4
Waste Treatment and Stabilization
This chapter deals with the central feature of the committee's task to review and evaluate the state of development of the final forms of treated wastes as they arise from current and emerging treatment technologies. The first section of the chapter presents the Mixed Waste Focus Area's (MWFA's) approach to identifying appropriate technologies for treating and stabilizing mixed waste, namely the division of the U.S. Department of Energy (DOE) Office of Environmental Management's (EM's) mixed waste inventory into five groups so that the wastes in each group have similar treatment requirements. Plans for obtaining the technical services of private contractors (privatization) to treat portions of the inventory are described. The second section gives an overview of the current and emerging treatment technologies for each waste group. Waste forms that result from the treatment processes or that can be made from the treated waste are described in the third section. Needs in technology identified by the MWFA that are directly related to waste form development are discussed in the fourth section. The committee's findings and recommendations are presented in the final section.
The MWFA methodology to identify and provide mixed waste treatment technologies in support of EM's cleanup goals were presented in two documents, both entitled "Mixed Waste Focus Area Technical Baseline Report" (DOE, 1996a and DOE, 1997a). The Technical Baseline Reports provide information about the state of development of technologies for the treatment of mixed waste and production of acceptable
waste forms. Most of the information in this chapter was taken from the Technical Baseline Reports and presentations made to the committee by MWFA representatives. All numerical data were taken from the 1997 Baseline Report.
Treatment Groups
The present inventory of mixed wastes under EM's responsibility is about 167,000 cubic meters (m3). Approximately two-thirds of the waste is mixed low-level waste (MLLW) and the remainder is mixed transuranic (MTRU) waste. The inventory is expected to grow to about 250,000 m3 during the next few years, and that inventory will be further increased by waste resulting from EM's site remediation activities (Kolts, 1996). While these additions will increase the total inventory, the current inventory is believed to be representative of all the various types of mixed wastes that must be treated and stabilized. EM's mixed waste inventory was described in Chapter 2.
The MWFA has categorized the current inventory of EM's mixed waste into five groups, based on waste characteristics that require similar handling, treatment, and associated activities. This allows the assignment of generalized treatment technologies (for example, waste water treatments) to an entire category of wastes rather than to individual waste streams. The percent of the total inventory that each group comprises, by volume, is listed in the first column of Table 4. The next three columns summarize plans for assigning responsibility for waste treatment.
Obtaining waste treatment services through competitive procurements from private contractors (privatization) is an important part of DOE's strategy for treating mixed wastes. According to Table 4, there are firm plans to treat only about 7% of the inventory in DOE facilities. Present plans are for private contractors to design, build, and operate facilities for treatment of 38% of the inventory. This leaves 55% of the inventory without a current treatment plan. Of this unassigned inventory, MWFA expects that treatment contracts for 40% will be awarded to commercial contractors. All together this will result in about 60% of the
TABLE 4 Treatment Groups for EM Mixed Waste
|
Waste Group |
Percent of EM Inventory |
Percent to be Treated in an Existing DOE Facility |
Percent to be Treated via Private Contract |
Percent Unassigned |
|
Waste water |
4 |
2 |
|
2 |
|
Combustible organics |
1 |
1 |
|
|
|
Inorganic, homogeneous solids and soils |
47 |
2 |
7 |
38 |
|
Debris |
46 |
2 |
31 |
13 |
|
Unique |
2 |
|
|
2 |
|
Totals |
100 |
7 |
38 |
55 |
|
SOURCE: DOE (1997a). All percentages are by volume. |
||||
total waste inventory being treated through privatization.1 The Advanced Mixed Waste Treatment Project being constructed at the Idaho National Engineering and Environmental Laboratory (INEEL) by the private contractor British Nuclear Fuels, Ltd., exemplifies a major privatization initiative, as described in Box 3.
|
Box 3 Advanced Mixed Waste Treatment Project at INEEL INEEL is currently faced with the task of treating and disposing of 65,000 m3 of mixed wastes, under an agreement between the State of Idaho and DOE. Due to the technical and regulatory complexities associated with this waste, the DOE has contracted with a consortium of private companies to build and operate a treatment facility known as the Advanced Mixed Waste Treatment Project (AMWTP). The principal objective of the AMWTP is to produce stabilized transuranic (TRU) waste and MTRU waste that meets the requirements for being transported from INEEL to the Waste Isolation Pilot Plant (WIPP) and probably at least one other disposal facility. The WIPP is described in Chapter 3, Box 2. Disposal plans for non-TRU waste from INEEL are yet to be determined. |
|
1 |
The entire 38% in column 4 and 40% of the total in column 5, Table 4. |
|
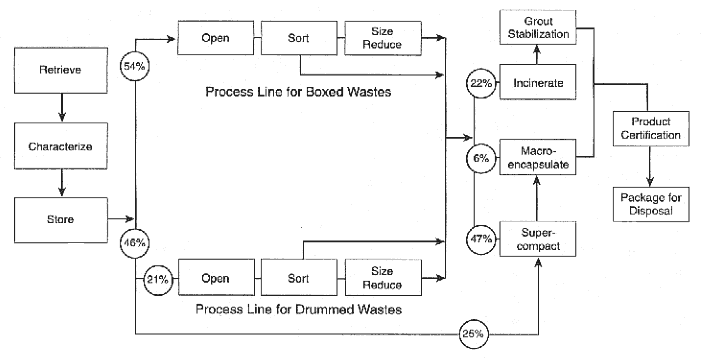
To meet its objective, the AMWTP is intended to achieve a 65% volume reduction of waste at INEEL, and to provide stabilized and packaged waste that complies with WIPP waste acceptance criteria, Department of Transportation TRUPACT II, and the U.S. Environmental Protection Agency (EPA) Land Disposal Restriction requirements. The AMWTP will be designed and constructed by a consortium of companies led by British Nuclear Fuels, Ltd. (BNFL). The initial value of the contract is for $876 million to design, permit, construct, retrieve the waste, and operate the facility to treat the 65,000 m3 of INEEL material. The estimated cost of the treatment facility alone is $270 million for design and construction. The facility is expected to open in 2003. In the intervening time, INEEL will package and ship 3,100 m3 of waste that already complies with waste characterization, transportation, and disposal regulations to the WIPP. The waste to be processed at the AMWTP consists of a wide variety of materials including contaminated soils, sludges and slurries, chemicals, and laboratory waste. TRU or MTRU waste that can be disposed in the WIPP comprises about two-thirds of the waste volume. Most of this waste is in boxes that cannot be shipped to the WIPP in the required TRUPACT II containers, and will have to be repackaged. In addition, much of this waste requires further characterization in order to meet the WIPP waste acceptance criteria. In its present form, the remaining one-third of the waste does not qualify as TRU waste because its concentration of transuranic nuclides is below 100 nanocuries per gram. Figure 4 shows the proposed treatment and stabilization process for the AMWTP. Waste received at the AMWTP will be characterized to determine how it is to be processed, then separated into one of two treatment trains depending on whether it is contained in drums or boxes. The initial processing will consist of opening the containers and separating the contents by size. Approximately 25% of the waste is believed to be sufficiently homogeneous that it does not require this step, and will be subjected to volume reduction through supercompaction. Once sorted, the waste will be treated or stabilized directly. Wastes containing high concentrations of organics or hazardous compounds such as solvents and PCBs will be incinerated. The ash will be grouted to meet EPA requirements. Debris and lead-containing wastes will be macroencapsulated, although the method of macroencapsulation has not yet been chosen. Most of the waste to be treated by the AMWTP will be classified as contact handled waste providing a total exposure of less than 200 millirem per hour. |
Treatment Technologies
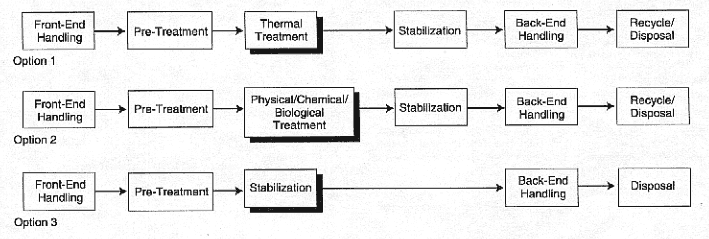
The MWFA has taken current site treatment plans2 and assembled block treatment diagrams that illustrate the treatment possibilities for each treatment group. Figure 5 provides an example block diagram of the three treatment options that MWFA has identified for the waste water treatment group. In addition to the block diagrams, basic flowsheets and processing technologies have been proposed for the treatment of about 90% of the waste inventory. Flowsheets for the remainder are yet to be proposed. Much of this remainder is in the debris group, a major fraction of which is expected to be treated through privatization. Most of the treatments described in the Baseline Report (DOE, 1996a, 1997a) are derived from processes developed for sanitary wastes or for hazardous wastes under the Resource Conservation and Recovery Act (RCRA) or Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).3 A large number of treatment processes and a considerable number of possible waste forms resulting from these processes are applicable to mixed wastes. Table 5 summarizes the treatment and waste form options that MWFA has identified for its five mixed waste groups.
An important part of the treatment strategy is to remove, stabilize, or destroy hazardous components of each waste stream, especially removal of heavy metals and destruction of organic materials (DOE, 1996a, 1997a). The treatment strategy must lead to a waste form that satisfies the requirements discussed in Chapter 3. Volume reduction is also desired for most wastes. In an initial or pre-treatment step, solid materials may undergo size reduction and aqueous streams may be filtered to remove solids. After pre-treatment, the waste can be converted to its final form directly with such techniques as grouting and polymer encapsulation, to be described in the following section. Alternatively, the waste can be treated by thermal, physical, chemical, or biological
|
2 |
Site treatment plans are required by the Federal Facility Compliance Act (FFCA) to be prepared by each DOE site. Each plan lists the wastes at the site and the treatment or disposal methods planned to bring the site into compliance with regulations. The FFCA and regulations that apply to mixed waste are discussed in Chapter 3. |
|
3 |
The RCRA and CERCLA are discussed in Chapter 3. |
TABLE 5 Summary of Treatment and Waste Form Options for MWFA Waste Groups
|
Waste Group |
Hazardous Characteristics |
Typical Hazardous Components |
Treatment Goal |
Treatment Method |
Available Waste Forms |
|
Waste water (<1% organic) |
Corrosive, toxic |
Cr, Pb, Cd, Hg |
Volume reduction organic removal |
Incineration, traditional water treatments: (reverse osmosis, neutralization, precipitation) none |
Grout, polymer, glass, Hg-amalgamation |
|
Combustible organics |
Ignitable, corrosive, toxic |
Halogenated; non-halogenated solvents Cr, Cd, Pb, Hg, PCBs |
Destroy organics volume reduction |
Incineration thermal oxidation |
Grout, polymer, glass, Hg-amalgamation |
|
Inorganic, homogeneous solids and soils (<60 mm particles) |
Toxic |
Electroplating waste, solvents, Pb, Cr, Cd |
Volume reduction meet disposal requirements |
Incineration thermal oxidation none |
Grout, polymer, glass, sulfur cement |
|
Debris (>60 mm pieces) |
Toxic |
Pb, solvents |
Volume reduction meet disposal requirements |
Thermal: incineration, melting, plasma-torch non-thermal: cutting, sorting, segregating, compaction |
Grout, polymer, glass, Hg-amalgamation, direct disposal of object or compacted material |
|
Unique |
Ignitable, reactive, toxic |
Reactive metals, compressed gases, explosives |
Hazard reduction |
Specific treatments for individual wastes or waste steams |
Grout, polymer, glass, Hg-amalgamation, direct disposal |
|
SOURCE: DOE (1997a). |
|||||
methods to remove or concentrate hazardous and radioactive constituents and to put them into a form that meets regulations. These treatment processes may produce secondary wastes that themselves must be treated. Thermal treatment, for example, produces an off-gas stream that may contain metals, organic vapors, acids, or radioactively contaminated particles. These must be trapped, stabilized, and eventually disposed.
Assurance that a proposed treatment process will operate safely is required under DOE Order 5820. This order requires documentation of the waste stream analyses, treatment options, rational for process selection, and a thorough safety analysis (Safety Analysis Report). Documentation of operating and maintenance procedures, operator training, and emergency response plans is also required. The order applies to all DOE sites, their contractors, and subcontractors.
Baseline Treatment Technologies
The treatment processes described below are general descriptions taken from experience with sanitary and hazardous (i.e., RCRA) wastes. In MWFA planning, these processes are presented as available baseline technologies that may be applied to mixed waste (DOE, 1997b).
Waste water
The waste water treatment group shown in Table 5 includes aqueous liquids and slurries containing less than 1% organic material. Waste water comprises about 4% of the EM waste inventory. Volume reduction and organic removal are the major objectives for treatment of waste water. About 40% of aqueous wastes are proposed to be pre-treated and stabilized directly with cements, polymers, and, in the case of some transuranic (TRU) wastes, with glass.
Physical, chemical, and biological treatment may apply to about 48% of the waste waters. In this series of processes, organics are removed by standard water treatment techniques, such as reverse osmosis, chemical or biochemical oxidation, steam stripping, and activated carbon. Volume reduction and metals separation occur by neutralization,
precipitation, filtration, evaporation, ion exchange, and other waste water treatment methods. The resulting concentrates are proposed to be grouted or incorporated into polymer matrices. Mercury is to be separated and amalgamated.
Pre-treatment and incineration are proposed for the remaining 12% of the waste water. In this process, organic compounds are destroyed, leaving an ash residue containing inorganic salts, metals, and radionuclides. Secondary wastes from this process include filtered solids, volatilized particulates and mercury, activated carbon, and ion exchange resins. All of these secondary wastes must be treated and disposed. Ash residues can be stabilized with grout, polymers or glass, and mercury can be amalgamated.
Combustible Organics
This treatment group comprises only about 1% of the EM inventory and includes organic liquids and sludges. The primary objectives for their treatment are to destroy the organic materials and reduce volume. About 93% of these wastes can be destroyed by thermal oxidation, such as incineration or an equivalent technology, and about 4% of the wastes can be oxidized by non-thermal methods. Ash residues from these wastes can be stabilized with grout, polymer, or glass. Mercury can be amalgamated.
Inorganic, Homogeneous Solids and Soils
These solid wastes, about 47% of total EM inventory, are generally soil, process sludges and particulates. A large portion of this waste group is inorganic sludge. Much of this sludge has been neutralized and partially stabilized. Contaminated soils comprise about 10% of this group, or about 5% of the EM inventory. The treatment objectives are to produce a material suitable for disposal at approved sites, and secondarily to reduce the final disposal volume. The majority of the homogeneous solids will be stabilized, with or without pre-treatment, with grout, polymer, glass, or sulfur cement. About 4% of the waste can be treated thermally through incineration, thermal desorption, or vitrification. Secondary wastes from off-gas treatment will also require treatment. The
wastes resulting from thermal treatments can be stabilized with grout, polymer, or glass.
Debris
Debris is defined under RCRA as solid material greater than 60 mm in particle size that is intended for disposal. Debris can include manufactured objects, plant or animal matter, or natural geologic material. The debris treatment group represents about 46% of EM's mixed waste. Because of its large quantity and its heterogeneity, the group presents a considerable treatment challenge.
For the majority of the materials in this group, the proposed treatment method is undetermined, but it will be selected to meet shipping and disposal requirements. Potential treatment methods are well established and include sorting and segregation, size reduction, removal of hazardous components by washing or mechanical means, and grouting of liquids. Direct stabilization of debris by macroencapsulation or grouting is also possible. Mercury can be removed by desorption and amalgamated. According to present plans about 13% of this waste may be treated thermally.
Unique Wastes
Unique wastes are those that do not fit into the other treatment groups. Waste streams comprising this group are generally small, and overall, this group constitutes only about 2% of EM's inventory. However, treatment of these wastes presents a considerable challenge in that treatment processes may have to be tailored for each waste stream. The primary objective in treating these types of wastes is detoxification in preparation for disposal.
Lab packs (mixed waste from laboratory operations) are expected to be oxidized thermally or chemically and may be also be treated by chemical precipitation. Waste products from this processing can be stabilized with grout or polymer. Mercury can be amalgamated. Metals such as lead, cadmium, or beryllium can be macroencapsulated after their surfaces have been cleaned to remove easily mobilized contamination. Reactive metals can be deactivated and residues can be stabilized.
Treatment processes for common batteries involve surface decontamination, liquid and solid separation, neutralization, and stabilization. Explosives and propellants will be incinerated or otherwise thermally oxidized or deactivated chemically. Compressed gases and aerosols will be incinerated, chemically reduced, or oxidized.
New Treatment Technologies
During the committee's review period, a primary goal of the MWFA was to demonstrate three technologies that had the potential to treat 90% of EM's mixed waste inventory. A key requirement was that the technologies produced a waste form that met all RCRA stability and leaching criteria and would not be significantly degraded by radiation damage. The technologies of interest were the following:
- Joule-heated melting;
- plasma-torch melting; and
- macroencapsulation.
According to presentations by the MWFA to the committee, tests of these technologies confirmed their potential for treating most of the mixed waste inventory. However, the tests also showed that actual application of the technologies to treat specific waste streams would require that each of the technologies be demonstrated, operating parameters be identified and optimized, and each design be adapted for the waste stream. The extremely broad physical, chemical, and radiological properties of EM's mixed waste may preclude the use of generically designed treatment processes. Waste forms that result from use of these technologies are described, along with other available waste forms, in the next section.
In addition to the above broadly targeted technologies, three other technologies were reported by the MWFA to be in an advanced stage of development and demonstration. These include molten metal processes, non-thermal treatment processes, and improved methods to stabilize mercury.
Molten Metals
Metallic pools with dissolved reagents (e.g., oxygen) and often with liquid slags, have been used to process mixed wastes (Evans, et al., 1997; EPRI, 1997a; EPRI, 1997b). The high temperature and highly reactive environment completely destroys organic waste components, and the metallic constituents partition between the slag and the bulk metal. Metals such as iron, copper, and nickel have been used as metallic baths and commercial scale mixed waste processing has been demonstrated. The high temperature of the process requires close attention to the off-gas streams, depending on the composition of the feed to the process. The aqueous corrosion behavior of product metals, while complex, may be extrapolated with modest confidence, especially if the metals are simple, that is, not complex alloys. The robust nature of the metals and the ceramic slags points to relatively reliable extrapolation of confinement loss and release rates. The process was demonstrated at Oak Ridge. Several demonstrations on non-radioactive wastes have also been reported.
Non-Thermal Treatment Processes
DOE recently completed an evaluation of alternative nonflame technologies for destruction of organic waste and organic constituents in aqueous waste (Schwinkendorf, 1997). Many of the technologies (e.g., supercritical water oxidation) were only applicable to aqueous wastes. Some of the alternative technologies, including electrochemical or biochemical decomposition, may have niche applications to streams for which incineration or other high temperature processes are inappropriate. The evaluation recommended further work on the more aggressive technologies: steam reforming (pyrolysis at 300 °C to 1200 °C in the presence of steam), direct chemical oxidation using peroxidisulfate, and a co-catalyzed wet oxidation process using acidic ferric chloride. If these processes were to become commercially attractive, the waste products resulting from these processes would still require immobilization.
Mercury Stabilization
Treatment of mercury-contaminated waste and disposal of mercury are among EM's highest priority mixed waste problems. At least some mercury is found in all five MWFA treatment groups. As discussed in Chapter 3, the U.S. Environmental Protection Agency (EPA) prescribes treatments, referred to as Best Available Demonstrated Technology (BDAT), for mercury-contaminated wastes. Wastes that contain mercury at contamination levels less than 260 parts per million (ppm) require stabilization so that mercury released from the resulting waste form by the Toxicity Characteristic Leaching Procedure (TCLP) is below 0.2 ppm. For solids contaminated with more than 260 ppm total mercury, EPA requires that the mercury be thermally separated from the waste and recovered (retorted). This is based on the premise that the mercury would be recyclable. Since the mercury recovered from mixed waste is expected to remain radioactively contaminated, it cannot be recycled. The MFWA is working with the EPA to develop appropriate treatment standards to replace the requirement for retorting.4
Near the end of the committee's review period the MWFA was evaluating two promising methods of providing waste forms for elemental mercury (DOE, 1997b). The first, amalgamation, was being demonstrated on five elemental mercury wastes from four DOE sites by two private subcontractors. The second method, stabilization of elemental mercury with sulfur polymer cement, was being demonstrated at Brookhaven National Laboratory. The development of direct stabilization technologies to provide waste forms for disposing of elemental mercury recovered from mixed wastes is a priority item in the MWFA technology development needs list described later in this chapter and reproduced in Appendix C.
Available Waste Forms
A wide variety of waste forms is available for stabilizing the products of the treatment processes described in the preceding section.
Selection of the most appropriate waste form for a waste stream at a DOE site is a key step in that site's overall waste management strategy. In identifying waste forms for mixed waste, the MWFA has used the considerable experience in both the private sector and DOE. The literature contains many examples of waste forms that have been developed outside the MWFA for sanitary, hazardous, and low- and high-level radioactive waste that are nevertheless applicable to mixed waste (Perret, 1998; Gilliam and Wiles, 1996; Lutze and Ewing, 1988; Donald, et al, 1997). Waste forms in MWFA's repertoire of available baseline technologies will be described in this section. The basic classes of waste forms include:
- Grout
- Glass
- Polymers
- Crystalline ceramics
- Vitreous ceramics
- Compacted wastes
For most treated mixed wastes in the EM inventory, one or more of the above waste forms can meet the requirements of chemical durability, for example, leach resistance and long-term stability, physical strength and fracture resistance, and resistance to radiation damage5 (Mayberry and Huebner, 1993; Ewing, et al., 1995).
Compatibility with the waste stream is a primary consideration in selecting among the available waste forms. In some cases, lack of compatibility limits the selection. For example, organic liquids are usually incompatible with grout and can degrade the physical properties of the grout matrix even when present in small amounts. Similarly, soluble inorganic salts leach from grout and, in some cases, from polymer-encased waste forms. If compacted waste containing paper, clothing, rubber gloves, and other materials is placed in the disposal facility,
|
5 |
Performance requirements for waste forms are discussed in Chapters 5 and 6. Radiation damage can cause loss of chemical durability and physical strength. In some waste forms radiation can produce gases. Recent reviews of radiation effects from HLW in ceramics and in glass can be found in Weber, et al., 1997, and Weber, et al., 1998. |
organic decay products will form gradually. Since many of these organic compounds can chelate heavy metals and radionuclides, increased mobilization of the waste compounds could result. In the following parts of this section, each waste form will be described, along with its advantages, disadvantages, and state of development.
Cement-Based Grout
Grout stabilization is now the process of choice for most of the routine mixed waste stabilization operations at DOE sites.6 Historically, grout has been one of the most commonly used materials for solidifying and stabilizing low-level radioactive wastes, and its technology is at a mature stage of development. Grout stabilization of RCRA heavy metals is standard technology for producing waste forms that meet EPA requirements (Kalb, et al., 1997; Conner, 1990). Chemical and physical properties of concrete are well known, and experience with concrete in construction is extensive. However, to achieve optimum physical properties and leach resistance for wastes, grout forms must be formulated taking waste composition into account.
Grout commonly is used in making waste forms because of its low cost and ease in preparation. Grouting is accomplished by mixing the waste, water, cement, and additives in appropriate proportions at room temperature and letting the mixture harden. Chemical processes in the mixture include hydration to form colloids that coagulate into gels and colloid precipitates (the setting process), followed by gel drying and crystallization (the hardening phase). In addition to portland cement, a variety of such materials as limes, blast-furnace slags, and pozzolans (volcanic rock, clays, and diatomites), and fly ash will form cementitious
solids. These materials have different chemical compositions of calcium, aluminum, and silicon oxides and provide different waste encapsulation properties. Under optimum conditions, inorganic materials are microencapsulated into the gels and become part of the crystal structure of the cement. Waste is protected inside the low-permeability grout mass. Properly formulated grout waste forms are physically strong. Compressive strengths suitable for construction can be attained.
Although grouts can be made to have low permeabilities, the grout matrix is relatively more porous than other waste form matrices (notably glass and ceramics, to be discussed later). Its ability to retain some of the more important waste constituents depends on their insolubility if water comes into contact with the grout. For example, the RCRA metals and TRU elements (e.g., Pu and Am) are retained very well by grout because they have low solubility in the alkaline pH range of 10–12. This is the typical pH range of ground water that is in contact with grout because of calcium and aluminum oxides in the grout itself.7
Cesium and strontium, on the other hand, are soluble in alkaline ground waters and are considerably more leachable from grout than are heavy metals. Anions such as chloride or sulfate are readily leachable, and they also affect the setting and strength of concrete. Organic wastes usually must be excluded from grout, because they interfere with the hydration chemistry, retard setting, lower the strength of the waste form, and are leachable. However, some wastes that contain organic contamination have been successfully treated by various grouting technologies (Means, et al., 1995).
For grout waste forms, the increase in waste volume caused by the cement itself and any required additives may be a factor in their selection. The cost penalty for disposing of a greater volume of waste forms that contain low waste loading can be evaluated against the cost of additional treatment to remove troublesome constituents or selecting a different matrix that is more compatible with the waste stream.
Glass
After over 40 years of research and evaluation, glass has become the material of choice for immobilizing radionuclides contained in high-level radioactive waste. Vitrified waste forms generally are considered to be more stable and leach resistant than cement-based forms, but their production requires high-temperature processing in specialized equipment with careful control of chemical reduction and oxidation (redox) in the melter. Glass forms are desirable because they offer low release rates and mechanical and thermal stability in the near-surface disposal environment. Because most waste constituents dissolve in the molten glass at typical processing temperatures ranging from 1100°C to 1500°C, high waste loading is possible, and the waste is retained in the resulting matrix, even if the waste form is disturbed or mechanically damaged. The high processing temperature destroys organic materials and volatilizes some inorganic salts (e.g., nitrate salts), resulting in a volume reduction of the waste stream. Other salts, such as sulfates and some RCRA metals, do not dissolve well in the glass, and they can pose difficulties in producing a homogeneous glass product with high waste loading. The decision to use glass as a waste matrix is generally based on economics, compatibility of glass with the waste stream, and the required product performance (NRC, 1996a).
Borosilicate glass composed of 35–55% SiO2, 10–20% alkali metal oxides (primarily sodium), and 7–20% B2O3 is the most well-known glass for waste stabilization (NRC, 1996a). Phosphate and aluminosilicate glasses also have been formulated for this purpose. In the process, waste and glass formers are mixed at high temperatures to produce a melt that becomes an amorphous solid when cooled. Leach resistance, compressive strength, and solubility of some waste constituents in the solid product can be modified by changing processing conditions or the composition of the glass.
Heating of the mixtures of waste and glass can be done by:
- Joule melters that heat by passing an electrical current through the glass;
- combustion melters using fuel to generate heat;
- graphite arc furnaces that generate heat by passing a current from a graphite electrode to the melt or another electrode; and
- high-frequency melters that use microwave energy or induction heating.
Even though glass is a robust matrix, the vitrification process and the quality of the resulting waste form depend on matching the waste composition with the appropriate glass-forming additives and on the operating temperatures. Key parameters such as waste loading, viscosity, melt temperature, and durability are interrelated. Higher temperature increases the solubility of practically all waste constituents in the molten glass and, within limits, increases the achievable waste loading and homogeneity of the resulting waste form, but higher temperature also increases corrosion of the processing equipment. In addition, higher temperature increases the volatilization of constituents like mercury and radioactive iodine and cesium, which must be trapped by an efficient off-gas system. This increases the expense, complexity, and amount of secondary waste. Organic materials will be destroyed at high temperatures in an oxidizing environment, but redox conditions must be controlled or else some of the less soluble constituents may precipitate from the melt. Crystals of insoluble inorganic salts or metals that may precipitate during the melting stage can affect the final properties of the glass and cause process problems, such as non-uniform heating and melter pluggage. For these reasons waste must be well characterized and the entire process thoroughly tested to assure that the vitrification system will perform as designed.
Polymer-Encapsulated Forms
Polymer encapsulation involves embedding waste materials in an organic polymer. The waste is either dispersed as a powdered solid (microencapsulation) or the waste is surrounded by a coating of polymer (macroencapsulation). Polymeric matrices, such as bitumen, polyethylene, epoxy resins, or polyesters are used in macro- and microencapsulation (Kalb, et al., 1997). The cost of preparing this waste form is generally between that of grout and glass, and it provides a high degree
of retention until the polymer itself is degraded. The variety of polymer systems allows matching waste form performance to a specific waste within economic constraints.
Macroencapsulation envelops debris in a polymer block or in a thick protective polymer coating and is an accepted treatment technology for debris waste. Macroencapsulation complies with EPA's requirements for disposal of EPA-defined debris wastes and other wastes where macroencapsulation has been designated as BDAT. Macroencapsulation of radioactive lead solids in polyethylene has been carried out at Envirocare as part of MWFA's technology development program and is now operational.
Microencapsulation8 uses polymer matrices to coat small particles of waste that have been prepared by size reduction. For example, polyethylene at 130–150 °C can be mixed with dry waste particles ranging in size from 75 microns to 3 mm and extruded into pellets. Other plastics (e.g., thermosets such as vinyl acetate and styrene) provide similar capabilities but at a considerably higher cost than polyethylene. Bitumen is used outside of the United States. Sulfur cement is a recently developed polymer consisting of 95% sulfur and organic monomer that may be especially useful for mercury.
Microencapsulated wastes are less likely to retain their dimensional stability than grout waste forms, and they generally require secondary containers to provide physical strength. The secondary containers also can act as additional barriers against release of the waste constituents. Both biological effects and radiation are known to degrade organic materials. Higher levels of can produce radiolytic gases. Polyethylene appears to have reasonable stability to radiation typically associated with MLLW (Kalb, et al., 1997). Because waste constituents are not chemically bound to the matrix, grinding the sample as required by the TCLP partially destroys the effectiveness of encapsulation. This may cause microencapsulated waste forms to fail the TCLP for some wastes. As a consequence, this technology presently has limited applicability.
Crystalline Ceramics
Ceramic materials have traditionally been produced by firing clays or hot pressing similar inorganic materials at high temperatures. Ceramics have been considered as waste forms for high-level radioactive waste (Lutze and Ewing, 1988), however, there has been little deployment of this technology for waste management at DOE sites. Phosphate-bonded ceramics that can be made at room temperature and show promise for immobilizing mixed wastes have recently been developed with financial support of the MWFA (Wagh, et al., 1997; Singh, et al., 1998).
Synthetic rock (synroc) is an example of a traditional hot-pressed ceramic waste form consisting of three titanate ceramic phases (zirconolite, hollandite, and perovskite) into which 10–25% waste is incorporated at about 1200 °C. The waste components are trapped in the molecular structure of the crystals (better-known examples are the natural and synthetic zeolites that trap metal ions and are used as catalysts in the chemical industry). Because the ions or molecules that comprise the waste constituents must fit into the molecular cages in the minerals, the composition of the ceramic and the waste must be carefully matched. Contact with ground water having a high chemical activity of silica can lead to phase alteration. Otherwise, this material is highly resistant to leaching.
Phosphate-bonded ceramics are formed by treating calcined magnesium oxide with monopotassium phosphate or phosphoric acid. The process does not require an elevated temperature, and it appears to be sufficiently inexpensive for practical application to mixed wastes. However, these ceramics are a new development and had not been thoroughly evaluated by the end of the committee's review period.
Ceramics are comprised of crystalline phases, some of which are similar to minerals, so their long-term leaching behavior and stability can be reasonably estimated from geologic and geochemical analogue data. Retention of waste constituents in properly formulated ceramics is very good. Radiation or biological activity is unlikely to affect the inorganic host matrix. For high-temperature ceramics, operational problems are similar to other high-temperature processes, such as vitrification or incineration. Although promising, the phosphate-bonded ceramics have
yet to be demonstrated superior to grout in an integrated industrial-scale process for immobilizing mixed waste.
Vitreous Ceramics
Vitreous ceramics are a combination of glasses and crystalline solids. As waste forms, they are generally produced at high temperatures in plasma-heated systems. Such plasma systems have a greater tolerance for waste composition variability than glass melters. However, the higher process temperatures require close attention to volatilized waste constituents and off-gas system design. The mixture of crystalline and amorphous components in a vitreous ceramic makes assurance of product quality and durability more difficult than for homogeneous waste forms.
As discussed previously, the apparent advantage of plasma processes to produce vitreous ceramics from a wide variety of wastes led to the MWFA's interest in their potential use for heterogeneous and debris wastes and for wastes that do not form glass readily. While demonstrations of this technology were considered successful by the MWFA, the process required optimization for each waste stream. The need for waste characterization could not be avoided to the extent hoped, and therefore the process showed no overall advantage compared to the more established technologies for making grout or glass waste forms (John Kolts, personal communication to NRC staff, October 1998). Although plasma-heated systems may have applications at some DOE sites, their widespread deployment for mixed waste treatment is no longer being pursued by the MWFA.
Compacted Debris
For slightly contaminated solid waste, compaction with a hydraulic press can provide large volume reductions and produce waste forms suitable for disposal. In simple systems, wastes are compacted by pressing them into a 55-gallon drum. More powerful compaction equipment, which can easily crush metallic scrap and entire 55-gallon waste drums, is commercially available. In the latter case, several crushed 55-
gallon drums can be placed in an overpack drum of about 80-gallon capacity. Large quantities of the debris group are planned to be compacted with supercompactors to achieve large volume reductions. The compacted material will be placed in overpack drums for disposal. Some metallic debris, such as radioactive metal shielding, drums, and process equipment, will be decontaminated by cleaning the surface, compacting, and macroencapsulating. This process has been applied at Envirocare to contaminated lead ingots and will be used at the INEEL Advanced Mixed Waste Treatment Project for all debris waste.
Technology Needs
As a part of the process of developing its technical baseline report, the MWFA has identified areas where technologies necessary for mixed waste treatment are deficient or missing. These needs are derived mainly from input from the Site Technology Coordinating Groups (STCG)9 at each DOE site. The input was prioritized by MWFA and presented in the Technical Baseline Report (DOE, 1996a, 1997a). The MWFA list of 24 technology needs is reproduced in Appendix C. The top four needs in the prioritized list are important to MWFA's task of assuring that adequate waste forms are available for DOE's mixed waste inventory.
The first priority is waste characterization. It was pointed out in Chapter 2 that the committee considers quantitative knowledge of the EM mixed waste inventory to be deficient, and that definition of detailed flowsheets for waste treatment and stabilization is not possible without this information. If waste to be treated is not well defined, only the most robust processes, such as vitrification or formation of vitreous ceramics, can be selected with confidence, and the process must be designed for conservative (possibly low and less efficient) waste loadings, thus increasing the volume of waste and cost of disposal.
Mercury and salt stabilization are the second and third priorities. Most treatments described in the previous section include provisions for
removing mercury from the primary or secondary waste stream. However, these methods to remove and stabilize mercury require further testing and development. In addition to mercury stabilization, there are four other mercury-related technology needs on the list. The difficulty of stabilizing inorganic salts was also described in the previous section. Salts cause problems with most waste form matrices, including grout, glass, and polymers. Presently, these problems can be overcome only by reducing the waste loading in these waste forms. A similar need to improve waste loading for incinerator ash is listed as priority 10.
The fourth priority is the need for assessing the behavior of waste forms in the disposal environment. Without an objective, defensible means to evaluate waste form performance in the disposal environment, waste managers may be required to use the most advanced and expensive waste forms available. Conversely, in the absence of tests to assess the waste forms, disposal facility designs may take no credit for the waste form and rely entirely on other features of the disposal site, including engineered barriers and site attributes to assure safety. The topics of waste form characterization and performance assessment are discussed in Chapters 5 and 6 of this report.
Findings, Discussion, And Recommendations
In following its statement of task, the committee gave special attention to assessing the state of technologies that MWFA has selected or developed for treating EM's inventory of mixed waste and producing waste forms for disposal. Based on MWFA's presentations to the committee (Appendix B), information in the section on Available Waste Forms, and the judgments of its members, the committee found that available classes of treatment methods and waste forms are sufficiently developed to accommodate DOE's current and expected mixed waste inventory. There is, however, a continuing need for improved engineering adaptation of these technologies to the actual mixed waste streams they are intended to treat.
The committee found the following:
- EPA hazardous waste regulations have been a major driver in technology selection and development by the MWFA. Other drivers (e.g., economics) have received less attention in MWFA's programs.
- Physical properties of the waste (e.g., solid, liquid, debris) or broad chemical properties (e.g., combustibility) have been the basis for selecting treatment technologies through the categorization of EM's mixed waste into five treatment groups. This has provided an efficient means of defining generalized flowsheets for waste treatment and identification of generally compatible waste forms, but it is not sufficient for engineering design and optimization of treatment processes, nor does it reflect the hazards posed by the various portions of the inventory.
- The selection of waste forms has been linked primarily to their compatibility with proposed treatment processes, rather than factors such as the disposal environment. The committee recognizes that this is because of, at least in part, a lack of realistic tests of long-term waste form performance in the disposal environment, and the minor role assigned to the waste form in current performance assessments. These factors will be discussed in Chapters 5 and 6.
- MWFA has recognized the need for better characterization of EM's mixed waste inventory, but it has not explicitly addressed the trade-off between detailed characterization and robust treatment processes that could accept more heterogeneous waste streams.
- MWFA has not given sufficient attention to the engineering work necessary to adapt existing or new technologies to operation with radioactive materials and to demonstrate these technologies on a production scale.
- Privatization is emphasized in EM's planning for mixed waste management. Even with privatization, technology development will still be required where there are deficiencies in available treatment or waste form technologies. The division of responsibility for technology development among MWFA and
- contractors is not clear, nor are the mechanisms for sharing results of technology development efforts well defined.
MWFA presentations and reports reviewed by the committee show that there are ample treatment technologies and waste forms to accommodate the wide variety of mixed wastes in the EM inventory. Although some wastes categorized as unique may require development of specific treatment methods, the committee believes that existing classes of waste forms are suitable for these wastes.
The limited inventory data that are available, as discussed in Chapter 2, suggest that treatment of solvent- and mercury-contaminated wastes should receive priority. Technologies for treating these wastes exist but need considerable development in application to mixed wastes. Optimization of existing waste form technologies to allow higher waste loadings and provide less expensive production methods certainly is possible, and potential cost savings justify continued effort toward process optimization. In particular, higher waste loadings (e.g., ash and salt) would reduce the volume of waste to be disposed and the concomitant disposal costs.10 Mercury stabilization is one of the MWFA's top priorities for technology development.
Better characterization of EM's mixed wastes can reduce uncertainties in the composition of the waste streams to be treated. This in turn should allow design of simpler treatment processes that accept a more narrow range of waste compositions. The process should be less expensive to build and operate than those that must accept poorly characterized wastes. Processes that treat well-characterized waste streams can be optimized to produce higher quality waste forms and less secondary waste.
The committee noted that, whereas many valuable treatment technologies have been identified by the MWFA, development steps were sometimes bypassed and technology deployment has not always been successful. Two examples, in the committee's opinion, were molten metal technology and the plasma torch. The committee viewed the plasma torch as an advancement over vitrification due to its potential to treat wastes of widely varying compositions. In spite of the major
technical difficulties encountered in implementing this technology at INEEL, the committee believes that a more methodological, stepwise development program and more careful adaptation to real wastes might have led to successful deployment of the plasma torch. If properly developed, the plasma torch could play a useful role by treating a portion of the EM mixed waste inventory that is poorly characterized.
Molten metal technology appeared to be a case where private vendors promoted development of a technology expected to be similar to the plasma torch in its applicability. Molten metal technology, while applied in a limited scope (EPRI, 1997a; Evans, 1997), was not pursued by MWFA. A planned visit by the committee to the molten metal demonstration at Oak Ridge was canceled by DOE, and the committee received no first-hand information about the practical results of the demonstration tests.
The promise of improved process technology must be weighed against the time frames (milestones) mandated in regulatory agreements, the overall goals of EM's "Paths to Closure" (DOE, 1998c), and cost of technology development. The many steps between identifying a new technology and its deployment include demonstrating safe operation, economic viability, and effective processing capability with actual waste streams, as well as acceptance by site operators. The committee cautions that problems in technology transfer may arise if a technology developer simply hands off new technologies to the user. It is the experience and judgment of the committee that considerable interaction with the user and support by the technology developer will be necessary to assure successful implementation.
EM expects privatization to yield cost savings, schedule acceleration, and other advantages for many of its cleanup projects (DOE, 1998c). Although assessing privatization was not within the committee's task, plans for the Advanced Mixed Waste Treatment project were presented to the committee and the committee learned that EM expects a large fraction of the mixed waste inventory to be treated by private contractors. Privatization may offer advantages in managing and implementing complex projects. However, the extensive reliance on private contractors to design and operate mixed waste treatment facilities
raised some concerns among committee members.11 One concern of the committee is how technologies that have been established by years of operating experience in DOE can be transferred successfully to a private contractor having relatively limited experience with DOE wastes. Another concern is how to assure that adequate knowledge of the characteristics of mixed waste to be treated is available to a bidder. A third concern how a contractor may trade-off "best" technology versus "minimum acceptable" technology.
Recommendations
The committee's general recommendation is that MWFA should no longer emphasize the development of new classes of waste forms. After reviewing the technologies available to treat EM's mixed waste inventory, and considering the resulting waste forms, it is the committee's judgment that no new classes of waste forms are required. Clearly no single form is appropriate for all wastes, but, collectively, the variety of available waste forms and well-established waste form production technologies make it unlikely that any totally new class of waste forms will be necessary to complete EM's planned cleanup program. Grout waste forms, for example, can accommodate essentially all mixed wastes in the inventory, although pre-treatment is required for some wastes (e.g., organics) before grouting. Where grout may be inadequate for either technical or regulatory reasons (e.g., failure of the TCLP), glass or polymers can be used. As discussed in this chapter, the advantages and disadvantages of each of these principal waste forms and the technologies for making them are well known. Vitreous ceramics comprise another well known class of waste forms that could accommodate most or all of the inventory, but at the present time the MWFA has no established technology for making these forms.
MWFA should now emphasize the engineering design, integration, and scale-up of its proposed treatment processes and their demonstration and deployment, as needed, at the DOE sites. Technology development and deployment must consider the overall EM waste
management strategy that is described in "Paths to Closure" (DOE, 1998c).
The committee recommends the following:
- MWFA should integrate individual treatment technologies being developed for its five treatment groups into an overall mixed waste management system.
- MWFA should demonstrate new treatment technologies on at least the pilot plant scale using real wastes or realistic surrogates before the technology is designated as ready for deployment.
- MWFA should continue to address technology deficiencies that it has identified through input from the Site Technology Coordinating Groups and update its Technical Baseline Report to reflect progress in addressing these deficiencies.
- MWFA should continue to provide research funding for developing robust processes such as the plasma torch that can treat and stabilize waste of poorly defined or variable composition.
- MWFA should continue basic research related to the understanding of the physical and chemical attributes of waste forms.