4.6 Separation of Transplutonium Elements
Schädel and coworkers [Sch88b, Sch89] have developed a microcomputer-controlled automatic rapid chemistry apparatus (ARCA) for carrying out fast, repetitive, high-performance liquid chromatographic (HPLC) separations. The nuclear-reaction products were carried by a helium jet containing KCl aerosol and were deposited on a frit. After collection, the frit was moved to the chemistry position; simultaneously, a second frit collected reaction products. Ion-exchange or extraction chromatographic columns were used along with an HPLC system for the separations. Electropneumatic valves were used for controlling and diverting the flow of fluids, and the entire process was controlled by a microprocessor. With the improvements achieved in reducing the dead volume of chromatographic columns to 35 µL, the separation could he completed in 35 s. Kratz et al. used this system to study the anion exchange characteristics of the halide complexes of element 105 produced by the reaction 249Bk(18O,5n); details are given under procedure Z=105, 105-3 in section 2.
5. Delivery Systems for Continuous Techniques
As pointed out in Sec. 3, both autobatch and continuous techniques have been used for the study of short-lived nuclides. Continuous techniques are preferable for nuclides with half-lives on the order of a second or less because of their higher efficiency (see Sec. 3). Two major components are essential for the continuous production and isolation of short-lived nuclides:
-
A method to continuously produce and deliver radioactive nuclides to a separation system in a short time (comparable with the half-life of the nuclide under investigation).
-
A system capable of continuously separating the nuclides of interest and delivering them to a detector system.
Gas-jet systems have been routinely used in particle accelerators to transport reaction products from the production site to a detector position for counting; in some cases, the products are delivered to a chemical separation system. The gas-jet system has been adapted for continuous delivery of fission products from a target chamber to a separation system where chemical or mass separation can be carried out. Volatile products (formed directly or by interaction of recoiling product with carrier gas) can be carried by a gas, whereas efficient transport of nonvolatile products requires the presence of aerosols or clusters in the carrier gas. Different kinds of aerosols have been used for carrying nonvolatile products.
Two different approaches have been used to carry out continuous chemical separations:
-
Conventional solvent-extraction procedures to chemically isolate the element of interest using fast-phase separations attainable with high-speed centrifuges.
-
Gas-phase separation techniques where products of interest are isolated either by using selective gas-phase reactions or by thermochromatography of volatile species.
In the following subsections, the use of gas-jet systems for radiochemical isolation of short-lived nuclides is discussed. The use of different kinds of aerosols in the gas-jet system and their relative merits are described in Sec. 5.1. Measurement of transport time of reaction products in nonpulsing nuclear reactors is described in Sec. 5.2; Sec. 5.3 cites some typical examples. Continuous radiochemical separation techniques will be discussed in Sec. 6 and Sec. 7.
5.1 Gas-Jet Systems
In order to transport nuclear-reaction products generated using accelerators to a detector located in a low-background area, gas-transport systems have been used for quite some time. Volatile products are allowed to either thermalize in the target and diffuse into a carrier-gas
stream, or to recoil into the gas, which thermalizes and carries them. Nonvolatile products are thermalized in a carrier gas and transported. Helium is generally used as a carrier gas, and polyethylene or Teflon capillary tubes are used for carrying the gas. Tubes as long as 200 m have been used successfully to transport reaction products. MacFarlane and McHarris [Mac74a] have reviewed in detail the use of gas-jet systems in the study of short-lived nuclides.
Perhaps the earliest application of a carrier gas to transport radioactivity was done in 1900 by Rutherford [Rut00] in his experiments on emanation from thorium compounds. A thick layer of thorium oxide enclosed in paper was placed in a long metal tube. The paper was thick enough, according to Rutherford, “to cut off the regular radiation almost entirely, but allowed emanation to pass through.” The metal tube was attached to a large, insulated cylindrical vessel with an electrode connected to an electrometer. By passing a stream of air, he showed that emanation collected in the cylindrical vessel and ionized the gas. By stopping the air flow, he was able to follow the decay of emanation collected in the vessel.
Transport of a nonvolatile product by gas was used by Ghiorso and coworkers [Ghi58] in their attempt to identify nobelium. A curium target containing 244Cm and 246Cm was bombarded with monoenergetic 12C ions. The recoiling product atoms were thermalized in helium gas; the resulting positively charged atoms were transported to a moving, negatively charged metallic belt. The products deposited on the belt were used for the identification of nobelium. The use of gas to transport nuclear reaction products produced in accelerators increased in the following decades.
In the 1960s and early 1970s, a number of research groups experimented with helium gas-jet systems for thermalizing and carrying reaction products from targets in accelerators. The transport efficiency obtained was low and not reproducible; hence, operating a gas-jet system and obtaining good transport efficiency seemed to be considered more “black magic” than science.
Junglas and coworkers [Jun71] studied the thermalization and transport of 8Li produced by deuteron bombardment of 7Li by 99.999% pure helium. They found that 8Li was attached to large molecular clusters in the helium with mass in the range of 106 to 108. The clusters were found to consist of 59% neutral, 25% positively charged, and 16% negatively charged components. They concluded that the intense ionization of the deuteron beam probably produced charged species; impurity molecules present in helium (e.g., H2O) could build clusters and thermalized reaction products could attach to clusters.
5.1.1 Role of Aerosols
Transport efficiency was uniformly low when reaction products were produced in a surrounding where intense ionization was not produced. For example, Aystö and Valli [Ays73] showed, using recoiling decay products from a 227Ac source, that pure commercial-grade helium transports less than 1% of the products over distances longer than 1 m. At distances shorter than 20 cm, transport efficiencies from 13 to 36% were obtained, depending on other conditions (e.g., target chamber pressure, diameter of the capillary). Cooling helium to liquid-air temperature increased transport efficiency to about 18%, even for a distance of 20 m [Ays74a], while addition of oil vapor to helium increased the efficiency to 75% [Ays74b]. Wien and coworkers [Wie72] found that commercial helium gas carried less than 1% of the thermalized fission fragments produced by spontaneous fission of 252Cf. They found that seeding helium with water vapor and irradiating it with ultraviolet radiation before its introduction into the 252Cf chamber increased the transport efficiency to nearly 55%.
Dautet and coworkers [Dau73] also found that when pure helium or nitrogen was used as a carrier in a gas-jet system to transport fission fragments produced in 14-MeV neutron irradiation of natural uranium, the yield was quite low. Addition of ethylene was found to increase efficiency to nearly 70%. Wilhelm and coworkers [Wil74] studied the effect of different clusters on the transport efficiency of 252Cf fission fragments and 232Th decay products. They mixed the vapor of
a cluster material with helium and subjected it to ultraviolet radiation. Maximum transport efficiency of nearly 80% was observed with ethanol and carbon tetrachloride, while trichloroethylene and water gave efficiencies of 53% and 39%, respectively.
Schmidt-Ott and coworkers [Sch73] also found that addition of carbon tetrachloride to carrier gas increased the yield, while Feldstein and Amiel [Fel73] found that the addition of carbon tetrachloride instead of water decreased the efficiency by a factor of 5. These results established the necessity of clusters for transporting reaction products over long distances in capillary tubes. The discrepancies in the reported results for similar conditions indicated that a better understanding of the system is essential to obtain reproducible results.
The next phase of research on gas-jet transport systems focused on understanding the formation of clusters, the size of clusters, and the influence of clusters on efficiency of transport.
Using gold clusters formed in electrical discharge, Zirnheld [Zir74a, Zir74b] found that cluster size and the carrier-gas flow rate are important factors that influence the transmission of reaction products through capillary tubes. Wiesehahn and coworkers [Wie73] investigated extensively the role of clusters in the transport of radioactivity in a gas-jet system. They showed that impurities in ethylene play only a minor role in carrying radioactivity; they also showed that a neutron flux of 108 n cm−2 s−1 is unlikely to produce sufficient cluster density for transporting fission products. They carried out experiments with a number of different gases and found that clusters present in gases near critical temperature and pressure most likely are responsible for the transport of reaction products. The clusters may also be formed when density fluctuations occur as the gas expands as it flows through a regulator.
Mayer and Mayer [May61], Hirschfelder, Curtiss, and Bird [Hir64], and Lee, Barker, and Abraham [Lee73] have discussed the formation of clusters near the critical point. The formation of droplets in flowing gas is discussed by Wegener [Weg69] and was studied experimentally with ethyl alcohol by Clumpner [Clu71].
Light-scattering experiments performed by Wiesehahn and coworkers [Wie74] showed the presence of clusters in ethylene gas flowing from a gas cylinder at room temperature when the cylinder pressure was 68 atm; clusters were absent when the pressure was 38 atm or when the temperature was below the critical temperature (for ethylene, the critical pressure was 50.5 atm; critical temperature was 9.9 °C). The clusters had diameters between 0.1 and 1.0 µm, a concentration of 105 cm−3, and a lifetime of about 10 min.
Loss of clusters in the capillary leads to lower transport efficiency. Reviewing the losses due to Brownian motion, sedimentation, and gas-flow pattern, Wiesehahn, Bischoff, and D'Auria [Wie75b] concluded that the cluster should be 1 to 2 µm in diameter for optimum transport. They also showed [Wie75c] that many substances (pump oil, water, ethanol, benzene, carbon tetrachloride, dichloroethylene, and trichloroethylene) can be used to generate clusters; all of them provided efficient transport in a gas-jet system as long as the cluster size was appropriate.
Results reported in the last several paragraphs summarize the information available on the formation of clusters (especially ethylene clusters), their size, the optimum size required for efficient transport of radioactive products (produced in accelerators, reactors, or by decay of radioactive parent nuclides), cluster density, and cluster lifetime. Similar results have been reported by a number of research groups [Wo175, Sch75, Wol76, Jun76, Maz76, Sil77].
5.1.2 Techniques of Aerosol Production
Wollnik and coworkers [Wol77] have discussed different methods of aerosol generation and their relative merits. The clusters generated in gases such as ethylene, discussed in the previous paragraphs, are generated by the Joule-Thomson effect. Aerosols can also be generated using an atomizer. For example, Schmidt-Ott and coworkers [Sch77b] used n-decane and a mixture of 70% decanol – 30% methanol in an atomizer to generate aerosols. They obtained 70 to 90% transport
yield in their gas-jet system (to carry deuteron-induced and 84Kr-induced recoils). Further, their experiments showed that sufficient aerosols were generated by condensation of n-decane in the presence of the ion beam. A third technique is to generate aerosols using the principle of Sinclair-LaMer, i.e., aerosols are formed by condensation of a vapor around some condensation nuclei.
Imanishi and coworkers [Ima80] used the Sinclair-LaMer principle for the generation of aerosol in a helium-jet system. Their aerosol-generation setup consisted of a nebulizer that produced dioctyl phthalate (DOP) particles; the particles were vaporized in a reheater and mixed well with an NaCl seed, which was introduced from a nucleator. Helium was the carrier gas. The mixture (DOP vapor and NaCl seed carried by helium) was then passed through a condenser kept at room temperature; the DOP condensed on the NaCl seeds and grew into aerosol particles. They used the gas jet to carry fission products produced by spontaneous fission of 252Cf and by 110-MeV alpha-particle bombardment of 232Th. The efficiency was nearly 100% (except for iodine and rare gases); the system was stable over a period of 2 weeks and was reproducible for more than 6 months.
Kawade and coworkers [Kaw82] used Geissler tubes to generate nuclei for aerosol particles in which DOP vapor and vacuum-pump oil were used as the aerosol materials. They measured a transport efficiency of 60% for 235U fission products.
Wollnik and coworkers [Wol77] studied all three techniques of generating aerosols, tagged the aerosol particles with 212Pb, and studied the activity distribution as a function of particle size. The Sinclair-LaMer generator produced aerosols of nearly uniform size that transported efficiently through long capillary tubes; this technique of production of aerosols gave the highest overall efficiency. The Joule-Thomson effect produced a large number of particles of small size that were lost most readily during transport; the overall efficiency was smallest for transport using aerosols generated using this technique. The atomizer technique gave results that were in between.
5.1.3 Types of Aerosols
Several different types of aerosols have been used in gas-jet systems. Quite often, condensable gases like carbon dioxide, ethane, and ethylene generated by the Joule-Thomson effect were used for transporting reaction products. Oil vapor, decane, decanol, methanol, and similar liquids were used with atomizers. In the Sinclair-LaMer technique, DOP was commonly used. A number of inorganic materials have also been used. In the following paragraphs, we present a brief summary of information regarding the use of such aerosol materials.
Sodium chloride was used in a helium-gas jet by Ghiorso and coworkers [Ghi74] to carry reaction products in their experiment to synthesize element 106 by bombardment of a 249Cf target by 18O. Helium at 800-mm Hg pressure passed through a furnace containing sodium chloride kept at 700°C. The gas, loaded with aerosol, flowed through the target chamber, collected reaction products, and deposited them on a rotating drum. Aystö and coworkers [Ays76] studied the efficiency of a sodium-chloride-loaded helium jet and found that a maximum efficiency of 70 to 80% for 252Cf fission product was obtained if the sodium chloride was kept between 700 and 800°C; the efficiency dropped when the temperature was above 800°C. The yield was found to be independent of pressure between 2.0 and 3.0 atm. They also found the efficiency to be lower for reaction products with smaller recoil energies. Similar results with KCl and NaCl aerosols were reported by Mazumdar and coworkers [Maz76, Maz80].
A detailed study of alkali halide aerosols in gas-jet systems was performed by Stender and coworkers [Ste80]. Nitrogen gas was passed through a quartz tube containing the alkali halide in a boat maintained at a specific temperature. The gas coming from the furnace passed through a 6-mm-i.d., 15-m-long polypropylene tube arranged in a circle to filter out small particles by collision with the walls of the tube; big particles were retained by a glass frit. The gas then
passed through the target chamber containing 235U located in a beam hole in the reactor. The halides investigated (KF, KCl, KBr, NaCl, and NaBr) showed a plateau for yield as a function of furnace temperature; the plateau was about 80°C wide and occurred 100 to 150°C below the respective melting points. Their results showed that the alkali halides produced aerosols of optimum size over a narrow temperature range.
Mazumdar and coworkers [Maz81 ] determined, efficiencies for ZnBr 2, PbCl2, MgCl2, CdBr2, and PbF2 for transporting fission products. All the materials showed narrow peaks for efficiency as a function of oven temperature. All materials except PbF2 showed an efficiency of nearly 60%; PbF2 showed lower efficiency. Other materials used for aerosol were AgCl, silver, and tellurium (all of which showed 60% yield) and cadmium, with a 40% yield [And81 ]. Thus, it is evident that a variety of inorganic materials can be used as efficient carriers for reaction products.
The selection of aerosols for a gas jet depends on the nature of the work to be performed with the collected reaction products. Ethylene left a sizeable deposit. The deposit left by n-decane was nearly one-tenth of that observed from ethylene [Sch77b]. If alpha or fission-fragment activity is to be determined, then of course an aerosol leaving minimum deposit should be used. If chemical separations are to be performed on the products transported by gas jet, the nature of chemical separation dictates the proper aerosol. For aqueous chemistry or gas-phase synthesis involving high temperature, the inorganic aerosols (especially alkali halides) offer several advantages. They provide complete dissolution in aqueous solutions and also will allow high-temperature (~900° C), gas-phase chemistry. Silva and coworkers showed that organic clusters resulted in incomplete dissolution [Sil77].
5.2 Transport Time
Gas-jet transport systems are used in particle accelerators and nuclear reactors for transport and study of short-lived radionuclides. It is essential to know the transport-time characteristics of the system and the influence of different parameters on transport time. Terms like transport time, transit time, and sweepout time have been used in the literature with different meanings. In this paper, we will use the following definitions:
-
“Transport time” will be used to refer to the total time taken by the product generated to reach the detector position.
-
“Sweepout time” is the time taken by the recoiling product to appear outside the target chamber.
-
“Transit time” is the time taken by the product to travel through the capillary tube from the outlet of the target chamber to the detector position.
Several authors have calculated the transit time using gas dynamics. Details of calculations will not be discussed here; the reader is referred to the articles by Zirnheld [Zir74a] and Weiffenbach and coworkers [Wei75]. A number of different techniques have been used for measuring the transport time. Dautet and coworkers [Dau73] measured the transport time as a function of flow rate of ethylene by injecting a pulse of helium into the carrier gas and using a helium-leak detector to signal the arrival of helium at the collection chamber. They observed a smooth decrease in transport time with increasing flow rate of the carrier gas.
The most common technique for the measurement of transport time utilizes the pulsing capability of accelerators and reactors (if available). For example, Weiffenbach and coworkers [Wei75] measured the transport time by recording the total activity observed at the collector in multiscaling mode; the multiscaling was initiated by the pulse which turned on the cyclotron beam. The counting was repeated several times, each with a new collector surface, to obtain good statistics. The results of a typical measurement are shown in Fig. 22. They found a decrease in transport time with increase in target-chamber pressure. Mazumdar and coworkers [Maz80] used the reactor in the pulsed mode to determine the transport time in the gas-jet system coupled to an
isotope separator. Similarly, Skarnemark and coworkers [Ska80] determined the transport time in the gas-jet system coupled to a continuous chemistry system by pulsing the reactor and measuring the total activity in a multiscaling mode. All these measurements were carried out on-line.
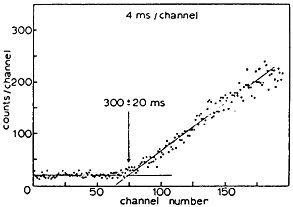
Figure 22. A typical measurement of the time of arrival of activities at the collection chamber after initiation of cyclotron bombardment. [Wei75; reprinted with permission from Nucl. Instrum. Methods]
An off-line technique was used by Zirnheld and coworkers [Zir79] to measure the transport time of the gas-jet system coupled to a mass separator. A spark chamber with volume identical to that of the fission chamber in the gas-jet system had two electrodes with gold tips. The helium-gas flow was maintained under conditions similar to that in the gas-jet system. A spark was flashed through the chamber, and the current of gold ions collected at the mass separator was recorded as a function of time.
Microscopic time distribution of the transfer of radioactive product was studied by Schmeing and coworkers [Sch76]. They used a 50-ms accelerator pulse to bombard a target at 875-ms intervals; 16 sequential energy spectra of protons emitted from the reaction product were recorded. By analysis of the data, they concluded that half of the activity generated by an accelerator pulse was transported to the catcher in 150 ± 40 ms, whereas no product arrived at the catcher after 300 ± 50 ms. Even though the values reported are applicable to the specific system used, the information gives a general clue to the nature of microscopic transfer of reaction products.
As mentioned in previous paragraphs, the transport time decreased with increase of flow rate and increase of target-chamber pressure. However, if the flow changes from laminar to turbulent, a change in the trend of transport time as a function of flow rate or target-chamber pressure occurs. Figure 23 shows the transport time determined by Macias and coworkers [Mac74b] as a function of inlet helium pressure, flow rate, and Reynolds number. A well-defined minimum is evident from the figure; the increase in transport time beyond the minimum, in spite of the increase in mass throughput, was attributed to the onset of turbulence. For gas jets in accelerators and pulsing-type reactors, the triggering pulse can be used to determine the zero point in transport time measurement. For systems in reactors that cannot be pulsed, the zero reference time must be determined. Two different techniques have been reported in the literature: an increased-flux technique [Kaw82, Oka81] and a stopped-flow technique [Ren85b].
In the increased-flux technique, Kawade and coworkers [Kaw82, Oka81] determined the zero time by moving the target towards the reactor core by 35 cm, thereby increasing the neutron flux by a factor of 9; the changing operation was performed in 0.2 s. The fission products were collected on a continuously moving tape, and the beta particles emitted were counted in a multiscaling mode. The counting rate started to increase after a certain delay (from the time of changing the target position); the delay time corresponded to transit time. Activity increased continuously and reached a constant value; the time taken to achieve a constant count rate
corresponded to sweepout time. Figure 24 shows the multiscaling data obtained by them. This technique is useful only if the position of the target chamber can be changed quickly. (Please note that in Fig. 24 Kawade and coworkers have used “transit time” to represent the time to change the target chamber from low-flux position to high-flux position.)
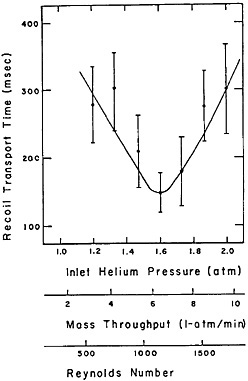
Figure 23. Dependence of recoil transport time on inlet helium pressure, mass throughput, and Reynolds number. [Mac74b; reprinted with permission from Nucl. Instrum. Methods]

Figure 24. A multiscaling spectrum of [β-ray counts of transported fission products for the measurements of sweepout and transit times through an 11.5-m capillary with 1.0-mm inner diameter at a helium pressure of 1.1 atm. The volume of the target chamber is 30 cm3.The solid line is drawn to guide the eye. [Kaw82; reprinted with permission from Nucl. Instrum. Methods]
The stopped-flow technique [Ren85b] is of general applicability for the measurement of transport time. In this technique, the time is determined by controlling the gas flow and measuring the fission-product activity collected at a quartz-wool trap. A schematic of the experimental setup is shown in Fig. 25. The gas mixture (nitrogen and ethylene) passed through an electrically operated, three-way valve to the target chamber. The valve was connected in such a way that when power was applied, gas would flow to the target chamber. The outlet from the target chamber was connected to a two-way, electrically operated valve. The fission products carried by ethylene clusters passed through the valve and were retained by a quartz-wool trap in front of a detector. The gas from the filter passed through another two-way valve to pump and exhaust. The activity of the trap was recorded by a multichannel analyzer in the multiscaling mode. The power for all three valves was applied by the operation of a single switch; the switch also initiated the multiscaling. The valves were opened while the reactor was operating steadily; the gas flow adjusted to the desired value, and the system was allowed to reach equilibrium. The valves were closed, the quartz-wool trap was replaced, and flow was initiated by opening the three valves simultaneously, initiating multiscaling. The valves were closed when the multiscaling ended. The cycle was repeated to improve counting statistics. The transport time obtained using this technique was compared with that obtained using the pulsing technique. Figure 26 shows the results obtained using both techniques. The values obtained are in good agreement with each other. A slight modification of this technique made use of a dual multiscaler based on a microcomputer for timing measurements [Gri85].
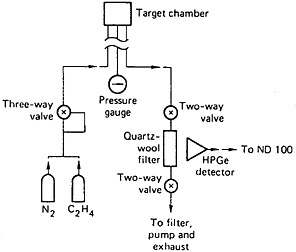
Figure 25. Schematic of the experimental setup for transport time measurements. [Ren85b; reprinted with permission from Nucl. Instrum. Methods A]
5.3 Examples of Gas-Jet Systems
A large number of gas-jet systems are used in conjunction with on-line isotope separators. There are only a few systems coupled to fast chemical separation systems. In the following paragraphs, selected examples of systems are described.
A helium-jet transport system coupled to an on-line isotope separator has been set up at the Research Reactor Institute of Kyoto University [Oka81]. Figure 27 shows a schematic of the system. The target was 93% enriched 235U electrodeposited as uranium oxide on aluminum; the weight and thickness of the target were 9.0 mg and 0.5 mg cm−2, respectively. The target was covered with approximately 2 mg cm −2 aluminum foil. The system was set up in the through tube of the reactor; the target chamber can be moved along the tube and thus can be in a neutron flux ranging from 1010 to 3 × 1012 n cm−2 s−1. The aerosol generator used dioctyl phosphate or pump oil. The output of the target chamber was fed to the ion source.
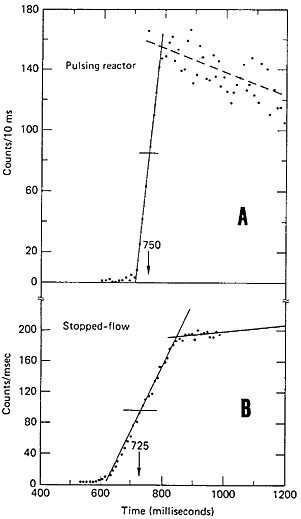
Figure 26. (A) Count rate observed as a function of time after pulsing the reactor. (B) Count rate observed as a function of time after opening the gas-flow valves (stopped-flow technique). In both experiments, the target chamber pressure was 25 psi and the gas flow was 2.6 L/min (at 1 atm and 22°C). [Ren85b; reprinted with permission from Nucl. Instrum. Methods]
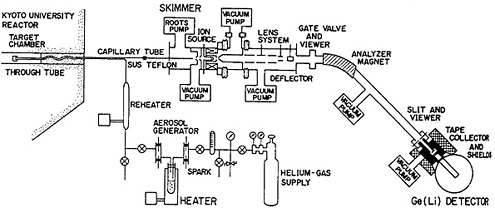
Figure 27. Schematic diagram of the He-jet-type ISOL at the Kyoto University Research Reactor Institute. [Oka81; reprinted with permission from Nucl. Instrum. Methods]
A gas-jet system at the University of Mainz has been used with a number of continuous fast chemical separation systems. Various fissioning nuclides have been used as target material. The target chamber was located in one of the beam holes close to the core of their TRIGA reactor. Plugs filled with boric acid and paraffin provided the shielding. The fission products from the target chamber were transported through 7 m of capillary tubing. A transport time of 1 s from the target chamber to the end of the capillary was observed at a flow rate of 20 cm3/s [Tra75].
Figure 28 shows the schematic of the gas-jet system installed in the beam port of Ford Nuclear Reactor at the University of Michigan [Ren86b]. The target was 1 mg of 235U chemically plated on an aluminum backing. The target was covered with aluminum foils of total thickness 2.75 mg/cm2. This arrangement, as reported by Zendel and coworkers [Zen87], provided an enhancement factor of 100 for light-fission fragments. The target chamber is located inside an aluminum tube. A series of modular (60 cm or 30 cm) concrete cylinders, with spiral conduits, was inserted in the aluminum tube for shielding, and three tubes, passing through the conduits, were connected to the target chamber. The aluminum tube housed in the beam port could be withdrawn about 1 m when the gas jet was not being used. The aluminum tube is surrounded by water, which provides the necessary shielding. In its nonirradiation position, the target chamber was shielded by a 1-m thickness of water.
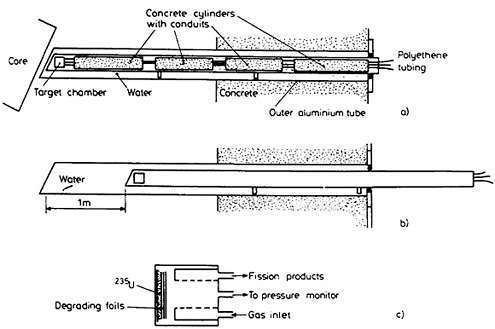
Figure 28. Schematic of the target chamber and its location relative to the reactor core; a) sketch of gas-jet system in irradiation position, b) sketch of gas-jet system in non-irradiation position, c) target chamber. [Ren86b; reprinted with permission from J. Radioanal. Nucl. Chem.]
Gas targets have been used for study of short-lived materials. For such studies, gas-target systems and circulation systems have been developed. Details of these systems will not be










