2
Ozone Photochemistry
Mitigation of the ozone-pollution problem is complicated by the fact that ozone (O3) is a secondary pollutant; that is, it is not emitted directly into the atmosphere, but is produced by photochemical reactions involving primary pollutants and modulated by meteorological conditions. The problem is further confounded by the complex nature of the photochemical mechanism responsible for producing ozone and the intricate array of precursors that can participate in this photochemical mechanism. These complexities are briefly reviewed in this chapter.
VOC Limitation vs NOx Limitation
As noted in Chapter 1, ozone is formed by chemical reactions involving volatile organic compounds (VOCs) and carbon monoxide (CO) in the presence of nitrogen oxides (NOx) and sunlight. One might expect, therefore, that the severity of ozone pollution in a given region can be reduced by lowering the emissions of VOCs, CO, NOx, or any combination thereof. However, mitigation of ozone pollution is not so straightforward. It turns out that the rate of ozone formation is a complex and variable function of the concentrations of VOC and NOx as well as meteorological conditions. As a result, establishing the relative benefits of VOC and NOx emissions controls can be a difficult and challenging task. The source of the complexity can be elucidated through an examination of
Figure 2-1, which is a schematic of the photochemical smog mechanism. Ozone production occurs as a result of a series of reactions initiated by the oxidation of VOCs or CO by the hydroxyl radical (OH). For example,
where RH represents a generic hydrocarbon (or VOC), R is a hydrocarbon
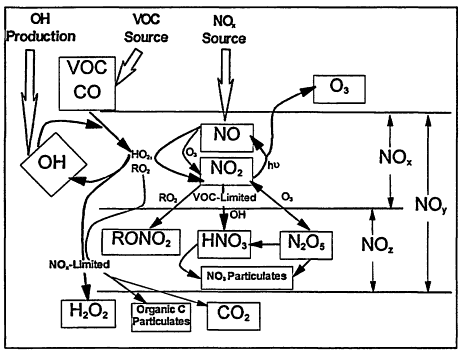
Figure 2-1
Schematic of the photochemical pathways leading to the production of ozone and the termination steps that dominate under NOx-limited and VOC-limited regimes.
radical (e.g., CH3CH2 for RH = ethane), M is a nonreactive, energy-absorbing third body (N2, O2), and h v represents energy from solar radiation (it is the product of Planck's constant h, and the frequency, v, of the electromagnetic wave of solar radiation). Of note in this sequence is that VOCs are consumed, whereas both OH/HO2 and NOx act as catalysts. Moreover, the by-product labeled "carbonyl" is itself a VOC and can, in general, react and produce additional ozone molecules. It is important to note that although OH is removed in Reaction 2-1, it is regenerated in Reaction 2-5.
Termination of the above ozone-generating cycle occurs when the catalysts are removed. Two important paths are
In general, the rate of ozone production can be limited by either VOCs or NOx. The existence of these two opposing regimes, often schematically represented in a so-called EKMA (Empirical Kinetic Modeling Approach) diagram (Figure 2-2), can be mechanistically understood in terms of the relative sources of OH and NOx (Kleinman 1994, in press). When the rate of OH production is greater than the rate of production of NOx, termination of the reaction chain that produces ozone is dominated by Reaction 2-8 (see Figure 2-1). Under these conditions, NOx is in short supply; as a result, the rate of ozone production is NOx-limited (i.e., ozone is most effectively reduced by lowering NOx). Therefore, ozone concentrations are most effectively reduced by lowering NOx emissions, and subsequent concentrations of NOx, instead of lowering emissions of VOCs. When the rate of OH production is less than the rate of production of NOx, on the other hand, termination of the ozone-forming chain proceeds predominately via Reaction 2-9 (see Figure 2-1), NOx is relatively abundant, and ozone production is VOC-limited (i.e., ozone is most effectively reduced by lowering VOCs). Because this region is characterized by rapid loss of OH via Reaction 2-9, it is also referred to as being the radical-limited regime. Finally, between these two extremes (i.e., the NOx-and VOC-limited regions) lies a transitional region, sometimes referred to as the ridge in an EKMA diagram. In this transitional region, ozone is about equally sensitive to VOCs and NOx, but, compared within its sensitivity to VOCs in the VOC-limited region and its sensitivity to NOx in the NOx-limited region, ozone is relatively insensitive to both.
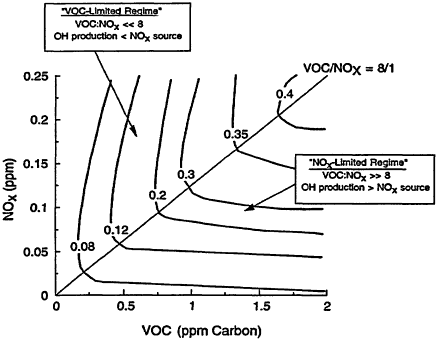
Figure 2-2
Typical EKMA (Empirical Kinetic Modeling Approach) diagram showing contours (or isopleths) of 1-hr maximum ozone concentrations (in parts per million by volume (ppm)) calculated as a function of initial VOC and NOx concentrations and the regions of the diagram that are characterized by VOC limitation or NOx limitation. "OH production" refers to the rate of OH photochemical production and "NOx source" refers to the rate at which NOx is emitted into the boundary layer.
A further complication arises from the fact that VOC and NOx limitation is not uniquely defined by location or emissions. Instead, it is a chemical characteristic of an air parcel that varies dynamically with transport, dispersion, dilution, and photochemical aging. For example, consider the results of a series of photochemical box model calculations illustrated in Figure 2-3. In each calculation, a boundary-layer air parcel was assumed to have initial VOC and NOx concentrations at 0800 hr and then allowed to react over the course of a single day while mixing with relatively dean air from aloft at varying rates. For simplicity, processes such as surface deposition and horizontal dispersion are not included. Although these simulations greatly simplify the photochemical smog
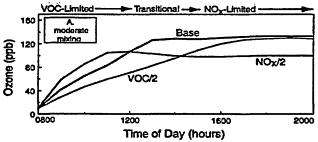
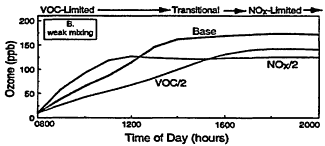
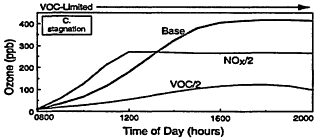
Figure 2-3
Model-calculated evolution of ozone as a function of time in an air parcel for various initial urban-like mixtures of VOCs and NOx at 0800 hr under summertime conditions and three rates of vertical mixing and free tropospheric entrainment. For each mixing rate, simulations for three initial VOC and NOx concentrations are presented: "Base" with initial VOC = 0.15 ppm and NOx = 1.5 ppm; "VOC/2" with initial VOC = 0.75 and NOx = 0.15 ppm; and "NOx/2" with initial NOx = 0.075 and VOC = 1.5 ppm. Note the characteristic tendency for the system to evolve from VOC limitation to NOx limitation with time and for the point of transition to be delayed as mixing decreases. Also note that varying the initial conditions of either precursor in EKMA implicitly changes the emissions after 0800 hr by the same percentage.
phenomenon, they nevertheless capture much of the essence of the relationship between ozone and its precursors and are, therefore, useful to illustrate some key points.
In the first example (Figure 2-3A), a moderate amount of vertical mixing during a typical summer day is assumed. For these conditions and the high initial concentrations of VOCs and NOx adopted for the Base case, the model predicts a rapid rise in ozone reaching a peak of about 130 ppb around mid-afternoon—an ozone variation that is characteristic of many moderate urban air-pollution episodes in the United States. (If the effects of dispersion and surface deposition are included, the peak concentration would have been somewhat depressed and the decay following the peak more pronounced.)
A key feature of the results illustrated in Figure 2-3A is the varying response of ozone to assumed decreases in the initial concentrations and emissions of VOCs and NOx. Because of the nature of urban VOC and NOx emissions, air parcels exposed to these emissions are usually initially within the VOC-limited regime. Thus, in Figure 2-3A, halving the initial VOC concentration is much more effective in reducing ozone than halving NOx during the first ˜5 hr of this particular simulation. In fact, during the first few hours of the simulation an "NOx disbenefit" appears, that is, an increase in ozone results from a decrease in NOx. This effect is caused by the conversion of more NO to NO2, and by an increase in the fraction of OH radicals which react with VOCs (and thereby leading to RO2 and HO2 radicals, which convert NO to NO2) compared with reaction with NO2. For these conditions, a decrease in NOx leads to more OH, more oxidation of VOCs (e.g., via Reaction 2-1), and thus an ozone increase.
Because NOx is processed and removed rapidly, the NOx disbenefit tends to be fairly short-lived. Moreover, as NOx concentrations continue to fall, the air parcel begins to move from VOC limitation to the transitional region and often reaches NOx limitation within many areas of the country. For the conditions adopted in the simulation illustrated in Figure 2-3A, ozone is more effectively reduced by halving NOx than by halving VOCs after about 1400 hr. Another important feature of the calculations illustrated in Figure 2-3A, which is also characteristic of the photochemical smog system in general, is that the peak ozone concentration is reached when the air parcel is in the transitional region between VOC limitation and NOx limitation. The formation of organic nitrates (including peroxyacetyl nitrate (PAN)) also affects ozone formation by
removing NOx from the system which would otherwise lead to ozone formations. Depending on the temperature, PAN formation can lead to a temporary reduction in ozone formation.
Other processes can further complicate and confound the relationship between ozone and its precursors. One of these is vertical mixing. As illustrated in Figure 2-4, vertical mixing has a direct impact on ozone concentrations: in the early morning hours it tends to contribute positively to ozone accumulation by bringing ozone-rich air from aloft into the boundary (or surface) layer, but in late morning and afternoon it tends to depress ozone by diluting surface air now laden with newly formed ozone with air from aloft. As a result, as the amount of mixing decreases and stagnation sets in, the severity of air-pollution episodes is exacerbated. That is illustrated in Figures 2-3B and 2-3C, in which higher peak ozone concentrations are generated as less vertical mixing and more stagnation occur. However, vertical mixing has another indirect, but still very important, effect on ozone. In addition to depressing peak ozone, vertical mixing also tends to depress NOx concentrations in the polluted boundary layer by diluting it with cleaner air from aloft. For this reason, stagnation tends to slow the rate of transition from VOC limitation to NOx limitation. If vertical mixing is extremely weak (i.e., conditions assumed for Figure 2-3C), the sun might set before NOx is sufficiently processed to allow the parcel to make the transition from VOC limitation. Thus, the efficacy of VOC and NOx controls is, in general, critically dependent upon the meteorological as well as the chemical conditions that prevail during any given episode.
The distribution of NOx emissions can also affect where and if air parcels within a given airshed make the transition from VOC limitation. Like stagnation, the presence of dispersed NOx sources in a large metropolitan area or megalopolis can lead to high NOx concentrations throughout an area, fostering continuous VOC limitation.
Reaction Pathways of Ethanol and Methyl Tertiary-Butyl Ether
For both methyl tertiary-butyl ether (MTBE) and ethanol, the important atmospheric loss processes are by reaction with the OH radical. Reaction of ethanol with the OH radical leads to the formation of acetaldehyde (CH3CHO) in 100% or close to 100% yield (Atkinson 1994; Atkinson in
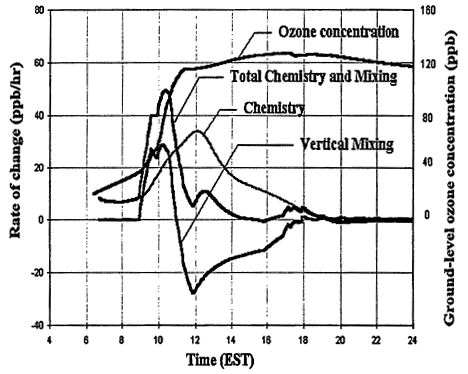
Figure 2-4
The relative contributions of vertical mixing and chemical production processes to the ground-level ozone concentration as a function of time during the day based on a one-dimensional model. No deposition processes are included in this simulation. The left scale is for the rate of change in ppb/hr and the right is for the ozone concentration in ppb.
Source: Adapted from Zhang and Rao (In Press).
press), with the major reaction pathway (˜90% of the overall OH radical reaction) proceeding by
For MTBE, the products of the OH radical reaction in the presence of NO are tert-butyl formate ((CH3)3COCHO), ˜75%; formaldehyde, ˜45%; methyl acetate (CH3C(O)OCH3), ˜15%; and acetone, ˜3% (see Atkinson 1994, and references therein). tert-Butyl formate reacts only slowly with the OH radical, with a half-life due to gas-phase reaction
with the OH radical of around 11 days for a 24-hr average OH radical concentration of 1 × 106 molecules·-3. Wet and dry deposition of tert-butyl formate may also be important.
Summary
To the extent that these calculations can be generalized to represent the evolution of a plume as it advects from an urban center (or other concentrated sources of anthropogenic emissions of VOC and NOx) to suburban and then rural areas (with time essentially representing distance from an urban core), they suggest that: (1) in isolated large urban cores and similar source regions, ozone concentrations during severe air-pollution episodes are most effectively reduced by reductions in VOC emissions and might even increase as a result of NOx-emission controls; (2) ozone concentrations in rural areas and over large regional expanses are most effectively reduced by reductions in NOx emissions from the pollution sources that affect that area or region (e.g., upwind urban sources and important local sources); and (3) the highest ozone concentrations during an episode generally occur in locations somewhat removed from the major precursor source areas (i.e., suburban areas) and tend to occur when the chemistry of the system is in a transitional stage between VOC limitation and NOx limitation. Where the peak ozone concentration will occur during any given episode and whether it will occur when the chemistry is, in fact, transitional or is VOC-limited are determined by myriad factors including the meteorological conditions and distributions as well as intensities of emissions.
The fact that ozone formation can vary from VOC limitation to NOx limitation is highly germane to the topic of this report. As discussed in more detail in Chapter 3, ozone-forming potential has historically been used to characterize the ability of VOCs to produce ozone. Thus the relevance of using existing methods to assess the ozone-forming potential of various reformulated gasoline blends will be largely limited to those areas and episodes characterized by VOC limitation (or at least transitional chemistry).
A further complication in assessing the efficacy of emission controls for VOC and NOx arises from the fact that VOCs comprise a rich and varied assortment of compounds. Two principal VOC categories are those that arise from anthropogenic sources and those that arise from natural or biogenic sources (e.g., isoprene from trees and other vegetation).
Natural VOCs can participate in the photochemical reactions that produce ozone, but they cannot, in principle, be directly controlled like those from anthropogenic sources. In regions where natural VOCs represent a significant fraction of the total reactive VOCs, NOx controls might be needed to reduce ozone substantially even if the oxidant chemistry is VOC-limited.
Moreover, the compounds that make up the general category of anthropogenic VOCs can be quite varied with widely different chemical characteristics and reactivities that lead to different rates of ozone formation. Thus, ton-for-ton, the reduction in the emissions of one VOC might lead to more or less reduction in ozone than the reduction of another VOC. The concept of ozone-forming potential, discussed in the next chapter, attempts to account for the differing chemical characteristics of VOCs as they relate to ozone photochemical production.










