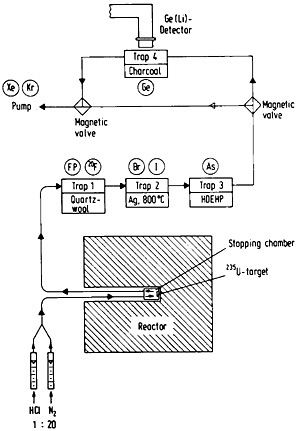
Figure 46. Experimental setup for the continuous separation and on-line measurement of germanium isotopes from fission products (not to scale). By placing the detector at the arsenic absorption position, the same setup can be used to measure arsenic isotopes. [Zen81; reprinted with permission from Radiochim. Acta]
Continuous gas-phase separations have been utilized in a variety of applications in the study of short-lived fission products. Its potential usefulness in the measurement of fission yields has been demonstrated [Ren82d]. Ground-state decay branchings for short-lived selenium isotopes have been measured using this technique by Lin and coworkers [Lin 82]. The decontaminations obtained, especially using thermochromatographic techniques, are often not as high as those obtained with continuous solvent-extraction techniques. However, gas-phase separations, especially those based on volatile species produced in the target chamber, provide a very important opportunity for chemists to study the chemical reactions taking place in a radiation environment.
8. Future of Ultrafast Chemistry
On-line isotope separators have been used in accelerators as well as in reactors to obtain isobaric separation of nuclear-reaction products. Recent developments in ion-source technology have extended the range of accessible elements by use of selective chemistry within the ion source of an on-line mass separator. Piotrowski and coworkers [Pio84], as well as Brüggar and coworkers [Brü85], have reported development of high-temperature sources that provide beams of alkaline earth and lanthanide elements. Gill and Piotrowski [Gil85] have developed a new FEBIAD-type ion source for use with on-line sources used in reactors. Figure 47 shows a survey of yields obtained from the ion source. The near-complete chemical suppression of elements such as selenium, zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium, and
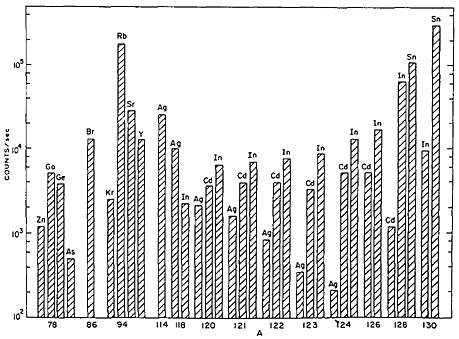
Figure 47. Survey of yields of some elements with the TRISTAN FEBIAD ion source. The results are based on g-ray spectra and are corrected to saturation activities. [Gil85; reprinted with permission from Nucl. Instrum. Methods Phys. Res.]
tellurium is notable [Gil85]. The yields reported from the high-temperature ion sources also show low or no yield for many elements [Pio84]. For study of short-lived nuclides of these elements, either new ultrafast ion-source techniques or ultrafast chemical techniques are essential.
Isotope separators provide isobaric beams; ideally, an additional separation will provide the best possible sources for far-from-stability spectroscopy studies. Rudstam and coworkers have investigated the possibility of subjecting the isobaric beam obtained from an isotope separator to thermochromatographic separation [Wes69, Gra73, Rud73]. They utilized such a combination to study neutron-rich zinc and cadmium isotopes [Rud81]. In the future, gas-phase chemical separations can be expected to provide Z separation following A separation provided by on-line isotope separators. In addition, production of volatile species in target chambers with a variety of reactive gases may be studied to understand the chemical reactions taking place with energetic nuclear-reaction products.


