BEYOND DISCOVERY
THE PATH FROM RESEARCH TO HUMAN BENEFIT™
This article was adapted by Ron Cowen from an article written by F. Sherwood Rowland for Beyond Discovery: The Path from Research to Human Benefit™, a project of the National Academy of Sciences. The Academy, located in Washington, D.C., is a society of distinguished scholars engaged in scientific and engineering research, and dedicated to the use of science and technology for the public welfare. For over a century, it has provided independent, objective scientific advice to the nation. Visit our Web site, http://www2.nas.edu/bsi
© 1996by theNational Academy of Sciences
bsi@nas.edu(202) 334-1575
April 1996
THE OZONE DEPLETION PHENOMENON
Like an infection that grows more and more virulent, the continent-size hole in Earth's ozone layer keeps getting bigger and bigger.
Each year since the late 1970s, much of the protective layer of stratospheric ozone above Antarctica has disappeared during September, creating what is popularly known as the ozone hole. The Antarctic hole now measures about 9 million square miles, nearly the size of North America. Less dramatic, but still significant, depletion of ozone levels has been recorded around the globe. With less ozone in the atmosphere, more ultraviolet radiation strikes Earth, causing more skin cancer, eye damage, and possible harm to crops.
What is ozone? How did researchers discover its role in Earth's atmosphere and the devastating consequences of its depletion? The following article, adapted from an account by Dr. F. Sherwood Rowland, a pioneering researcher in the field who shared the 1995 Nobel Prize in Chemistry for his work, attempts to answer these and other questions. In doing so, it dramatically illustrates how science works and, in particular, how basic research—motivated by a desire to understand nature—often leads to practical results of immense societal benefit that could not have been anticipated when the research first began.
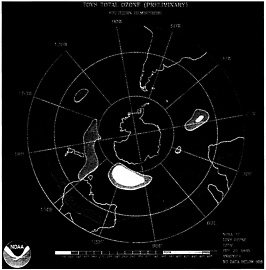
A satellite image of the ozone hole (pink area) over Antarctica taken on September 25, 1995.
The Problem
For four months of every year, Antarctica's McMurdo Research Station lies shrouded in darkness. Then the first rays of light peek out over the horizon. Each day, the sun lingers in the sky, just a little longer and the harsh polar winter slowly gives way to spring.
Spring also brings another type of light to the Antarctic, a light that harms instead of nurtures. In this season of new beginnings, the hole in the ozone layer reforms, allowing lethal ultraviolet radiation to stream through Earth's atmosphere.
The hole lasts for only two months, but its timing could not be worse. Just as sunlight awakens activity in dormant plants and animals, it also delivers a dose of harmful ultraviolet radiation. After eight weeks, the hole leaves Antarctica, only to pass over more populated areas, including New Zealand and Australia. This biologically damaging, high-energy radiation can
cause skin cancer, injure eyes, harm the immune system, and upset the fragile balance of an entire ecosystem.
Although, two decades ago, most scientists would have scoffed at the notion that industrial chemicals could destroy ozone high up in the atmosphere, researchers now know that chlorine creates the hole by devouring ozone molecules. Years of study on the ground, in aircraft, and from satellites has conclusively identified the source of the chlorine: human-made chemicals called chlorofluorocarbons (CFCs) that have been used in spray cans, foam packaging, and refrigeration materials.
All About Ozone
Ozone is a relatively simple molecule, consisting of three oxygen atoms bound together. Yet it has dramatically different effects depending upon its location. Near Earth's surface, where ozone comes into direct contact with life forms, it primarily displays a destructive side. Because it reacts strongly with other molecules, large concentrations of ozone near the ground prove toxic to living things. At higher altitudes, where 90 percent of our planet's ozone resides, it does a remarkable job of absorbing ultraviolet radiation. In the absence of this gaseous shield in the stratosphere, the harmful radiation has a perfect portal through which to strike Earth.
Although a combination of weather conditions and CFC chemistry conspire to create the thinnest ozone levels in the sky above the South Pole, CFCs are mainly released at northern latitudes—mostly from Europe, Russia, Japan, and North America—and play a leading role in lowering ozone concentrations around the globe.
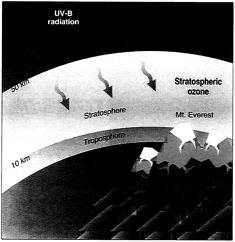
Stratospheric ozone occupies the region of the atmosphere between 10 and 50 kilometers from Earth's surface and provides a shield against damaging ultraviolet radiation.
Worldwide monitoring has shown that stratospheric ozone has declined for at least two decades, with losses of about 10 percent in the winter and spring and 5 percent in the summer and autumn in such diverse locations as Europe, North America, and Australia. Researchers now find depletion over the North Pole as well, and the problem seems to be getting worse each year. According to a United Nations report, the annual dose of harmful ultraviolet radiation striking the northern hemisphere rose by 5 percent during the past decade.
Although, two decades ago, most scientists would have scoffed at the notion that industrial chemicals could destroy ozone high up in the atmosphere, researchers now know that chlorine creates the hole by devouring ozone molecules.
During the past 40 years, the world has seen an alarming increase in the incidence of malignant skin cancer; the rate today is tenfold higher than in the 1950s. Although the entire increase cannot be blamed on ozone loss and increased exposure to ultraviolet radiation, there is evidence of a relationship. Scientists estimate that for each 1 percent decline in ozone levels, humans will suffer as much as a 2 to 3 percent increase in the incidence of certain skin cancers.
Exploring Earth's Atmosphere
Like many lines of scientific inquiry, research leading to the prediction and discovery of global ozone depletion and the damaging effects of CFCs followed a path full of twists and turns. Investigators did not set our to determine whether human activity affects our environment nor did they know much about chemical pollutants. Instead, they began with basic questions about the nature of Earth's atmosphere—its composition, density, and temperature distribution.
The composition of our planet's atmosphere fascinated humans long before chemistry became a formal science. “The storm thundered and lightened, and the air was filled with sulfur,” Homer wrote in the Odyssey, referring to the sharp odor, created during thunderstorms, of what later became known as ozone.
By the late 1800s, atmospheric scientists had isolated carbon monoxide and inferred the existence of a second combustible gas in the air, which they tentatively identified as methane, the simplest hydrocarbon. But in attempting to further analyze the composition of the atmosphere, researchers at the turn of the century faced a major stumbling block: virtually all gases, except for molecular nitrogen and oxygen, exist in such minute concentrations that available equipment could not detect them.
Help, however, was on the way. During the 1880s, scientists had begun perfecting a new, highly precise method of identifying a compound by recording a special kind of chemical fingerprint—the particular pattern of wavelengths of light it emits or absorbs. Scientists call this pattern a spectrum.
In the 1920s, G.M.B. Dobson developed a spectrometer that could measure small concentrations of ozone. By measuring the spectrum of air, the Belgian scientist M.V. Migeotte demonstrated in 1948 that methane is a common constituent of the atmosphere with a concentration of about one part per million by volume. Soon, scientists had the tools to detect other atmospheric gases that occur in concentrations one-tenth to one-hundredth as great as that. By the 1950s, researchers had identified 14 atmospheric chemical constituents.
Despite this progress, researchers were still missing a major piece of the atmospheric puzzle. All of the compounds detected possessed an even number of electrons, a characteristic which typically gives chemical stability. Other less common compounds with an odd number of electrons—known as free radicals—readily undergo chemical reactions and do not survive for long. These compounds play crucial roles in such phenomena as urban smog, the loss of stratospheric ozone, and the global removal of atmospheric impurities.
Scientists had not detected free radicals because they reside in the atmosphere at concentrations well below the part-per-million level that state-of-the-art equipment in 1948 could detect. But unrelated research in an entirely different field, analytical chemistry, soon came to the rescue. Analytical chemists had begun developing a cavalcade of new instruments and methods to measure minute quantities of compounds in the laboratory.
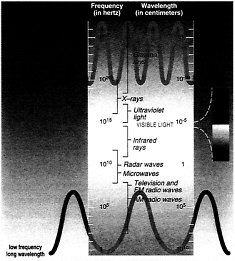
Cancer-causing ultraviolet radiation occupies the region of the spectrum between visible light and even higher frequency radiation such as x-rays and gamma rays.
Such research spurred advances on two fronts: a substantial increase in the precision and accuracy of measurements of atmospheric gases and a striking decrease in the minimum concentration of a compound that must be present to be detected. As a result, the number of atmospheric compounds identified by scientists has increased from 14 in the early 1950s to more than 3,000 today. Detectors today routinely measure compounds at concentrations below one part per trillion, and some can record gases that occur in concentrations one-thousandth as great at that.
The hole lasts for only two months, but its timing could not be worse. Just as sunlight awakens activity in dormant plants and animals, it also delivers a dose of harmful ultraviolet radiation.
Even in some of the most remote locations on Earth, scientists have detected hundreds of compounds. Curiously, some of the substances that occur in the smallest concentrations rank as some of the biggest players in altering the atmosphere. A case in point: the group of chemicals known as CFCs.
Enter the CFCs
CFCs were invented about 65 years ago during a search for a new, nontoxic substance that could serve as a safe refrigerant. One of these new substances, often known by the DuPont trademark Freon, soon replaced ammonia as the standard cooling fluid in home refrigerators. It later became the main coolant in automobile air conditioners.
The 1950s and 1960s saw CFCs used in a variety, of other applications: as a propellant in aerosol sprays, in manufacturing plastics, and as a cleanser for electronic components. All this activity doubled the worldwide use of CFCs every six to seven years. By the early 1970s, industry used about a million tons every year.
Yet as recently as the late 1960s, scientists remained unaware that CFCs could affect the atmosphere. Their ignorance was not from lack of interest, but from lack of tools. Detecting the minuscule concentrations of these compounds in the atmosphere would require a new generation of sensitive detectors.
After developing such a detector, the British scientist James Lovelock, in 1970, became the first to detect CFCs in the air. He reported that one of these compounds, CFC-11, had an atmospheric concentration of about 60 parts per trillion. To put that measurement in perspective, the concentration of methane (natural gas) is 25,000 times greater. Twenty years earlier, merely detecting methane had been considered a major feat.
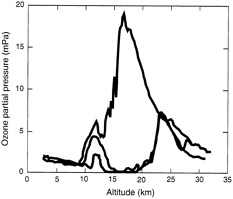
Ozone loss over the South Pole in 1995 (in green) compared with 1993 (in red). The blue line shows values before ozone destruction began.
SOURCE. National Oceanic and Atmospheric Administration.
Lovelock found CFC-11 in every air sample that passed over Ireland from the direction of London. That was not surprising, because most major cities, including London, widely used CFCs. However, Lovelock also detected CFC-11 from air samples
Advances in Atmospheric Science and Policy Decisions Through 1996
This timeline shows the chain of events leading to prediction of the ozone depletion phenomenon, recognition of its consequences, and eventual actions to avert a threatened disaster. It is rich in examples of how basic research often contributes to unanticipated outcomes of immense societal benefit.
1840
Christian Friedrich Schönbein identifies ozone as a component of the lower atmosphere and names it.
1881
W.N. Hartley identifies ozone as the substance that absorbs ultraviolet radiation from the sun at wavelengths below 290 nanometers. He also shows that ozone resides primarily at high altitudes.
1913–1932
C. Fabry and M. Buisson show that the total amount of ozone in a vertical column of the atmosphere can be measured and that it equals (in modern units) 300 Dobson units.
1924
G.M.B. Dobson sets up a regular program of ozone measurements at Oxford with his newly developed spectrophotometer.
1930
Sydney Chapman explains how sunlight striking molecular oxygen in the atmosphere generates ozone.
1957
As part of the International Geophysical Year, four to five research stations in Antarctica begin making regular ozone measurements.
1970
The Nimbus series of satellites begins making ozone measurements.
1970
James Lovelock uses his electron capture detector to measure chlorofluorocarbons(CFCs).
1973
Richard Stolars and Ralph Cicerone disco stratospheric chlorine chain reaction.
directly off the North Atlantic, uncontaminated by recent urban pollution.
This unexpected discovery prompted Lovelock to do further studies. Accordingly, he asked the British government for a modest sum of money to place his apparatus on board a ship traveling from England to Antarctica. His request was rejected; one reviewer commented that even if such a measurement succeeded, he could not imagine a more useless bit of knowledge than finding the atmospheric concentration of CFC-11.
But Lovelock persisted. Using his own money, he put his experiment aboard the research vessel Shackleton in 1971. Two years later the British researcher reported that his shipboard apparatus had detected CFC-11 in every one of the more than 50 air samples collected in the North and South Atlantic. Lovelock correctly concluded that the gas was carried by large-scale wind motions. He also stated that CFCs were not hazardous to the environment, a conclusion soon to be proven wrong.
Ozone Loss: The Chemical Culprits
In 1972, the life of atmospheric scientist F. Sherwood Rowland took a critical turn when he heard a lecture describing Lovelock's work. Like other researchers at the time, Rowland had no inkling that CFCs could harm the environment, but the injection into the atmosphere of large quantities of previously unknown compounds piqued his interest. What would be the ultimate fate of these compounds? Rowland, joined by Mario Molina, a colleague at the University of California, Irvine, decided to find out.
The scientists showed that CFCs remained undisturbed in the lower atmosphere for decades. Invulnerable to visible sunlight, nearly insoluble in water, and resistant to oxidation, CFCs display an impressive durability in the atmosphere's lower depths. But at altitudes above 18 miles, with 99 percent of all air molecules lying beneath them, CFCs show their vulnerability. At this height, the harsh, high-energy ultraviolet radiation from the sun impinges directly on the CFC molecules, breaking them apart into chlorine atoms and residual fragments.
If Rowland and Molina had ended their CFC study with these findings, no one other than atmospheric scientists would ever have heard about it. However, scientific completeness required that the researchers explore not only the fate of the CFCs, but also of the highly reactive atomic and molecular fragments generated by the ultraviolet radiation.
In examining these fragments, Rowland and Molina were aided by prior basic research on chemical
74
Sherwood Rowland and Mario Molina discover that CFCs can destroy ozone the stratosphere.
1976
The National Academy of Sciences releases its report verifying the Rowland-Molina finding.
1976
The Food and Drug Administration and the Environmental Protection Agency announce a phase-out of CFCs in aerosols.
1978
CFCs used in aerosols are banned in the United States.
1984
A British research group led by Joseph Farman detects a 40 percent ozone loss over Antarctica during spring in the southern hemisphere.
1985
NASA satellite data confirm the existence of the ozone hole over the Antarctic.
1987
The Montreal Protocol is signed, calling for eventual worldwide CFC reduction by 50 percent.
1988
The United States ratifies the Montreal Protocol in a unanimous vote.
1988
Scientists present preliminary findings of a hole in the ozone layer over the Arctic.
1996
Complete ban on industrial production of CFCs goes into effect.
1995
F. Sherwood Rowland, Mario Molina, and Paul Crutzen awarded the Nobel prize for their work in atmospheric chemistry.
kinetics—the study of how quickly molecules react with one another and how such reactions take place. Scientists had demonstrated that a simple laboratory experiment will show how rapidly a particular reaction takes place, even if the reaction involves the interaction of a chlorine atom with methane at an altitude of 18 miles and a temperature of −60 degrees Fahrenheit.
Rowland and Molina did not have to carry out even a single laboratory experiment on the reaction rates of chlorine atoms. They had only to look up the rates already measured by other scientists. Basic research into chemical kinetics had reduced a decade's worth of work to two or three days.
After reviewing the pertinent reactions, the two researchers determined that most of the chlorine atoms combine with ozone, the form of oxygen that protects Earth from ultraviolet radiation. When chlorine and ozone react, they form the free radical chlorine oxide, which in turn becomes part of a chain reaction. As a result of that chain reaction, a single chlorine atom can remove as many as 100,000 molecules of ozone.
Unknown to Rowland and Molina, the same chlorine atom chain reaction had been discovered a few months earlier by Richard Stolarski and Ralph Cicerone. In 1974, Rowland and Molina made a disturbing prediction: If industry continued to release a million tons of CFCs into the atmosphere each year, atmospheric ozone would eventually drop by 7 to 13 percent.
Even in some of the most remote locations on Earth, scientists have detected hundreds of compounds. Curiously, some of the substances that occur in the smallest concentrations rank as some of the biggest players in altering the atmosphere.
To make matters worse, other scientists had demonstrated that an entirely different group of compounds could further reduce ozone levels. Paul Crutzen first showed in 1970 that nitrogen oxides react catalytically with ozone, playing an important role in the natural ozone balance. Soil-borne microorganisms produce nitrogen oxides as a decay product, and Crutzen's work spotlighted how microbe-rich agricultural fertilizers might lead to reduced ozone levels. His research and that of Harold Johnston also focused attention on the effect of nitrogen oxides spewed by high-altitude aircraft. These emissions may also reduce ozone levels in the stratosphere.
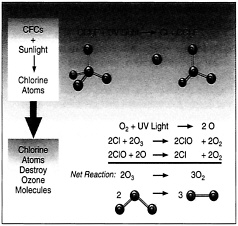
Chlorine atoms induce the decomposition of two ozone molecules into three oxygen molecules in a net chain reaction in which the chlorine atoms are regenerated so that decomposition of ozone continues.
Earlier studies, which had investigated whether exhaust emissions from the supersonic transport and other high-speed aircraft could damage the environment, had already begun to document the effects of ozone loss. Compiled because of the perceived threat from these aircraft, the data were brought to bear on the very real threat from CFCs and nitrogen oxides.
With less ozone in the atmosphere, more ultraviolet radiation reaches Earth. Scientists estimated that increased exposure would lead to a higher incidence of skin cancer, cataracts, and damage to the immune system and to slowed plant growth. Because some CFCs persist in the atmosphere for more than 100 years, these effects would last throughout the twenty-first century.
Concluding that such long-term hazards were unacceptable, Rowland and Molina called for a ban on further release of CFCs. Alerted to this clear and present danger, the United States, Canada, Norway, and Sweden in the late 1970s banned the use of CFCs in aerosol sprays.
The Ozone Hole Emerges
As it turned out, the ozone problem was far worse than Rowland and Molina could have imagined. The first warning signs of a bigger crisis did not appear until the late 1970s, but the studies that uncovered these findings had their roots in research dating back nearly a century.
In the 1880s, W.N. Hartley discovered that a broad band of ultraviolet light reaches Earth almost unimpeded. This band, known as UV-A, has wavelengths just slightly shorter than ordinary visible light. The ozone layer partly absorbs another ultraviolet band, known as UV-B, before it can reach Earth. During the 1920s, G.M.B. Dobson managed to measure the ratio of UV-A to UV-B in incoming sunlight. By doing so, he determined for the first time the total amount of ozone in the atmosphere.
Dobson had hoped his study would lead to a new method of predicting the weather. Instead, he became interested in the seasonal variations in ozone concentrations. An instrument that he developed, the Dobson spectrometer, has become the standard for monitoring ozone from the ground.
When chlorine and ozone react, they form the free radical chlorine oxide, which in turn becomes part of a chain reaction. As a result of that chain reaction, a single chlorine atom can remove as many as 100,000 molecules of ozone.
The rapid development of new scientific tools after World War II—many of them based on wartime instrumentation—led to a flowering of studies in earth science. In 1957–1958, this led to a worldwide scientific effort known as the International Geophysical Year (IGY). IGY sparked an international outpouring of research on the oceans, the atmosphere, and unexplored land areas of the planet.
Monitoring ozone levels in the south polar region, researchers found them to be consistently about 35 percent higher in late spring than in winter. Annual monitoring showed the same seasonal pattern through the late 1970s.
But in 1978 and 1979, the British scientists found something different. In October, the beginning of spring in the southern hemisphere, the researchers detected less ozone than had been detected during the past 20 years. During the next several years, October ozone levels continued to decline.
In 1984, when the British first reported their disturbing findings, October ozone levels were about 35 percent lower than the average for the 1960s. The U.S. satellite Nimbus-7 quickly confirmed the results, and the term Antarctic ozone hole entered popular language.
The Evidence Mounts
By the mid 1980s, scientists had become expert in measuring the concentration of chlorine-containing compounds in the stratosphere. Some monitored the compounds from the ground; others used balloons or aircraft. In 1986 and 1987, these scientists, including Susan Solomon and James Anderson, established that the unprecedented ozone loss over Antarctica involved atomic chlorine and chlorine oxide radicals.
At the same time, measurements in the lower atmosphere established that CFC levels had increased steadily and dramatically since the first recordings taken by Lovelock in 1970. The conclusion was clear: The prime sources of the ozone-devouring chlorine atoms over Antarctica were the CFCs and two other pollutants, the industrial solvents carbon tetrachloride and methylchloroform.
A satellite operated by the National Aeronautics and Space Administration appears to have removed any possible doubt about the role of CFCs. Data collected over the past three years by the Upper Atmosphere Research Satellite revealed these compounds in the stratosphere. Moreover, the satellite has traced the worldwide accumulation of stratospheric fluorine gases, a direct breakdown product of CFCs. The quantitative balance of CFCs and its products eliminates the possibility that chlorine from volcanic eruptions or other natural sources created the ozone hole.

The Outcome: Potential Catastrophe Averted
Painstaking research on ozone and the atmosphere over the past 40 years has led to a global ban on CFC production. Since 1987, more than 150 countries have signed an international agreement, the Montreal Protocol, which called for a phased reduction in the release of CFCs such that the yearly amount added to the atmosphere in 1999 would be half that of 1986. Modifications of that treaty called for a complete ban on CFCs which began in January 1996. Even with this ban in effect, chlorine from CFCs will continue to accumulate in the atmosphere for another decade. It may take until the middle of the next century for ozone levels in the Antarctic to return to 1970s levels.
More globally, ozone depletion is expected to remain a fact of life for several decades to come, but thanks to the research that led to early recognition of the problem and steps that have been taken to address it, the potential consequences are much less severe than they otherwise would have been.
Scientists estimate, for example, that if active research in stratospheric chemistry had not been in place at the time the ozone hole was discovered in 1985 and confirmed in 1986, global ozone depletion, measuring 4 percent today, would be close to 10 percent by the year 2000. Even larger ozone depletion would have been observed over the United States and Eastern Europe, substantially exceeding the current measurements there of about 10 percent loss in winter and spring and 5 percent in summer and autumn. These larger losses have been avoided because basic research in the atmospheric sciences had already advanced to a level where it was able to explain the chemical reactions occurring in the ozone layer. That knowledge allowed other informed political and regulatory decisions to be made
In 1995, the Royal Swedish Academy of Sciences awarded Rowland, Molina, and Crutzen the Nobel prize in chemistry for showing “how sensitive the ozone layer is to the influence of anthropogenic emissions of certain compounds.” In explaining the chemical mechanisms that affect the thickness of the ozone layer, “the three researchers have contributed to our salvation from a global environment problem that could have catastrophic consequences,” the academy noted.
As lawmakers and the public face new challenges in the struggle to protect the environment, they will increasingly rely on basic research to open new vistas and suggest new solutions about these pressing concerns.








