
FIGURE 1: The U.S. balance of trade. The plots illustrate the continued net positive contribution of the chemical industry to the overall U.S. position. Source: U.S. Department of Commerce, Bureau of the Census.
The U.S. chemical industry added about $153 billion in value to the approximately $137 billion worth of raw materials it processed in 1990. 2
The U.S. chemical trade balance, which has been consistently positive in the last decade, grew to a surplus of $19 billion in 1991; by contrast, the United States had a net trade deficit of more than $65 billion in that same year. 3 (See Figure 1.)
|
1 |
U.S. Department of Commerce, Bureau of the Census, Industry Division. |
|
2 |
U.S. Department of Commerce, Bureau of the Census, 1990 Annual Survey of Manufacturers, M90(AS)-1. |
|
3 |
Chemical & Engineering News, December 9, 1991, pp. 41, 43; June 29, 1992, p. 62. |

Hybrid resins provide a unique combination of strength, stiffness, and a smooth, corrosion-resistant surface for manufacturing applications such as the sailboard shown here.

Research on polymers that resist high temperatures has resulted in the development of lightweight synthetic fabrics for the protective clothing of firefighters.
What about research and development (R&D), our investment in future economic growth? The U.S. chemical industry spent $12.7 billion on R&D in 1990, 12 percent of total U.S. industrial R&D expenditures; 78,000 scientists and engineers were engaged in chemical industry R&D activities in that year. 4 Moreover, the chemical industry has been steadily increasing its R&D expenditures, a strategy that undoubtedly contributes to its international competitiveness. Thus, R&D in the chemical industry appears to play a major role in the economic strength of the nation. But chemical research activities go far beyond the activities of the chemical industry alone. For example, the petroleum industry is a major employer of chemists and chemical engineers in its research laboratories.
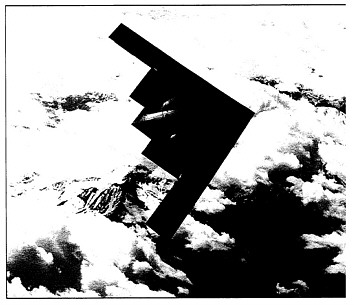
Carbon fiber reinforces the precision-contoured skin of the B-2 advanced-technology aircraft. The combination of high strength and light weight of the carbon fiber material contributes significantly to the performance of this unique “flying wing” aircraft.
The products of the chemical process industry are quite diverse; some of the more important ones are bulk chemicals (organic and inorganic), bulk polymers, fuels and lubricants, fertilizers, pesticides and fungicides, paints and coatings, elastomers, soaps and detergents, specialty chemicals, propellants and explosives, ceramics, plastic films, membranes (organic and inorganic), drugs and diagnostic products, catalysts and adsorbents, electronic and photonic materials, functional polymers (e.g., adhesives), biomaterials, food products, and synthetic fibers. These products have a broad influence on U.S. commerce. Consider, for example, polymers and the effects that they have on our everyday lives. For automobiles, polymers need to be stronger, lighter, and more readily recyclable. In aerospace applications, lighter and stronger polymer composites would offer fuel savings, enhanced safety, and higher performance. In defense applications, new polymers will lead the way as materials of construction for many new weapons systems components. Improved polymers for food packaging permit safe delivery of food to a larger fraction of the world's hungry, while recyclability and
|
4 |
Chemical & Engineering News, August 19, 1991, pp. 53, 55. |

Modern fabric for athletic clothing. Water vapor from perspiration can pass through the fabric, but water from rain or snow does not penetrate the water-repellant finish.
degradability of these packaging materials will help reverse the unwanted buildup of solid waste in industrialized societies.
New products must be developed to eliminate environmental problems, to meet legislative requirements in the energy and transportation sectors, and to enable further medical advances. The electronics industry is in need of new synthetic materials for the production of electronic components, and all segments of industry are encountering the increasing pressures of international competition.
Chemical technologies pervade our lives. They are essential for our economic well-being and competitiveness, for our security, and for our health. Chemical process technologies and their corresponding products are integral parts of the highly competitive international marketplace.
Plastic Components for Automobiles
The plastics content of automobiles is currently about 8 percent of vehicle weight, a percentage that is growing. The development of new polymer materials and advances in polymer processing are contributing to this transition from metals to plastics. One driving force for the change is the need for weight reduction to obtain improved fuel economy. New high-performance plastic materials have the toughness, hardness, and weather resistance that are required for rigid structural components. The development of improved reinforcements and adhesive bonding technologies will hasten the continued introduction of plastic components.
Because plastics can be injection-molded, they offer a wider range of complex shapes than can be obtained with metals. This leads to a reduction in the number of parts that must be assembled, affording greater simplicity and reduced assembly labor requirements. This favorable impact on
manufacturability also affects the quality of assembled products. The use of fuels containing methanol requires new plastics for use in fuel systems; these plastics must exhibit both chemical resistance and low-temperature mechanical flexibility. Plastic body panels require a smooth surface finish, fast demolding, and resistance to stress cracking. Improved paint systems with lower solvent emissions are also needed to meet volatile organic compound emission requirements during manufacture. Plastics used in lighting fixtures require melt-flow stability, heat resistance, and stability to ultraviolet (UV) light.
Now coming to market are new materials that meet these requirements. Some examples are thermoplastic bumpers that eliminate the need for metal reinforcements and polypropylene fiber for trim applications that are colorfast and UV-stable.

Bumper fascias on this 1992 automobile are molded from copolyester thermoplastic elastomer that offers heat resistance and paintability.
Plastics recycling and recyclability are being considered early in the design process both in terms of material selection and disassembly techniques. Efficient recycling requires the use of plastics that are resistant to process-induced degradation, a property achieved by the addition of chemical antioxidants. Recycling of mixed plastics alloys and blends must be better understood before recycled components can be manufactured for demanding applications. Alternatively, reducing the number of different plastics used in automobile manufacturing could also result in increased use of components made of blends of recycled plastics.
Manufacturing of plastic parts relies on such research areas as polymer melt flow rheology, structure-property relationships, and the kinetics of the curing process. Optimizing the manufacturing process for productivity, product quality, and durability of plastics relies on the inputs from extensive research, much of it by chemists and chemical engineers.
Petrochemicals
The organic chemical industry was originally developed on the basis of coal chemistry. The increasing availability of petroleum and natural gas, together with advances in catalytic processing, eventually permitted a nearly complete conversion to chemicals based on these new raw materials. The important products of the petrochemicals industry include chemical building blocks for polymers, synthetic fibers, pharmaceutical intermediates, and fertilizers. Among the major U.S.-based developments is the heterogeneous catalytic ammoxidation of propylene to form acrylonitrile; this large-volume chemical intermediate is a source of synthetic acrylic fibers and of the important structural polymer acrylonitrile-butadiene-styrene (ABS) that is used, for example, in telephone and computer housings. More recently, a new homogeneous catalytic process has been commercialized for the reaction of butadiene and hydrogen cyanide to form adiponitrile, a building block for nylon.

Cracking unit produces ethylene from petroleum feedstock.
Concern about environmental pollution has prompted many important improvements in petrochemical processes. For example, aromatic amines (starting chemicals for a type of synthetic fibers) are formed by catalytic nitration using concentrated sulfuric acid in the liquid phase. This reaction is not very selective and results in a number of by-products that require disposal. Solid acid catalysts, currently under development, may result in improved yield and reduced pollution at the same time. An exciting recent development is the catalytic conversion of the common hydrocarbon butane to isobutylene, a compound that is key to the manufacture of methyl tertiary-butyl ether (MTBE), an oxygen-containing ingredient in reformulated gasoline that was introduced to meet recent legislative requirements for gasoline composition.
The list of breakthrough opportunities in the chemical processing industries is long, and their potential economic impact is staggering. Dehydrogenation of ethane to produce the important petrochemical feedstock ethylene, methane oxidative coupling to ethylene, and the direct partial oxidation of methane to methanol all depend on yet-to-be-made breakthroughs in catalysis research.
Ceramics for Engines
The term ceramics usually conveys an image of pottery, porcelain, or even fine china. Inorganic nonmetallic materials, ceramics can have remarkable properties. Modern ceramics, although often brittle, can survive very high temperatures and chemically aggressive environments, have exceptional resistance to wear from friction, and exhibit extreme ranges or combinations of electrical and heat conduction. Their individual characteristics depend on both their chemical compositions and the processes by which they are manufactured.
Ceramics have been used for years in the spark plugs of gasoline engines and for jet engine igniters, mainly because of their ability to act as electrical insulators while withstanding high temperatures. Recently, they have begun to replace some metal components in engines: aluminum titanate ceramics in exhaust port liners to reduce cooling system mass and volume, silicon nitride glow plugs in diesel engines for improved starting, and low-inertia silicon nitride turbocharger rotors for faster throttle response. Two major auto firms now produce over 100,000 cars annually with silicon nitride rotors. Ceramic valves are already employed in some race car engines because they have low inertial mass, and ceramic coatings on pistons are also under study to improve engine heat management.
A molten ceramic is poured into a mold for further processing. Applications include specialty materials for electronics, such as ceramic-based die-attach adhesives to replace products based on the use of precious metals.
Beyond just replacing metal components, ceramic materials have even greater potential when incorporated into basic engine design. The U.S. Army, for example, has successfully demonstrated an “adiabatic ” diesel engine in a truck. In such engines, the ceramic coatings or linings on the piston caps, cylinder walls, and cylinder head result in increased fuel efficiency and eliminate the need for the water-cooling system that is a significant source of engine failures. Dramatic improvements could also be made in other types of engines; reductions in size as well as increases in specific power output, together with fuel efficiency enhancements of up to 25 percent, have been demonstrated with ceramic turbines for cars, trucks, buses, and power-generating stationary gas turbines.
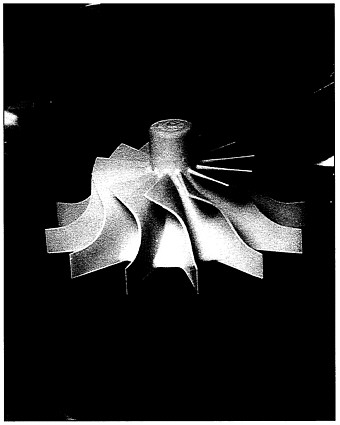
Prototype gas turbine rotor made of injection-molded monolithic silicon nitride.
For ceramics to move into broader use in engines, additional research and development are required in the basic chemistry of ceramic materials. The primary aim of such research is reduction of the brittleness that can lead to catastrophic component failure, without compromising the advantages that ceramic materials offer. What are their precise molecular structures? How do the different components of a composite interact with each other at the molecular level? Which molecular structures correlate with specific physical properties? How do the different components of ceramic composites—ceramic particles, whiskers, or fibers in a ceramic matrix—interact with each other at the microscopic level?
Processing and fabrication techniques must also be improved; the necessary advances can be made through a better understanding of the chemistry and morphology of ceramic powders and through the consolidation of these powders into ceramic components.
Ceramic materials technologies may offer automobile buyers engines that are smaller and last longer, while providing sizable savings in fuel economy in comparison with that of today's all-metal engine construction technology.
Silicon nitride powder is formed in the luminous flame produced by a carbon dioxide laser.
Coating Technologies
Several manufacturing technologies rely on controlled processes to create thin layers or films. In film casting, a viscous layer of polymer solution is laid down on a large, highly polished stainless steel drum a few feet to a few yards in diameter. As the drum is coated with solution, the solvent evaporates, leaving a thin polymer layer on the drum; this “skin” is then removed for further processing. Because film casting is solvent-based, a high degree of process control is required to minimize organic solvent emissions to the atmosphere.
Recently, extrusion technology has come into widespread use for producing photographic film support. In this methodology, the polymer melt, without any solvent, is extruded through a thin slot to produce a film that is stretched to the desired thickness and width. Cheaper and less polluting than solvent-casting methods, extrusion technology can be used to combine different polymers in a multilayer film with several desirable properties. Plastic bottles for carbonated beverages are fabricated in this manner. The plastic film that forms the bottle can be co-extruded from two different polymers, providing the necessary physical strength while preventing loss of the carbon dioxide that gives the beverage its “fizz.”

TOP: New approaches to coating technologies provide increased safety and environmental protection. Here, a special spray gun electrostatically charges the paint so that it is drawn to the airplane, resulting in fewer paint particles in the air.
Today's technology can produce thin-film materials of greater complexity than was previously possible. An example is photographic color film, in which multiple film layers are coated onto the clear plastic base to provide the storage elements for the recorded image. With a total coating thickness of about 20 microns, the individual film layers can be as thin as 1 micron, or 0.001 millimeter.
The coating machines that produce photographic film and other film products operate with high precision at speeds of up to several thousand feet per minute. As many as 10 layers of material are coated in a single pass without intermixing of the layers. This technology is the result of extensive and ongoing research by chemists and chemical engineers to continually improve film quality. Further advances will require increased fundamental knowledge about viscous flow of fluids and numerous unique characteristics of polymers, including surface properties and wetting, drying, and setting characteristics.

BOTTOM: This magnified cross section shows the dye layers in a segment of photographic color film that has been exposed and developed. Layers can be produced with very sharp boundaries and precise uniformity at thicknesses as small as 1 micron (µm).
Computer-aided Process Design
World-scale chemical processes are the design work of chemical engineers. Computer-based methods for process design emerged soon after the advent of computers that were able to carry out the needed computations. The first major users of linear programming were the oil companies in the late 1950s; ever since, the operations of petroleum refineries have been guided by linear programming to maximize profits by optimizing the product spectrum. Simulation systems capable of analyzing the performance of complete chemical processes also appeared in the late 1950s. Examples for the use of computer-based process design include the design of plants for the efficient, large-scale manufacture of major chemical intermediates or end products such as methanol, ethylene, and ammonia.
Research efforts in the late 1960s to have the computer automatically design the structure of heat exchanger networks led to design methodologies that are used today throughout the industry to create more energy-efficient processes that include power generation, steel production, and food processing. In the late 1980s, chemical engineering researchers discovered how to solve a class of scheduling problems 100 times larger than those previously possible; this work led to computer codes that saved many millions of dollars. Some of the best programming algorithms have resulted from research efforts by chemical engineers to search automatically among the possible alternatives for configuring a complete process.
Major research efforts in this area are being stimulated by the need to carry out computations for predicting the behavior of complex fluid mixtures such as the flow patterns in reactors or the flows involved in the processing of plastics. The tasks are to capture, save, and retrieve enormous amounts of data from operating plants; to aid teams of designers who create, capture, and share information on designs; and to diagnose the root causes of process upsets or plant equipment failures.
Food Packaging and Preservation
A significant fraction of the world's food production spoils before it ever reaches the table. In underdeveloped countries, the loss may be as large as 50 percent, and the food that is left may be severely compromised in its nutritional value, appearance, and taste. Food destruction can be caused by biological agents such as bacteria, fungi, rodents, and worms, by chemical agents, including oxygen and moisture, or by sunlight. Drying, addition of salt and preservatives, boiling and pasteurization, refrigeration, and protective containers of metal, ceramics, or glass have all been used to protect food from spoilage.

TOP: Barrier bags and laminated polymeric films provide protection and customer appeal for smoked and processed meats.
Highly advanced polymer films that can bar oxygen, moisture, and carbon dioxide are chemistry's best answer to the traditional metal container and are often used to retard spoilage and loss of quality in packaged food. Absolute barriers are typically used to preserve processed food, but in some cases selectively permeable polymer films provide an even better solution to food packaging. Food is a biological product that must “breathe.” To keep the desired red color of hemoglobin in meat from turning to purple, a “smart” package is needed that can prevent the loss of water while allowing relatively free exchange of oxygen and carbon dioxide with the outside air. Similarly, the packaging of fruits and cheese also requires the exchange of carbon dioxide and oxygen but not of water.

BOTTOM: Heat-set process for molding plastic bottles allows them to withstand the temperatures at which cranberry juice is bottled.
Food packaging films need other special properties in addition to selective permeability. A good barrier must be strong enough to preclude tears, punctures, and pinholes. Films used to package carbonated beverages must be able to contain carbon dioxide under pressure. “Retortable pouches” for cooking food are sterilized by boiling water or microwaving and require heat-resistant films. Other films should melt with heat so that they can be heat-sealed. And, of course, polymer films used in food packaging should be nontoxic and should contain no impurities or additives that might cause adverse health effects.
A film made of a single polymer will rarely exhibit the array of properties that might be desired for a given package, and so two or more kinds of film are often laminated together. One may retard the flow of oxygen, and another the flow of moisture; one layer may melt for heat-sealing applications, and others may serve as adhesives that bond different layers together.
Protection of the environment requires that large-volume food packaging be recycled or degraded in the environment. For example, a film might be produced with a biodegradable component such as starch, so that a package discarded by the roadside will be degraded more easily by nature.
Materials for Housing
Traditional materials have long enabled the housing industry to construct sturdy, high-quality buildings. Nevertheless, the development of new materials through chemistry and materials science could be of enormous value to the nation.
Energy-efficient buildings. Aerogels—porous silica foams—are as fire-resistant as fiberglass but are better insulators. These materials, nicknamed “solid smoke,” are now being introduced into aircraft and other high-value systems. If their price can be reduced sufficiently, they could be used in housing. Since the raw material for these lightweight gels is sand, their larger-scale manufacture depends on the development of cost-effective processes.
Cost-effective housing. There is a need for new materials that could replace wood in housing —materials that cost less, are stronger and more functional, and are aesthetically pleasing. Such materials may be developed based on new polymer or cementlike composites. Continued research efforts will result in lighter-weight concrete with improved tensile strength, with both waterproofing and fireproofing directly incorporated into these new materials.
Fire-safe buildings. Both challenges and opportunities are presented by the need to design and engineer paints, construction materials, and electrical and thermal insulators that will neither burn nor generate toxic gases when exposed to heat from the combustion of wood or other materials. Cost is the major issue, and the most practical approach for today is probably through additives and modifiers. “Intumescent” inorganic films that foam on heating and provide insulation, protective barrier coatings, improved fire retardants, catalysts that promote nontoxic products on pyrolysis, and flame-quenching systems—all are possibilities for future improvements in building fire safety.
“Smart” buildings. Significant opportunities exist for developing interactive materials and systems that could introduce entirely new functions into buildings. Electrochromic or photochromic glasses or plastics permit the transparency of windows to be varied in response to light or human input. Sensors can control air quality, humidity, and temperature and can detect

Engulfed by flames, the twelfth floor of the First Interstate Bank building in Los Angeles was consumed in this 1988 fire. The building 's steel structure survived this inferno because it was protected by a fireproof coating that prevented the steel's temperature from reaching metallurgically critical levels.
pollutants, smoke, or intruders. Although these capabilities are based on today's technology, they must be made affordable, durable, and practical so that they can be adopted by the building industry for future construction.
New construction techniques. With new materials, labor-intensive procedures such as roofing, cladding, and fabricating structural components could be carried out by robots or by mass production in automated factories. Constructing a high-rise structural steel building by robots has already been demonstrated. The adaptation of robotics could greatly increase the productivity of the construction industry while allowing for variation in design; waste could be minimized and automatically sorted. These enhancements in future construction practices will require interdisciplinary research in which chemists and chemical engineers will continue to play an important role.
MATERIALS FOR THE FUTURE
Throughout much of human existence, only naturally occurring materials were available. Progress in civilization can be measured by advances in the development of new materials: for example, the Bronze Age gave way to the more technologically sophisticated Iron Age. Today we have a great variety of man-made materials that did not exist 100 years ago, particularly in the area of polymers. But as great as the advances have been, we can continue to took forward to new and exciting developments. 1

Chemically stable, active matrix liquid-crystal-display glass is used for portable TV sets and for the video-display screens of protable computers. Further research is aimed at glass that can withstand higher processing temperature, offering higher performance at lower cost.
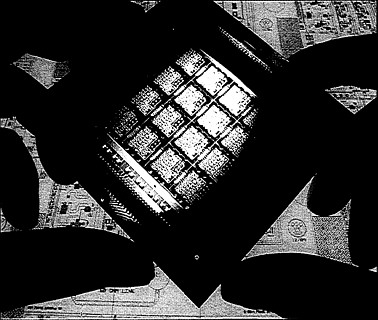
Wafer-scale integration of high-speed gallium arsenide microwavecircuits offers improved airborne radar technology.
Materials of the future will be characterized by improvements in production. Materials will be produced at lower cost and with less pollution, and they will be much more readily recycled. Improved production methods will afford materials with greater reliability and with greater diversity, in the sizes, shapes, and forms of components made of them. Such advances will be particularly important in ceramics, intermetallics, and a wide range of composites. A key component of increased process capability will be the use of more diverse processing methods and greatly enhanced process control, including in-process feedback systems to provide a high level of quality assurance.
Better matching of materials performance to increasingly broadening design needs will require new materials. An improved understanding of the relationship of materials performance with microstructural and macrostructural composition will allow more comprehensive and precise models of performance. These research advances will depend in part on the development of new diagnostic tools for characterizing materials.
Great progress can be anticipated in the development of materials that exhibit new types and ranges of performance. High-temperature superconductors and the recently discovered carbon clusters known as “buckyballs” provide but a glimpse of the possibilities. Today we know only a modest fraction of all the forms and combinations of materials achievable from elements in the periodic table. Opportunities abound, not only in the typical arena of materials made in the highly diverse world of organic molecules, but also in the realm of mixed organic and inorganic materials. A remarkable breadth is emerging in the
|
1 |
National Research Council, Materials Science and Engineering for the 1990s: Maintaining Competitiveness in the Age of Materials, National Academy Press, Washington, D.C., 1989. |

One of the fastest single-chip microprocessors is put through 1 of100 fabrication steps in a class-one, ultraclean manufacturing facility.
inorganic sector as well. At present, our use of inorganic materials is heavily weighted toward simpler compounds such as alumina, silica, and silicon nitride. There is much less emphasis on complex inorganic species, except where they occur naturally and except for a few materials such as those being developed as high-temperature superconductors. Significant developments will rely on an expansion of our understanding of structure-property relationships.
A key component of new materials will be composites with new compositional and microstructural relationships. A major new area is likely to be composites that contain two or more levels of composite structure, rather than the single level that is typically employed now. Such composites would afford better performance for a given function and also would provide for more diverse performance by combining multiple functions in a single material. One application is for adaptive or “smart” materials that will be capable of changing their physical shape, properties, or both in response to their environment in a way that improves their functionality.
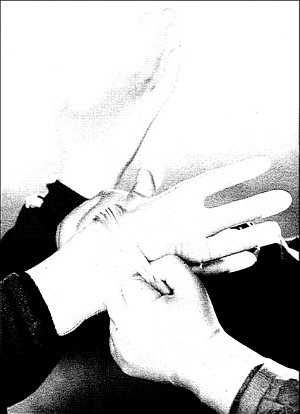
Continued research is aimed at developing protective clothing and related products such as the medical glove liners shown here, designed to be worn under latex gloves, protecting surgeons and other medical personnel from accidental cuts and exposure to blood-borne diseases such as hepatitis and AIDS.
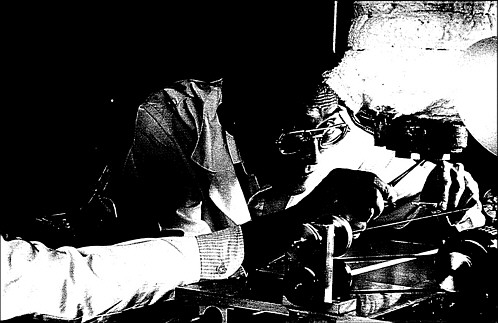
Research scientists investigate properties of composite fibers.
















