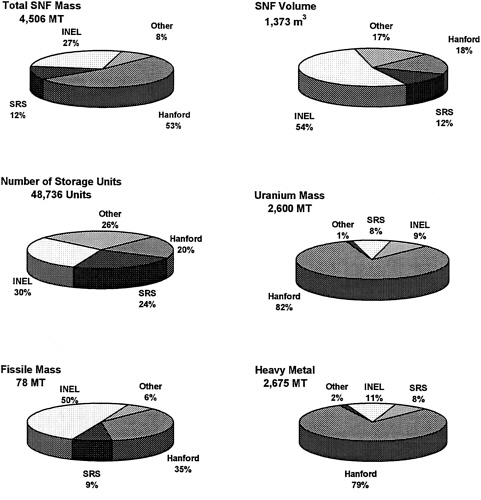
FIGURE 4 Distribution of DOE SNF throughout all DOE sites, according to six different characteristics. MT: Metric tons. INEL: Refers to the physical location and includes inventory belonging to the Idaho National Engineering Laboratory, ANL-West, and the Naval Research Facility. SRS: Savannah River Site. Other: Includes other DOE sites, non-DOE domestic reactor fuel, and research reactor fuel that will be owned and managed by DOE. (Data from DOE Spent Nuclear Fuel Technology Integration Plan, Department of Energy report SNF-PP-FS-002, December, 1994.)
In early 1995 the DOE set the following milestones for activities to address these concerns:6
-
Fuel removal from K-basins at Hanford to be completed by December 1999;
-
Fuel consolidation, processing of SNF and target assemblies, and stabilization of resultant uranium solutions at Savannah River by April 2000; and
-
Removal of all fuel from CPP-603 by December 2000.
In their present form and condition the EBR-II and N-reactor fuels are not suitable for direct geologic disposal, and even long-term interim storage would require repackaging or some other treatment. 7 The principal goal for treating these fuels would be to put them into a safe and stable condition for interim storage until their ultimate disposition can be decided. Because the proposed Yucca Mountain repository cannot be available until after 2010, at the earliest, to receive any spent fuel consigned to geologic disposal, there is an unavoidable need to attend to these two categories of fuel (about 80% by mass of the DOE's total spent fuel inventory), as well as other spent fuels not already suitable for storage, to permit their interim storage for an estimated 20 to 40 years prior to final disposition.
POSSIBLE CANDIDATE FUELS FOR ELECTROMETALLURGICAL PROCESSING
The electrometallurgical technology developed by ANL is potentially applicable to a fairly wide variety of spent fuel types. In principle, electrometallurgical separations in alkali metal chloride media could be used for most of the DOE SNF, if used in conjunction with appropriate head-end processes. The purpose of specially tailored head-end processes would be to convert the fuels to metal and free them from elements such as aluminum and carbon that, above certain threshold-level concentrations, are incompatible with the electrometallurgical process as currently conceived.
The DOE has already determined that the EBR-II driver fuel and at least half of the blanket fuel will be treated via the electrometallurgical process. It is expected that, in addition to processing EBR-II fuel, the electrometallurgical technology could process undamaged, metallic fuels from the Hanford N-reactor without any lithium-reduction head-end treatment. N-reactor fuels constitute about 80% by mass of DOE's SNF inventory and EBR-II fuels about 1%.
However, a significant fraction of the N-reactor fuel elements have experienced a breach in their cladding, and these failures have resulted in oxidation of the uranium metal as well as the zirconium cladding. These failed fuel elements (and the accompanying sludge that has formed in Hanford's K-basin East) would have to be treated by head-end processing such as chemical purification and oxide reduction
|
6 |
“Defense Nuclear Facilities Safety Board Recommendation 94-1 Implementation Plan,” Department of Energy, Feb. 28, 1995. |
|
7 |
Uranium and zirconium metals are chemically reactive in water and in air, and powdered forms of these metals are pyrophoric. In the reaction with water, hydrogen is also produced, and under conditions of limited oxygen, uranium hydride may form as well. Initially, uranium dioxide forms a protective coating on the metal surface that greatly retards further oxidation, but after an indeterminate time, the protective layer cracks and crumbles, allowing further oxidation to occur. Fuel elements with breached cladding will oxidize after contact with water, and the resulting physical changes at the uranium surface can lead to additional cladding damage and release of radioactivity into the water. |
prior to electrorefining. The extent of fuel damage has been estimated from two different visual surveys of the open-topped canisters in K-basin East. The first was carried out in 1981 on approximately 500 canisters, and the second, in 1991, on only 100 canisters. The results of the first survey indicated that a minimum of 6% of the fuel elements had cracked cladding and that 50% of the canisters contained an element with cracked cladding. The cladding of 1% of the elements was cracked sufficiently to be considered broken. From the second survey it could be concluded that at least 12% of the fuel elements in the sample were damaged and that at least 90% of the canisters contained at least one damaged fuel element.8 K-basin East contains 1146 MTHM of N-reactor fuel in 3667 open canisters plus 0.4 MTHM of single pass reactor (SPR) fuel. K-basin West contains 954 MTHM of N-reactor fuel in 3815 closed canisters plus 0.1 MTHM of SPR fuel. The water in K-basin East is known to be contaminated, while that in K-basin West is not. In addition, the West basin does not have any accumulation of contaminated sludge on the floor, because the canisters are closed.
In addition to N-reactor fuel, Hanford has a wide variety of other SNFs stored in various places on site. These fuels amount to only about 1.5% of the mass of N-reactor fuel, but the amount is not negligible (33 MT). About half of this by mass is the Shippingport PWR Core II, treatment of which by the electrometallurgical process would require a special head-end step to remove a layer of graphite from the fuel wafers that contain the fissile material. Another (small) portion of the Hanford SNF contains aluminum that is metallurgically bonded to the uranium. It is unlikely that this fuel could be processed directly by the electrometallurgical process without additional head-end treatment to remove the aluminum. Aluminum's tendency to form low-melting eutectic compounds and volatile species would significantly challenge containment and effluent treatment for the electrorefining process.
ALTERNATIVE TECHNOLOGIES FOR TREATING DOE SPENT NUCLEAR FUEL
At present, the most promising alternatives to the electrometallurgical approach for treating DOE's SNF are direct disposal and Purex. In addition, DOE is supporting development of several other approaches for converting SNF and other nuclear materials to forms suitable for disposal.
Direct Disposal
In the direct disposal option, the SNF would receive minimum stabilization treatment and be canned directly in repository containers. Many variations of this option are currently under study by the DOE.9 Information to date is insufficient to make a meaningful comparison between electrometallurgical processing and direct disposal.
Purex
Purex is a proven process for treating SNF. The DOE has successfully operated Purex facilities at SRS, INEL, and Hanford, but these facilities have been shut down. Commercial facilities at West Valley, New York, were operated for several years before their shutdown in 1973. The Purex facility at SRS may become
|
8 |
“Hanford Spent Fuel Inventory Baseline,” K.H. Bergsman, Spent Fuel Management Project, 2C200, June 1994. |
|
9 |
Performance Assessment of the Direct Disposal in Unsaturated Tuff of Spent Nuclear Fuel and High-Level Waste Owned by U.S. Department of Energy, 3 volumes, Rob P. Rechard, editor, Sandia National Laboratories report SAND 94-2563, March, 1995. |
operational again, but the Purex facility at Hanford was permanently shut down in 1993. France and the United Kingdom have operating Purex facilities for SNF. France recycles all of its own spent fuel through Purex and also that of other nations (e.g., Japan, Germany, and Sweden). The United Kingdom has processed spent Magnox fuel for years in Purex facilities at Sellafield and has recently built a thermal oxide reprocessing plant (THORP), complete with waste treatment facilities, at Sellafield.
Despite its success, Purex suffers the disadvantages of high cost and generation of large amounts of waste, although the volumes are less when the acidic output streams are not neutralized. Two earlier reports10 compared Purex and the related electrometallurgical processing proposed for the Integral Fast Reactor (IFR) and concluded that the latter processing might not avoid these disadvantages. However, the first of these reports, dealing with costs, was based on an assumed annual throughput of 1,500 MTU, which is much higher than needed for DOE SNF, and it noted that only limited economy of scale could be achieved for an electrometallurgical facility of that size. The second report indicated that the amounts of waste might be comparable for Purex and IFR processing but noted that estimates of waste generation “are very contingent upon assumptions . . .” and that “these results need further review.” A firm comparison between Purex and electrometallurgical processing is not possible because such a comparison would require demonstration of the latter process on a production scale, and indeed much of the development, including engineering and scale-up of the various unit processes, remains to be done. The separation factors needed for the proposed electrometallurgical process are less stringent than those of the earlier Purex operations, which further complicates comparisons of the methods.
When Hanford's Purex facility was used to process N-reactor fuel, approximately 8 MT of high-level liquid waste was generated per metric ton of uranium feed processed.11 This waste was stored in underground tanks. Approximately 230 MT/MTHM of secondary waste was also generated and was sent to cribs, and other chemical process wastes amounted to about 900 MT/MTHM. ANL estimates electrometallurgical processing effluent streams, including the uranium and TRU products, would be about 2 MT/MTHM, with only modest generation of secondary wastes.
In 1985, ANL published cost estimates for a commercial-scale IFR pyroprocessing facility for a 1200- to 1400-MW reactor.12 An electrometallurgical processing facility would have similar costs, with some increases if oxide reduction were included. To compare these estimated costs to those for a Purex facility with the same throughput capability, 35 MTHM/yr, DOE commissioned an independent study by ORNL and the Hanford Engineering Development Laboratory. 13 The results of the two conceptual design studies suggest that a Purex facility might be roughly five times the size of an electrometallurgical facility of comparable throughput, in terms of both building volume and the amount of concrete required. This suggests in turn that the size and cost of a new electrometallurgical facility might be much less than those for a new Purex facility, although the comparison is at best a very rough estimate. ANL's electrometallurgical process would have to be fully developed before meaningful cost comparisons could be made. The committee did not have access to data on operating and maintenance costs for an electrometallurgical facility of this size; estimates in the EPRI report were very preliminary ones for a
|
10 |
The Cost of Processing Irradiated Fuel from Light Water Reactors, report NP-7264, and Projected Waste Packages Resulting from Alternative Spent-Fuel Separation Processes, report NP-7262, both from Electric Power Research Institute, Palo Alto, Calif., 1991. |
|
11 |
Data from G.K. Allen, D.C. Hedengren, L.L. Jacobs, R.D. Fox, and D.W. Reberger, Purex Flowsheet Reprocessing N-Reactor Fuels, Hanford internal report PFD-P-020-00001, Sept. 16, 1985. |
|
12 |
ANL-IR-25, “Commercial-size IFR Fuel Cycle Facility: Conceptual Design and Cost Estimate,” October, 1985. |
|
13 |
The results of the Purex study are given in ORNL/TM-9840. |
facility some 40 times larger. It should also be noted that electrometallurgical facilities, in consideration of criticality controls, would achieve scale-up by replication of electrorefiners rather than by increasing their size. In contrast, scale-up of a Purex plant could benefit from economies of scale, but only a small Purex facility would be needed for processing DOE SNF. These considerations would further affect any cost comparison.
4
DISPOSAL OF ELECTROMETALLURGICAL EFFLUENT STREAMS
The following is a brief survey of the potential suitability for direct disposal—placement in a geologic repository (e.g., Yucca Mountain or an alternate)—of each of the output streams from electrometallurgical processing. It is difficult to assess the adequacy of planned studies to confirm the performance of these waste forms under simulated repository conditions; a waste forms test plan is apparently in the final stages of preparation at ANL but was not available for review prior to completion of this report.
As noted in Chapter 2, ANL's proposed electrometallurgical separation process would produce four output streams, each of which is a potential waste stream:
-
A uranium ingot, which the cathode processor produces by removing entrained electrolyte (i.e., LiCl/KCl) from the uranium solid deposited on the electrorefiner's steel cathode;
-
A metallic stream (from the cadmium cathode) containing most of the TRUs and some uranium and rare-earth elements;
-
A molten salt stream containing fission products that would be recycled after capture of the fission products by a synthetic zeolite to produce a waste form for disposal; and
-
A metallic stream, remaining at the anode in the dissolver basket, that would contain cladding hulls and noble metal fission products.
Additional output streams would be expected if any head-end processes were added.
According to ANL's proposal, the first two output streams would be directed to interim storage rather than to final geologic disposal. Nonetheless, there are attendant safety and proliferation issues with respect to the surface storage of such materials for an unspecified duration. The third and fourth output streams are intended as waste streams for final geologic disposal.
URANIUM METAL FRACTION
Removal of the uranium metal fraction from SNF is cited in ANL's proposal (see Appendix A, p. A-6) as a key advantage of the electrometallurgical processing technique because of the expected reduction in the volume of the streams to be directed to geologic disposal. (The total mass of DOE SNF, though, is just a small fraction of the 70,000 MTHM of mostly commercial SNF that is to be disposed of in the first repository.) The processed uranium metal fraction is asserted by ANL to be pure uranium, with an isotopic composition the same as that of the original spent fuel that is processed. The levels of impurities in this uranium metal fraction resulting from routine batch processing have not been established.
This uranium is a reactive metal and consequently does not appear to be a candidate for direct geologic disposal. Accordingly, the uranium-metal fraction leaving the cathode processor would either be recycled as reactor fuel, be sent to interim surface storage, or be treated to produce a form suitable for geologic
disposal. In the last case—which follows if the uranium fraction is deemed to be a waste stream —additional processing steps (for example, oxidation) would be required.
An issue with respect to the uranium metal fraction is that some of it is enriched. ANL has indicated plans for blending this material down to low enrichment levels in order to minimize proliferation concerns.
TRU METAL FRACTION
The electrometallurgical process would generate a separate TRU metal fraction composed of approximately 30% uranium by weight, with the remaining mass composed of unfractionated actinides, primarily plutonium, and some of the rare-earth elements from the processed fuel. As a reactive metal, it is unlikely that this fraction would be acceptable for direct geologic disposal, so that either interim surface storage or additional treatment for disposal would be required.
Deep geologic repositories are the internationally preferred option for disposal of high-level waste (HLW) because associated release of radiation to the biosphere would be unlikely. This characteristic is important because the generally accepted standard of risk for public exposure to radioactivity is expressed in terms of radiation doses1 that are calculated by taking into account actual pathways from repository to biosphere, and their likelihoods, when estimating the probabilities of radionuclide releases to the biosphere.
While removal of actinides from the waste stream might result in a reduction in the volume of waste bound for storage in a geologic repository,2 removal of actinides appears to yield little accompanying reduction in the overall risks from the repository. This is because, in evaluating the comparative radiation dose risks associated with actinides as compared to fission products in HLW, safety analyses by international repository programs3 and for the U.S. HLW repository4 suggest that long-lived, soluble, weakly sorbed fission products (e.g., Se-79, Tc-99, I-129) would dominate the long-term dose risk.
An emerging alternative for disposal of the TRU metal fraction, mentioned by ANL toward the end of this study but not considered in detail by the committee, would involve a modification of the electrometallurgical process such that the TRU metal fraction would not be deposited electrolytically in a cadmium cathode. Instead, the TRU metal fraction would be incorporated into the zeolite waste fraction, described below.
|
1 |
L.C. Hebel et. al., 1978, Rev. Mod. Phys., 50(1), Part II. |
|
2 |
Ibid. |
|
3 |
See, e.g., NAGRA, 1985. “Project Gewahr 1985/Nuclear Waste Management in Switzerland: Feasibility Studies and Safety Analyses,” NGB 85-09, National Cooperative for the Storage of Radioactive Waste, Baden, Switzerland; SKB 91: “Final Disposal of Spent Nuclear Fuel. Importance of the Bedrock for Safety, ” Swedish Nuclear Fuel and Waste Management Co., Stockholm, Sweden; YJT, 1992, “Final Disposal of Spent Fuel in the Finnish Bedrock: Technical Plans and Safety Assessments,” YJT-92-31E, Nuclear Waste Commission of Finnish Power Companies (YJT), Helsinki, Finland. |
|
4 |
Electric Power Research Institute, 1992, “Demonstration of a Risk-Based Approach to High-Level Repository Evaluation: Phase 2,” EPRI TR-100384, Electric Power Research Institute, Palo Alto, Calif.; “Total-System Performance Assessment for Yucca Mountan —SNL Second Iteration (TSPA-1993),” SAND93-2675, Sandia National Laboratories, Albuquerque, N. Mex. |








