C
ARGONNE NATIONAL LABORATORY RESPONSES TO QUESTIONS SUBMITTED ON DECEMBER 4, 1995, BY THE COMMITTEE
-
How many waste streams will result from plutonium immobilization?
In this concept, plutonium immobilization will be done in conjunction with treatment of spent fuel; therefore, the same two waste streams will obtain (mineral waste form and metal waste form). The waste streams include salt from the electrorefiner that will be absorbed on zeolite, and metal cladding and noble metal fission products that remain in the anode basket. In the reference case, the plutonium and other transuranium elements will remain in the salt waste stream to be absorbed in the zeolite and ultimately become part of the mineral waste form. The third stream emanating from the process is pure uranium; its ultimate disposition will be determined by National policy.
-
Where does Pu enter the flow sheet?
There are three entry points, depending on the materials being immobilized. Metals will be placed directly into the electrorefiner, oxides will be reduced to metals in the lithium reduction step, and plutonium-bearing chlorides will be mixed with salt from the electrorefiner and absorbed directly onto the zeolite (with appropriate pretreatment to remove or solubilize oxide or metal particulates).
-
Is there a possible Pu metal (cladding) waste? Would this require a cathode?
One of the options being considered is to place the plutonium in the metal waste form. This metal waste form option would require fission product cesium, both from spent fuel and from the cesium capsules, to be placed in the metal waste form in order to provide a degree of self-protection against unauthorized recovery of the plutonium. The challenges with this option are handling the plutonium-bearing chloride residues and incorporation of cesium into the metal waste form.
This option would require the use of a liquid-cadmium cathode to recover the transuranium elements as metals (together with some uranium) for placement into the metal waste form. They would be alloyed with the cladding hulls and noble metal fission products together with the external source of cesium.
-
For Pu, do we use just the one flow sheet? Are there alternative flow sheets?
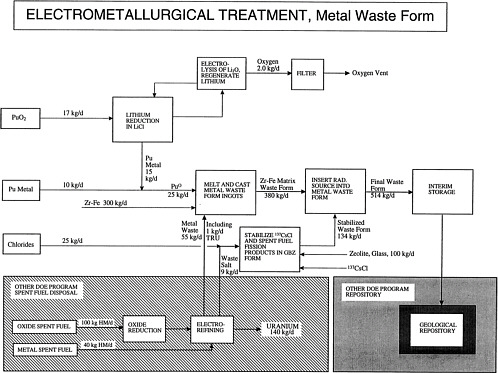
The flow sheet for the metal waste form option is attached [p. 58]. The mineral waste form is the preferred option at this time, but the metal waste form option is being retained as a backup.
-
Should the committee use 14 wt% Pu or 6-10 wt%? Both have been discussed.
We are not sure of the context of the numbers quoted here. It is possible that the 14 wt% is for plutonium concentration in the waste salt before absorption on the zeolite, and the 6-10 wt% refers to the concentration in the final waste form. The committee should realize, also, that the concentration of transuranics in the salt (and the corresponding lower concentration in the final waste form) has not yet been established. The concentration will depend on several factors, including criticality considerations for the process vessels as well as the waste form, and loading achievable in the zeolite.
-
What are the consequences of Si and other impurities in Pu feed?
Silicon is troublesome because it tends to form intermetallic compounds with plutonium, thus lowering its activity and making it difficult to recover. As part of a previous study sponsored by
-
Lawrence Livermore National Laboratory (the plutonium resource recovery, PuRR, program), we studied the effect of silicon on plutonium recovery from some of the production residues. We found that addition of zirconium, which forms very stable compounds with silicon, carbon, nitrogen, etc., tied up the silicon and allowed the plutonium to be extracted.
Magnesium is another element that can interfere with operation of the electrorefiner, because the stability of MgCl2 is similar to that of uranium and plutonium. Therefore, magnesium will electrotransport along with uranium and plutonium in this system. The presence of magnesium in these “products” should be of little consequence, because the objective in this application is not to make pure uranium or plutonium. If the uranium product is contaminated with magnesium, this should not interfere with disposal of the uranium as a low-level waste, because the magnesium contaminant is not radioactive. It could make the recycling of the recovered uranium as LWR fuel material somewhat more complicated.
-
Is there any objection to inclusion of the Questions and Answers as an Appendix?
No objection. We would like to revise one response to the questions posed by the Committee on November 3, 1995: the response to Question 13(c). The revised response is as follows:
(c)Page 32 shows the Zr-Fe binary phase diagram; what are the consequences to this diagram of additional stable (e.g., Cr) and radioactive (e.g., U, Pu) components at the several wt% level? Because Pu is found to partition preferentially into the Zr-rich phase (page 35), what techniques will be used to separately evaluate the long-term corrosion behavior of this phase, rather than the bulk intergrowth of Zr- and Fe-rich phases?
A preprint1 of a paper dealing with the effects of the additional metals on the basic Fe-Zr system is attached. We are currently preparing other papers on this topic, but they are not yet available as preprints; they deal with actinides in the metal waste form, as does the attached presentation, which is to be given at the SPECTRUM 96 meeting. In this presentation, it is reported that the actinides seem to follow the zirconium. The corrosion behavior of this phase cannot be evaluated separately from the matrix in which it exists, because the matrix largely determines the corrosion rate. However, the behavior of the actinide-rich phases is being and will be carefully followed during corrosion testing, using all the appropriate tools at our disposal including all the usual metallographic and surface analysis methods as well as special corrosion tests such as applied potential studies and testing at extreme pH. We will provide a pre-publication copy of the paper as soon as internal reviews are complete.
-
What is zeolite's stability with respect to conversion of zeolite A to sodalite at 500°C? (ORNL/TM 12515, Petek, et al.)
The question of the stability of zeolite A under the conditions that we use or anticipate using is of major interest to us. Determination of the allowable times and temperatures for hot pressing is being addressed along two separate lines of work, which have been assigned highest programmatic priority. Zeolite stability under other conditions (high water contents, for example, or other salt compositions) is also of interest, but much lower on our priority list. In particular, at least some of the ORNL salts contain 5% or more of fluoride, which is known to destroy the zeolite structure.
We have observed, by X-ray diffraction, that zeolite remains as zeolite in the mineral waste form that had been uniaxially hot-pressed at temperatures in excess of 700°C for 1/2 h at 7,000 psi.
|
1 |
S.W. McDeavitt, D.P. Abraham, D.D. Keiser, Jr., and J.Y. Park, “Stainless Steel-Zirconium Alloy Waste Forms,” to be presented at SPECTRUM International Conference on Nuclear and Hazardous Waste Management, Seattle, Washington, August 18-23, 1996. The preprint was supplied to the committee but is not included in this appendix. |
-
More recently, we have observed conversion to sodalite at 715°C for 1 h at 25,000 psi; this appears to be sensitive to the kindof glass used as binder, but may also be related to the pressuresused.
We have also recently observed, however, that zeolite remains as zeolite for up to 4 h at 700°C. We have never observed zeolite conversion in blending operations at up to 550°C for two days, nor do we ever see conversion at temperatures up to 550°C in the columns. All these materials are routinely examined by X-ray powder diffraction; other techniques are being investigated for use.
-
What temperatures, pressures and holding times will be used for hot pressing?
-
We are varying the pressing variables (including composition) in a systematic manner to determine optimum conditions and penalties for off-optimum operation. The baseline temperature, pressure, and time for hot isostatic pressing are 700°C, 25,000 psi, and 1 h.
-
Regarding question #18 [Appendix B], what is the basis for stating that some binders are acceptable?
-
The basis for assigning acceptability is just that those binders that have not yet shown visible decrepitation are still deemed acceptable. Other criteria may arise as we gain experience. We did initial screening by placing the pellets in molten salt for 24 h or longer; about half failed. Yet other materials were rejected because they are no longer commercially available. Not all of the provisionally acceptable materials have been tested in the column, but the few that have been tested in the column have survived.
-
What is the effect of any oxidic material introduced into the zeolite? How complete is the reduction process?
-
We do not intend to introduce particulate oxides (such as PuO2) into the zeolite. A PuO2 separate phase would not be a desirable waste form configuration. The intention for this process is to form soluble plutonium phases, such as PuCl3 or PuOCl, in the electrorefiner electrolyte salt (LiCl-KCl eutectic). These soluble species would ion-exchange with the zeolite to form stable forms that would then be immobilized in a glass matrix.
We have conducted many lithium reduction experiments in LiCl salt at various temperatures and various simulated spent fuel compositions. These experiments have shown consistent PuO2 reduction effectiveness of>99.9% and, in some cases, >99.99%.
Particulates that are not amenable to absorption in the zeolite column will be removed prior to infiltration of the salt through the column by passing the salt through a porous metal filter to remove the particles. Our plan is to dispose of these particulates by melting the filter and making it part of the metal waste form.
-
How do you prevent migration of chloride from zeolite into glass? How is it demonstrated?
-
We don't make any special effort to prevent chloride migration to the glass. However, we do not observe chloride migration with the relatively simple energy-dispersive X-ray imaging that we have been able to do to date. We certainly plan to do more extensive characterization as resources become available.
-
What is ANL's program to develop testing of waste forms?
-
The goal of the program is to develop a testing protocol that can be used to establish a repository acceptance standard for the two waste forms: glass-bonded zeolite and metal. The overall approach is to utilize the experience gained in the testing of both borosilicate glasses and glass-ceramics as a guide to the development of these test protocols. We must also remain cognizant of the likely mechanistic differences that may exist between the reactions of glass, glass-ceramics, zeolite-based materials, and metals. We are seeking to identify environmental conditions that may influence zeolite and metal reactions, and to establish testing procedures that provide a link
-
between the short-term test performance of the waste forms and their long-term behavior in a waste disposal setting.
In the case of the metal waste form, we can capitalize to some extent on the experience gained in the conduct of corrosion tests on borosilicate glass canister materials, but we realize that the functions of the canister material and the waste form matrix are somewhat different. We have done considerable testing now on metal waste form corrosion behavior; we are rather hampered by the fact that this waste form does not corrode at rates that are measurable in short-term tests. The testing to date has involved (1) immersion corrosion tests, a modified version of the standard MCC-1 test, using J-13 well water, deionized water, and acidic solutions as the leachants; (2) electrochemical polarization tests, also in the same leachants; and (3) vapor hydration tests in J-13 well water. We have also explored the use of a standardized pitting susceptibility test, another electrochemical testing procedure. Because the waste form is too durable to permit determination of a realistic reaction rate, we are forced into the use of accelerated test procedures that may or may not be appropriate for establishing mechanisms of waste form degradation. Considerable effort is going into resolution of this situation.
The general approach for the zeolite-based waste form is to follow ASTM Procedure C-1174-91, “Standard for the Prediction of Long-Term Behavior of Waste Package Materials.” We are assuming that a wide range of environmental conditions may exist in a geologic repository, and that such variations may influence the reactivity of the waste form. Attribute characterization and accelerated test methodologies are included in this program, with the goal of these tests being to identify the specific environmental conditions that would compromise the integrity and/or radionuclide retentive properties of the waste form, as well as to identify the mechanisms for degradation of the waste form.
Initial attribute characterization studies are being conducted to determine mineral waste form material properties such as waste form mineralogy, composition, structure, element partitioning, product consistency, and waste element loading. Characterization and accelerated tests are being used to provide such information as forward reaction rates, alteration mechanisms, alteration product formation, reaction kinetics, reaction thermodynamics, component interactions, and radiation damage effects. The characterization and accelerated tests include MCC-1 tests, Product Consistency Tests (PCT), vapor hydration tests, and flow-through testing formats.