6
The Effects of Reformulated Gasoline On Ozone and Its Precursors
The ability to distinguish the air-quality benefits of one reformulated gasoline (RFG) blend from that of another depends, to a substantial degree, on the overall magnitude of the effect of RFG on air quality. If the RFG effect is large, then the effect of two blends of RFG might be quite discernible. If on the other hand, the RFG has a lesser effect on air quality, it is likely to be very difficult to identify which of two RFG blends is preferable from an air-quality point of view, let alone to reliably quantify these effects. As a prelude to Chapter 7, in which an attempt is made to quantify and compare the ozone-forming potential of eight different RFG blends, this chapter assesses available information on the overall impact of the RFG program on ozone and its precursors as deduced from measurements.
The steps taken in the approach to make that determination are illustrated in the "Decision Tree" depicted in Figure 6-1. The chain of inference proceeds from the tests of the emissions from a limited sample of motor vehicles in the laboratory to determinations of the influences of the use of RFGs in light-duty vehicles (LDVs) on air quality. The figure also indicates the types of findings at each step along this chain, from considerations of the currently available and published observations to the reduction of ozone concentrations and other air-quality issues. The sequence of questions addressed are listed below.
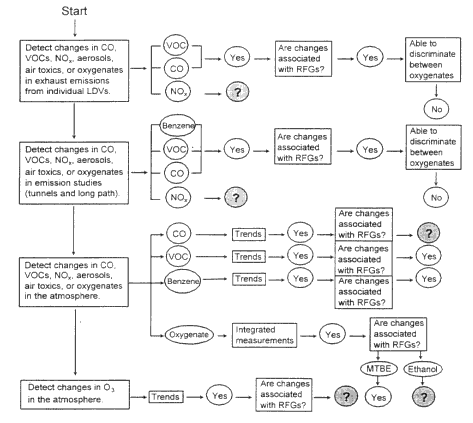
Figure 6-1
The Decision Tree illustrates the steps taken in an effort to quantify and compare the ozone-forming potential of various RFG blends. The figure indicates the types of findings at each step that resulted from the committee's considerations of the currently available observations that are pertinent to the reduction of ozone concentrations and other air-quality issues. When comparing different RFG blends, such as a blend containing ethanol versus a blend containing MTBE, it is desirable to account for as many differences as possible between the RFG blends.
- What changes in motor-vehicle exhaust emissions of VOCs, NOx, CO, or air toxics are observed in laboratory tests when RFGs are used?
- Have the changes in emissions from RFGs indicated by laboratory studies been observed in emissions studies using tunnels and remote sensing of tailpipe exhaust?
- Are there data to support meaningful analysis of atmospheric data to determine the effect of RFGs?
- Have changes in the concentrations of air toxics or oxygenates been observed in the atmosphere and can these changes be related to the use of RFGs?
- Have changes in the concentrations of CO been observed in the atmosphere and can these changes be related to the use of RFGs?
- Have changes in the concentrations of ozone been observed in the atmosphere and can these changes be attributed to the use of RFGs?
This analysis proceeds from the information concerning the measurements of exhaust and evaporative emissions from individual vehicles to the observation of the effect of those emissions on atmospheric composition. When comparing two RFG blends, it is desirable to account for as many differences as possible between the RFG blends.
What Changes in Motor-Vehicle Exhaust Emissions of Vocs, NOx, CO, or Air Toxics are Observed in Laboratory Tests When RFGs are Used?
Probably the most extensive single data set on the emissions of motor vehicles using RFG blends is that compiled from the Auto/Oil Air Quality Improvement Research Program (AQIRP).1 This study included over 3,000 emissions tests. In Phase I of AQIRP, different sets of 26 reformulated fuels and 2 reference gasolines were tested in fleets composed of 20 then-current (1989) LDVs (cars and light-duty trucks) and 14 older vehicles (1983-1985). Further, two methanol blends (10% and 85% methanol in gasoline) and one industry-average fuel were tested in 19 flexible-fueled and 5 variable-fuel passenger vehicles. In Phase II of AQIRP, fuels were prepared in several sets, or matrices, to study the effects of individual fuel properties: (1) the composition set tested the effects of aromatic content, olefin content, T90 (temperature at which 90% of mass of the fuel has evaporated), T50 (temperature at which 50%
has evaporated), and the addition of methyl tert-butyl ether (MTBE)); (2) the RVP-oxygenate set tested the effects of Reid vapor pressure (RVP), as well as the addition of ethanol, ethyl tert-butyl ether (ETBE), and MTBE; (3) the methanol set tested various methanol-gasoline mixtures; and (4) the sulfur-series set tested effects of varying the sulfur content of the fuel. The properties of the RFGs used in the AQIRP compositional and sulfur tests (and those used in MTBE and ethanol blends discussed in Chapter 7 of this report) are summarized in Table 6-1.
Exhaust emissions were measured from the various vehicles as they ran on a dynamometer under the Federal Test Procedure (FTP) protocol. Gas chromatographic and high-performance liquid chromatographic analyses of the exhaust emissions were made for all measurable components, including 140 structurally different hydrocarbons with from I to 12 carbon atoms, as well as ethers, methanol, ethanol, and 12 different aldehydes and ketones. Samples of exhaust emissions were segregated according to the point in the cycle of engine operation (cold start, hot stabilized, hot start, and composite) to reconstruct the emissions inventories for various vehicular operating scenarios. For some fuel-vehicle combinations, evaporative emissions were tested (modes of operation, hot soak, diurnal, and running loss).
Emissions of Toxics
Many of the RFG blends used in the AQIRP studies showed significantly lower total mass emissions of toxics than the industry-average gasoline. This is illustrated in the comparisons shown in Figure 6-2 for industry-average gasoline (A) and one of the RFG blends studied (C2). The comparison is made for the older fleet, the current fleet, federal Tier 1 vehicles, and vehicles with "advanced technology." With the exception of formaldehyde,2 the RFG blends showed significantly lower toxic emissions for every class of vehicle when compared to emissions resulting from the industry-average gasoline.
TABLE 6-1 Properties of Some of the Research RFG Blends Used in AQIRP and California Studies
|
Codea |
Composition Identifierb |
Aromatics (vol %) |
Oxygenates (vol %)c |
Olefins (vol %) |
T50 (°F) |
T90 (°F) |
RVP (psi) |
Sulfur (ppm by wt) |
|
AQIRP Phase I |
||||||||
|
A |
Industry average |
32.0 |
0 |
9.2 |
218 |
330 |
8.7 |
339 |
|
B |
Certified |
29.9 |
0 |
4.6 |
220 |
309 |
8.7 |
119 |
|
C |
AMot |
43.8 |
15.4 (M) |
3.3 |
213 |
288 |
8.7 |
284 |
|
D |
amOT |
20.7 |
0 |
22.3 |
218 |
357 |
8.5 |
316 |
|
E |
AMOT |
43.7 |
14.8 (M) |
17.2 |
220 |
357 |
8.7 |
267 |
|
F* |
amot |
20.0 |
0 |
3.2 |
197 |
279 |
8.8 |
290 |
|
G |
AmOt |
44.3 |
0 |
17.4 |
214 |
286 |
8.8 |
317 |
|
H |
aMOt |
20.2 |
14.6 (M) |
20.2 |
168 |
286 |
8.5 |
312 |
|
I |
AmoT |
42.9 |
0 |
4.1 |
239 |
353 |
8.9 |
261 |
|
J |
aMoT |
21.4 |
14.9 (M) |
4.0 |
208 |
356 |
8.6 |
297 |
|
K |
Amot |
45.7 |
0 |
4.9 |
208 |
294 |
8.8 |
318 |
|
L |
AmOT |
47.8 |
0 |
17.7 |
236 |
357 |
8.5 |
266 |
|
M |
aMOT |
18.0 |
14.5 (M) |
21.8 |
193 |
356 |
8.7 |
301 |
|
N |
aMot |
21.4 |
13.9 (M) |
5.7 |
164 |
292 |
8.8 |
294 |
|
O |
AMOt |
46.7 |
14.6 (M) |
19.3 |
204 |
283 |
8.6 |
288 |
|
P |
amOt |
20.3 |
0 |
18.3 |
190 |
284 |
8.5 |
333 |
|
Q |
amoT |
21.5 |
0 |
4.8 |
234 |
357 |
8.6 |
310 |
|
R |
AMoT |
46.0 |
15.2 (M) |
4.0 |
225 |
354 |
8.4 |
279 |
|
S |
|
21.2 |
0 |
3.8 |
199 |
280 |
8.0 |
297 |
|
T |
|
18.1 |
9.7 (E) |
3.6 |
174 |
276 |
9.8 |
246 |
|
U |
|
19.1 |
9.7 (E) |
3.1 |
171 |
278 |
9.6 |
278 |
|
Codea |
Composition Identifierb |
Aromatics (vol %) |
Oxygenates (vol %)c |
Olefins (vol %) |
T50 (°F) |
T90 (°F) |
RVP (psi) |
Sulfur (ppm by wt) |
|
AQIRP Phase I |
||||||||
|
MM |
|
22.2 |
14.8 (M) |
5.4 |
167 |
289 |
8.0 |
345 |
|
AQIRP Phase II |
||||||||
|
C1 |
|
22.7 |
0 |
4.6 |
208 |
297 |
6.9 |
38 |
|
C2 |
|
25.4 |
11.2 (M) |
4.1 |
202 |
293 |
6.8 |
31 |
|
Y4 |
|
24.9 |
10.9 (M) |
1.2 |
201 |
298 |
9.1 |
44 |
|
Y5 |
|
24.3 |
11.1 (M) |
1.3 |
200 |
299 |
9.0 |
138 |
|
Y6 |
|
24.6 |
10.7 (M) |
1.1 |
200 |
297 |
8.9 |
258 |
|
Y7 |
|
24.9 |
10.6 (M) |
1.1 |
201 |
299 |
8.8 |
350 |
|
Y8 |
|
24.6 |
10.7 (M) |
1.0 |
201 |
300 |
8.8 |
443 |
|
B2 |
|
26.7 |
0 |
2.5 |
220 |
318 |
8.9 |
49 |
|
Y2 |
|
26.1 |
0 |
2.3 |
220 |
316 |
8.8 |
466 |
|
California Studies |
||||||||
|
63 |
|
23.4 |
11.6 (M)d |
5.0 |
196 |
296 |
6.8 |
32 |
|
64 |
|
23.3 |
11.2 (E)d |
4.8 |
188 |
297 |
7.8 |
34 |
|
a Fuel mixtures A-R are the compositional matrices for RFGs used in AQIRP Phase I; Y4-Y8 are sulfur matrices (with MTBE) from AQIRP Phase II; B2 and Y2 are from sulfur-varied fuels used in AQIRP Phase I with no added MTBE. b Composition indicator: A/a, high/low aromatics; M/m, high/low MTBE; O/o, high/low olefins; T/t, high/low T90. c Oxygenates added are indicated with letters: MTBE (M), ethanol (E). d For these two fuels, the oxygenate composition is given in mass %. |
||||||||
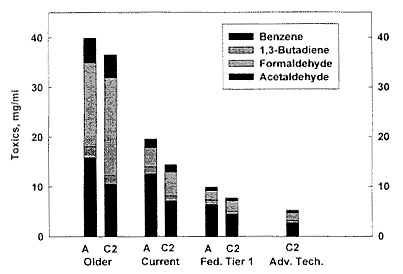
Figure 6-2
Comparison of the mass of exhaust toxics: acetaldehyde, formaldehyde, 1,3 butadiene, and benzene (mg/mi) from the industry-average fuel (A) and an RFG (C2), using the FTP composite. Chemicals are displayed from top to bottom as follows: acetaldehyde, formaldehyde, 1,3-butadiene, and benzene. On the x-axis, the results are divided into those for older, current, federal Tier-1-control, and advanced-technology cars. Source: Adapted from AQIRP Technical Bulletin No. 17, 1995.
Emissions of VOCs
The specific and total reactivity (using the MIR scale) of VOCs in exhaust, evaporative (i.e., diurnal and hot soak), and running-loss emissions from current-fleet vehicles using several of the AQIRP-tested reformulated gasolines as well as the industry-average gasoline are shown in Figure 6-3. Speciation and reactivity data on exhaust emissions were obtained from Hochhauser et al. (1992); data on evaporative emissions and running losses were obtained from Burns et al. (1992). In the case of each type of emission, the ordering of the fuels has been adjusted to show the progression of emissions from the lowest-emitting fuel to the highest-emitting fuel. In viewing these figures, it should also be borne in mind that the ozone-forming potential of VOC emissions is determined by the total mass of the emissions as well as the reactivity of the species that are emitted. The relative contribution of each of these factors can be inferred by comparing the specific and total reactivities of the emissions because the specific reactivity is a measure of the amount of ozone
formed per unit mass of VOC emitted and the total reactivity is the product of the specific reactivity and the mass of VOC (and CO) emitted per mile traveled (see Table 3-9). Finally, it should noted that in addition to emissions data for current fleet vehicles, AQIRP data exists for emissions from older fleet vehicles. Although the older-fleet data differ somewhat from that of the current fleet (e.g., the ordering of the fuels with increasing reactivity), the basic conclusions concerning the nature and magnitude of the emissions reductions that might be obtained from RFG do not.
Inspection of Figure 6-3 indicates that rather substantial changes in the reactivity of VOC emissions can result from variations in gasoline formulation. In the case of exhaust emissions for example, the specific reactivities of the fuels tested vary by a factor of 1.4, and total reactivities by a factor of about 2 (Figure 6-3A). The variability in the reactivities of diurnal and hot soak emissions are of a similar magnitude, although the ordering of the fuels changes significantly (Figure 6-3 B and C). By comparison, the range of running-loss reactivities is considerably larger (i.e., factor of 2 variability in specific reactivity and a factor of almost 70 in total reactivity) (Figure 6-3D).3 However, the maximum reduction in the reactivity of the VOC missions obtained by switching from the industry-average formulation to the most favorable of the RFGs tested is, in each case, considerably smaller. For exhaust, diurnal, and hot soak emissions, the reduction in specific and total reactivity from the industry average is about 25% or less. In the case of running losses, the reduction is more substantial; i.e., a factor of about 2 for the total reactivity.
Of course the most important parameter to consider here is the composite reactivity of all the LDV emissions; i.e., the reactivity obtained from the gases emitted by all exhaust, evaporative, and other loss processes. An example of such composite reactivities is given in Figure 6-4. In this case, composite, specific reactivities were calculated for each fuel using the AQIRP measurements of exhaust emissions (weighted for all cycles of operation), evaporative emissions, running losses, resting losses, and refueling losses from LDVs using the EMFAC-7E emissions model and the measured vapor pressures of the fuels. The relative
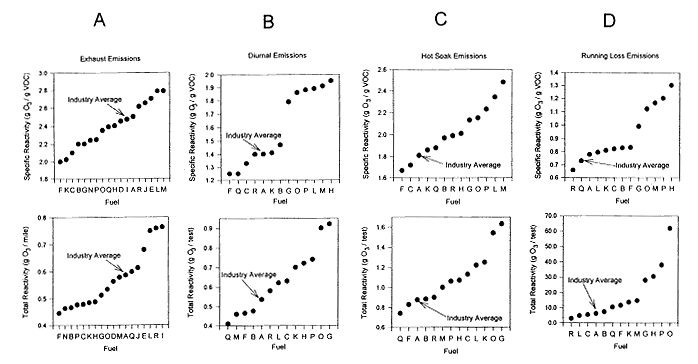
Figure 6-3
AQIRP current fleet vehicles using various RFGs (see Table 6-1) and industry-average fuel. Reactivities are expressed using the MIR scale. (A) Exhaust emissions; (B) diurnal evaporative emissions; (C) hot-soak evaporative emissions; and (D) running-loss emissions. Source: Bums et al. 1992 and Hochhauser et al, 1992.
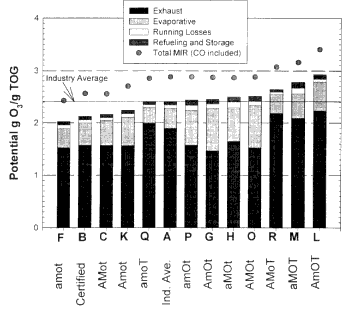
Figure 6-4
Comparison of the specific reactivity (potential g O3/g VOC for the total VOC emissions) with the contribution of using industry-average fuel A and various RFGs. (TOG (total organic gas) is considered to be interchangeable with VOC.) The properties of the fuels and the compositional abbreviations shown on the x-axis are described in Table 6-1.
Emissions are displayed in the bars from top to bottom as follows: refueling and storage, running losses, evaporative, and exhaust. For data represented by circles, the mass of CO emissions is not included in the denominator of the specific reactivity values plotted. The addition of CO reflects the importance of a very low reactivity compound that is emitted along with the VOCs. Source: Adapted from AQIRP Technical Bulletin No. 12, 1993.
weighting of the various emissions to produce a composite emission was made to simulate the conditions present in Los Angeles, California in 1995. Here again we find substantial differences in the reactivities resulting from the fuels tested. The fuel range of reactivities from the least reactive fuel (F) to the most reactive fuel (L) is a factor of about 1.5. However, the reactivity resulting from the least reactive fuel is only about 20% less than that obtained with the use of the industry-average fuel.
Another interesting facet of the reactivities in Figure 6-4 relates to the role of CO. Note in the figure that the circle above each of the bars represents the specific reactivity for the appropriate fuel when the reactivity of the CO emissions is included. The average increase in the reactivities from the inclusion of CO is 18 ± 2%; the ordering of the fuels is also changed somewhat. These results dearly demonstrate the need to include CO emissions when assessing the ozone-forming potential of LDV emissions.
Exhaust Emissions of NOx
The AQIRP data suggest that the effect of RFG on exhaust emissions of NOx will vary depending upon the specific properties of the blend. For example, NOx emissions were lowered by 6 ± 1.9%4 by reducing olefin content from 20 to 5%, while reducing T90 from 390°F to 280°F increased NOx emissions by 5 ± 2.4%, and the impact of lowering aromatic VOC content did not have a statistically significant effect (i.e., NOx emissions were lowered by 2.1 ± 2.0%).
The effect of adding oxygenates to the fuel tended to produce a small increase in NOx emissions. For example, increasing ethanol from 0 to 10% gave rise to a 5 ± 4.1% emissions increase. On the other hand, while adding 15% MTBE and 17% ETBE also resulted in an emissions increase, the increase was not statistically significant (i.e., 3.6 ± 5.4% for MTBE and 5.5 ± 6.4% for ETBE). The average of experiments with added oxygenates was a statistically significant increase of 4.8 ± 2.9%.
By far the largest decrease in NOx emissions were achieved by lowering the sulfur content of the fuel. This effect is discussed in more detail in the next section.
Effect of Fuel Sulfur Content of RFGs on Exhaust Emissions
Dramatic changes in exhaust emissions of all ozone precursors (i.e., VOC, CO, and NOx) were obtained from the sulfur set of AQIRP tests (see Table 6-1). In these tests, the fuel's sulfur content was varied while the
aromatic and alkene VOCs and MTBE compositions, as well as the T90 values, were kept relatively constant (e.g., fuels Y4 to Y8). Figure 6-5 shows that the mass of hydrocarbons (HCs) and CO, and NOx in the exhaust gases generally increase with increased sulfur in the fuel. In Figure 6-6, it can be seen that the mass (milligrams per mile) of the total toxics, benzene, 1,3-butadiene, and acetaldehyde tend to increase with increasing sulfur content of the fuel, and that of formaldehyde decreases somewhat.
Thus, the data from the Auto/Oil Study suggest a very clear effect on the emissions of most reactive organic species as well as NOx, CO, and toxics with the use of low sulfur-containing fuels. In contrast, the effects of sulfur content of the fuel on engine-out emissions (i.e., no flow through the catalysts) were found to be very small. This suggests that the sulfur effect is related to a temporary decrease in catalyst efficiency, most likely because the sulfur reacts with and alters the catalyst surface. However, these effects appear to be largely reversible as sulfur in the fuel is decreased.
Effect of RVP on Emissions
Another major contributor to the reduction of LDV emissions is lowering a fuel's RVP, which significantly reduces evaporative VOC emissions. (Exhaust emissions are also reduced, to some extent, by compositional changes made to RFG blends to lower their RVP.) Overall, lower RVP appears to be the major contributor to lowered VOC emissions resulting from the use of RFG. It is important to note that before implementation of the RFG program, reductions in RVP were mandated and likely led to a significant decrease in VOC emissions. However, appropriate monitoring networks were not in place at that time, and it has been difficult to quantify the impact of RVP reduction.
Have the Changes in Emissions from RFG Blends Indicated by Laboratory Studies Been Observed in Emission Studied Using Tunnels and Remote Sensing of Tailpipe Exhaust?
The laboratory tests of the AQIRP indicate that RFG can result in significant decreases in the emissions of the ozone-forming precursors (reactive
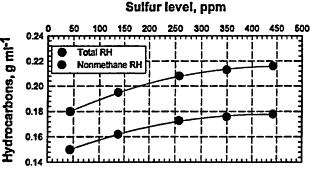
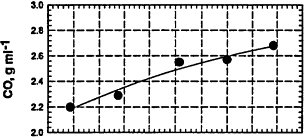
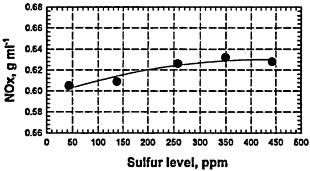
Figure 6-5
Tailpipe emissions (g/mi) for HCs, CO, and NOx from current-fleet vehicles fueled with various RFGs that have very similar hydrocarbon compositions but contain different amounts of sulfur compounds (fuels Y4 to Y8 in Table 6-1). In contrast, the engine-out emissions show very little effect of the sulfur content of the fuel, consistent with the importance of sulfur-catalyst interactions that lower the effectiveness of the catalyst. For the plot of hydrocarbons versus sulfur level, the upper curve corresponds to total hydrocarbons (total RH) and the lower curve corresponds to nonmethane hydrocarbons.
Source: AQIRP Technical Bulletin No. 8, 1992.
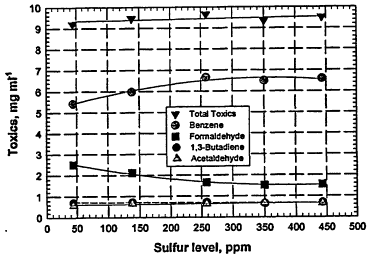
Figure 6-6
Tailpipe emissions (mg/mile) for the toxic compounds (acetaldehyde, formaldehyde, 1,3-butadiene, and benzene) from current-fleet vehicles fueled with various RFGs that have very similar hydrocarbon compositions but contain different amounts of sulfur compounds (fuels Y4 to Y8 in Table 6-1). Source: AQIRP Technical Bulletin No. 8, 1992.
VOC, NOx, and CO), as well as toxics. In its second level of investigation, the committee focuses on whether the effects of RFG blends seen in these laboratory tests are also found in the emissions of motor vehicles operating under actual driving conditions. Emissions studies of this nature are typically carried out in two ways: (1) measuring aggregate emissions from motor vehicles within a tunnel; and (2) measuring tailpipe emissions of individual motor vehicles using remote sensing. It is important to recognize that these types of measurements do not provide a comprehensive measurement of emissions from motor vehicles. Tunnel measurements are conducted in a restricted environment, and as such, they are neither air-quality measurements nor emissions measurements. The emissions data from tunnel studies only measure exhaust plus evaporative running losses, are highly aggregated, and represent a snapshot of on-road emissions of a representative vehicle fleet for specialized driving conditions. Remote-sensing of tailpipe exhaust, on the other hand, largely measures exhaust emissions. Despite these limitations, tunnel studies and remote-sensing provide an important calibration point between automotive emissions tests (such as those carried out in the AQIRP) and studies that attempt to identify a signal from ambient measurements.
Tunnel Studies
A series of tunnel studies was conducted by Kirchstetter et al. (1996, 1997, 1999a,b) for the period from 1994 to 1997 when fuels in California changed from Phase 1 RFG to Phase 2 RFG. In 1994, measurements were performed in August and in October for vehicles operating on California Phase 1 RFG fuels with and without the addition of winter oxygenates, thus allowing for assessment of the effects of oxygenates on emissions. In each year, intense measurements were conducted for 10 days or more during late July and early August. The vehicle fleet, ambient conditions, driving conditions through the tunnel, the tunnel, and the ambient air quality are described in detail in Kirchstetter et al.(1999a). This research affords one of the best opportunities to examine the effects of various types of RFGs on emissions.
Vehicular emissions were measured in the Caldecott tunnel, a heavily used commuter tunnel that runs in the east-west direction through the Berkeley Hills near Berkeley, California, connecting Contra Costa County residential areas to San Francisco. The tunnel has three two-lane bores, and on weekdays, traffic through the central bore is switched from the downhill westbound direction in the morning to the uphill eastbound direction in the afternoon. The tunnel is about 0.7 mi (1.1 kilometer) long, has a nearly constant grade of +4.2% in the eastbound direction, and has fully transverse ventilation provided by adjustable pitch fans.
Sampling was conducted between 4:00 p.m. and 6:00 p.m. when vehicles were traveling in the uphill eastward direction. The nearest on-ramp providing access to the center bore of the tunnel is located more than 0.6 mi away, ensuring that all vehicles in the center bore were in the warmed-up mode. Vehicle counts per hour during the sampling period averaged approximately 4,200. The mean model year for the fleet driving through the tunnel was 1989.3 for the 1995 study, 1990.1 for the 1996 study, and 1990.9 for the 1997 study. Averages were slightly less than the median values. Approximate average fleet composition was 67% cars, 33% vans and sport-utility vehicles, and less than 0.3% heavy-duty trucks; however, light-duty trucks increased from 31% to 35% during the period from 1994 to 1997, with cars exhibiting a corresponding decline. Vehicles traveling through the tunnel were in the hot stabilized mode and averaged 37 mph. Average vehicle speed at the entrance was 32 mph and at the exit was 43 mph. Instrumented vehicular measurements performed during extensive drive-through in 1996
provided additional information about driving conditions. Heavy acceleration and stop-and-go driving were seldom observed. Most of the driving in the tunnel occurred within a small range of speeds and accelerations that is largely within the FTP domain.
Continuous measurements of CO, CO2, and NOx were made in the tunnel exhaust air at a location close to the exit. Background gas concentrations were determined by making measurements in the in-coming ventilation air. Concentrations of CO, NOx, and VOCs were typically 25, 30, and 10 times higher in the tunnel air compared with background air. Two-hour integrated air samples for quantifying hydrocarbons and carbonyls were taken concurrently with the continuous measurements, and analyzed within 48 hr by gas chromatography and high-performance liquid chromatography.
The 1994 Caldecott Tunnel Studies
The 1994 studies of Kirchstetter et al. (1996) are described separately because they afford an opportunity to examine the effects of California Phase 1 gasoline with and without the addition of winter oxygenates. Average properties of gasoline used during various segments of the 1994 study are given in Table 6-2. Unfortunately, in addition to the changes in oxygen content, other fuel properties changed as well. For example, there is a small increase in both sulfur and RVP in the winter gasoline. Each of these tends to result in increased VOC emissions. The increased sulfur content also tends to increase CO and NOx emissions (see Figure 6-5).
The data from the study suggest that the addition of oxygenates (in the form of MTBE) to the fuel during October appeared to lead to a reduction in CO and VOC emissions of 21 ± 7% and 18 ± 10%, respectively. A similar reduction in CO emissions (16 ± 3%) was measured during the Colorado oxygenated fuels program (Bishop and Stedman 1990). NOx emissions showed no change during the two sampling periods. In the case of toxics, formaldehyde emissions increased by 13 ± 6% and benzene emissions decreased by 25 ± 17%, but no significant change on acetaldehyde emissions was observed.
The addition of MTBE also appeared to lead to changes to the relative abundances of individual VOCs and thus might have affected the reactivity of the emissions. However, analysis of the data indicated that
TABLE 6-2 Average Properties of California Bay Area Phase 1 RFG for August and October 1994
|
|
Sampling Periodb |
|
|
Fuel Propertya |
August 1994 (Low Oxygenates) |
October 1994 (High Oxygenates) |
|
Oxygen content (wt %) |
0.3 ± 0.4 |
2.0 ± 0.2 |
|
Sulfur (ppm by wt) |
54 ± 47 |
90 ± 53 |
|
Reid vapor pressure (psi) |
7.2 ± 0.2 |
7.7 ± 0.3 |
|
Paraffins (vol %) |
47 to 54 |
38 to 46 |
|
Aromatics (vol %) |
34 to 43 |
26 to 35 |
|
Olefins (vol %) |
0.4 to 7.3 |
4.3 to 13.4 |
|
Naphthenes (vol %) |
2.9 to 10.4 |
4.1 to 9.6 |
|
Benzene (vol %) |
1.7 to 5.1 |
1.0 to 3.6 |
|
a Gasoline composition was determined by the California Air Resources Board (CARB), and was based upon averaging 65 samples during the August period and 54 samples during the October period. On an oxygen weight basis, 80% of the oxygenate was MBTE and 20% was ethanol. b Errors are reported as 1 o (standard deviation) of the mean. Source: Adapted from Kirchstetter et al. 1996. |
||
the normalized VOC reactivity, using the MIR scale (see Chapter 3), did not change significantly from the low-oxygenate to the high-oxygenate period.
The 1994-1997 Studies
During the five sampling periods of this study, California gasoline changed composition from the 1994 summer and fall values indicated in Table 6-2 to California Phase 2 RFG. The evolution of the average summer gasoline properties during the study is summarized in Table 6-3. Emissions of all pollutants decreased by between 20% and 40% over the 1994 to 1997 study period (Kirchstetter et al. 1999a). However, attributing these changes to a specific cause such as an RFG blend is problematic because of the difficulty in separating the effects of fleet turnover from those of fuel changes. Using a statistical time-series analysis to separate these two effects, Kirchstetter et al. concluded that the effect of
TABLE 6-3 Summer (July and August) Gasoline Properties in California from 1994 to 1997
|
|
RVP (psi) |
API Gravity |
Oxygen (wt %) |
Sulfur (ppm wt) |
Saturates (vol %) |
Olefins (vol %) |
Aromatics (vol %) |
MTBE (vol %) |
Benzene (vol %) |
ASTM D-87 |
Density (kg/m3) |
||
|
Year |
|
|
|
|
|
|
|
|
|
T10 |
T50 |
T90 |
|
|
1994 |
|||||||||||||
|
Avg. |
7.4 |
54.4 |
0.51 |
131 |
57.4 |
7.9 |
31.9 |
2.7 |
1.56 |
136 |
214 |
334 |
761 |
|
sd |
0.1 |
1.9 |
0.32 |
41 |
4.8 |
4.4 |
2.1 |
1.7 |
0.39 |
3 |
8 |
8 |
8 |
|
1995 |
|||||||||||||
|
Avg. |
7.4 |
54.7 |
0.21 |
81 |
56.5 |
8.8 |
33.7 |
1.0 |
1.54 |
136 |
218 |
341 |
760 |
|
sd |
0.1 |
1.0 |
0.18 |
36 |
5.1 |
3.5 |
3.3 |
0.9 |
0.45 |
3 |
4 |
8 |
4 |
|
1996 |
|||||||||||||
|
Avg. |
7.0 |
58.9 |
1.96 |
16 |
62.6 |
3.3 |
23.5 |
10.7 |
0.42 |
138 |
199 |
300 |
743 |
|
sd |
0.1 |
0.6 |
0.30 |
9 |
2.5 |
0.9 |
1.4 |
1.7 |
0.08 |
2 |
4 |
4 |
2 |
|
1997 |
|||||||||||||
|
Avg. |
7.1 |
59.5 |
1.57 |
12 |
65.4 |
3.4 |
22.7 |
8.2 |
0.43 |
138 |
200 |
299 |
741 |
|
sd |
0.1 |
1.3 |
0.60 |
11 |
3.7 |
1.2 |
1.4 |
3.7 |
0.05 |
2 |
3 |
6 |
5 |
|
Abbreviations: Avg., average; sd, standard deviation. Source: Adapted from Kirchstetter et al. 1999a. |
|||||||||||||
an RFG was greater on VOCs than on NOx. No significant change was observed in acetaldehyde emissions, whereas the effect of RFG blends on benzene was estimated to be a 30% to 40% reduction, and on formaldehyde, a 10% increase.
Kirchstetter et al. (1999b) attempted to characterize evaporative emissions that are affected by gasoline vapor pressure (i.e., those due to refueling, running loss, and diurnal evaporation). Bay Area gasoline was analyzed to determine composition, and headspace vapor composition was estimated using the Wagner equation (see Reid et al. 1987). The individual compound vapor pressures were determined from the vapor pressure of the pure species, its mole fraction, and activity. Combining that information with the emissions data from the tunnel, the change to California Phase 1 RFG was estimated to cause a 13% vapor-phase reduction in evaporative emissions, and the change to Phase 2 caused a further 9% reduction, giving rise to a net reduction of evaporative emissions from California RFGs of 20%. (Normalized reactivity of liquid gasoline and headspace vapors decreased by 23% and 19%, respectively.) Combining that result with those for exhaust emissions indicated that the ozone-forming potential (measured as total reactivity by the MIR scale) of all on-road emissions decreased by 8% or less as a result of RFG blends. The total reactivity decrease was less than that of evaporative mass emissions because of increased weight fractions of highly reactive iso-butene and formaldehyde in the exhausts (from the combustion of MTBE).
Collectively, the Caldecott tunnel studies suggested that there were significant reductions in the motor-vehicle emissions of all pollutants (except formaldehyde) between 1994 and 1997. These decreases, summarized in Table 6-4, are attributable to a combination of the use of RFG and fleet turnover effects, with RFG most likely making a significant contribution. However, some caution should be exercised before using these results to characterize the overall effect of RFGs on motor-vehicle emissions. As noted above, any number of factors can have a significant effect on emissions from motor vehicles (e.g., age, stop-and-go driving, and cold-start conditions) that are minimally represented in the Caldecott tunnel.
Finally, Kirchstetter et al. observed high concentrations of ethene and acetylene in the tunnel, which are indicative of reduced catalytic-converter activity that results in high-emitting vehicles. Because decreases in sulfur concentration in gasoline have little effect on such high-emitting vehicles, one interpretation of the study results suggests that high emitters, such as older-technology vehicles or vehicles with faulty
TABLE 6-4 Decrease in Emissions 1994-1997 Inferred from the Caldecott Tunnel Studiesa
|
Emission |
% Decrease |
|
CO |
31 ± 5 |
|
Nonmethane VOC |
43 ± 8 |
|
NOx |
18 ± 4 |
|
a A fraction of these decreases are attributable to RFG and a fraction to fleet turnover. |
|
catalytic converters, might be responsible for a disproportionate share of the VOC emissions in the tunnel. As discussed below, a similar conclusion was reached by Beaton et al. (1995) using on-road remote sensors in urban locations in California.
Remote-Sensing Studies
Remote sensing has been used to infer the amount of ozone precursors (CO, NO and total VOCs) in exhaust emissions from individual in-service LDVs relative to the emission of CO2 in the exhaust (Bishop and Stedman 1989, 1990; Bishop et al. 1989; Guenther et al. 1991, Zhang et al. 1993, 1996a,b, Stedman et al. 1994, 1997, Butler et al. 1994). In this technique, light at specific wavelengths in the infrared OR) or ultraviolet (UV) spectrum is passed through the exhaust plumes of passing motor vehicles. The measurement and analysis, which is based on the amount of light absorbed by the compounds contained in the exhaust, have been shown to quantitatively determine CO emissions in the exhausts to within ±5% and VOC emissions to within ±15% (Lawson et al. 1990; Stephens and Cadle 1991; Ashbaugh et al. 1992).
In typical studies, the technique is deployed at frequently traveled roadways, such as freeway entrances and exits. Used that way, the technique measures exhaust emissions under nominal roadway operation. The technique has been deployed at a variety of locations in the United States and abroad. Provision can be made to record the identity of individual vehicles to determine the emissions demographics of the vehicle fleet. The technique has been extensively used to establish the trends in emissions as a function of vehicular age, to monitor the effectiveness of vehicular maintenance and inspection programs, and to measure the effect of the addition of oxygenated compounds to fuel to reduce emissions of CO and VOCs.
Remote-sensing measurements show that, in every model year from pre-1971 to 1991 for the given fuel supply (Zhang et al. 1993; Beaton et al. 1995), there has been a steady reduction in exhaust emissions of VOCs and CO (see Figure 6-7 for data on VOC emissions). The measurements also indicate that, although there has been a steady decline in exhaust emissions in the more recent models, the exhaust emissions for each model year are dominated by a relatively small number of high emitters (i.e., the vehicles in the fifth quintile in Figure 6-7). The results show that, for each model year, properly maintained vehicles provide only a small contribution to the emissions from that model year compared with poorly maintained or malfunctioning vehicles.
The remote-sensing technique has been used extensively in Denver to study the efficacy of the use of oxygenated fuels to reduce CO exhaust emissions from LDVs. In Denver, CO pollution is most severe during the winter and the Colorado program specifically targeted reductions in winter CO emissions from LDVs by the addition of oxygenated compounds to the gasoline sold in Colorado. In the first Denver study, the CO emissions were measured from approximately 60,000 vehicles at a freeway on-ramp during and after the oxygenated fuel season. Bishop and Stedman (1989) reported a 6 ± 2.5% reduction in CO attributable to the use of oxygenated fuel containing 2.0 wt % oxygen. In a second Denver study, Bishop and Stedman (1990) analyzed vehicular emissions from more than 117,000 vehicles at two Denver locations (a freeway on-and off-ramp) before, during and after a winter season when oxygenated fuels were mandated (November 1988 through February 1989). They reported a 16 ± 3% decrease in CO emissions from the use of oxygenated fuel at 2.0% oxygen.
A followup study using this technique was carried out in Denver to determine the effectiveness of the 1991-1992 winter Colorado oxygenated fuels program (PRC 1992). Based on the results, the percentage reduction of CO emissions was nearly the same for all vehicles, and most of the reduction in CO emissions attributed to oxygenated-fuel use were from the highest-emitting vehicles (Figures 6-8 and 6-9). Even though a small portion of the vehicles tested were high emitters, those vehicles contributed a substantial portion of the CO emissions. The study indicated a comparable result for the reduction in exhaust emission of VOCs.
An important finding of these remote-sensing measurements was that most of the overall CO and VOC emissions and the reductions in these emissions from the use of RFGs are associated with emissions from high emitters and, more specifically, from vehicles with malfunctioning emissions controls. The studies (Bishop and Stedman 1989, 1990, 1995;
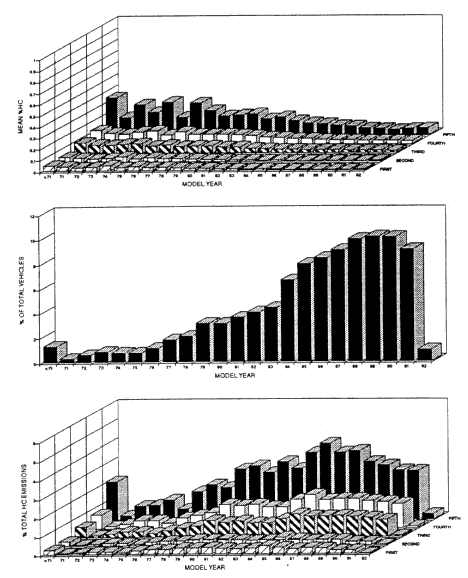
Figure 6-7
Location-specific data from the Denver area using remote sensing. Part A shows emission factors by model year divided into five groups (quintiles) in ascending order of emissions. Part B shows the vehicle age distribution of the measured fleet. Part C is the product of data from Parts A and B; percentage of total HC (or VOC) emissions is shown for each quintile of each model year.
Source: Zhang et al. 1993. Reprinted with permission from Environmental Science and Technology, copyright 1993, American Chemical Society.
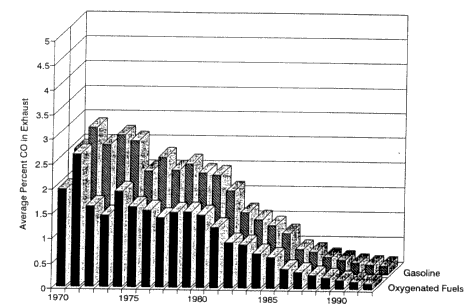
Figure 6-8
CO emissions by model year of motor vehicle as recorded at a specific location in the Denver area. Source: PRC 1992.
Bishop et al. 1989; Guenther et al. 1991; PRC 1992; Zhang et al. 1994, 1996a,b; Beaton et al. 1995; Stedman et al. 1997) find that the addition of oxygenated fuels reduces the exhaust emissions of CO and VOCs by approximately 20% for all model years in LDVs with the largest emissions. For example, Beaton et al. (1995) placed on-road remote sensors of exhaust CO and VOC emissions at various urban locations in California. They found that 7% of the vehicles accounted for more than 50% of CO emissions and 10% of the vehicles accounted for more than 50% of the VOC emissions. This group probably involves LDVs that were not well maintained or have otherwise improperly functioning emissions control systems. Because this finding was independent of the model year, it implies that a relatively small percentage of vehicles with the highest exhaust emissions will be the principle sources of the exhaust emissions of CO and VOCs and that the use of oxygenated fuels in those vehicles will be of the greatest benefit by reducing the exhaust emissions of CO and VOCs by approximately 20%.
The remote sensing methods, by virtue of their deployment, observe LDVs under "cold-start" conditions, and emissions estimates based on the method will fail to account for the cold-start fraction of the total emis-
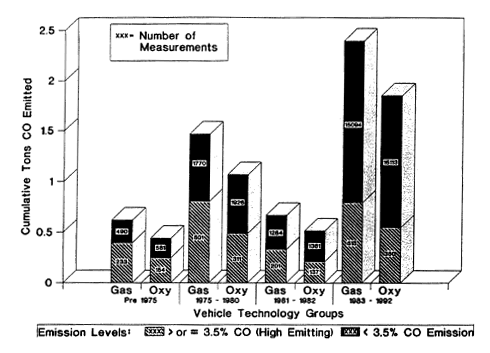
Figure 6-9
CO emissions contribution from high-emitting vehicles (hatched) and lower-emitting vehicles (black) by vehicle-technology grouping. High emitters are defined as having emissions with more than 3.5% CO; lower-emitting vehicles have emissions with less than 3.5% CO. The number on each bar segment refers to the number of cars recorded in the sample of vehicles measured at a specific location in the Denver area. Source: PRC 1992.
sions. Because catalytic converters will operate at reduced effectiveness under cold-start conditions, the relative importance of high emitters under these conditions might be less. If cold-start emissions represent a sizable fraction of the total exhaust emissions, the benefits of oxygenated fuels may be more uniformly spread across the light-duty vehicle fleet.
Are There Data to Support Meaningful Analysis of Atmospheric Data to Determine the Effect of RFGs?
The tunnel studies and remote-sensing measurements discussed in the previous section have provided useful information concerning the effects of RFG blends on the emissions of a variety of ozone precursors, including CO from LDVs. However, it is difficult to relate these snapshot assessments of LDV exhaust and running losses to the actual net effect
of RFGs on air quality. To accomplish this, the use of atmospheric measurements as an assessment must be examined.
The attribution of the trends of ambient ozone concentrations and those of its precursors to specific control policies is complicated by the presence of confounding influences such as meteorological fluctuations (NRC 1991; Rao et al. 1992, 1998; Cox and Chu 1993; Milanchus et al. 1998). Therefore, answering questions such as "what portion of the change in ambient ozone concentrations can reasonably be attributed to a particular emissions control policy?" requires the existence of high-quality, long-term concentration data gathered in a carefully designed network. Therefore, it is critical that a spatially well-designed monitoring network be in place to measure precursors as soon as possible. When time series of ozone and precursor data covering the pre- and post-implementation time periods are available for the regions where the control program is in effect and where it is not in effect, one can apply space-time analyses and change-point detection techniques as suggested by Rao et al. (1998), Hogrefe et al. (1998), and Zurbenko et al. (1996) to observe the effects of the emission control strategy on ambient pollutant levels.
During the last 30 years, there have been extensive data sets acquired from integrated field measurements and the estimation of long-term trends from those measurements. However, these measurements were not aimed at determining specifically the effectiveness of particular air-quality regulations. The measurements were aimed at assessing the reductions in concentrations of criteria pollutants or w determine the processes or sources of the primary emissions that limit these reductions. In the case of ozone, which is formed by secondary photochemical reactions, these measurements were not designed to determine the alteration of ozone concentrations that results from the RFGs. Unfortunately, when the planning of integrated field measurements or monitoring fails to include directed observations to document a particular aspect of air-quality regulations, it is generally not possible to isolate these effects from the data that have been acquired for other purposes. For these reasons, the ability to discern an "RFG signal" in the ambient data sets is quite limited. At this time, researchers are only able to even attempt such an analysis for a limited set of relevant species: RFG oxygenates, toxics, CO, and ozone. Rao et al. (1998) concluded that a goal of trend assessment should be to isolate and characterize long-term (greater than 1-year concentrations of pollutants) information based on multi-variate analyses of ambient weather, climate, and emissions. All
long-term variation should be considered without regard to a particular trend model (e.g., linear, step, or ramp).
Have Changes in the Concentrations of AIR Toxics or Oxygenated Been Observed in the Atmosphere and Can These Changes be Related to the USE of RFG?
Benzene is both an ozone precursor and an air toxic and, as a result, regulations have specifically targeted its reduction. LDV emissions of benzene are derived directly from benzene and from higher aromatics in the fuel. The RFG programs, with their prescribed reductions in benzene and other aromatics (see Table 5-3), are intended to reduce ambient benzene concentrations. Although reductions have been observed in the atmospheric concentrations of benzene over the past several years (EPA 1998), the observations are not capable of attributing these reductions to a particular control strategy or to differentiate between different oxygenates used in fuels. Because oxygenated compounds were added to RFGs specifically to replace benzene and other aromatic compounds, it is reasonable to assume that at least part of the observed reduction in ambient concentrations is associated with the reduction in vehicular emissions as a source. To date, although reductions are observed at many locations in various VOC concentrations including larger aromatic compounds, the trends are not sufficiently consistent to draw definite conclusions.
Both MTBE and ethanol have been observed to be present in the atmosphere. These compounds can serve as ozone precursors, but because their atmospheric reactivity is low, they are not expected to be as effective as more-reactive VOCs in generating ozone in urban environments. However, like benzene and CO, they might be more effective in ozone formation farther downwind of the source of their emission. Because the only identified use for MTBE is as a motor-fuel additive, it is reasonable to assume that its presence in the atmosphere is associated with the emissions from LDVs using fuels with an MTBE additive. In this connection, MTBE could serve as an important tracer to determine the influence of its addition to motor fuel on the other compounds of interest.
By contrast, ethanol has many natural and anthropogenic sources. To date, no analysis has yet been carried out to determine if or how
much of the burden of ethanol in the atmosphere is associated with its use as a fuel additive.
Have Changes in the Concentrations of CO Been Observed in the Atmosphere and Can These Changes be Related to the USE RFGs?
Because motor vehicles are the primary source for CO, the U.S. Environmental Protection Agency (EPA) required that urban areas classified as nonattainment for CO use oxygenated fuels in gasoline-fueled engines during the winter season beginning in 1992. CO is primarily a winter problem because low surface temperatures limit the dispersion of the pollutant and enhance its emissions from cold engines. As outlined in Table 5-4, oxygenated fuels in most CO nonattainment areas are blended to contain a minimum of 2.7% oxygen by weight.
The Oxygenated Fuels Program has now been in effect for at least five winters in several different metropolitan areas, a time interval that might be long enough to begin an assessment of whether or not this program has or has not been effective. In fact, recently, a number of researchers have attempted to assess the impact of fuels on ambient CO concentrations (Mannino and Etzel 1996; Cook et al. 1997; Dolislager 1997; Whitten et al. 1997). Those studies have generally concluded that the oxygenated fuels program has resulted in a discernable downward trend in ambient CO concentrations. However, in the committee's view, the studies are not conclusive. The Oxygenated Fuels Program was initiated in the midst of other control programs and technological improvements designed to lower CO emissions. Colorado, for example, places restrictions on both wood-burning stoves and driving times when CO concentrations are likely to be high. Most likely, such other programs and improvements have had some downward effects on CO emissions. Discerning the portion of the downward CO trend in an area that is specifically attributable to oxygenated-fuel use is a challenging problem. Another problem arises from inhomogeneities and discontinuities in the way in which CO data are reported. During the 1990s, the reporting of CO data in the United States was changed from rounding to the nearest 1 ppm to the nearest 0.1 ppm. Such discontinuities can produce an artifact in a trend analysis that confounds identification of an impact of a control program.
To illustrate such problems, CO data from areas using oxygenated fuels were analyzed. It is important to note that this analysis is not intended to be a comprehensive assessment of the relationship between oxygenated fuel and ambient CO but just an illustration of the difficulties such analyses can encounter. The regions analyzed are broadly the states of New York, New Jersey, and Connecticut (NY/NJ/CT); Colorado; and California. Within the NY/NJ/CT region, the New York City Metropolitan Statistical Area (NYCMSA), implemented its program during the winter of 1992-1993. Colorado implemented a statewide oxygenated fuels program in 1988, and California during the winter of 1992-1993.
All data used in the analysis presented here were obtained from the EPA's Aerometric Information Retrieval System (AIRS). The monitoring sites considered are listed in Table 6-5. There are 2 sites in Connecticut, 10 sites in New Jersey, 8 sites in New York, 16 sites in California, and 10 sites in Colorado. With the exception of California, these sites were chosen because of the length of their CO time-series records. The sites in California are the same as those used by Dolislager (1997), and are sites which have reasonably complete records that include violations of the 8-hr standard for CO during 1990-1993.
There are some problems with the raw hourly data because of the way in which the lowest values (detection limits) were reported. For the northeastern-states sites and Colorado sites, retaining only the daily maxima of 1-hr concentrations eliminates this problem. However, for the sites in California, the hourly data were first rounded to the nearest part per million prior to extracting the maxima. Rather than examining the reported daily maxima, their logarithms were examined for this report to help stabilize the variability due to seasonal variation.
The various physical processes reflected in each of the CO time-series were separated into three components that contribute independently to the overall trend. These components are a short-term component (attributable to fluctuations in weather and day-to-day emissions); a seasonal-variation component (attributable to the Earth's rotation around the Sun); and a long-term component (attributable to secular, or lasting, changes in climate or emissions). It is this last component that is most important here, because the effect of control policies must be manifested in this component.
Table 6-5 shows the amount contributed by each component to the total variance of the data at each monitoring site. In all three of the regions studied here, the short-term component contributes the most to the total variance, especially in the NY/NJ/CT region. The contribution of
TABLE 6-5 AIRS Data from Selected Sites in California, Colorado, and Northeastern States (1980-1997)
|
AIRS ID |
Station Location |
Percent Contribution to the Total Variance |
Implementation Date (yymmdd) |
% Improvement (∆) |
2σ -Level for ∆ (%)a |
|||
|
Short-term |
Seasonal |
Long-term |
||||||
|
090010004 |
Bridgeport |
CT |
74.4 |
6.6 |
15.5 |
921101 |
1.4 |
5.2 |
|
090010020 |
Stamford |
CT |
49.3 |
9.4 |
36 |
921101 |
-22.2 |
12.2 |
|
340035001 |
Hackensack |
NJ |
75.6 |
5.4 |
16.1 |
921101 |
7.6 |
4.8 |
|
340051001 |
Burlington |
NJ |
60.6 |
12.7 |
21.4 |
N/A |
11.2 |
5.0 |
|
340070003 |
Camden |
NJ |
76.3 |
10.5 |
8.9 |
N/A |
-7.1 |
5.5 |
|
340071001 |
(Not in a city) |
NJ |
63.8 |
14.5 |
15.6 |
N/A |
-5.8 |
8.5 |
|
340171002 |
Jersey City |
NJ |
74.5 |
1.6 |
22.4 |
921101 |
9.4 |
5.6 |
|
340232003 |
Perth Amboy |
NJ |
62.8 |
3.8 |
30.1 |
921101 |
-3.0 |
7.8 |
|
340252001 |
Freehold |
NJ |
63.9 |
5.3 |
27.7 |
921101 |
-7.8 |
3.9 |
|
340270003 |
Morristown |
NJ |
64.7 |
3.6 |
28.7 |
921101 |
4.6 |
3.7 |
|
340292001 |
Toms River |
NJ |
72.9 |
5.4 |
17.6 |
N/A |
1.8 |
5.7 |
|
340390003 |
Elizabeth |
NJ |
79.0 |
2.6 |
16.6 |
921101 |
7.9 |
6.3 |
|
360290005 |
Buffalo |
NY |
81.8 |
2.5 |
13.4 |
N/A |
-7.1 |
3.2 |
|
360290016 |
Buffalo |
NY |
77.4 |
2.5 |
17.6 |
N/A |
-8.9 |
4.8 |
|
360551004 |
Rochester |
NY |
84.7 |
2.9 |
10 |
N/A |
-11.6 |
8.2 |
|
360556001 |
Rochester |
NY |
67.5 |
5.1 |
23.8 |
N/A |
6.4 |
5.8 |
|
360590005 |
(Not in a city) |
NY |
78.6 |
6.0 |
12.2 |
921101 |
9.3 |
3.7 |
|
AIRS ID |
Station Location |
Percent Contribution to the Total Variance |
Implementation Date (yymmdd) |
% Improvement (∆) |
2σ -Level for ∆ (%)a |
|||
|
Short-term |
Seasonal |
Long-term |
||||||
|
360610062 |
New York City |
NY |
49.6 |
3.3 |
43.9 |
921101 |
-4.2 |
10.6 |
|
360632006 |
Niagara Fails |
NY |
80.4 |
2.9 |
13.6 |
N/A |
5.7 |
6.5 |
|
360930003 |
Schenectady |
NY |
73.5 |
11.2 |
10.4 |
N/A |
0.1 |
11.8 |
|
060190008 |
Fresno |
CA |
49.1 |
37.4 |
1.3 |
921101 |
-4.3 |
13.0 |
|
060371002 |
Burbank |
CA |
49.3 |
32.7 |
8.3 |
921101 |
-1.7 |
5.2 |
|
060371103 |
Los Angeles |
CA |
63.7 |
26.1 |
2.2 |
921101 |
1.3 |
3.0 |
|
060371201 |
Reseda |
CA |
57.5 |
26.7 |
7.4 |
921101 |
-7.5 |
7.4 |
|
060371301 |
Lynwood |
CA |
44.2 |
41.8 |
1.4 |
921101 |
1.8 |
5.4 |
|
060372005 |
Pasadena |
CA |
57.6 |
29.1 |
3.8 |
921101 |
-3.9 |
5.3 |
|
060375001 |
Hawthorne |
CA |
51.1 |
37.4 |
1.5 |
921101 |
1.6 |
4.7 |
|
060590001 |
Anaheim |
CA |
45.9 |
35.4 |
7.7 |
921101 |
-3.0 |
9.1 |
|
060591003 |
Costa Mesa |
CA |
49.0 |
37.8 |
1.6 |
921101 |
4.7 |
8.6 |
|
060595001 |
La Habra |
CA |
49.4 |
36.1 |
4.4 |
921101 |
12.2 |
6.1 |
|
060670006 |
Sacramento |
CA |
67.0 |
21.6 |
3.7 |
921101 |
1.0 |
5.4 |
|
060670010 |
Sacramento |
CA |
61.6 |
26.7 |
1.6 |
921101 |
2.4 |
7.1 |
|
060771002 |
Stockton |
CA |
65.4 |
24.2 |
2.1 |
921101 |
11.2 |
5.2 |
|
060850004 |
San Jose |
CA |
48.9 |
35.7 |
4.1 |
921101 |
24.3 |
5.6 |
|
AIRS ID |
Station Location |
Percent Contribution to the Total Variance |
Implementation Date (yymmdd) |
% Improvement (∆) |
2σ -Level for ∆ (%)a |
|||
|
Short-term |
Seasonal |
Long-term |
||||||
|
060950004 |
Vallejo |
CA |
59.0 |
29.5 |
1.2 |
921101 |
3.1 |
4.3 |
|
060990005 |
Modesto |
CA |
59.2 |
27.8 |
2.8 |
921101 |
16.0 |
5.9 |
|
080050002 |
Littleton |
CO |
64.2 |
19.0 |
9 |
880101 |
-3.0 |
11.6 |
|
080131001 |
Boulder |
CO |
56.0 |
18.2 |
19.4 |
880101 |
9.4 |
13.2 |
|
080310002 |
Denver |
CO |
55.3 |
14.6 |
25 |
880101 |
32.5 |
6.8 |
|
080310013 |
Denver |
CO |
61.1 |
16.0 |
17.4 |
880101 |
4.4 |
3.7 |
|
080310014 |
Denver |
CO |
58.1 |
22.7 |
11.8 |
880101 |
5.7 |
5.1 |
|
080410004 |
Colorado Springs |
CO |
64.0 |
15.0 |
15.4 |
880101 |
5.3 |
6.8 |
|
080410006 |
Colorado Springs |
CO |
72.3 |
12.0 |
10.9 |
880101 |
11.8 |
5.8 |
|
080590002 |
Arvada |
CO |
60.3 |
19.7 |
13.4 |
880101 |
23.7 |
7.0 |
|
080691004 |
Fort Collins |
CO |
61.7 |
22.6 |
9.1 |
880101 |
3.7 |
12.2 |
|
081230007 |
Greeley |
CO |
56.4 |
29.4 |
5.2 |
880101 |
15.6 |
4.2 |
|
a A positive value corresponds to a decrease in CO levels; a negative value corresponds to an increase. |
||||||||
the long-term component is large in NY/NJ/CT and Colorado, but very small in California.
The behavior of the CO rime-series at Riverside, California, is presented in Figure 6-10. (Note that Riverside is not included in Table 6-5.) A change in the detection limits and resolution or dam-reporting practices around 1994 is apparent from an inspection of the lower values shown in Figure 6-10A. The strongest decline in CO levels has occurred since 1987 (see Figure 6-10D). It should be noted that California introduced Phase 2 RFG in 1996 and winter oxygenates in 1992. The presence of strong downward trends in CO throughout the time series in the post-1987 period complicates evaluation of mid-series changes to regulatory policy. Examination of CO concentrations before and after implementation of the oxygenated fuels program might very well indicate a decrease in CO, but this decrease may be indicative of the overall downward trend that began well prior to the implementation of the program as opposed to the program itself.
To discern the contribution of oxygenated fuels to a trend such as that depicted in Figure 6-10, an analytical approach is needed that attempts to identify an abrupt "break" or change in the trend line at the time the program was first implemented. One such approach uses a linear regression on the long-term component (i.e., trend) for the period prior to the program implementation. That linear trend, prevailing prior to implementation of the program, is removed from the long-term component of the entire time series. Linear trends are then estimated for the detrended data for the pre- and post-implementation time periods. (By definition, the slope and intercept of the trend for the pre-implementation time period are zero.) The change in the intercept (∆) in the pre-implementation time period at the date of fuels program implementation is an estimate of the percent change in CO concentrations attributable to that program. A confidence level (2σ) for the change, ∆, is also computed. ∆ is positive (negative) for a decrease (increase), i.e., improvement (deterioration), in CO levels.
Values of ∆ for each site included in this study are listed in Table 6-5 along with their respective 2σ confidence intervals. When ∆ is greater than 2σ, the value derived for ∆ is statistically significant at the 95% confidence level; when ∆ is smaller than 2σ, the effects of the oxygenated fuels program on CO at that site cannot be discerned reliably from the data.
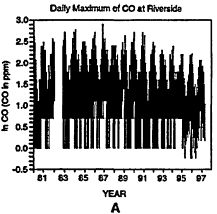
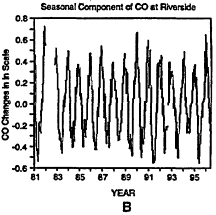
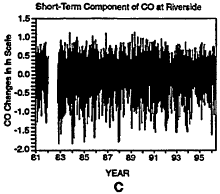
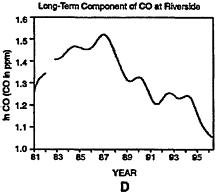
Figure 6-10
Daily maxima of CO concentrations at Riverside, CA, from 1980 to 1997 (A). Three components of the overall trend are seasonal (B), short term (C), and long term (D).
Examination of the ∆ and 2σ values in Table 6-5 reveals varied results for the sites.5 Most sites had positive ∆ values (indicative of a benefit from the oxygenated fuels program). However, a substantial fraction (14 out of 46) of the sites had negative ∆ values, and for many of the sites (23 out of 46) the ∆ values were not significant at the 2σ (or
95%) confidence interval. Thus, while this analysis suggests that the oxygenated fuels program probably has had some small ameliorative effect on CO concentrations, its impact does not appear to be spatially uniform and in many cases is too small to discern with a high degree of statistical confidence.
A very similar conclusion was reached in a report of the National Science and Technology Council (NSTC 1997). The NSTC report reviewed various studies relating to the ambient air-quality effects of oxygenated fuels. It concluded that CO concentrations in urban areas have been decreasing at a rate of 2.8% per year for the last 10 years. This decrease is attributable primarily to EPA-mandated motor-vehicle emissions standards and improved vehicular emissions control technology. However, the NSTC report concluded that the benefits of oxygenated fuels on ambient air quality in cold climate areas could not be confirmed. (See Anderson et al. (1994) for additional information on the influence of oxygenated fuels on ambient CO.)
Have Changes in the Concentrations of Ozone Been Observed in the Atmosphere and Can These Changes be Related to the USE of RFGs?
Assessing the effects of RFG on ambient ozone air quality involves challenges similar to those discussed above for CO. For example, Larsen and Brisby (1998) attempted to assess the effect of California's cleaner-burning gasoline program on ozone concentrations. In that study, for the Sacramento, South Coast, and San Francisco Bay areas, Larsen and Brisby reported ozone decreases of 14%, 17%, and 4%, respectively. However, the contribution of cleaner-burning gasoline to this decrease is uncertain because of the presence of many other ongoing ozone-mitigation efforts. To address this problem, Larsen and Brisby assumed that the contribution of the cleaner-burning fuels program to the overall ozone decrease was proportional to the estimated percent reduction in the precursor emission inventory resulting from the program. Thus, even though the Larsen and Brisby study was based on ambient ozone concentrations, the attribution of a portion of the observed ozone decrease to the use of cleaner-burning gasoline was derived from an emission inventory and does not constitute empirical verification of program effectiveness.
To further illustrate some of the difficulties with applying trend analysis to ambient ozone data, consider the log-transformed ozone concentrations from Riverside, California, presented in Figure 6-11.6 As in the CO analysis, the data are decomposed into its long-term, seasonal, and short-term components. Because the information from the moving-average filter (Zurbenko et al. 1996) used here is not reliable at the beginning and at the end of the time-series, data for the first and last years are not included in these figures. At this site, the long-term, seasonal, and short-term components contribute about 2%, 63%, and 34%, respectively, to the total variance of the ozone data.
To examine whether the use of RFGs in California had an impact on ambient ozone concentrations, data during the 1980-1997 period from several locations in the Los Angeles Air Basin of California were also analyzed. As was the case for the CO analysis in the previous section, an overall downward trend in ozone over the past 15-year period is evident in the long-term component at Riverside (Figure 6-11D). Between 1981 and 1996, ozone has decreased by about 30% at Riverside; the largest decrease of about 20% in ozone concentrations occurred between 1989 and 1993. Ozone then increased slightly in 1994, and then decreased again in 1995.
Whereas the oxygenated fuels program was implemented in California in 1992, the RFG program was implemented in 1996. Figure 6-11 indicates the presence of a strong downward trend in ozone before these programs were implemented. Unfortunately, data for the time period after the RFG program was implemented are not yet available for this type of analysis to dearly discern the impact of this control strategy on ozone air quality. For example, if an abrupt change of 10% in the middle of ozone time-series data illustrated in Figure 6-11 were introduced, it would contribute only about 0.5% to the total variance. This illustrates that the detection of any abrupt change of the order of 10% or less and its attribution to a specific control of an emission is a formidable task.
These results demonstrate the difficulty in linking a particular emissions-control policy to a change in ozone concentrations. Clearly, the problem of assessing the effectiveness of a particular air-pollution control program requires further development.
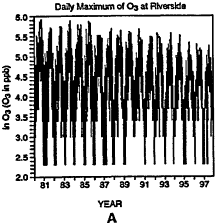
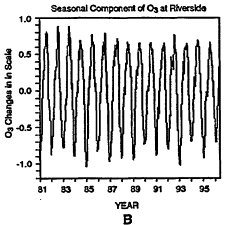
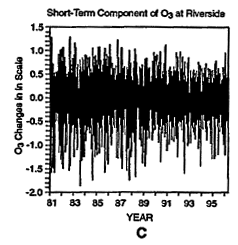
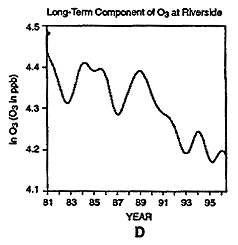
Figure 6-11
Daily maxima of ozone concentrations at Riverside, CA, from 1980 to 1997 (A). Three components of the overall trend are seasonal (B), short term (C), and long term (D).
Documentation of RFG Effects in a Future Observational Program
On January 1, 2000, federal Phase II reformulated gasoline (RFG) will be required in commercially available LDVs operating in areas classified as being in severe nonattainment of the National Ambient Air Quality Standard (NAAQS) for ozone. On the basis of estimates from the Com-
plex Model, EPA expects that this action will result in reductions in both exhaust and evaporative emissions of VOCs and some air toxics from LDVs, as well as LDV exhaust emissions of CO and NOx. It is further believed that these emissions reductions will help alleviate the severity of the ozone pollution in the severe nonattainment areas where the program is to be implemented, although, for the reasons discussed above, these effects are not expected to be large or even observable.
Will the projected air-quality benefits of Phase II of the federal RFG program be met? As with any regulatory program, the committee recommends that a complete and comprehensive RFG program should include—part and parcel—a plan for documenting the impact of the program and assessing to what extent the expected benefits are realized. The committee further recommends that this plan be organized around addressing a progression of three scientific questions7 that attempt to document the effect of Phase II RFG on ozone precursor compounds and their ozone-forming potential. (Ideally, such a plan would include a fourth question that addresses the effect of the Phase II RFG on ozone concentrations. However as discussed above, it is unlikely that such a signal in ambient ozone concentrations could be discernible given the relatively large variability in ozone, the myriad factors that affect ozone concentrations, and the rather small overall impact RFG is projected to have on ozone.) The three questions recommended here for consideration are briefly discussed below.
Question 1: Do in-use Phase II RFG blends decrease the emissions from LDVs?
This first question can be addressed in much the same way that the potential air-quality benefits of RFG were initially assessed in studies such as the AQIRP and California Ethanol Testing Program (see Chapter 7). Representative vehicles can be selected and then subjected to emissions tests using dynamometers, etc. In this case, however, actual, in-use Phase II RFG would be used instead of prospective RFG formulations. Fungibility issues, such as that related to in-tank blending of RFGs, could then, in principle, be directly tested and assessed.
|
7 |
These questions tend to mirror the progression of questions included in the Decision Tree in Figure 6-1. |
Question 2: Are changes in emissions resulting from the use of Phase II RFG blends observable under driving conditions?
Although measurements of LDV emissions in a laboratory setting are informative, they do not necessarily represent the emissions of LDVs in operation under actual driving conditions. Confirmation that laboratory-measured emission reductions also occur on the road can be obtained through tunnel studies and remote sensing of tailpipe emissions. As noted earlier in this chapter, these measurements characterize LDV emissions under a limited set of conditions and, as such, do not comprehensively quantify LDV emissions. Nevertheless, they do provide a real-world test of the emissions and as such are an important step in linking laboratory-measured LDV emissions to an ambient concentrations signal.
Question 3: Are changes in emissions resulting from the use of Phase II RFG blends observable as a signal in the ambient concentrations of ozone precursor compounds?
Establishing the connection between changes in LDV emissions and the ambient concentrations of the compounds contained in those emissions is a more-formidable task. The most-straightforward approach for accomplishing such a task is through the use of time-series analyses of a long-term record of ambient concentrations of VOC, CO, and NOx to isolate a signal that can be associated with Phase II RFG. However, this approach presents a variety of challenging problems. The time-series record must encompass a period significantly before as well as after initiation of Phase II RFG and the data set must include highly accurate and precise measurements. Even under those circumstances, identification of a shift in the time series of the quantity of interest due to RFG can be obscured by other transient factors (e.g., meteorological variations or. implementation of other emissions control programs). Therefore, there is a need to develop and evaluate techniques for detecting ambient effects of a control program separately from the effects of meteorological variability.
For those reasons, it is recommended that an alternative approach be taken to document the effect of Phase II RFG usage on ambient precursor concentrations. This alternate approach would be to use measurements of various tracers in conjunction with measurements of
VOC, CO, and NOx to (1) characterize the contributions of LDV emissions to the concentrations of ozone-precursor compounds; (2)estimate the ozone-forming potential of these compounds through the application of various observation-based methods (e.g., Cardelino and Chameides 1995); and (3) document the change in this contribution that can be attributed to the use of RFG. Tracer species that would be useful in this regard include those that could be used to identify LDVs emissions (e.g., acetylene for LDV exhaust), as well as those that could serve as a fingerprint of emissions from LDVs using RFG (e.g., MTBE). These measurements would ideally be made in a variety of locations within and surrounding each severe nonattainment area to document effects occurring on regional scales as well as local or urban scales. Especially important in this regard would be the enhancement of monitoring capabilities in rural areas of the United States.
Summary
The first investigation in this chapter focused on determining if changes in ozone precursors (NOx or VOCs, CO, air toxics, and oxygenates) have been observed in the emissions studies done on individual vehicles tested under controlled conditions in the laboratory. The most comprehensive study undertaken to date of the effects of varying gasoline properties, the Auto/Oil Air Quality Improvement Research Program (1989-1995), indicated that substantial ozone-precursor emissions reduction benefit should be achieved by RFG. Decreases in the ozone-forming potential (as measured by the MIR scale) of emissions from LDVs of as much as 20% appear to be possible. The most dramatic effects on ozone-precursor exhaust emissions seen in the various gasoline compositional matrices studied were those due to lowering the fuel's RVP and the amount of sulfur-containing compounds. Only slight reductions, less than 10%, in the CO and VOC emissions can be ascribed to the addition of either MTBE or ethanol.
The second investigation focused on determining if changes in NOx or VOCs, CO, air toxics, and oxygenates, have been observed in the emissions studies done in tunnels or from remote sensing of exhaust. From a qualitative point of view, these studies appear to be consistent with the laboratory tests. Reductions were observed in the LDV emissions of NOx, VOCs, CO, and various toxics, and they appear to be at
least partially attributable to the introduction of RFGs. Formaldehyde emissions were found to increase—most likely from the combustion of MTBE. These studies also indicated that high-emitting vehicles are responsible for a disproportionate share of the VOC and CO emissions. The tunnel studies and remote-sensing measurements also indicated that the addition of oxygenates to fuel substantially reduced the emissions of CO and VOCs from these high emitting vehicles, perhaps because these high-emitters are operating with faulty or nonfunctioning catalytic converters. However, the data from these studies could not be used to discern the relative air-quality benefits of fuels using MTBE or ethanol.
The third and final investigation sought to identify RFG effects in the atmosphere by analyzing ambient data. Such an undertaking is easily confounded by competing and offsetting interferences (e.g., meteorological variations and the existence of other contemporary control programs), and statistically significant trends specifically attributable to the RFG program could not be identified. Several areas of the country have seen significant improvements in air quality, including reductions in ambient CO and ozone concentrations. In the case of CO, it appears that some portion of the decrease can be attributed to the addition of oxygenates to fuels but the magnitude of the oxygenate effect is not spatially uniform and in some areas is too small to discern with statistical confidence. In the case of ozone, it is not clear if any portion of the concentration decrease can be directly associated with the addition of oxygenated compounds to motor fuel or the development and use of RFG.
Thus, it would appear that RFGs have an impact on ozone-precursor emissions from LDVs by reducing both the mass and ozone-forming potential of these emissions. However, discerning a statistically significant effect of RFGs on ambient ozone concentrations has thus far proven to be quite difficult. This is most likely because ambient ozone concentrations tend to be quite variable from year to year and the RFG program is but one of a multitude of ozone-mitigation programs underway in the nation whose impact on ozone is of a similar or larger magnitude. Thus, air-quality models—which are themselves subject to significant uncertainty—present the only avenue for estimating the magnitude of the effect of RFG on ozone concentrations. As described in Text Box 6-1, simulations using these models indicate that the overall reduction in ozone from the implementation of the RFG program is likely to be a few percent. This finding should not be interpreted to mean that RFG use is
|
Text Box 6-1 Model Predicted Effects of RFG On Ground-Level Ozone Laboratory tests and tunnel studies suggest that the use of RFGs in LDVs lowers the ozone-forming potential (as measured by the MIR scale) of an individual vehicle's emissions using an RFG blend with the lowest MIR by about 20% (see Rigure 6-4). Yet, analysis Of ambient data is unable to identify a discernible impact on ground-level ozone concentrations. Does that indicate an inconsistency or gap in our understanding of the processes that lead to the formation and accumulation of ozone pollution? Not necessarily. In the first place, ozone concentrations generally do not respond in a linear fashion to decreases in VOCs (see discussion in chapter 2). Moreover, emissions from LDVs represent only a fraction of the total VOC emissions in an airshed, Thus, it might be expected that the effect on ambient ozone of a ˜20% decrease in the reactivity of motor-vehicle. emissions would be considerably less than 20%. A more quantitative assessment of the probable impact Of RFGs on ozone can be made using air-quality models. One could ask, Are changes in emissions resulting from the use of RFG blends observable in air-quality models, and has the performance of those models been evaluated? A version of the gridded Urban Airshed Model was exercised as part of the AQIRP Study to do just such an assessment (AQIRP 1997a). In this study, the Urban Airshed Model was used to simulate ozone Concentrations when different RFG fuels were used for conditions typical of Los Angeles, New York, and Chicago-Milwaukee. Simulations were first carried-out for a historical ozone episode in each metropolitan area (Los Angeles, August 26-28, 1987; New York, July 9-11, 1988; and Chicago-Milwaukee, June 24-28, 1991). RFG effects on ozone were then estimated using the same meteorological conditions that occurred during the historical episode and emissions projections for 2000 and 2010 that included the emissions reductions for motor vehicles predicted by the data from the Auto/Oil study. Table 6-6 lists the predicted change in peak ozone for each simulation for changes in T50, T90, and sulfur content of the fuels. As might be expected, lowering these fuel properties does in fact lead to a decrease in peak ozone concentrations. However, the ozone decrease is quite small—about 1 part per billion by volume (ppb) or less—although in many cases still |
|
An independent model assessment of the impact of the federal RFG program was carried out by the New York State Department of Environmental Conservation using the emissions inventory prepared by the Ozone Transport Assessment Group (OTAG). The study involved a regional-scale application of the Urban Airshed Model (UAM-V) with a model. domain covering much of the eastern third of the continent. The regions where the RFG program was implemented during 1995 is presented in Figure 6-12. A comparison of model simulations of a multi-day ozone episode during July 7-18, 1995, with and Without the RFG program indicates ozone decreases up to 3 ppb over Chicago, Lake Michigan, and along the northeastern corridor (see Figure 6-13). Of course it should be recognized that air-quality models simulations are themselves uncertain because of the uncertainties in both the algorithms (e.g., the chemical mechanisms) and the input data (e.g., the emission inventories) used to run the models. Even recognizing these Uncertainties, it seems unlikely that the RFG program could result in ozone decreases of more than 10 ppb. For example, even if the mobile source emissions used in the model simulations were underestimated by a factor of 2, the maximum ozone decrease would probably be less than 10% at most because peak ozone concentrations generally respond nonlinearly to Changes in ozone precursor concentration. Thus, model simulations predict that RFG has a beneficial effect of a few percent On Overall Ozone concentrations. It is therefore not surprising · that discerning an RFG-signal in the ambient ozone data has proven to be. difficult. It also suggests that it will be difficult to discern the impacts of two RFG blends with subtle differences in their properties. This issue is addressed as a case study in Chapter 7. |
ineffective. As noted earlier, reduction of RVP in gasoline prior to the RFG program is thought to have had a significant air-quality benefit. As discussed in the next chapter, such a reduction size limits the ability to document the benefits of RFGs and to reliably distinguish between the ozone-forming potentials of different RFG blends.
TABLE 6-6 Predicted Effects on Peak Hourly Ozone Concentrations Expected Due to Changes in Certain Fuel Composition Variables in Three Cities As Estimated by Using the Urban Air Shed Modela
|
|
Change in Fuel Variableb |
||
|
City, Year, Episode Dayc |
T50d(215ºF to 185ºF) |
T90(325ºF to 280ºF) |
Sulfur (320 to 35 ppm) |
|
|
Change in Peak Ozone (ppb) from That of the Historical Episode |
||
|
Los Angeles, August 28, 1987 |
|
|
|
|
2000 |
-0.3 ± 0.3* |
-0.9 ± 0.3* |
|
|
2010 |
-0.1 ± 0.2 |
-0.1 ± 0.2 |
|
|
New York, July 11, 1988 |
|
|
|
|
2000 |
-0.1 ± 0.1 |
-0.4 ± 0.1* |
-0.4 ± 0.1* |
|
2010 |
0.0 ± 0.1 |
0.1 ± 0.1 |
-0.4 ± 0.1* |
|
Chicago-Milwaukee, June 26, 1991 |
|
|
|
|
2000 |
-0.8 ± 0.7* |
-1.2 ± 0.9* |
0.0 ± 0.9 |
|
2010 |
-0.2 ± 0.7 |
-1.0 ± 0.8* |
0.4 ± 0.8 |
|
Chicago-Milwaukee, June 27, 1991 |
|
|
|
|
2000 |
-0.6 ± 0.5* |
-0.9 ± 0.7* |
-0.2 ±0.7 |
|
2010 |
-0.1 ±0.4 |
-0.5 ± 0.4* |
0.1 ± 0.4 |
|
Chicago-Milwaukee, June 28, 1991 |
|
|
|
|
2000 |
-0.3 ± 0.2* |
-0.5 ± 0.3* |
-0.2 ± 0.3 |
|
2010 |
-0.1 ± 0.2 |
-0.3 ± 0.2* |
0.0 ± 0.2 |
|
a The predicted effects may not be reflective of the greatest change in gasoline composition such as changes from the late 1980s to when California Phase 2 RFG began to be used. b Main effects are shown with 95% confidence intervals. An* denotes statistically significant effects. c Data from the location and date that was used to establish meteorological conditions employed in each simulation. d The effects of T50 on ozone may be underestimated because only the effects on emissions from lower exhaust emitters are included. The effect of T50 on emissions from higher emitters could not be estimated from the available data and are assumed to be zero. Source: AQIRP Technical Bulletin No. 21, 1997a. |
|||