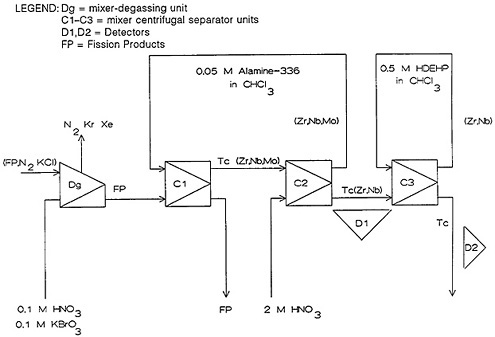
Figure 38. Schematic of the SISAK system used for the separation of technetium from fission products. [Reproduced with permission from G. Skarnemark]
7. Continuous Gas-Phase Chemistry
A continuous supply of products has been used in batch processes; i.e., gas-phase separations have been used for volatile elements like nitrogen, noble gases, bromine, and iodine. Dropesky and Schardt [Dro56], in their study of the decay of 24Ne, passed the irradiated neon gas through an activated carbon trap cooled in dry ice; the impurities were retained by the trap, and pure neon flowed through. For some elements, volatile products have been produced by chemical reaction between the recoiling product and a chemical present in the irradiation chamber. For example, Denschlag and Gordus [Den67] allowed recoiling fission-product halogens to react with methane in an irradiation vial; the CH3Br and CH3I formed were separated using gas chromatography. Kratz and Herrmann [Kra73, Kra78] also prepared CH3Br and CH3I in a similar manner and separated iodine and bromine by selective retention on AgNO3 and AgCl traps maintained at different temperatures. One of the earliest gas-phase chemistry studies of a gas-jet system was the work of Gauvin and coworkers [Gau73]. They found that the mercury isotopes produced by 144Sm(Ar,xn) reaction could not be deposited on a collector surface. However, in later experiments they added iodine vapor to helium, and found that mercury could be efficiently deposited; the efficient collection was attributed to the reaction of recoiling mercury atoms with iodine atoms present along with helium [Cab75].
For continuous gas-phase chemistry in a gas-jet system, two different approaches have been used. In one, the clusters would be decomposed and the selected group of elements would be
allowed to form volatile compounds (by the addition of a reactive gas, if necessary). The element of interest could be separated by selective adsorption. Thermochromatography has also been used to separate the volatile products formed. A second approach is to form volatile compounds of elements of interest by allowing the recoiling products to chemically react with a reactive gas used in the gas jet; selective adsorption of the products by different traps could lead to the separation of the element of interest. The following sections give a summary of techniques used and specific examples of procedures used for some elements.
7.1 Production Outside the Target Chamber
Fission-product selenium and tellurium have been studied by Zendel and coworkers [Zen78] using volatile products generated outside the target chamber. An ethylene-nitrogen gas mixture thermalized and carried fission products from the target chamber of a gas-phase chemistry system that was in the beam port of a reactor. Tellurium was carried by clusters, while selenium, bromine, and iodine formed volatile products and were carried by the gas stream along with krypton and xenon. A schematic of the setup used for the continuous separation of tellurium from fission products is shown in Fig. 39. The gas stream from the target chamber was passed through a charcoal trap that retained selenium, bromine, and iodine. The gas mixture was then passed through a quartz spiral heated to 860°C. The clusters were decomposed at this temperature; tellurium formed volatile compounds that were carried away by the gas stream, and all other fission products were deposited in the spiral. The gas stream passed through a charcoal trap in front of a Ge(Li) detector that retained tellurium while krypton and xenon passed through.
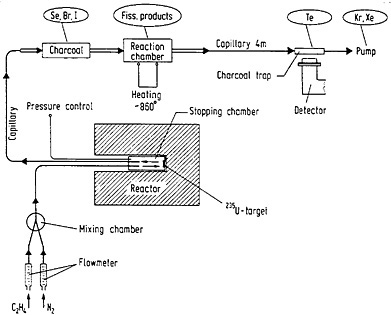
Figure 39. Setup for the continuous separation of tellurium from fission products (not to scale). By replacing the first charcoal trap by two paper filters, the same setup can be used for the separation of selenium. [Zen78; reprinted with permission from Nucl. Instrum. Methods]
In order to separate selenium, the first charcoal trap was replaced by two filters of 2-µm pore size; the filters retained the clusters while selenium, bromine, iodine, and the noble gases passed through to a quartz spiral kept at 860°C. Bromine and iodine stayed in the spiral; the gas stream carried the volatile products of selenium and the noble gases. Selenium was retained by a trap containing charcoal on quartz powder coated with silver.
The chemical separations of selenium and tellurium are the only reported procedures where the volatile products are formed outside the target chamber without the addition of another reactive gas. The volatile products formed resulted from the reaction of tellurium with ethylene at elevated temperature. Selenium formed volatile species by reaction with ethylene in the target chamber; it might have undergone further modifications at the elevated temperature in the quartz spiral.
Volatile compounds of elements of interest are often generated outside the target chamber by the addition of a reactive gas. The volatile products generated are frequently separated using the thermochromatographic technique (see Sec. 2.7). Zvara and coworkers [Zva75] used an on-line thermochromatographic apparatus to produce volatile bromides of reaction products carried by a helium gas jet. Figure 40 shows a schematic of their apparatus. Recoiling nuclear-reaction products, produced by 12C or 11B bombardment on lanthanide targets, were stopped and carried by a mixture of helium and bromine or of helium, bromine, and boron bromide. The products carried by the gas entered a thermochromatographic column with a temperature gradient. The distribution of activities was measured after 2- to 10-h runs. They studied the deposition of selenium, arsenic, germanium, and zinc activities produced from the nickel backing of the target as well as hafnium and tantalum activities produced from the rare-earth target. They established the usefulness of this technique and discussed its application for the study of element 105.
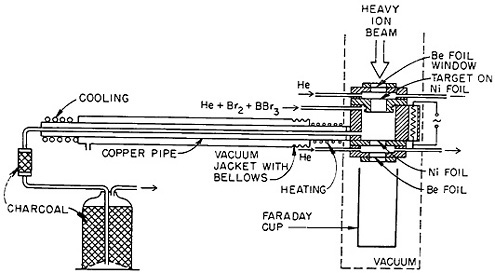
Figure 40. Schematic diagram of the apparatus for continuous bromide processing of recoils from the cyclotron-bombarded target. [Zva75; reprinted with permission from J. Chromatog.]
Zvara and the Dubna group have done pioneering work in the utilization of thermochromatography for the study of transplutonium elements. They have used SO2Cl2, TiCl4, NbCl5, and ZrCl4 as chlorinating agents in their study of element 104 [Zva66, Zva71, Zva72]. They utilized volatile bromine species produced with Br2 and BBr3 vapor as brominating agents in
their study of element 105 [Zva76]. Dry and moist air were used to produce volatile oxides of element 107 [Dom83]. In all cases, track detectors were placed at collection points to provide evidence for spontaneous fission events. For additional information, also see the earlier discussion in Sec. 2.7.
Bächmann, Bögl, and coworkers have investigated the use of oxides, chlorides, oxychlorides, and bromides in the gas phase for the separation of fission products. The fission products recoiling from a 252Cf source were carried by a nitrogen stream and mixed with different reactive gases. Each mixture passed through a transfer tube (0.6-cm diameter, 150-cm length) that could be heated to 400°C. The mixture then passed through an adsorption trap in front of a Ge(Li) detector, where the trap temperature was varied from −45 to 150°C. They used O2, H2O, Cl2, HCl, SOCl2, ZrCl4, and Br2 as reactive gases. The chlorinating agents were found to transport zirconium, niobium, molybdenum, technetium, ruthenium, tin, antimony, tellurium, and iodine. The gas-phase transport characteristics of technetium [Bög74a, Bög74b], ruthenium [Bög74c, Bög74d], tellurium [Bög74e], and iodine [Bög74e] were studied as a function of chlorinating agent and temperatures of the transport tube and trap. The transport of volatile oxides of technetium [Bäc75], ruthenium [Bög75a], tellurium [Bög75b], and iodine [Bög75b] using oxygen as the reactive gas was also studied as a function of the temperatures of the transport tube and trap. Further, their experiments showed that, by using a temperature gradient and selective chemisorption, a number of elements could be separated and deposited in different regions of a thermochromatographic column [Bäc76a, Bäc76b, Bög76].
Hickmann and coworkers [Hic80] coupled a potassium chloride aerosol carrying fission products in a gas jet with a thermochromatographic column and used it to study the usefulness of chlorides and oxychlorides for fast on-line separation. A schematic of the thermochromatographic system they used is shown in Fig. 41. The gas stream carrying the fission products was mixed with chlorinating agent and fed through a quartz-wool plug heated to 920°C. The clusters were destroyed at this temperature. The gas stream leaving the plug passed through a 60-cm-long thermochromatographic column having a negative temperature gradient of 15.5°C/cm. The deposition along the column was analyzed using gamma-ray spectroscopy. By comparing different chlorinating agents, chlorine and hydrogen chloride were found to be the most efficient, followed by carbon tetrachloride and thionyl chloride. A typical chromatogram obtained with chlorine is shown in Fig. 42. Lanthanides and alkaline earths deposited in the high-temperature region, while elements like antimony, molybdenum, technetium, selenium, and halogens were deposited in the low-temperature region. The exposure times were varied from 90 to 160 min and were shown to have no effect on the relatively nonvolatile chlorides such as those of the lanthanides and alkaline-earth elements. Cerium and cesium deposited in lower-temperature regions with increase of exposure time; other elements showed broader deposition regions. Hickman and coworkers have discussed possible applications of this technique.
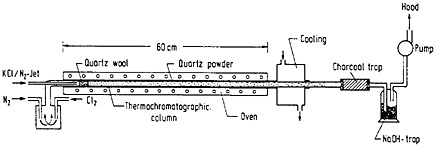
Figure 41. Schematic setup of the thermochromatographic system. [Hic80; reprinted with permission from Nucl. Instrum. Methods]
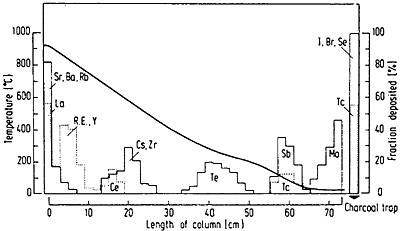
Figure 42. Element distribution in the thermochromatographic column under standard conditions with 7 vol% of chlorine in the carrier gas. [Hic80; reprinted with permission from Nucl. Instrum. Methods]
7.2 Production in the Target Chamber
Recoiling nuclear-reaction products are in a highly excited state and are capable of chemically reacting with materials around them. As pointed out in the introduction to this section, Denschlag and Gordus [Den67] utilized the reaction of recoiling fission-product halogen atoms with methane to produce CH3Br and CH3I and separated them by gas chromatography. Strickert and coworkers [Str74] showed that recoiling fission-product tellurium reacted with carbon monoxide and formed volatile compounds, possibly TeCO. They also concluded that the reaction probability was greater for tellurium formed directly in fission compared to tellurium atoms formed by beta decay. These results established the possibility of continuously producing volatile species in the target chamber and their use in gas-phase separations.
The use of volatile species produced by the chemical reaction of recoiling fission products with a gas present in the target chamber, for continuous gas-phase separation, has been established [Zen78, Ren82a, Ren82c], and a schematic of a typical setup is shown in Fig. 43 (taken from [Ren82c]). In this gas-jet system, the recoiling fission products were stopped in a mixture of nitrogen and ethylene. The ethylene clusters carried all the nonvolatile fission products; however, selenium and the halogens reacted with ethylene, forming volatile species. A quartz-wool filter stopped the clusters and allowed the volatile species to pass through. Bromine vapor was allowed to react with the mixture before it passed through a second quartz-wool trap in front of a Ge(Li) detector. Selenium was retained by the trap, while other volatile products passed through.
The nature of chemical reactions taking place in the target chamber is very complex and not fully known. However, ethylene can be expected to form a variety of free radicals in the radiation field present at the target chamber. Rice and Glasebrook [Ric34] have shown that free radicals readily react with elements such as selenium and tellurium. In the case of tellurium, Glasebrook and Pearson [Gla36, Gla37] found that free-radical reactions lead to the formation of tellurides and ditellurides of type R2Te and R2Te2, respectively (R = CH3, C2H5, etc.). Fission-product selenium can be expected to form compounds of the type CH3SeH, (CH3)2Se, (C2H4)2Se, (C2H5)2Se, etc., all of which are low-boiling liquids that will be volatile under experimental conditions. The boiling points of CH3SeH, (CH3)2Se, and (C2H5)2Se are 25.5, 58.2, and 108°C, respectively [Kau61]. The volatile selenides carried by the gas jet can react with bromine vapor to form additional
compounds of the type R2SeBr2 [Irg74], which are high-boiling liquids; the addition compounds can be expected to deposit on quartz wool.
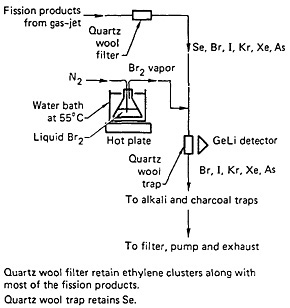
Figure 43. Schematic of the selenium chemistry system configuration. [Ren82c; reprinted with permission from Nucl. Instrum. Methods]
Evidence for the formation of similar organometallic compounds in a nuclear reaction chamber has been obtained by Tsuneyoshi and coworkers [Tsu83]. Recoiling 63Zn and 116Sb produced by 60Ni(α,n), 63Cu(p,n) and 116Sn(p,n) reactions were stopped in acetone vapor. The experimental arrangement they used is shown in Fig. 44. Acetone gas flowed through the target chamber and to a thermal decomposition port (TDP) in front of a Ge(Li) detector. The activity produced was studied as a function of acetone gas pressure and the thermal decomposition temperature. From the data obtained, they concluded that 116Sb formed stable Sb(CH3)3, which was decomposed at the TDP while 63Zn formed unstable species under the experimental conditions.
By using ethylene in a gas jet, continuous gas-phase separation of bromine has been achieved [Ren82b, Ren86b, Ska86a]. A schematic diagram of a typical bromine chemistry apparatus is shown in Fig. 45. Aluminum foils, which covered the uranium target, provided the primary separation of bromine from its heavier homolog, iodine [Zen87]. The nonvolatile fission products carried by ethylene clusters were retained by a quartz-wool trap. Selenium was retained by another quartz-wool trap coated with silver nitrate. A cotton-wool trap in front of a Ge(Li) detector preferentially retained bromine activity, providing further decontamination from iodine [Ren86b]. The chemistry responsible for the preferential retention of bromine is not known.
Continuous gas-phase procedures for the separation of germanium and arsenic from fission products have been developed by Zendel and coworkers [Zen81]. The experimental arrangement they used is shown in Fig. 46. The recoiling fission fragments were stopped in nitrogen containing hydrogen chloride. The gas mixture passed through a series of traps. The first trap, containing quartz wool, retained clusters carrying nonvolatile fission products as well as 20F produced by activation of fluorine in the Teflon coating of the target chamber. The gases passed through a second trap made of silver-coated quartz spiral, which was kept at 800°C. In this trap, the volatile bromine and iodine compounds were decomposed and retained by the silver. Arsenic
was adsorbed by di-(2-ethylhexyl)orthophosphoric acid coated on polystyrene beads in trap 3. Germanium was retained by charcoal in the last trap.
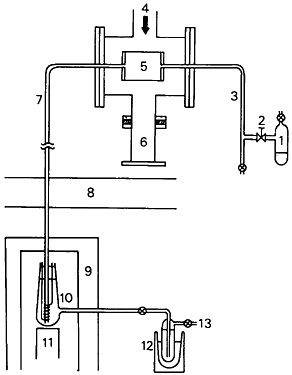
Figure 44. Apparatus for on-line methylation: 1. Acetone reservoir; 2. Variable leak valve; 3. Quartz glass tube; 4. Beam; 5. Target and methylation cell; 6. Faraday cup; 7. Teflon tube; 8. Concrete shield; 9. Lead shield; 10. Heater for thermal decomposition of methyl compounds (TDP); 11. Ge(Li) detector; 12. Liquid-nitrogen trap; 13. Rotary pump. [Tsu83; reprinted with permission from Radiochim. Acta]
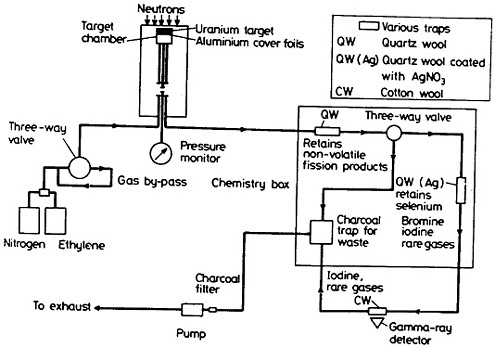
Figure 45. Schematic diagram of the setup used for the separation of bromine from fission products. [Ren86b; reprinted with permission from J. Radioanal. Nucl. Chem.]







