ENERGY AND TRANSPORTATION
Until the mid-nineteenth century, the combustion of renewable resources, primarily wood, served as the principal source of energy for human needs. But the massive increases in energy consumption that fueled the industrial revolution could no longer be satisfied by wood and inexhaustible power sources such as wind and flowing water. The world turned to coal and continued to rely on it until early in this century, when inexpensive petroleum became readily available.

As we approach the start of a new century, we must first recognize that our supply of fossil fuels is limited. Conventional petroleum resources in particular appear likely to become insufficient during the first half of the twenty-first century. Research into new catalytic processes will eventually lead to new technologies for producing liquid fuels from natural gas and coal. Even so, we cannot ignore the steady increase in the atmospheric burden of carbon dioxide that would result from the continued use of fossil fuels.

Extensive chemical and chemical engineering research has been essential to the development of reliable and powerful propulsion systems for our space program.
There are two ways to decrease the environmental impact of fossil fuels and to defer their ultimate exhaustion at the same time. The first is to increase the efficiency of our current fossil energy use, and the second is to develop alternative, non-fossil energy sources such as geothermal, wind, water, solar, and nuclear energy. If energy consumption continues its worldwide growth, the only alternative to our current use of petroleum may be the careful development of nuclear power. In the meantime, all of these alternative energy sources represent worthwhile areas of continued investigation. The following sections discuss the roles of chemical and chemical engineering research and their impact on the development of specific technologies associated with energy and transportation.
Designer Gasoline
The technology for conversion of fossil fuels into portable and storable transportation fuels is chemical in nature. Most molecules in gasoline, diesel fuel, or jet fuel have been chemically transformed by a catalyst in a process that changes the original molecule into one of different size and properties. Broad research efforts by chemists and chemical engineers are aimed at optimizing these chemical reactions to allow more efficient combustion, reduce volatility, or remove atoms such as sulfur and nitrogen that contribute to air pollution when the fuel burns.
Refined petroleum satisfies the present demand for clean transportation fuel at low cost. Large hydrocarbon molecules are broken down into smaller ones in the refining process known as catalytic cracking, and the amount
of gasoline and diesel fuel that can be produced from a barrel of oil depends on the cracking efficiency. As the single largest catalytic process (in terms of tons of catalyst used), the highly efficient conversion of crude oil to gasoline and diesel fuel by catalytic cracking has had a significant positive effect on the U.S. balance of payments. The future is less clear, however. As the once abundant sources of light, low-sulfur crude oil dwindle, heavier crude oils and those with higher nitrogen and sulfur contents are increasingly being used. Conversion of these heavier crudes (some of which are quite waxy, while others contain high concentrations of asphaltenes and metals) presents a challenge to chemists and chemical engineers for the design of new, more efficient catalysts. For example, catalysts with larger pore openings must be constructed to accommodate the larger molecules present in heavier crude oils. These zeolite-type catalysts are porous oxide materials that allow molecules to diffuse into their cavities, where the chemical reactions take place.

TOP: Laboratory research on designer gasoline for automobile engines.
The term designer gasoline refers to reformulated gasolines that are being developed to meet legislation on future gasoline composition. Such legislation, aimed at reducing pollution, prescribes a reduction of the percentage of aromatic hydrocarbons in gasoline and the blending of organic oxygenates into the base gasoline stock. One such oxygenate is methyl tertiary-butyl ether (MTBE); it compensates for the loss in octane number caused by a reduction in aromatics, it reduces the vapor pressure (and thus

BOTTOM: The MTBE (an oxygen-containing organic chemical) produced in this completely automated unit in a Kentucky refinery is blended into gasoline to improve its octane rating, decrease its rate of evaporation, and reduce tailpipe emissions of pollutants.
evaporative emissions) of the fuel, and it provides some reduction in vehicle tailpipe hydrocarbon and carbon monoxide emissions. Current trends suggest that the demand for such oxygenates as MTBE and ethanol may approach 1 million barrels per day by the year 2000, a level equivalent to nearly 10 percent of current U.S. petroleum production. Most of the oxygenates produced currently are derived from by-products of petroleum refining, but increases in the price of petroleum may eventually require the development of new catalysts for producing oxygenates from coal or natural gas as well.
Liquid Fuels from Natural Gas, Coal, and Shale
The United States has large reserves of natural gas, which is mainly methane. We may someday be forced to follow the lead of New Zealand, where up to one third of the liquid fuel required can now be obtained from chemical plants that convert natural gas to gasoline with zeolite catalyst technology that was originally developed in the United States. Another technology for converting natural gas into liquid fuels first uses partial oxidation to convert the natural gas into carbon monoxide and hydrogen, which are then catalytically recombined to form larger hydrocarbon molecules. New catalysts are the key for increasing the efficiency of these processes.
This plant converts natural gas to methanol, which is then processed into gasoline by a zeolite-based catalytic technology.
Intensive catalytic research is aimed at finding ways to directly convert methane into methanol or liquid fuels, without any intermediate steps.

Two different laser beams are employed for the spectroscopic determination of chemical species present in a turbulent diffusion flame. Research on chemical processes that occur during combustion can lead to more efficient use of our energy resources and to less pollution.
The world's most abundant fossil energy resource is coal, with reserves estimated at 153,000 quads (a quad equals 1015 British thermal units) or 71 percent of the world's total fossil fuel resources; 25 percent of the world's coal reserves are located in the United States. Depending on competitive technologies and on the cost of meeting environmental regulations, coal may be used increasingly for future electric power generation and for conversion to liquid fuels, replacing the less abundant petroleum resources, which total only 3,100 quads worldwide.
Shale oils, available from vast deposits in the western United States, can be converted to clean-burning liquid fuels by retorting the raw shale and then using catalysis to upgrade the shale oil liquids. Major scientific challenges lie in the development of more efficient retorts and catalysts that could allow this process to compete economically with existing petroleum-based technologies.
Fuel-efficient and Low-emission Vehicles
U.S. motorists drove their automobiles about 1.5 trillion miles in 1990; their vehicles consumed nearly 73 billion gallons of fuel in the process. The fuel crisis of the mid-1970s led to federally mandated regulations for average fuel economy in new passenger automobiles, with a floor currently set at 27.5 miles per gallon. Between 1974 and 1991 the new-car average fuel economy rose by 107 percent for the domestic fleet and by 34 percent for the imports. Fuel economy must be improved further in the years ahead through research and development to provide cost-effective, lightweight polymeric and composite structural materials, improvements in combustion efficiency, efficient storage and reuse of energy now lost through friction and waste heat, and development of emission-control technologies that permit better fuel economy while meeting increasingly stringent environmental regulations.
Motor vehicles have become less polluting as well as more fuel-efficient. With respect to pre-1974 technologies, the catalytic converter and other advances in emission control have reduced hydrocarbon and carbon monoxide emissions by 96 percent and nitrogen oxide emissions by 76 percent. Nevertheless, the 1990 Clean Air Act amendments call for further substantial reductions in emissions from gasoline-fueled vehicles. The state of California has taken a leading role in this area, adopting even more stringent vehicle emission standards, to be phased in over the 1990s and to result

This prototype electric vehicle, first displayed at the Los Angeles Auto Show in 1990, is a “zero-emissions” vehicle as mandated by California law for the late 1990s. Research and development on advanced batteries for electric vehicles must focus on improved energy density and battery lifetime. The source of pollution is transferred if the electricity for the batteries comes from a fossil-fuel-fired power plant, but the overall pollution is decreased.
ultimately in “ultralow-emission vehicles” with almost a factor-of-10 reduction in tailpipe hydrocarbon emissions in comparison with today's federal standard.
New technology is needed to meet these regulations. Durable catalysts must be developed that remove more of the hydrocarbons and carbon monoxide emitted at low temperatures, particularly right after start-up of the vehicle. Diagnostics for sensing and controlling the operation of the catalyst must be further developed. The new regulations also mandate the use of reformulated gasoline, requiring a better understanding of the relationship between fuel composition and engine exhaust composition.
Concerns over air pollution are directing increasing interest to vehicles that use such alternate fuels as methanol or compressed natural gas. New emission control devices will be needed to handle their very different exhaust hydrocarbon constituents, including partially oxidized compounds such as aldehydes. The most dramatic change in automobile power systems is that mandated by the California requirements for a “zero-emissions” vehicle or electric car, which must make up 2 percent of all vehicles sold in the state by 1998. To meet that target, aggressive research is required on low-cost, long-life, high-energy-density batteries.
Portable Electric Power
Numerous technologies demand mobile electric power supplied by batteries. Improvements in batteries have enabled the development of new products, many having large markets. Examples include camcorders as well as laptop and notebook computers, complete with bright displays and large-capacity hard disks; long-lived cardiac pacemakers that make frequent surgical replacement unnecessary; cordless power tools that enhance freedom of mobility in the workplace; intelligent, motorized cameras for high-performance photography; blood sugar analyzers no larger than a fountain pen
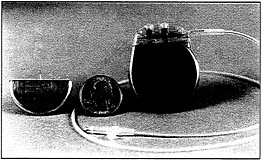
A cardiac pacemaker containing reliable batteries that are the product of research by electrochemists and materials scientists.
that improve the quality of life for diabetics; cellular telephones that allow sophisticated communications from almost any place; and electric vehicles with zero tailpipe emissions.
The definition of a “better” battery will depend on the application. High energy-storage capacity, small size, ability to sustain recharge over many recharge cycles, and long shelf life are all important goals in battery technology. Additional challenges include the need to make discarded batteries less harmful to the environment and the need to increase safety requirements as the energy density of batteries is increased.
Fuel Cells
A promising alternative for converting fuel to electricity is by way of direct oxidation in a fuel cell. Here, in contrast to combustion engines and power plants, the conversion process is electrochemical in nature, and efficiency is not limited by the Carnot-cycle thermodynamics of heat engines. Current research and development have afforded fuel cells that operate with 45 to 60 percent overall energy efficiency (compared with approximately 37 percent for fossil-fuel-fired power plants) and that offer several additional advantages: they can be recharged simply by filling the fuel tank, there is no need to store an oxidizing agent, and combustible fuels can be used without generating noxious nitrogen oxides.
High-temperature, solid-state fuel cells can use hydrocarbon fuels for the net production of water and carbon dioxide plus a flow of electricity through an external circuit. In addition, their hot exhaust is suitable for the co-production of steam, further boosting their overall thermodynamic efficiency. Fuel cells typically require methanol or hydrogen as the fuel. Hydrogen is usually produced chemically, starting with fuels such as methane, propane, and methanol. In a fuel cell system, many cells must be connected in series to produce high voltage. Considerable additional research will be required for the development of more practical and economical fuel cells for terrestrial applications. But if improved catalysts for the
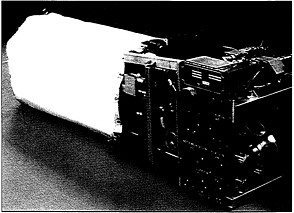
Space Shuttle 12-kilowatt fuel cell, used for the catalytic conversion of chemical energy to electricity.
electrochemical processes and improved materials for construction can be developed, fuel cells offer outstanding potential for cleaner and more efficient energy; much of that research will be done by chemists and chemical engineers.
Nuclear Energy: Promise and Problems
Nuclear fission, which produces energy as a by-product of neutron-induced cleavage of uranium nuclei, accounts for approximately one fifth of the electricity generated in the United States, substantially less than in other industrialized countries. France, for example, derives about three quarters of its electrical power from nuclear energy. Highly publicized accidents such as those at Three Mile Island and Chernobyl have raised public apprehension about nuclear power, however.
If the United States continues with a commitment to water-cooled reactors using enriched fuels, it will eventually become necessary to build new uranium enrichment facilities. Whether these enrichment facilities are the current gaseous diffusion type or use more environmentally friendly alternatives depends on advances in research associated with isotope separation. Laser chemistry may find applications in isotope separation, not only for nuclear fuels, but also for the low-cost separation of stable isotopes for medical and research purposes.
Breeder reactors, in which the nonfissioning but more abundant isotope of uranium is converted to fissionable reactor fuel, remain under development. This technology requires reprocessing of the nuclear fuel because the buildup of fission products decreases the efficiency of the nuclear reaction. A proposed new type of reactor would employ molten-salt electrochemical processing of metal fuel rather than aqueous reprocessing of metal oxide fuel; successful implementation would require continued chemically related research and development in fields ranging from chemical metallurgy and electrochemical engineering to waste processing.
Nuclear fusion, the source of the sun's energy, is a potentially inexhaustible energy resource because the raw materials—the hydrogen atoms of water—are in abundant supply. Moreover, this means of energy production would not produce large quantities of radioactive waste. The major goal at present is proof of the principle, and practical power generation remains on the far horizon. Current research is focused on fusion of two isotopes of hydrogen, deuterium and tritium. Deuterium can be separated from seawater, and tritium, which is radioactive, could be generated from neutron bombardment of lithium in a fusion reactor. Chemists and chemical engineers are heavily involved in nuclear power-related research ranging from tritium generation to high-temperature heat transfer.
Solar-Electric Power Generation
Solar energy is ubiquitous, free, and continuously replenished. In principle, it can be converted to electricity or a fuel such as hydrogen by processes that have no adverse environmental impact. The use of photovoltaic devices to convert solar energy directly to electricity, due to its high cost, is currently limited to specialty niche applications. Large-scale plants for photoelectric power or fuel production require a large collection area and reliable sunshine. With currently available solar cells that are about 12 percent efficient in converting sunlight into electricity, approximately 100 square feet of them are needed to generate 1 kilowatt at noon on a sunny day; the construction costs for a 1,000-megawatt power station, which would require nearly 4 square miles of solar cells, would be prohibitive in today's economy. However, power companies are exploring the use of smaller photovoltaic arrays as auxiliary power sources for peak periods of demand.
Specialized applications of photovoltaic power generation are also becoming more common as the cost of solar cells decreases and the cost of electricity from conventional sources goes up; solar panels, formerly restricted to spacecraft and other high-technology applications for which higher costs can be tolerated, are becoming increasingly common in such applications as exterior illumination, remote communications facilities, and rural power generation.

This array of solar cells, a ground-mounted, single-axis tracking system, illustrates the large area of photovoltaic material needed to generate significant amounts of electricity.
It is likely that research and development aimed at efficient and reliable solar photovoltaic systems will continue to be driven by the demands of space exploration. In 1958 the Vanguard 1 satellite was powered in part by silicon cells with 5 percent efficiency. Since that time, research on silicon photovoltaic devices has improved our understanding of material properties, device structures, and manufacturing procedures, resulting in some arrays with efficiencies as high as 23 percent.
Ongoing research indicates that materials other than silicon may provide even higher efficiencies. For example, multibandgap solar cells employ several different semiconductors to use light over a broader range of the solar spectrum. Although they are currently very expensive, gallium arsenide solar cells have steadily increased in efficiency from 13 to 26 percent under terrestrial conditions, and efficiencies approaching 30 percent have been obtained in space solar simulators.
Continued improvements in solar spectral efficiency, reductions in material and manufacturing costs, and increasing environmental burdens attributable to polluting technologies may accelerate the penetration of solar cells in terrestrial applications. One interesting example is their use in cathodic corrosion protection of the steel reinforcing bars in concrete highway bridge decks, where solar cells produce the protective electric potential.
Solar Photoelectrochemical Cells
Can energy conversion devices duplicate the efficient capture and use of solar energy by green plants? A number of laboratories are investigating photoelectrochemical cells that, in some respects, do just that. Hydrogen gas is regarded as a nonpolluting fuel because it burns in oxygen to yield water as the only product; an ultimate quest of solar energy research is to devise a practical system to use sunlight to drive the reaction backwards and split water into this useful and lightweight fuel.
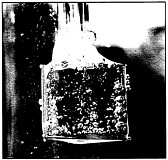
Hydrogen gas evolves from an illuminated photoelectrode in this photoelectrochemical cell.
For the present generation of photoelectrochemical cells, efficiencies for conversion of solar to electrical energy fall in the range of 15 to 20 percent. But photovoltaic cells with comparable efficiency are less complex and cost less to manufacture than photoelectrochemical cells. More importantly, the performance of photoelectrochemical cells degrades with time, a problem caused by corrosion, adsorption of impurities, and other surface phenomena that occur at the semiconductor-liquid interface. Research aimed at overcoming these obstacles involves approaches such as using nonaqueous solvents to suppress corrosion and improving the efficiency of stable materials such as titanium dioxide by using several dyes that combine to capture more of the solar spectrum.
CATALYTIC CRACKING
Crude oil in itself is a very poor fuel for internal combustion engines, and considerable chemical transformation is necessary to convert it to gasoline. Modern refineries contain, in addition to distillation columns, process units such as thermal and catalytic crackers (to make smaller molecules out of larger ones), hydrotreaters (to convert undesired sulfur compounds into sulfur-free compounds), reformers (to make aromatics for high-octane fuel), and alkylation and isomerization units (to make higher-octane molecules). Central to the modern refinery is the catalytic cracker. Improved zeolite cracking catalysts, developed since the early 1960s, have allowed the United States to squeeze more gasoline from each barrel of crude oil, saving to date the equivalent of about 60 percent of the total production from Alaska's North Slope. Over 6 mil
Framework of a zeolite catalyst used in the catalytic cracking process for gasoline production. Because only molecules of a particular size can fit into their pores and channels, zeolites have highly selective chemical activity.
lion barrels of feed-stock are processed in fluid catalytic cracking units in the United States daily, with more than 70 percent of the cracked products ending up as transportation fuel. Cracking catalysts greatly benefit our balance of payments: the zeolite-based cracking catalysts have allowed the United States to reduce crude-oil imports by more than 400 mil lion barrels per year (relative to pre-zeolite technologies).
The first cracking catalysts were introduced in 1936, when acid-washed natural clays were employed. Over the years, amorphous silica-alumina catalysts were developed, and the introduction of fluid cracking technology enabled scaling of the process to large units that processed over 100,000 barrels per day. The new zeolite-based cracking catalysts have regular microscopic channels as part of their molecular structure.
Because the dimensions of these channels are very similar to the size of the hydrocarbon molecules that constitute gasoline, the zeolite catalysts are very efficient in converting the much larger molecules of crude oil to the desired range of small hydrocarbons for gasoline.
Zeolitic cracking catalysts have had a major impact on the design and operation of modern fluid catalytic cracking units; these units were extensively redesigned to take full advantage of the higher activity and selectivity of the zeolites as well as their ability to process heavier feeds. A growing research challenge for chemists and chemical engineers is the design of catalysts that can reduce the emissions of oxides of nitrogen and sulfur during refining, and can at the same time produce cleaner-burning transportation fuels. Another major challenge is to modify cracking catalysts to accommodate the changing process needs of reformulated fuels dictated by environmental considerations. As an example, catalysts that yield increased quantities of isobutylene and isoamylene may be desirable for producing oxygenated blending stocks that can replace more environmentally harmful gasoline additives.











