2
Current State of Knowledge About Europa
Before outlining the major outstanding scientific issues and questions regarding Europa, COMPLEX in this chapter summarizes the current state of knowledge about Europa. This summary, which includes discussion of the reasons for thinking that Europa has the potential for both liquid water and biological activity, focuses on the geology and composition of the surface, the structure and composition of the deep interior, the likelihood of a liquid-water mantle, the properties of Europa's tenuous atmosphere, and the possibility of conditions favorable for life. The final section of this chapter summarizes the outstanding scientific questions about Europa.
GEOLOGY AND COMPOSITION OF THE SURFACE
Europa displays a complex and diverse surface history involving disruption of its icy crust by fracturing, impact cratering on a wide range of scales, and possible eruptions of materials onto the surface from the interior. Although suggestions of these processes were provided by the Voyager spacecraft in the late 1970s and early 1980s,1,2 image resolution was only about 2 km/pixel at best and was usually substantially poorer.
The Galileo spacecraft entered orbit around Jupiter in 1995 and has performed repeated flybys of Europa, returning images with resolutions as high as 6 m/pixel. (Table 2.1 summarizes Galileo's instrumentation.) Coupled with other remote-sensing data and measurements that provide insight into the interior, the Galileo mission has revealed that Europa is a remarkable object.
Early indications of Europa's geologic characteristics were derived from Earth-based telescopic observations indicating that the surface is predominantly water ice.3,4,5 Determination of its density suggested that Europa is a rocky object, but with a substantial volume of ice. Theoretical studies have suggested that Europa, like its neighbor Io, experiences interior heating from tidal stresses. These ideas, coupled with the limited Voyager imaging data, contributed to making Europa a primary target for exploration by Galileo in both the prime mission and the extended phase, the Galileo Europa Mission (GEM; Box 2.1), which undertook a general global reconnaissance of Europa's geology, composition, and magnetic environment.
Geology
Recent measurements from the Galileo imaging experiment have had a dramatic impact on our understanding of Europa. As its average density, strong infrared signature of surface water ice, and moment of inertia (see below)
TABLE 2.1 Galileo Science Instruments and Investigators
|
|
Instrument |
Description |
Investigator/ Team Leader |
Objectives |
|
Remote Sensing (Despun) |
SSI Solid-State Imaging camera |
A 150-cm focal length narrow-angle telescope (inherited from Voyager), with an image sensor, filter wheel, focal plane shutter, and electronics |
Michael Belton, National Optical Astronomy Observatories |
Geology of Galilean satellites; atmospheric motions and structures of small-scale clouds |
|
|
NIMS Near-Infrared Mapping Spectrometer |
A 22.8-cm diameter (f/3.5), 80-cm focal length, Ritchey-Chretien telescope with a spatial scanning secondary mirror and diffraction grating spectrometer |
Robert Carlson, Jet Propulsion Laboratory |
Surface/atmospheric reflection/emission |
|
|
PPR Photo-polarimeter Radiometer |
A Cassegrainian Dall-Kirkham telescope with a 10-cm aperture, a 50-cm focal length, and a 2.5-milliradian instantaneous field of view |
James Hansen, Goddard Institute for Space Studies |
Atmospheric particles, thermal/reflected radiation |
|
|
UVS/EUV (spinning) Ultraviolet Spectrometer |
The UVS is a Cassegrainian Dall-Kirkham telescope with a 5.03 x 5.28-cm aperture and a 25.0-cm focal length. The telescope is the front end to a standard 12.5-cm focal length. Ebert-Fastie scanning spectrometer. |
Charles Hord, University of Colorado |
Atmospheric gases, aerosols, etc. |
|
|
and |
|
and |
|
|
|
Extreme Ultraviolet Spectrometer |
The EUV is an objective grating spectrometer with a mechanical collimator; it is a modified Voyager spare ultraviolet spectrometer with an electrical interface to adapt it to the Galileo command and data bus. |
A. Ian F. Stewart, University of Colorado |
|
|
Fields and Particles (Spinning) |
MAG Magnetometer |
An electronics box and two sets of ring core triaxial fluxgate sensors |
Margaret Kivelson, UCLA |
Strength and fluctuations of magnetic fields |
|
|
EPD Energetic Particles Detector |
Divided into two systems: the Low-Energy Magnetospheric Measurements System (LEMMS) and the Composition Measurements System (CMS), both contained in one package, with bi-directional, solid-state detector telescopes mounted on a platform that rotates via a stepper motor to eight positions |
Donald Williams, Johns Hopkins University Applied Physics Laboratory |
Electrons, protons, heavy ions |
|
|
PLS Plasma Subsystem |
A concentric set of four spherical-plate electrostatic analyzers and three miniature magnetic mass spectrometers |
Louis Frank, University of Iowa |
Composition, energy, distribution of ions |
|
|
Instrument |
Description |
Investigator/ Team Leader |
Objectives |
|
Fields and Particles (Spinning) |
PWS Plasma Wave Subsystem |
One 6.6-m tip-to-tip electric dipole antenna (mounted on the end of the 10.6-m magnetometer boom) and two search coil magnetic antennas (mounted on the high-gain antenna feed) |
Donald Gurnett, University of Iowa |
Electromagnetic waves and wave particle interactions |
|
|
DDS Dust Detector Subsystem |
An impact ionization detector and an electronics box containing signal conditioning and spacecraft interface electronics |
Eberhard Grun, Max Planck Institut fur Kernphysik |
Mass, velocity, charge of submicron particles |
|
Engineering Experiment |
HIC Heavy Ion Counter |
Two solid-state detector telescopes called the Low-Energy Telescopes (LET B and LET E) |
Edward Stone, California Institute of Technology |
Spacecraft charged-particle environment |
|
Radio Science |
Celestial Mechanics |
The team sampled the downlink in a frequency bandwidth of 2500 Hz at a rate of 5000 samples per second. For satellite occultations the spacecraft downlink is in the residual carrier (i.e., non-suppressed) mode and referenced to the on-board Ultra Stable Oscillator (USO). The Radio Science Digital Signal Processor (DSP-R) is also required for these experiments. |
John Anderson, Jet Propulsion Laboratory |
Masses and internal structure of bodies from spacecraft tracking |
|
|
Propagation |
|
H. Taylor Howard, Stanford University |
Jupiter/satellite radii and atmospheric structure from radio propagation |
attest, Europa clearly has differentiated. It has a fairly pure ice crust estimated to be some 80 to 170 km thick and a denser deep interior. Within the interior, theoretical models suggest that dissipation of tidal energy may lead to melting of the water ice at relatively shallow depths and the presence of a global ocean.6 The very small number of impact craters observed (Figure 2.1) indicates that Europa's surface must be fairly young by geologic standards.
The Galileo mission has provided substantial indications either that there is a liquid-water ocean on Europa or, at a minimum, that a soft, ductile layer has underlain the ice crust quite near the surface at some relatively recently time. Most spectacularly, disruption of the surface in some of the so-called "mottled" terrain clearly shows that small blocks (sizes near 5 km) have become detached from adjacent "ice sheets" and have been translated and rotated to new positions (Figure 2.2). These high-resolution images provide the best evidence that the ice crust of Europa was thin at the time that the disruption took place.7 New varieties of pits, domes, and spots observed in the mottled terrain are consistent with the occurrence of solid-state convection near the surface and are interpreted as the surface expression of upwelling masses of warm ice or diapirs. 8
The age and orientation of varying large-scale linear features seen in global images of Europa change systematically, as would be expected if the surface ice were decoupled from the interior by a liquid or ductile layer.
|
Box 2.1 Galileo Europa Mission (GEM) Description Galileo was originally scheduled to end its exploration of the jovian system on December 7, 1997, but NASA and Congress approved the extension of the mission through the last day of 1999. The Galileo Europa Mission (GEM), as it is called, was designed as both a streamlined, low-cost extension to Galileo's exploration of the jovian system and as a precursor to future missions to Europa and lo. GEM encompasses 14 orbits of Galileo around Jupiter and is divided into three phases, each with its own tightly focused objectives; the Europa, Perijove Reduction, and lo campaigns. Mission Phases and Major Science Objectives Europa Campaign — A 1-year intensive study of Europa comprising eight consecutive close encounters. Europa's crust, atmosphere, and possible subsurface ocean are studied using imaging, gravity, and space physics data gathered by Galileo's full complement of remote-sensing and in situ instruments. The design of this phase of GEM allows for a number of unique imaging opportunities. These include high-resolution imaging and spectral observations (<50 m/pixel for images) and stereo imaging of selected topographic features and views of Europa's polar regions. Careful tracking of Galileo during this phase of GEM yielded geophysical information, such as Europa's moment of inertia, that will allow refinement of knowledge on the interior configuration of the satellite. Magnetospheric data obtained during close flybys and other periods chosen to provide maximum spatial coverage will help to further refine understanding of Europa's ionosphere and possible internally produced magnetic fields, and the satellite's interaction with the jovian plasma disk. Although data were not collected during one encounter. (E-13) because it occurred during solar conjunction, additional observations of Europa are planned for the final scheduled orbit (l-25) of the lo Campaign. Perijove Reduction Campaign — A series of four encounters with Callisto designed to modify Galileo's orbit sufficiently to enable close flybys of lo. In addition to observations of Callisto, a major focus of this phase of GEM is observation to characterize the lo plasma torus, including studies of satellite/magnetosphere interactions. lo Campaign — Close flyby of lo in October 1999 with the possibility of a second flyby 6 weeks later. The scientific focus of these flybys is high-resolution imaging as well as in situ observations of los' volcanic processes, atmosphere, and magnetospheric environment. |
Such decoupling leads to non-synchronous rotation of the surface ice shell with respect to the interior and appears to be expressed in stress fractures of the surface as the shell changes shape.9 The non-synchronous rotation may arise because the balance between the torque exerted by Jupiter, tending to speed up Europa, and a resisting torque, associated with a slight departure of Europa's minimum-moment-of-inertia axis from the Jupiter line, cannot be permanently maintained if the material of the satellite can adjust in some way to bring the minimum-moment-of-inertia axis back into alignment with Jupiter. This may be the case on Europa if the ice shell is decoupled from the underlying rock. If so, the rate of non-synchronous rotation depends on the viscosity of the ice, which determines the readjustment time of the shell. The absence of any perceptible offsets in the positions of features imaged by both Voyager and Galileo implies that the current non-synchronous rotation period must be in excess of 10,000 years.10
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Smaller-scale surface features, such as the arcuate lineaments known as cycloidal features, provide additional reason to believe that the surface-ice shell is relatively thin. These features, first spotted in Voyager images, consist of chains of arch-shaped segments, each some 100 km in length. Their distinctive shapes have been accurately reproduced by modeling the migration of tidal stresses across Europa's surface.11 In this interpretation, each cycloidal segment marks the surface expression of the changing orientation of tidal stresses during the course of a single europan day. However, this model only works if the amplitude of the diurnal tides is some tens of meters, the value predicted for a relatively thin ice shell decoupled from the satellite's interior by a liquid layer.
The higher-resolution Galileo images have revealed many additional small impact craters. Although varying interpretations of cratering statistics and surface age dating leave the absolute age of the europan surface uncertain, current analysis suggests that the surface is quite young (10 million to 50 million years old) and therefore that these
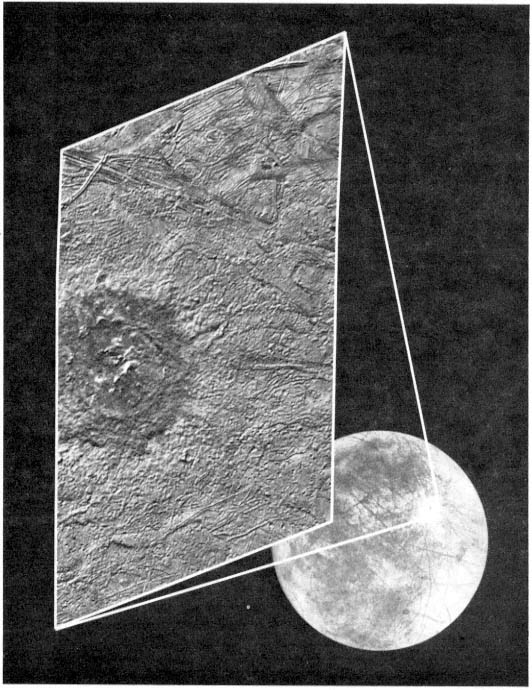
FIGURE 2.1 Although Europa's surface is remarkably free of craters, it does exhibit some large impact features. This composite of two Galileo images shows the area surrounding the 26-km-diameter crater Pwyll. The bright rays radiating from Pwyll in the global image indicate that this crater is geologically young. The close-up image reveals that, unlike most fresh impact craters that have deep floors, Pwyll's crater floor is at approximately the same level as the surrounding terrain. North is to the left, and the Sun illuminates the close-up image from the upper left. Pwyll is located at approximately 26 degrees south and 271 degrees west, and the close-up image covers an area some 125 by 75 km. The resolution of the close-up image is about 250 m/pixel. Images courtesy of the Jet Propulsion Laboratory.

FIGURE 2.2 This view of the Conamara Chaos region of Europa shows an area where the icy surface has been broken into many separate plates that have moved laterally and rotated with respect to each other. North is to the top left of this image, and the Sun illuminates the surface from the east. This image, centered at approximately 8 degrees north and 274 degrees west, covers an area approximately 4 by 7 km. The resolution is 9 m/pixel. Image courtesy of the Jet Propulsion Laboratory.
resurfacing and crustal disruption events have occurred relatively recently. The young geologic age of this mobile surface leaves the potential for a liquid or ductile layer to exist near the surface of Europa to the present day.
Galileo observations reveal that the mid-latitude plains units can be distinguished based on their visible and near-infrared spectral properties, with differences attributed to varying grain sizes of water ice. Numerous new varieties of linear features have also been recognized. These features include triple bands that either "pinch out" or emerge from simple bands that can be either bright or dark; some bands become discontinuous, a string of dark or light pearls. New topographic features have been identified, such as the complex ridge structures of triple bands (Figure 2.3), usually with a central groove, and a few low-relief mountains and plateaus. Finally, features suggesting resurfacing from flows or material extruded onto the surface are seen in Galileo's images. The bilateral symmetry in wedge-shaped bands suggests that upwelling material emerges along a central zone as crustal blocks move apart, similar to terrestrial seafloor spreading. 12
Surface Composition
Knowledge of the composition of Europa's visible surface comes primarily from ultraviolet, visible, and near-infrared reflectance spectra. These have been obtained by ground-based telescopes, the International Ultraviolet Explorer, and the Hubble Space Telescope in orbit around Earth, the Voyager flyby spacecraft, and, most recently, the Galileo spacecraft as it orbits Jupiter. From the early studies,13,14,15 we know that water ice is the most abundant material on the surface of Europa and that the ice at the very surface has a fluffy, or porous, texture.16 The surface of the leading hemisphere of Europa is brighter than that of the trailing hemisphere, presumably because the trailing hemisphere receives more impacts by charged particles from the jovian magnetosphere than does the leading side. Frozen sulfur dioxide (SO 2 frost) has been observed, especially on the trailing hemisphere where bombardment by the plasma is strongest;17,18 the source of this sulfur appears to be volcanic eruptions and sputtering from Io. Some spectroscopic features are thought to be produced by elemental sulfur.19
The two spectrometers and a color camera carried by the Galileo spacecraft have increased our knowledge of the surface composition (see Table 2.1). Initial results from the Ultraviolet Spectrometer (UVS) confirmed the leading/trailing asymmetry of the ultraviolet albedo and the SO2 frost absorption on the trailing hemisphere.20 The Solid-State Imager (SSI) has distinguished water-ice grain size differences among plains units on Europa. Cross-cutting relationships among the linear features show that the youngest areas are dark and that they lighten with exposure to the space environment. This has been interpreted as indicative of weathering of sulfur to SO2 frost or that solid, clear ice (such as that seen in frozen lakes) is being damaged by radiation and broken down into finer, brighter particles.
The data from Galileo's Near-Infrared Mapping Spectrometer (NIMS) show that Europa's water-ice absorption features are distorted in comparison with those seen in pure ice. This is true even in the brightest regions that were inferred to be nearly free of "contaminants." While this has commonly been interpreted as implying a contribution from hydrated minerals such as evaporite salts or clays in all regions of the satellite, it has been shown that light reflected from fairly clear ice with entrained bubbles or defects also exhibits band shifts similar to those observed on Europa. Many of Europa's dark regions exhibit spectral characteristics that appear distinct from those of water ice, and these have been interpreted as being consistent with the spectral characteristics of water-bearing salts such as hexahedrite (MgSO4• 6H2O), epsomite (MgSO4• 7H2O), and natron (Na2 CO3• 10H2O). It is not yet known how plasma-bombardment damage may affect the near-infrared absorption features of water ice. However, the detection of H2O2 by NIMS suggests that Europa's surface chemistry is dominated by radiolysis.21
At the spatial scales observed by NIMS (typically 10 to 20 km/pixel), it is likely that water ice of varying textures and clarity, and the presence of minerals, contribute to the observed spectral features. This view has, however, recently been challenged by an alternative interpretation based upon a radiolytic cycling of sulfur between sulfuric acid, sulfur dioxide, and sulfur polymers.22 In this model, the dark features are radiolytically altered sulfur polymers and, if correct, indicate that sulfuric acid may play an important role in Europa's geologic activity. Other possible surface constituents such as ammonia and carbon dioxide ice have been searched for but have not yet been detected.23
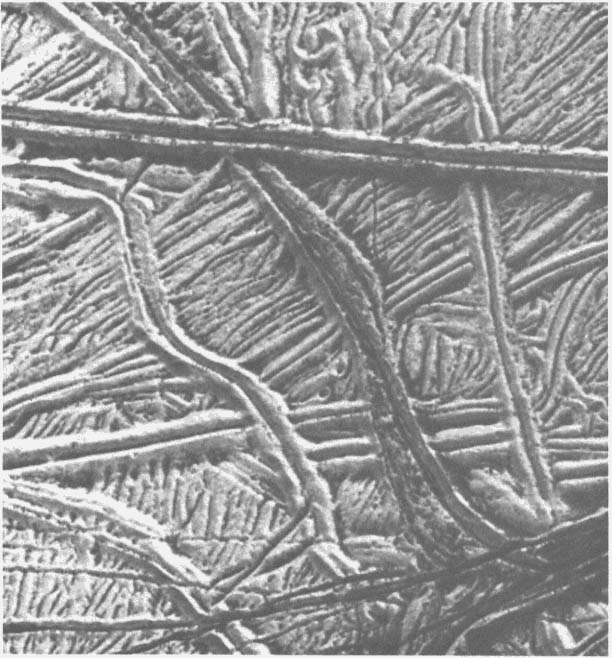
FIGURE 2.3 The complexity of the ridged plains on Europa is demonstrated by this Galileo image of several cross-cutting triple bands. Much of this surface is very bright, with the darker material concentrated primarily in the valleys between the ridges. Indeed, the most prominent ridges have dark deposits along their margins and in their central valleys, suggesting that the dark material may have moved down the flanks of the ridges and piled up along their bases. North is to the right, and the Sun illuminates the surface from the upper right. This image, centered at approximately 14 degrees south and 194 degrees west, covers an area approximately 20 km across. The resolution is 26 m/pixel. Image courtesy of the Jet Propulsion Laboratory.
STRUCTURE AND COMPOSITION OF THE DEEP INTERIOR
Prior to the Galileo mission there were two competing models for the internal structure of Europa.24 In one model, Europa consisted of an anhydrous rocky core with a density like that of Io or the Moon, surrounded by a layer of water ice or liquid water that could be more than 100 km thick. In the other model, most of the water in Europa was retained in a hydrated silicate interior surrounded by a thin water-ice layer. The possibility that Europa might have a differentiated metallic core was not considered. Instead, debate centered on the extent to which Europa's interior was dehydrated and, in the fully dehydrated model, whether the outer water layer was completely frozen or had a melted liquid layer beneath an outer solid layer of ice.
Today, as a consequence of the Galileo measurements of Europa's gravitational field, the model of Europa with a thin ice shell above a largely hydrated silicate interior is no longer tenable. Moreover, the density of Europa's deep interior is high enough that it argues strongly for a metallic core at the center of the satellite. Unfortunately, we still do not know with certainty whether there is a liquid-water ocean beneath Europa's icy surface. Neither do we know if the metallic core is solid or liquid.
The gravitational field of Europa has been measured in four flybys of the satellite by the Galileo space-craft.25,26 The measurements have yielded quite accurate determinations of the degree-two spherical harmonic contributions to Europa's gravity. On the assumption that the shape of Europa's gravitational field results from a physical distortion caused by its spin and by the tidal forces it experiences as it orbits around Jupiter in synchronous rotation, the gravitational field can be used to infer Europa's axial moment of inertia C and tell us about the distribution of mass in Europa's interior. Normalized to MR2 (M is the mass of Europa and R is its radius), the moment of inertia is C/MR2 = 0.346 ± 0.005.27 This value of C/MR2 is substantially less than 0.4, the value for a uniform-density sphere, and requires that the density of the interior increase toward the center of Europa.
The implications of Europa's mean density and moment of inertia for the structure of its interior have been explored in terms of simple two-and three-layer models of the satellite.28 Two-layer models of Europa with an ice outer shell and a uniform silicate/metal inner region are possible, but only if the interior density is greater than about 3800 kg m-3. This structure is considered implausible because the interior density would be higher than Io's mean density, and because it is likely that radiogenic heating in such an interior would cause a metallic core to differentiate.29 Therefore, Europa must have a three-layer structure with an Fe or Fe-FeS core at its center, a rock mantle surrounding the metallic core, and a water-ice or liquid-water shell around the rock. The size of the core is between 40 and 50% of Europa's radius, depending on its composition. The thickness of the outer shell of water must lie in the range of about 80 to 170 km, with a value of some 100 km being the most likely.30 The gravity data do not allow any conclusion regarding the physical state (i.e., liquid or solid) of either Europa's metallic core or its outer water shell. Lack of detection of a europan magnetic field also precludes any unique inference about the physical state of Europa's metallic core.31
EVIDENCE FOR THE PRESENCE OF A LIQUID-WATER MANTLE
Thermal models of Europa provide additional insight into the possibility that a liquid-water ocean may exist under Europa's surface ice.32 Modeling suggests that accretional and radiogenic heat sources are large enough to have dehydrated Europa early in its evolution, leaving the satellite covered with a layer of liquid water 100 km or more thick. Early models, which considered only the conductive cooling and freezing with time of the outer layer of water,33 resulted in the presence today of liquid water beneath the ice shell. However, other models showed that the outer layer of ice would become unstable to convection with sufficient thickening, thereby promoting heat transfer through the ice and the cooling and solidification of the underlying water.34 These models predicted the complete freezing of the outer layer of water in a small fraction of geologic time. However, the predicted freezing of Europa's ocean by efficient subsolidus convection (i.e., the slow deformation and flow of a material below its melting temperature in response to a source of heat) assumed only radiogenic heating in Europa's silicate interior. Other models included the additional heating produced by tidal dissipation in the ice shell and indicated that this heat source could offset the subsolidus convective cooling of the ice and prevent complete solidification of the water ocean.35 Basically, a steady state could be achieved in which the balance between the dissipative heat
source and the convective cooling would leave the ice layer with a constant thickness. Modifications to the latter model resulted in a reduction in the estimated amount of tidal heating, again opening the question of whether the water layer on Europa would freeze completely over geologic time.36 Various other thermal models have been constructed, including those that took into account the effects of an insulating regolith on the stability of the ice shell.37
The competition between the tendency of tidal heating to maintain a liquid-water ocean and that of subsolidus ice convection to freeze the ocean has now been analyzed for nearly two decades without a definitive conclusion having been reached. The major uncertainty in the modeling is the uncertain rheology of ice and of its control of both convection and dissipation.38 Both dissipative heating and convective cooling involve nonlinear feedback mechanisms associated with the dependence of rheology on temperature and the dependence of temperature on the heating and cooling mechanisms. The amount of tidal heating in the ice depends on the rheology of the ice at tidal periods and on the magnitude of tidal deformation, the latter in turn depending on the internal structure and, in particular, on whether there is a liquid ocean beneath the ice layer and on the ice thickness.
Other properties of the ice are also both important and highly uncertain. The thermal conductivity of the ice is dependent on the temperature and physical state of the ice (its density and the distribution of cracks, for example). A thermally insulating layer at the surface of Europa would promote stabilization of a liquid-water ocean.39 The occurrence of minor constituents in the ice and ocean such as salts and ammonia would affect both the rheology of the ice and the freezing temperature of the ocean.40 Tidal heating along major faults in Europa's ice shell may be important,41 and tidal heating due to forced circulation in a thin liquid-water ocean sandwiched between the rock interior and the overlying ice may prevent complete solidification of the ocean.42 Tidal heating is too dependent on many unknown or poorly known properties of Europa's ice shell, therefore, to settle the debate on the existence of a liquid-water ocean beneath the ice of Europa theoretically, without the benefit of direct observations.
ATMOSPHERE AND IONOSPHERE
Europa's Neutral Atmosphere
That the moons of Jupiter should have transient atmospheres, undergoing continual production and loss, is one of the many enigmas of the complex jovian system. While volcanism is accepted as the ultimate source of Io's atmosphere, other processes must be responsible for Europa's atmosphere.
To date, remote-sensing techniques have identified two constituents of Europa's atmosphere — molecular oxygen (O2) and atomic sodium (Na). The former is inferred from ultraviolet emissions,43 while the latter comes from detection of light scattered at visible wavelengths. 44 The vertical column abundances (the number of molecules sitting above a unit of area on the surface) are estimated to be ~1015 cm-2 for O2 and ~ 1010 cm-2 for Na, with number densities just above the surface of ~108 cm-3 and ~70 cm-3, respectively. Although sodium is far less abundant than oxygen, it is more easily observed and has been traced out to distances of more than 20 times the radius of Europa. Other gases must also be present with greater or lesser abundances than these and, collectively, may serve as important tracers of the chemical composition of Europa's surface; they have not been detected, however.
While some atmospheric constituents may arise from the impact of micrometer-sized dust grains or from simple evaporation of surface materials, the dominant source for Europa's O2 and Na atmospheres is thought to be sputtering (i.e., the ejection of molecules or atoms from a surface resulting from the impact of ions or electrons) by energetic (100- to 1000-keV) magnetospheric ions (Figure 2.4). The oxygen comes from water ice at the surface. Laboratory experiments have confirmed that ice subjected to ion bombardment yields gaseous H2O, H2, and O245; the water vapor quickly freezes again at Europa's surface temperatures, the hydrogen quickly escapes to space due to the weak surface gravity (with a surface acceleration of about 1.3 m s-2), and the oxygen remains to form a bona fide atmosphere, albeit one subject to loss by subsequent ionization and transport processes. Sodium, on the other hand, comes from impurities (such as salts) intrinsic to the icy surface material, as well as from Io, where sodium escapes and can be implanted subsequently onto Europa's surface. The eventual detection of additional species,
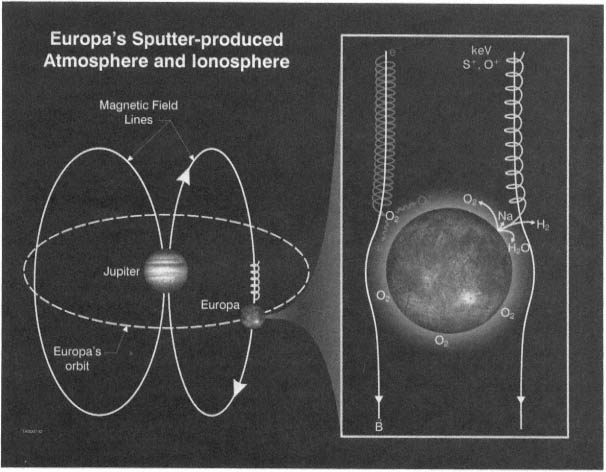
FIGURE 2.4 Europa's neutral atmosphere and ionosphere are produced by a complex chain of interactions between energetically charged particles trapped in Jupiter's magnetic field (B) and the elements in Europa's icy surface. Sampling the neutral gases and plasma in Europa's atmosphere will reveal the chemical composition of the ice on its surface. Image courtesy of Southwest Research Institute, San Antonio, Texas.
such as potassium, calcium, and magnesium (and, potentially, organic molecules) will allow determination of the chemical makeup of Europa's icy terrain.
Europa's Ionosphere
The presence of a tenuous neutral gas surrounding Europa leads naturally to an ionospheric plasma. The ionization sources are collisional ionization and photoionization. The energetic magnetospheric plasma that sputters the surface materials to form the neutral atmosphere also is able to ionize the gases once they are in the atmosphere. While energetic ions are probably the dominant sputtering agent, it is the energetic electron population incident upon O2 that can lead to the formation of plasma. Since incoming ions and electrons can penetrate the atmosphere all the way to the surface, the peak neutral and ionospheric densities are probably just above the surface. This is in marked contrast to having a well-defined ionospheric layer with peak density (Nmax) at some
height (hmax) significantly above the surface, as found in more-typical planetary ionospheres. A photoionization source in the ultraviolet region of the spectrum will act similarly, and thus both processes act to some (unknown) degree to produce Europa's ionosphere. Preliminary results from Galileo suggest an Nmax~ 104 e- cm-3 just above the surface.46
Once produced, the ionospheric plasma has a short residence time at Europa since there is no intrinsic magnetic field or dense atmosphere to keep it bound to the satellite. Rather, the ions and electrons will immediately begin to gyrate about the jovian magnetic field. Since the magnetic field is coupled to Jupiter's rapid rotation, the ionospheric plasma becomes entrained in the corotating magnetospheric plasma that impinges on the trailing side of Europa (upstream in the magnetospheric flow) and is swept away downstream, ahead of the satellite in its orbit.
The current state of knowledge of Europa's neutral atmosphere and its embedded ionosphere is extremely rudimentary. The day-to-day variability of both and their responses to the stresses caused by electrodynamical interactions with the magnetosphere in which they reside are essentially unknown at this time. However, in contrast to the terrestrial situation, the atmosphere and ionosphere on Europa are highly representative of surface materials, and therefore the detection of all possible species is important.
MAGNETIC-FIELD AND ENERGETIC-PARTICLE ENVIRONMENT IN THE VICINITY OF EUROPA
Europa is located in the inner magnetosphere of Jupiter (at a radial distance of ~ 9.5 RJ), a region populated mainly by plasma derived from the Io plasma torus. The plasma there consists of protons, oxygen and sulfur ions, and their corresponding electrons. The background magnetic field of Jupiter is quite strong near Europa's orbit (~ 500 nT), and the fluxes of energetic electrons and ions are among the highest found in the solar system. The plasma is nearly corotational, being dragged around Jupiter by the magnetic field as Jupiter rotates; with the magnetic field rotating at a faster rate than Europa revolves around Jupiter, the plasma hits Europa with a relative velocity of ~ 120 km/s on Europa's orbital trailing hemisphere. Most of the plasma resides in a thin plasma sheet (with a half-thickness ~ 2RJ), located approximately in the plane of Jupiter's magnetic equator. Because Jupiter's magnetic field is tilted by 10 degrees relative to its rotation axis, the plasma sheet and the magnetic equator appear to move up and down as seen from Europa, with a period of 11.2 hours (i.e., the synodic period corresponding to the 9.9-hour rotation rate of Jupiter's magnetic field and Europa's 3.6 day rotation rate). This relative motion produces dramatic changes in the charged particle fluxes and the magnetic field experienced by Europa.47
Though several spacecraft have traveled interior to the orbit of Europa, Galileo is the first to have provided in situ field and plasma measurements near Europa. Galileo collected data in Europa's vicinity four times during its primary mission and, ultimately, an additional seven times during the subsequent Galileo Europa Mission (see Box 2.1). Plasma measurements from Galileo show that Europa acts in a manner similar to a cometary source of plasma; while it both absorbs and emits charged particles, it is a net source of plasma emitted into the magnetosphere. Measurements from the E-4 and E-6 orbits show that the plasma density was enhanced by a factor of two or more within a large volume around Europa. Simultaneous measurements from the energetic-particle detectors showed that the radiation environment near Europa is extremely variable, changing by an order of magnitude between orbits. These are the energetic particles that bombard the surface of Europa to produce its transient neutral atmosphere and ionosphere and to cause resurfacing and migration of material on its surface.
When Europa is located above or below the central plasma sheet, the fluxes of charged particles are relatively low and sputtering is at a minimum. During this time, the variations in the background magnetic field that result from the rotation of the tilted jovian dipole produce a response from Europa.48,49 The oscillating jovian field is somewhat ''neutralized," apparently by the presence of a conducting material within Europa that produces an eddy current in its surface. Simple modeling calculations suggest that the induced field is dipolar in nature and that its magnitude at its pole is equal and opposite to that of the applied oscillating field (thereby canceling it out). The electrical conductivities of the ionosphere (< 10-4 S/m) alone, however, or of the ice crust (< 10-6 S/m) are too small to shield out the oscillating field in this manner. A possible explanation for the presence of a more-conductive medium is that there is a global liquid-water ocean and that it contains dissolved salts.50,51 The
conductivity of salt water is high (~ 1 S/m), and a wave driven by a changing magnetic field with a period of just over 11 hours would have a penetration skin depth of ~ 100 km. Thus, if the ocean thickness is in the range of tens of kilometers or larger, it would be able to produce an induction response to the varying background field. In this limit, the strength of the induced response depends only on the depth of the conducting layer below the surface.
A second type of response to the field and plasma conditions occurs when Europa is at the center of the plasma sheet. There, the much higher fluxes of energetic particles produce a large escaping flux of sputtered and ionized material; this flux can be greater than 50 kg/s. Europa is similar to a comet in such a situation, and the newly picked-up plasma affects the background magnetic field at distances as great as 8 Europa radii from Europa. In this situation, the magnetic field drapes around the cloud of plasma and its strength increases upstream of Europa and decreases downstream. Measurements from Galileo's E-12 orbit showed that the field indeed increased by more than 400 nT upstream of Europa. The expected induction response was of the order of 40 nT during the E-12 flyby and could not be separated from the very large comet-like response.52
POTENTIAL FOR BIOLOGICAL ACTIVITY
If liquid water exists beneath the surface ice layer on Europa, then one of the environmental requirements for life will have been met. If, in addition, the satellite has provided a source of energy for metabolism and access to the requisite biogenic elements, then it is possible that life may have originated on Europa independently of life on Earth, and even that it may exist now.
On Earth, organisms use either sunlight (via photosynthesis) or chemical reactions (via chemosynthesis) as energy sources for their metabolic processes. However, plausibility arguments based on the phylogenetic tree of all life on Earth suggest that chemosynthesis likely predates photosynthesis.53,54 The chemosynthetic microorganisms that branch most deeply in the tree are autotrophs; they gain energy from inorganic chemical reactions such as reduction of sulfur to hydrogen sulfide or formation of methane (methanogenesis) from carbon dioxide and hydrogen. Because these microorganisms do not require sunlight as a source of energy and carry out reduction reactions involving inorganic compounds, they suggest both the type of life that might thrive beyond Earth and the first kind of organism that might form in an energetic extraterrestrial environment. On Earth, many of these microorganisms are "hyperthermophiles" that require temperatures above 70°C for growth and live in hot springs and hydrothermal systems.55,56 It is not yet known whether high temperatures are a necessary condition for the origin or early evolution of life, but there are many indications that hydrothermal systems are ideally suited for providing geochemical sources of metabolic energy and may be sites of organic synthesis. 57,58
Chemosynthesis is possible on Earth owing to numerous environments that are not in a state of chemical equilibrium. In many of these environments, the chemical interaction of water with rocks, and the movement and circulation of the resulting solutions between regions of differing temperatures, establish disequilibrium states by bringing together compounds that are in different oxidation states. For example, water-rock reactions in rocks containing ferrous silicates (like basalts, peridotites, and other igneous rocks) can lead to a small amount of H2 production from H2O as some of the ferrous iron is oxidized to ferric iron. The H2 can be generated in solutions that contain bicarbonate, leading to a mixture that is thermodynamically unstable and that should react to form methane. However, the inorganic reaction by which methane forms is extremely slow, providing a situation in which methanogenesis by organisms becomes a viable energy-producing metabolic strategy.59 Autotrophic methanogenesis illustrates how chemosynthetic biological systems can be fueled by geochemically generated reduction-oxidation (redox) disequilibria. If there are sources of redox disequilibria on Europa, then energy-producing chemical reactions may occur there and may have the potential to support life (see Box 2.2).
Water-rock interactions on Europa seem plausible given the possible presence of liquid water surrounding an underlying rocky mantle that is likely to contain ferrous silicates near the boundary. In addition, the rocky interior by itself would be nearly comparable in size to the Moon and may have been volcanically active in the past, and may even be so at the present; circulation of water through volcanically heated rock in the form of hydrothermal systems can provide access to energy.60 Finally, a geologically active interior could provide access to the biogenic elements in a form that would allow their utilization in prebiological or biological chemical reactions.
Other sources of energy might also be available on Europa to support metabolism. For example, although
|
Box 2.2 Redox Chemistry and Its Role in Biological Systems Redox is the chemical process by which a reaction between two chemical species can result in the oxidation of one of the reactants and, simultaneously, the reduction of the other. For example, H2 and O2 are out of redox equilibrium in the modern terrestrial environment, and they can be driven to react chemically by a small input of energy (from, for example, a spark) to form H2O, releasing energy in the process. The hydrogen is said to have become oxidized and the oxygen reduced. The essence of the redox process is the transfer of electrons from the oxidized species to that which is reduced (it is the charge on the electron receptor that is being "reduced"). The energy released in such reactions can be used to drive other chemical reactions. As such, redox chemistry plays an important role in most biological systems. Any species out of equilibrium can, in principle, react by exchanging electrons until they are in equilibrium. Moreover, in the proper situation, just about any chemical species can act as either an oxidizing (electron acceptor) or reducing (electron donor) agent. Common electron donors include H2, CO, Fe, Mn, CH4, S, NO2, H2S, and NH3. Common electron acceptors include O2, CO2, CO, S, NO2, Mn, and Fe. The diversity of donors and acceptors means that a wide variety of different redox systems can power life. |
photosynthesis is thought to have developed later than chemosynthesis, organisms in a europan ocean might have access to sunlight in regions of recent eruption of liquid water to the surface. Some organisms might be able to survive by metabolizing organic molecules that arrive in meteoritic or cometary debris and become entrained into an ocean or are remnants of organic molecules previously produced in situ or of prior living organisms. Still others might utilize H2 and CO 2 in the formation of acetogen.61 Organisms utilizing each of these metabolic strategies are found on Earth and might be able to survive in an oceanic environment on Europa.
Recent calculations have cast doubt on the idea that sufficient energy is available to sustain life in a europan ocean. Researchers have argued that the water in a closed europan ocean would rapidly become chemically reduced due to interactions with hot rocks in hydrothermal systems.62 If this view is correct, a europan ocean would not be an energetically favorable environment for life. This view has, however, been challenged. Additional calculations have indicated that even if the chemical nature of the ocean is reducing, abundant redox chemistry can still take place and, thus, provide an energy source for metabolism. 63 Nevertheless, it is still far from clear whether or not a europan ocean contains sufficient energy to support an origin of life. Moreover, if life did originate at some time, it is unclear if it survived to the present day.
SUMMARY OF OPEN ISSUES REGARDING EUROPA
The major outstanding questions about Europa that remain unanswered at the close of the Galileo Europa Mission are whether Europa has a liquid-water ocean beneath its icy surface and whether there is the potential for the existence of life there. The former is amenable to investigation by spacecraft in the immediate future, whereas the latter will require development of new technological approaches and implementations.
The question of the presence or absence of a europan ocean is central to our understanding of the satellite as a whole. It provides the intellectual underpinning for our understanding of the geology, geophysics, atmosphere, and history of the satellite. Though Europa's gravity field may be known to spherical-harmonic-degree three or better through analysis of Galileo tracking data, knowledge of the properties of this field will not tell us if there is liquid water beneath the surface.
The two most intriguing arguments in favor of a subsurface liquid-water ocean on Europa at present or in the recent geologic past are the geologic evidence for rafting of blocks of ice floating on an underlying fluid and the detection by the Galileo magnetometer of an electromagnetic induction response in Europa that appears to be
explainable only by the presence of a salty ocean.64,65,66 The problem with accepting the latter as a definitive detection of a europan ocean is that a similar electromagnetic response is seen at Callisto, yet this moon displays no surface morphological evidence for the existence of a subsurface ocean. Could such an ocean exist on Callisto without being reflected in the geologic processes that have created its surface?
A summary, then, of the major outstanding scientific issues and questions for Europa includes the following:
-
Is there liquid water on Europa and, if so, what is its spatial distribution? If there is a globally distributed "ocean" of water, how thick is the layer of ice that covers it, and what are the properties of the liquid? If there is not liquid water today, has there been any in the relatively recent past, and what is the time dependence of its occurrence?
-
Are the kilometer-scale ice rafts seen on Europa's surface a product of the movement of ice on an underlying liquid-water sea or through a warm, soft (but not necessarily melted) ice? What is the overall relationship between the surficial geologic units and the history of liquid water?
-
What is the composition of the deep interior of Europa, including both the presumed silicate mantle and the iron (or FeS) deep core? Is the core solid or liquid, and is it actively convecting to get rid of heat? Is there a global magnetic field produced by motions of the core? What are the dynamics of the interior, especially regarding the possible physical decoupling of the rotation of the surface ice from the deep interior and the possible non-synchronous rotation of the surface or of the entire satellite? What is the magnitude of tidal heating of the interior, and how is the heating distributed within the interior?
-
What is the composition of the non-ice component (such as salts) of the surface materials that are seen in imaging and spectroscopic investigations? How do they vary over the surface? What are the source and history of these materials, and how do they relate to the geologic history of the surface and the potential for the at-least-intermittent presence of liquid water?
-
What is the nature of the ice-tectonic processes that have affected the surface, and how are they reflected in the features that are seen (such as triple bands and spots)? Is there active or ongoing cryovolcanism? What are the absolute ages of the various surface geological units?
-
What is the composition of the neutral atmosphere and of the ionosphere? What are the sources and sinks of these species? What are the spatial and temporal variations in the atmosphere, and how do they relate to the physical processes that might control them? What is the composition of the magnetospheric ions that can sputter the surface, and of the sputtering products?
-
What are the characteristics of the radiation environment at the surface of Europa (currently and in the past), and what are the implications for organic/biotic chemistry and the survival of life on the surface?
-
What is the abundance of geochemical sources of energy that could support an origin of life on Europa or its continued existence? Is there extant life, or has there been life in the past? If there has been liquid water, access to biogenic elements, and a source of energy but there is no life present, what factors might explain the lack of occurrence of life, and does the potential exist for an independent origin of life in the future?
These fundamental science questions about the nature and evolution of Europa can be addressed through an ongoing program of telescopic and spacecraft exploration. Some of these issues may be tackled in part or in full by NASA's proposed Europa Orbiter mission. It is almost certainly the case that they cannot all be addressed by a single, short-lived spacecraft mission. Rather, answering these questions will require an ongoing program that progresses from flybys to orbiters to landers to subsurface penetrators. In the following chapters, COMPLEX discusses how these scientific questions can be addressed.
REFERENCES
1. B.K. Lucchitta and L.A. Soderblom, "Geology of Europa," in Satellites of Jupiter, D. Morrison, ed., University of Arizona Press, Tucson, Arizona, 1982.
2. M.C. Malin and D.C. Pieri, "Europa," in Satellites, J.A. Burns and M.S. Matthews, eds., University of Arizona Press, Tucson, Arizona, 1986.
3. G.P. Kuiper, Astronomical Journal 62: 245, 1957.
4. V.I. Moroz, Soviet Astronomy-AJ 9: 999, 1965.
5. T.V. Johnson and B.B. Pilcher, "Satellite Spectrophotometry and Surface Compositions," Planetary Satellites, J.A. Burns, ed., University of Arizona Press, Tucson, Arizona, 1977, page 232.
6. P.M. Cassen, S.J. Peale, and R.T. Reynolds, "Structure and Thermal Evolution of the Galilean Satellites," in Satellites of Jupiter, D. Morrison, ed., University of Arizona Press, Tucson, Arizona, 1982.
7. See, for example, M.H. Carr et al., "Evidence for a Subsurface Ocean on Europa," Nature 391: 363, 1998.
8. R.T. Pappalardo et al., "Geological Evidence for Solid-State Convection in Europa's Ice Shell," Nature 391: 365, 1998.
9. P.E. Geissler et al., "Evidence for Non-Synchronous Rotation of Europa," Nature 391: 368, 1998.
10. G.R. Hoppa et al., "Rotation of Europa: Constraints from Terminator Positions," Lunar and Planetary Science Conference Abstracts 28: 597, 1997.
11. G.V. Hoppa, B.R. Tufts, R. Greenberg, and P.E. Geissler, "Formation of Cycloidal Features on Europa," Science 285: 1899, 1999.
12. R. Sullivan et al., "Episodic Plate Separation and Fracture Infill on the Surface of Europa," Nature 391: 371, 1998.
13. C.B. Pilcher, S.T. Ridgeway, and T.B. McCord, "Galilean Satellites: Identification of Water Frost," Science 178: 1087, 1972.
14. R.N. Clark, F.P. Fanale, and M.J. Gaffey, "Surface Composition of Natural Satellites," in Satellites, J.A. Burns and M.S. Matthews, eds., University of Arizona Press, Tucson, Arizona, 1986.
15. W. Calvin, R.N. Clark, R.H. Brown, and J.R. Spencer, "Observations of the Icy Galilean Satellites from 0.2 to 5 Microns: A Compilation, New Observations, and a Recent Summary," Journal of Geophysical Research 100: 19041, 1995.
16. D. Domingue and B. Hapke, "Disk Resolved Photometric Analysis of Europan Terrains," Icarus 99: 70, 1992.
17. A.L. Lane, R.M. Nelson, and D.L. Matson, "Evidence for Sulphur Implantation in Europa's UV Absorption Band," Nature 292: 38, 1981.
18. K.S. Noll, H.A. Weaver, and A.M. Connella, "The Albedo Spectrum of Europa from 2200 Å to 3300 Å," Journal of Geophysical Research 100: 19057, 1995.
19. J. Spencer et al., "CCD Spectra of the Galilean Satellites: Molecular Oxygen on Ganymede," Journal of Geophysical Research 100, 19049, 1995.
20. A. Hendrix et al., "Europa: Disk-Resolved Ultraviolet Measurements Using the Galileo Ultraviolet Spectrometer," Icarus 135: 79, 1998.
21. R.W. Carlson, "Hydrogen Peroxide on the Surface of Europa," Science 283: 2062, 1999.
22. R.W. Carlson, R.E. Johnson, and M.S. Anderson, "Sulfuric Acid and the Radiolytic Sulfur Cycle," Science 286: 97, 1999.
23. T.B. McCord, G. Hansen, F.P. Fanale, R.W. Carlson, D. Matson, T.V. Johnson, W. Smythe, J.K. Crowley, P.D. Martin, A. Ocampo, C.A. Hibbits, J.C. Granahan, and the NIMS Team, "Salts on Europa's Surface Detected by Galileo's Near Infrared Mapping Spectrometer," Science 280: 1242, 1998.
24. G. Schubert, T. Spohn, and R. Reynolds, "Thermal Histories, Compositions and Internal Structures of the Moons of the Solar System," in Satellites, J.A. Burns and M.S. Matthews, eds., University of Arizona Press, Tucson, Arizona, 1986, pages 224-292.
25. J.D. Anderson et al., "Europa's Differentiated Internal Structure: Inferences from Two Galileo Encounters," Science 276: 1236, 1997.
26. J.D. Anderson et al., "Europa's Differentiated Internal Structure: Inferences from Four Galileo Encounters, Science 281: 2019, 1998.
27. J.D. Anderson et al., "Europa's Differentiated Internal Structure: Inferences from Four Galileo Encounters, Science 281: 2019, 1998.
28. J.D. Anderson et al., "Europa's Differentiated Internal Structure: Inferences from Four Galileo Encounters, Science 281: 2019, 1998.
29. J.D. Anderson et al., "Europa's Differentiated Internal Structure: Inferences from Four Galileo Encounters, Science 281: 2019, 1998.
30. J.D. Anderson, W.L. Sjogren, and G. Schubert, "Galileo Gravity Results and the Internal Structure of Io," Science 272: 709, 1996.
31. M.G. Kivelson et al., "Europa and Callisto: Induced or Intrinsic Fields in a Periodically Varying Plasma Environment," Journal of Geophysical Research, submitted 1998.
32. G. Schubert, T. Spohn, and R. Reynolds, "Thermal Histories, Compositions and Internal Structures of the Moons of the Solar System," in Satellites, J.A. Burns and M.S. Matthews, eds., University of Arizona Press, Tucson, Arizona, 1986, pages 224-292.
33. See, for example, G.J. Consolmagno and J.S. Lewis, "Structural and Thermal Models of Icy Galilean Satellites," in Jupiter, T. Gehrels, ed., University of Arizona Press, Tucson, Arizona, 1976, page 1035.
34. R.T. Reynolds and P. Cassen, "On the Internal Structure of the Major Satellites of the Outer Planets," Geophysical Research Letters 6: 121, 1979.
35. P.M. Cassen, R.T. Reynolds, and S.J. Peale, "Is There Liquid Water on Europa?" Geophysical Research Letters 6: 731, 1979.
36. P.M. Cassen, S.J. Peale, and R.T. Reynolds, "Tidal Dissipation in Europa: A Correction," Geophysical Research Letters 7: 987, 1980.
37. G.W. Ojakangas and D.L. Stevenson, "Thermal State of an Ice Shell on Europa," Icarus 81: 220, 1989.
38. W.B. Durham, S.H. Kirby, and L.A. Stern, "Rheology of Planetary Ices," in Solar System Ices, B. Schmidt et al., eds., Kluwer Academic Publishers, Dordrecht, the Netherlands, 1998, pages 63-78.
39. M.N. Ross and G. Schubert, "Tidal Heating in an Internal Ocean Model of Europa," Nature 325: 133, 1987.
40. J.S. Kargel, "Brine Volcanism and the Interior Structures of Asteroids and Icy Satellites," Icarus 94: 368, 1991.
41. D.J. Stevenson, "Heterogeneous Tidal Deformation and Geysers on Europa," abstract, Europa Ocean Conference, San Juan Capistrano Research Institute, San Juan Capistrano, California, November 12-14, 1996.
42. C.F. Yoder and W.L. Sjogren, "Tides on Europa," abstract, Europa Ocean Conference, San Juan Capistrano Research Institute, San Juan Capistrano, California, November 12-14, 1996.
43. D.T. Hall et al., "Detection of an Oxygen Atmosphere on Jupiter's Moon Europa," Science 373: 677, 1995.
44. M.E. Brown and R.E. Hill, "Discovery of an Extended Sodium Atmosphere Around Europa," Nature 380: 229, 1996.
45. R.E. Johnson, "Sputtering of Ices in the Outer Solar System," Reviews of Modern Physics 68: 305, 1996.
46. A.J. Kliore et al., "The Ionsophere of Europa from Galileo Radio Occultations," Science 277: 355, 1997.
47. M.G. Kivelson et al., "Europa's Magnetic Signature: Report from Galileo's Pass on 19 December 1996," Science 276: 1239, 1996.
48. K.K. Khurana et al., "Induced Magnetic Fields as Evidence for Subsurface Oceans in Europa and Callisto," Nature 395: 777, 1998.
49. M.G. Kivelson et al., "Europa and Callisto: Induced or Intrinsic Fields in a Periodically Varying Plasma Environment," Journal of Geophysical Research, submitted 1998.
50. K.K. Khurana et al., "Induced Magnetic Fields as Evidence for Subsurface Oceans in Europa and Callisto," Nature 395: 777, 1998.
51. M.G. Kivelson et al., "Europa and Callisto: Induced or Intrinsic Fields in a Periodically Varying Plasma Environment," Journal of Geophysical Research, submitted 1998.
52. M.G. Kivelson et al., "Europa and Callisto: Induced or Intrinsic Fields in a Periodically Varying Plasma Environment," Journal of Geophysical Research, submitted 1998.
53. C.R. Woese, "Bacterial Evolution," Microbiology Review 51: 221, 1987.
54. R. Pace, "A Molecular View of Microbial Diversity and the Biosphere," Science 276: 734, 1997.
55. K.O. Stetter, "Hyperthermophilic Procaryotes," FEMS Microbiology Review 18: 149, 1996.
56. J.A. Baross, "Do the Geological and Geochemical Records of the Early Earth Support the Prediction of Global Phylogenetic Models of a Thermophilic Cenancestor?" in Thermophiles: The Keys to Molecular Evolution and the Origin of Life?, J. Wiegel and M.W.W. Adams, eds., Taylor and Francis, 1998, pages 1-18.
57. E.L. Shock and M.D. Schulte, "Organic Synthesis During Fluid Mixing in Hydrothermal Systems," Journal of Geophysical Research 103: 28513, 1998.
58. E.L. Shock, T. McCollom, and M.D. Schulte, "The Emergence of Metabolism from Within Hydrothermal Systems," in Thermophiles: The Keys to Molecular Evolution and the Origin of Life?, J. Wiegel and M.W.W. Adams, eds., Taylor and Francis, 1998, pages 59-76.
59. E.L. Shock, "Geochemical Constraints on the Origin of Organic Compounds in Hydrothermal Systems," Origins of Life and the Evolution of the Biosphere 20: 331, 1990.
60. B.M. Jakosky and E.L. Shock, "The Biological Potential of Mars, the Early Earth, and Europa," Journal of Geophysical Research 103: 19359, 1998.
61. J.R. Leadbetter, T.M. Schmidt, J.R. Graber, and J.A. Breznak, "Acetogenesis from H2 Plus CO2 by Spirochetes from Termite Guts," Science 283: 686, 1999.
62. E.J. Gaidos, K.H. Nealson, and J.L. Kirschvink, "Life in Ice-Covered Oceans," Science 284: 1631, 1999.
63. T.M. McCollom, "Methanogenesis as a Potential Source of Chemical Energy for Primary Biomass Production by Autotrophic Organisms in Hydrothermal Systems on Europa," Journal of Geophysical Research, 1999, in press.
64. M.H. Carr et al., "Evidence for a Subsurface Ocean on Europa," Nature 391: 363, 1998.
65. K.K. Khurana et al., "Induced Magnetic Fields as Evidence for Subsurface Oceans in Europa and Callisto," Nature 395: 777, 1998.
66. M.G. Kivelson et al., "Europa and Callisto: Induced or Intrinsic Fields in a Periodically Varying Plasma Environment," Journal of Geophysical Research, submitted 1998.


















