ENVIRONMENT
The growing public concern about environmental protection is having a significant impact on political and economic activity, as manifested in a growing number of environmental regulations of increasing severity. As they begin to understand the environmental fate of various products, scientists are searching for ways to make them “greener.” How can commercial products and industrial waste materials be made more environmentally benign?

How can we reduce the amount of waste that is produced? And how can we close the loop by redirecting spent materials and products into programs of recycling? All of these questions must be answered through careful research in the coming years as we strive to keep civilization in balance with nature.
Alteration of long-established practices to enhance environmental protection can involve serious financial expenditures. The possibility that these costs could bankrupt local and even regional governments and put U.S. industries at a competitive disadvantage makes it imperative that the best possible science be brought to bear on environmental problems. It also places a premium on finding creative, environmentally beneficial alternatives to existing products and processes. In many instances, industries are finding unanticipated payoffs from process redesign; greater efficiencies save money as well as reduce pollution, and waste streams can sometimes be turned into useful products. In the long run, the benefits of investing in environmental protection will be recognized as environmental awareness grows around the globe.
Atmospheric Chemistry
Since the time 3,000 years ago when Homer noted the smell of ozone created by lightning, people have been fascinated by the chemical behavior of the atmosphere. New techniques of analytical chemistry have fostered a rapid pace of discovery and understanding over the past few decades, allowing separation, identification, and quantitative measurement of chemical species at ever more sensitive levels. Other than nitrogen, oxygen, argon, and water vapor, all other components of air have average atmospheric concentrations below 0.1 percent of the total. Between 0.1 and 0.0001 percent, five more chemical species are added: carbon dioxide, methane, helium, neon, and krypton. Now that modern instruments have lowered these detection limits by up to a factor of 1 trillion during recent years, we know of many other substances whose atmospheric chemistry is both scientifically fascinating and globally important. At least 3,000 different chemical species have been identified in the atmosphere, and more than 50 of these have been detected in locations as remote as the South Pole.
Coal-burning power plants, as well as some natural processes, deliver sulfur compounds to the stratosphere, where oxidation produces sulfuric acid particles that reflect away some of the incoming visible solar radiation. In the troposphere, nitrogen oxides produced by the combustion of fossil fuels combine with many organic molecules under the influence of sunlight to produce urban smog. The volatile hydrocarbon isoprene, well known as a building block of synthetic rubber, is also produced naturally in forests.
And the chlorofluorocarbons, better known as CFCs, are inert in automobile air conditioners and home refrigerators but come apart under ultraviolet bombardment in the mid-stratosphere with devastating effect on the earth's stratospheric ozone layer. The globally averaged atmospheric concentration of stratospheric ozone itself is only 3 parts in 10 million, but it has played a crucial protective role in the development of all biological life through its absorption of potentially harmful short-wavelength solar ultraviolet radiation.
During the past 20 years, public attention has been focused on ways that mankind has caused changes in the atmosphere: acid rain, stratospheric ozone depletion, greenhouse warming, and the increased oxidizing capacity of the atmosphere. We have known for generations that human activity has affected the nearby surroundings, but only gradually have we noticed such effects as acid rain on a regional and then on an intercontinental scale. With the problems of ozone depletion and concerns about global warming, we have now truly entered an era of global change, but the underlying scientific facts have not yet been fully established.
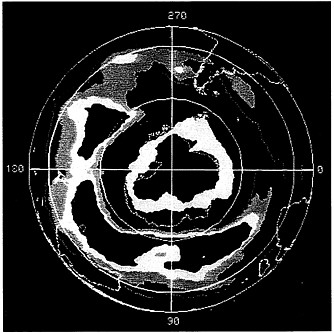
Data from NASA's Nimbus-7 satellite show a distinct ozone hole coded black and pink over the South Pole. Higher ozone concentrations are coded yellow, green, and red.
The data needed to understand the chemistry of the atmosphere must be obtained by high-precision chemical analysis. Scientists and the general public are both aware that the earth's atmosphere is changing. High-precision data show that atmospheric concentrations of carbon dioxide now average about 355 parts per million by volume (ppmv), a 13 percent increase over the 315 ppmv of 1958. Superimposed on this long-term increase are seasonal variations resulting from changes in photosynthetic activity. Similar data conclusively support the increases in atmospheric methane, nitrous oxide, CFCs, and bromofluorocarbons, and the decreases (especially over Antarctica) of stratospheric ozone.
Striking confirmation of the unprecedented rapidity of these recent atmospheric changes has been obtained through the examination of “old air” from bubbles trapped in the ice masses of Antarctica and Greenland. Such bubbles from a 2-kilometer-deep antarctic ice core have shown that
carbon dioxide and methane concentrations have oscillated with the ice ages over the past 200,000 years, only to break the long-established upper bounds in the latter half of the twentieth century.
Much of the research that afforded current data about the earth's atmosphere simply could not have been done prior to recent technological advances in analytical chemical instrument sensitivity and precision. A pertinent illustration is the interaction of CFCs with stratospheric ozone, and especially of atomic chlorine formed by the decomposition of CFCs. Without major improvements in the sensitivity limits of 1 part per billion that prevailed up to 1970, the atmospheric presence of these compounds would still be undetected. Even now, the atmospheric concentration of the most abundant of the CFCs is only 0.5 parts per billion. The dramatic discovery of the ozone hole over Antarctica has had a significant public impact, and the United Nations' globally negotiated Montreal Protocol is already taking effect in reducing the yearly emissions of CFCs.
Life Cycle Analysis
Every stage of a product's life cycle has an environmental impact, starting with extraction of raw materials, continuing through processing, manufacturing, and transportation, and concluding with consumption and disposal or recovery. Technology and chemical science are challenged at every stage. Redesigning products and processes to minimize environmental impact requires a new philosophy of production and a different level of understanding of chemical transformations. Environmentally friendly products require novel materials that are reusable, recyclable, or biodegradable; properties of the materials are determined by the chemical composition and structure. To minimize waste and polluting by-products, new kinds of chemical process schemes will have to be developed. Improved chemical separation techniques are needed to enhance efficiency and to remove residual pollutants, which in turn will require new chemical treatment methods in order to render them harmless. Pollutants such as radioactive elements and toxic heavy metals that cannot be readily converted into harmless materials will need to be immobilized in inert materials so that they can be safely stored. Finally, the leftover pollution of an earlier, less environmentally aware era demands improved chemical and biological remediation techniques.
To know which corrective measures will be effective, we need to understand the way chemicals are transformed in nature. Attempted solutions based on incomplete knowledge may only increase the problem. Early efforts to reduce smog backfired, for example, when automobile engine ignition temperatures were increased in order to reduce the emission of
unburned hydrocarbons: the higher combustion temperatures led to greater nitrogen oxide (NOx) emissions. The formation of smog depends on a complex relationship between hydrocarbons and NOx, and control of only one of the pair was not successful in reducing smog formation.
Knowledge of chemical transformations can also help in the discovery of previously unknown environmental problems. The threat to the ozone layer posed by CFCs was correctly anticipated through fundamental studies of atmospheric chemistry, eventually leading to international agreements for phasing out the production of these otherwise useful chemicals in favor of equally functional but environmentally more compatible alternatives. On the other hand, the appearance of the ozone hole over the Antarctic came as a surprise to scientists and only subsequently was traced to previously unknown chlorine reactions occurring at the surface of nitric acid crystals in the frigid antarctic stratosphere. Thus it is critically important to improve our understanding of the chemical processes in nature, whether they occur in fresh water, saltwater, soil, subterranean environments, or the atmosphere.
Risk and Impact Analysis
Large economic dislocations can result from unfounded popular perceptions of risk, but ignorance of a real hazard can be just as costly. The evaluation of potential risks to the environment and to public health, however, is a highly complex process. Risk prediction frequently requires that extrapolations be made beyond existing knowledge. Furthermore, the public tolerance for risk is variable and difficult to rationalize. We have a higher tolerance

The land around a former chemical plant site has been developed into a 5,000-acre wildlife preserve. The site includes 200 acres of former settling ponds, which are now desirable wetlands and home to 160 species of birds.
for the risks posed by traffic accidents, for example, than for those associated with contaminated foods.

An argon laser beam illuminates a nuclear magnetic resonance (NMR) sample tube in an experiment on laser-enhanced NMR spectroscopy. This is one of many research efforts to develop more sensitive analytical techniques that can be used in risk assessment.
Understanding the interaction of chemistry and the biosphere is especially important because environmental damage is expressed in this interaction. We need a better grasp of how toxic chemicals behave, especially at low levels. After release to the environment, a substance can be transformed into another species with a strikingly different degree of risk. Whereas photochemical transformations of some volatile organic compounds generate smog, many other organic materials are metabolized to carbon dioxide and water when released to the ground.
Communicating risk is as important as evaluating it, and public education should be a key objective of environmental research and development. We live in a chemical world in which natural chemical cycles establish the conditions for sustaining life; yet most people associate the term chemical with man-made chemicals, especially the hazardous ones. This perception presents a serious obstacle to understanding the true character of environmental issues and to evaluating proposed solutions. People should know, for example, that some of the plants we commonly eat produce and contain their own pesticides. They should realize that the toxicity of a chemical depends on its molecular structure, not on whether it is made by plants or by humans. If a complete analysis of the environmental dangers we actually face could be better communicated to the general public, they could in turn support environmental regulation that weighs these dangers appropriately. This would help the development of new, environmentally benign technologies.
Risk assessment requires research in a diversity of areas. In health effects research, for example, the fields range from molecular and cellular biology to studies of exposed populations. Deliberate exposure of humans to toxic substances is unacceptable, and so data on humans are limited; risk analysis must rely on animal studies coupled with the fundamentals of biochemistry. An important development here is the discovery of biomarkers, chemical and biochemical species that indicate when a person has been exposed to a particular agent and whether the exposure has had a significant impact on the individual. Most risk analyses are based on statistical models, but a particular person may have a much higher or lower tolerance than the average. Biomarkers can provide personalized risk assessment.
Sampling and analysis play key roles in risk assessment. Preventing environmental damage often requires careful monitoring of the manufacturing facility, which can reduce waste, improve efficiency, and reduce costs.
The combination of lasers with other analytical instruments has led to major advances in this area. From the laser analysis of a single strand of human hair, it is possible to determine the cumulative exposure of an individual to radon. Similarly, microscopic sensors can now provide real-time monitoring of specific contaminants in air and water. The ongoing development of such devices relies on research in surface electrochemistry, surface photo-physics, and materials and polymer science.
Manufacturing with Minimal Environmental Impact
Discharge of waste chemicals to the air, water, or ground not only has a direct environmental impact, but also constitutes a potential waste of natural resources. Early efforts to lessen the environmental impact of chemical processes tended to focus on the removal of harmful materials from a plant's waste stream before it was discharged into the environment. But this approach addresses only half of the problem; for an ideal chemical process, no harmful by-products would be formed in the first place. Any discharges would be at least as clean as the air and water that were originally taken into the plant, and such a process would be “environmentally benign.”
Increasing concern over adverse health effects has put a high priority on eliminating or reducing the amounts of potentially hazardous chemicals used in industrial processes. The best course of action is to find replacement chemicals that work as well but are less hazardous. For example, N-methylpyrrolidone appears to be a benign and effective substitute for methylene chloride, a widely used industrial solvent that is suspected of being a weak carcinogen.
If a substitute cannot be found for a hazardous chemical, then a promising alternative strategy is to develop a process for generating it on-site and only in the amount needed at the time. On-demand generation can greatly reduce the potential exposure of workers and neighboring communities by obviating the need for shipment and storage of bulk quantities of the chemical. A significant advance in environmentally benign technology is an electrolysis kit that uses ordinary electric current and delivers a precisely metered amount of arsine gas, a highly poisonous raw material used for the manufacture of gallium arsenide semiconductors. The arsenic metal electrodes from which the gas is generated are nonvolatile and easy to handle, and thus the overall risk in processes that use arsine is substantially reduced.
Innovative new chemistry has begun delivering environmentally sound processes that use energy and raw materials more efficiently. Recent advances in catalysis, for example, permit chemical reactions to be run at
lower temperatures and pressures. This change, in turn, reduces the energy demands of the processes and simplifies the selection of construction materials for the processing facility.
Novel catalysts are also being used to avoid the production of unwanted by-products. In the pharmaceutical industry, such catalysts are being used to selectively prepare the desired form of chiral molecules, molecules that exist in two mirror-image forms, analogous to a pair of hands. Left and right hands look similar, but only the left hand will fit into a left-handed glove. Similarly, the desired therapeutic effect of a drug often occurs exclusively for just the right- or left-handed form of the molecule.
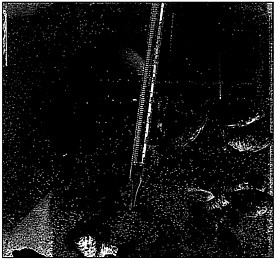
Researcher observes fish that are unharmed by newly developed, nontoxic hydraulic oil.
Before the development of chiral synthesis procedures, many chiral pharmaceuticals were distributed as the 50-50 mixtures of mirror-image forms that result from traditional chemical synthesis. In the case of thalidomide, one of these isomeric forms is a sedative, and the other isomer was belatedly and tragically found to cause birth defects. But now, chiral catalysts are being developed for the synthesis of the desired isomer of many drugs, including, for example, the common antiinflammatory drugs naproxen and ibuprofen. In the case of naproxen, one isomer is beneficial, but the other is toxic and must be separated out or, preferably, not produced in the first place. In the case of ibuprofen, the unwanted isomer is not toxic, but it is only marginally or not at all beneficial. Direct synthesis of only the desired isomer will allow a reduction by 50 percent of the amount of chemical given to the patient. This means that people will no longer need to ingest 7.5 million pounds of the inactive form of ibuprofen each year worldwide and that the resources used to produce that quantity of the chemical will therefore not go to waste.
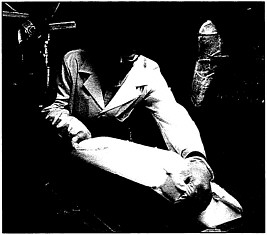
Water-based release agents provide an effective substitute for solvent-based products, allowing solvent emissions to be greatly reduced in the process of “demolding” polyurethane foam.
Control of Power Plant Emissions
About 70 percent of the electricity in the United States is generated by combustion of fossil fuels, either in boilers or in gas turbines. Coal-, oil-, and natural-gas-fired power generation facilities contribute to the emissions of carbon monoxide, hydrocarbons, nitrogen oxides, and a variety of other undesired by-products such as dust and traces of mercury. A rapidly increasing array of technologies are now available to reduce the emissions of unwanted species to meet national or local standards. Chemists and chemical engineers have made major contributions to the state of the art, and catalytic science is playing a critical role in defining the leading edge.
Nitrogen oxides, for example, can be reduced to nitrogen by using a selective reduction catalyst and injecting ammonia into the exhaust gas stream. The ammonia selectively reacts with the nitrogen oxides on the catalyst's surface (rather than with the large excess of oxygen in the flue gas), producing water and nitrogen gas. This technology is now widely used in Japan and Germany, and domestic use is increasing. Significant research programs are under way to develop new and improved catalysts that will further enhance the economics of this process.
The simultaneous control of more than one pollutant is the aim of some recently developed catalyst or sorbent technologies. For example, catalytic methods allow carbon monoxide to be oxidized at the same time that nitrogen oxides are being chemically reduced in gas turbine exhaust. Other research efforts are aimed at pilot-plant evaluation of the simultaneous removal of sulfur and nitrogen oxides from flue gas by the action of a single sorbent and without the generation of massive volumes of waste products.
The frontiers of nitrogen oxide abatement catalysis are represented by fundamental research into the direct decomposition of these pollutants into nitrogen and oxygen without the use of a reducing agent. Although this reaction is thermodynamically favored, efforts have so far failed to identify a catalytic surface with the required activity and resistivity to water and sulfur oxides in the feed stream. The race is on, in the United States and abroad, to make the next breakthrough in emission control catalysis.
Environmentally Friendly Products
Increased understanding of the fate of products in the environment has led scientists to design “greener” products. A significant early example comes from the detergent industry. In the 1940s and 1950s, new products were introduced that were based on synthetic surfactants called branched alkyl-benzene sulfonates. These detergents had higher cleaning efficiency, but it was subsequently discovered that their presence in waste water caused
foaming in streams and rivers. The problem was traced to the branched alkylbenzene sulfonates; unlike the soaps used previously, these were not sufficiently biodegraded by the microbes in conventional sewage treatment plants. An extensive research effort to understand the appropriate biochemical processes permitted chemists to design and synthesize another new class of surfactants, linear alkylbenzene sulfonates. The similarity in molecular structure between these new compounds and the natural fatty acids of traditional soaps allowed the microorganisms to degrade the new formulations, and the similarity to the branched alkylbenzene sulfonates afforded outstanding detergent performance.
Another example of a green replacement product is the herbicide glyphosate, which controls weeds by blocking the activity of EPSP synthase, a plant enzyme. Since this activity is required for plant survival, spraying a plant with glyphosate will cause it to die. Before planting a new crop, a farmer can first spray the field with glyphosate, ignoring existing weeds because the herbicide is nonselective and kills virtually all existing green plants. But glyphosate is rapidly degraded by soil microorganisms into benign chemicals, including carbon dioxide, nitrogen, water, and phosphate, and so a new crop can be planted quickly and safely. As a consequence, land need not be tilled before planting, and glyphosate treatment can help protect against the erosion common in sloped farms. Because EPSP synthase is not found in animals, glyphosate is inactive in animals, thereby providing an additional safety feature.

A controlled-release fertilizer, formulated for strawberry growers, is released more rapidly at warmer soil temperatures. This results in more efficient use of fertilizer and reduced nitrogen loss from leaching into groundwater.
Novel biochemistry is also helping farmers reduce the use of insecticides. Cotton plants, for example, are being genetically modified to make them resistant to the cotton bollworm. A single gene from a naturally occurring bacterium, when transferred into cotton plants, prompts the plant to produce a protein that is ordinarily produced by the bacterium. When the bollworm begins to eat the plant, the protein kills the insect by interrupting its digestive processes. This protein has been used for more than 40 years to control garden insects, but the new approach is more effective. Not only is the protein in insect-resistant cotton present whenever it is needed, but it also has no effect on beneficial insects that do not eat cotton plants. The development of this new approach to controlling the cotton bollworm required extensive research, including the complete chemical synthesis of the 3,500-nucleotide gene. The bacterial gene was relatively ineffective when incorporated into the cotton plant, but the chemical synthesis was

Soap bubbles. Research into environmentally compatible products and processes is an important challenge for the $ 13 billion detergent industry.
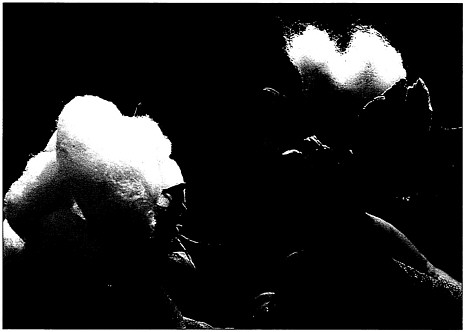
Environmentally friendly insecticides. The cotton plant on the left was protected by a genetic modification, causing it to produce a protein that is toxic to cotton bollworms.
followed by minor chemical modifications of the nucleotide sequence to produce a gene that is more plantlike, exhibiting a thousandfold enhancement in its production of the identical protein. The potential impact of this unique way to control pests is astounding when placed in the context that cotton accounts for nearly 40 percent of all pesticide use in the United States.
Potent yet environmentally friendly products can be extracted from plants growing in nature. An example is azidirachtin, a powerful pesticide that can be extracted from the seeds of the neem tree, indigenous to India and certain regions of Africa.
Recycling
Increasing problems associated with waste disposal have combined with the recognition that some raw materials exist in limited supply to dramatically increase interest in recycling. Recycling of metals and most paper is technically straightforward, and these materials are now commonly recycled in many areas around the world. Recycling of plastics presents greater technical challenges. Even after they are separated from other types of waste, different plastic materials must be separated from each other. Even then, the different chemical properties of the various types of plastic will require the development of a variety of recycling processes.
Some plastics can be recycled by simply melting and molding them or by dissolving them in an appropriate solvent and then reformulating them into a new plastic material. Other materials require more complex treatment,
such as breaking down large polymer molecules into smaller subunits that can subsequently be used as building blocks for new polymers. Indeed, a major program to recycle plastic soft drink bottles by this route is now in use.

Research efforts are finding ways to effectively use recycled motor oil.
A great deal of research by chemists and chemical engineers will be needed to successfully develop the needed recycling technologies. In some cases, it will be necessary to develop entirely new polymers with molecular structures that are more amenable to the recycling process. New instrumentation will also be needed that can distinguish among different plastics on-line so that the plastics can be automatically sorted or properly blended.
Separation and Conversion for Waste Reduction
New processes are needed to separate waste components requiring special disposal from those that can be recycled or disposed of by normal means. Development of these processes will require extensive research to obtain a fundamental understanding of the chemical phenomena involved.
Metal-bearing spent acid waste. Several industrial processes produce acidic waste solutions in large quantities. Could this waste be separated into clean water, reusable acid, and a sludge from which the metals could be recovered? Such processes would preserve the environment, and their costs could be competitive with disposal costs and penalties.
Industrial waste treatment. The hazardous organic components in industrial wastewater could be destroyed with thermocatalytic or photocatalytic processes. A promising line of research employs “supercritical” water at high temperatures and pressures. Under these conditions, water exhibits very different chemical and physical properties. It dissolves and allows reactions of many materials that are nearly inert under normal conditions.
High-level nuclear waste. Substantial savings would be achieved if the volume and complexity of nuclear waste requiring storage could be significantly reduced; this reduction would require economic separation of the radioactive components from the large volumes of other materials that accompany the nuclear waste. The hazardous chemical waste might then be disposed of separately. The current national strategy for radioactive waste management requires conversion to a form that would remain stable over extremely long periods of time. For example, solid waste could be loaded into melted borosilicate glass, poured into metal canisters, and
allowed to cool and harden. The radioactive materials would then be encapsulated within the resulting glass “logs,” greatly reducing any threat to the environment. The disposal of nuclear waste will require major research and development efforts over many years.
Membrane technology. Separations involving semipermeable membranes offer considerable promise. These membranes, usually sheets of polymers, are impervious to some kinds of chemicals but not to others. Such membranes are used to purify water, leaving behind dissolved salts and providing clean drinking water. Membrane separation techniques also permit purification of wastewater from manufacturing. Membrane separations are also applicable to gases and are being used for the recovery of minor components in natural gas, to enhance the heating value of natural gas by removal of carbon dioxide, and for the recovery of nitrogen from air. Research challenges include the development of membranes that are chemically and physically more resilient, that are less expensive to manufacture, and that provide better separation efficiencies to reduce processing costs.
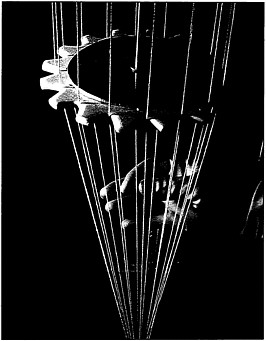
Hollow fiber membranes, essential for many biotechnology and pharmaceutical applications, are produced in a high-speed fabrication process.
Biotechnology. Scientists have turned to nature for help in destroying toxic substances. Some microorganisms in soil, water, and sediments can adapt their diets to a wide variety of organic chemicals; they have been used for decades in conventional waste treatment systems. Researchers are now attempting to coax even higher levels of performance from these gifted microbes by carefully determining the optimal physical, chemical, and nutritional conditions for their existence. Their efforts may lead to the design and operation of a new generation of biological waste treatment facilities. A major advance in recent years is the immobilization of such microorganisms in bioreactors, anchoring them in a reactor while they degrade waste materials. Immobilization permits high flow rates that would flush out conventional reactors, and the use of new, highly porous support materials allows a significant increase in the number of microorganisms for each reactor.
Cleaning Up Contaminated Sites
During the last two decades, the United States has undergone a dramatic change in its approach to the use and disposal of chemical waste. The once-acceptable approaches of putting waste material into the ground are now recognized as both undesirable and potentially dangerous. Many sites,
particularly those given the “Superfund” label, must now be cleaned up at a high cost to our national economy. Such expenditures demand the best possible technology, and this in turn will require major research and development efforts by chemists and chemical engineers.
Most large-scale contamination requires on-site cleanup. The site must be carefully characterized by a variety of techniques, with extensive chemical analysis and by determining the mobility of contaminants in the soil and groundwater. A remediation strategy must then be developed to remove the identifiable pollutants or convert them to nontoxic materials.
Groundwater remediation can take advantage of ion-exchange processes to remove certain pollutants, but many more can be rendered nontoxic by chemical oxidation. Current research and development efforts show great promise, including low-temperature catalytic oxidation and oxidation in supercritical water.
Cleanup of contaminated soil represents a major challenge. Methods that rely on combustion of the contaminated materials may, as an example, suffer from slag formation, which fouls the reactor. Among the innovative approaches now being investigated are in situ soil washing techniques, in which the contaminants can be dissolved in water or other extractants and brought to the surface for further treatment. These techniques require a thorough understanding of such matters as the chemical interactions of the contaminants with the soil and the flow patterns of liquids through the soil. The use of surfactants can change the way that contaminants are bound to the soil, making it possible to bring them to the surface or rendering them accessible to in situ destruction by microorganisms.
Whether chemical or biological methods are employed, remediation efforts must be based on an understanding of the chemistry of natural systems. Research by chemists and chemical engineers is essential to the development of effective ways to attack this pressing national problem.
CATALYSTS FOR CONTROL OF AUTOMOBILE EXHAUST
The Clean Air Act amendments of 1970 established a series of air quality standards that dramatically influenced the operations and philosophy of U.S. manufacturing. Arguably, the most important technology that has been developed for control of air quality is the catalytic destruction of pollutants. After extensive research in catalysis, new catalysts were introduced for the control of automobile exhaust emissions in the fall of 1974, and today the catalytic converter is a common feature on automobiles around the world. The provisions of the 1990 Clean Air Act amendments have placed even greater restrictions on automobile exhaust emissions.
In an ideal world, combustion of fossil fuels such as gasoline would not cause pollution. These fossil fuels, being hydrocarbons, would burn to form water and carbon dioxide, two compounds that are components of the life cycle of the earth's biosphere, as their only chemical products. (However, the accumulation of carbon dioxide in the atmosphere is raising concern over its potential contribution to long-term climate changes.) But in reality, the combustion process inside an engine does not work perfectly. Incomplete combustion of hydrocarbon molecules produces carbon monoxide in the engine's exhaust. At the high temperatures of gasoline combustion, nitrogen, to some small extent, undergoes reaction with oxygen to form nitrogen oxides. These have been implicated in such environmental problems as smog, photochemical ozone in the lower atmosphere, and acid rain.

Electrically heated converter. By electrically heating the foil on which the catalyst is coated, this catalytic converter is effective even while the automobile's engine is cold.
Catalysts are materials that facilitate (accelerate) chemical reactions without being consumed in the process; they take part in the chemical transformations by reducing the “energy barrier” but are regenerated at the end of each reaction cycle. The catalytic converters in automobile exhaust systems include a honeycomb material made of metal or ceramic that carries a thin coating of alumina that disperses the noble-metal catalyst. When hot engine exhaust gases come into contact with the catalytic surface, carbon monoxide, hydrocarbons, and nitrogen oxides are converted to carbon dioxide, nitrogen, and water.
The environmental improvements afforded by automobile catalytic converters can be readily seen from data on emissions. Before the introduction of catalysts, a typical U.S. automobile released about 10 grams of hydrocarbons for each mile driven. But any car meeting today's environmental standards will produce less than 0.4 gram of hydrocarbons per mile. The next great technological breakthrough will be required by the mid-1990s, when certain state regulations specify that hydrocarbon emissions for new automobiles must be below 0.04 gram per mile.
Research efforts are already well under way to overcome this next challenge. In the “Federal Test Procedure,” a driving schedule that simulates city-suburban traffic, nearly 80 percent of the hydrocarbon and carbon monoxide emissions are produced in the first 8 minutes. The catalyst becomes fully effective only after the engine has run for a few minutes and the entire system has reached a normal operating temperature. With this knowledge in hand, researchers have developed an electrically heated converter, which quickly reaches its high-efficiency operating temperature. The net result is greatly reduced emissions of carbon monoxide and hydrocarbons, with the promise of providing a technical solution for challenges such as California's “ultralow-emission vehicle ” standards for the late 1990s.
Illustrations courtesy of:
Abbott Laboratories, 34
Adolph Coors Company, 47
Air Products and Chemicals, Inc., 62 (bottom)
Allied-Signal, Inc., 19 (top left, right)
American Cyanamid Company, 39 (top)
Amoco Corporation, 6, 10,* 33 (bottom)
Ashland Oil, Inc., 22 (bottom), 66
AT&T Bell Laboratories, 44 (inset), 45 (top), 54
The Boeing Company, 13 (top)
Bristol-Myers Squibb Co., 33 (top)
California Institute of Technology, 29
Chedoke-McMaster Hospitals, 41*
Ciba-Geigy Corporation, 32
Corning Incorporated, 18 (bottom), 44 (bottom)
Dow Chemical Company, 19 (bottom)
Du Pont Merck Pharmaceutical Company, 38
Eastman Kodak Co., 13 (bottom)
E. I. du Pont de Nemours & Company, 7 (top)
Emory University, 50*
Exxon Corporation, 22 (top), 64 (top)
General Motors Corporation, 9, 25
W. R. Grace & Co., 11, 15 (top), 17, 47, 67, 69
GTE Laboratories Incorporated, 12 (top)*
Hoechst Celanese Corporation, 8
Hughes Aircraft, 47
IBM Corporation, 44 (top), 51, 53
International Fuel Cells, 26 (bottom)
Johnson Controls, Inc., 15 (bottom)
Massachusetts Institute of Technology, 12 (bottom),* 39 (bottom)
Mobil Corporation, 23, 30, 62 (top)
Mobil Solar Energy Corporation, 28*
Monsanto Company, 59,* 65
National Aeronautics and Space Administration, 21
Northrop Corporation, 7 (bottom)
Perkin-Elmer Corporation, 57*
Princeton University, 60
Promean-Meditronics, 26 (top)
Sandia National Laboratory, 24*
Siemens Corporation, 44 (top)
Sterling Winthrop, Inc., 36*
University of Texas, Austin, 45 (bottom)
Washington University, 42
Westinghouse Electric Corporation, 18 (top)
3M, 40
Credits for specific photographs: E. S. Garnett, 41;* Gladstone Studio, Ltd., 19; Jon Love (copyright 1992, reprinted with permission), 62 (top); The MIT Report, 12 (bottom);* Niedorf Photography, 40; R. Schliepman, 28;* Uniphoto, Inc., 46,* 64 (bottom).
* Illustrations marked above with an asterisk also appear on the cover.

















