5
Reformulation of Gasoline
For the vast majority of light-duty vehicles (cars and smaller trucks), whose engines are spark-ignited, the propulsion fuel is gasoline. As described in Chapter 4, the properties of vehicles and how they are driven influence the quantity of emissions they can generate. The makeup of the fuel that powers a given vehicle can also have a major impact on the emissions, from both a mass and a component speciation point of view. By extension, such a change could exacerbate or mitigate the effects of chronic human exposure to primary and secondary mobile-source air pollution. A brief overview of the toxicology of several oxygenates in fuels is presented in Text Box 5-1.
This chapter reviews recent state- and federal-regulatory efforts to protect human health and the environment by means of the modification, or reformulation, of motor gasoline—emphasizing the requirements for the addition of oxygen and how that oxygen is provided. A discussion then follows on the properties and laboratory-measured performance of these reformulated gasolines (RFGs) with respect to the amount of ozone precursor emissions (volatile organic compounds (VOCs), oxides of nitrogen (NOx), and carbon monoxide (CO)) generated by the vehicles that use them. This discussion is intended to serve as preface for the review of actual in-use case studies and real-world observations of air-quality effects presented in Chapter 6.
|
TEXT BOX 5-1 Toxicological Considerations of Oxygenates in Fuels Although this report focuses on the effects of motor-vehicle fuel composition on formation of tropospheric ozone, earlier reports dealt in considerable detail with the toxicological and health effects related to fuel composition. Two reports that focused specifically on the effects of oxygenates in fuels are Toxicological and Performance Aspects of Oxygenated Motor Fuels (NRC 1996) and Interagency Assessment of Oxygenated Fuels (NSTC 1997). The NRC report reviewed a draft of the interagency assessment, and recommended a number of refinements and improvements in the assessment of potential human health risks associated with prolonged exposure to gasoline containing MBTE and in the assessment of the comparative risks associated with oxygenated and nonoxygenated fuels. The NRC report concluded that "until these recommendations are acted upon, no definitive statement can be made regarding these health-risk issues. Based on the available analysis, however, it does not appear that MTBE exposure resulting from the use of oxygenated fuels is likely to pose a substantial human health risk. It appears that MTBE-containing fuels do not pose health risks substantially different from those associated with nonoxygenated fuels, but this conclusion is less well established and should become the centerpiece for the government's comprehensive assessment." The interagency assessment report concluded that "it is not likely that the health effects associated with ingestion of moderate to large quantities of ethanol would occur from inhalation of ethanol at ambient levels to which most people may be exposed from use of ethanol as a fuel oxygenate. Potential health effects from exposure to other oxygenates are not known and require investigation if their use in fuels is to become wide-spread." In a related issue, in 1998, U.S. Senator Dianne Feinstein (California) requested an investigation possible contamination of the nation's ground-water by MTBE and sought help from EPA in dealing with potentially serious MTBE issues confronting California, namely, water contamination in the state. Moreover, Senator Barbara Boxer had requested that EPA phase out MTBE because of mounting evidence of MBTE contamination of California's drinking water. EPA announced in November 1998 that it will undertake a pilot site-remediation demonstration project in California. On March 25, 1999, California Governor Gray Davis issued Executive Order D-5-99, which requires the phase out of MTBE from California gasoline by no later than December 31, 2002. |
Basic Properties
Irrespective of any regulation of their content, the composition and properties of motor-vehicle fuels are routinely tailored to meet the requirements of the existing and the emerging fleet of automobiles and trucks. Cost and general availability are obviously major considerations; in the past, a fuel's specifications were established by vehicle manufacturers, together with the fuel's producers. Increased concern about air pollution and health effects from the use of motor-vehicle. fuels have brought the federal and some state governments, through their environmental regulatory agencies, into an increasingly prominent role in determining fuel composition. Within this regulatory context, fuel composition has typically been defined by specifications set as a range of properties, each having a maximum or minimum or both stipulated.
Volatility and Distillation Curve
Fuel volatility and distillation are related to the composition of vapors in the gasoline tank and in the fuel delivery system. They are critical to the proper operation of the engine. For example, a sufficiently high "front end volatility" is required for cold starting a vehicle and is generally higher in the winter than in the summer. Fuel volatility is often expressed in terms of the Reid vapor pressure (RVP), which is defined as the vapor pressure (or gauge pressure) of a liquid at 100°F, as measured in a standardized apparatus, or "bomb" (in pounds per square inch (psi)). A distillation curve can be characterized by the temperatures (usually in °F) at which 10%, 50%, and 90%, respectively, of the fuel is distilled (or evaporated). Those temperatures are represented by T10, T50, and T90.
Octane Number
Octane number is a measure of the tendency of a fuel to detonate during combustion in a standardized variable-compression-ratio "knock"-test engine in which the compression ratio1 is increased until knock is de-
tected. The test results for a fuel are scaled to an octane number of zero for n-heptane and 100 for isooctane (2,2,4-trimethylpentane). The sets of measurement conditions generally applied for determining each octane-rating component are summarized in Table 5-1.
With the phasing out of tetraethyl lead from motor gasoline, changes in composition were necessary to maintain the octane number of the unleaded gasoline so that the current and future fleets of passenger cars could operate properly. This was accomplished by increasing the content of high-octane hydrocarbons such as alkylated aromatics, olefins, and branched paraffins. Oxygenated compounds (e.g., alcohols and ethers) are also high-octane blending components, and their use as octane enhancers began as early as the late 1960s.
Oxygenates in Fuels
The major components of gasoline are hydrocarbons, whose elemental make-up includes only carbon and hydrogen. For a variety of reasons, including a desire to minimize motor-vehicle pollutant emissions, a small amount of chemically-combined oxygen is sometimes incorporated into the fuel by adding an oxygenated organic compound to the blend. The two oxygenated compounds most commonly used as additives in gasoline today are MTBE (CH3OC(CH3)3) and ethanol (C2H5OH).
The amount of oxygen in a fuel is usually expressed in terms of the percent of oxygen in the fuel by weight (i.e., wt % oxygen) or the percent by volume of the oxygenated additive (i.e., vol % additive). Table 5-2 presents the values for vol % of ethanol and MTBE that correspond to a range of wt % oxygen contents that are typical of RFG blends. Note that because ethanol contains more oxygen on a per-gram basis than
TABLE 5-1 Test Parameters for Octane Measurement
TABLE 5-2 Amounts of Ethanol and MTBE Needed to Produce a Given Oxygen Content in RFG
|
Wt % Oxygen |
Vol % Ethanol |
Vol % MTBE |
|
1 |
2.85 |
5.6 |
|
1.5 |
4.3 |
8.3 |
|
2 |
5.7 |
11.2 |
|
2.5 |
7.1 |
13.9 |
|
3.0 |
8.6 |
16.7 |
|
3.5 |
10.1 |
18.9 |
does MTBE, about 50% less ethanol (by volume) is required to produce a given % wt of oxygen in a fuel than in the case of MTBE. As discussed later in this chapter, the federal RFG program mandates a minimum 2 wt % oxygen in all RFG blends. In Table 5-2, it is shown that meeting such a requirement takes a little less than 6 vol % ethanol and a little more than 11 vol % MTBE. It turns out however, that when ethanol is present in fuel at concentrations of a few vol % to about 10 vol %, it tends to significantly enhance the fuel's RVP.2 As a result, there is a general tendency for ethanol-containing blends to contain more oxygen (on a wt % basis) than MTBE-containing blends.
Because the octane numbers for both ethanol and MTBE. are relatively high, they are attractive additives for use in lead-free gasoline. Except where mandated by law, however, oxygenate producers compete with conventional refining processes for producing high-octane hydrocarbons that can be added to gasoline. These conventional processes include the following:
- Catalytic cracking to increase the amount of components with boiling points in the range of that of gasoline and to produce high-octane olefins and aromatics.
- Catalytic reforming to convert naphthenes and some paraffins to high-octane aromatics.
- Isomerization and alkylation to produce branched paraffins.
In general, those processes can be more economical than those that produce oxygenates; and thus, oxygenates were not initially the additive of choice for enhancing octane number in fuels, as discussed later in this chapter. However, in addition to enhancing octane number, oxygenates in gasoline can provide air-quality benefits. For example, as discussed in Chapter 4, use of oxygenates can lower emissions of CO during open-loop operation (such as warm up) in modern vehicles (i.e., those with closed-loop feedback control) and in vehicles that do not have closed-loop controls. There is also some indication that oxygenates can lower the mass and reactivity of VOC exhaust emissions in some cases (see Chapters 6 and 7). The presence of oxygenates in reformulated gasoline has been mandated by law and regulation, and this provides the incentive for using oxygenates to boost octane number instead of using components produced by conventional processes.
All things being equal, the choice of which specific oxygenate to use would be dictated by economic factors; that is, which oxygenate can produce the desired gasoline characteristics (e.g., high-octane number) at the least cost. The principal production method for ethanol used in gasoline is fermentation of carbohydrates from grain (mostly corn):
Ethanol is also produced in petrochemical facilities through ethane-ethene synthesis:
MTBE, on the other hand, is produced in a two-step process, with petrochemical synthesis employed to manufacture methanol from natural gas:
2-Methylpropene is manufactured from 2-methylpropane:
MTBE is then produced by reacting methanol with 2-methylpropene:
This multistep process makes use of readily available inexpensive feedstock and enables MTBE to be produced at a cost that is generally less than that of producing ethanol by grain fermentation. However, in the United States, tax subsidies have made ethanol production via fermentation competitive with MTBE production. Because the committee was not asked to address this aspect of the RFG issue, the economic implications of using MTBE versus ethanol as an oxygenated additive are not discussed in this report. A discussion of the potential air-quality benefits of the two oxygenates is presented in Chapter 7.
Sulfur in Gasoline
Sulfur (combined chemically in the organic components of the fuel) is a trace impurity of gasoline. Reductions in gasoline sulfur content can substantially improve catalytic-converter performance (AQIRP 1992), as well as lower sulfur dioxide (SO2) emissions. Sulfur's effect in impairing the function of a catalytic converter by poisoning the catalyst is believed to be reversible. Removal of sulfur to a low weight-percent of gasoline (i.e., ![]() parts per million (ppm) by weight) can be accomplished by hydro-desulfurization of catalytic, thermal, and virgin naphtha.
parts per million (ppm) by weight) can be accomplished by hydro-desulfurization of catalytic, thermal, and virgin naphtha.
Federal and California Regulation of Gasoline Properties
History of Federal Actions Before 1994
The first federally mandated gasoline reformulation in recent history was the staged removal of the octane-enhancing additive tetraethyl lead from all motor gasolines. In general, the function of the oxidizing exhaust catalyst of a vehicle is impaired when the vehicle is operated with leaded gasolines. In anticipation of the introduction of catalysts to the light-duty motor-vehicle fleet in 1975, the U.S. Environmental Protection Agency (EPA) began phasing out leaded gasoline in the early 1970s (EPA
1973). A subsequent EPA rule restricted the lead content of any gasoline to a maximum 0.1 grams per gallon (g/gal) as of January 1, 1996, to achieve reductions in the inhalation exposure of humans (especially young children) residing in urban areas to airborne lead. Up to 1995, trace amounts of lead (up to 0.05 g/gal) could still be included in gasolines, but thereafter gasolines in the United States were mandated to be essentially lead-free.
Because lead had been in gasoline for many years to enhance combustion performance Coy increasing its octane rating or antiknock index), a comparably effective substitute additive was desired. Initially, lower paraffins, such as butane, offered the combination of octane enhancement and cost-effectiveness that refiners sought because they boosted the rating sufficiently at relatively low concentrations. However, butane in particular evaporated readily, having an RVP of about 58 psi and also volatilized other reactive hydrocarbons in the gasoline. The result was an industry-average gasoline with an RVP as much as 2 to 3 psi higher during the ozone season than that of the EPA certification test gasoline.
Through about 1987, discrepant volatility was not an issue because excursions of the 1-hr ambient ozone concentration standard of 0.12 ppm in most locations had been in steady decline. However, the summer of 1988 witnessed some of the worst ozone excursions on record (see Chapters 4 and 6). These excursions were widespread and often of long duration because of unusually protracted hot and sunny conditions and air stagnation over much of the nation. The ozone excursions led to speculation that evaporation of the then-common high-volatility summer gasoline, in use and in bulk storage, was a major contributor to the mass of VOC emissions giving rise to these ozone episodes. A seminal compendium of peer-reviewed research results, at that time, identified reduction of gasoline volatility as the most effective means then available to reduce anthropogenic VOC emissions attributable to mobile-source activity (NAPAP 1991).
The air-regulatory structure created under the National Environmental Protection Act (Public Law 91-190) of 1969 and the Clean Air Act (CAA) Amendments of 1970 had sought to substantially reduce transportation's contribution to the ozone problem through an almost exclusive programmatic focus on motor-vehicle manufacturers (Chapter 4). The core of this structure was a set of increasingly stringent per-vehicle emissions standards (shown in Tables 4-1 and 4-2) called the Federal Motor Vehicle Control Program. Beginning in 1989, the structure ex-
panded to encompass the fuels industry, especially petroleum producers, in the quest for greater control of emissions from gasoline-powered vehicles. Following initiatives taken by individual states, such as Colorado, EPA promulgated a rule that set upper RVP limits for gasoline sold during the ozone season throughout the nation (EPA 1989). The limits were determined, in part by meteorology, but largely by average summer temperatures. These limits were subsequently redefined and made more stringent for 1992 and later years (EPA 1990). This initial foray by the federal government into using fuel properties to aid in ozone mitigation efforts was then substantially expanded by the passage of the Clean Air Act Amendments of 1990, which mandated the federal RFG program. The key aspects of this program are discussed later in this chapter.
Corresponding California Actions
Various regions of California exceed the air-quality standards for ozone several times per year, and the Los Angeles area is generally recognized as having the most severe ozone pollution problems in the nation. Perhaps, for this reason, California has often led the nation in the promulgation of new and creative approaches to ozone-pollution mitigation, and regulation of gasoline is no exception. Requirements for fuel modifications in California have existed since 1971 when RVP limits were mandated. Through the 1970s, requirements were also promulgated for quantities of lead, sulfur, and manganese-phosphorous in gasoline and sulfur in diesel fuels.
The California Clean Air Act of 1988 imposed additional requirements on mobile sources to (1) achieve maximum emissions reductions of VOCs and NOx by the earliest practicable date; (2) achieve feasible reductions in particulate mass (PM), CO, and toxic-air contaminants; and (3) adopt the most effective control measures on all classes of motor vehicles and their fuels. In response to this, the California Air Resources Board (CARB) adopted the California RFG regulations to require cleaner-burning gasoline. This program is a critical component of California's State Implementation Plan (SIP) to reduce air pollution, and will also meet the requirements of the federal RFG program some 3 to 4 years earlier than that mandated in the CAA Amendments of 1990. Motor-vehicle-exhaust emissions standards were further specified under California's Low Emission Vehicles and Clean Fuels Program.
The Auto/Oil Study
A key principle first manifested in the concept of an RFG program is the concept that a vehicle and its fuel are an integrated system for which emissions controls should be fashioned to derive the optimum benefit from each of the system's components. In acknowledgment of this principle, the auto and oil industries initiated the Auto/Oil Air Quality Improvement Research Program (AQIRP) in 1989. The purpose of AQIRP was to develop data on potential improvements in vehicular emissions and air quality that could be realized through the use of RFG, various alternative fuels, and the development of automotive technology (Burns et all. 1992).3
AQIRP sought to identify those fuels and formulations that could be most effective in reducing ozone precursors without compromising driveability or substantially increasing the cost (per gasoline or diesel equivalent range) of driving. The program was motivated in part by the perception that the crafting of gasoline should be completely rethought, such that the entire range of its potentially health-harmful constituents, including sulfur, aromatics, and reactive olefins, should be subject to limits. The AQIRP findings have served as the cornerstone for the design of both the federal and California RFG programs, and are discussed in depth in Chapter 6.
What is Reformulated Gasoline?
There are currently in the United States two RFG programs: a federal program mandated in Section 211(k) of the CAA and a California program. The California program precedes the federal program by about 3 to 4 years. Both the federal and California programs are to be implemented in two phases. (To avoid confusion, Arabic numerals are used in this report to identify Phases 1 and 2 of the California program, and Roman numerals are used to identify Phases I and II of the federal program.) The general characteristics of the two programs are outlined
in Table 5-3 Parts 1 and 2. (The tables are not intended to provide a comprehensive presentation of the programs' requirements.)
The federal and California RFG programs are specifically aimed at mitigation of the ozone-pollution problem through the reduction of light-duty-vehicle (LDV) emissions of VOCs, CO, and NOx. These programs should not be confused with oxygenated fuels programs, such as the Federal Oxygenated Fuels Program (see Table 5-4), which seeks to lower motor-vehicle emissions of CO to avoid nonattainment of the National Ambient Air Quality Standard (NAAQS) for CO. Because CO pollution is typically most severe in the winter months, the oxygenated fuels program generally seeks to regulate fuel composition during those months. By contrast, the RFG programs tend to prescribe content and volatility of gasoline sold during the summer ozone season.
Federal RFG Program
In general terms, the federal concept of RFG, as of January 1, 1998, is gasoline blended such that, on average, the exhaust and evaporative emissions of VOCs and air toxics (chiefly benzene, 1,3-butadiene, polycyclic organic matter (POM), formaldehyde, and acetaldehyde) resulting from RFG use in motor vehicles are significantly and consistently lower than such emissions resulting from use of conventional gasolines. In a legal context, a gasoline is reformulated if the EPA administrator has certified that it meets all specifications of the CAA. Section 211 of the CAA codifies the redefinition of gasoline to be sold in areas failing to achieve ambient air-quality standards for air pollutants linked to emissions of CO, nonmethane hydrocarbons (NMHCs), and NOx. As described in Chapter 2, all three are precursors for tropospheric ozone formation. (CO and nitrogen dioxide (NO2) are also subject to ambient-concentration standards because of their direct impact on human health.)
As indicated in Table 5-3 Part 1, nine metropolitan areas are specified for application of the federal RFG program. Before passage of the CAA Amendments of 1990 that codified these requirements, EPA had already concluded that those areas would require an arsenal of new weapons to combat their ozone problems, and that changes in the composition of motor fuels would play a key role. Subsection 211(k) (10)(D) officially defined those areas as the "covered areas" for use of
TABLE 5-3 Part 1: California and Federal Reformulated Gasoline Programsa
|
California RFG Program, Phase 1 (1992-1996) |
Federal RFG Program, Phase I (1995-1999) |
|
|
|
|
|
|
California RFG Program, Phase 2 (1996-) |
||
|
|
||
|
|
7.0 psi (gauge) 40 ppm (vol) 0-2.7% (wt) 6.0% (vol) 25% (vol) 1.0% (vol) |
|
|
|
||
TABLE 5-3 Part 2: Future Reformulated Gasoline Program
|
Federal RFG Program, Phase II (2000-) |
|
|
|
a Opt-in areas are listed in Table 5-5. |
TABLE 5-4
Federal Oxygenated Fuels (Oxyfuels) Program (1992- )
|
|
|
a California began using oxygenates in November 1992 to comply with federal requirements for the control of CO, and implemented a modified form of the Federal Oxygenated Fuels Program to reduce ambient CO in 1992 in approximately 40 nonattainment areas (Kirchstetter et al. 1996). The periods in which oxygenates were required depended on location, but all were in the range of October 1 through February 29. b California's adopted requirement is lower at 1.8% to 2.2% due to concern about NOx formation. |
summer RFG. In addition, the subsection allowed for participation of any other nonattainment areas wishing to opt in to the gasoline content regulations. Table 5-5 lists these so-called RFG voluntary opt-in areas as of May 1, 1998.
The intent of Congress in formulating the federal RFG program was to ensure the participation of oxygen and oxygenate constituents in the modified-gasoline composition. The addition of oxygen at a minimum of 2.7% content by weight to winter-blended gasoline as a means to control CO emissions was required on a national basis for CO nonattainment areas outside California in CAA Section 211(m)(2). Further, as part of the federal RFG program, the EPA Administrator was instructed by CAA Section 211(k)(2)(B) to set content requirements for oxygen, benzene, and aromatics in any gasolines. For oxygen, the section requires a minimum of 2.0% by weight; for benzene, a maximum of 1.0% by volume; and, for aromatics, a maximum of 25% by volume. Such gasolines were also prohibited from containing heavy metals and from excluding detergent additives. Subsection 211(k)(7) also provides for a determination of credits for refining gasolines certified to a greater stringency (i.e., less benzene or aromatics, more oxygen) than that stipulated by the stated limits.
In developing regulations to implement the requirements of the CAA Amendments of 1990, specific per-gallon emissions-reduction targets for RFGs were set for each of two VOC-control regions: those generally in the northern tier of states, with summer baseline gasolines in the 9.0-psi RVP range, and those generally in the southern tier, where summer gasoline has RVPs of 8.0 psi and lower. Such a distinction reflects the
TABLE 5-5 Federal RFG Opt-in Areas As of July 1998
|
Connecticut (entire state) |
Massachusetts (entire state) |
|
Delaware (entire state) |
Boston-Lawrence-Worcester portion, MA and NH |
|
Washington, DC (including MD and VA suburbs) |
New Jersey (entire state) |
|
Louisville, KY |
Duchess and Essex Counties, NY |
|
Auburn and Portland, ME |
Rhode Island (entire state) |
|
Lewiston, Knox, and Lincoln Counties, ME |
Dallas-Ft. Worth, TX Richmond, VA |
|
Kent and Queen Anne's Counties, MD |
Norfolk, Virginia Beach, and Newport News, VA |
historical industrial practice where southern gasoline had lower RVP than northern gasoline to compensate for higher ambient temperatures. (The covered areas outside California that fall into the southern tier include only the Houston-Galveston-Brazoria, Texas area, and the recent opt-in areas of Dallas-Ft. Worth, Texas; and the Richmond and Tidewater metro areas of Virginia.) A target of 15% reduction for toxics and at least 15.6% for VOCs was defined for northern-tier ozone control regions; the southern-tier VOC-reduction target was 35.1%. As described below, these targets are to be computed using the so-called "Complex Model." Following the mandatory introduction of Phase II RFG after December 31, 1999, the targets rise to 20% minimum reduction for toxics and 25.9% reduction of VOCs (or at least 23.4% on a year-round averaged basis for certified RFGs) for northern-tier RFGs; and a 27.5% reduction of VOCs (at least 25.0% on a year-round averaged basis) for southern-tier RFGs. Phase II RFG is also required to reduce exhaust emissions of NOx by 5.5% in both VOC-control regions, relative to a defined baseline gasoline.
California RFG Program
In 1991, shortly after passage of the CAA Amendments of 1990 and the establishment of the federal RFG program, CARB adopted the California RFG regulations to require cleaner-burning gasoline. This program is now a critical component of California's SIP (State Implementation Plan) to reduce air pollution and meet the requirements of the CAA. Phase 1 requirements, effective January 1, 1992, required: (1) an RVP limit of 7.8 psi; (2) deposit-control additives to prevent and reduce deposits; and (3) elimination of leaded gasoline from on-road motor vehicles.
Refiners were required to begin making California Phase 2 RFG in March 1996 (Cal EPA 1996) and the gasoline was introduced statewide in the summer of 1996. As indicated in Table 5-3 Part 1, California Phase 2 RFG was required to limit specific fuel properties: RVP to reduce evaporative VOCs; sulfur content to avoid catalyst poisoning and thereby reduce VOCs, NOx, CO, and toxic emissions; aromatics content to reduce the atmospheric loading of mono- and polycyclic hydrocarbons linked to ozone formation; olefins to reduce VOC emissions reactivity; and benzene content to decrease emissions of this regulated toxic substance.
Phase 2 also has an oxygen requirement of 1.8% to 2.2% by weight during the winter or CO-control season.4 (Recall that there is also a minimum winter oxygenate content standard of 1.8% by weight for those areas that have to comply with federal RFG requirements for CO (see Table 5-4)).
Introduction of the federal Phase II RFG program will begin in the year 2000. Because the California Phase 2 RFG program was initiated in 1996 and satisfies the requirements of the federal Phase II program, it offers an opportunity to determine the effect of Phase II RFG before its more general introduction. This aspect of the RFG program is addressed in Chapter 6.
California allows four methods for determining the fuel properties for RFG. First, there is a fiat-limit provision (listed in Table 5-2 Part 1) whereby all of the fuels from a given refiner at all times must meet the standard. Second, there is an average-limit provision, whereby the refiner can average gasoline properties over time, but with caps (upper limits) on properties that cannot be exceeded in blending. Third, refiners can produce market-equivalent gasoline formulations evaluated with the California Predictive Model (Cleary 1998). Fourth, refiners can produce market-equivalent formulations evaluated by emissions testing. The emissions-testing option for alternative property limits requires testing 20 vehicles in four technology classes with different vehicles and considering only exhaust emissions. (For reasons of practicality, refiners tend to rely on the Predictive Model instead of emissions testing.) Cap limits and constant RVP were imposed on the property limits. In principle and in contrast to the federal Phase II RFG requirements, a fuel can be certified for Phase 2 of the California RFG program through emissions testing without having any oxygenates.
Federal Requirements for RFG Under the Complex Model
In a final rule of February 16, 1994 (EPA 1994), EPA articulated requirements for RFG blends eligible for sale during the ozone season in the
covered and opt-in areas. These were based upon Simple and Complex Models to determine the allowable maxima and minima of regulated constituents. Either model could be used for determining formulation of gasoline for federal RFG through December 31, 1997. After that date, only the Complex Model could be used for Phase I and then for Phase II, which begins in the year 2000. Because the Simple Model is no longer being used, only the Complex Model will be discussed here.
The Complex Model provides values of emissions due to modification of fuel properties with respect to RVP, oxygen by weight-percent, benzene content, sulfur, olefins, and distillation fraction (at 200°F and 300°F). The values are generated by a set of regression relationships derived by EPA from tests on differing fuel formulations performed in the 1980s and early 1990s on gasoline-fueled cars and trucks of numerous model years (and emissions-control technologies). Many hundreds of tests were involved. The regulation defines both summer and winter baseline fuel properties with respect to eight content variables, and from these derives baseline exhaust emissions of VOCs, NOx, toxics, and polycyclic organic matter, and evaporative emissions of VOCs and benzene as the basis of comparison for both federal Phase I and Phase II RFG. There is further allowance for the difference between normal and high emitters, such that emissions-reduction credit for each season and RFG phase must be weighted between them. The complete set of performance calculation equations for federal Phase II RFG summer emissions in the Complex Model is provided in Appendix C. The appendix shows the various (flat and variable) fuel-content standards and emissions standards of performance to be computed using the Complex Model that Phase II RFG must meet.
The Complex Model is complex because it separates the consideration of fuel properties and the calculation of a fuel's emissions performance into many different classification bins that vary by geographical region, season, RFG phase, emitter category, and range of parameters of fuel constituents. All emissions comparisons rest on EPA's definitions of baseline fuel properties and emissions (Table 5-6).
The California Predictive Model
The California Predictive Model is based only upon exhaust emissions measurements. Because evaporative emissions represent a substantial fraction of the total VOC emissions from LDVs, their omission represents
TABLE 5-6 Base and Example Ozone Season Phase II RFG Requirements
|
Fuel Parameter |
Baseline Value |
Qualifying Phase II RFG Value |
|
Benzene (vol %) |
1.53 |
<1.00 |
|
Oxygen Content (wt %) |
0.0 |
>2.0 |
|
RVP (psi) |
8.7 |
6.8 (expected average) |
|
Aromatics content (vol %) |
32.0 |
—a |
|
Sulfur (ppm) |
339 |
—a |
|
Olefins (vol %) |
9.2 |
—a |
|
200°F distillation fraction |
0.41 |
—a |
|
300°F distillation fraction |
0.83 |
—a |
|
Emission category |
Baseline value (mg/mi) |
Required % reduction from baseline computed from Complex Model for (a) per gallon or (b) pooled average over all of any refiner's RFG output |
|
Exhaust VOCs |
907.0 |
((a) > 27.5, (b) >29.0 (southern)) sum of exhaust VOCs + nonexhausta VOCs |
|
Nonexhaust VOCs |
559.31 (for southern states) |
((a) > 25.9, (b) >27.4 (northern)) sum of exhaust VOCs + nonexhausta VOCs |
|
|
492.07 (for northern states) |
|
|
NOx |
1,340.0 |
NOx minimum reduction requirements removed effective 1/1/98 |
|
Exhaust benzene |
53.54 |
—c |
|
Nonexhaust benzene |
6.24 (southern) |
—c |
|
|
5.50 (northern) |
—c |
|
Acetaldehyde |
4.44 |
—c |
|
Formaldehyde |
9.70 |
—c |
|
1,3-Butadiene |
9.38 |
—c |
|
Emission category |
Baseline Value (mg/mi) |
Required % reduction from baseline computed from Complex Model for (a) per gallon or (b) pooled average over all of any refiner's RFG output |
|
Polycyclic organic matter |
3.04 |
—c |
|
Additional Fuel-Content Requirements |
||
|
Oxygen content (wt %) |
(a) >2.0; (b) >2.1 |
|
|
Per-gallon minimum O2 under option b (wt %) |
1.5 |
|
|
Year-round maximum O2 (wt %) |
2.7 (MTBE) |
|
|
|
|
3.5 (ethanol) |
|
Benzene content (max. vol %) |
(a) 1.00; (b) 0.95 |
|
|
Per-gallon maximum benzene under option b (vol %) |
1.5 |
|
|
a Any combination of aromatics content, sulfur, olefins, 200°F distillation fraction, or 300°F distillation fraction, that collectively results in the target fuel meeting the performance levels for the pollutants shown in the table. b If option b is selected, per-gallon percent reduction requirements of 25.0 (southern states) and 23.4 (northern states) still apply. c Collective percent reduction requirement for benzene in exhaust and nonexhaust, acetaldehyde, formaldehyde, 1,3-butadiene, and polycyclic organic matter is |
||
a serious limitation of this model. The model is derived from data collected from 20 different test programs that investigated the relationship between fuel properties and exhaust emissions. In the course of these studies, over 1,000 vehicles were tested using 200 different fuels. In spite of the rather large numbers, many fuels were evaluated on a rather small set of vehicles. Only two vehicle types were modeled: 1980-1985 model years (i.e., the Tech 3 class), and post-1985 model years, (the Tech 4 class). Caps limited the range of fuel-property values, and RVP was held constant and not treated as a variable in the regression formula. Perhaps holding RVP constant was the basis for the neglect of evaporative emissions; however, neglecting those emissions biases the overall emissions estimates for the vehicle fleet. The resulting model consists of a series of regression equations that describe the exhaust emissions of NOx, VOCs, and potency-weighted toxics as a function of various properties of the fuel blend. For example, the NOx and VOC emissions for the Tech 4 class (in units of percent reduction from a California Phase II reference fuel) are given by
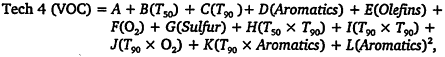
where the coefficients are given in Table 5-7.
There are different formulas for Tech 3 vehicles; these are not included here for brevity. For a fuel to qualify for the program, the model-predicted emissions for the proposed blends must be less than the California default limits. Refiners use the model to validate their blends and to adjust limits of fuel properties to fit refinery operations.
Performance and Reliability of Complex and Predictive Models
The Complex Model and Predictive Model were designed to predict reductions in the mobile-source emissions of NOx, VOC, and toxics as a result of the use of RFG. The models are used to certify a candidate fuel for the federal RFG program (Complex Model) or California RFG program (Predictive Model). Because of their limitations as discussed in this
TABLE 5-7
Coefficients for the California Predictive Model for Tech 4 Vehicles
|
Coefficient |
Tech 4(NOx) |
Tech 4(VOC) |
|
A |
6.82 × 10-1 |
-1.16 |
|
B |
1.95 × 10-3 |
7.64 × 10-2 |
|
C |
-8.20 × 10-3 |
3.89 × 10-2 |
|
D |
4.14 × 10-3 |
1.37 × 10-1 |
|
E |
2.59 × 10-2 |
-6.87 × 10-3 |
|
F |
-8.99 × 10-3 |
-1.04 × 10-2 |
|
G |
5.01 × 10-2 |
1.17 × 10-1 |
|
H |
-5.79 × 10-3 |
2.58 × 10-2 |
|
I |
1.35 × 10-2 |
1.82 × 10-2 |
|
J |
|
1.51 × 10-2 |
|
K |
|
1.21 × 10-2 |
|
L |
|
-1.20 × 10-2 |
report, these models are not used routinely to generate input data for regulatory air-quality models to assess the ozone reductions. In Chapter 7 of this report, the Complex and Predictive Models were used to evaluate the relative benefits of RFG with and without oxygenates and with various amounts and types of oxygenates. For these reasons, some discussion of the reliability of the models and their attendant uncertainties is in order.
Both the Complex and Predictive Models are based on statistical analyses of a large number of tests and the data used to develop both models are similar. Nevertheless, substantial differences exist. Some of these differences make comparison between the models cumbersome. For example, the Complex Model yields mobile emissions from a given RFG in units of milligrams per mile and the Predictive Model yields the percent reduction in the emissions from a given RFG blend relative to the so-called California Phase 2 reference fuel. There are also more-substantive differences. Probably most glaring of these is the fact that the Predictive Model ignores evaporative emissions. There are also differences of a more-subtle nature—e.g., the Predictive Model adopts a linear relationship between NOx emissions and olefin content, and the Complex Model includes a linear and a quadratic term. As illustrated below, these differences can produce significant discrepancies between the results of the two models.
Figure 5-1 compares the Predictive and Complex Models' calculated reductions of NOx and VOC emissions for four illustrative RFG formulations. (A more detailed discussion of how various RFG formulations fare using the Predictive and Complex Models is presented in Chapter 7.) Note that in all four cases and for both models, the use of RFG is predicted to lead to substantial emissions reductions relative to the federal baseline fuel (see Table 5-6). This is perhaps not surprising because RFG is intended to perform better than baseline fuels. However, this does not have to be the case a priori, and, in fact, there is evidence that an increase in emissions can result in some instances (Weaver and Chan 1997).
Inspection of Figure 5-1 indicates a good deal of consistency between the two models. For example, both models predict substantial benefits from the use of low sulfur fuel (i.e., ˜90% reduction in sulfur relative to baseline fuel). (Indeed it appears that the use of low sulfur fuel will be critical to meeting the Phase II RFG requirements for VOC and NOx emissions.) On the other hand, the models tend to diverge in their simulations of the effects of oxygenates. In the case of NOx, the Complex Model shows a slight increase in emissions with the use of oxygenates, but little difference between the moderately and highly oxygenated fuels. However, the Predictive Model produces a varying effect, with little change with a moderate amount of oxygen and an increase in emissions with a high amount of oxygen. It is likely that this difference arises from the aforementioned different formulas used by the models to represent the effect of oxygenates on NOx emissions. In other words, while both models are based on similar data, their different sets of covariates and associated parameters have apparently generated small, but non-negligible inconsistencies between model results. In the case of VOC emissions, the differences in the model results are far more substantial. For example, the Predictive Model yields greater emissions reductions as the oxygen content is increased, while the Complex Model predicts decreasing emissions reductions with the use of fuel with high amounts of oxygen.
The discrepancies illustrated in Figure 5-1 point to the possible existence of specific problems with one or both of the models. There are also some more-general concerns that need to be borne in mind. The use of the Complex and Predictive Models requires a substantial extrapolation of measured emissions from a sample set of motor vehicles operating under controlled test conditions to real-world emissions from a fleet of motor vehicles using one or more RFG blends. As discussed in
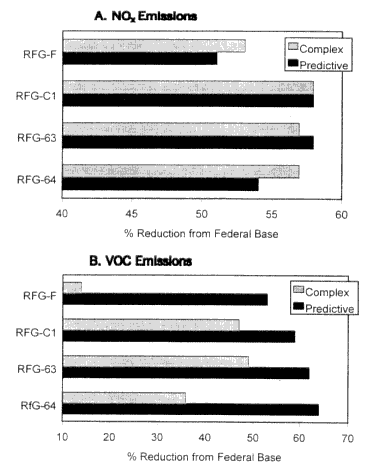
Figure 5-1
Recent reductions in the mobile-source emissions from four illustrative RFG blends relative to the federal base fuel (see Table 6-1) as predicted by the EPA Complex Model and the California Predictive Model. The reductions were determined assuming emission rates from federal base fuel of 1340, 907, and 500 mg/mi for NOx, exhaust VOC, and evaporative VOC, respectively, and emissions from California Phase 2 reference fuel of 569 or NOx and exhaust VOC, respectively. Abbreviations: F, low aromatics; C1, low sulfur; 63, low sulfur plus moderate oxygen using MTBE; 64, low sulfur plus high oxygen using ethanol.
Chapter 4, there are myriad factors that can affect real-world vehicle emissions and confound attempts to produce a mobile-source-emissions model using statistics and regression models. Moreover, the characterization of the relationship between emissions measured in a controlled,
testing program and those resulting from on-road driving remains a scientific and technological challenge. A potential source of error in both models arises from their treatment of high-emitting vehicles. As discussed in Chapter 6, a large portion of motor-vehicle emissions come from high-emitting vehicles. However, the emissions from these vehicles are likely to be quite variable and thus difficult to characterize through sampling a small subset of the total population. All the above issues will tend to limit our ability to use these models to assess the benefits of oxygenated RFGs. See Chapter 7 for additional discussion of results obtained from the Complex and Predictive models.
Specification Flexibility and Downstream Control in Federal Phase II RFG
In the year 2000, Phase I RFG blends sold under the federal RFG program in the nine severe nonattainment areas and all present and future opt-in areas will be replaced by Phase II RFG. The targets for fuel content and exhaust emissions reductions relative to conventional 1990 baseline gasoline are summarized in Table 5-6. In light of the focus of this report, it is relevant to note the Phase II requirement for a minimum oxygen content of 2% by weight.
The rules for meeting the requirements for Phase II are codified in 40 CFR 80.41 (e-f) and have been amended by subsequent action by removal of the per-gallon minimum NOx reduction requirements for refiners using the pooled-averaging method. Other limits vary by whether an area lies in a northern- or southern-tier state. A refiner may select whether to meet product performance requirements on a per-gallon or pooled-average basis, as under California regulations. For example, with respect to benzene, if the former option is chosen, no single gallon of gasoline produced can contain more than 1% benzene by volume. If the latter is chosen, the pooled sample of all a refiner's RFG sold in nonattainment areas must average no more than 0.95%, meaning at least some of the gasoline must have less than the per-gallon maximum. Similarly, the oxygen content of an RFG sold by a refiner opting for the pooled-average method must average at least 2.1% by weight at any time, with no individual gallon falling below 1.5% but not exceeding 2.7% in winter if VOC-control requirements are in place.
There remains a concern about potential abuse of the process of
adding oxygenate to gasoline downstream of a refinery. This practice, called "splash blending," involves mechanical mixing of finished gasoline or gasoline blending stock having front-end volatility set at a typical warm-season value (RVP of 7 to 8 psi) with a liquid oxygenate (such as ethanol). Splash blending, unlike refinery-performed match blending that renormalizes product output to the required properties of an RFG, can change the proportional constituents of a gasoline by diluting (replacing) their mass and volumetric share in each gallon. It also has the potential to increase the quantity of the total fuel that evaporates from vehicles if the fuel's resulting RVP is significantly higher. EPA sought to obviate this possibility by requiring the type of oxygenate that can be added be stipulated at the refinery and thus maintain RVP integrity. It also assures that even in the "worst case," with respect to volumetric displacement of benzene and other aromatics by an oxygenate (i.e., about 6% ethanol by volume in an ethanol-gasoline blend), Complex-Model content limits can be maintained by blending-stock planning at the refinery. EPA has instituted enforcement procedures to assure correct blending stock labeling, and the entire process for maintaining downstream RVP control is documented in the February 16, 1994, rulemaking on RFG standards (EPA 1994).
The possibility of an increase in the volatility of gasoline after leaving the refinery is expected to be low. Because refiners are held liable for the performance of their gasolines tested during EPA's in situ sample audits, most refiners now blend oxygen into summer RFG at the refinery (adding it in a controlled process to base gasoline at very low RVP, e.g., 6.7 psi or less). This is done to ensure that it matches Phase I property specifications. Because formulation stringency will increase for Phase II gasoline, this practice is likely to persist. Thus, splash blending should become a nonissue as applied to RFG formulation and sale during the ozone season. In fact, the demonstrated consistency of refining practice year-round has prompted EPA to remove the distinction between gasolines designated as "oxygenated fuels program reformulated gasoline" (OPRG) and those designated as non-OPRG, effective November 6, 1997 (EPA 1997b).
A related issue has to do with the fungibility of the gasoline supply (i.e., different blends of gasoline that comply with RFG requirements can be mixed freely in the distribution system as far downstream as the vehicle's tank and the resulting mixtures themselves comply with the requirements). The RVP of an ethanol blend can increase slightly if the
volumetric share of the ethanol falls to a value between 5% and 10%. Thus mixing of Phase II RFGs with and without ethanol could lead to an in-use blend that does not meet Phase II RFG requirements. Recognizing that nonlinearities in the relationships between specific fuel properties and emissions could give rise in the gasoline distribution chain to a mixture of fuels that independently meet RVP specification but in combination violate it, EPA conducted extensive parametric variation testing within the Complex Model. Its conclusion was that use of various RFG blends within an area, would not give rise to scenarios in which application of the Complex Model showed nonconformity with specified emissions-performance requirements. (EPA 1994, pp. 7731-7732)
Modeling Evaporative Voc Emissions from RFG for SIP Development
Although EPA requires that the Complex Model be used to certify the properties of RFG, a meteorologically driven air-quality model is specified to derive the mobile-source emissions from vehicles using RFG for the purpose of assessing the air-quality benefits of the RFG program and demonstrating attainment of air-quality standards. In this method, the current version of the MOBILE emissions-factor algorithm is to be used as the basis for determination of the mass-emissions rate for exhaust and evaporative VOCs, CO and NOx from highway vehicles that is appropriate to the local climatological regime and type of gasoline sold. Air-quality regulatory and planning organizations do not directly use the Complex Model in their forecasts, but may, for sophisticated air analyses, apply the Complex Model gasoline-property results obtained from refiners for the gasoline sold locally.
For purposes of complying with planning requirements for attainment of the ambient ozone standard, the values of the key variables needed for computation of evaporative VOC emissions in MOBILE (ambient temperature and gasoline RVP) should accurately represent the average conditions for the ozone "design-value" day. These are the conditions observed on the day that the regulated maximum ozone concentration—the datum from which ambient concentration reduction requirements is computed for purposes of SIP (State Implementation Plan) commitments—was recorded. MOBILE computes separate emissions factors for four nonexhaust components of nonmethane hydrocarbons (hot soak plus resting, diurnal, running, and refueling losses).
The Complex Model's determination of nonexhaust (evaporative) emissions of Phase II RFG also involves four separate computations. However, in each case, RVP (and distillation temperatures) are used to characterize these emissions (i.e., temperature is specifically not included as an independent variable) (40 CFR 80.45 (c)(2-3)). The results of these four computations are summed to yield the nonexhaust component of the overall VOC-emissions-performance equation. The temperature conditions input to the Complex Model were based on average temperatures observed on ozone exceedance days which were estimated by EPA to be 72-92°F for the northern United States and 68-95°F for the southern United States. Thus, the Complex Model is broadly representative of high ozone conditions in the areas where RFG is sold. Accounting for the effects of variations in temperature on program implementations would involve considerations that are outside the scope of this study, such as the possible nonuniformity of RFG certification standards and possible complications with the distribution of RFG.
The absence of any temperature dependency in evaporative emissions computations in the Complex Model has raised concern that the model might assign too low a value to the nonexhaust VOC component of the RFG compliance calculation. At the very least it is quite possible that the VOC emissions derived from the Complex Model to certify fuels will be inconsistent with the emissions derived from MOBILE and used by regulatory agencies in the development of their SIPs. However, in preparing their SIPs, states can use the MOBILE model to estimate RFG effects on evaporative emissions by using more accurate local temperatures.
Is this discrepancy important? Certainly, as discussed in Chapter 4, high ambient temperature (and the magnitude of daily temperature rise) plays a role in the quantity of evaporative VOC emissions produced, and it is possible that current emissions-certification procedures underestimate the contribution of hot soaks to total evaporative emissions. Moreover, with low-volatility fuels such as RFGs, it appears that RVP differences, other things being equal, still dominate differences in total evaporative emissions for most relevant urban ozone nonattainment cases given current on-board emissions controls. Another concern is that refiners are limited, by current requirements, in their ability to craft fuels with lower total reactivity, if they chose to do so, because they might exceed exhaust plus evaporative mass-emissions targets. It is also the case, as alluded to above, that regression models inevitably introduce smoothing and other simplifying approximations that might be inappro-
priate in specific nonattainment areas, especially if they were developed from a data base different from that used to build any of the other regulatory models associated with the ozone-compliance process.
Summary
Gasoline has been reformulated to adjust its basic properties for various reasons over a very long period of time, before, in fact, air quality became a major issue. The relatively recent emphasis on the control of ozone precursor emissions and toxic emissions has prompted a new and comprehensive gasoline reformulation strategy. This strategy involves: (1) reduction in summer volatility (expressed as RVP); (2) reduction in reactive gasoline components (e.g., olefins) during the summer to reduce the ozone-forming potential of motor-vehicle emissions; (3) reduction in benzene and other aromatic content of gasolines year-round, and (4) addition of oxygenates as a means to help control emissions and to maintain octane rating using nontoxic constituents. The first three of these are formally included in the federal and California reformulated gasoline programs.
The adoption and use of the Complex Model and Predictive Model have been driven by a need for establishment of a level playing field for all refiners, as well as an easy and inexpensive fuel certification procedure that allows mixing of different batches thereby facilitating fuel distribution. The models appear to meet those needs. However, the methods used in those models to predict the in-use performance of gasolines reformulated to meet the criteria of the reformulated gasoline programs, are based on results from large and diverse, but nonetheless limited, data bases. They might not accurately represent what actually occurs in specific nonattainment areas, especially where a high summer-temperature rise produces relatively high evaporative VOC emissions. They might even deny refiners the ability to formulate fuels that could be more beneficial on the basis of atmospheric reactivity—an issue that is addressed in Chapter 7.






























