1
Global Investment and Research Needs
Nitrogen is an essential plant nutrient. It is the nutrient that is most commonly deficient, contributing to reduced agricultural yields throughout the world. Molecular nitrogen or dinitrogen (N2) makes up four-fifths of the atmosphere but is metabolically unavailable directly to higher plants or animals. It is available to some species of microorganism through Biological Nitrogen Fixation (BNF) in which atmospheric nitrogen is converted to ammonia by the enzyme nitrogenase. Microorganisms that fix nitrogen are called diazotrophs.
BNF requires energy. Those microbes that fix nitrogen independent of other organisms are called free living. The free-living diazotrophs require a chemical energy source if nonphotosynthetic, whereas the photosynthetic diazotrophs utilize light energy. The free-living diazotrophs contribute little fixed nitrogen to agricultural crops. Associative nitrogen-fixing microorganisms are those diazotrophs that live in close proximity to plant roots (that is, in the rhizosphere or within plants) and can obtain energy materials from the plants. They may make a modest contribution of fixed nitrogen to agriculture and forestry, but quantification of their potential has not been established. The symbiotic relationship between diazotrophs called rhizobia and legumes (for example, clover and soybean) can provide large amounts of nitrogen to the plant and can have a significant impact on agriculture.
The symbiosis between legumes and the nitrogen-fixing rhizobia occurs within nodules mainly on the root and in a few cases on the stem. A similar symbiosis occurs between a number of woody plant species and
the diazotrophic actinomycete Frankia. The plant supplies energy materials to the diazotrophs, which in turn reduce atmospheric nitrogen to ammonia. This ammonia is transferred from the bacteria to the plant to meet the plant's nutritional nitrogen needs for the synthesis of proteins, enzymes, nucleic acids, chlorophyll, and so forth.
JUSTIFICATION FOR RESEARCH INVESTMENT
Biological nitrogen fixation is an essential natural process that supports life on this planet. Higher plants and animals obtain nitrogen ultimately from nitrogen-fixing organisms or from nitrogen fertilizers (including nitrogen compounds formed during lightning strikes). Available soil nitrogen, which originates from decomposing plant residues and microorganisms, is normally deficient for intensive crop production. This is the compelling reason to improve our understanding of BNF for application to agriculture and forestry production worldwide. In addition, the projected doubling in population over the next 50 years will put increasing pressure on food production, the environment, and the need for fixed nitrogen. Growing concerns about the environment, energy, nutrition, and agricultural sustainability make the need for BNF research even more compelling.
Concerns about the cost and supply of fossil-based energy were major reasons for the expansion of BNF research in the 1970s. Environmental quality and sustainability are equally compelling concerns in the 1990s.
Nitrogen fixation occurs both biologically and non-biologically. Asymbiotic and symbiotic biological systems fix an estimated 100-175 million metric tons of nitrogen annually (Burns and Hardy, 1975), and this probably has not changed substantially during the last century. Non-biological nitrogen fixation occurs through the effects of lightning, and in industry primarily by the Haber-Bosch process, which requires high levels of fossil fuel. Worldwide, lightning may fix 10 million metric tons of nitrogen a year, a value that probably has not changed over time. Industrial fixation for fertilizer nitrogen has increased from 3.5 million tons in 1950 to 80 million tons in 1989 (Figure 1) (Hardy, 1993) in response to the needs of high-yielding crops.
By the year 2050, world population is expected to double from its current level of more than 5 billion. It is reasonable to expect that the need for fixed nitrogen for crop production will also at least double. If this is supplied by industrial sources, synthetic fertilizer nitrogen use will increase to about 160 million tons of nitrogen per year, about equal to that produced by the biological process. This amount of nitrogen fertilizer will require burning some 270 million tons of coal or its equivalent. However, expanded exploitation of BNF could reduce, and in the longer
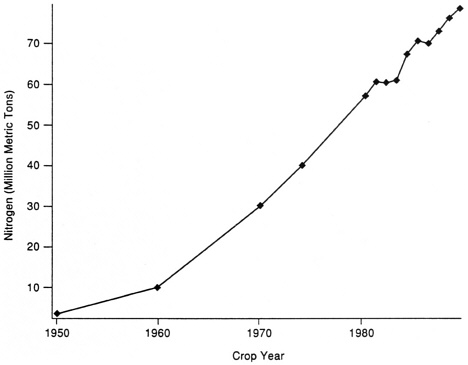
FIGURE 1 World Fertilizer Consumption of Nitrogen Totals, 1950–1989 (Based on Fertilizer Facts and Figures. 1990. The Fertilizer Institute, Washington, D.C.)
term substantially replace, the need for industrially produced fertilizer nitrogen.
The Environment
There are several significant environmental reasons to seek alternatives to chemically fixed nitrogen fertilizer: it affects the balance of the global nitrogen cycle, pollutes groundwater, increases the risk of chemical spills, and increases atmospheric nitrous oxide (N2O), a potent “greenhouse” gas.
Global Nitrogen Cycle
Until relatively recently, the contribution of nitrogen fixation to the global nitrogen cycle probably had not changed for centuries, having been in approximate balance with the denitrification process that converts combined nitrogen back to atmospheric nitrogen. Fixation did not
occur to excess because BNF is inhibited by the presence of mineral nitrogen. During the past 40 years, the global nitrogen cycle has been affected by the increase in industrial fixation of nitrogen, but the environmental impacts are yet to be measured and assessed. It would be prudent to minimize the further perturbation of the global nitrogen cycle, a major natural cycle. About two tons of industrially fixed nitrogen are needed as fertilizer for crop production to equal the effects of one ton of nitrogen biologically fixed in a legume crop. Thus, fixed nitrogen from BNF will affect the global nitrogen cycle substantially less than will industrially fixed nitrogen.
Groundwater Pollution
Fertilizer nitrogen is used inefficiently by crops. Up to 50 percent becomes part of the current crop; of the other 50 percent, or more, some is stored in soil organic matter, where it becomes available to subsequent crops, some is converted back to atmospheric nitrogen through denitrification, and some is leached and pollutes the groundwater as nitrate (NO3). For example, in Iowa, the increasing nitrate content of groundwater from the 1950s to 1980s paralleled the increasing rates of usage of nitrogen fertilizer. Nitrate pollution of groundwater is a significant environmental problem of high-production-crop agriculture. Limitations are already being established, or considered, on the maximum amounts of nitrogen fertilizer to be applied to crops such as corn. In contrast, all of the biologically fixed nitrogen is assimilated by a legume crop.
Transportation of Anhydrous Ammonia
Industrially produced anhydrous ammonia (NH3) must be transferred in large amounts to distribution centers and taken by road to farms, with the risk of spills. This is a small but real environmental hazard. Ammonia is highly caustic, and spills could result in significant risk to humans and livestock and damage to the environment. Consequently, handlers of anhydrous ammonia must purchase liability insurance.
Greenhouse Gas
Nitrous oxide (N2O), along with carbon dioxide (CO2), methane (CH4), and chlorofluorocarbons (CFCs) is a “greenhouse” gas that traps reflected sunlight and may cause global warming. The energy reflectivity per mole of nitrous oxide is about 180 times that of carbon dioxide. Denitrification of nitrate produces about 90 percent nitrogen gas and 10 percent nitrous oxide. The tropospheric nitrous oxide increase during
the 1980s correlates with the increase in the use of fertilizer nitrogen more than with the increase in fossil fuel combustion. At present, the global budget for nitrous oxide appears to be out of balance, with sources exceeding sinks by 30-40 percent; the concentration of nitrous oxide in the atmosphere is increasing at 0.25 percent per year (Vitousek and Matson, 1993). The production of nitrogen fertilizer by industrial nitrogen fixation not only depletes our finite reserves of fossil fuels, but also generates large quantities of carbon dioxide, the principal greenhouse gas. Thus, increased use of fertilizer nitrogen may contribute substantially to the potential for global warming, and early replacement with BNF is desirable.
Energy
Research in BNF has been aimed primarily at elucidating the process per se and at increasing its application for the economical and environmentally sound production of food. In the 1970s, concern over the rapidly increasing prices and dwindling supplies of fossil fuels, especially natural gas, focused attention on BNF research. A major energy cost for production of a highly fertilized crop such as corn is the natural gas consumed during the industrial fixation process (Pimentel et al., 1973).
The green revolution, with its dramatically improved productivity of wheat and rice, was achieved by breeding short, lodging-resistant cultivars, applying high levels of nitrogen fertilizer and other nutrients, and utilizing irrigation and improved pest control. Because these grains are primary components of diets in many developing countries, their enhanced productivity did much to alleviate hunger.
The Green Revolution was accompanied by a huge increase in the application of fertilizers, particularly nitrogen. The rupture of the stable triple bond of molecular nitrogen (N≡N) is very energy demanding and is achieved at high temperatures and pressures. Typically, natural gas is used to supply hydrogen for the reduction of the nitrogen, and combustion of natural gas furnishes the high temperature. When supplies of natural gas become limited, industry will undoubtedly return to coal.
The energy requirements of BNF, although large, are met by renewable sources such as plant-synthesized carbohydrates rather than from nonrenewable fossil fuels such as natural gas.
When soil nitrogen is depleted, associative nitrogen fixers, for example Azotobacter spp. and Azospirillum spp., function vigorously when supplied with an energy source. However, they are considered of minor agricultural significance. Recently, two other free-living diazotrophs, Acetobacter diazotrophicus and Herbaspirillum spp., were found to live endophytically in the vascular tissue of sugarcane, where there is access to
abundant sucrose as a possible source of energy for nitrogen fixation (Döbereiner et al., 1993). This finding may explain the large positive nitrogen budgets measured with some cultivars of sugarcane in Brazil (Urquiaga et al., 1992). The fixation of nitrogen in these cultivars reduces the energy required for production of ethanol from sugarcane.
In the 1970s, as a result of shortages of oil and natural gas, energy costs rose dramatically. Without question that situation will recur, with concomitant increase in the cost of fertilizer nitrogen produced by the Haber-Bosch process. Those exploiting BNF for crop production will be in a relatively favorable position. Because the world population is increasing rapidly, there will be additional pressure on food production, and in turn, on nitrogen fixation, especially in the poorer developing countries.
Improvement in agricultural sustainability will require the exploitation of BNF as a major source of nitrogen for plants. Mixed cropping and crop rotations of nonlegumes with legumes were employed for centuries to capitalize on BNF. Nonlegumes deplete the soil of nitrogen, whereas leguminous crops can restore nitrogen, primarily as organic forms that are not readily leachable. However, improved crops, microbes, and appropriate management practices must be developed if higher levels of production and sustainability are to be realized.
In much of the developing world, nitrogen is the nutrient most commonly limiting agricultural productivity. But in many situations, nitrogen fertilizer is too expensive, or not available. Under these circumstances, the exploitation of solar energy by nitrogen-fixing legumes in crop rotations is particularly appealing.
Nutrition
In many countries, human nutrition is highly dependent on grain legumes for protein. There is marked consumer preference for various seed types: for example, some groups prefer the black form of common bean, whereas others favor the navy or kidney bean. The nutritional differences among these is not great, so preference is based on custom (as are cooking methods that reduce antidigestive activity) and the local adaptation of specific legumes.
There are more than 13,000 described species of legumes. Of the approximately 3,000 species examined, more than 90 percent form root nodules (in which nitrogen fixation presumably occurs in symbiosis with rhizobia). Because few have been exploited for food, there is the prospect that the utilization of legumes could be expanded substantially. It is estimated that about 20 percent of food protein worldwide is derived from legumes. The highest consumption occurs in the former Soviet
Union, South America, Central America, Mexico, India, Turkey, and Greece. The dietary use of legumes is quantitatively in the following order: dry bean (Phaseolus vulgaris), dry pea (Pisum sativum), chickpea (Cicer arietinum), broad bean (Vicia faba), pigeon pea (Cajanus cajan), cowpea (Vigna unguiculata), and lentil (Lens culinaris) (Agostini and Khan, 1986). Peanut (Arachis hypogaea) and soybean (Glycine max) are dominant sources of cooking oil and protein. They are also major food sources in some regions.
Symbiotic nitrogen fixation in legumes allows them to grow well without the addition of fertilizer nitrogen. However, it may be necessary to apply phosphorus and other deficient nutrients, as well as lime to alleviate soil acidity.
The amino acid components of leguminous seed proteins commonly show deficiency in cysteine and methionine, but when consumed in combination with cereal protein offer a complete nutritional balance. Thus, the legume complements amino acid deficiencies in cereal grains.
Because of concerns over coronary heart disease and certain types of cancer, Americans have been admonished to reduce fat intake and increase intake of plant products, as stated in Diet and Health (National Research Council, 1989):
Diets high in plant foods—i.e., fruits, vegetables, legumes, and whole grain cereals—are associated with a lower occurrence of coronary heart disease and cancer of the lung, colon, esophagus, and stomach. . . . By using plant products (e.g., cereals and legumes), instead of animal products, as sources of protein, one can also reduce the amount of saturated fatty acids and cholesterol in the diet. . . . Foods highest in dietary fiber include (unrefined) grains and breads made from them, legumes, vegetables, fruits, nuts, and seeds.
The importance of legumes in animal feed should not be overlooked. Alfalfa (Medicago sativa), clovers (Trifolium spp.), stylosanthes (Stylosanthes spp.), desmodium (Desmodium spp.), and other forages are grown extensively. They are either grazed or fed as hay or silage. Alfalfa silage furnishes not only roughage and high-quality protein, but also a variety of vitamins, minerals, and other nutrients. The anaerobic ensiling process supports a rapid fermentative acidification of the plant material, serving to preserve nutritional quality.
Soybean is used for production of oil, and the residual meal is an excellent and relatively inexpensive source of protein. Although a small percentage of the meal is incorporated into human foods, most of it is used for feeding livestock and pets.
Sustainability
Advances in agricultural sustainability will require an increase in the utilization of BNF as a major source of nitrogen for plants. Ladha et al. (1992), in the symposium on Biological Nitrogen Fixation For Sustainable Agriculture, commented as follows:
Long-term sustainability of agricultural systems must rely on the use and effective management of internal resources. The process of biological nitrogen fixation offers an economically attractive and ecologically sound means of reducing external nitrogen input and improving the quality and quantity of internal resources.
Richard Harwood (1990) defined sustainable agriculture as “an agriculture that can evolve indefinitely toward greater human utility, greater efficiency of resource use, and a balance with the environment that is favorable both to humans and to most other species. ”
Clearly, it is not realistic to consider sustainable agriculture on a broad scale in the absence of BNF; research is needed to optimize the contribution of BNF to sustainable agriculture.
Nonleguminous crops grown under all but the most arid conditions respond well to nitrogen fertilizer when other nutrients are adequate. This, and the demand for food and feed, has been a major factor in the substitution of nitrogen fertilizer for biologically fixed nitrogen and in the adoption of monocultures for crop rotations. Millions of tons of chemically fixed nitrogen are applied annually, in amounts that have been increasing since the 1940s. The primary feedstock and energy source for the manufacture of nitrogen fertilizer is natural gas. Petroleum and coal could also be used to supply the energy and hydrogen required to convert atmospheric nitrogen to ammonia. These fossil fuels are exhaustible resources. Thus, modern agricultural production systems are not sustainable.
Until the middle of the twentieth century, agricultural production depended mainly on soil nitrogen that had accumulated over time or was recently added by BNF and natural atmospheric deposition or animal manures rather than fertilizer. Soil organic matter, manures, and unharvested crop residues, especially those of legumes, can meet crop nitrogen needs in many circumstances where appropriate management is exercised. Because significant amounts of nitrogen reside in the unharvested portion of crops, it is important for sustainability to return these by-products to the soil. The need for fertilizers can be reduced by nitrogen supplied by BNF in the field, or by recycling through animals back to the soil, including human waste.
In modern agriculture, less than half to as little as a quarter of animal manure and almost none of human waste is effectively returned to the
soil. Political, economic, and attitudinal norms have led the industrial nations to embrace systems of agriculture that are not sustainable, and developing nations are now doing the same.
Increasing the contributions of BNF will be a significant challenge. Nitrogen-fixing legumes are no less susceptible to environmental stresses than those plants dependent on mineral nitrogen, and in some instances they are even more adversely affected. Extremes of temperature, moisture and pH, and high salinity adversely affect the formation and function of root nodules. The range of genetic diversity for tolerance to these stresses is poorly understood for both symbionts. Development of stresstolerant combinations of rhizobia and legumes is needed.
Cereals and other nonlegumes usually require heavy applications of fertilizer nitrogen for good yields. If these species could be genetically manipulated to form effective symbiotic or associative nitrogen-fixing systems, it would decrease or remove our major dependence on fertilizer nitrogen. The transformation of plants and microorganisms through molecular genetics offers promise for development of new symbiotic and associative BNF systems. However, such studies are in their infancy.
As a society, we must find ways to reduce dependency on fossil fuels for economic, environmental, and geopolitical reasons. The development of sources of renewable energy will be particularly important for agriculture. BNF will be a major component in the improvement of agricultural sustainability. Its advantages are renewability and independence of fossil-based energy, the costs and supply of which are influenced by international politics and the infrastructure required for fertilizer manufacture, distribution, and use.
Economic Benefit
There must be positive net economic or other benefits to induce the farmer to use a new technology. Farmers are most likely to adopt BNF when it reduces production costs and/or increases yields, and also when it reduces, or at least does not increase, risk. Early adopters will receive the greatest rewards.
Reduced production costs or higher yields of food and feed lead to increased production and, ultimately, to lower food prices. Thus, the development and adoption of new BNF technology can become a real engine for economic growth, with society as a whole the beneficiary.
The chief problem in evaluating the economic benefits of BNF is in assigning prices to inputs and outputs (Heinz and Welsch, 1991). For the farmer, actual costs and income are critical. For society as a whole, the price may be influenced more by the government than by market forces. Fertilizer may be supplied to the farmer below cost. Moreover, market
price rarely reflects costs to society; for example, the often ignored cost of groundwater pollution. In developed countries, prices of products may be supported above world market levels as a means of protecting farm income, whereas in developing countries, prices may be controlled below world market levels to benefit urban residents and the industrial sector.
Given these problems, the only way to estimate the economic advantage of a BNF technology is by analysis of cropping systems that use it as a source of nitrogen. If livestock are involved, then the analysis should be based on the entire farm operation. Tauer (1989) modeled five separate scenarios for increased nitrogen fixation by plants in the United States. His analysis indicated that increasing BNF would have a high economic value for the United States.
Social Impact
Nitrogen-fixation research has produced a large number of highly trained and qualified biologists, biochemists, and molecular geneticists, who have participated in a variety of government, academic, and industrial scientific activities. BNF is an area of research that has been useful for training young scientists in developing countries, since it is directly applicable to local crop-production problems and can be approached with relatively simple techniques and tools.
The social impacts of BNF are just emerging. The question arises as to who will benefit from increased BNF: the scientist creating the research breakthrough, the agribusiness firm developing and marketing it, the farmer adopting it, the farm laborer utilizing it, the financial institution providing the capital, the agribusiness processor or retailer selling the product, the consumer, or society in general? Previous analyses of the impacts of agricultural technology have focused almost exclusively on the producer, although in developed countries the consumer has been the major benefactor through the reduced cost of food. A number of factors determine the benefits from BNF technology to the producer including conditions on the farm, regulations at the local, regional, and national levels, access to market and technology, community and regional infrastructure, and cultural values.
A new technology may have effects beyond the farm extending to the community as a whole. The roles of family members and divisions of labor may change, with significant consequences for the way the farm operates and for nonfamily farm labor. At the community level, the technology may require infrastructure with financial institutions called upon to provide support, possibly requiring bank-policy changes. Education of the producer and/or of the community (for example, county extension agents and farm advisers) may be necessary.
Future analyses will require understanding of the history and social origins of the technology and of the cultural values shaping the criteria for evaluating the technology, with goals and purposes of the assessments clearly defined.
Policy Constraints
There are at least three major policy constraints on adoption of BNF technology in crop production. The first is underpricing of industrially produced fertilizer nitrogen—for example, some governments subsidize the cost of producing or importing fertilizer nitrogen. The second is the overpricing of some farm products to keep farm income high. The third is the underpricing of farm products so as to lower the cost of food to urban people. The first and second both lead to overuse of fertilizer nitrogen and underuse of BNF worldwide. The third leads to underuse of all sources of nitrogen and a decrease in the food supply. All three result in economic-policy actions disfavoring systems employing BNF technology.
Underpricing of fertilizer nitrogen in industrialized countries ignores environmental and social costs, including the large amounts of energy consumed in production and the pollution of groundwater and of estuarine and coastal marine ecosystems. Policymakers around the world are becoming aware of the environmental and social implications of many of their policies, but insufficient “political will” often prevents appropriate change.
RESEARCH DIRECTIONS
Twenty years ago, the spiraling costs of energy and, as a consequence, the costs of nitrogen fertilizers, resulted in a new momentum for research in BNF. Ambitious predictions were made about the impacts that BNF, a “free” inexhaustible source of nitrogen, would have on world agriculture. Research support for BNF expanded and the knowledge base increased. However, for the most part, the impacts were less than the inflated expectations. Inoculants do not play a major role in the production of some of the enormously important food legumes, yet soybean production in South America has succeeded in large part due to the use of inoculants on adapted cultivars. Associative nitrogen fixation, a new field of research in the 1970s, has not led to major application. While the measurement of large amounts of atmospheric nitrogen fixed by the Azolla-Anabaena azollae association held great promise for rice production, that potential has not been realized.
Although our efforts in BNF over these 20 years have not resulted in
the creation of nitrogen-fixing plants via the transfer/expression of the nitrogen-fixation (nif) genes into the plant nucleus, we have gained a much better understanding of the problems at hand and generated much new information with which to solve these problems. For example, based on a large number of biochemical and (molecular) genetic studies, it has been recognized that we must develop a better understanding of the molecular basis of rhizobium-plant interactions before attempting to extend the nitrogen-fixing symbiosis to currently non-nodulated plants such as cereals (de Bruijn and Downie, 1991).
The 1990s bring a pressing sense of the need to preserve the environment while supporting a burgeoning global population. BNF represents a process for supplying nitrogen needed for economic, sustainable, and environmentally acceptable agricultural production; it involves production technology applicable both to the impoverished farmer in developing countries and to the farmers in developed countries who must cope with decreasing profitability. Research for novel efforts in the area of BNF is imperative.
RESEARCH AREAS: SCIENTIFIC CHALLENGES
To make BNF more useful, progress must be made to meet numerous scientific challenges. A few judged to be of the highest priority are mentioned below.
-
No BNF microbial associations or symbioses are known that produce significant amounts of fixed nitrogen for the major cereals—corn, wheat, barley, and sorghum. The symbiotic genes in legumes are being identified. Might these be transferred to cereals to enable them to have nitrogen-fixing symbioses? Endophytic nitrogen-fixing microorganisms found in sugarcane may provide significant amounts of nitrogen. Can such endophytes be made to contribute substantial amounts of nitrogen to important cereals? The nitrogenase enzyme is energy demanding and requires about 32 adenosine triphosphate (ATP) molecules per dinitrogen molecule reduced. This energy requirement for nitrogen fixation in a legume nodule requires the complete metabolism of 12 grams of glucose to support fixation of one gram of nitrogen to ammonia. Can these requirements be met if nitrogen-fixing capacity is transferred to nonfixing plants?
-
In general, the more efficient nitrogen-fixing strains of rhizobia compete poorly with the rhizobia already in the soil. Can ways be devised to improve the competitive ability of inoculant rhizobia?
-
Fertilizer nitrogen generally inhibits BNF in both symbiotic and
-
asymbiotic systems. Can this inhibition be reduced to obtain large contributions of nitrogen from BNF?
-
Can the factors causing low nitrogen-fixation activity in legumes (for example in common bean) be identified and activity improved in desirable cultivars?
-
Packaging or formulating rhizobial inoculants to minimize adverse effects of high temperatures and other stresses encountered in the distribution to farmers, particularly in tropical countries, poses problems. What inexpensive packaging or formulations can be devised to protect the rhizobia?
-
There are many environmental stresses that negatively affect nodulation and nitrogen fixation such as acid and alkaline soils, nutritional deficiencies, salinity, high temperature, and presence of toxic elements. Can cultivar-strain combinations resistant to these stresses be developed for stressed field conditions?
-
The rhizobia have a high specificity for host legumes. Broader host range would simplify production, distribution, and grower use of rhizobia. Can this specificity be overcome without decreasing nitrogen-fixing ability?
-
The blue-green algae Azolla association with the aquatic fern Anabaena azollae has the potential to fix nitrogen in paddy rice, but needs improvements to be useful as a green manure. Can such improvements in production and efficiency be made?
-
In addition to legumes, a number of nonlegume woody plants such as casuarinas (Casuarina spp.) and alders (Alnus spp.) fix nitrogen symbiotically with Frankia spp. Research on this system is in its infancy. Advances could be beneficial to forestry and agroforestry. How much nitrogen is fixed by these systems? Are there other nitrogen-fixing systems not yet discovered?
Clearly, there are many challenges to be met in maximizing the use of BNF for the common good. An expanding knowledge base and the use of the powerful tools of biotechnology should help.
Research opportunities abound to use BNF to improve crop and tree production in environmentally friendly ways, to conserve energy resources, and to improve and maintain sustainability, particularly in developing countries.
Providing financial and human resources for these tasks would represent a sound investment, with returns expected over the near and long term. Particular areas of research opportunities are discussed below.
Basic Biology
The process of BNF has provided a number of useful paradigms for both basic and applied research. BNF research is an important scientific subdiscipline that has made several original and broadly applicable contributions to science. This, in turn, provides a valid rationale for expanded investment in BNF research during the 1990s and beyond.
Particularly intriguing is the so-called oxygen paradox inherent in BNF. The reaction in which nitrogen is reduced to ammonia is catalyzed by nitrogenase. It is energy intensive. Many nitrogen-fixing organisms, and in particular the symbiotic bacteria, generate the energy via respiration, which requires the availability of an ample oxygen supply. However, the nitrogenase enzyme complex is exceedingly oxygen sensitive; it is denatured and irreversibly inactivated even at low oxygen concentrations. This paradox led to the development of model systems for study of mechanisms of control of cellular oxygen level and of regulation of genes for nitrogen fixation in response to cellular oxygen (de Bruijn and Downie, 1991). Research on rhizobia has resulted in the elucidation of the first signal transduction pathway for a microbial response to an environmental factor—in this case, oxygen (Gillis-Gonzales et al., 1991).
The two nitrogenase components provide a challenging system for protein X-ray crystallographers and structure biochemists. Only recently, the three-dimensional structure was elucidated, providing data useful for study of other proteins with similar active sites and for catalyst chemistry (Kim and Rees, 1992). Will chemists be able to synthesize effective mimics of nitrogenase? The multiple genes responsible for nitrogenase have been cloned and sequenced from a number of bacterial species, providing important new tools for gene transfer and for a better understanding of gene evolution (Merrick, 1992).
The isolation and characterization of diverse nitrogen-fixing organisms provide important germplasm resources. Studies on free-living nitrogen-fixing organisms from both terrestrial and aquatic habitats have provided useful models for the discipline of microbial ecology.
Experiments on the agronomic effects of associative and symbiotic microorganisms on crops have provided useful model systems for rhizosphere studies and plant-microbe communication. The chemical structures of plant and bacterial signal molecules were first elucidated with the nitrogen-fixing symbioses between Rhizobium and Bradyrhizobium spp. and their respective host legumes (Fischer and Long, 1992). Rhizobial genes known to control host specificity and nodule induction fall into two groups: the structural nod genes and the regulatory nod genes. The structural nod genes are expressed in response to biochemical signals from the plant, usually flavonoid compounds, and a transcriptional activator
produced by nodD regulatory genes. The proteins encoded by nodD genes are activated by particular flavonoid compounds; thus, nodD genes are partial determinants of strain/host specificity. Expression of structural nod genes results in the production of specific extracellular lipo-oligosac-charide compounds that elicit root-hair deformation, cortical cell division, and other responses in the susceptible legume root.
This work has provided important paradigms for studies on plant-pathogen interactions, biocontrol agents, and prokaryote-eukaryote cellular communication. Also, parallels between symbiotic plant-microbe interactions and signaling, and pathogenic invasion of mammalian tissues have been identified.
During the 1970s, ambitious predictions were made regarding the immediate application of recombinant DNA research to the production of transgenic nitrogen-fixing plants, which resulted in increased funding for BNF research. However, the technological base for achieving such goals did not exist at that time. Today, these goals are more realistic, thanks to advances in molecular genetics and an improved understanding of nitrogen-fixing processes.
The rationale for investment in BNF research has grown with recognition of the negative environmental impact of overuse of chemical fertilizers and the increasing need for sustainable agricultural practices. These changes have substantially increased the need for BNF research with appropriate funding to attract and retain creative scientists. We can expect that the next 10 years of research will yield many original contributions to science and technology in general and contribute ecologically sound agricultural and forestry applications.
To increase fundamental knowledge of systems of particular importance in local agriculture, researchers in the developing countries should be encouraged to initiate or continue basic research. Collaboration of developing country laboratories with compatible units in the United States should be encouraged, especially for the purpose of exchanging techniques and information. While it is realized that many developing countries cannot divert appreciable resources to basic research, their involvement is important because many biological systems are unique to the tropics. These BNF systems could include already identified nitrogen-fixing microbes and/or partner plants, particularly those not being used or extensively studied elsewhere. The discovery and initial characterization of the stem-nodulating (in some cases photosynthetic) rhizobia from the tropical legumes Sesbania rostrata and Aeschynomene spp. included research in Africa and India. Moreover, the observation of chromosomal rearrangements (genome plasticity) in rhizobia and the classification of the bean-nodulating species Rhizobium tropici came from a laboratory in Mexico.
More work is needed on the genetics of the legumes, not only from a breeding standpoint, but also to increase understanding of the plant genes involved in nodule development and nitrogen fixation. A total of about 30 plant sym genes are now known to be involved in the formation of fully functional root nodules. At the various steps of nodule ontogeny, different sets of nodule-specific (nodulin) genes are expressed in the host legume (Nap and Bisseling, 1990). The comprehension of nodulin functions will help elucidate the complex interaction between the host legume and its microbe. These genes are being mapped in peas and eventually should be cloned and sequenced.
The eventual transfer of nodulation capacity to nonlegumes will probably require an understanding of the genes responsible in rhizobia and legumes, of the structural chemical bases of rhizobia/legume communication, and of the signal transduction pathways responsible for the finely orchestrated induction of the symbiosis-specific genes in both plants and microbes (de Bruijn and Downie, 1991; Fischer and Long, 1992).
Control of Nitrogenase
Nitrogen fixation is energy demanding. Most diazotrophs have a control mechanism that decreases nitrogenase activity in less than a minute after ammonium ions are supplied. This allows the bacteria to use the ammonium ion when available and to conserve the energy it would use in fixing nitrogen. The nitrogenase is restored as soon as the ammonium ions are metabolized. It would be useful to overcome this inhibition of nitrogenase so that appreciable fixation can occur even in soils with high available nitrogen levels.
It is now feasible to effect rational specific changes in nitrogenase structure by genetic alteration, and to determine how these specific changes alter enzymatic activity. For example, changing arginine 101 to tyrosine in one of the two subunits of dinitrogenase reductase effects a dramatic reduction of the switch on/off control of nitrogenase. Similar manipulations may well increase nitrogenase activity, although improvement always is more difficult than impairment. Electron generation and transfer, and ATP generation and coupling are other possible targets for improvement.
Nitrogenase has an iron-molybdenum cofactor, and its removal and reinsertion to restore activity encourages attempts to improve the catalysis. The demonstrations that vanadium-iron and iron-only active sites can substitute for the iron-molybdenum unit are of great interest, although these alternatives are less active catalytically than the molybdenum-iron center.
More than 20 genes have been identified as controlling the structure and function of the nitrogenase system, and much functional detail has been defined. Unquestionably, future study will center on the manipulation of these genes to elucidate nitrogenase function and improve its effectiveness. The production of hydrogen by nitrogenase is obligatory in the reaction, dissipating at least 25 percent of the energy. At present, there is no hint as to how hydrogen formation can be decreased, but genetic manipulation offers new approaches.
The development of systems to make nitrogenase less sensitive to oxygen, or to protect nitrogenase from exposure to oxygen, could expand its use to a wider spectrum of plant species.
Trees
Several nitrogen-fixing tree and shrub species are particularly advantageous for reforestation and agroforestry in having the ability to grow fast, tolerate soil acidity and withstand regular coppicing (National Research Council, 1993). Acioa baterii, Anthonotha macrophylla, Gliricidia sepium, Leucaena leucocephala, and Erythrina poeppigiana are commonly recommended for agroforestry systems in the humid tropics. They provide a variety of products including fruit, forage, fuelwood, fodder, and fence and timber material and are used in soil improvement, land reclamation, and forestry programs.
Research on nitrogen-fixing trees in general has lagged behind that on food, feed, and forage crops. Some shrub and tree species form a symbiosis with Frankia, a nitrogen-fixing actinomycete that only relatively recently has been cultured in vitro. The other nitrogen-fixing trees are legumes and form a symbiosis with rhizobia. Only limited research has been conducted on such topics as inoculation, breeding, stand establishment, tissue culture, fertilization, ecology, disease control, marketable products, and interaction with other microorganisms. The major research need to quantify nitrogen fixation by trees is still at the methodological stage. This is clearly of particular importance to developing countries.
Rhizosphere Ecology
The establishment of genetically improved strains of rhizobia for nitrogen fixation will be unsuccessful if introduced organisms do not compete well with less-efficient symbiotic organisms already in the soil. In addition, inoculation practices do not always result in a good distribution of microbes in the root zone. Therefore, studies on the ecology of nitrogen-fixing microbes in the rhizosphere is an essential component of BNF research. We need a better understanding of the microbial genes,
plant genes, and other soil and plant factors influencing microbial ability to develop and function in the soil adjacent to (rhizosphere) and away from plant roots. We also must study the mechanisms involved in microbial responses to nutrient limitations and other environmental stresses. The interaction of roots in mixed-cropping systems is poorly understood. The genetic bases of the beneficial interaction of nitrogen-fixing rhizobia with mycorrhizae and other microbes involved in biocontrol or plant growth promotion are not understood. Coinoculation of mycorrhiza, rhizobia, and other beneficial organisms needs to be evaluated and relationships defined. The manipulation of “biased rhizospheres,” consisting of genetically modified plants secreting a selective nutritional mediator in conjunction with beneficial microbes that contain the genes to catabolize the mediator, should be further investigated. Development of convenient genetic markers for the identification, enumeration, classification, and tracking of microorganisms in the soil and rhizosphere will assist in the achievement of these goals. Research is particularly needed in developing countries with soils, systems, and species that are locally unique.
Fuels
Population pressure in many developing countries has led to deforestation because of the increasing need for wood, the traditional fuel for cooking. Important research opportunities for fast-growing nitrogen-fixing trees and shrubs have been mentioned previously.
There is a possibility that BNF may play a role in developing countries in the production of fuel of another kind: ethanol for internal combustion engines, especially in automobiles. Brazil has demonstrated the potential for enormous reductions in gasoline imports by substituting ethanol fermented from sugarcane. Some cultivars of sugarcane are capable of maintaining high yields year after year without addition of appreciable amounts of fertilizer nitrogen. Experiments utilizing the 15N-dilution method indicated that certain sugarcane cultivars can accrue significant amounts of nitrogen—up to 150 kg N/ha/year, apparently from BNF (Urquiaga et al., 1992). When these high-yielding sugarcane cultivars are grown with modest application of nitrogen, up to 2.5 units of ethanol energy are produced for each unit of fossil energy used for production. Using media with high sucrose content, endophytic diazotrophic bacteria have been isolated from the roots, stems, and leaves of sugarcane. Acetobacter diazotrophicus, isolated from leaf sheaths, was found to excrete nitrogenous compounds into a nitrogen-free sugar medium in vitro, suggesting that a similar transfer of nitrogen from the endophyte, for example within xylem tissue, may occur in sugarcane
(Döbereiner et al., 1993). If so, this opens new approaches for conferring cereals and other nonlegumes with BNF capability.
Biofuels are also produced from vegetable oils. Soybean and palm oils are being formulated into biodiesel to substitute for diesel fuel. Studies of these systems as practical fuel sources should be continued.
Bioenergy alternatives are particularly attractive for developing countries in the tropics, where sunshine is dependable and of high intensity. Moreover, many tropical species have the C4 photosynthetic pathway, which more efficiently utilizes radiant energy.
Charcoal can be produced from Pennisetum spp., a tropical grass with C4 photosynthesis, with a yield up to three times more per hectare than from eucalyptus, a fast-growing tree (V.N.G. Mazarello, personal communication). Of particular interest is the accrual of nitrogen presumably from associative BNF observed with some Pennisetum species.
There is a clear need to quantify BNF in the above systems utilizing isotopic methods. The amounts fixed may be substantial or trivial, but at this time there is insufficient data to make a judgment.
Industrial Uses
Research is needed to develop nonfood uses from nitrogen-fixing crops to provide needed products and to broaden market opportunities for growers. Some potential areas for research follow.
-
Medicinal. Traditional herbal medicines in Thailand make use of legumes including 19 species of Caesalpiniaceae, 39 species of Fabaceae, and 13 species of Mimosaceae. Biologically active compounds from three species of Fabaceae inhibit bacteria in vivo. Coumarin, used as a blood thinner, was derived from sweet clover.
-
Cosmetics and flavors. This specialized market requires knowledge of customer preference as well as of potential sources of useful compounds. Soy protein is used in the formulation of shampoos and lotions, for example.
-
Other products. Soybean-oil-based printing ink has superior qualities and is environmentally friendly. Other products are similarly environmentally benign; for example, plastics made from soybean. The opportunities are many, but research is necessary to identify opportunities and develop specific products.
-
Rhizobia with added characteristics. Genes for the production of substances for promotion of plant growth and control of plant pests or diseases could be added via molecular methods. Rhizobia with these characteristics would have increased value as commercial inoculants.
Sustainable Agriculture and Forestry
Over the past 40 years, agriculture in the developed world has become increasingly dependent on chemical fertilizers and pesticides for achievement and maintenance of the high yields possible with modern crop types. In recent years, documentation of the adverse effects of these chemicals on the rural population, the environment, and on food safety, has demonstrated the need for changes in agricultural production methods, with the objectives of sustainability, economic production, conservation of natural resources, and reduction in the use of synthetic chemicals. The challenge will be in instituting sustainable methods without compromising food-production levels, particularly in view of the need to increase agricultural yields worldwide to accommodate population growth.
The increased use of legumes offers the potential for a significant decrease in the need for fertilizer nitrogen, and therefore is a key component of sustainable agricultural systems. Site- and situation-specific research will be necessary to develop new and varied crop rotations, to optimize nitrogen fixation and other nutritional resources, and for pest control. Accrual of nitrogen can be attained by using legumes as green manures, and by returning legume crop residues to the soil.
Research should lead to the development of methods and systems that minimize the use of nitrogen fertilizer while maximizing the production of food, fodder, fuel, timber, and fiber.
Legumes are important components of many cropping systems and are grown commonly in areas where cereals, such as paddy and upland rice, maize, wheat sorghum, and millet, are the staple crops. Legumes are not only grown in pure stands, but often are interplanted with other species in many parts of Africa, Asia, and Latin America. Cowpea, peanut, soybean, chickpea, common bean, pigeon pea, and rice bean are often intercropped with cereals. The determination of the optimum combination of species, cultivars, and environment is complicated, requiring much research. Intercropping two or more species more effectively exploits environmental resources. The possible combinations of crops are determined by the length of growing season and environmental adaptation, and early- and late-maturing crops may be combined to ensure efficient utilization of the entire growing season. Superior competitive ability of the nonlegume for soil nitrogen can result in a stimulation of nitrogen fixation with an increased nitrogen yield in the intercrop relative to the legume and cereal monocrops.
Estimates of nitrogen fixation by food legumes range from 0 to 300+ kg N/ha/year. The level of fixation depends on species, cultivar, water supply, inoculation, crop management practices, soil conditions, and fer-
tility. There is a strong inverse relationship between the amount of available soil nitrogen and nitrogen fixation.
A diverse range of herbaceous forage and tree legumes is increasingly being employed for livestock production in developing countries. The woody perennial legumes reclaim degraded land, retard erosion, and provide fodder, shade, fuelwood, mulch, and green manure. There is a real need to explore the potential for the integrated use of these species in alley–cropping systems where arable crops are grown in the spaces between rows of planted trees or shrubs that are coppiced to provide green manure.
Despite the large number of genera and species available for forage and green manure in the tropics and subtropics, there are relatively few estimates of nitrogen fixation by herbaceous legumes in these systems. Assessments of nitrogen fixation by woody legumes are particularly needed. Quantitative methods of measurement of BNF are yet to be devised for plants older than 3 years.
Substitution of BNF for fertilizer nitrogen in crop production is a plus for the environment, particularly if energy is conserved. In developing countries there is a need to maximize the contribution of BNF. This may be achieved by agroforestry, mixed cropping, green manuring, or rotations that optimize the use of legumes. There is also a need to assess the utility of underused species with high nitrogen-fixing capabilities (for example, Sesbania rostrata). It is important to examine each cropping system for the potential to include BNF as a major source of nitrogen.
For centuries, the Azolla-Anabaena azollae association has been used in Southeast Asia to supply fixed nitrogen to paddy rice. After incorporation as a green manure, nitrogen released by the decomposition of the azolla supports rice growth. The current technology is labor intensive and requires management skills to grow and maintain large amounts of vegetative azolla between growing seasons. AID-BOSTID-funded research was successful in inducing reliable sporocarp formation in one species. These sporocarps are stable and serve as an effective and low mass method of culture maintenance; sporocarps must be induced to germinate consistently. Research is needed to determine ways to obtain reliable, abundant sporocarp production in important species. Because rice is the number one food crop in the world, a biological system to supply its nitrogen requirement could have a dramatic impact, warranting high priority in research and application.
Legume Improvement
The legume, with its symbiotic root nodule bacteria, is the most commonly used BNF system in agriculture. However, it generally has a sec-
ondary role in cropping systems because its yield potential, food energy, and often its profitability is lower than that for cereals. Furthermore, in the tropics, legume production is often not maximized because of limiting nutritional factors, disease, insect predation, and environmental stresses to which the plants may be more susceptible than those of other crops. If BNF is to be effectively exploited, factors that constrain nodulation, nitrogen fixation, and the growth and yield of legumes must be addressed. Increased yields will make legumes more competitive with cereals as crops.
High yield, disease and insect resistance, and tolerance of environmental stresses are required in legumes that are high in nitrogen-fixing ability. Improved cultivars should be developed using both conventional and molecular plant-breeding techniques. All legume breeding must be done on low-nitrogen soils without nitrogen fertilizer application to ensure preservation of optimal nodulation and nitrogen-fixing capacity. To exploit the advances in molecular biology, routine transformation systems for legumes will be needed.
The common bean (Phaseolus vulgaris) is the most important legume for human nutrition in developing countries, but it usually responds strongly to nitrogen fertilizer in the field—that is, it fixes nitrogen only poorly. There is a pressing need to understand why the common-bean root-nodule symbiosis is generally poor, with the objective of increasing levels of nitrogen fixation and thus improving yields without fertilizer nitrogen. When effectively nodulated, other legumes, for example broad bean (Vicia faba), respond poorly to nitrogen fertilizer in the field—that is, they fix nitrogen abundantly and can derive most of their nitrogen from fixation. An understanding of why the broad bean symbiosis is so effective could provide guidance for improving the common bean and other legumes.
The yield potential of locally important legumes should be measured in the field using quality seed, controlling disease and insects, applying sufficient nutrition (fertilizer), employing irrigation when needed, controlling weeds, and using optimum plant spacing and the best management practices known. The yield potential with effective inoculation of superior strains should be compared with and without a high rate of nitrogen fertilization.
It is important to develop legume cultivars that obtain most of their nitrogen from fixation without sacrificing actual and potential yield levels. Cultivars that are disease and insect resistant as well as tolerant of environmental stresses also must be developed. This will entail the use of the best combination of conventional and molecular plant-breeding techniques with trials on low-nitrogen soils and including rhizobia inoculation.
Over the past decade, the nodules that form on stem-borne root primordia of a few legume species such as Sesbania rostrata have been shown to fix nitrogen effectively. A number of strains of rhizobia that nodulate the stem-borne roots of Aeschynomene spp. have recently been found to possess bacteriochlorophyll a. The energy sufficiency of these stem nodules containing photosynthetic rhizobia (Hungria et al., 1992) invites efforts to induce agriculturally important legumes to form nodules containing photosynthetic strains on stems and branches. A fundamental question in nodule development is why only the root is receptive to infection by rhizobia.
The factors that limit legume production must be identified and quantified, and ways devised to overcome them. Opportunities must be explored to include legumes that are not currently in use, for example, stem-nodulated species as green manures in water-logged soils.
Research in legume improvement for BNF must always recognize that the goal involves maximization both of yield and of the proportion of the nitrogen in the plant derived from fixation.
Survival and Competition of Nitrogen-Fixing Organisms
The introduction of genetically modified microorganisms for improved nitrogen fixation (or biocontrol or plant-growth promotion) has often failed because of poor competitiveness with the indigenous flora. This problem is paramount in developing and developed countries alike. Related research needs include studies of microbial ecology and development of inoculation practices and modification of microbes for good distribution on the root surface. Competitive advantage may be gained by inducing rhizobia to excrete antibiotic compounds, for example, by molecular genetic modification.
Studies on the (molecular) ecology of nitrogen-fixing microbes in the rhizosphere must be a component of BNF research for and in developing countries. There is a need to develop increased understanding of the microbial genes and plant and soil factors involved in rhizosphere and saprophytic competence. Also needed are studies on the mechanisms involved in microbial responses to nutrient limitations and other environmental stresses, especially those germane to the tropics.
It is necessary to increase understanding of the biological basis of the beneficial interactions of nitrogen-fixing rhizobia with mycorrhizae and with microbes that suppress root diseases. The potential of mixed inocula for plant growth stimulation needs further examination. The utility of “biased rhizospheres,” consisting of plants secreting a selective nutritional mediator (or otherwise influencing microbial communities in the rhizo-
sphere), to favor beneficial (for example, nitrogen-fixing) microbes able to catabolize these mediators, should be investigated.
Novel convenient methods for the rapid identification, enumeration, classification, and tracking of microorganisms in the soil and rhizosphere will expedite the achievement of these goals.
BNF Systems for Cereals
The cereals—rice, maize, wheat, sorghum, and millet—are the major food crops in the world. In the 1960s and '70s, the Green Revolution greatly increased yields of rice and wheat through breeding and greater inputs of fertilizer nitrogen and irrigation. The yields of maize have also risen steadily, largely because of use of hybrids and increased inputs of fertilizer nitrogen.
To accommodate the world's expanding population, which is projected to double by 2050, an ever-increasing production of food crops will be necessary. This must be achieved primarily by increasing productivity of currently farmed areas, since suitable new land is very limited. Concomitantly, total usage of fertilizer nitrogen and application rates per unit area will increase, with accompanying environmental deterioration, unless alternative technologies are developed. An obvious goal of BNF research is to find ways to enable the major cereal crops to utilize BNF directly as a partial or major source of their nitrogen needs.
Research promising great rewards would focus on the transfer of legume genes to cereals to enable nodule formation and nitrogen fixation by rhizobia. Most of the genetic material and techniques are now available for this research. Research on pea (Pisum sativum) has led to the identification of about 30 sym genes (Lu et al., 1993), the locations of which are being mapped on the legume chromosomes. Research is needed to microlocate these genes, clone them, and transfer them to cereals. The term sym is used for legume genes required for the formation and functioning of nitrogen-fixing root nodules.
Another, but less-developed, alternative lies in the exploitation of the recently discovered nitrogen-fixing endophytic diazotrophs that colonize several cereals (Döbereiner et al., 1993). The introduction of such bacteria into various cereals with the aid of mycorrhizae (Paula et al., 1992) should be investigated further. Another possibility is the transfer of nitrogen-fixation (nif) genes to bacteria that are endophytic in cereal species or to induce nitrogen-fixing bacteria to become endophytic.
Another approach, although with a high risk of failure, is to transfer and express nif genes from a free-living microorganism directly into a cereal. A major problem with this approach is still the difficulty of com-
partmenting of the nitrogenase enzyme within the cereal cell to afford protection from oxygen while providing an adequate supply of energy.
Cropping Systems Using Nitrogen-Fixing Plants
Legumes can contribute nitrogen to cropping systems in several ways. A gain in nitrogen will accrue to the soil if the total nitrogen in the plant residues left after harvest is greater than the total amount of nitrogen absorbed from the soil. In general terms, the less nitrogen available in the soil and the lower the nitrogen-harvest index of the legume crop, the greater will be the nitrogen gain by the system.
To maximize the nitrogen contribution from a legume crop, the total crop must be incorporated in the soil as a green manure. This can be achieved by conventional means or by alley cropping (with legume shrubs) or agroforestry (with legume trees) approaches. Regular coppicing of the shrubs and trees provides foliage for incorporation into the soil as a green manure or for application as mulch. Sesbania rostrata, a legume shrub that is tolerant of waterlogging, forms nodules on stems, and has a high nitrogen-fixing capacity. It has been used to good effect as a green manure in paddy fields in Thailand and Senegal. Similarly, in some parts of China and Southeast Asia, Azolla is allowed to grow in paddy water and is then incorporated into the soil as a green manure.
Legumes are often a component of intercropped systems in tropical agriculture, and the possibility of direct benefit to the nonlegume as the result of nitrogen excretion by the legume has been a contentious issue. Data in the literature show that nitrogen exchange does occur in certain circumstances, but it can be detected only under conditions of very low availability of soil nitrogen because it occurs only in small amounts. There is evidence that mycorrhizal connections between the intercropped components may provide a route of nitrogen transfer. Such nitrogen benefit to an intercropped cereal would be significant only under low-yielding conditions. When parts or all of the legume senesces and decomposes, the associated crop can obtain nitrogen in larger quantities.
More information is needed on: (1) nitrogen cycling in agricultural systems; (2) the yield-improving effects of rotations including nutritional factors other than nitrogen; and (3) the effects of tillage practices on nitrogen cycling, as well as on how nitrogen fixation is affected by various sources of nitrogen and by quantity and quality of soil organic matter including composts and crop residues.
Although there are data available from model-system studies on the factors that affect nitrogen fixation by legumes, there is little understanding of how the various environmental stresses may interact to affect the total nitrogen available to the plant.
Culture Collections
The successful establishment of nonindigenous legume crops, such as alfalfa and soybean in areas in the United States, clovers in areas of Australia, and recently soybean in Brazil, required the application of rhizobial inoculants to seed at planting to ensure adequate root nodulation and nitrogen fixation. Currently, several international and national institutes and individual researchers accumulate and store microbial isolates for research on nitrogen fixation and other plant-microbe interactions. These collections, which constitute a basic resource for utilizing BNF to improve crop productivity, include rhizobia, associative nitrogen-fixing organisms —for example, Azospirillum and Azotobacter spp. and Azolla spp.—and endo/ectomycorrhizae.
In the past, collections have been lost as a result of changes in research personnel and programs, and through cessation of funding. Therefore, it is essential to establish permanent storage facilities to conserve these critically important germplasm resources. To eliminate any possibility of loss, the collections must be maintained at several locations, preferably with working collections at additional sites.
This system would afford the scientific community access to microbial germplasm in a fashion similar to that now provided by the CGIAR institutions for crop germplasm. The UNESCO-coordinated Microbiological Research Centers (MIRCENS) currently perform a useful role as regional repositories and suppliers of germplasm, but they are not intended to provide permanent storage.
The microbes in these collections should be characterized in accordance with standardized procedures to improve their scientific and agronomic utility.
Of particular importance to the developing countries would be the development of user-friendly diagnostics such as DNA fingerprinting, phage typing, and antibiotic resistance to assist in classification and in ecological studies. It is essential to investigate more efficient and effective methods of preservation at the lowest cost and to develop a list of key characteristics for stored germplasm. Research is needed to develop storage methods that minimize genetic change in the cultures.
Inoculants
The inoculation of legumes with rhizobia has been practiced for the past century, primarily in industrialized countries. Inoculant production and use, both small- and large-scale, are well documented in the literature (Smith, 1987). Putting legume inoculation into practice requires substantial infrastructure for production, storage, and distribution, some-
times under stressful local conditions. The technology for producing effective inoculant can be implemented readily in developing countries, but extension of information and training is needed to teach growers proper handling and use of inoculants (Sims et al., 1984).
Continued research is needed to identify the best rhizobial strains available for improved varieties of legumes. Despite our extensive experience, we need a better understanding of the questions of when, where, and how much inoculant to apply to seed or soil to ensure effective colonization of roots in competition with soil rhizobia (Thies et al., 1991).
Mixed inoculants containing rhizobia and microbes that control pests or provide growth promoters are feasible, but effectiveness needs to be determined, improved, and stabilized, and production techniques developed. Inoculant storage and distribution, and inoculation practices, require adaptation to local conditions. Fermentation tanks for culturing rhizobia are rather simple, but in many countries the search for suitable local inoculant carriers must continue (Smith, 1992).
Quality assurance for inoculants is a continuing concern. There is a need to adapt and adopt methods that are rapid, accurate, and provide the user with reliable information. Development of DNA probes, serological tests, and selective media should make identification and enumeration of viable, superior microbes in inoculant more feasible.
Mycorrhizal inoculants are limited in their use to transplanted crops such as trees and grapes because inoculum must be generated under greenhouse conditions on plant roots. Research and development are required to make feasible the economic production and separation of mycorrhizal spores on a large scale for the technology to be more broadly implemented.
To allow the expansion of the Azolla Anabaena symbiotic system in aquatic systems, the mass production and maintenance of Azolla spores would need to be developed. Disease, another major limitation to the field use of this system, must be reduced.
Socioeconomics
The end goal of BNF research is to improve the well-being of people worldwide over the long term. This suggests that the net effect of BNF is positive on society and on most sectors of the economy. BNF is in competition with industrial production of fixed nitrogen. Currently, the immediate economic advantage to the farmer often lies with the industrial product. However, the finite limit of resources needed, environmental concerns, and research progress in BNF shifts the balance. Research is needed to quantify these aspects.
In many countries, infrastructure requirements for development and
adoption of BNF have generally developed as needed. This includes the nature, organization, and support for applied BNF research and development; the technology-transfer system, including the information and education subsystems; links between research and extension; increased educational levels of producers and agribusiness support personnel; availability of consultants; and strengthening of farmers' organizations. Research on these systems might provide information to improve efficiency and survival.
The adequacy of organizational structures and capital markets (for financing production and distribution of BNF inputs) also should be assessed, as well as marketing systems for current products and new products that may be forthcoming. In addition, the policy environment needed for enhancing the adoption and utilization of BNF should be studied.
BNF-induced changes at the farm and infrastructure level may well trigger changes in the structure of the agricultural sector, such as number and size of farms, input production and distribution industries, product-marketing firms, and rural institutions.
Research is needed to develop a policy that facilitates structural changes and eases the transitional costs and problems of the people involved. Widespread adoption of farm-level BNF technology is also likely to lead to changes in land use. Thus, existing land-use policies and practices should be examined for their incentive or disincentive effects on BNF technology adoption and on the farm profitability and well-being of rural communities.




























