4
Earth-based Studies and Technology Development
In addition to in situ and remote-sensing measurements of Europa and its environment that must be made from spacecraft at or near Europa, studies that can be done on Earth or from Earth-orbiting telescopes will be essential for understanding the overall nature of Europa. These terrestrial studies include field studies of Earth analogs to Europa's environment, laboratory studies of processes that might occur on Europa, theoretical analyses of Europa's physical processes and of their ramifications for its interior, and development of new technology that will allow us to explore Europa. These issues are discussed in this chapter.
TERRESTRIAL FIELD AND REMOTE-SENSING STUDIES
Earth's ice cover is distributed in glaciers, ice caps, and ice sheets that are found from pole to pole. From the thickest and coldest ice deep within the Antarctic Ice Sheet, to the seasonal and thermodynamically active ice cover of the polar oceans, the structural and physical properties of Earth's ice cover are highly variable and can be very complex. These different environments can provide challenging places to test instrument design and data processing and analysis concepts for sounding Europa's icy shell.
Terrestrial Analogs
Below, COMPLEX summarizes categories of terrestrial ice cover that may prove suitable for proof-of-concept studies for exploring Europa-like environments. Examples include polar and temperate glaciers and ice sheets and ice resting on either bedrock or liquid water. A range of terrestrial analogs will be needed for proof-of-concept studies that emphasize technologies, measurement techniques, and analysis methods planned for Europa missions. It is useful to keep in mind that the process of conducting proof-of-concept studies may lead to new techniques and information for studying Earth's own ice cover. This potential argues for a strong programmatic linkage between the terrestrial and planetary science communities in a joint Europa venture.
Temperate Glaciers and Ice Caps
Temperate glaciers contain ice that is everywhere near the melting point. Free water may be present in inclusions that are centimeters to tens of centimeters in size. The inclusions may eventually connect to form
drainage channels within the glacier. The glacier itself may be several hundreds of meters thick; its structure may be further complicated by the presence of medial moraines, composed of rocky debris that snake across many valley glaciers. The moraine patterns are good indicators of past dynamical instabilities.
Extensive geophysical studies have been conducted on temperate glaciers in the Pacific Northwest such as the Blue and Columbia Glaciers. The Bering and Malaspina Glaciers, located along the Gulf of Alaska, are examples of surging glaciers with highly crevassed surfaces, complex subglacial hydrology, and surface and internal moraines. Each has been intensively studied with surface, airborne, and spaceborne remote-sensing techniques.
Polar Ice Sheets
Greenland and Antarctica are blanketed by the last of Earth's great ice sheets. Continental in size, the ice sheets are characterized by complex dynamics driven in part by external climate forcing and by spatial and temporal variations at the glacier bed and at internal boundaries. The dynamical processes manifest themselves on the ice sheet surface by the presence of exotic structures such as ice streams. These are rivers of ice within the ice sheet, hundreds of kilometers long, that discharge ice from the interior ice sheet toward the floating ice shelves and eventually to sea. The margins of ice streams are heavily crevassed and are strong targets for microwave radar, and they can effectively attenuate signals from high-frequency radar (Figure 4.1).
Antarctic ice streams ride over a bed lubricated by subglacial water. The nature of the bed enables the ice streams to move at speeds of several hundreds of meters per year, whereas nearby ice frozen to the bed may move at speeds of only tens of meters per year. Greenland ice streams (such as the Jacobshavn Glacier) apparently flow via the deformation of a basal layer of relatively warm ice — the combination of warm basal ice and the presence of extensive surface crevassing makes Jacobshavn one of the last important glaciers to resist detailed sounding of the glacier bed.
Ice sheets preserve an important stratigraphic record of past changes in climate and dynamics. The record takes the form of vertical and horizontal gradients in density, temperature, crystal size, crystalline fabric, impurity content, and deformation rate. Local vertical variations in these properties can lead to stratigraphic horizons that are detectable and apparently continuous for more than hundreds of kilometers (Figure 4.2).
Polar Ice Shelves
Ice shelves are enormous slabs of floating ice that are fed by a combination of ice flow from the interior ice sheet and accumulation on the ice sheet surface. The largest ice shelves are found in Antarctica. Both the Ross and Filchner-Ronne Ice Shelves are about the size of Texas. Ice thickness ranges from about 800 m near the grounding line to about 250 m near the calving margin. Water-layer thickness beneath the ice shelves varies from a few meters near the grounding line to hundreds of meters.
A few ice-shelf-like environments have been identified recently in northern Greenland. For example, Peterman Glacier occupies a long fjord. Much of the length of Peterman Glacier is floating on ocean water that fills the fjord. Pockets of water upstream of the grounding line are also believed to exist based on the strength of radar returns from deep subglacial valleys (Figure 4.3).
The interior structure of ice shelves can be complex. Moraine material deposited on the surface of East Antarctica's outlet glaciers, for example, is carried downstream and buried, only to show up as a strong scattering layer in radio-echo sounding data. Rifts through the ice shelf can form near grounding lines or around ice rises. Upwelling brine is forced horizontally through lower-density firn (i.e., granular ice formed by the recrystallization of snow) near the surface, forming a layer nearly opaque to radar. These brine layers are carried downstream and can completely obscure the ice bottom from radar. Bottom and surface crevasses can tear through a significant thickness of ice. Once identified, the crevasses can be useful indicators of the stress regime within the ice shelf (Figure 4.4).
Ice-thickness gradients of the ice shelf and currents within the subglacial ocean can plate large thicknesses of sea ice onto the base of the ice shelf. Direct measurement has shown a 6-m-thick layer of briny sea ice on the bottom of the southeastern Ross Ice Shelf. Several hundred meters of sea ice are believed to be accreted onto the
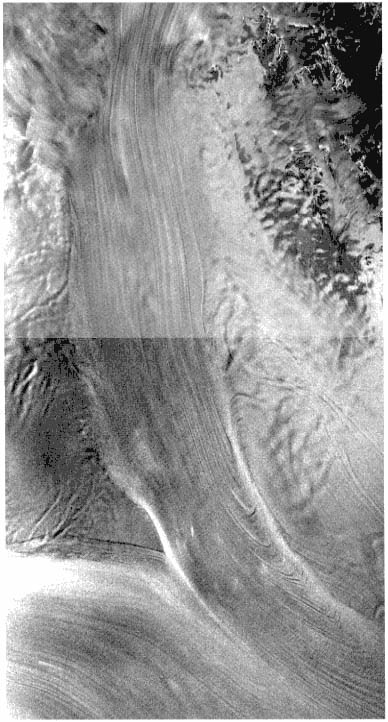
FIGURE 4.1 This synthetic-aperture radar image of the confluence of West Antarctic Ice Streams A and B was obtained by the Radarsat-1 spacecraft. Ice Stream A appears vertically oriented in this image. The area imaged is about 100 km wide, and north is roughly toward the bottom. Streaming flow is evidenced by the regular patterns of flow stripes and the bright shear margins. Arc-shaped features on the right flank of Ice Stream A are suggestive of non-steady flow. Image courtesy of K.C. Jezek.
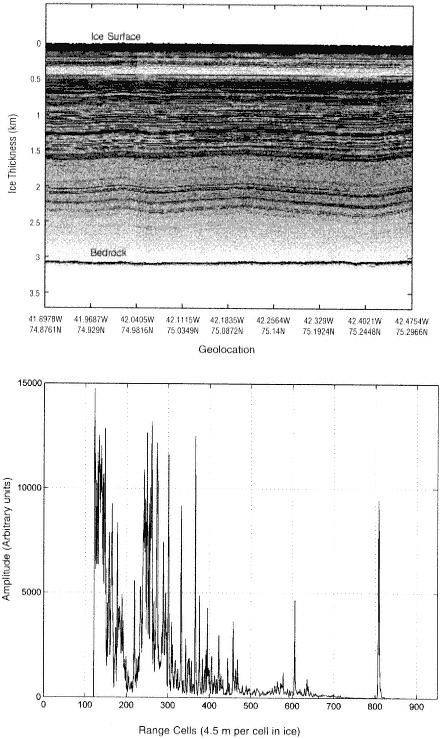
FIGURE 4.2 Radio-echo sounding of North Central Greenland. Internal layers and the ice bottom are illustrated by the radar profile shown in the upper panel. A single echogram is shown in the lower panel. (From S. Gogineni et al., "An Improved Coherent Radar Depth Sounder," Journal of Glaciology 44: 659, 1998.)
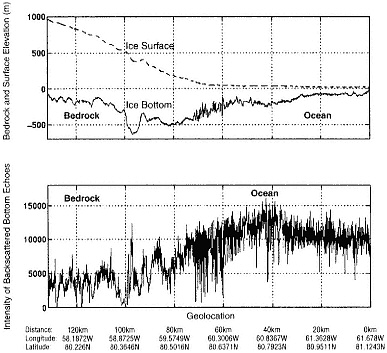
FIGURE 4.3 A radar profile of the Peterman Glacier in northeastern Greenland. The ice goes afloat at approximately 70 km inland from the calving front. This is demonstrated by the increased intensity associated with the glacier's transition from a rocky bed to ocean (lower panel). It is also demonstrated by the sequence of rapid changes in ice thickness which are, in fact, bottom crevasses opened in the presence of seawater. The data illustrate how radar measurements of ice structure and basal reflectivity can be used to infer information about the glacier bed. (From S. Gogineni et al., ''An Improved Coherent Radar Depth Sounder," Journal of Glaciology 44: 659, 1998.)
base of the central Filchner-Ronne Ice Shelf. The brine in the sea ice is a strong internal reflector of radio frequency energy and a strong absorber of energy that enters the sea ice. Consequently, the sea ice has resulted in the misinterpretation of Filchner-Ronne ice thicknesses.
The combination of relatively thick fresh ice, the presence of a basal saline ice layer, and an underlying ocean ecosystem suggest that ice shelves are attractive sites for proof-of-concept studies. Results from previous borehole campaigns on Antarctic ice shelves can be used as direct validation of results acquired from Europa-like systems.
Lake Vostok and Other Subglacial Lakes
Lake Vostok, discovered during radio-echo sounding campaigns over Antarctica in 1974-1975, is one of many bodies of water buried under the Antarctic Ice Sheet.1,2 Located in East Antarctica, Lake Vostok is the largest subglacial lake so far discovered. The lake itself is probably composed of fresh water and may be 250 m or more deep (Figure 4.5).
Exploration of Lake Vostok offers a unique opportunity to develop and test new technologies for the exploration of Europa. Similarities in the requirements for the characterization of Lake Vostok and a europan ocean include the following:
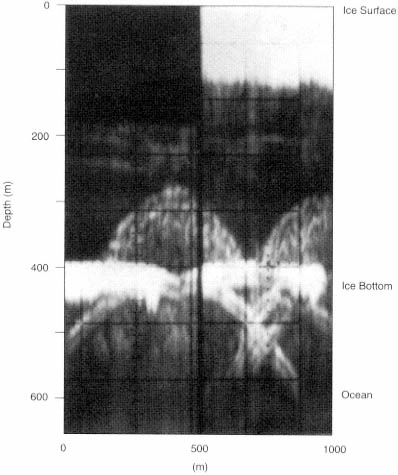
FIGURE 4.4 Radar diffraction hyperbolae from bottom crevasses are shown here in these results from high-frequency radio-echo sounding measurements at a location in the interior of the Ross Ice Shelf in Antarctica. The top of the 1-km-wide image represents the surface of the ice shelf, and the thick horizontal band in the lower center of the image is the ice bottom. The ice is about 420 m thick and is floating over some 200 m of ocean water. Crevasses, formed by strong extensional stresses, penetrate up approximately 120 m from the bottom of the ice shelf. These bottom crevasses appear as diffraction hyperbolae located in the center and right-hand side of this image. The basal edges of the roughly 100-m-wide crevasses also appear as hyperbolae with vertices near the ice-bottom echo. Note the missing data in the upper right-hand corner of this plot. (From K.C. Jezek, C.R. Bentley, and J.W. Clough, "Electromagnetic Sounding of Bottom Crevasses on the Ross Ice Shelf," Journal of Glaciology 24: 321, 1979.)
-
Potential for use of remote sensing (radar) to determine the extent of water;
-
Requirement for physical penetration of a considerable thickness of ice to access the water;
-
Application of in situ measurements to determine the chemical and physical properties of the aqueous environment; and
-
Possible presence of microbiological organisms and the need to practice appropriate planetary protection policies and procedures (lest investigators contaminate the lake, in this case, with surface organisms).
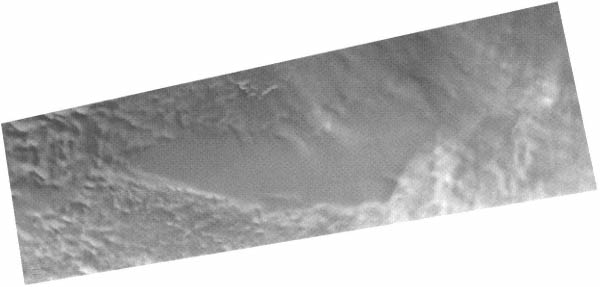
FIGURE 4.5 This mosaic of synthetic-aperture radar images from Radarsat-1 shows the surface expression of subglacial Lake Vostok. Located in East Antarctica, this lake is buried beneath almost 4000 m of ice and is roughly the size of Lake Ontario. Image courtesy of K.C. Jezek.
These similarities make the exploration of Lake Vostok an invaluable tool for evaluating and testing techniques for exploring physically buried bodies of water such as might be found on Europa. In situ analysis of the aqueous environment might be accomplished using a cryobot/hydrobot,3,4 a development of the so-called thermal or Philberth probes developed more than 30 years ago for polar and glacial studies on Earth.5 Appropriate measurements to be made of the water would include salinity, pressure exerted by the overlying ice, light-scattering properties, and acidity. Temperature profiling will allow a search for geothermal inputs, which are thought to be critically important for long-lived biotic systems functioning in isolation from sunlight. Measurement of chemical gradients in key redox compounds have proven to be good indicators of functioning ecosystems. Such stratified chemical fingerprints would include oxygen, hydrogen, methane, carbon dioxide, nitrogen and sulfur compounds, and dissolved iron and manganese. The presence of various classes of organic compounds, and even microorganisms themselves, might be determined using fluorescence spectroscopy.
A desired analysis strategy would include measurements made at various depths in the water column and in the bottom sediments (although it is unclear whether an ocean on Europa would be floored by sediments, or even whether the ocean floor could be reached). It would also be desirable to be able to make measurements in Lake Vostok after stirring the bottom waters and/or sediments to monitor possible effects of anaerobic activity. Finally, it would be useful to operate the hydrobot on the sediment interface for an extended time, to monitor changes in variables over time that would result from biological activity.
The above in situ experiments could demonstrate that even unknown biological systems might be capable of being discovered and studied using relatively simple chemical and physical measurements. Moreover, characterization of the habitability of Lake Vostok would be as useful for testing strategies for exploring a completely foreign ecosystem (including appropriate planetary protection issues) as for testing the required technology. Environments to be evaluated include ancient systems with extinct biota, contemporary functioning ecosystems, and "extremophile" populations in the overlying ice. All activities relating to the exploration of Lake Vostok and other pristine environments should be conducted only within the framework of an appropriate awareness of the ecological sensitivity of such endeavors.
Ice-Probing Technology Development and Demonstration
Radar sounding can provide high-resolution, global mapping of the internal structure of Europa's ice shell. Given knowledge about Europa's depth-varying dielectric properties, ice thicknesses could be estimated to better than several hundred meters using a system operating between 1 and 100 MHz. A source of uncertainty in the interpretation of radar data is the lack of information about the dielectric properties of the ice that are needed for estimating both propagation velocities and attenuation. An estimate of the thickness of Europa's ice accurate to 10% is not possible unless the ice's average bulk dielectric properties are known to the same degree of certainty.
Within terrestrial glaciers, and presumably on the europan surface ice, there will be physical structures that can scatter radar energy. These can take the form of a surface roughened by physical stress, compositional variations associated with the presence of rocky debris or salt deposits, or even meteoritical debris deposited onto the surface. Moreover, because radar is a ranging instrument, energy from targets distributed at different locations, but at the same electrical range, are unresolvable with a simple radar. It is particularly challenging to separate clutter owing to the surface-illuminated footprint of the radar from weaker signals reflected from a deeper ice-water interface. Indeed, ice-sounding radars have not been successfully used from orbit because of the clutter-separation problem. Clutter rejection algorithms must be tested over complicated terrain (such as Jacobshavn Glacier, Greenland) as part of proof-of-concept studies.
Exploration of Life in Extreme Environments
Earth-based field studies of life in extreme environments have identified deep subsurface organisms that thrive in the dark on chemical reactions, organisms that conduct redox reactions in hydrothermal systems at temperatures in excess of 100° C and that are energetically independent of photosynthesis, and organisms that live in ice or rock or in thin films of liquid water stabilized below 0° C. All of these discoveries of extremophiles contribute to the notion that life may be possible on Europa.6 As more is learned about life in all of these extreme environments, the results will continue to inform ideas about whether there is or can be life on Europa. Progress in studying these environments is likely to be brisk now that the National Science Foundation has initiated the Life in Extreme Environments (LExEn) program and NASA has established its Astrobiology Institute.
Life is found in an enormous diversity of surface and deep environments on Earth, many of which were long thought to be sterile.7 It now appears that life may be present anywhere on Earth that there is a source of energy, and, at least occasionally, liquid water. In recent years microorganisms have been found living in the ice at both poles,8 at the bottom of the deepest oceanic trenches, in continental hot springs, in submarine hydrothermal systems where elevated pressure keeps water from boiling at 100° C,9 at pH values from 0.4 to 12, in brines and saturated salt solutions,10 within rocks at the surface, and deep in the continental crust (up to several kilometers deep) and oceanic crust (to a depth of at least 700 m).11 As a result, it is currently known that life thrives at temperatures ranging from several degrees below 0° C to nearly 115° C, and at pressures up to at least 1000 bars. Several investigators expect to find life at temperatures up to 150° C or so, and at several kilobars pressure. 12,13
Both high-and low-temperature environments on Earth provide analogs for what may be possible on Europa. The potential for life in plausible environments on either planetary body can be tested by assessing the forms of available energy, the quantity of energy, and the extent to which that energy can be focused. An enormous contribution to this effort can be made by quantifying the availability of chemical energy forms in extreme terrestrial environments that support life.
Much of the effort to quantify energy sources could profitably focus on autotrophs, organisms that capture energy from the environment to generate organic compounds from inorganic forms of carbon, nitrogen, sulfur, phosphorus, and other elements. In the case of chemoautotrophs (organisms that gain their energy from chemical reactions), recent progress has been made in quantifying the geochemical energy available in terrestrial environments if an organism can mediate reactions and take advantage of this energy.14,15,16 Recent progress provides the ability to calculate the thermodynamic data at almost any temperature and pressure of interest.17,18,19,20,21 What is generally lacking are adequate analytical data appropriate to the environment of interest.
Carbon, sulfur, nitrogen, iron, and other redox-sensitive elements exist in more than one oxidation state, and
these various forms can be present at concentrations that are far from chemical equilibrium. As an example, H2S in contact with Earth's O2- rich atmosphere is unstable and should react to form sulfate. If the reaction is slow, as this oxidation reaction is at low temperatures, then coexisting H2S and O2 represent food to a sulfide-oxidizing microorganism. At high temperatures, terrestrial geochemical constraints necessitate that most energy-yielding reactions are reduction reactions, like the formation of CH4 from bicarbonate and H2 in submarine hydrothermal systems.22 In any event, the thermodynamic calculations require analytical data on iron, sulfur, carbon, nitrogen, and other elements that can exist in variable redox states.
Quantitative inventories of available energy in terrestrial environments can be complemented with laboratory studies of the energetic demands of microorganisms. It will then be possible to answer questions about the amount of biomass that can be supported by a geochemical process in a surface or subsurface environment.23 At present, microbial growth experiments on extremophiles focus on optimum temperatures, and ranges of salinity, pH, and temperature, at which an organism can live. Missing from this approach is an assessment of the amount of energy provided by chemical reactions that is required to grow and maintain a given cell concentration. For example, although we know that Methanococcus jannaschii is an autotrophic methanogen living optimally at 85° C in submarine hydrothermal systems, we do not know how many cells of this organism can be supported on the disequilibrium between hydrogen and bicarbonate present in the natural system.
In addition, little is known about the energy requirements of the metabolic pathways used by chemolitho-autotrophs and other extremophiles at the actual temperatures and pressures where they live. Does a hyperthermophile require more or less energy to make peptide bonds than is required by a low-temperature organism? What is the energy yield of ATP hydrolysis at the high temperatures and pressures encountered deep in hydrothermal systems? How do the redox potentials of important biochemical processes change with temperature and pressure? Although hints about the answers to these questions are currently available, more definitive results could help identify likely metabolic strategies used by novel organisms in unfamiliar environments.
A unifying theme of energy availability and energy demand, if quantified, would enable transport of microbial growth data from the laboratory to the study of natural environments. It would then be possible to test whether a given geochemical process can yield enough energy to support a given metabolic strategy, and, if it can, how many organisms can be supported. These studies would permit quantitative estimates of the potential for life on Europa or in terrestrial environments that might be close analogs to conditions on Europa.
LABORATORY STUDIES
Space Weathering of the Surface Materials
There is considerable observational evidence that it is necessary to understand space weathering in order to interpret reflectance properties and ages of surface features on Europa. The surface materials are weathered by plasma ions and electrons from the jovian plasma torus, solar ultraviolet photons, and micrometeorite bombardment, all occurring in the presence of a tenuous oxygen atmosphere that is itself a product of ion bombardment. These are processes that also occur on the surfaces of other objects that have tenuous or no atmospheres. Whereas laboratory studies directed toward lunar materials are extensive, however, the materials of interest for Europa (ices, salts, and organics) have been little studied. The principal need is data on irradiation effects — plasma and ultraviolet weathering of these materials. The types of data required pertain to the effects of weathering on the production of gas-phase molecules (sputtering and decomposition), changes in reflectance, and chemical alterations induced in the surface. These effects are clearly interrelated. Because of the detection of S (as SO2) and Na, the chemistry of an H2O-S-Na system subjected to a radiation environment needs to be understood.
In the last 10 years there has been a considerable effort to understand the ion bombardment of water ice. This process results in redistribution of H2O molecules across the surface of Europa and also in the production of O2 that contributes to Europa's atmosphere and H2 that escapes into space. These data, along with those for the sputtering and chemistry of other frozen volatiles of potential importance (e.g., SO2 and CO2), have been summarized in Solar System Ices.24 However, there is still a dearth of data on the spectral changes linked to irradiation of these ices, including the absorbance properties produced by implantation of S and Na ions from the jovian torus
into water ice, which are especially relevant to Europa. Careful laboratory measurements are required to allow derivation of ages of surface materials from absorption band depths and ion fluxes.
Over the same period, data on the effects of irradiation on organic materials have increased, in part motivated by space science studies, but also owing to the fact that heavy energetic-ion bombardment ejects whole molecules into the gas phase, a process of interest in the study of biomolecules. However, key pieces of data are missing. Surprisingly, there is much more data on the spectral properties of irradiated organics than there are absolute yields for small-molecule production (e.g., CO2, HCN, and HCO) due to decomposition induced by ion bombardment. Such data are key to understanding the ambient gas and local plasma that can, in principle, be detected either by the Hubble Space Telescope or by a mass spectrometer on an orbiter. A key issue is to identify decomposition products that are indicative of prebiotic materials brought to the surface. In addition, because the dominant sputtering agents at Europa are the energetic heavy ions (~100 keV S+ and O+), large fragments or whole molecules can be ejected. However, measurements of absolute yields of these large molecules and/or fragments are needed.
The most urgent need is data on the sputtering and irradiation alteration of salts and hydrated minerals. The presence of materials such as magnesium and sodium sulfate or carbonate hydrates is suggested by modeling and observations by Galileo's NIMS. Although it is known that bombardment will lead to ejection of H2O from these hydrated minerals and Na is a principal product of Na-containing minerals, other ejecta are less certain. In an oxidizing environment, SO2 from the sulfates and CO2 from carbonates might be expected as decomposition products. A longitudinally correlated dark stain seen in images of Europa is inferred to be due to polymerized sulfur, but in high-resolution images the younger linea are darker and seem to lighten with age. Therefore experiments on the alteration of minerals by irradiation are critical for understanding how the effects of sulfur implanted by the plasma compete with the effects of sulfates created by sputtering of tectonically emplaced subsurface materials. Finally, also, almost no data exists on isotopic effects in radiation-induced alterations.
Rheological Properties of Impure Water and Ice
Abundant theories exist to explain Europa's obvious differentiation and the subsequent tectonic mobility of its surface. Extensive studies on the viscosity, density, and shear strength of pure water ice have been conducted.25 However, Galileo's images have established cross-cutting relationships that suggest that the most recent, upwelling material may not be pure ice but is, rather, rich in sulfur, sulfur-bearing minerals, or other dark, red materials, based on the visual albedo. Galileo has resolved linear features and lineaments that are strings of splotches, as well as larger patches of mottled terrain, all of which show that surface tectonism is widely variable in both style and color, with an implied concomitant compositional heterogeneity.
Some theoretical and laboratory work has been done on the MgSO4-H2 O system that is applicable to Europa.26,27 However, early theoretical predictions that Europa's ice crust should contain abundant sulfates seem to have been countered by subsequent measurements suggesting that, in the MgSO4-H2O system, pure water ice nucleates first and this pure ice should be more buoyant than the solution. Condensed sulfate solids should "sink" within the solution, so it remains enigmatic how such impure systems become sufficiently unstable gravitationally to emerge in the observed dark lineaments on the surface. It may be that systems akin to terrestrial sea ice are a better Europa model, where nearly pure water ice forms a crust on the saline "ocean," increasing the salinity of the underlying liquid in the process. Local circulation controls the temperature and hence the stratification of this layer, and tidal flexure or circulation in the underlying liquid creates cracks through which the saline solutions appear. Alternately, localized heating from the rocky core may create "hot spots" that are then convectively unstable and push to the surface to create the more lobate or splotchy forms seen in Galileo images. Surface weathering processes then erode or redistribute the emergent material. In all likelihood a combination of scenarios is required to explain the diversity of geologic forms observed on Europa.
Clearly more observational work on impure ice systems at europan temperatures is required to help assess whether Europa's crust is stratified either as a solid/liquid or as compositional stratification in solid and/or possibly liquid layers. In addition to the MgSO4-H 2O system, observations of both sulfur-and silicate-H2O systems are important. Other relevant properties that have not been determined include the effects on strength properties of the
inclusion of various amounts of impurities — e.g., is a salty ice-block crust more susceptible than pure ice to tidal flexure and heating? Would such a system preserve an interior "ocean" to the present day, or are the disturbed terrains only frozen-in remnants from a tectonically active period earlier in the satellite's history?
Geochemical Processes That Influence the Composition of a Potential Ocean
Compounds can reach oceans on Europa in essentially two ways: melting or overturning of the overlying and surrounding ice, or reaction of water with the underlying rock. Besides water and salts (or other compounds brought out from the interior during cryovolcanism), ice melting and overturning would deliver the other volatile constituents of the ice (such as NH3, CO2, and organic compounds) to the oceans, along with their alteration products resulting from sputtering processes or photochemistry. In addition, the ice would also contain embedded ions responsible for the sputtering processes (largely S+, O+, and H+ from Io and/or Jupiter), as well as dust, meteorites, and other exogenous material. Water-rock reactions will contribute soluble compounds to the solution and are likely to strongly influence the pH, oxidation state, and concentrations of the major ions. Depending on the temperature and oxidation state of the water-rock system, there may be a potential for hydrothermal organic synthesis.
Sputtering experiments on ice-salt mixtures that could be appropriate to the study of the surface of Europa are lacking. Nevertheless, there are speculations that sputtering of sodium and magnesium sulfates in ice could produce compounds like NaOH and H2SO4, either of which could affect the pH if the ice were to melt. Redox conditions in the melt would be affected if potential sputtering products like SO2, MgS, or O2 were released to the solution. In addition, sputtering of organic compounds in ice from exogenous or endogenous sources may lead to ejection of small molecules like CO or HCN, and to cross-linking reactions in more refractory residues. Sputtering of hydrocarbons can lead to the production of H2, CH4, larger molecules, and a carbonized residue,28 and experiments on other organic compounds have yielded CO, CO2, HCN, HCO, other fragments, larger molecules, and carbonized residue.29,30,31 The consequences of hydration reactions in the molten equivalents of these ices are unexplored.
Laboratory simulations of water-rock reactions appropriate to conditions on Europa are lacking. The suggestion that water on Europa would be a sulfate-rich solution if the composition of the rock were like that of carbonaceous chondrites may be corroborated once better resolution of NIMS spectra is achieved.32,33 These measurements also suggest the presence of natron or other carbonate minerals in the ice of Europa. Experiments are needed that test the production of salt solutions during heating, dehydration, and decarbonation of model compositions for the interior of Europa.
The presence of sulfur as sulfate may require that the water-rock reactions occur at relatively low temperatures. As temperature increases, water-rock reactions should be capable of reduction of the sulfate. The range of temperatures at which sulfate reduction can occur depends largely on the composition of the rock, and to a lesser extent on the water-to-rock ratio. For example, calculations of water-rock reactions using the Murchison meteorite's bulk composition show that sulfate reduction is possible above about 100° C at low water-to-rock ratios.34 However, the rates of sulfate reduction may be extremely slow at low temperatures even if they are strongly favored by thermodynamics. Although many hydrothermal sulfur redox experiments have been conducted in terrestrial systems, experiments designed to represent processes that can occur in icy satellites are lacking.
THEORETICAL STUDIES
Thermal History of Europa
Previous studies of Europa's thermal history argue convincingly that enough heat is generated by radioactive decay in the rocks inside Europa that separation of rock and metal from water must have occurred early in the evolution of the satellite. There is also enough energy from radiogenic heating for early differentiation of a metal core from the silicate-metal mixture. The present structure of metal core, rock mantle, and water outer shell must
have been set early in the thermal history of Europa. Further thermal history modeling is needed, however, to assess whether Europa's core could have remained liquid and convective to the present and whether the outer water shell could have been prevented from completely freezing. Models that have already addressed the issue of freezing of the outer water shell have been equivocal in their results and have oversimplified or neglected important aspects of the problem.
The thermal history of Europa is, firstly, not only a thermal history but also a dynamical history. The two evolutionary aspects are coupled in an essential way because of the potential importance of tidal heating in the evolution of Europa. The dynamical problem involves not only Europa, but Io and Ganymede as well because of the dynamical resonance these bodies are in today — the Laplace resonance — and the possible role of other resonances in the past. The coupled dynamical evolution of these satellites depends on the tidal dissipative heating in all the satellites, particularly Io, so the thermal evolution of all the satellites is tied together. Researchers must therefore calculate the coupled thermal and dynamical evolution of Io, Europa, and Ganymede as a single system. Still other nonlinear couplings and feedbacks contribute to the challenge of modeling the thermal-dynamical history of the system through the dependence of the dissipative heating rates on the temperature-dependent and deformation-dependent rheologies of the satellites and their dynamically dependent and rheologically dependent deformations. The problem is a formidable one, and only isolated aspects of the evolutionary calculation have been attempted so far. The solution will require code development and significant computational resources by today's standards. Adding to the difficulties is the uncertainty in the relevant rheological behaviors and the parameter values of potentially applicable constitutive laws. The numerical code will have to be extremely efficient to allow the exploration of broad ranges of parameter space.
Theoretical Exploration of the Biological Potential of Europa
Theoretical studies can constrain the abundance of energy available on Europa for life, once some additional compositional data are available. Only the most general tests are possible with the observational data currently available, even when augmented with results of condensation models for the jovian nebula. As compositional and geophysical data from Europa missions become available, the results of theoretical models may be considerably different than at present.
Europa's volatile budget depends on the kinetics in the protojovian nebula. If hydration of silicates occurred in this nebula,35 then Europa's ice layer/ocean may be a product of the dehydration of the silicate interior. Otherwise, Europa's ice may have condensed directly from the protojovian nebula, either locally or in more distant, cooler regions, and then been scattered inward gravitationally. One particular set of thermochemical models of the composition of ice in the protojovian nebula suggests that melting europan ice could yield an aqueous solution with abundant NH3, together with traces of HCN and HCO3-.36 This assumes maximum radial mixing in the nebula, and that such mixing may have extended as far in as Europa at some stage of condensation. This solution composition is relatively depleted in carbon, but it is unlikely that Europa accreted without at least a few percent nonvolatile carbon phases, as in carbonaceous chondrites. During initial heating and dehydration of Europa's silicate interior, and subsequent volcanism, this carbon would most likely have been driven out as CO2 (similar to the case of Triton), 37 and the europan ocean has been estimated to contain as much as ~0.3 mole % CO2.38 Greater CO2 concentrations would result in plating out of CO2-clathrate at the base of the ocean, which if sufficiently thick might seal the ocean from hydrothermal interaction.
Results of theoretical studies of water-rock reactions show that the single most important factor in determining the potential for hydrothermal organic synthesis is the oxidation state imposed by the composition of the rocks that host hydrothermal systems.39 It follows that rigorous constraints on the compositions of the outermost layer of silicates, and their influence on the compositions of hydrothermal fluids, are major determinants in testing the potential for hydrothermal organic synthesis on Europa. Preliminary results suggest that hydrothermal systems on Europa are likely to generate altered rocks that have many similarities with altered basalts in the oceanic crust of Earth. 40 On the other hand, the fluid compositions, which may be extremely reduced or have highly alkaline pH, may be dramatically different from those in black smokers (i.e., terrestrial submarine hydrothermal vents). In addition, if the potential for organic synthesis during hydrothermal alteration is considerable it may have implica
tions for the composition and physical properties of the lower-most layers of ice, as well as the possibility that living systems could emerge in the dark using chemical energy supplied by the disequilibrium between hydrothermal fluids and molten ice.
Although essential, characterization of geophysical, petrological, and geochemical properties is not sufficient to reveal the difference between a hydrothermal system and a hydrothermal ecosystem. The possibility that a hydrothermal system can support life depends on how well it can meet the demands that life places on it. Is the supply of carbon sufficient for biosynthesis? Are nutrients available at useful concentrations? Is sufficient energy available in usable forms? As mentioned above, the energetic requirements of hyperthermophilic organisms are largely unknown. Given sufficient analytical data, it is possible to evaluate the geochemical energy available from various inorganic reactions used by autotrophs in terrestrial hydrothermal ecosystems. As an example, it is now possible to determine the amount of energy that can be obtained by a methanogen in a seafloor hydrothermal system at the precise temperature and pressure at which it lives. However, we do not know why this is enough energy, because the energetics of many biochemical reactions and metabolic processes have not been studied at elevated temperatures and pressures.
Dynamics of Ice in the Europan Environment
Terrestrial ice sheets deform under the force of gravity in a fashion determined by boundary conditions at the sides and bottom. On short time scales, ice behaves elastically and, for large stresses, ice undergoes brittle fracture. For low stresses, operating over long times, ice undergoes creep deformation.
The terrestrial sea-ice pack moves under the influences of ocean currents and winds that frequently exert forces strong enough to fracture individual floes. The effective rheology of the polar ice pack is governed primarily by consequent macroscopic processes such as ridging, rafting, and lead formation rather than the intrinsic rheology of the sea ice itself.
The surface of Europa seems to be composed of features suggestive of icebergs locked in a sea-ice matrix, block rotation and displacements, brittle fracture, and, perhaps, creep deformation as evidenced by flow-stripe-like structures on the surface of some blocks. Consequently, and perhaps at different times, the surface of Europa may have behaved somewhat like terrestrial ice sheets and sea ice cover. More enigmatic are the banded ridges running for hundreds of kilometers across the surface. These features, more reminiscent of terrestrial tectonic processes, have no apparent analog in the terrestrial ice environment and may be related to the tidal stresses exerted on Europa by Jupiter.
Observations such as these lead to several questions that could be explored by modeling of ice dynamics. These include the following:
-
What long-term processes might lead to creep deformation?
-
Where and how did the ice sheet fracture, and did fracturing result in an upwelling of liquid water?
-
What forces caused rotation of the ice blocks? and
-
Is convection possible within the ice sheet?
TELESCOPIC OBSERVATIONS
Earth-based telescopic observations have played an important role in advancing current understanding of Europa (see Chapters 2 and 3). The key advantages of future Earth-based (as opposed to spacecraft) data collection are the ability to look for long-term (yearly or decadal) variability, the capability of using very-high-spectral-resolution spectroscopy, and the advantages afforded by telescopes with large apertures.
At ultraviolet wavelengths, the Hubble Space Telescope (HST) will continue to provide high-resolution spectral capability, along with moderate spatial resolution. These are the most important for studies of the atmospheric composition and the morphology of the gas distribution. Detection of magnesium, for example, could rule out Io as a source of Europa's tenuous atmospheric constituents. Additional HST observations of the oxygen atmosphere could provide limits on its temporal variability.
An explosion of large-telescope construction heralds a new era of Earth-based observations of Europa at visible and near-infrared wavelengths. More than a dozen new large telescopes are expected to have first light by 2003, and virtually all of them are expected to have instrumentation with spectroscopic capability in the visible and near infrared. Even moderate-sized telescopes, when combined with the power of high-spectral-resolution spectroscopy, can provide valuable observations of Europa. For example, long-slit high-resolution spectroscopy of the sodium 589-nm line made in 1995 revealed a large-scale atmospheric component sputtered from the surface.41 Subsequent observations suggest that the sodium originates on Europa and may be coming from surface salts.42 With the new larger-aperture telescopes, similar observations for potassium and calcium should be feasible, giving good geochemical constraints on the presence of trace species on the surface. In particular, ratios of abundances of sodium, potassium, and calcium, combined with HST measurements of magnesium, will help to constrain the types and sources of salts on the surface. Calcium observations in particular could be used to definitively rule out Io as a source of europan atmospheric elements.
Perhaps most exciting will be advances in near-infrared technology for telescopes with large (36 m) and very large (310 m) apertures. A 10-m telescope using adaptive optics at 2 microns has a diffraction-limited resolution of 170 km on Europa, or more than 20 resolution elements across the planet's diameter (Figure 4.6). This is sufficient to resolve diverse surface units for spectral mapping. Longward of 2.5 microns, Europa is faint, and thus a large-aperture telescope provides substantial gains in signal-to-noise ratio, even compared to observations from spacecraft located near Europa. Although many of the spectral signatures of frozen volatiles tend to appear in the near infrared, features do continue beyond 3 microns. When combined with the capability for high-spectral resolution (for example, using a Fabry-Perot imaging system or an infrared image slicer), the large apertures of these new telescopes will provide a useful tool for probing surface composition on Europa.
Other Earth-based investigations include the thermal infrared and radar observations. In the thermal infrared, observations have revealed a featureless spectrum at the 3% level.43 Further investigation, at higher spectral resolution and looking for temporal variability, may be warranted. Expected resources include the Space Infrared Telescope Facility, the Stratospheric Observatory for Infrared Astronomy, and several of the large ground-based telescopes. With the newly refurbished Arecibo telescope, radar mapping of Europa may be revisited. Past observations have revealed the unique character of ice satellite radar reflection and scattering properties;44 these observations can now be done with higher spatial resolution, potentially allowing regional terrains to be identified.
A key factor enabling large, ground-and space-based telescopes to observe Europa and other solar system objects will be their ability to track objects moving at a non-sidereal rate. Solar system objects move relative to the stars and more distant bodies that exhibit only the sidereal motion caused by Earth's rotation. Thus, telescope guidance systems must be able to lock onto a guide star moving at the sidereal rate and generate the necessary nonlinear corrections to enable the telescope to track the independent motion of a planetary object. The correction can vary from an arc second per hour for a distant comet to an arc second per second for a near-Earth object. Europa's motion is closer to the former than to the latter extreme.
In principle, implementing such a capability is not technically difficult provided that the need for it is recognized early in the design of a telescope and its software. Unfortunately, in some notable cases this was not done. The guide-star systems on many ground-based telescopes are ill equipped to handle moving targets. Moreover, the procedures to enable tracking of solar system objects were not in place when the Hubble Space Telescope was launched, and work over several years was required to implement this capability. HST can now observe Europa relatively easily using linear approximations of the actual non-linear tracking rates. The rates are low enough that errors from the approximations are small. New ground-and space-based facilities must have a non-sidereal tracking capability with an accuracy analogous to that of HST.
TECHNOLOGY DEVELOPMENT FOR FUTURE EUROPA MISSIONS
Europa presents a challenging target for spacecraft exploration, a challenge that is multiplied by the complexity of the scientific tasks researchers are motivated to undertake there. While many of the scientific instruments needed for the successful exploration of Europa already have a heritage in past planetary missions, all will,
nevertheless, require technology development in many areas. These developments include (in approximate order of the relative priority assigned to each):
-
Low-mass, radiation-hardened instrumentation to be used on orbiting spacecraft and on surface-deployed packages (including landers, rovers, and penetrators);
-
Low-mass, compact-size, wavelength-tunable radar systems, or other subsurface remote-sensing systems, with a broad range of capabilities for measuring the thickness and structure of an ice layer with unknown and poorly constrained dielectric and mechanical properties;
-
High-capacity communications to enable sophisticated application of microscopic and spectroscopic imaging techniques;
-
Robotic systems capable of physically penetrating through substantial thicknesses of ice that may contain some unknown fraction of admixed rock with unknown size distribution;
-
Robotic systems capable of in situ study of the organic chemistry and, perhaps, biochemistry of deep ice cores and/or subsurface liquid water;
-
Robotic systems capable of reaching and exploring a subsurface layer of liquid water; and
-
Robotic systems capable of returning samples of deep ice cores and/or subsurface liquid water to Earth.
Radiation hardening of sensitive electronic components presents, perhaps, the greatest near-term technological impediment to the future exploration of Europa. As discussed, the critical measurements of Europa's topography and gravitational and magnetic fields that provide the best hope for determining the existence of a global ocean require orbital measurements lasting for approximately a few tens of europan days (i.e., a few months). In this period, a spacecraft in a low orbit about Europa will accumulate a total radiation dose of more than 2 megarads. While many electronic components can be hardened to survive in such a radiation environment, it is unlikely that all can and, thus, they will have to be shielded. Shielding will increase the mass of the spacecraft or, more likely, reduce its payload capacity.
Thus, the design of an orbiter mission requires that a complex balance be struck between competing factors, including mission lifetime, instrument complement, and the scientific capability of those instruments. If the right balance is not found, an orbiter may not be able to provide a definitive answer to the fundamental question — Does Europa possess an internal ocean? — which is key to determining the priority assigned to future studies of this body.
If the existence of a europan ocean is confirmed by an orbiter, then attention will inevitably shift to sophisticated delivery vehicles such as cryobots and hydrobots, which offer the promise of eventual in-depth exploration of Europa. Penetrating to significant depths within the ice will be extremely difficult, but may be required to provide definitive answers to questions about europan life. The design, delivery, emplacement, and operation of such vehicles present many technical challenges, not the least of which is relaying data from a vehicle through many kilometers of ice.45 Much preliminary exploratory, experimental, and theoretical work needs to be done prior to their final design, outfitting, and deployment, however. Most direct measurements of europan properties in the foreseeable future will be made above, at, or on the surface, or at very shallow depths within the ice. Much about the interior can be learned from measurements from orbit and by detailed examination of materials at the surface.
REFERENCES
1. A. Kapitsa et al., ''A Large Deep Freshwater Lake Beneath the Ice of Central East Antarctica," Nature 381: 684, 1996.
2. R.E. Bell and D.M. Karl, eds., Lake Vostok Workshop — Final Report — Lake Vostok: A Curiosity or a Focus for Interdisciplinary Study? National Science Foundation, Arlington, Virginia, 1999.
3. W. Zimmerman et al., "Europa Cryo-Hydro Integrated Robotic Penetrator System (CHIRPS) Feasibility Study," Jet Propulsion Laboratory, Pasadena, California, 1998.
4. Space Studies Board, National Research Council, A Scientific Rationale for Mobility in Planetary Environments, National Academy Press, Washington, D.C., 1999, pages 25 and 43-44.
5. H.W.C. Aamot, "Instrumented Probes for Deep Glacial Investigations," Journal of Glaciology 7: 321, 1968.
6. See, for example, Space Studies Board, National Research Council, Evaluating the Biological Potential in Samples Returned from Planetary Satellites and Small Solar System Bodies, National Academy Press, Washington, D.C., 1998.
7. See, for example, K. Horikoshi and W.D. Grant, eds., Extremophiles: Microbial Life in Extreme Environments, John Wiley and Sons, New York, 1998.
8. See, for example, D.J. Kushner, ed., Microbial Life in Extreme Environments, Academic Press, London, 1980.
9. See, for example, David M. Karl, ed., The Microbiology of Deep-Sea Hydrothermal Vents, CRC Press, Boca Raton, Florida, 1995.
10. See, for example, A. Oren, ed., Microbiology and Biogeochemistry of Hypersaline Environments, CRC Press, Boca Raton, Florida, 1998.
11. See, for example, P.S. Amy and D.L. Haldeman, eds., The Microbiology of the Terrestrial Deep Subsurface, CRC Press, Boca Raton, Florida, 1997.
12. R.M. Daniel, "Modern Life at High Temperatures," in Marine Hydrothermal Systems and the Origin of Life, N. Holm, ed., special issue of Origin of Life and Evolution of the Biosphere 22:33, 1992.
13. A.H. Segerer, S. Burggraf, G. Fiala, G. Huber, R. Huber, U. Pley, and K.O. Stetter, "Life in Hot Springs and Hydrothermal Vents," Origin of Life and Evolution of the Biosphere 23:77, 1993.
14. T.M. McCollom and E.L. Shock, "Geophysical Constraints on Chemolithoautotrophic Metabolism by Microorganisms in Seafloor Hydrothermal Systems," Geochemica et Cosmochemica Acta 61:4375, 1997.
15. J.P. Amend and E.L. Shock, "Energetics of Amino Acid Synthesis in Hydrothermal Ecosystems," Science 281: 1659, 1998.
16. J.P. Amend, "What Does Pyrodictium Really Metabolize in the Natural Habitat?" Nature, 1998 (in preparation).
17. E.L. Shock, "Organic Acids in Hydrothermal Solutions: Standard Molal Thermodynamic Properties of Carboxylic Acids, and Estimates of Dissociation Constants at High Temperatures and Pressures," American Journal of Science 295: 496, 1995.
18. E.L. Shock, D.C. Sassani, M. Willis, and D.A. Sverjensky, "Inorganic Species in Geologic Fluids: Correlations Among Standard Molal Thermodynamic Properties of Aqueous Ions and Hydroxide Complexes," Geochemica et Cosmochemica Acta 61: 907, 1997.
19. J.P. Amend and H.C. Helgeson, "Group Additivity Equations of State for Calculating the Standard Molal Thermodynamic Properties of Aqueous Organic Species at Elevated Temperatures and Pressures," Geochemica et Cosmochemica Acta 61: 11, 1997.
20. J.P. Amend and H.C. Helgeson, "Calculation of the Standard Molal Thermodynamic Properties of Aqueous Biomolecules at Elevated Temperatures and Pressures," Journal of the American Chemical Society 93: 1927, 1997.
21. H.C. Helgeson, C.E. Owen, A.M. Knox, and L. Richard, "Calculation of the Standard Molal Thermodynamic Properties of Crystalline, Liquid, and Gas Organic Molecules at High Temperatures and Pressures," Geochemica et Cosmochemica Acta 62:985, 1998.
22. T.M. McCollom and E.L. Shock, "Geophysical Constraints on Chemolithoautotrophic Metabolism by Microorganisms in Seafloor Hydrothermal Systems," Geochemica et Cosmochemica Acta 61: 4375, 1997.
23. B.M. Jakosky and E.L. Shock, "The Biological Potential of Mars, the Early Earth, and Europa," Journal of Geophysical Research 103: 19359, 1998.
24. B. Schmidt et al., eds., Solar System Ices, Kluwer Academic Publishers, Dordrecht, the Netherlands, 1998.
25. W.B. Durham, S.H. Kirby, and L.A. Stern, "Creep of Water Ices at Planetary Conditions: A Compilation," Journal of Geophysical Research 102: 16293, 1997. (See also, 102: 28725, 1997.)
26. J.S. Kargel, "Brine Volcanism and the Interior Structures of Asteroids and Icy Satellites," Icarus 94: 368, 1991.
27. D.L. Hogenboom et al., "Magnesium Sulfate-Water to 400 MPa Using a Novel Piezometer: Densities, Phase Equilibria, and Planetological Implications," Icarus 115: 258, 1995.
28. G. Strazzulla, "Chemistry of Ice Reduced by Bombardment by Energetic Charged Particles," in Solar System Ices, B. Schmidt, C. de Berg, and M. Festou, eds., Kluwer Academic Publishers, Dordrecht, the Netherlands, 1998, pages 281-300.
29. M.H. Moore and R.K. Khanna, "Infrared and Mass Spectral Studies of Proton Irradiated H2O+CO2 Ice: Evidence for Carbonic Acid," Spectrochimica Acta 47a: 255, 1991.
30. R.E. Johnson and B.U.R. Sundqvist, "Electronic Sputtering: From Atomic Physics to Continuum Mechanics," Physics Today 45: 28, 1992.
31. A. Benninghoven, F.G. Rudenauer, and H.W. Werner, Secondary Ion Mass Spectrometry, John Wiley and Sons, New York, 1987.
32. J.S. Kargel, "Brine Volcanism and the Interior Structures of Asteroids and Icy Satellites," Icarus 94: 368, 1991.
33. T.B. McCord, G. Hansen, F.P. Fanale, R.W. Carlson, D. Matson, T.V. Johnson, W. Smythe, J.K. Crowley, P.D. Martin, A. Ocampo, C.A. Hibbits, J.C. Granahan, and the NIMS Team, "Salts on Europa's Surface Detected by Galileo's Near Infrared Mapping Spectrometer," Science 280: 1242, 1998.
34. E.L. Shock and M.D. Schulte, "Organic Synthesis during Fluid Mixing in Hydrothermal Systems," Journal of Geophysical Research 103: 28513, 1998.
35. B. Fegley, Jr., and R.G. Prinn, "Solar Nebula Chemistry: Implications for Volatiles in the Solar System," in The Formation and Evolution of Planetary Systems, H.A. Weaver and L. Danly, eds., Cambridge University Press, Cambridge, U.K., 1989, pages 171-211.
36. R.G. Prinn and B. Fagley, Jr., "Kinetic Inhibition of CO and N2 Reduction in Circum-Planetary Nebulae: Implications for Satellite Composition," Astrophysical Journal 249: 308, 1981.
37. E.L. Shock and W.B. McKinnon, "Hydrothermal Processing of Cometary Volatiles — Applications to Triton," Icarus 106: 464, 1993.
38. G.D. Crawford and D.J. Stevenson, "Gas-Driven Water Volcanism and the Resurfacing of Europa," Icarus 73: 66, 1988.
39. E.L. Shock, "Chemical Environments of Submarine Hydrothermal Systems," Origins of Life and the Evolution of the Biosphere 22: 67, 1992.
40. E.L. Shock, M.D. Schulte, and W.B. McKinnon, "Coupled Organic Synthesis and Mineral Alteration in Hydrothermal Systems on Europa," Europa Ocean Conference, San Juan Capistrano Research Institute, San Juan Capistrano, California, 1996, page 63.
41. M.E. Brown and R.E. Hill, "Discovery of an Extended Sodium Atmosphere Around Europa," Nature 380: 229, 1996.
42. M. Brown, "Observations and Modeling of Europa's Sodium Atmosphere," 1999, in preparation.
43. F.P. Mills and M.E. Brown, "Thermal Infrared Spectroscopy of Europa and Callisto," Journal of Geophysical Research — Planets, submitted.
44. S.J. Ostro et al., "Europa, Ganymede, and Callisto — New Radar Results from Arecibo and Goldstone," Journal of Geophysical Research — Planets 97: 18227, 1997.
45. Space Studies Board, National Research Council, A Scientific Rationale for Mobility in Planetary Environments, National Academy Press, Washington, D.C., 1999, page 44.