreign markets, the United States needs to accelerate research on synthesis and processing equipment and to strengthen the manufacturing industry for this equipment. A strong machine and equipment component is essential to improving the synthesis and processing component of the technology base for any industry.
It is also important to improve the scientific foundations underlying U.S. manufacturing processes. Processing efficiency not only is essential to U.S. industrial competitiveness, but also poses many intellectual problems. Basic research directed at increasing the understanding of crystal growth, vapor deposition, sintering, phase transformations, rheology, and other generic processes key to manufacturing could have profound effects on national productivity. This research will be particularly valuable when it can be extended to the development of real-time process models that can be used in process control. In recent years, robust nondestructive sensors have been developed to measure materials properties during processing. For example, sensors are now available to check the thickness, grain size, and texture of thin-sheet-rolled metals as they are being processed. Figure 4.6 depicts a sensor that measures various properties of an aluminum rod during extrusion processing. The introduction of such sensors earlier in the process stream
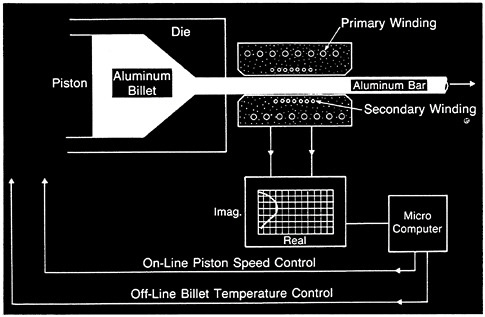
FIGURE 4.6 Schematic representation of a prototype eddy current sensor for measuring the diameter, electrical conductivity, and temperature of an aluminum rod during extrusion processing. The control system uses sensor-acquired temperature measurements in a feedback loop, performing off-line control of the initial temperature of the billets and online control of the speed of extrusion (itself a heat-generating process). This system will result in improved product quality and reduction of rejected output through in-process temperature and measurement control. (Courtesy National Institute of Standards and Technology.)
enables improved control and/or rejection of bad products at minimum value-added process steps. When coupled with process modeling and elements of artificial intelligence, these sensors promise to usher in a new era of intelligent processing of materials. Developments in this field will require the combined efforts of experts in sensor development, materials modeling, the relationships between process variables and product structure, and artificial intelligence (with a strong emphasis on expert systems). To maximize the impact of such an effort, collaboration between industrial, university, and government laboratories must be achieved at the outset. An expanded effort by funding agencies and national laboratories directed to the intelligent processing of materials could have an immediate impact on the quality of processing of conventional materials and could hasten the introduction of advanced materials. Such an effort would also have the benefit of focusing academic
attention on processing and of stimulating the interest of a new generation of highly qualified engineering and science students in this vital area.
In the United States, a long-standing tendency to view synthesis and processing as a service function that is not an elite activity of science or engineering has often led to a weak linkage between synthesis and processing and the other elements of materials science and engineering. Even though this viewpoint is generally understood to be invalid in today’s world, it persists in the structure of scientific institutions—particularly of universities—and in many industries, where production is not viewed as a route to senior positions. Furthermore, because academia has often given comparatively little attention to synthesis and processing, there has been a shortage of qualified scientists and engineers in these areas (this issue is discussed further in Chapter 5). There is no more practical argument for the necessity of a unified view of materials science and engineering than this relative neglect of synthesis and processing. This lack of support exists not only in the federal government and universities but also in many industries, and it spans the complete range of materials-related activities, from science to engineering and from the creation of new materials to the manufacture of products using materials.
Because of the crucial role the committee sees for synthesis and processing, the remainder of this chapter provides examples of areas for pioneering research in synthesis and processing, most of which extend across several of the materials classes.
Artificially Structured Materials
The development of new materials and of materials systems structured on an atomic scale is a recent phenomenon that will have many important applications. For example, the processes used for producing artificially structured materials make it possible to combine optically active materials with electronic circuitry in ways that should lead to qualitatively new kinds of optoelectronic devices. Artificially structured materials can be produced by a variety of techniques, including molecular beam epitaxy (MBE), liquid-phase epitaxy (LPE), chemical vapor deposition (CVD), vacuum evaporation, sputter deposition, ion beam deposition, solid-phase epitaxy, chemical beam epitaxy (CBE), metallo-organic molecular beam epitaxy (MOMBE), and low-pressure chemical vapor deposition (LPCVD).
Techniques for growing thin films epitaxially, such as MBE, have been used to produce artificially structured materials with levels of purity and structural perfection that seemed impossible only a few years ago. Layered semiconductor systems with layer thicknesses of atomic dimensions and with atomically smooth interfaces are now grown. The gallium-arsenide/gallium-aluminum-arsenide (GaAs-GaAlAs) system has received the most attention
to date, with structures of widely varying electronic properties produced by control of composition. Carrier mobilities in excess of 106 cm2/V-s have been achieved in systems with layer thicknesses of the order of 10–6 cm.
An artificially structured material generally can be expected to exhibit novel and useful properties when the length scale of the structure is comparable to the characteristic length scale of the physical phenomenon of interest. Examples of interesting microscopic length scales include the de Broglie wavelengths of electrons, the wavelengths of phonons, the mean free paths of excitations, the range of correlations in disordered structures, characteristic diffusion distances, and the like. These distances can vary from a few atomic spacings to microns. The least explored area, and the one with the greatest potential interest for processing technology, lies between the atomic and macroscopic sizes.
To date, most of the interest in the field of artificially structured materials has been focused on semiconductors, but there are many opportunities for new and useful combinations involving metals, insulators, and even polymers. The processing technologies now available are capable of producing both equilibrium and novel nonequilibrium phases, including amorphous structures and extended solid solutions. The range of possibilities in this area is truly remarkable.
Ultrapure Materials
New synthetic methods and new procedures for handling materials during synthesis are now yielding substances of unprecedented purity and performance. The synthesis of very pure substances is becoming increasingly important both in microelectronics and in the development of new structural materials. The need for extremely pure silicon in microelectronic devices is well known. More recently, very pure and atomically perfect III–V semiconductor crystals are being used in advanced electrooptical devices. In the area of structural materials, it is becoming clear that oxygen and carbon impurities can limit the strength of fibers used in composites. Crack initiation and growth in solids can be attributed to impurities and defects.
The success of molecular precursors in solid-state synthesis often depends on the use of ultrapure molecular materials. For example, a promising new method for producing ceramic fibers starts with synthesis of a preceramic polymeric material that can be processed into a fiber and then pyrolyzed to form the ceramic. The purity of the preceramic polymer determines the strength of the ceramic fiber. In another example, very pure organometallic precursors can be used in synthesizing complicated ternary and quaternary III–V compounds for use in the preparation of device-quality materials. Lasers with unusually low threshold currents have been produced in this way. Fi-
nally, molecular precursors to metal lines and thin films may be useful in device fabrication.
Organic nonlinear optical materials provide another illustration of the opportunities for research in the synthesis of ultrapure substances. It is becoming widely appreciated that the nonlinear optical properties of organic and organometallic molecules can be superior to those of inorganic solids. Preparation of new organic and organometallic molecular solids for use in nonlinear optics represents a special opportunity for academic chemists, because the synthetic techniques are relatively commonplace in major chemistry departments in the United States. What makes this a new opportunity is the need to prepare organic and organometallic solids of a purity and optical quality seldom demanded in typical chemical applications. New strategies are needed for designing and preparing organic and organometallic solids with good optical properties.
One of the most critical and pervasive needs for ultrapure materials is in fundamental physical studies of structure-property relationships. For example, very pure samples of polymers with narrow distributions of molecular weights are needed to test modern theories of the behavior of polymers in dense fluids. Thus, for both fundamental and applied purposes, laboratories dedicated to the preparation of ultrapure materials must have high priority in programs aimed at upgrading U.S. capabilities in materials science and engineering.
In metals, the importance of purity with respect to ductility, resistance to corrosion, strength, and other properties is increasingly evident. Toughness at low temperatures (e.g., in steel for pipelines in cold climates) is obtained through the use of high-purity carbon steels with very low sulfur and phosphorus levels. Vacuum induction melting, vacuum arc remelting, and electron beam melting are used to obtain structural metals (especially superalloys and titanium) of extremely low impurity contents. Reduction of iron impurities significantly below usual commercial specifications results in dramatic improvement in the corrosion resistance of magnesium alloys. Purification of copper through zone melting imparts to the metal the ductility necessary to draw it economically to the extremely fine diameters needed for interconnects in very large scale integrated circuits.
New Structures
The systematic search for new and potentially useful structures, based on theory, empiricism, or usually a combination of both, is an essential activity of materials science and engineering. It is the domain of synthetic chemists, who work to develop new polymeric molecules, ceramic superconductors, and fast-ion conductors. It is also the domain of solid-state scientists, processors, and others, who find and develop, for example, new metastable
structures, structures that provide new strengthening mechanisms, and new superhard materials.
History shows that the search for radically new structures is an important goal for materials science and engineering, even when no immediate application is in sight. Fifty years ago, scientists in England and Germany were developing techniques to obtain fully columnar microstructures in castings; today, these same techniques are used in production of the most advanced turbine blade materials. The quasi-crystalline structures, the nano-crystalline structures, and the new metastable structures being produced today may find equally important uses in the decades ahead.
Solidification
Most engineering materials pass through the molten state at some stage during their processing, and the transformation from liquid to solid is an area ripe for fundamental research and technological development.
Nucleation and growth processes have been important areas of research for more than 30 years. Today, important research topics on nucleation deal with how to avoid it to achieve high undercoolings and, hence, nonequilibrium structures in materials, and with how to promote it to achieve fine grain sizes. Topics in growth deal with interfacial phenomena, dendritic growth mechanisms, nonequilibrium processes, and formation of heterogeneities such as lattice defects, segregation, porosity, and inclusions.
In recent years, great advances have been made in growing single crystals; a notable example is provided by the semiconductor industry, in which, during the last 20 years, the size of silicon single crystals grown from the melt has increased from 1 in. (2.5 cm) in diameter to more than 6 in. (15 cm) in diameter (with further increases expected in the future), while the dislocation content of these crystals has dropped from 100 to 1000/cm2 to practically zero.
Solidification processing can be configured to achieve not only the desired bulk material properties but also components in almost the desired form (a process known as near-net-shape forming). Newer foundry techniques often exploit solidification processing to achieve reduced costs. But the process is also used to achieve special structures with unique properties. One example is the directionally solidified turbine blades used on high-performance jet engines (see Figure 2.1); another is composites made by semisolid forming. Casting of steel strip is a continuous near-net-shape casting process that is attracting worldwide attention. It is used today for metals with lower melting points, and it appears to be only a matter of time until it is perfected for steel.
In conventional casting processes, cooling rates are on the order of 1 K/s, and they are much lower in some cases. In rapid solidification pro-
cesses, cooling rates of from 102 to 108 K/s are obtained; at the higher ends of the scale, crystallization may be wholly prevented, even in metals and low-viscosity ceramics. A process for making rapidly solidified powder is illustrated in Plate 3. A more detailed discussion of the promises of rapid solidification processing is given in Appendix B.
Rapid solidification technology has led to amorphous materials with new and useful combinations of magnetic properties. Their unique soft magnetic properties will lead to applications in electronics, power distribution, motors, and sensors. New permanent magnets produced by rapid solidification will be useful in building compact, powerful motors. Rapid solidification has also led to new fine-grained and homogeneous crystalline materials with improved properties and performance. The materials that have responded well to this processing technology include high-strength aluminum and magnesium alloys, tool steels of high toughness, nickel-based superalloys, and oxide abrasive materials. Many thousands of tons of rapidly solidified alumina zirconia abrasives are now produced and sold each year.
Rapid solidification recently played a key role in the remarkable discovery of the so-called quasi-crystalline phases. These phases were first produced accidentally during rapid solidification of aluminum-manganese alloys. The scientific interest in these phases arises from the fact that they display long-range order—they are not amorphous or glassy—but the symmetry of the order is not consistent with the heretofore accepted rules defining the allowable symmetries of crystals. The discovery of quasi-crystals has led to an ongoing reexamination of the basic principles of crystallography, a science that now will have to be reformulated in a more general framework. It is not known, at present, whether these new phases will have interesting and useful properties, but this entirely new phenomenon clearly calls for intense investigation. It is notable that a study of structure and properties made possible by rapid solidification processing has led to a major discovery in crystallography.
Vapor Deposition and Surface Processing
Vapor-solid processing is becoming an increasingly important tool for achieving ultrafine structures, epitaxial layers, surface coatings, and bulk forms in single shapes. The list of processes used is very long and includes physical vapor deposition, CVD, plasma-assisted vapor deposition, metalloorganic chemical vapor deposition, MBE, and ion beam deposition. Vapor deposition processes are used extensively in the electronic materials industry to build chip structures. They also have wide applications in other areas.
Chemical and physical vapor deposition processes have long been used to coat high-temperature materials, notably turbine blades. Plastic parts are sometimes coated with a metal by vapor deposition so that they appear to
be metallic. The backs of gemstones are sometimes coated with a metal to increase their luster. Vapor processes are involved in metal reduction or purification of many metals. Solid shapes (e.g., of “pyrolytic” carbon) are formed by “vapor forming.”
Many broad research opportunities evident in this field involve nucleation, growth, and materials transport. These processes can be carried out far from equilibrium, providing the opportunity to obtain new and nonequilibrium structures and compositions. Co-deposition processes can produce unique and layered structures. Most vapor-coating processes involve some degree of intermixing of the coating with the substrate; this intermixing is enhanced in processes such as ion beam deposition that provide new approaches to fabricating new structures.
Solid-State Forming Processes
Solid-state forming processes include those that involve extensive materials flow (e.g., injection molding, rolling, forging, and calendaring) and those that involve cutting and grinding. Important new processes that continue to be developed in these areas can influence productivity in important ways and can also result in production of materials with new structures and new properties. Figure 4.7 illustrates technologies for studying strain in deformed metal sheets.
Continuous rolling and annealing of steel sheet has been made possible in recent years by advances in equipment and especially in instrumentation and computer technology. The result is less expensive sheet of greatly improved quality. Injection molding, previously the domain of polymers, is being used increasingly for ceramics and metals. Forming of semisolid (semimolten) metals is a new process, now reaching commercialization, that is a hybrid between forging and casting. In addition to process innovation and process control, process modeling presents important opportunities for research in this field.
Joining, Consolidation, and Materials Removal
Joining processes range from welding of structural steel plate to soldering of the nearly countless interconnects between electronic chips. Both extremes provide important areas of research in process fundamentals and process innovation. Figure 4.8, which shows an example of process fundamentals, illustrates the effects of substrate crystallography and beam direction on the microstructure of an electron beam weld.
Consolidation processes include processing and manufacture of composite materials by a variety of techniques, especially pressing and sintering. Im
FIGURE 4.7 Metal coupons with dot patterns such as these are used to measure the strain in a deformed metal sheet up to (top) and including (bottom) ductile tearing. (Reprinted from National Materials Advisory Board, The Impact of Supercomputing Capabilities on U.S. Materials Science and Technology, National Academy Press, Washington, D.C., 1987.)
portant new consolidation processes involve hot pressing, hot forging (as applied to ceramics), and hot isostatic pressing.
Materials removal involves a wide range of processes, including cutting, grinding, machining, etching, and ion bombardment; it ranges in scale from the scarfing of large ingots to the manufacture of semiconductor chips. Lasers, advanced ceramic materials, and new synthetic diamond materials now provide a new dimension for cutting and grinding.
Important research topics, many with far-reaching economic implications for a range of materials, exist in all these areas.
Electrolytic Processing
Electrolytic processing is an important segment of the broader, $28 billion electrochemical industry. The processing segment includes metal production, plating, semiconductor processing, and chemical production. In recent years, new materials, new processes, and process modeling have had a dramatic influence on the course of the industry, and they promise to further alter its character in the years to come.
An important topic for research in electroprocessing is the electrosynthesis of advanced materials. Co-deposition is one method. For example, the ternary
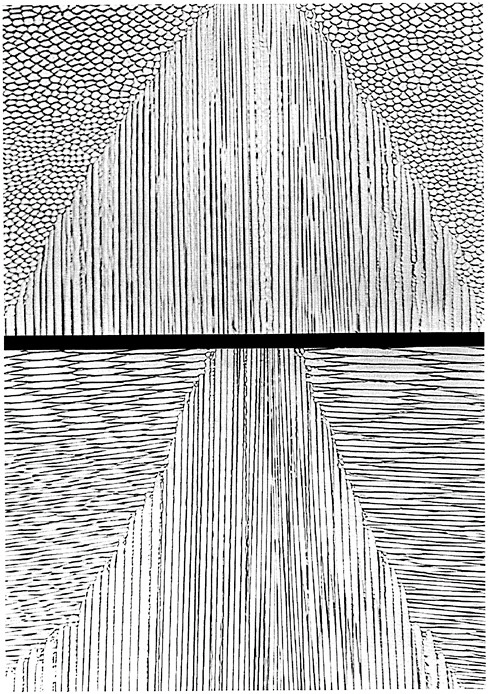
FIGURE 4.8 Electron beam welds made along the (110) (top) or (100) (bottom) directions of a single crystal Fe-15Ni-15Cr alloy, revealing the influence of beam direction and crystallography on the solidification substructure (original magnification: ×500, top; ×400, bottom). (Courtesy Oak Ridge National Laboratory, operated by Martin Marietta Energy Systems, Inc., for the U.S. Department of Energy.)
magnetic alloy neodymium-iron-boron has been produced by co-deposition from a molten salt. A wide range of compounds—from electronic and photonic materials such as gallium arsenide and cadmium telluride to wear-resistant materials such as tungsten carbide to electron emitters such as Ianthanum hexaboride—have been deposited. Opportunities exist to develop new nonaqueous chemistries to extend the window of electrochemical potential and thereby give greater access to highly reactive materials.
Electrolysis is a nonequilibrium process that has the capability of generating nonequilibrium structures such as coatings, epitaxial layers, or powders. Compositionally modulated structures such as that illustrated in Figure 4.9 are readily produced electronically.
Electrodeposition is carried out at or near room temperature in aqueous and organic electrolytes, at elevated temperatures in molten salts, and at low temperatures in cryogenic liquids. Cryogenic electroprocessing offers a new window of opportunity in a temperature region in which kinetic processes occur at rates quite different from those that occur at room temperatures or elevated temperatures, providing new opportunities for achieving metastable structures.
COMMON THEMES
Two important themes cut across all four of the elements of materials science and engineering. The first is the importance of instrumentation in performing and controlling synthesis and processing; characterizing structure, composition, and properties; and analyzing performance (see Appendix D). The second is the increasing importance of analysis and modeling (see Appendix E), which, together with increased computational abilities and judicious experimentation, are helping to make materials science and engineering increasingly quantitative.
Instrumentation
The instrumentation used in materials science and engineering has become increasingly sophisticated and expensive. The cost of analytical instruments such as electron microscopes ranges from $50,000 to $850,000, and the cost of dedicated process equipment, such as MBE and ion implantation equipment, is in the range of $1 million to $2 million. Furthermore, these costs are rising rapidly, at well above the rate of inflation. With the increasing use of powerful computers to simulate the synthesis and processing, structure and composition, properties, and performance of materials, instrumentation has become important for theoretical as well as experimental materials research. Cost-effectiveness rules out having such facilities at every institution, making new sharing mechanisms a necessity.

FIGURE 4.9 Scanning electron microscope micrograph of an electrochemically produced copper-nickel compositionally modulated alloy grown on a (111) copper single crystal with the layer thickness continuously graded from 15 nm to 1500 nm. This gradation in layer thickness results in gradations in those properties—such as flow stress, hardness, corrosion resistance, magnetic properties, and wear resistance—that depend on the layer thickness. (Courtesy National Institute of Standards and Technology.)
Low levels of research support during the past decade and a half, as described in Chapter 6, have made it impossible for universities to modernize their facilities or even to maintain and use existing facilities effectively. As a result, much of the equipment used for basic research in materials science and engineering is quite old and not always fully functional. According to the 1986 report of the White House Science Council’s Panel on the Health of U.S. Colleges and Universities, more than 45 percent of the instrument systems for materials science at universities are more than 10 years old, about 20 percent are between 6 and 10 years old, and about 35 percent are from 1 to 5 years old. In fact, the instrument systems for materials science were older than the systems for any other scientific area sampled. This agedness of materials research equipment in universities is a major problem.
Very recently, the National Science Foundation (NSF), the Department of Energy (DOE), and the Department of Defense (DOD) have undertaken special initiatives to upgrade instrumentation in materials research. In FY1987, the NSF spent almost 17 percent of its budget for research facilities, and its division of materials research devoted about 22 percent of its budget—or about $22 million—to these purposes. Other agencies are also devoting an increasing fraction of their resources to instrumentation. Yet these amounts are dwarfed by the magnitude of the need. According to the aforementioned White House panel study, the materials research community in universities requires $100 million to $400 million in new instrumentation to replace aging equipment and to address new opportunities.
In addition to the need for instrumentation to support ongoing materials research at universities, there is a pressing need in the United States for the development of new instrumentation. Increasingly, the development of new instruments is taking place in foreign countries. American laboratories now depend primarily on foreign suppliers for various types of essential equipment, ranging from apparatus used for growing crystals to electron microscopes and superconducting magnets used in a variety of spectroscopic and low-temperature measurements. In these areas of research, more instrument companies exist in other countries than in the United States, and these companies work more closely with government laboratories and universities. In these same areas, national laboratories abroad also play a much more important role in instrument development than they do in the United States.
A particular example from surface science is relevant. Twenty years ago, the United States was the dominant player in the development of surface science instrumentation, as demonstrated by development of the Auger spectrometer and the low-energy electron diffraction display system. Universities, industrial laboratories, and instrument companies were all actively involved in the development of such instruments. Since then, the United States has lost almost completely the dominance it once enjoyed. Innovative designs or unique applications of instruments are still being pursued in the United
States, but these developments are not being converted into commercial instruments. The commercial development of instrumentation is occurring increasingly in foreign laboratories able to sustain complicated and expensive instrument development programs.
Another important example involves processing equipment. Research on processing equipment in many technologies related to materials science and engineering has subsided in the United States, with a commensurate loss of equipment markets to foreign competition. Notable examples include the markets for machine tools and semiconductor processing equipment. Yet a leading position in materials processing technology requires leadership capability in the machinery and equipment sector and a close collaboration with materials processing. Figure 4.10, a scanning electron micrograph of polystyrene, illustrates the use of advanced instrumentation to explore materials morphology and growth processes. Figure 4.11, which shows fiber pullout and bridging across a crack in a fiber-reinforced resin, illustrates the importance of microscopic analysis in performance research.

FIGURE 4.10 Scanning electron microscope micrograph of rosettelike crystalline aggregates of isotactic polystyrene grown from dilute polymer solutions. The splaying-layered morphology of these objects has a direct bearing on aspects of the growth processes that govern the morphological evolution of spherulites of this polymer grown from a molten state. (Courtesy National Institute of Standards and Technology.)

FIGURE 4.11 Fiber pullout and bridging across a crack in a fiber-reinforced resin. (Courtesy E.I.du Pont de Nemours & Co., Inc.)
Federal funding agencies have not made instrument development a prominent component of their programs. The Division of Materials Research at the NSF spends only about 1 percent—about $1 million per year—for the development of new instrumentation. The DOE supports instrument development only on a limited, project-specific scale, and the DOD has no documented case of instrument development in materials science having been funded through its University Research Initiative program. This neglect of instrument development ignores the research leverage resulting from early access to novel equipment.
Several consequences are likely if instrument development is not pursued in American universities. First, the lag between demonstration of a new instrument and its transfer into American research or industrial laboratories will increase. In surface science, there is often a 5- to 10-year lag between an instrument’s demonstration in a foreign laboratory and its availability in the United States from a commercial instrument company. Second, fewer students will be trained in instrument development. Third, foreign companies will command an increasing portion of the commercial instrument business, especially for small, specialized equipment.
Many scientists and administrators contend that instrument development is not compatible with the operation of American universities. The period for promotion to tenure might be too short for young scientists embarking on a difficult instrument development program, and a project might require more time than a graduate student’s education. But these problems can be overcome, as demonstrated by laboratories in Europe and Japan and by different departments (such as high-energy physics, astrophysics, or biology) within the American university system.
The potential for development of new instrumentation is particularly great at present. For instance, materials processing equipment could combine computer control of such technologies as MBE, plasma deposition, and particle beam lithography. Synergistic combinations of emerging technologies could give birth to new and exciting instruments.
Analysis and Modeling
Recent advances in theoretical understanding and computational ability are changing the nature of materials science and engineering. Two complementary forces are driving these changes. The first is the unprecedented speed, capacity, and accessibility of computers. Problems in mathematics, data analysis, and communication that seemed untouchable just a few years ago can now be solved quickly and reliably with modern computational systems. The second is the growing complexity of materials research. The latter change has occurred in large part because instruments are now available to make highly detailed and quantitative measurements and because the computational ability exists to deal with the resulting wealth of data. Underlying all of these developments are advances in the theoretical understanding of materials properties and the mathematical ability to devise accurate numerical simulations.
Analysis and modeling find application in each of the four elements of materials science and engineering. For instance, in performance research, advances in analysis and modeling have made it possible to develop quantitative methods for solving some of the major problems in the field. In particular, accurate codes may become available to enable reliable predictions of the lifetimes of structural materials in service. Such observations also apply to the application of analysis and modeling in synthesis and processing and in characterization of the structures, compositions, and properties of materials. An example of computer analysis of copolymer systems is shown in Plate 4.
Analysis and modeling in materials research traditionally have been divided into roughly three different areas of activity. The most fundamental models—those used primarily by condensed-matter physicists and quantum chemists—deal with microscopic length scales, where the atomic structure of materials plays an explicit role. Much of the most sophisticated analysis is carried out at intermediate length scales, where continuum models are appropriate. Fi-
nally, modeling of complex industrial processes aids in improving process reliability and control, and in reducing an important aspect of performance—the initial cost of the material or components. There is work done at macroscopic length scales in which the bulk properties of materials are used as inputs to models of manufacturing processes and performance. Historically, research in each of these three areas has been carried out by separate communities of scientists. However, modern developments are tending to blur the distinctions between these research areas.
The need for advanced analysis and modeling provides an especially clear argument in favor of unified support for materials research. For example, in the past it has sometimes been difficult for managers of engineering programs to support accurate experimental or theoretical investigations of simple model systems; both the models and the precision appeared to be irrelevant for technological purposes. At the same time, many such projects have seemed scientifically uninteresting in the absence of a technological motivation. It is now apparent, however, that the simulations needed for advanced applications may make no sense without scientific input. Carefully controlled measurements, critically evaluated data, and calculations are essential to test the basic assumptions being built into simulations of complex situations. Moreover, the problems often turn out to be unexpectedly challenging from a scientific point of view.
In the future, science-based numerical simulations in combination with new methods for storing, retrieving, and analyzing information may make it possible to optimize not only the properties of specific substances but also entire processes for turning raw materials into useful objects. Materials considerations are important throughout the life cycles of most products—from design through manufacturing to support and maintenance and, finally, to disposal or recycling. Significant improvements in quality, reliability, and economy might be realized at all stages of this cycle if quantitative models of processing and performance could be applied throughout this process.
FINDINGS
Materials science and engineering deals with the synthesis and processing, structure and composition, properties, and performance of materials and with the interrelationships among these elements of the field. It encompasses all materials classes. To a degree unique among technical fields, it gains great strength from drawing on the full spectrum of science and engineering.
-
In evaluating the status of materials science and engineering, it is important to recognize not only that each of the elements of the field must be strong, but also that the elements of the field are increasingly interdependent. Only by viewing the field as a coherent whole can the interrelationships among its elements be distinguished and strengthened.
-
Synthesis and processing is an element of materials science and engineering offering great promise as new techniques for the preparation of materials enable nearly atom-by-atom synthetic flexibility. It is an element of special importance also, because it bears directly on questions of industrial productivity and international competitiveness. However, the United States suffers from a serious weakness in synthesis and processing, particularly in that aspect required to translate scientific promise into commercial success: process technology.
-
Structure and composition can now be characterized with unprecedented accuracy and resolution. The challenge is to give practitioners of materials science and engineering broad access to the increasingly expensive equipment—including major national facilities—required to perform this characterization.
-
Unprecedented variability of materials properties is now available in new materials as a result of research in the areas of synthesis and processing and of properties, offering designers almost unlimited variability in design choices. Often, use of such new materials is limited by the lack of evaluated design data on their properties.
-
The performance of materials in actual systems depends on their response to the combined stimuli of stress in various forms, including mechanical and electromagnetic stress. The integration of understanding about materials performance and the design of devices and systems for full life cycles is an emerging phenomenon in industry that needs to be reflected in programs at universities and federal laboratories—where only limited activities can be identified today.
-
Instrumentation for characterization and processing of materials is an increasingly important issue in materials science and engineering. Replacement of aging equipment, acquisition of new equipment, particularly for research on process technology, and funding for research on and development of new equipment are areas that deserve increased attention by funding agencies.
-
Analysis and modeling find application in each of the four elements of materials science and engineering. High-speed computation has ushered in a new era in the use of these techniques, from the design of new materials to their ultimate use in products. Significant improvements in quality, reliability, and economy are the promise offered by increased application of analysis and modeling to all phases of materials science and engineering.