7
Calculation and Comparison of the Health Benefits and Differential Costs Associated with Candidate Vaccines
This chapter presents the method used to calculate and compare the reductions in morbidity and mortality that could result from the vaccine candidates evaluated in this report. The proposed method has two principal components: (1) calculation of values for the annual health benefits that would occur at a time when such benefits have reached a steady state, and (2) adjustment of these values to reflect the probability of their occurring and the times at which they may occur. Procedures are also presented whereby expenditures associated with the purchase of vaccines for immunization programs may be calculated and combined with the annualized cost of development.
This chapter presents a central analysis; Chapter 9 discusses sensitivity analyses involving some of the factors used in the base case.
PROCEDURES
The steps for calculating potential health benefits from the vaccine candidates are listed below and illustrated in Figure 7.1.
-
Calculate the total disease burden values (TDBVs) for each candidate disease. These values incorporate both disease burden estimates and a factor expressing the undesirability of the conditions. Infant mortality equivalence (IME) values, described in Chapter 4, quantify these value judgments. The set of IME values used throughout this analysis is the median of perspectives derived from a poll of public health experts in developing countries: it is for illustrative purposes only. Adopting alternative perspectives in these calculations would have effects similar to those described in Chapter 4. Derivations of disease burden estimates for the various diseases are described in Appendixes D-1 through D-19.
-
Estimate the proportion of the total disease burden that is vaccine preventable. This is defined as that portion that could theoretically be prevented by immunization of the entire target population (at the anticipated age of administration) with a hypothetical vaccine that is 100 percent effective against the strains/types included in it. This factor is influenced by the distribution of the disease in
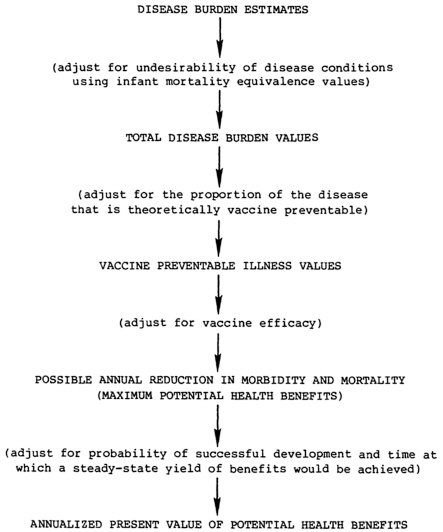
FIGURE 7.1 Calculation of potential health benefits.
-
relation to the age of anticipated vaccination, by whether any disease falls outside the target population, and by the ability to formulate a vaccine effective against all strains/types causing disease. Factors influencing the estimate of the proportion of vaccine preventable illness are discussed in relevant sections of Appendixes D-1 through D-19.
-
Calculate vaccine preventable illness (VPI) values for each disease/vaccine combination. In this analysis, VPI values are calculated from TDBVs using the estimate of the proportion of the disease burden that is potentially preventable. A more exacting approach is to estimate for each disease in each age group/morbidity category combination, the number of cases, complications, sequelae, or deaths that could theoretically be prevented annually (in a steady state of vaccine use) and then use IME values to calculate VPI values in a manner similar to that for deriving TDBVs as described in Chapter 4.
-
Calculate the possible reduction in morbidity and mortality (PRMM) for each vaccine. These figures represent vaccine preventable illness values adjusted for the predicted efficacy of the vaccine. For
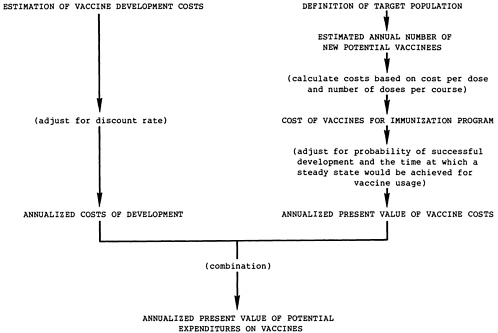
FIGURE 7.2 Calculations of expenditures on vaccines.
-
diseases in which vaccine efficacy is expected to be the same for all conditions, calculation of PRMM simply entails multiplying the vaccine preventable illness values (one for each IME perspective) by the efficacy. If the vaccine is expected to have different efficacies for various conditions, the calculations are more complex. PRMM values must be calculated separately for each morbidity category/age group combination and then added together.
-
Calculate the annualized present value of potential health benefits. These values represent an adjustment of the possible reductions in morbidity and mortality values to account for the probability of a vaccine’s successful development and the time at which a steady-state yield of benefits would be achieved. As discussed in Chapter 6, it is assumed for this analysis that there are no differences in the utilization of vaccine candidates.
-
Calculate the vaccine expenditures necessary to achieve the possible reductions in morbidity and mortality, as illustrated in Figure 7.2.
A more detailed explanation of some elements in the calculations and a discussion of certain adopted assumptions appear below.
Vaccine Characteristics
The vaccine characteristics used in this comparison are described in Chapter 5, Tables 5.1. and 5.2. Detailed information on specific vaccines is included in Appendixes D-1 through D-19.
Target Population
Target populations for the vaccines are described in the relevant appendixes and briefly outlined in Table 7.1. The number of new potential vaccine recipients entering the target population each year must be determined to calculate the vaccine costs for each immunization program. These calculations are based on the envisaged target population and the 1984 population projections (see Chapter 4). The basis for the calculation of the number of new potential recipients of each vaccine is shown in Table 7.1 and described more fully in relevant appendixes.
In addition to indigenous populations at risk from the various diseases in developing countries discussed here, it is likely that travelers and personnel from developed countries who are stationed overseas would also be given some vaccines. Relative to the indigenous target populations, the number of such potential vaccinees is judged to be insignificant. However, decision makers may wish to consider the size of these groups and the benefits that would result from their protection when making ultimate choices on vaccine priorities.
Vaccine Preventable Illness
For each disease, an estimate of the potential vaccine preventable illness is needed. This can be expressed either as a proportion of the total burden of illness, or developed as estimates of the numbers of cases, complications, sequelae, and deaths that result from each disease. These estimates are derived from the distribution of the disease burden; the envisaged target population; the characteristics of the vaccine (e.g., the number of doses necessary to achieve full protection) and the likely age(s) for vaccine delivery; and for some diseases, where appropriate, the proportion of the disease affecting an identified high-risk group or target population.
In this analysis, VPI was expressed as a proportion of the total disease burden. The factors used for each vaccine/disease combination to estimate VPI are discussed in Appendixes D-1 through D-19.
Trends in Disease Burden and Population Numbers
Calculations of morbidity, mortality, and costs assume that the effects of trends in disease burden and population size (between 1984 and the achievement of steady-state benefits) would not be of sufficient magnitude to obscure differences between diseases. The effects of such
trends could be examined, if desired, within the model proposed. These assumptions apply only to diseases under study and the current population projections: if other disease candidates are added to the list, the assumptions should be reexamined. Because of the trend in most developing countries toward greater relative numbers of individuals in the younger age groups, the major effect of adopting these assumptions would be to somewhat underestimate the benefits of vaccines reducing disease in these age groups, assuming incidence rates remain constant.
Adverse Reactions
Because no vaccine in this analysis is predicted to have serious side effects, the current calculations omit adverse reactions, if desired, estimation of each vaccination program’s adverse effects can be incorporated into the benefit calculations. The predicted incidence of adverse reactions, the annual number of potential new vaccinees, and the IME values for the types of adverse conditions predicted can be used to calculate values representing the vaccine-induced morbidity and mortality (if any). These values for adverse effects may be used as a correction to PRMM figures. In the analysis of vaccine priorities for the United States, it was calculated that anticipated occurrence of adverse reactions necessitated only a very small adjustment of PRMM (i.e., less than 1 percent of PRMM).
For the reasons described above, adverse reaction values (and their costs) are not included in the tables and discussion that follow in this chapter. If other vaccine candidates are added to the analysis, however, their potential for adverse reactions should be evaluated.
The Times for Occurrence of Vaccine-Associated Health Benefits and Cost Savings
The purpose of the accelerated vaccine development program is to expedite the realization of the benefits theoretically possible with various vaccines. It is appropriate, therefore, to account for the times at which benefits and costs associated with vaccine development and use would occur. Usually this is done by a process termed discounting (Weinstein and Stason, 1977), which can be applied both to the health benefits (of morbidity and mortality averted) and the costs incurred in vaccine development and use.
Time to Licensure
Factors affecting the predictions of the time to develop the vaccine candidates are discussed in Chapter 5. The predictions are related to probability of success and other issues discussed in that chapter and shown in Table 5.1.
TABLE 7.1 The Basis for Estimating the Annual Number of New Potential Vaccinees and the Delay in Vaccination Benefits
|
Pathogen (Target Population) |
Type of Vaccine |
Individuals Entering Target Populationa |
Delay in Vaccination Benefits |
|
Dengue virus (Infants and children in endemic areas; travelers to endemic areas) |
Attenuated live vector virus containing gene for broadly cross-reacting protective antigen |
Birth cohort in endemic areas: 48 million |
Probable age of vaccination (<1 year) to peak of DHF/DSSb (6 years), i.e., approx. 5 years Vaccinate to peak age of dengue fever (adolescents and adults) |
|
Escherichia coli (enterotoxigenic) (Infants<6 months) |
A combination of purified colonization factor antigens and possibly other antigens |
Birth cohort: 115.1 million |
Probable age of vaccination (<1 year) to peak age of disease (1–2 years), i.e., approx. 1 year |
|
Genetically engineered attenuated strains |
|||
|
Hemophilus influenzae type b (Infants) |
Conjugated polysaccharide |
Birth cohort: 115.1 million |
Probable age of vaccination (<1 year) to peak of serious disease (2–3 years), i.e., approx. 2 years |
|
Hepatitis A virus (Susceptibles of all ages; routine for preschool children) |
Attenuated live virus |
Birth cohort: 115.1 million |
Probable age of vaccination (<1 year) to peak age of disease in LTDCs (2–4), i.e., approx. 2 years |
|
Polypeptide recombinant vaccine produced in yeast |
|||
|
Hepatitis B virus (Areas with high perinatal infection: all infants at birth (if possible). Other areas: all infants, simultaneous with other vaccinations, at earliest possible age) |
Polypeptide produced by recombinant DNA technology |
Birth cohort: 115.1 million |
Probable age of vaccination (<1 year) to mid-point between peak of acute disease (10 years) and serious chronic illness (35–40 years), i.e., approx. 30 years |
|
Japanese encephalitis virus (Children in epidemic and endemic areas; foreign visitors to epidemic regions) |
Inactivated virus produced in cell culture |
Birth cohort in endemic/ epidemic areas: 64.8 million |
Probable age of vaccination (<1 year) to peak age of disease (approx. age 8), i.e. approx. 7 years |
|
Mycobacterium leprae (Immunoprophylactic: all children in endemic areas. Immunotherapeutic: all recently infected individuals) |
Armadillo-derived M. leprae |
Immunoprophylactic—birth cohort in endemic areas: 44.8 million Immunotherapeutic—incidence approx. 1.5 million |
6 years, based on estimated incubation period |
|
Pathogen (Target Population) |
Type of Vaccine |
Individuals Entering Target Populationa |
Delay in Vaccination Benefits |
|
Neisseria meningitidis (Infants, 3 to 6 months) |
Conjugated capsular polysaccharides, Groups A,C,Y, and W135 |
Endemic—birth cohort: 115.1 million Epidemic—birth cohort in areas subject to epidemics: 13.1 million |
Endemic—probable age of vaccination (<1 year) to peak of disease (2 years), i.e., approx. 1 year. Epidemic—probable age of vacc. to peak of disease (approx. 11 years), i.e., approx. 6 years |
|
Parainfluenza viruses (Infants) |
Trivalent, subunit vaccine (which must contain fusion proteins) |
Birth cohort: 115.1 million |
Probable age of vaccination (<1 year) to peak of illness (2 years), i.e., approx. 1 year |
|
Plasmodium spp. (All infants at risk, military personnel, travelers) |
Plasmodium falciparum, synthetic or recombinant sporozoite antigen preparation |
Birth cohort in major areas of occurrence: 78 million |
Probable age of vaccination (<1 year) to peak of most severe consequences (i.e., death in young children 2–5 years) i.e., approx. 3 years |
|
Multivalent synthetic or recombinant sporozoite antigen preparation (P. falciparum, P. vivax, P. ovale, P. malariae) |
|||
|
Rabies virus (Individuals at high risk, plus post-exposure prophylaxis) |
Vero cell |
Yearly number of individuals requiring post-exposure prophylaxis as estimated by WHO: 5.6 million |
Less than 1 year |
|
(As above) |
Glycoprotein produced by rDNA technology in mammalian cells |
Yearly number of individuals requiring post-exposure prophylaxis as estimated by WHO: 5.6 million |
Less than 1 year |
|
(Birth cohort in areas of high risk) |
Attenuated live vector virus containing gene for protective glycoprotein antigen |
Birth cohort in high risk areas: approximately 53 million |
Probable age of vaccination 2–4 years Highest risk group is assumed to be teenagers; delay in benefits is assumed to be 10 years |
|
Respiratory syncytial virus (Infants at earliest possible age) |
Polypeptides produced by recombinant DNA technology |
Birth cohort: 115.1 million |
Probable age of vaccination (<1 year) to peak of illness (2 years), i.e., approx. 1 year |
|
Attenuated live virus |
Time to Adoption and Steady-State Yield of Benefits
After licensure, the utilization of a vaccine increases until it reaches a steady state. Vaccination program costs also increase to a steady state (as would the numbers of adverse effects, if applicable). The rate at which a vaccine is adopted depends on provider and target population attitudes toward the new vaccine and other issues discussed in Chapter 6.
Predictions of the time to adoption for the various candidate vaccines are shown in Table 7.2. The major factor considered in estimating these times was the perception in the developing world of the seriousness of the disease threat. The times may be affected by such factors as governmental or donor purchase programs, or by the combination of new vaccines with vaccines currently delivered via the World Health Organization Expanded Program on Immunization (WHO-EPI). These factors were not considered in arriving at the times in Table 7.2, but the effects of adopting alternative values could be evaluated easily (see Appendix F).
The time between the probable age of vaccine administration and the probable age of disease occurrence without vaccination is termed the delay of vaccination benefits. This time must be determined separately for each vaccine (consider the difference between the vaccines for Hemophilus influenzae type b and hepatitis B virus) and is a component of the total time to the steady-state yield of vaccine benefits. Information used to determine the delay is shown in Table 7.1, and the derivation of the time to steady-state yield of benefits is shown in Table 7.2.
In the calculations presented later in this chapter, the present values of vaccine costs are calculated on the assumption that costs are incurred from the time at which steady-state vaccine use is achieved. The present values of health benefits and expected reduction in morbidity costs are calculated on the assumption that the benefits and reduced costs occur at the time of steady-state yield of vaccine benefits. Equations for deriving present values are given in Chapter 3.*
|
* |
If desired, a more exact procedure can be used for these calculations—one that accounts for the presumably linear increase from zero at the time of licensure to the values at the steady state. This is accomplished by substituting in the discounting process (Chapter 3) an adjusted time, T*, for the time to adoption or for the time after licensure to a steady-state yield of benefits (time to adoption plus delay of vaccination benefits). The general equation defining T* is T*=−1/r 1n[1/2 (1+e−rT)], where T is the time to adoption or the time from licensure to steadystate yield of benefits, and r is the discount rate, in this analysis, the discount rate adopted is 0.05, so T*=−20 1n[1/2 (1+e−0.05T)]. |
TABLE 7.2 Times Associated with Vaccine Use and Benefits
|
Pathogen (Target Population) |
Type of Vaccine |
Time to Licensure (years) |
Time After Licensure to Adoption (years) |
Total Time to Steady State of Vaccine Use (years) |
Delay of Vaccination Benefits (years) |
Total Time to SteadyState Yield of Benefits (years) |
|
Dengue virus (Infants and children in endemic areas; travelers to endemic areas) |
Attenuated live vector virus containing gene for broadly cross-reacting protective antigen |
10 |
2 |
12 |
5 |
17 |
|
Escherichia coli (enterotoxigenic) (Infants < 6 months) |
A combination of purified colonization factor antigens and possibly other antigens |
10 |
2 |
12 |
1 |
13 |
|
Genetically engineered attenuated strains |
10 |
2 |
12 |
1 |
13 |
|
|
Hemophilus influenzae type b (Infants) |
Conjugated polysaccharide |
3 |
5 |
8 |
2 |
10 |
|
Hepatitis A virus (Susceptibles of all ages; routine for preschool children) |
Attenuated live virus |
4 |
5 |
9 |
2 |
11 |
|
Polypeptide recombinant vaccine produced in yeast |
5 |
5 |
10 |
2 |
12 |
|
|
Hepatitis B virus (Areas with high perinatal infection: all infants at birth (if possible). Other areas: all infants, simultaneous With other vaccinations, at earliest possible age) |
Polypeptide produced by recombinant DNA technology |
1 |
2 |
3 |
30 |
33 |
|
Japanese encephalitis virus (Children in epidemic and endemic areas; foreign visitors to epidemic regions) |
Inactivated virus produced in cell culture |
7 |
2.5 |
9.5 |
7 |
16.5 |
|
Pathogen (Target Population) |
Type of Vaccine |
Time to Licensure (years) |
Time After Licensure to Adoption (years) |
Total Time to Steady State of Vaccine Use (years) |
Delay of Vaccination Benefits (years) |
Total Time to Steady-State Yield of Benefits (years) |
|
Mycobacterium leprae (Immunoprophylactic: all children in endemic areas. Immuno therapeutic: all recently infected individuals) |
Armadillo-derived M. leprae |
10 |
5 |
15 |
6 |
21 |
|
Neisseria meningitidis (Infants, 3 to 6 months) |
Conjugated capsular polysaccharides, Groups A,C,Y, and W135 |
5 |
5 |
10 |
3 |
13 |
|
Parainfluenza viruses (Infants) |
Trivalent, subunit vaccine (which must contain fusion proteins) |
5 |
5 |
10 |
1 |
11 |
|
Plasmodium spp. (All infants at risk, military personnel, travelers) |
Plasmodium falciparum, synthetic or recombinant sporozoite antigen preparation |
6.5 |
2 |
8.5 |
3 |
11.5 |
|
Multivalent synthetic or recombinant sporozoite antigen preparation (P. falciparum, P. vivax, P. ovale, P. malariae) |
9 |
2 |
11 |
3 |
14 |
|
|
Rabies virus (Individuals at high risk, plus post-exposure prophylaxis) (As above) |
Vero cell |
3 |
3.5 |
6.5 |
1 |
7.5 |
|
Glycoprotein produced by rDNA technology in mammalian cells |
3 |
3.5 |
6.5 |
1 |
7.5 |
|
|
(Birth cohort in areas of high risk) |
Attenuated live vector virus containing gene for protective glycoprotein antigen |
10 |
2 |
12 |
10 |
22 |
|
Respiratory syncytial virus (Infants at earliest possible age) |
Polypeptides produced by recombinant DNA technology |
5 |
2 |
7 |
1 |
8 |
|
Attenuated live virus |
5 |
2 |
7 |
1 |
8 |
|
|
Rotavirus (Infants at earliest possible age. preferably with oral polio vaccine) |
Attenuated high passage bovine rotavirus |
2 |
2 |
4 |
1 |
5 |
|
Attenuated low passage bovine rotavirus |
5 |
2 |
7 |
1 |
8 |
|
|
Rhesus monkey rotavirus |
5 |
2 |
7 |
1 |
8 |
|
|
Salmonella typhi (Children; young adults at risk; travelers from developed countries to endemic areas) |
Attenuated ga1E mutant S. typhi strain TY21a |
1 |
2 |
3 |
13 |
16 |
|
Aromatic amino acid dependent strains of S. typhi |
6.5 |
2 |
8.5 |
13 |
21.5 |
|
|
Shigella spp. (Infants at birth or earliest possible age; elderly for epidemic strains) |
Probably plasmid mediated outer membrane protein invasion determinant (a small number of options need investigation to determine best approach) |
10 |
2 |
12 |
1 |
13 |
|
Streptococcus A (Children, < 3 to 4 years) |
Synthetic M protein segment (excluding portions cross-reacting with human tissue) |
7 |
3.5 |
10.5 |
9 |
19.5 |
|
Streptococcus pneumoniae (Infants) |
Conjugated polysaccharides, polyvalent |
5 |
2 |
7 |
2 |
9 |
|
Vibrio cholera (Children, especially < 2 years) |
Genetically defined live mutant V. cholerae (A−B+ or A−B−) with respect to toxin subunit synthesis |
6 |
2 |
8 |
2 |
10 |
|
Inactivated antigens |
4 |
2 |
6 |
2 |
8 |
|
|
Yellow fever virus (Young children) |
Attenuated live virus produced in cell culture |
3 |
2 |
5 |
14 |
19 |
Costs
Ideally, calculation of the annual costs associated with each of the vaccine candidates would include three major components: (1) the reduction in morbidity costs, (2) the vaccination program costs, and (3) the vaccine development costs. The costs of adverse reactions also should be calculated, if applicable. Such a comprehensive calculation can be attempted where reliable estimates of treatment costs can be made. However, as discussed in Chapter 4, the committee judged that attempting to incorporate the average cost of disease treatment(s) in the developing world into the calculations would be unrealistic. Additionally, the committee assumed that all vaccine candidates would, if developed, be delivered through the WHO-EPI; hence, possible differential utilization or delivery costs would not be a factor in the priority selection of vaccine candidates.
Because of these judgments, the cost comparisons in this analysis are somewhat simplified, including only those expenditures on the candidate vaccines necessary to achieve the potential health benefits. Table 7.3 shows these expenditures: vaccine development costs and vaccine costs for the immunization programs. The annual number of new potential vaccinees is derived as shown in Table 7.1, and Table 5.1 indicates the predicted cost per dose (price) for each vaccine and the number of doses required.
THE TREATMENT OF VACCINE IMPROVEMENT PROJECTS
Some clarification of the assumptions underlying vaccine improvement projects may be useful. In these cases, the incremental benefit should be used in comparisons.
For all diseases, the TDBV represents the burden of illness presently occurring with the current vaccine usage. However, in some cases (cholera, yellow fever, Japanese encephalitis, N. meningitidis), the current vaccine use may not be as widespread or, because of vaccination timing or efficacy, as likely to prevent as large a proportion of the disease as would the universal pediatric approach with the improved vaccine assumed in this analysis. Hence, the current disease burden reasonably represents the disease amenable to further control.
For other diseases (H. influenzae type b) the currently available (PRP) vaccine may not be used widely in developing countries either because most disease occurs in age groups (under 2 years) for which the vaccine is ineffective or because the vaccine is relatively new. Hepatitis B vaccine is also not yet widely used in developing countries, probably because it is relatively new and expensive. For these diseases, the present total disease burden is a reasonable starting point for calculations.
Developing an improved vaccine for Streptococcus pneumoniae represents a situation where the assumptions may greatly affect the potential health benefit calculations. The current vaccine is not widely used in the developing world, probably because it is immunogenic
in children 2 years of age or older, but not in infants. It could be argued that the health benefits of an improved pneumococcal vaccine are simply the incremental benefit that could be obtained from extending protection to the child population under 2 years of age who would respond to the conjugated improved vaccine. However, a more reasonable assumption is that the current vaccine would not be used in the developing world for the reason stated above and because the delivery requirement (children 2 years and older) could not conveniently be added to existing vaccination schedules. Additionally, it is doubtful the existing vaccine would be used if the improved version becomes available. Hence, the potential benefits of an improved vaccine are calculated using the entire existing disease burden as a starting point. The proportion of the TDBV that is vaccine preventable is discussed in Appendix D-17.
This analysis does not include calculation of the potential benefits of improving any vaccine that is in widespread use in the developing world. For such calculations it is necessary to estimate the incremental benefits (e.g., in efficacy, disease proportion amenable to vaccine prevention by virtue of effectiveness at younger ages, etc.) or costs associated with its use as compared to the existing vaccine. The analyses of potential benefits for improved influenza and pertussis vaccines presented in the committee’s first report illustrate the approach needed in such calculations (Institute of Medicine, 1985).
RESULTS
The results of the central analysis, presented below, are based on the following assumptions:
-
the probability of successful development and other vaccine characteristics, e.g., efficacy, described in Chapter 5
-
the times to licensure and adoption, and the delay of vaccination benefits, as shown in Tables 5.1 and 7.2
-
a discount rate of 0.05
-
the IME perspective representing the median of values derived from public health professionals in various developing countries (as described in Chapter 4)
-
the uniformity of utilization rates across target populations (Chapter 6)
Health Benefits
Table 7.4 shows values representing the possible health benefits resulting from the development of each vaccine candidate. Total disease burden values represent the burden of illness resulting from the pathogen(s) against which the vaccine is directed.
Vaccine preventable illness values represent the burden of illness that could be averted by delivering a hypothetical vaccine that is 100
TABLE 7.3 Expenditures to Achieve Health Benefits for Various Vaccines
|
Pathogen (Target Population) |
Type of Vaccine |
Probability of Successful Development |
Cost of Development (dollars) |
Minimum Time to Steady State of Vaccine Use |
|
Dengue virus (Infants and children in endemic areas; travelers to endemic areas) |
Attenuated live vector virus containing gene for broadly cross-reacting protective antigen |
0.75 |
25,000,000 |
12 |
|
Escherichia coli (enterotoxigenic) (Infants < 6 months) |
A combination of purified colonization factor antigens and possibly other antigens |
0.50 |
25,000,000 |
12 |
|
Genetically engineered attenuated strains |
0.70 |
37,500,000 |
12 |
|
|
Hemophilus influenzae type b (Infants) |
Conjugated polysaccharide |
0.90 |
15,000,000 |
8 |
|
Hepatitis A virus (Susceptibles of all ages; routine for preschool children) |
Attenuated live virus |
0.95 |
15,000,000 |
9 |
|
Polypeptide recombinant vaccine produced in yeast |
0.95 |
25,000,000 |
10 |
|
|
Hepatitis B virus (Areas with high perinatal infection: all infants at birth (if possible). Other areas: all infants, simultaneous with other vaccinations) |
Polypeptide produced by recombinant DNA technology |
0.99 |
5,000,000 |
3 |
|
Japanese encephalitis virus (Children in epidemic and endemic areas; foreign visitors to epidemic regions) |
Inactivated virus produced in cell culture |
0.50 |
50,000,000 |
9.5 |
|
Mycobacterium leprae (Immunoprophylactic: all children in endemic areas. Immunotherapeutic: all recently infected individuals) |
Armadillo-derived M. leprae |
0.50 |
25,000,000 |
15 |
|
Neisseria meningitidis (Infants, 3 to 6 months) |
Conjugated capsular polysaccharides. Groups A,C,Y, and W135 |
0.50 |
30,000,000 |
10 |
|
Parainfluenza viruses (Infants) |
Trivalent, subunit vaccine (which must contain fusion proteins) |
0.80 |
25,000,000 |
10 |
|
Plasmodium spp. (All infants at risk, military personnel, travelers) |
Plasmodium falciparum, synthetic or recombinant sporozoite antigen preparation |
0.50 |
25,000,000 |
8.5 |
|
Multivalent synthetic or recombinant sporozoite antigen preparation (P. falciparum, P. vivax, P. ovale, P. malariae) |
0.50 |
35,000,000 |
11 |
|
Annual Number of New Potential Vaccinees |
Doses/ Course |
Cost/Dose (dollars) |
Cost/Course (dollars) |
Expected Annual Cost of Vaccination Programs (dollars) |
Annualized Present Value of Vaccination Program Costs (dollars) |
Annualized Present Value of Total Expenditures to Achieve Health Benefits (dollars) |
|
48,000,000 |
1 |
12.00 |
12.00 |
576,000,000 |
240,553,765 |
241,803,765 |
|
115,100,000 |
3 |
7.50 |
22.50 |
2,589,750,000 |
721,034,852 |
722,284,852 |
|
115,100,000 |
1 |
1.50 |
1.50 |
172,650,000 |
67,296,586 |
69,171,586 |
|
115,100,000 |
1 |
7.50 |
7.50 |
863,250,000 |
525,853,421 |
526,603,421 |
|
115,100,000 |
1 |
15.00 |
15.00 |
1,726,500,000 |
1,057,271,429 |
1,058,021,429 |
|
115,100,000 |
3 |
20.00 |
60.00 |
6,906,000,000 |
4,027,700,683 |
4,028,950,683 |
|
115,100,000 |
3 |
30.00 |
90.00 |
10,359,000,000 |
8,859,008,746 |
8,859,258,746 |
|
64,800,000 |
2 |
15.00 |
30.00 |
1,944,000,000 |
611,459,820 |
613,959,820 |
|
44,800,000 |
1 |
25.00 |
25.00 |
1,120,000,000 |
269,369,575 |
270,619,575 |
|
115,100,000 |
2 |
10.00 |
20.00 |
2,302,000,000 |
|
|
|
115,100,000 |
2 |
15.00 |
30.00 |
3,453,000,000 |
|
|
|
78,000,000 |
3 |
12.50 |
37.50 |
2,925,000,000 |
|
|
|
78,000,000 |
3 |
12.50 |
37.50 |
2,925,000,000 |
|
|
|
Pathogen (Target Population) |
Type of Vaccine |
Probability of Successful Development |
Cost of Development (dollars) |
Minimum Time to Steady State of Vaccine Use |
|
Rabies virus (Individuals at high risk, plus post-exposure prophylaxis) (As above) |
Vero cell |
0.90 |
5,000,000 |
6.5 |
|
Glycoprotein produced by rDNA technology in mammalian cells |
0.85 |
4,000,000 |
6.5 |
|
|
(Birth cohort in areas of high risk) |
Attenuated live vector virus containing gene for protective glycoprotein antigen |
0.50 |
15,000,000 |
12 |
|
Respiratory syncytial virus (Infants) |
Polypeptides produced by recombinant DNA technology |
0.80 |
25,000,000 |
7 |
|
Attenuated live virus |
0.80 |
25,000,000 |
7 |
|
|
Rotavirus (Infants, 0 to 6 months) |
Attenuated high passage bovine rotavirus |
0.90 |
10,000,000 |
4 |
|
Attenuated low passage bovine rotavirus |
0.80 |
20,000,000 |
7 |
|
|
Rhesus monkey rotavirus |
0.80 |
30,000,000 |
7 |
|
|
Salmonella typhi (Children 5 to 18; young adults; travelers from developed countries to endemic areas) |
Attenuated ga1E mutant S. typhi strain TY21a |
0.90 |
2,000,000 |
3 |
|
Aromatic amino acid dependent strains of S. typhi |
0.50 |
2,000,000 |
8.5 |
|
|
Shigella spp. (Infants at birth; elderly for epidemic strains) |
Probably plasmid mediated outer membrane protein invasion determinant (a number of promising options need investigation to determine best approach) |
0.70 |
37,500,000 |
12 |
|
Streptococcus A (Children, < 3 to 4 years) |
Synthetic M protein segment (excluding portions cross-reacting with human tissue) |
0.80 |
50,000,000 |
10.5 |
|
Streptococcus pneumoniae (Infants) |
Conjugated polysaccharides, polyvalent |
0.80 |
30,000,000 |
7 |
|
Vibrio cholera (Children, especially < 2 years) |
Genetically defined live mutant V. cholerae (A−B+ or A−B−) with respect to toxin subunit synthesis |
0.75 |
25,000,000 |
8 |
|
Inactivated antigens |
0.65 |
10,000,000 |
6 |
|
|
Yellow fever virus (Young children) |
Attenuated live virus produced in cell culture |
0.95 |
15,000,000 |
5 |
|
Annual Number of New Potential Vaccinees |
Doses/ Course |
Cost/Dose (dollars) |
Cost/Course (dollars) |
Expected Annual Cost of Vaccination Programs (dollars) |
Annualized Present Value of Vaccination Program Costs (dollars) |
Annualized Present Value of Total Expenditures to Achieve Health Benefits (dollars) |
|
5,600,000 |
4 |
10.00 |
40.00 |
224,000,000 |
|
|
|
5,600,000 |
4 |
10.00 |
40.00 |
224,000,000 |
|
|
|
53,000,000 |
1 |
1.00 |
1.00 |
53,000,000 |
|
|
|
115,100,000 |
2 |
15.00 |
30.00 |
3,453,000,000 |
|
|
|
115,100,000 |
1 |
15.00 |
15.00 |
1,726,500,000 |
|
|
|
115,100,000 |
1 |
10.00 |
10.00 |
1,151,000,000 |
|
|
|
1,15,100,000 |
1 |
10.00 |
10.00 |
1,151,000,000 |
|
|
|
115,100,000 |
1 |
10.00 |
10.00 |
1,151,000,000 |
|
|
|
115,100,000 |
2 |
2.00 |
4.00 |
460,400,000 |
357,939,747 |
358,039,747 |
|
115,100,000 |
2 |
2.00 |
4.00 |
460,400,000 |
152,053,450 |
152,153,450 |
|
115,100,000 |
1 |
2.00 |
2.00 |
230,200,000 |
89,728,782 |
91,603,782 |
|
115,100,000 |
2 |
5.00 |
10.00 |
1,151,000,000 |
551,667,844 |
554,167,844 |
|
115,100,000 |
1 |
20.00 |
20.00 |
2,302,000,000 |
1,308,790,738 |
1,310,290,738 |
|
22,200,000 |
1 |
2.00 |
2.00 |
44,400,000 |
22,538,751 |
23,788,751 |
|
22,200,000 |
2 |
2.00 |
4.00 |
88,800,000 |
43,071,553 |
43,571,553 |
|
24,800,000 |
1 |
5.00 |
5.00 |
124,000,000 |
92,299,382 |
93,049,382 |
TABLE 7.4 Health Benefits Associated with Various Vaccines
|
Pathogen (Target Population) |
Type of Vaccine |
Probability of Successful Development |
Time to Steady-State Yield of Vaccine Benefits |
Total Disease Burden Value |
|
Dengue virus (Infants and children in endemic areas; travelers to endemic areas) |
Attenuated live vector virus containing gene for broadly cross-reacting protective antigen |
0.75 |
17 |
34,365 |
|
Escherichia coli (enterotozigenic) (Infants < 6 months) |
A combination of purified colonization factor antigens and possibly other antigens |
0.50 |
13 |
978,248 |
|
Genetically engineered attenuated strains |
0.70 |
13 |
978,248 |
|
|
Hemophilus influenzae type b (Infants) |
Conjugated polysaccharide |
0.90 |
10 |
471,336 |
|
Hepatitis A virus (Susceptibles of all ages; routine for preschool children) |
Attenuated live virus |
0.95 |
11 |
30,229 |
|
Polypeptide recombinant vaccine produced in yeast |
0.95 |
12 |
30,229 |
|
|
Hepatitis B virus (Areas with high perinatal infection: all infants at birth (if possible). Other areas: all infants, simultaneous with other vaccinations) |
Polypeptide produced by recombinant DNA technology |
0.99 |
33 |
2,394,256 |
|
Japanese encephalitis virus (Children in epidemic and and endemic areas; foreign visitors to epidemic regions) |
Inactivated virus produced in cell culture |
0.50 |
16.5 |
18,075 |
|
Mycobacterium leprae (Immunoprophylactic: all children in endemic areas. Immunotherapeutic: all recently infected individuals) |
Armadillo-derived M. leprae |
0.50 |
21 |
657,349 |
|
Neisseria meningitidis (Infants, 3 to 6 months) |
Conjugated capsular polysaccharides. Groups A,C,Y, and W135 |
0.50 |
13 |
68,252 |
|
Parainfluenza viruses (Infants) |
Trivalent, subunit vaccine (which must contain fusion proteins) |
0.80 |
11 |
145,954 |
|
Plasmodium spp. (All infants at risk, military personnel, travelers) |
Plasmodium faleiparum, synthetic or recombinant sporozoite antigen preparation |
0.50 |
11.5 |
2,111,795 |
|
Multivalent synthetic or recombinant sporozoite antigen preparation (P.falciparum, P.vivax, P.ovale, P.malariae) |
0.50 |
14 |
2,111,795 |
|
Proportion of Disease that is Vaccine Preventable |
Vaccine Preventable Illness Value |
Predicted Vaccine Efficacy |
Possible Reduction in Morbidity and Mortality |
Annualized Present Value of Health Benefits |
|
1.00 |
34,365 |
0.85 |
29,210 |
9,558 |
|
0.65 |
635,861 |
0.75 |
476,896 |
126,454 |
|
0.50 |
489,124 |
0.80 |
391,299 |
145,260 |
|
0.90 |
424,202 |
0.90 |
381,782 |
210,943 |
|
1.00 |
30,229 |
0.90 |
27,206 |
15,112 |
|
1.00 |
30,229 |
0.90 |
27,206 |
14,392 |
|
0.50 |
1,197,128 |
0.90 |
1,077,415 |
213,192 |
|
1.00 |
18,075 |
0.80 |
14,460 |
3,232 |
|
1.00 |
657,349 |
0.75 |
493,012 |
88,481 |
|
0.95 |
64,839 |
0.80 |
51,872 |
13,754 |
|
0.80 |
116,763 |
0.80 |
93,411 |
43,692 |
|
0.99 |
2,082,019 |
0.80 |
1,665,615 |
475,190 |
|
1.00 |
2,111,795 |
0.80 |
1,689,436 |
426,640 |
|
Pathogen (Target Population) |
Type of Vaccine |
Probability of Successful Development |
Time to Steady-State Yield of Vaccine Benefits |
Total Disease Burden Value |
|
Rabies virus (Individuals at high risk, plus post-exposure prophylaxis) (as above) |
Vero cell |
0.90 |
7.5 |
67,821 |
|
Glycoprotein produced by rDNA technology in mammalian cells |
0.85 |
7.5 |
67,821 |
|
|
(Birth cohort in areas of high risk) |
Attenuated live vector virus containing gene for protective glycoprotein antigen |
0.50 |
22 |
67,821 |
|
Respiratory syncytial virus (Infants) |
Polypeptides produced by recombinant DNA technology |
0.80 |
8 |
183,326 |
|
Attenuated live virus |
0.80 |
8 |
183,326 |
|
|
Rotavirus (Infants, 0 to 6 months) |
Attenuated high passage bovine rotavirus |
0.90 |
5 |
925,042 |
|
Attenuated low passage bovine rotavirus |
0.80 |
8 |
925,042 |
|
|
Rhesus monkey rotavirus |
0.80 |
8 |
925,042 |
|
|
Salmonella typhi (Children; young adults at risk; travelers from developed countries to endemic areas) |
Attenuated ga1E mutant S. typhi strain TY21a |
0.90 |
16 |
1,308,121 |
|
Aromatic amino acid dependent strains of S. typhi |
0.50 |
21.5 |
1,308,121 |
|
|
Shigella spp. (Infants at birth; elderly for epidemic strains) |
Probably plasmid mediated outer membrane protein invasion determinant (a small number of promising options need investigation to determine best approach) |
0.70 |
13 |
828,068 |
|
Streptococcus A (Children, < 3 to 4 years) |
Synthetic M protein segment (excluding portions cross-reacting with human tissue) |
0.80 |
19.5 |
811,477 |
|
Streptococcus pneumoniae (Infants) |
Conjugated polysaccharides, polyvalent |
0.80 |
9 |
6,612,261 |
|
Vibrio cholera (Children, especially < 2 years) |
Genetically defined live mutant V. cholerae (A−B+ or A−B−) with respect to toxin subunit synthesis |
0.75 |
10 |
229,217 |
|
Inactivated antigens |
0.65 |
8 |
229,217 |
|
|
Yellow fever virus (Young children) |
Attenuated live virus produced in cell culture |
0.95 |
19 |
32,887 |
|
Proportion of Disease that is Vaccine Preventable |
Vaccine Preventable Illness Value |
Predicted Vaccine Efficacy |
Possible Reduction in Morbidity and Mortality |
Annualized Present Value of Health Benefits |
|
1.00 |
67,821 |
0.99 |
67,143 |
41,910 |
|
1.00 |
67,821 |
0.95 |
64,430 |
37,983 |
|
0.75 |
50,866 |
0.95 |
48,322 |
8,260 |
|
0.66 |
120,995 |
0.80 |
96,796 |
52,412 |
|
0.75 |
137,495 |
0.80 |
109,996 |
59,559 |
|
1.00 |
925,042 |
0.80 |
740,034 |
521,852 |
|
1.00 |
925,042 |
0.90 |
832,538 |
450,795 |
|
1.00 |
925,042 |
0.90 |
832,538 |
450,795 |
|
1.00 |
1,308,121 |
0.80 |
1,046,497 |
431,471 |
|
1.00 |
1,308,121 |
0.85 |
1,111,903 |
194,745 |
|
0.85 |
703,858 |
0.85 |
598,279 |
222,096 |
|
0.90 |
730,329 |
0.80 |
584,263 |
180,513 |
|
0.50 |
3,306,131 |
0.80 |
2,644,904 |
1,363,943 |
|
1.00 |
229,217 |
0.90 |
206,295 |
94,986 |
|
1.00 |
229,217 |
0.65 |
148,991 |
65,548 |
|
1.00 |
32,887 |
0.90 |
29,598 |
11,127 |
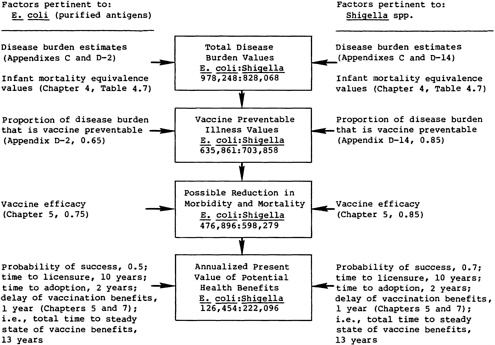
FIGURE 7.3 The sequence of calculations for comparing the potential health benefits of two vaccine candidates.
percent effective to the entire target population, at the time envisaged for immunization. The values for the possible reduction in morbidity and mortality take into account the vaccine’s predicted efficacy.
The annualized present value of the expected health benefits incorporates the probability of successful development and the time at which benefits would occur. Use of these values to compare health benefits from these vaccines is discussed in Chapter 9.
An Illustration of the Process
Figure 7.3 illustrates the sequence of calculations in the proposed method for comparing the health benefits expected from two vaccine candidates. Chapter 3 and the foregoing text describe the specific computations involved at each stage.
Costs
The vaccine expenditures associated with achieving each vaccine candidate’s benefits are shown in Table 7.3.
The annualized present value of vaccine costs incorporates the annual number of potential new vaccines; the cost per dose; and the number of doses required, adjusted for the probability of successful vaccine development and the time at which steady-state vaccine use would be achieved. The annualized present value of vaccine expenditures incorporates the vaccine cost for the immunization program and the annualized vaccine development cost (i.e., the discount rate multiplied by the cost of development).
These cost calculations can be used to compare vaccine candidates from various viewpoints, as discussed in Chapter 9. Sensitivity analyses described in that chapter also show the effects on the priority rankings of assumptions other than those in the central analysis.
The health benefit values and the vaccine expenditures necessary to achieve these benefits do not incorporate adjustments for utilization, which was judged to be uniform in this analysis. Thus, values represent potential benefits and expenditures.
REFERENCES
Institute of Medicine. 1985. New Vaccine Development: Establishing Priorities, Volume I. Diseases of Importance in the United States. Washington, D.C.: National Academy Press.
Weinstein, M.C., and W.B. Stason. 1977. Foundations of cost-effectiveness analysis for health and medical practices. N. Engl. J. Med. 296(13):716–721.
























