discussed here. Readers are referred to the supersonic-jet gas-target system described by Becker and coworkers [Bec82], to the recirculating gas-target system developed by Hamel and coworkers [Ham84], and to the references given in those articles.
6. Continuous Liquid-Phase Chemical Separations
Continuous liquid-phase chemical separation techniques generally have used a gas-phase delivery system. In early work, recoil species that were carried by a gas jet were deposited on a surface coated with a thick NH4Cl film. The NH4Cl was dissolved, and the solution was used for chemical separation. For example, Silva and coworkers [Sil70b] deposited recoil products carried by the helium jet on a platinum foil coated by ammonium chloride; the deposit was dissolved in a small volume of ammonium α-hydroxyisobutyrate and used for loading on an ion-exchange column. Puumalainen and coworkers [Puu73] used helium gas containing a carrier vapor to transport the decay products of 227Ac. The gas bubbled through a mixture of an aqueous solution of HCl (pH 3) and a CCl4 solution of dithizone. The bubbling helium mixed the two phases effectively. The carbon tetrachloride extracted bismuth as dithizone complex and thus separated it from its parent lead.
Kosanke and coworkers [Kos74] attempted batch as well as continuous on-line chemistry with the helium gas-jet system set up to carry cyclotron-produced activities. The nuclear reaction products were trapped by bubbling the helium through 2M HCl solution in a beaker. A schematic of their apparatus for continuous on-line chemical separation of gallium from copper and zinc is shown in Fig. 29. The helium gas carrying the clusters was mixed with flowing 8M HCl containing carriers; the mixing was turbulent. The mixture entered a reservoir on top of an anion-exchange column. The HCl solution flowed through the column, while the helium escaped from the reservoir. A Ge(Li) detector recorded the activity of the ion-exchange column. Continuous separation of gallium from copper and zinc was achieved by this procedure. Aumann and Weismann [Aum74] showed that fission products carried by a gas-jet stream can be trapped in aqueous solution. They performed a separation of barium from the mixed fission products collected in the aqueous solution.
6.1 High-Speed Centrifuge for Phase Separation
One of the most common techniques used for radiochemical separations, as discussed in Sec. 2.4, is solvent extraction. This is because of the selectivity it offers and the wealth of literature data available. However, for ultrafast separation, the slowness of phase separation has been a problem. Rydberg and coworkers investigated rapid phase-separation techniques using high-speed centrifuges for their studies in solvent-extraction equilibria. The incomplete phase separation provided by commercially available units led them to develop the “H-centrifuges.” These units had much smaller hold-up volume compared to the commercial centrifuges, and ran at a speed of about 16,000 rpm, developing 15,000 g-force [Rei67]. Rydberg and coworkers used the system, termed “AKUFVE” (the Swedish inventor's abbreviation for “apparatus for continuous measurement of distribution factors in solvent extraction”), to study a Cu(II) acetylacetone-benzene system [Ryd69, Rei69, And69]. The system was first utilized by Aronsson, Ehn, and Rydberg [Aro70] for continuous separation and study of 13.6-s 116Pd. The centrifuge and the 116Pd experiments are described in the following paragraphs.
The details of the H-centrifuge, which provides absolute phase separation, are given by Reinhardt and Rydberg [Rei69]. The liquid mixture enters the internal chamber of the centrifuge and is accelerated by the high-speed rotation of the unit. The liquid mixture enters a separation volume, which consists of eight chambers symmetrically arranged around the axis; the chambers
are totally separated from each other. The peripheral partition walls and the baffle ridges force a zigzag motion for the flowing liquid. There are two collection chambers, one above and one below the separation volume; the light phase is discharged from the upper collection chamber, whereas the heavy phase is discharged from the lower chamber. Different types of pump wheels are used for discharge. The centrifuge was constructed of pure titanium (later models have the titanium pacified with palladium that has been diffused into the titanium surface) and was driven by a pneumatic motor or a variable-speed induction motor. The central bowl volume was 120 mL, and the holdup time was about 3 s. The system gave excellent phase separation.
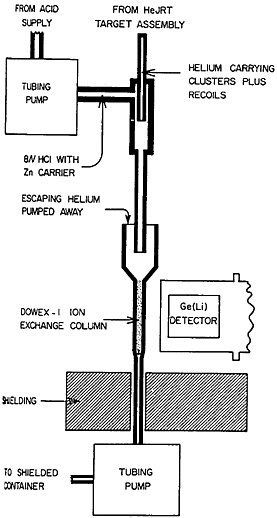
Figure 29. Sketch of the experimental apparatus used for on-line separation of gallium from zinc and copper activities after these had been transported to a low-background area with an HeJRT system. [Kos74; reprinted with permission from Nucl. Instrum. Methods]
Aronsson, Ehn, and Rydberg [Art70] used the H-centrifuge to achieve fast, continuous separation of 116Pd produced by 14-MeV neutron-induced fission of 238U. Figure 30 shows a schematic of the equipment they used. Uranyl nitrate (2M) in 1M nitric acid solution containing acetylacetone (0.1M) passed through the irradiation cell and a delay line and was then mixed with toluene and fed to the first centrifuge. Palladium isotopes formed in fission were complexed by acetylacetone and were extracted by toluene. The toluene phase from the centrifuge was passed through a delay line; 116Pd decayed to 116Ag during the delay. The organic phase was then mixed
with 0.001M HNO3 and fed to a second centrifuge. The silver daughter products were stripped by HNO3; the aqueous phase was passed through a filter containing preformed AgI, which retained the Ag activities. They identified 116Pd through its daughter 116Ag and determined its half-life to be 13.6 s.
Aronsson and coworkers utilized the rapid phase-separation power of the H-centrifuge to develop new, rapid, continuous radiochemical separations and called the technique “SISAK,” i.e., short-lived isotopes studied by the AKUFVE technique [Aro74a]. The general characteristics of the technique and a two-detector, dynamic-flow method for the determination of half-life are discussed in their paper. In the early applications of the technique for the study of short-lived, neutron-rich lanthanides, they used uranium adsorbed on Dowex-1 as the target; the lanthanides produced by fission were continuously eluted using 0.02M (NH4)2SO4 at a pH of 4.5. Typically,a three-stage solvent-extraction cycle using three H-centrifuges [Aro74b, Aro74c, Aro74d] was used.
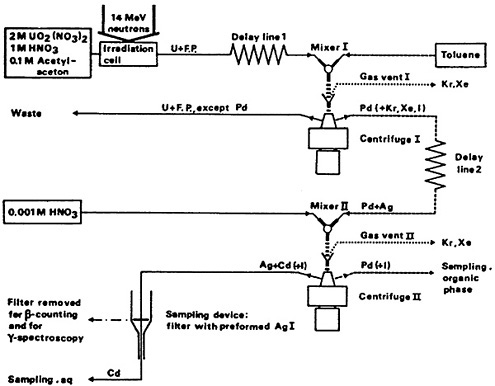
Figure 30. Equipment used for separation and identification of 116Pd. Solid and dashed lines refer to aqueous and organic flows, respectively. Uranium solutions are at 50°C. [Aro70; reprinted with permission from Phys. Rev. Lett.]
A gas-jet system was coupled to a SISAK system by Trautmann and coworkers in their study of short-lived lanthanum, cerium, and praseodymium nuclides [Tra75, Bjö77a, Bjö77b, Ska76,
Ska77a]. They used a mixture of nitrogen and ethylene in the gas jet. The gas carrying the fission products was thoroughly mixed with 1M HNO3, degassed before contact with organic solvent. Examples of these separation procedures are given in Sec. 6.3.
A smaller version of the H-centrifuge has been developed by Rydberg and coworkers [Ryd80]. The new version, termed an “H-10 centrifuge, ” has a much smaller hold-up volume of 12 mL, and the hold-up time is reduced to 0.40 s. Operation of the H-10 centrifuge is similar to that of the H-centrifuge. The development of the H-10 centrifuge has made it possible to use multistage chemical separations and on-line detection that have a transport time of a few seconds from target to detector. Skarnemark and coworkers [Ska80] describe the SISAK-2 technique and its application for the study of lanthanides.
Recently Persson and coworkers [Per89] have redesigned the H-centrifuge and reduced the hold-up time to 0.05 s. Figure 31 shows a cross section of the H-0.3 centrifuge unit. The separation chambers consist of four sets of elliptical holes of approximately 2 mm2 area each, thus reducing the volume significantly (to 0.3 mL). Flow rates of 0.1 to 3.3 mL s−1 per phase can be used without affecting the phase purity. The optimal speed of rotation of the centrifuge varied from 20,000 to 30,000 rpm, depending on the flow rate. The new system, SISAK-3, will be used in the study of exotic nuclides.
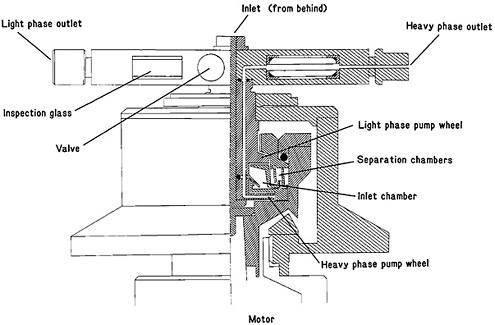
Figure 31. Cross section of the H-0.3 centrifuge unit. [Ska86b; reprinted with permission from Physica Scripta]
Other types of centrifuges have been used in fast chemical separations. Hellmuth and Valli [Hel75] used a continuous centrifuge with a mean hold-up time of about 5 s to separate 211Pb and 211Bi formed in the decay of 227Ac and carried by a gas jet. Another type of centrifugal contactor
used by Meikrantz and coworkers [Mei81] in the work on short-lived palladium isotopes (see Sec. 4.3) is described by Meikrantz [Mei80] and by Siczek and coworkers [Sic80]. These centrifugal contactors, unlike the H-centrifuges, allow mixing of the two phases in the device. These units can be used in continuous chemical separations.
6.2 Transfer from Gas-Jet System to Aqueous Phase
As described at the beginning of Sec. 6, early attempts at on-line chemistry involved transferring radioactive products carried by the gas jet to aqueous phase by simply bubbling the gas through an aqueous solution. It is known that nuclear-reaction products are being carried by clusters present in the gas jet (see Sec. 5.1.1). The reproducibility and the reliability of on-line aqueous chemistry depends on the efficient destruction of clusters and transfer of nuclear-reaction products carried by clusters to an aqueous solution. Skarnemark [Ska77b] found that by using a nitrogen-ethylene gas jet and performing the dissolution with HNO3 at 22°C, the cerium extracted by the organic solvent contained large amounts of lanthanum. If the dissolution was done with HNO3 at 90°C, the cerium contained almost no lanthanum. At intermediate temperatures, the lanthanum contamination was found to be at intermediate values.
According to Skarnemark, the ethylene clusters are not completely decomposed at temperatures below 70 to 80°C. The undecomposed clusters are transported with the liquid phase and, because of their hydrophobic nature, are extracted by the organic solvent. The higher level of lanthanum contamination observed at lower dissolution temperatures is most likely due to the extraction of undecomposed clusters.
The example given above illustrates the importance of the technique for transferring nuclear-reaction products from the gas-jet system to the aqueous phase. When clusters of organic molecules are used for carrying the products, it is essential to decompose the clusters completely before attempting any chemical separation. As pointed out by Stender and coworkers [Ste80], the difficulty can be nearly eliminated by using inorganic clusters, especially alkali halides.
Another aspect that affected the overall phase separation achieved with H-centrifuges and the radiochemical purity is the presence of gas in the system. It is essential that the aqueous solution be degassed before it is mixed with the organic solvent and fed into the centrifuge. A typical degassing unit consists of a tube with baffles and a conical bottom. The gas-liquid mixture is fed tangentially, and the baffles break the rotation. The liquid is withdrawn from the conical bottom, and the gas escapes from the top. The degassing removed more than 95% of the noble gases produced in fission.
A new centrifugal device was developed by Broden and coworkers [Bro80] for the separation of gas and liquid phases after dissolution of the clusters. The device has further been modified and improved by Persson and coworkers [Per89]. Figure 32 shows a cross section of the HG-0.1 degassing unit. The liquid is mixed with the gas from the gas jet in the static gas-liquid mixer. The mixture then enters the inlet chamber and is accelerated to the rotational speed. The mixture is forced into the separation chamber through the connecting three holes. There are three separation chambers; in each of these, the liquid is forced along the wall and then downward into a liquid-collection chamber. A stationary pump wheel in the collection chamber pumps the liquid to the outlet. The gas is forced towards the center and to the outlet. The centrifuge has a volume of 0.1 mL, and the hold-up time is 0.3 s. The system is reported to have a degassing efficiency of ≥99.5%.
6.3 Examples
Continuous on-line chemical separations have been used for the study of short-lived nuclides of a number of elements; many of the chemical separations developed were aimed at the study of
neutron-rich fission products. Nearly all published procedures use the SISAK techniques described in Sec. 6.1.

Figure 32. Cross section of the HG-0.1 degassing unit. [Ska86b; reprinted with permission from Physica Scripta]
The first application of the H-centrifuge for the study of short-lived radioactive nuclides was by Aronsson and coworkers [Aro70] in their characterization of 116Pd. The details of the procedure were described in Sec. 6.1. A number of procedures were developed in the 1970s. Early experiments to study neutron-rich lanthanides used UO2(SO4)2− complex sorbed on Dowex-1 resin as the target and 14-MeV neutrons as projectiles. Fission products produced were continuously removed by elution with 0.015M (NH4)2SO4 solution. The H-centrifuge with a hold-up volume of about 100 mL was used. The total time taken for the product to reach the detector varied from 10 to 20 s, depending on the number of steps in the chemical procedure.
Development of the H-10 centrifuge, with its smaller volume, has reduced the hold-up time to 0.25 s; the time required for each centrifuge stage is reduced to 1 s. This improvement has increased the potential of SISAK-2 techniques for the study of nuclides with half-lives in the range of 1 to 5 s. Broden and coworkers [Bro81] developed procedures for the separation of zirconium, niobium, technetium, bromine, and iodine from fission products. Depending upon the element, the mean time required for the product to reach the detector was 5 to 9 s. The bromine procedure was utilized by Skarnemark and coworkers [Ska86a] in their study of the beta decay of 88Br. Table 11 gives a list of known continuous aqueous chemical separation procedures arranged alphabetically according to the element; the table also gives the separation time, the extraction reagent, and the procedure number.
Recently, Skarnemark and coworkers [Ska86b] have reviewed the use of the SISAK system for nuclear studies. Figure 33 shows a schematic drawing of a SISAK system consisting of four parts, namely, the gas jet (including the target), the degassing unit, the chemistry system with the centrifuges, and the detection and data collection system, as depicted in their review article. Their
article also gives a summary figure showing the elements for which the SISAK system has been used, an outline of procedure, and the nuclides studied. In the following subsections, we describe some specific examples of the procedures using the SISAK techniques.
Table 11. Continuous solvent-extraction procedures.
|
Element |
Reagenta |
Timeb |
Procedure # |
|
Arsenic |
CHCl3 |
3 or 4 s |
As-6 |
|
Arsenic |
HDEHP |
1.0 s |
As-11 |
|
Barium |
Crown ethers |
<1 min |
Ba-2 |
|
Bromine |
CHCl3 |
~5 s |
Br-9 |
|
Cadmium |
MIBK–CHO |
~2 s |
Cd-3 |
|
Cerium |
HDEHP |
<5 s |
Ce-3,4,5 |
|
Copper |
Oxime |
20 s |
Cu-1 |
|
Indium |
MIBK–CHO |
~2 s |
In-2 |
|
Iodine |
CCl4 |
~5 s |
I-4 |
|
Lanthanum |
HDEHP |
<5 s |
La-2,3,5 |
|
Lead |
Crown ethers |
<1 min |
Pb-4 |
|
Neptunium |
HDEHP |
10 s |
Np-1 |
|
Niobium |
HDEHP |
~9 s |
Nb-2 |
|
Praseodymium |
HDEHP |
10–20 s |
Pr-2 |
|
Ruthenium |
CCl4 |
5–6 s |
Ru-8 |
|
Silver |
MIBK–CHO |
~2 s |
Ag-7 |
|
Technetium |
Alamine–336 in CHCl3 |
5 s |
Tc-5,7,8 |
|
Uranium |
Alamine–336 |
<1 min |
U-1,2 |
|
Z = 114 |
Crown ethers |
<1 min |
114-1 |
|
Zirconium |
HDEHP |
~7 s |
Zr-2 |
|
a For the full name of the reagent refer to the procedure(s). b Time reported is for the fastest procedure. |
|||
The introduction of the SISAK technique has rejuvenated the study of short-lived nuclides by fast chemical processes. The technique is being continuously improved. As pointed out earlier, the design of H-0.3 centrifuges with smaller volume reduced the hold-up time to 0.05 s. Semkow and Wahl [Sem83] have developed a technique for continuous separation of indium from tin and compared it with batch techniques. Björnstad [Bjö81] has outlined the use of the technique for fission-yield measurements, and Semkow and coworkers [Sem83] have utilized it for such measurements.
A method for the determination of the half-life of short-lived nuclides in a liquid stream using a two-detector delay method has been demonstrated by Alstad and coworkers [Als86]. This technique was utilized in the identification of 1.85-min 243Np and 2.29-min 244Np [Tet86, Moo87]. A technique for preparing thin samples on-line from a continuous liquid flow has been developed by Novik and coworkers [Nov81]. Porous plastic membranes impregnated with ion-exchange resin or organic solvent and thin, preformed precipitate are used to obtain a sample of radionuclides of interest; thin samples prepared in this way are very useful for beta, x-ray, and low-energy gamma-ray spectroscopy. Persson and coworkers [Per89] have demonstrated that α-spectrometric
measurements can be performed using the output from the centrifuge. We can anticipate more exciting developments associated with, and interesting results from, the SISAK technique.
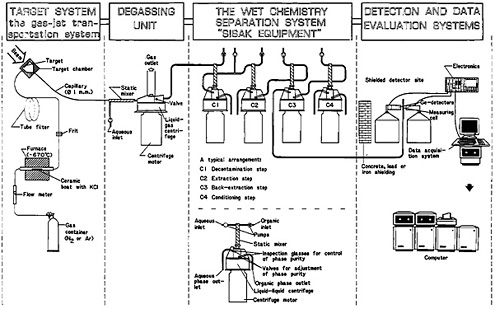
Figure 33. Schematic of a SISAK continuous-extraction system (not to scale). [Ska86b; reprinted with permission from Physica Scripta]
6.3.1 Arsenic
Skamemark and coworkers [Ska83] used a target that had 400 µg of 235U and was covered with a 2 mg/cm2 Teflon deposit. A gas mixture containing nitrogen and hydrogen chloride in the ratio of 20:1 was used to stop the recoiling fission fragments and to carry them to the chemistry system. Volatile chlorides of arsenic and germanium, along with bromine, iodine, krypton, and xenon, were carried out by the gas stream; nonvolatile fission products were deposited on the walls of the target chamber and capillary tube. A flow diagram of the arsenic chemical separation is shown in Fig. 34. The gas was mixed with a solution of HCl (3M) – HI (1M) at 70°C in a static mixer and fed into a degassing centrifuge. The solution flowing from the liquid outlet of the degassing unit contained arsenic, germanium, bromine, and iodine, and was fed into the first mixer-centrifugal separator unit (C1), where it made contact with chloroform. Arsenic was extracted by chloroform along with some noble-gas contamination. In the second mixer-centrifugal separator unit, arsenic was back-extracted with 0.1M HCl. The HCl solution was used for counting. The chloroform was recycled to C1. The total delay time from the target to the detector site was 4 s. The delay time could be reduced to 3 s if the chloroform phase from the first centrifugal separator containing noble-gas contamination could be used for counting.
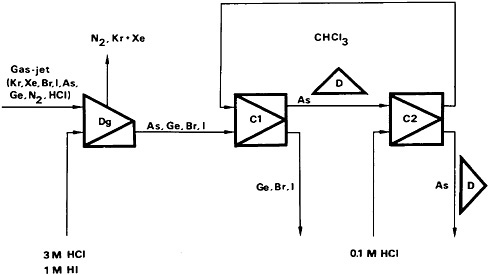
Figure 34. Flow sheet for the continuous separation of arsenic from fission products. Dg = degassing unit; C1, C2 = mixer-centrifugal separator units; D = detector positions. [Ska83; reprinted with permission from Radiochim. Acta]
6.3.2 Zirconium and Niobium
A fast continuous-separation procedure using the SISAK-2 technique has been reported by Broden and coworkers [Bro81]. Figure 35 shows a flow diagram of the zirconium and niobium separation procedure. The fission products generated in the target chamber were thermalized and carried by nitrogen gas containing KCl clusters. The fission products were dissolved by 1M H2SO4 in a static mixer, and the gas-liquid mixture was degassed, keeping the solution at 70°C. The aqueous phase was contacted with 0.1M Alamine-336 in Shellsol-T (an aliphatic kerosene) containing 5% n-dodecanol in the first mixer-centrifugal separator (C1). Zirconium and niobium were extracted by the organic solvent along with a small amount of technetium and other contaminants. In the second mixer-centrifugal separator (C2), zirconium and niobium were stripped by 0.3M HNO3, leaving technetium and other contaminants in the organic phase; the organic phase was recycled to the first unit (C1). The aqueous phase leaving the second unit was mixed with 5M H2O2 and 10M HNO3 to bring the aqueous solution entering the third mixer-centrifugal separator (C3) to 3M in HNO3 and 1M in H2O2. Zirconium was extracted into 1M di(2-ethylhexyl)orthophosphoric acid (HDEHP) in Shellsol-T, leaving niobium as the peroxide complex in the aqueous phase. The HDEHP phase was counted for zirconium. The niobium in the aqueous phase was contaminated with a small amount of zirconium. In the fourth mixer-centrifugal separator (C4), the zirconium contamination was removed by extraction with 1M HDEHP in Shellsol-T; the aqueous phase leaving C4 contained only niobium. The delay times were 7 s and 9 s for zirconium and niobium, respectively.
6.3.3 Lanthanum
Neutron-rich, short-lived lanthanum isotopes 144–146La were studied by Skarnemark and coworkers [Ska77a] using the SISAK-2 system for the separation of lanthanum from fission products. The flow diagram for this chemistry system is shown in Fig. 36. The nitrogen-ethylene
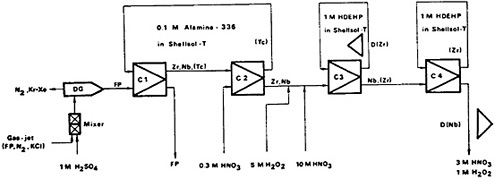
Figure 35. Flow sheet for the continuous separation of zirconium and niobium from fission products. DG = degassing unit; D(Zr) and D(Nb) = detector positions; C1, C2, C3 = centrifuge steps; FP = fission products. [Bro81; reprinted with permission from J. Inorg. Nucl. Chem.]
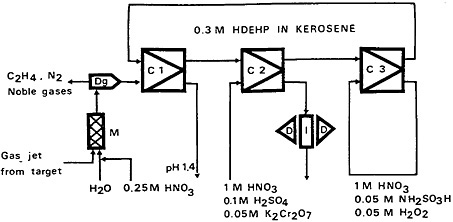
Figure 36. Flow sheet showing the chemical system used for the isolation of lanthanum isotopes. M = mixer; Dg = degassing unit; C1–C3 = mixer-centrifugal separator units; I = detector cell; D = detectors. [Ska77b; reprinted with permission from J. Inorg. Nucl. Chem.]
gas mixture carrying fission products was mixed with a nitric acid solution at a pH of 1.4 and at 90°C in a static mixture. The gas-liquid mixture was then passed on to a degassing unit, where the liquid and gas were separated. The liquid was then allowed to enter the mixer-centrifugal separator system consisting of three units (C1 to C3). In the first unit, all trivalent lanthanides were extracted along with other fission products (zirconium, niobium, molybdenum, etc.) into 0.3M HDEHP in Shellsol-T. The extract was contacted with an aqueous solution containing K2Cr2O7 in the second unit; all trivalent lanthanides were stripped into the aqueous phase, while cerium was oxidized to Ce(IV) and remained in the organic phase along with other fission products (zirconium, niobium, molybdenum, etc.). The activity in the aqueous phase leaving unit 2 was mostly due to lanthanum since the fission yields of praseodymium, neodymium, etc., are very low. In the third unit, the fission products were stripped from the organic solvent and the solvent was recycled to unit 1.
The SISAK system can be manipulated to study specific longer-lived nuclides. Björnstad and coworkers [Bjö77a] have reported the study of 14.2-min 143La using the SISAK system. The precursor of 143La has a half-life of 14 s, while 144La, the longest-lived heavier lanthanum isotope, has a half-life of 42 s. The chemical separation system they used is shown in Fig. 37. Three mixer-centrifugal separator units were used. As in the study of 144-146La, the fission products carried by the gas-jet stream were dissolved and degassed; in the first unit, the lanthanides and other fission products were extracted by 0.3M HDEHP. The aqueous phase from unit 1 containing the precursor of 143La flowed through a delay unit of 15 s before entering the second mixer-centrifugal separator unit. The delay allowed 50 to 60% of the 143Ba to decay to 143La. In the second unit, lanthanum was extracted into 0.3M HDEHP; the organic phase flowed through a 200-s delay line before entering the third separator unit. This delay allowed 144–146La to decay. In the third unit, the organic phase was contacted with an oxidizing aqueous phase; lanthanum (143La) was back-extracted, leaving cerium (144Ce) in the organic phase.
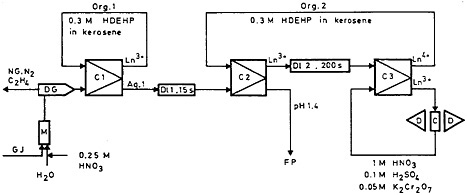
Figure 37. Chemical separation system for the isolation of 143La. C1, C2, C3 = mixer-centrifugal separation units; D11, D12 = delay lines; GJ = gas jet (N2+ C2H4); M = static mixer for gas and liquid; DG = degassing unit; NG = noble gases; FP = fission products; C = counting cell (Dowex-50W X4, 50–100 mesh); D = detectors. [Bjö77a; reprinted with permission from J. Inorg. Nucl. Chem.]
6.3.4 Technetium
The technetium procedure reported by Broden and coworkers [Bro81] shows the wide applicability of the SISAK technique in nuclear studies. Stachel and coworkers [Sta84] utilized the technetium procedure to perform γ-γ angular correlation measurements on 5-s 108Tc. Figure 38 shows a schematic of the technetium separation procedure they used. The fission products carried by the KCl aerosol in the gas jet were dissolved in 0.1 M HNO3 – 0.1 M KBrO3. The solution was heated to 80°C to accelerate the oxidation of technetium to pertechnetate. Technetium was extracted by 0.05M Alamine-336 in CHCl3 in the first mixer-centrifugal separator unit. The organic phase contained zirconium, niobium and molybdenum. In the second stage, technetium was stripped using 2M HNO3. Traces of zirconium and niobium were also stripped (<1%). The angular correlation measurements were performed with three detectors. The total transport time was 5 s. Additional decontamination can be achieved by including a mixer-centrifugal separator stage to extract zirconium and niobium with 0.5M HDEHP in CHCl3; this increased the transport time to 7 s.











