2  Technology in Daily Life
Technology in Daily Life
Here is a brief story about life today in the United States. It is fiction, but millions of episodes like it occur every day. Each event involves familiar technologies whose present state of development—or very existence— would have seemed extraordinary just a generation ago. The capitalized phrases in the story are links to sidebars on the facing pages that provide more information about some of these technologies.
The owner of a small business is driving her car to the airport. Many structural elements of her car and the airplane that she will be boarding are products of research in MATERIALS SYNTHESIS. She is on the way to visit a potential customer. As a seasoned business traveler, she has with her all the tools she needs for her normal daily business. She picks up her cellular telephone and dials her son's pager. The communications revolution represented by the telephone and the pager has been greatly enhanced by advances in COMPOUND SEMICONDUCT ELECTRONICS.

The woman's son is a college student who, at that moment, is rollerblading across the campus, listening to a compact disk that he has just recorded in his music course. His rollerblades are light and strong and run smoothly because of advances in the physics and chemistry of POLYMERS. The compact disk, containing over an hour of high-fidelity music, is a miracle in the development of OPTICAL STORAGE MATERIALS. The crucial component of the student's portable CD player is a SEMICONDUCTOR LASER.

Feeling the vibration of his pager, the student turns off his CD player and hurries to his dorm room to return his mother's call. The message is about his grandmother, who has been hospitalized following an accident. A CAT scan has indicated that she needs a hip replacement. Artificial bone replacements are possible because of research in BIOMATERIALS.
Fortunately, an additional MRI scan has ruled out any spinal injury. Magnetic resonance imaging (MRI) depends on SUPERCONDUCTING MAGNETS.
The attending physician believes that the surgery may be more complex than usual, so he has arranged a video consultation with a specialist in another part of the country before operating. Videoconsultation is an example of today's growing use of OPTICAL FIBERS for telecommunication.
The surgery has been successful, and the patient is resting comfortably.

The woman reaches the airport, parks her car, goes through security to the gate, and boards her flight. Only minutes after takeoff, her airplane is cruising at 500 miles per hour at an altitude of 30,000 feet. It will fly across the continent without refueling.
To achieve such power and efficiency, the turbine blades in modern jet engines must operate at very great speeds and high temperatures. To withstand such extreme conditions, they are made of SUPERALLOYS.
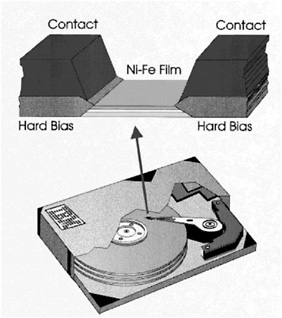
Once airborne, the businesswoman opens her laptop computer and reviews the presentation that she plans to make on her arrival. The computer 's monitor uses one of the new LIQUID CRYSTAL DISPLAY MATERIALS. Like almost every other technology in this story, the computer also depends on MAGNETIC MATERIALS.

|
SUPERALLOYS Superalloys are special combinations of metals that maintain high strength during prolonged exposure to elevated temperatures. This capability is essential for applications such as the turbine blades in a jet engine. Superalloys consist mostly of nickel, with smaller amounts of aluminum,titanium, chromium,and up to ten other elements. The idea behind the design of these alloys is the creation of stable, hard, small precipitates like Ni3Al or Ni3Ti in the nickel matrix to obstruct the motion of dislocations, the atomic-scale cause of undesirable deformation. The performance of alloys in turbine blades is further improved by eliminating crystal boundaries in each blade; those boundaries are the prime sites for the initiation of fracture. Through the development of a detailed understanding of the solidification process, it is now possible to cast an entire turbine blade, with the very intricate shape shown in Figure 2.9 , as a single crystal. Progress in alloy design and processing has led to a continuing increase in the allowable operating temperature of the turbine blades. The most recent improvements have resulted from the application of ceramic thermal barrier coatings to the outside of the blade. Increases in the operating temperature, together with improvements in the blade design (such as air cooling, through the passages seen in Figure 2.9 , made possible by sophisticated casting techniques) have greatly increased the efficiency of jet engines and decreased their weight for a given thrust. 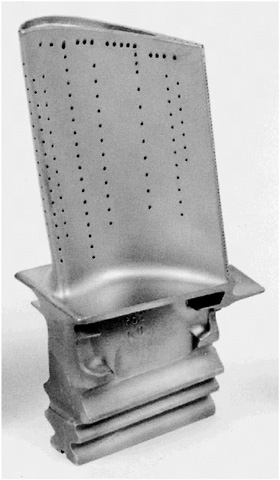 FIGURE 2.9 A turbine blade from a jet engine, cast as a superalloy single crystal. (Courtesy of GE Aircraft Engines.) |
The computer that analyzed the data from the MRI and CAT scans, and the computers that were involved in essentially every aspect of the airplane trip—making the reservations, assuring security at the airport, determining the flight path, and so on—are based on SILICON TECHNOLOGY.
Well into her flight and resting briefly before her important meeting, the woman relaxes in her seat and reflects on just how much of what she has taken for granted in the past several hours would have been unthinkable only a few years ago: modern cars and airplanes, telecommunications, modern medicine, the power and portability of computers. Our lives have been changed by CMMP research—and for the better.
She wonders what equally remarkable changes will take place during her son's lifetime. No one really knows, of course, but perhaps one hint can be found in new advances like INTEGRATED MICROSYSTEMS. Other ideas will arise from the scientific challenges discussed in Part 3. ![]()