B
ARGONNE NATIONAL LABORATORY RESPONSES TO QUESTIONS SUBMITTED ON NOVEMBER 3, 1995, BY THE COMMITTEE
Plutonium Disposition
-
What flow sheet is actually being proposed? On the basis of the presentations and information supplied, the committee is uncertain.
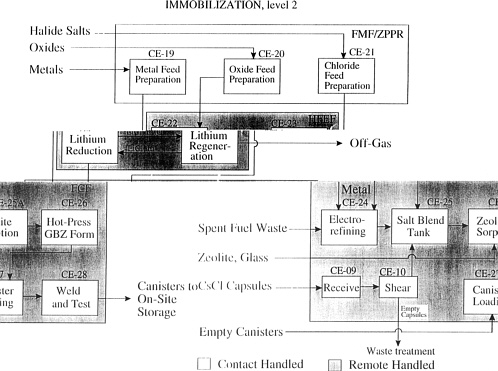
Two flow sheets were presented in the meeting; however, they are actually different levels of detail of the same flow sheet. Attached [p. 53] is the “Second-Level Flow sheet” that will appear in the “Alternative Team Technical Data Document”, Lawrence Livermore National Laboratory Report, L-20220-1, soon to be published. This “Second-Level Flow sheet” should be considered the actual proposed flow sheet for this process. The Plutonium Immobilization Task required that each option be presented as a “stand-alone” process, even though the Electrometallurgical Treatment Option was never presented as a sensible option in the absence of spent fuel treatment. The connection to the spent fuel treatment process is shown as an input of waste salt from the spent fuel treatment process. In fact, the plutonium immobilization process is visualized as being conducted in the same facilities, in the same electrorefiner, and at the same time as the spent fuel treatment. The waste salt input shown in the flow sheet is actually within the same electrorefiner vessel. The important features of this waste salt are the fission products from the spent fuel that contribute to the radiation source for meeting the “spent fuel standard”, and the UCl3 that helps convert metallic plutonium to PuCl3 for ion exchange with the zeolite.
-
What are the sources of the additional nuclides (e.g., EBR-II fuel, Cs-capsules, N-reactor fuel, etc.) that will be added to meet the “spent fuel standard”?
The sources of radioactivity for meeting the spent fuel standard will be obtained from treatment of spent fuels and from 137Cs from capsules now being stored at Hanford. The spent fuels assumed in the Plutonium Immobilization study include all those spent fuels now being stored at the Idaho National Engineering Laboratory (INEL), excluding the Naval spent fuel. This spent fuel is about 285 metric tons of heavy metal (MTHM).
-
Does the quantity of spent fuel to be processed match the quantity of plutonium to be disposed of through the electrometallurgical process?
Other than the spent fuel standard requirements (discussed below), there are no requirements concerning the ratio of uranium to plutonium to be processed. If UCl3 is used to convert plutonium metal to PuCl3, then the uranium-to-plutonium ratio must be at least unity, i.e., at least 50 MTHM of spent fuel would be required to process 50 MT plutonium. Otherwise, there is no need to match the spent fuel and plutonium quantities.
-
Is the radioactivity in the spent fuel commensurate with the amount of Pu to be denatured? Is there enough spent fuel in the DOE inventory? From what parts of the DOE inventory will it come?
No, there is not enough radioactivity. More radioactivity is needed than that available in the 285 MTHM mentioned above. The cesium capsules at Hanford will be used to supplement the radioactivity requirement. However, the “spent fuel standard” is not clearly defined. The radiation level associated with this standard has been variously specified as 100 R/h at 1 m and 1000 R/h at 1 m. The Savannah River glass concept is to use the radioactivity already present in the tank wastes, which may be even lower than the 100 R/h at 1 m specification, depending on
-
-
the amount of cesium that can be retained in the glass melter. So the quantity of cesium from the Hanford capsules, if any, that is needed for this process is not clear.
-
Is the half-life of the 137Cs long enough to permit the conclusion that the immobilization form meets the “spent fuel standard” if it is denatured with 137Cs instead of spent fuel?
The gamma radiation from spent fuel that has cooled more than 20 years is essentially all from decay of 137Cs (actually the 137mBa daughter). The second highest gamma source is 154Eu, which is only about 1 % of the 137Cs activity. All other significant radioactive isotopes are either alpha or beta sources. Therefore, the “spent fuel standard” really refers to 137Cs activity (after the first 20 to 30 years). The 137Cs activity (and the spent fuel standard activity) will decay away with a ˜30-year half-life.
-
Under what circumstances would it be necessary to add Cs, and how much Cs would be needed? Would sufficient Cs be available?
See the above discussion for the answers (and uncertainties) for the first part. The quantity of Cs required depends directly on the definition of the spent fuel standard. If the required gamma field is 1000 R/h at 1 m, we calculated that 20,000 Ci 137Cs would be required for each canister. The total activity of 137Cs available in the Hanford capsules is 54 MCi, so there is enough activity to protect about 2700 canisters. If the 50 MT plutonium were evenly divided among these 2700 canisters, there would be about 18.5 kg plutonium per canister.
The actual canister design, described in the above reference (L-20220-1), incorporates eight “ANL” canisters (9.0-in dia, 56.5-in tall) in a defense high-level waste (DHLW) canister. Each ANL canister would contain 6.5 kg Pu and 2500 Ci 137Cs, for a total of 52 kg Pu and 20,000 Ci 137Cs in the DHLW canister. Approximately 1000 such canisters will be needed to dispose of the 50 MT weapons-grade plutonium, so a total 137Cs activity of 20 MCi will be needed.
The gamma radiation from the spent fuel fission products could be used to reduce the number of cesium capsules needed, to reduce the amount of plutonium in each canister (by increasing the number of canisters), or to increase the gamma field around each canister. So, the answer to the question is: “Yes, there would be sufficient cesium available.”
-
If the proposed process is used ONLY for the EBR-II fuel, is there enough Cs to make the excess Pu meet the spent fuel standard?
The above discussion already answers this question. There is not enough activity in the spent fuel alone, in any case, but the radioactivity can be adequately supplied by use of the Cs from the Hanford capsules.
Waste Forms
-
Are there specific impurities (such as fluoride ion) that could adversely affect the zeolite structure and behavior?
Other than large quantities of fluoride ion at high temperatures, we have not seen adverse effects of any material on the zeolite structure. We have looked extensively for effects of rare-earth, uranium, and plutonium ions, but have seen none. Because the amount of fluoride expected in the waste is essentially nil, we have deferred the determination of the allowable fluoride level.
-
Are there specific impurities that adversely affect the performance of the glass matrix?
We have not seen any such effects, nor have we identified any materials that might be expected to have an adverse effect at “impurity” levels. In the course of waste form fabrication, the glass
-
does not become molten, although hot pressing does involve heating above the glass transition temperature for relatively short periods of time. The opportunity for incorporation of harmful major constituents (such as large quantities of chloride ion) is thus limited.
-
Are there specific impurities that adversely affect the metal waste form?
The metal waste form is composed of zirconium and stainless steel from the hulls as well as noble metal fission products and, in one variation of the flow sheet, transuranic elements. The metal waste form development task involves a systematic consideration of the (1) effects of changes in the ratios of the major constituents and (2) effects of noble metals and transuranics in various amounts up to and beyond those that would be found at any burnup level and at reasonable concentrations of the transuranics. The higher levels are being investigated to clarify effects that might not be seen at normal levels of concentration. No adverse effects have been seen to date, although the potential use of the metal waste form for transuranic disposition requires considerable further effort. Other materials of concern are inclusions of oxide slag and other foreign materials; because the waste form is not required to have mechanical properties typical of conventional structural materials, impurity or inclusion effects are not expected to be of major concern.
-
From committee discussions, it appears that there may be some chemical as well as physical changes to the zeolite during the vitrification process. What data are available?
This is a question to which we are devoting particular attention. No changes are seen by x-ray diffraction, but the sensitivity of this technique is limited to changes as large as 5-20%. Microscopy, short-term leach rate tests (including leachate analysis) for selected elements, density and porosity measurements are routine on all samples; thermogravimetric analysis and differential scanning calorimetry are being investigated for use in routine characterization as well. Selected samples receive more extensive characterization, including but not limited to electron microscopy and element mapping examinations, penetration porosimetry, and long-term leach rate/leachate testing. We believe that the leach tests are the best practical measure of the integrity of the structure, but we are continuing to seek better ways to characterize the waste form and to identify any possible changes associated with fabrication.
-
What permitting issues are anticipated with the several waste forms?
We believe that the metal and mineral waste forms must be fully qualified for repository disposal, just as the spent fuel and glass waste forms must be. The Waste Acceptance Systems Requirements Document identifies glass and spent fuel as “standard” waste forms. Although the mineral waste form meets the nominal composition requirements for a high-level waste glass, we are approaching the issue as though our waste forms must be qualified separately. In the case of the mineral waste form, this is due in part to the fact that the waste form has such a considerably lower waste volume per unit of fission product activity.
-
What process equipment configuration is planned for the zeolite loading operation?
Several zeolite columns (nominally three, at this time) will be employed in series, with replacement of the “upstream” column when it reaches maximum loading. The replacement column would be added at the “downstream ” end of the column set. The columns would have a large surface area/volume ratio (approximately 4-6 inches in diameter and 6 feet long, although prismatic configurations are being considered) to facilitate heat rejection.
-
If a column is being considered, what work has been done to determine the behavior of Pu in this type of configuration?
Initial measurements of plutonium distribution relative to the solvent and fission product ions have been made; plutonium is strongly retained (much like the rare-earth ions) and thus would concentrate at the head of the first column. If this is a criticality concern (not the case
-
-
with the EBR-II spent driver fuel) then the amount of material removed would have to be limited. Note that the column contents are homogenized and diluted with anhydrous zeolite in the hot blending operation prior to mixing with glass frit and hot-pressing.
-
Will the Pu concentrate as in conventional ion exchangers?
Yes. See (a) above.
-
What are the estimated radionuclide inventories (in either mass or activity of nuclide per gram of waste form) for the cladding-metal and zeolite waste forms from the different flow sheets being considered? (These nuclides, plus 90Sr and 137Cs, are those identified as relevant to safety in recent total system performance assessments for the Yucca Mountain repository.)
At this time, detailed fission product quantity estimates are available only for the EBR-II spent fuel inventory or for a “typical” PWR oxide fuel. The EBR-II estimate is about a year old. The EBR-II records are being converted to computerized form; improvements in the estimates should thus be anticipated.
There are differences in fission product distribution between fast and thermal reactor spent fuels, but we have not seen any differences that would affect the development program. We have therefore spent our limited resources only on a detailed breakdown for the EBR-II fuel. That breakdown is as follows, based on the fall 1994 inventory estimate (34.2 MTHM in driver fuel and blankets):
|
Nuclide |
Amount in Fuel, kg |
Amount in Zeolite*, kg |
Amount in Zeolite**, kg |
Amount in Metal, kg |
|
79Se |
2.59E−02 |
2.59E−02 |
2.59E−02 |
0 |
|
90Sr |
1.10E+00 |
1.10E+00 |
1.10E+00 |
0 |
|
99Tc |
2.45E+00 |
0 |
0 |
2.45E+00 |
|
129I |
5.94E−01 |
5.94E−01 |
5.94E−01 |
0 |
|
135Cs |
3.94E+00 |
3.94E+00 |
3.94E+00 |
0 |
|
137Cs |
2.28E+00 |
2.28E+00 |
2.28E+00 |
0 |
|
234U |
4.70E+00 |
2.29E−02 |
2.84E−04 |
0 |
|
235U |
4.79E+02 |
2.33E+00 |
2.89E−02 |
0 |
|
236U |
1.31E+01 |
6.38E−02 |
7.91E−04 |
0 |
|
238U |
3.31E+04 |
1.61E+02 |
1.90E+01 |
0 |
|
237Np |
6.79E−01 |
3.31E−03 |
0 |
0 |
|
239Pu |
4.80E+02 |
4.80E+02 |
9.60E−03 |
0 |
|
240Pu |
1.07E+01 |
1.07E+01 |
2.14E−04 |
0 |
|
242Pu |
2.88E−03 |
2.88E−03 |
0 |
0 |
|
241Am |
2.70E−01 |
2.70E−01 |
0 |
0 |
|
* assuming TRU in waste, Pu/U in electrolyte = 3 when electrolyte removed for treatment ** assuming TRU NOT in waste |
||||
-
What characterization data exist on “as-processed” zeolite that contains two or more actual or simulated waste elements (e.g., Pu and Cs)?
-
Are there any x-ray diffraction data of zeolite-A before and after contact with molten salt?
-
Yes; x-ray diffraction is a fairly routine measurement. The zeolite lattice is preserved, and no degradation products are seen. Occlusion of large amounts of salt swells the zeolite lattice somewhat. Limited neutron diffraction results support the x-ray findings.
-
What are the composition data (wt %) of major and minor components in zeolite-A before and after contact with molten salt?
The following table provides the compositions of a typical salt-occluded zeolite A and “anhydrous” zeolite A. The occluded zeolite compositions are experimentally-determined values, except for the oxygen content, which is calculated. The samples were free of additional salt phase. Zeolite A contains considerable water, depending on its history.
-
|
Element |
Concentration, weight % |
Ions per Unit Cell |
||
|
Anhydrous Zeolite A |
Occluded Zeolite A |
Anhydrous Zeolite A |
Occluded Zeolite A |
|
|
Cs |
0 |
3.74 |
0 |
0.79 |
|
K |
0 |
6.74 |
0 |
4.93 |
|
Li |
0 |
2.76 |
0 |
11.66 |
|
Ba |
0 |
0.54 |
0 |
0.12 |
|
Sr |
0 |
0.12 |
0 |
0.04 |
|
Ce |
0 |
4.20 |
0 |
0.85 |
|
La |
0 |
3.03 |
0 |
0.59 |
|
Nd |
0 |
4.74 |
0 |
0.98 |
|
Pr |
0 |
1.27 |
0 |
0.27 |
|
Sm |
0 |
0.59 |
0 |
0.12 |
|
Y |
0 |
0.16 |
0 |
0.05 |
|
Na |
16.18 |
0.58 |
12 |
0.74 |
|
Cl |
0 |
16.80 |
0 |
14.06 |
|
Si |
19.77 |
12.00 |
12 |
11.97 |
|
Al |
18.99 |
11.60 |
12 |
12.03 |
|
O |
45.05 |
27.43 |
48 |
48.00 |
-
What is the spatial distribution of waste elements in the “as-produced” zeolite, especially if produced by flowing molten salt through a zeolite column?
The elements are evenly distributed (insofar as we have been able to determine) when the zeolite is simply batch-contacted with salt and also after hot blending. We are striving to know the distribution of elements in the zeolite column ever more precisely. We have an isothermal equilibrium model that indicates that the distribution evolves as one would expect as more and more salt is passed through the column—“waves” of ions pass down the column as each ion is displaced by more strongly-retained ions until eventually the column would become entirely uniform and at equilibrium with the inlet salt composition. Unfortunately, some of the kinetics are slow and, of course, the fission products generate heat in the column. Therefore, we are developing a dynamic model that incorporates chemical kinetics as well as heat and mass transfer models. Only a few actual experimental column runs have been completed to date, and the analytical results are not yet available. Observed color changes were as expected, however.
-
What other data exist (from spectroscopic or other analytical techniques) on the location and bonding of waste elements and chloride within the “as-produced” zeolite waste form?
There are some data from neutron diffraction experiments that assign ion positions within the unit cell, but the data do not span a large variety of compositions; we think that this information is not particularly critical. Element-mapping data (from EDAX measurements) were presented to the Committee in October; they showed the location of key fission product elements and chlorine in the zeolite particles and not in the glass. More recent measurements support these observations. X-ray diffraction does not pick up any changes in structure associated with the consolidation operation.
-
What is known about (and what analytical approaches are proposed to investigate) transmutation effects (e.g., 137Cs+→137Ba2+)on zeolite stability and performance?
Essentially nothing is known about transmutation effects. We are not able to work with actual fission products in our Illinois facilities, and therefore are preparing equipment in Idaho for experiments in which we will investigate the Cs→Ba decay effects. The possibility of using 134Cs decay (2-year half-life) is being evaluated. We are also looking into accelerated tests of the effects of alpha-decay on the zeolite structure, using 238Pu. Various analytical approaches are planned, including x-ray diffraction structure analysis, neutron diffraction, and leach testing. We are also developing a molecular dynamics model that may provide some insights into the effects of transmutation processes.
-
What information, data, or calculations are available on the following issues with respect to geologic disposal of excess weapons Pu in zeolite and/or cladding-metal waste forms?
-
heat generation rate of waste form over time.
Calculations of heat generation have not been done specifically for the Pu disposition forms; however, calculations were done for heat generation by fission products and transuranic elements in the glass-bonded zeolite waste form for disposal of spent fuel wastes. The results of these calculations were published in the ANL Report, ANL-IFR-165, June 1992, for the calcium reduction system, and the calculations were updated in ANL-IFR-253, March 1995, for the lithium reduction system.
The heat generation rate from LWR spent fuel is, of course, dominated by cesium and strontium over the first 50 to 75 years of cooling, then the heat from the transuranium elements begin to dominate for the remaining decay time. The heating rate from 137Cs (and daughters) is about 5 mW/Ci, and that from the transuranium elements is about 25 W/kg. For the canisters described above, the heating rate will be about 1.3 kW/DHLW canister for plutonium and about 0.1 kW/DHLW canister for cesium, for a total of about 1.4 kW/DHLW canister. This is a lower heat loading than that planned for the LWR spent fuel waste form, and it should remain essentially constant for thousands of years.
-
estimates of mass loading of waste form into canisters after disposal.
The plutonium loading in the waste form is planned to be 5 wt%. (Recent experiments have shown that loadings as high as 26 wt% plutonium in zeolite are achievable.) The mass of waste form in the DHLW canister is planned to be 1040 kg total waste form, containing 52 kg plutonium.
-
estimates of centerline temperatures of canisters after disposal.
The centerline temperature calculated for the LWR spent fuel waste form case was 350°C. The heat load assumed in that case was about 4.5 kW. The plutonium disposal form heat loading is expected to be about 1.4 kW, so the centerline temperature will be much lower. However, the calculation has not yet been done for this case.
-
-
analysis of criticality safety of waste form, especially regarding limits on Pu loading.
The criticality potential of the waste form is, of course, a primary concern. The configuration of the disposal form and the presence of neutron poisons, such as gadolinium, have not yet been determined in detail. All potential plutonium disposal forms have the same criticality concerns, and these concerns have not yet been resolved. For example, one of the issues is differential leaching of either plutonium or the neutron poison. If the neutron poison is leached more rapidly, the remaining plutonium could evolve into an unsafe configuration. If the plutonium is leached more rapidly, it could reassemble in an unsafe configuration in the geological environment. These issues must be resolved for all potential disposal forms before plans are made to place the plutonium in any of the disposal forms.
-
With respect to John Ackerman's presentation to the committee (briefing slides dated October 4, 1995):
-
Page 6 cites “immobilize entire salt batch in zeolite” as an option; what is the fate of the LiCl processing salt? Is this salt expected to remain within the zeolite structure? How does the waste loading and volume of the “waste zeolite” compare to the zeolite produced from the “reuse of salt” option?
LiCl is incorporated (“occluded”) within the zeolite lattice in the same way as the rest of the salt. It is exchanged into the zeolite more strongly than sodium and potassium, but substantially less strongly than cesium, which in turn is retained much less strongly than the multiply-charged ions. All of the salt is expected to remain within the zeolite structure. Water exposure tests on blended zeolite powders (not consolidated waste form) show from 0.05 - 1.5 wt% loss depending on zeolite loading. The “reuse of salt” option has roughly one-tenth the waste volume of the “throwaway” option, assuming that heat generation limits are not exceeded. Excessive volumetric heat generation is not a problem with the EBR-II fuel because of its age. This applies as well to all of the spent fuel in the DOE inventory.
-
Page 27 cites conclusions from several tests on partitioning. What experimental procedures and measurements led to these conclusions?
These were batch measurements wherein anhydrous zeolite powder was exposed to molten salt. The following variables were examined: (1) length of exposure (from 4 hours to 15 days); (2) temperature of exposure (from 625 to 785K); (3) composition of salt (two kinds, differing mainly in amount of triply-charged ions); and (4) dilution of salt with LiCl-KCl (none to 90%). The salt was decanted from the zeolite and analyzed to establish a salt-phase composition. The zeolite was washed with water to remove free salt and analyzed for all exchangeable ions, silicon, aluminum, and chlorine. The resulting data were used to determine “exchange factors” in which concentrations (not activities) are plugged into the equilibrium constant expression for ion exchange. The table in the answer to question 11 is taken from the data analysis of one such zeolite sample.
-
Page 32 shows the Zr-Fe binary phase diagram; what are the consequences to this diagram of additional stable (e.g., Cr) and radioactive (e.g., U, Pu) components at the several wt % levels? Because Pu is found to partition preferentially into the Zr-rich phase (page 35), what techniques will be used to separately evaluate the long-term corrosion behavior of this phase, rather than the bulk intergrowth of Zr- and Fe-rich phases?
A preprint of a paper dealing with the effects of the additional metals on the basic Fe-Zr system is attached.1 As mentioned in the discussion of that paper, the actinides seem to follow
-
|
1 |
S.W. McDeavitt, D.P. Abraham, D.D. Keiser, Jr., and J.Y. Park, “Stainless Steel-Zirconium Alloy Waste Forms,” to be presented at SPECTRUM International Conference on Nuclear and Hazardous Waste Management, Seattle, Washington, August 18-23, 1996. The preprint was supplied to the committee but is not included in this appendix. |
-
the zirconium. The corrosion behavior of this phase cannot be evaluated separately from the matrix in which it exists, because the matrix largely determines the corrosion rate. However, the behavior of the actinide-rich phases is being and will be carefully followed during corrosion testing, using all the appropriate tools at our disposal including all the usual metallographic and surface analysis methods as well as special corrosion tests such as applied potential studies and testing at extreme pH.
-
Page 29 cites previous gamma irradiation of zeolite-A waste form; was this irradiation before or after processing and contact by molten salt? What was the composition of the irradiated sample? Was the sample contacted by water during or after the irradiation? What techniques were employed to confirm that there was “no damage” to the sample?
The irradiation tests were done on zeolite A powders that had been exposed to molten salt, and not on consolidated waste forms. The concentrations of major constituents in the sample tested were 14.7 Al, 2.43 Ba, 2.13 Cs, 7.38 K, 2.83 Li, 0.83 Na, 14.5 Si, 1.05 Sr, 5.8 Cl, and 34.0 O (all in weight percent). The sample was not contacted with water during the irradiation and not until it was leach-tested. The analytical techniques employed were x-ray diffraction, optical microscopy, and leach rate measurements. Color center formation was observed optically.
-
Comments:
-
Regarding nomenclature, the Linde Type A zeolite (referred to as the LTA framework structure in the terminology of the International Zeolite Association) is a synthetic zeolite. It is normally synthesized in the Na+form and these Na+cations are fully ion exchangeable in aqueous or molten salt ion exchange. This material is commonly referred to as NaA zeolite or as zeolite 4A. It is manufactured by UOP and many other companies worldwide.
-
The void space inside the crystals as synthesized (i.e., in the “micropores” of the zeolite) is filled with water. The as-synthesized powder particles are typically in the 1–10 micron diameter size range and each powder particle consists of an intergrown mass of several crystals.
-
For column ion exchange or adsorption applications these powder particles are bonded, often with clay binders (typically with about 80 wt zeolite and 20 wt binder, on an anhydrous weight basis) to form larger particles, e.g., 20 × 50 mesh particles for ion exchange, or 8 × 12 mesh or 1/16” pellets for gas phase adsorption applications. To “set” the clay binders the material is “fired” at elevated temperatures, e.g., 600-750°C; this thermal treatment drives most of the water off the zeolite and clay binder and the binder becomes amorphous. (The amorphous materials formed from the binder clays by this treatment normally have no significant residual ion exchange capacity.)
-
The anhydrous, fired zeolite is said to be in the “activated” (water-free) state with its micropores largely empty; however, it should be noted that, in equilibrium with completely dry air at atmospheric pressure and room temperature, the “activated” NaA zeolite will adsorb an appreciable amount of N2and some O2and CO2from the air. Similarly, the “activated” NaA zeolite will adsorb appreciable amounts of other gases, such as Argon, at atmospheric pressure and room temperature.
We agree with all these comments. We are not sure of the extent of ion exchange onto the binders under our conditions, especially with triply-charged ions in molten salt, and will determine the extent of such sorption in the coming year.
-
-
Comments
-
Typical clay binders are kaolin, montmorillonite or attapulgite (palygorskite) clays. Since they are natural minerals, they vary considerably in purity; most have small amounts of various other mineral impurities such as quartz, mica, feldspar, etc.
-
-
The bonded NaA zeolite is a very strong desiccant and its equilibration with ambient humid air will result in nearly complete filling of its micropores with water, which will displace most of the air gases from the micropores in the process. Heating in vacuum to about 400 °C will allow almost all of this water to be removed to “re-activate” the zeolite.
-
In the Argonne molten salt ion exchange process the K+and Li+ion-exchanged form of the Type A zeolite (i.e., the KLiA) is dehydrated and contacted with the molten LiCl, KCl salt at elevated temperature (e.g., 500°C) and subsequently with the molten salt containing Cs and other radioactive waste products. Then excess salt is removed and it is thermally treated at a still higher temperature (e.g., 700°C); in this step the zeolite crystal structure will be either completely or nearly completely converted to other more stable phases, e.g., crystalline phases having the same framework topologies as sodalite or pollucite.
-
There have been many proposals (and studies) in the technical literature for the use of sodalite, pollucite, and other similar structures, as low-solubility waste forms for radioactive wastes.
We agree with comments (a) and (b). We also agree with comment (d), and have developed a particularly effective synthesis of pollucite to immobilize the pure CsCl that exists in the DOE complex, should the decision be made to dispose of that material directly and not use it for imparting a radiation barrier in the disposal of surplus fissile materials.
With regard to comment (c), we do not (as yet) use K- and Li-loaded zeolites made by aqueous ion exchange, although we may do so in the future. Early on, we found that materials advertised to be Li-exchanged or K-exchanged still contained mostly Na. Because it is difficult to fully exchange Na for Li and K by aqueous methods (although it is easy in molten salt), and because of the impossibility of fully dehydrating the zeolite, we pre-treat carefully dried “anhydrous” zeolite 4A with molten LiCl-KCl to exchange out the bulk of the sodium and to remove as much residual water as possible.
We take exception to the last sentence in comment 15(c). Consolidation of the waste form takes place at high pressure and temperatures in excess of 700°C, but only for relatively short times. As mentioned in our presentation to the Committee in October, we are investigating the allowable time-temperature combinations that will not destroy the zeolite structure, so as to continue to stay well within the allowable envelope. This is being done with both glass-bonded zeolite waste form and with the salt-loaded zeolite. We do not destroy the zeolite structure in routine consolidation steps, as evidenced by x-ray diffraction and the various other characterization techniques. Note that in Comment 14(c), “. . . the material is ‘fired' at elevated temperatures, e.g., 600-750°C . . . .” This implies at least some ability of a closely related zeolite A material to withstand up to 750°C for at least some time.
-
Sodalite is a felspathoid. Its unit cell consists of a framework consisting of 6 AlSiO4moieties that contain 6 Na+ions and 2 NaCl's which completely fill the micropores in the crystal. The largest pores in the sodalite framework consist of 6-rings of oxygen atoms (vs. the 8-rings of oxygen atoms which form the largest pores in the Type A zeolite framework) and the NaCl that fills the pores of sodalite cannot be washed out of the structure at room temperature because the Cl−anions are too large to pass through the 6-rings. Have there been studies made of the ion exchange of sodalite at elevated temperatures?
As described for the Committee previously, we have done a substantial amount of work on sodalite synthesis from zeolite because there may be some advantages gained from collapsing the zeolite structure. That work has been de-emphasized due to funding constraints. As far as using sodalite as an ion exchanger, we did one or two experiments exposing large natural sodalite specimens to molten salt. The samples bleached and disintegrated. Only very limited ion exchange of cesium into the resulting powders was observed, and there was no ion exchange between sodalite and the multiply-charged fission product cations in the salt.
-
Pollucite, CsAlSi2O6, is technically a member of the zeolite group of minerals but in structure, chemistry, and paragenesis it is close to the felspathoids. The Cs completely fills the cavities in the crystal, so pollucite contains essentially zero water. The largest pores in the pollucite framework are also 6-rings, too small to allow the Cs+to pass through, so the Cs+cannot be ion-exchanged out of pollucite, at least not at room temperature. Have there been any studies of the ion exchange of Cs+out of pollucite at elevated temperatures?
We have not done any such studies with pollucite other than the development of a synthesis method as described in our response to comment 15(d). For the electrometallurgical treatment of DOE spent nuclear fuel, we need to have (preferably one) material to retain all the fission products, not just cesium, and we strongly prefer a material which will remove all of these fission products from the electrolyte salt.
-
What Type A zeolite samples have been most successful in the Argonne test? What non-proprietary information is available on supplier, product name or code, lot numbers, or other designations? What, if anything, is known about the binder composition and percentage in the bonded zeolite product?
For our purposes, we do not see much difference amongst the various pure zeolite A powders from vendor to vendor. On the other hand, there are tremendous differences among the bonded zeolite pellets. We have several materials from each of two vendors that are acceptable, but cannot divulge supplier, product names, codes, lot numbers, etc. for any particular material. We know very little about the binders from one supplier, and the binders from the other are being developed under a Cooperative Research and Development Agreement (CRADA) and are thus protected. We do know that the amount of binder in successful pellets varies from 10% by weight in some materials to 50% in others.
-
What physical properties, including attrition, have been measured on the bonded zeolites that have been most successful?
The test method we use is simply to expose the pellets to molten salt for several days and look for decrepitation. Most pellets fail this relatively mild test. Those that remain intact are being used in our zeolite column apparatus. When more is known about actual column conditions, we will establish quantitative requirements and do more detailed mechanical evaluations.
EBR-II Shutdown and R&D Program
-
With regard to the EBR-II fuel processing at ANL-West, what is the status? Have they started up yet?
Argonne has not yet (as of November 17, 1995) received approval from the Department of Energy for the initiation of irradiated driver fuel processing.
-
Is work under way for preparation and study of (1) the heat-treated zeolite of the composition expected in the final waste form and (2) the zeolite-free glass of the composition expected in the final waste form? For each case:
-
What is the thermal coefficient of expansion?
-
What are the leaching rates at room temperature and at the maximum expected temperatures in the final waste forms?
The meaning of “heat-treated zeolite” is not clear. We are preparing and/or studying (in addition to column work and partitioning studies) (1) the dehydration process, determining the dehydration conditions and maximum allowable water levels; (2) the required particle size distribution in ground column-origin material; (3) the blending process, determining the conditions required to obtain homogeneous blended zeolite, the relationship of salt loading to
-
-
waste form performance and properties, and the properties of the blended zeolite (especially thermal stability); (4) glass compositions, homogeneity, and physical properties (the optimum glass has yet to be selected, and we are characterizing and evaluating several glasses that appear to be acceptable); (5) the pressing process, including glass blending and canister loading/sealing as well as pressing time-temperature-pressure profiles; and (6) the properties of the final waste form including, among others, dimensions of structural features, chemical homogeneity, thermal conductivity, thermal expansion, and degradation modes in various media.
Until we have actual measurements later this year, we are using the provisional property values shown below. The values for zeolite are to be treated with particular caution; these values are based on anhydrous zeolite, and very little is known about salt-loaded zeolite. As mentioned in the October presentation to the Committee, equipment for specialized thermal property measurements is being assembled at the present time.
|
Property |
Glass |
Loaded Zeolite |
|
Thermal Conductivity, W*m−1*K−1 |
1.0 |
0.15 |
|
Coefficient of Thermal Expansion, K−1 |
1E−05 |
7E−06 |
|
Heat Capacity, J*g−1*K−1 |
1.0 |
0.8 |
We believe that the leach test data that were presented to the Committee in October (deionized water leachant at 90°C) approximate the “worst case” exposure of the waste form to water. The various brines, including J-13 well water, that have been tested are less aggressive than pure water, and 90°C is within a few degrees of the maximum temperature for liquid water at the proposed Yucca Mountain geologic repository location. We do not have room temperature data, and do not plan room temperature leaching studies in the immediate future.
Literature
-
What literature searches, reviews, or reports are available on molten salt ion exchange of zeolites? (The committee understands that studies of molten salt ion exchange have been performed at ORNL, LLNL, and LANL, as well as the ANL work on this project.) The committee is aware of one report—ORNL/TM-12515 (Dec. 1993) by Petek et al. of ORNL—and of a series of 11 papers (many by Liquornik and Marcus) on these topics.
Copies* of all the related work that we have been able to acquire are appended. We don't know of any publications on work that has been done at LLNL or LANL, although we are aware of inquiries for any available information from LANL, who are considering the electrometallurgical process in their Accelerator Transmutation of Waste (ATW) program, [Note: Copies of these articles were supplied to the committee but are not included in this appendix; a list of the literature citations is given below.]
-
Are there reviews, reports, or references available on molten salt combustion as applied to management of radioactive wastes?
We have not formally investigated this area, because we have not seen a direct application in the mainstream electrometallurgical treatment process. Because of our extensive experience with molten salt processing, however, we would be in a good position to evaluate such processes and would appreciate any guidance or suggestions from the Committee on possible applications.
-
What literature searches, reviews, or reports are available on the stability, solubility, leaching, etc., of the zeolite, sodalite, pollucite, or other phases identified (or thought to be likely final phases formed from the zeolite) in the final waste form?
-
There is a considerable amount of related literature on this subject; a few references are provided.* Please realize that we have yet to determine what the final degradation products of our waste form (not just the zeolite component) will be. We would only be speculating if we were to make a list of likely degradation products.
Callahan, C.M. 1966. Ion exchange in fused salts-II: The distribution of alkali metal and alkaline earth ions between Chabazite and fused LiNO3, NaNO3, and KNO3. Journal of Inorganic Nuclear Chemistry 28:2743-52.
Callahan, C.M., and M.A. Kay. 1966. Ion exchange in fused salts-I: A comparison of the ion-exchanger characteristics of five mineral exchangers in fused Sodium Nitrate with selected alkali-metal and alkaline-earth cations. Journal of Inorganic Nuclear Chemistry 28:233-44.
Lewis, M.A., D.F. Fischer, and L.J. Smith. 1993. Salt-occluded zeolites as an immobilization matrix for chloride waste salt. Journal of the American Ceramic Society 76 (September):2826-32.
Lewis, M.A., D.F. Fischer, and J.J. Laidler. 1993. Leach resistance properties and release processes for salt-occluded Zeolite A. Argonne National Laboratory294:95-101.
Liquornik, M., and Y. Marcus. 1968. Ion exchange in molten salts-I: The ion-exchange properties of Sodium Zeolite in molten NaNO3; Exchange reactions with alkali metal, Thallium, and silver cations Israel Atomic Energy Commission 72 (August):2885-88.
Liquornik, M., and Y. Marcus. 1968. Ion exchange in molten salts-II: The occlusion of Lithium, Sodium, Potassium and Silver Nitrates in the respective forms of Zeolite A. Israel Atomic Energy Commission 6:115-21.
Liquornik, M., and Y. Marcus. 1968. Ion exchange in molten salts-III: The ion-exchange properties of Sodium Zeolite A in molten NaNO3. The exchange with Calcium and Strontium cations. Israel Atomic Energy Commission 72 (December):4704-5.
Liquornik, M., and Y. Marcus. 1971. Ion exchange in molten salts-V: Potassium Zeolite A as an ion exchanger in Nitrate melts. Israel Atomic Energy Commission 75:2523-25.
Liquornik, M., B. Ale, and J.A.A. Ketelaar. 1973. Ion exchange in molten salts-VI: The occluded Sodium Nitrate in Zeolite A as an Anion exchanger. The Cl–NO3 exchange in molten Na(NO3, Cl) mixtures. Israel Atomic Energy Commission 77:1398-1400.
Petek, M., L.M. Toth, D.G. Brown, G.E. Michaels, and B.C. Chakoumakos. 1993. High level waste form development for molten salt waste streams. Oak Ridge National Laboratory (December):1-27.
Petranovic, N., and M. Šušic. 1974. Surface phenomena on Zeolite A in molten salt media-I: The mechanism of bivalent cation exchange through metal-nitrate complex formation in the zeolite cages. Journal of Inorganic Nuclear Chemistry 36:1381-85.
Šušic, M.V., N.A. Petranovic, and D.A. Mioc. 1971. The properties of Zeolite 4A treated in molten salts. Journal of Inorganic Nuclear Chemistry 33:2667-75.