3
The Concept of Ozone-Forming Potential and Its Quantification
In any given airshed, it is common to find hundreds of different VOC species, each with its own unique chemistry. In the simplest approach to ozone mitigation based on VOC controls, emission reductions are implemented on a mass basis without any regard to the unique chemistry of each of the VOCs. The principle behind ozone-forming potential or reactivity1 is the notion that, in addition to the amount of a specific VOC species emitted into a given airshed, the difference in the chemistry of each of the VOCs needs to be considered when assessing the impact of those species on ozone formation.
The utility of the concept of ozone-forming potential can be illustrated through a comparison of the impacts on ozone concentrations in an urban airshed of two ubiquitous VOC species: ethane and propene.
If one were to increase the total mass of VOC emissions in a city, such as Los Angeles, by 20% through additional emissions of ethane, ozone levels would increase slightly. However, if the same amount of propene were added instead, there would be a large increase in ozone. Why the big difference between the two, given that both are rather simple hydrocarbons? The primary cause of the difference is the differing rates at which these two species react in the atmosphere. Ethane has an atmospheric lifetime of weeks. Little of the ethane emitted in an urban area reacts within that area before it is transported away. Its contribution to ozone formation within the urban area is therefore very small. Propene, on the other hand, has a lifetime of hours. Most of it will typically react near its source and thus be able to contribute to the photochemical production of ozone in the area in which it is emitted (or immediately downwind). A secondary, but smaller, cause for the differing impacts of the two species is the different number of ozone molecules formed in that environment for each molecule of ethane and propene that reacts. Differences in ozone productivity arising from the first effect are often expressed in terms of the kinetic reactivity (KR), and differences from the second are expressed in terms of the mechanistic reactivity (MR).
Regulatory Application of VOC Ozone-Forming Potential
There is, in fact, a significant historical precedent for accounting for VOC reactivity in U.S. regulatory policy, albeit to a limited extent (see Dimitriades 1996, for a history of VOC regulation in the United States). During the early years of ozone mitigation, it was recognized that there were some organics, for example ethane, that did not contribute significantly to smog formation on urban scales, whereas others, such as propene, did. Thus, two categories of organic gases were defined for regulatory purposes: unreactive and reactive.2 (see Table 3-1). However, the term
TABLE 3-1 Acronyms and Names Used for Classifying Organic Compounds
|
Common Abbreviation |
Full Name |
Definition |
|
VOCa |
Volatile organic compound |
Organic compounds that are found in the gas phase at ambient conditions. Might not include methane. |
|
ROG |
Reactive organic gas |
Organic compounds that are assumed to be reactive at urban (and possibly regional) scales. Definitionally, taken as those organic compounds that are regulated because they lead to ozone formation. The term is predominantly used in California. |
|
NMHC |
Nonmethane hydrocarbon |
All hydrocarbons except methane; sometimes used to denote ROG |
|
NMOC |
Nonmethane organic compound |
Organic compounds other than methane |
|
NMOG |
Nonmethane organic gas |
Organic gases other than methane |
|
RHC |
Reactive hydrocarbon |
All reactive hydrocarbons; also used to denote ROG |
|
THC |
Total hydrocarbon |
All hydrocarbons, sometimes used to denote VOC |
|
OMHCE |
Organic material hydrocarbon equivalent |
Organic compound mass minus hetero-atom mass (i.e., carbon plus hydrogen mass only) |
|
TOG |
Total organic gas |
Total gaseous organic compounds, including methane. Used interchangeably with VOC |
|
a Unless noted otherwise, VOCs is the term used in this report to represent the general class of gaseous organic compounds. Source: U.S. Environmental Protection Agency at http://www.epa.gov/docs/OCEPAterms.7 |
||
unreactive is a misnomer, because even compounds such as ethane and methane do react and contribute to tropospheric ozone formation,
|
|
terms are listed in Table 3-1. Unless noted otherwise, VOCs is the term used in this report to represent the general class of gaseous organic compounds. |
though at much lower rates, on a per mass basis, than other compounds. Such low-reactivity compounds, particularly carbon monoxide (CO) and methane, do contribute to ozone formation, because emission rates of those compounds are very large. (The contribution of CO to ozone-forming potential is discussed further in Chapters 6 and 7.)
A complication in this two-category approach is deciding where to place the dividing line between unreactive and reactive VOCs. Somewhat arbitrarily, that dividing line has been chosen to be at the level of reactivity of ethane. In the United States, but outside of California, species with reactivities equal to or less than that of ethane are placed in the unreactive category.
California has been a leading force in the application of reactivity assessment to ozone mitigation efforts. For example, California uses ozone-forming potential in its Low Emission Vehicles and Clean Fuels Program (LEV/CF) to adjust and regulate the amount of emissions from vehicles (CARB 1991). A fuel with higher VOC emissions, but a lower net reactivity than the reference fuel, is permitted in the program, thus providing an incentive to develop fuels with less-reactive emissions. (The current CARB program is limited, however, to exhaust emissions, and, as discussed in Chapter 4, evaporative emissions can be quite important.) The use of reactivity in California's regulatory air-quality programs has been a major catalyst for continuing research on ozone-forming potential and its application to policy-making. As the understanding of how to define ozone-forming potential operationally has grown substantially in recent years, the use of ozone-forming potential to other regulatory issues (e.g., emissions from consumer products) is now under consideration.
Operational Definition of Ozone-Forming Potential Using Reactivity
The photochemical degradation of most VOC species is initiated by reaction with the OH radical (i.e., Reaction 2.1 in Chapter 2). Therefore, for most VOCs, the KR of a specific VOC is greater if its OH-radical reaction rate constant is greater. As seen in Table 3-2, these rate constants can vary by many orders of magnitude. A relatively simple type of reactivity scale, sometimes referred to as the OH-reactivity or the kOH scale, expresses the relative contribution of VOCs in terms of their rates of reaction with OH (e.g., Darnall et al. 1976; Chameides et al. 1992).
TABLE 3-2 OH Rate Constants (kOH) and Maximum Incremental Reactivity (MIR)a for Selected Compounds
|
Compound |
1012 × kOHb (cm3·molecule-1 s-1) |
MIRc O3 formed/g VOC emittedb |
|
Carbon monoxide |
0.21 |
0.065 |
|
Methane |
0.0062 |
0.016 |
|
Ethane |
0.25 |
0.32 |
|
Propane |
1.1 |
0.57 |
|
n-Butane |
2.4 |
1.18 |
|
n-Octane |
8.7 |
0.69 |
|
2,2,4-Trimethylpentane |
3.6 |
1.34 |
|
Ethene |
8.5 |
8.3 |
|
Propene |
26 |
11.0 |
|
trans-2-Butene |
64 |
13.2 |
|
Isoprene |
101 |
9.3 |
|
α-Pinene |
54 |
3.9 |
|
Benzene |
1.2 |
0.81 |
|
Toluene |
6.0 |
5.1 |
|
m-Xylene |
24 |
14.2 |
|
1,2,4-Trimethylbenzene |
32 |
5.3 |
|
o-Cresol |
42 |
2.5 |
|
Formaldehyde |
9.4 |
6.6 |
|
Acetaldehyde |
16 |
6.3 |
|
Acetone |
0.22 |
0.49 |
|
2-Butanone |
1.1 |
1.4 |
|
Methanol |
0.94 |
0.65 |
|
Ethanol |
3.3 |
1.7 |
|
Methyl tert-butyl ether |
2.9 |
0.73 |
|
Ethyl tert-butyl ether |
8.8 |
2.2 |
|
tert-Butyl formate |
0.75 |
No value cited |
|
a MIR combines kinetic and mechanistic reactivities of a specied compound for conditions that maximize the predicted reactivity of VOCs by making the reactive systems very NOx rich. b Rate constants at 298 K are taken from Atkinson (1994, 1997) and Le Calve et al. (1997) c From Carter (1997), http://www.cert.ucr.edu/˜carter/bycarter.htm. The MIR of the assumed urban mix used in the calculations was 4.06 g of O3 per gram of VOC emitted. |
||
This approach has some significant advantages. OH-rate constants for a large number of VOCs have already been well characterized by laboratory experiments, and many others can be estimated with a fair degree of reliability (e.g., Kwok and Atkinson 1995; Atkinson in press). Moreover, these constants are defined by the VOC species themselves and not the environment in which the VOCs are emitted (other than minor temperature dependencies). Thus, the OH reactivities for a wide range of VOC species can be readily calculated and compared. Combining these OH reactivities with data on the ambient concentrations of these VOCs provides a measure of the rate at which the various VOC species are oxidized and produce peroxy radicals (e.g., via Reaction 2-2 and Reaction 2-4 in Chapter 2), and thus provides a rough estimate of their relative potential roles in ozone-formation (Chameides et al. 1992).
There are, however, significant limitations to using the OH-reactivity scale to characterize the roles of VOCs: The method does not account for the potentially different yields of peroxy radicals formed from different VOCs, the different reactive pathways these peroxy radicals can take once they are produced, and the varying tendency of VOCs to enhance or inhibit radical levels, and thus influence the contribution of other VOC species to ozone formation. All of these factors can have a significant effect on the amount of ozone formed from the oxidation of a VOC (Carter and Atkinson 1989; Bowman and Seinfeld 1994; Carter 1994). For this reason, the OH-reactivity scale does not always correlate well with other measures of ozone-forming potential, particularly for the more rapidly reacting VOCs (e.g., Dodge 1984; Bergin et al. 1998a). For example, aromatics, which have strong NO x sinks and radical sources in their mechanisms, can have relatively high reactivities under conditions with low ratios of VOC to NOx, but negative values of reactivities when the VOC to NOx ratio is sufficiently high, because NOx, which (as NOx) would otherwise photolyze to form ozone, is removed from the system.
MR is used to account for this second influence on the ozone-forming potential of VOCs (Carter and Atkinson 1989). In general, the variability in mechanistic reactivities is substantially less pronounced than that of kinetic reactivities, and thus the species-to-species variability of reactivity scales that combine KR and MR tend to follow the variability in KR but not exactly (see Table 3-2).
If KR is defined as the number of molecules of a specific VOC that react within a given airshed (by photolysis, reaction with the OH radical, reaction with NO3 radical, or reaction with ozone) and MR is the number of ozone molecules that are formed for each VOC molecule in the system
that reacts, the total number of ozone molecules formed from a given VOC molecule is equivalent to the product of the two quantities, that is,
This way of dissecting the ozone-forming potential of a compound, although remarkably simple, is also quite powerful and instructive. However, it also has its limitations. For example, neither KR nor MR is a property inherent in a compound. Instead, both are dependent upon the protocol established to calculate them (e.g., the type of environments in which the VOC exists and the length of time used to assess the amount of the VOC that reacts and the ozone that is formed). Thus, the use of the relationship expressed in Equation 3-1 requires an operational definition for quantifying reactivities.
Quantifying Ozone-Forming Potential Using Reactivity
If ozone-forming potential is to be used in ozone mitigation programs, it is necessary to develop an operational definition for ozone-forming potential, and a protocol for quantifying it. One such definition is the incremental reactivity (IR) proposed by Carter and Atkinson (1989) and Carter (1994).3 IR is defined as the number of additional grams of ozone formed per gram of VOC compound added to a base mixture (the VOC compound could be present in the base mixture):
where IRi is the incremental reactivity of species i; ∆[O3] is the change in some ozone metric used to assess the impact of VOCs on air quality (e.g., the 1-hr peak or 8-hr averaged ozone concentration in an airshed) or the total human exposure to ozone above some threshold concentration); and ∆[VOCi] represents a change in the emissions of species i
(e.g., from an RFG blend). Defining IR in this way takes into account both the KR and MR of a given VOC species, and, in principle, the incremental reactivity can be broken into kinetic and mechanistic reactivities:
where KRi and MRi are, respectively, the kinetic and mechanistic reactivities of the species i.
The IR, as defined by Equation 3-2, is an absolute measure of ozone-forming potential (e.g., the number of grams of ozone per gram of VOC). A somewhat more useful quantity for developing ozone mitigation strategies is the relative incremental reactivity (RIR). RIR is defined as the reactivity of one compound normalized to the reactivity of a base mixture:

where IRi is the incremental reactivity of species i, fj is the fraction of species j in a base mixture containing n different VOCs so that the denominator in the above expression is the total incremental reactivity of a base mixture, such as an RFG blend. The advantages of working with relative incremental reactivities are threefold. First, in a policy-making context, comparisons of reactivities between species or VOC sources are often of most interest. Second, RIP, tends to be less sensitive to variations in ambient conditions and thus provides a more robust measure of reactivity. Third, RIR is often easier to develop from three-dimensional models, because there is no apparent absolute scale (e.g., the location and timing of how ozone changes is not uniform) (see McNair et al. 1994).
The two dominant methods that have been used to assess species' reactivities (IR and RIR) are via direct experimental measurement, for example, in an environmental (or smog) chamber, and numerical simulation using computer-based, air-quality models (Carter and Atkinson 1989; Carter 1994; Derwent and Jenkin 1991; Bowman and Seinfeld 1995; McNair et al. 1992; Yang et al. 1995; Bergin et al. 1996). Both methods have serious limitations. Smog chambers do not realistically represent the physics of pollutant transport and the impact of fresh emissions. Moreover, most do not operate over the full range of NOx
concentrations and VOC to NOx ratios typically encountered in the polluted atmosphere. Thus, the conditions inside a smog chamber do not reflect those of the ambient air. Given the sensitivity of many VOC reactivities to environmental conditions, smog chamber experiments, by themselves, provide reactivity estimates that are less applicable to atmospheric conditions than those derived from air-quality models. Furthermore, smog chambers have artifacts (e.g., chamber wall and background effects) that can affect the results, particularly if the compound reacts slowly or has radical sinks in its mechanism (Carter and Lurmann 1991). However, chamber experiments are necessary to develop (parameterized) chemical mechanisms for those VOCs for which product and mechanistic data are not yet available from laboratory studies. Data from those chemical mechanisms can then be included in chemical mechanisms for the assessment of their ozone-forming potentials.
Because models can be run for conditions that more accurately reflect actual atmospheric conditions, they can, in principle, provide a more appropriate measure of a species' reactivity than that obtained from a smog chamber. However, virtually all photochemical mechanisms used in current air-quality models are based on data from smog chambers. Thus, the ability of models to accurately simulate air quality depends critically upon reliable extrapolation of smog chamber data to atmospheric conditions and elimination of chamber wall and background effects. This has proven to be a very difficult task (Dodge in press). For these reasons, a level of uncertainty is inherent in any assessment of ozone-forming potential, A variety of approaches has been adopted that attempt to characterize and minimize this uncertainty and thus provide a foundation if reactivity were to be implemented in a policy-making context.
Chemical Mechanisms and Their Development
Because of the aforementioned limitations of smog chambers, air-quality models have played a central role in the quantification of VOC reactivity. Of the various components within air-quality models, the chemical mechanism, which attempts to reproduce the VOC-NOx-air photooxidation process discussed in Chapter 2, is perhaps the most critical component when these models are used to quantify reactivity. This section briefly reviews how these mechanisms are developed and discusses principal mechanisms currently in use.
Any chemical mechanism used in an air-quality model must be designed so that it can, at a minimum, reproduce the major features of the VOC-NOx-air photooxidation process. The principal chemical mechanisms used in current air-quality models, along with representative airshed modeling applications and their key attributes, are listed in Table 3-3. With the exception of the Harwell Master Chemical Mechanism, all the chemical mechanisms in use today include various kinds of parameterizations, approximations, and condensations to simplify the very complex chemical processes that actually occur when VOCs are oxidized in the atmosphere. There are hundreds of different organic compounds in the atmosphere, and from a numerical point of view, it is often impractical to explicitly follow each species. If this were attempted, the chemical mechanisms would be huge (e.g., the Harwell Master Chemical Mechanism (Jenkin et al. 1997) that has over 7,000 reactions) and would be computationally burdensome in three-dimensional models.
TABLE 3-3 Commonly Used Chemical Mechanisms for Air-Quality Modeling and Reactivity Studies
|
Mechanism |
Description |
Reference |
|
Statewide Air Pollution Research Center 1990 (SAPRC-90/93/97) |
Explicit for a large number of organics, but uses a lumped representation for reactive products. Designed, in part, for reactivity applications. |
Carter 1990, 1995, 1997 |
|
Carbon Bond IV (C84) |
Lumped by number of carbon bonds in compounds. Specified by EPA for regulatory purposes. |
Gery et al. 1989 |
|
Lurmann, Carter, Coyner (LCC) |
Earlier and more-condensed version of SAPRC-90. Used for the earlier CIT grid-model reactivity-assessment calculations. |
Lurmann et al. 1987 |
|
Regional Acid Deposition Model, version 2 (RADM-2) |
Developed for use in regional acid-deposition modeling. Similar to LCC in detail, except more detailed model for peroxide formation. |
Stockwell et al. 1990 |
|
Harwell |
Used in Europe. Very large number of compounds represented explicitly. |
Derwent and Hov 1979 |
|
Harwell Master Chemical Mechanism |
Detailed, explicit mechanism with over 7,000 reactions. |
Jenkin et al. 1997 |
Moreover, if it were practical, laboratory data are available for only a small subset of the relevant reactions, and for all others their rate constants and the products they form would have to be estimated by extrapolation or by analogy from the simpler, better-studied systems. Thus, preserving the full complexity of the atmospheric VOC chemical system in a model might not necessarily increase the reliability of the model's predictions. Chemical mechanisms in air-quality models, therefore, are typically based on the assumption that the atmospheric oxidation of complex VOCs can be simulated by analogy to simpler ones or by using parameterizations to describe the full suite of elementary reactions. To ensure that these simplifying assumptions are capable of adequately simulating the real world, chemical mechanisms should be, and generally are, tested against experimental data from smog chambers in which the relevant chemical processes are monitored under controlled and well-characterized conditions. These data are then used to tune the various parameterizations contained in the mechanism or to test whether model predictions using the mechanism match experimental results.
Various types of chamber experiments are used to test different aspects of the chemical mechanisms. Irradiations of single VOCs in the presence of NOx are used to test the mechanism's ability to simulate the oxidation of and ozone production from an individual VOC; NOx-air irradiations of more complex VOC mixtures test the performance of the model as a whole; and experiments in which the effect of adding single VOCs to irradiations of NOx and complex mixtures test model predictions of the VOC's incremental reactivity. Evaluation of chemical mechanisms with smog chamber data is complicated by uncertainties in chamber effects, and separate characterization experiments are needed to evaluate those effects.
Chamber data are currently available to test the mechanisms for only a subset of the many types of VOCs emitted into the atmosphere. For the other species, reactions are either derived by analogy with mechanisms for compounds that have been studied, or they are represented in the model as if they reacted in the same way as some other chemically similar species. Mechanisms are further simplified or extrapolated using an approach referred to as "lumping." In this approach, a single hypothetical (or pseudo) species is used in the model to represent a larger number of compounds assumed to react in the same way, or a group of model species is used to represent aspects of the reactions of various chemical compounds. The lumping approaches, and the approxi-
mations and inaccuracies they introduce, vary depending on the mechanisms (see Table 3-3).
Reactivity Assessments Using Smog Chambers
As mentioned above, one way to assess a VOC's reactivity is to measure its effect on ozone when irradiated in the presence of NOx and other VOCs in smog-chamber experiments. Although these results have limited applicability for the reasons discussed above, they can be quite valuable for evaluating and verifying reactivities calculated using air-quality models. Studies based on smog chambers include those of Carter at the University of California at Riverside, Kelly at the General Motors Research Laboratories, and Jeffries at the University of North Carolina. The results of the experiments have been encouraging.
Carter and Atkinson (1987) conducted a series of experiments in which the impact of adding a VOC to a base mixture of organics and NOx was compared with a similar experiment without the extra compound being added. This series was done at various NOx levels. Results of those and more recent series of experiments have been compared with the predictions of both the SAPRC-90 mechanism and SAPRC-93 mechanism. The reactivities calculated using the SAPRC-90 mechanism agreed reasonably well with the experimental results for most VOCs, except for the internal alkenes (e.g., 2-butene, 2-pentene). Reactivities calculated using the SAPRC-93 mechanism performed significantly better. In particular, the mechanisms performed quite well in simulating the effects of varying the NOx levels and the nature of the reactive VOC surrogate. However, neither mechanism performed particularly well in simulating reactivity differences among xylene and trimethylbenzene isomers.
In the experiments of Kelly et al. (1994, 1996), incremental reactivities of several representative VOCs were measured as a function of the amount of VOC added under conditions that tended to maximize the reactivity. Although the VOC mix used in the experiments only approximated the VOC mix simulated in a replicate modeling study, the experimental reactivity results correlated well with the modeled reactivity results.
Jeffries et al. (1997, 1998) used a large outdoor smog chamber to study ozone formation from various complex mixtures designed to closely duplicate components in vehicle exhausts, and Kleindienst et al.
(1996) performed Similar experiments using an indoor smog chamber to examine the reactivity of the exhaust from vehicles using alternative fuels. The purpose of the Jeffries et al. studies was to evaluate chemical mechanisms, and to compare, directly, ozone formation from various chemically realistic mixtures.
Although smog-chamber studies are essential for chemical-mechanism evaluation, incremental reactivities in smog chambers are not the same as incremental reactivities in the atmosphere (as discussed above). It is not practical to duplicate all the environmental conditions that affect a VOC's incremental reactivity in smog-chamber experiments, and, even if it were practical to do so, it would not be practical to use such information to investigate comprehensively how reactivities vary over the wide variety of conditions that occur in the atmosphere. For this, air-quality model calculations are required.
Air-Quality Models
Air-quality models are computerized representations of the atmospheric processes responsible for air pollution, which includes ozone formation (NRC 1991), These models integrate current understanding of the atmosphere's chemistry and meteorology with estimates of source emissions to predict how the composition of trace atmospheric species, such as ozone, respond to changes in emissions. Table 3-4 lists and describes some of the air-quality models that have been used to assess VOC reactivity and the ozone-forming potential of motor-vehicle emissions.
The models vary greatly in complexity, and thus also vary in the amount of input data and computational resources they require. In general, the major processes that affect the evolution of pollutants are parameterized within the models, including emissions releases, gas-phase chemical reactions (using chemical mechanisms as described above), transport, mixing, deposition, and scavenging. The equation upon which air-quality models are founded is a statement of chemical species conservation (Seinfeld 1986):
TABLE 3-4 Examples of Air-Quality Modelsa
where, on the left,
![]() represents the local time rate of change in ci, the concentration of species i, and
represents the local time rate of change in ci, the concentration of species i, and
![]() represents the rate of transport of species i by organized wind fields (i.e., advection); on the right,
represents the rate of transport of species i by organized wind fields (i.e., advection); on the right,
![]() represents the rate of transport due to turbulent mixing,
represents the rate of transport due to turbulent mixing,
Ri is the net rate of change in c1 through cn due to chemical reactions for time t and temperature T, and
Si represents emissions (sources) of compound i over a specified time and at a specified location.
The differences in air-quality models arise primarily from the varying degrees of complexity allowed in the treatment of the nonchemi-
cal processes and in the numerical techniques used to solve Equation 3-5. To date, model simulations of ozone formation and VOC reactivity have been performed using two types of air-quality models: (1) box or trajectory models and (2) three-dimensional Eulerian models.
The trajectory or box model represents the polluted atmosphere by a discrete air parcel. (This model is the kind used to illustrate aspects of ozone chemistry in Chapter 2.) Many trajectory models use a single cell to represent a column of boundary-layer air; others use discreet cells to subdivide the vertical column (e.g., the two-cell model used by Derwent and Jenkin 1991). The model's air parcel either is fixed in space (i.e., as a box over a city) or allowed to move over the air basin, following a trajectory calculated from the wind fields (i.e., a Lagrangian simulation). In either case, emissions, deposition, and meteorological changes can be included. However, box and trajectory models, by their very nature, greatly simplify transport and diffusion, provide very limited information on spatial variability, and thus cannot represent any particular pollution episode with great detail. However, they can represent chemical transformations in as great detail as is known. Further, they are readily applied and are not computationally intensive. For these reasons, box models have been used extensively to define reactivities. For example, the reactivity scale specified by the California Air Resources Board (CARB) in the California LEV/CF Regulations (CARB 1990) was developed using a single-cell model (see discussion below). To test how well these models predict reactivity in a specific airshed, and to examine the spatial and temporal aspects of VOC reactivity, a more physically detailed Eulerian model must also be applied.
Three-dimensional Eulerian models, also called grid or airshed models, divide a represented air mass into multiple vertical and horizontal cells. Grid models provide the most comprehensive representation of any airshed and provide the only means to predict observed pollution levels in real-world pollution episodes, particularly with respect to spatial and temporal variation. However, these models require large quantities of detailed input data and have relatively high computational demands. In addition to uncertainties in chemical mechanisms (a feature common to both box and grid models), grid models are also often limited by uncertainties in input data (e.g., emissions and wind fields). For these reasons, grid models are best applied to airsheds in which extensive, carefully examined input data are available. Results can then be compared with ambient pollutant observations to evaluate the accuracy of
model predictions. Although models are frequently only evaluated against observed ozone data, comparisons with observations of VOC and NOx concentrations are needed to assess a model's ability to accurately simulate the relationships between ozone and its precursor emissions.
Thus, box and grid models provide varying advantages and disadvantages. Because they are not computationally intensive, box models can be used to represent a wide variety of chemical conditions and to perform extensive, formal sensitivity analyses. Grid models, on the other hand, although not well suited to multiple scenario testing and comprehensive sensitivity analysis, provide an opportunity to comprehensively assess specific pollution scenarios with great spatial and temporal detail. Choosing which model is best suited for a specific application is often based on balancing the need for physical detail with computational limitations. For these reasons, the study of reactivity should, in principle, rely on both box- and grid-model predictions. In this case, results from both types of models can be compared to help assess the reliability of the predictions. Air-quality modeling studies conducted specifically for investigating VOC species reactivity are given in Table 3-5.
Box- and Trajectory-Model Reactivity Assessments
Carter and Atkinson (1989) used a box model and a detailed chemical mechanism to quantify the reactivities of a variety of VOCs. They found, not surprisingly, that the reactivity in terms of grams of ozone per grams of VOC varied significantly between compounds and also as a function of the VOC to NOx ratio. In follow-on work, Carter (1993, 1994) developed 18 separate reactivity scales for quantifying VOC reactivity under different conditions, in this case using the SAPRC-90 chemical mechanism in a single-cell trajectory model. Those reactivity scales were derived using nine different approaches for dealing with the dependence of reactivity on environmental conditions and on two methods for quantifying ozone impacts. Of the 18 reactivity scales, 3 have received the most attention: the maximum incremental reactivity (MIR) scale, the maximum ozone incremental reactivity (MOIR) scale, and the equal benefit incremental reactivity (EBIR) scale (see Table 3-6).
The MIR scale is the incremental reactivity (IR) of a VOC computed for conditions in which the compound has its maximum absolute IR value. This generally occurs at a low VOC-to-NOx ratio in which the
TABLE 3-5 Examples Of Compound-Reactivity Modeling Studies
|
Reference |
Model Type |
Mechanism |
Application |
|
Carter and Atkinson 1989 |
Trajectory |
SAPRC |
One-day simulation of reactivities under varying VOC-NOx conditions. |
|
Derwent and Jenkin 1991 |
Trajectory |
Derwent and Hov (1979) |
Two-layer 5-day trajectory simulations of reactivity. Photochemical ozone creation potential (POCP) scales. |
|
McNair et al. 1992 |
Three-dimensional (CIT) |
LCC |
Calculation of three reactivity scales for 11 lumped compounds. Simulations were performed for a 3-day period in the Los Angeles area (the SCAQS episode). |
|
Carter 1994 |
Trajectory |
SAPRC-90 |
Development of 18 reactivity scales (including the maximum incremental reactivity (MIR) and the maximum ozone incremental reactivity (MOIR)) for 117 compounds. Results are the average of 39 trajectory simulations for 10-hr periods. |
|
Yang et al. 1994 |
Trajectory and three-dimensional |
SAPRC-90 |
Review of rate constant uncertainties |
|
Yang et al. 1995 |
Trajectory |
SAPRC-90 |
Rate constant uncertainty calculations for the reactivities of 26 compounds under MIR- and MOIR-type conditions. One averaged trajectory was used rather than the 39 used in the Carter MIR and MOIR calculations. |
|
Bergin et al. 1995 |
Three-dimensional (CIT) |
SAPRC-90 |
Calculation of three reactivity scales for 27 compounds. Simulations were performed for the SCAQS episode. |
|
Jiang et al. 1996 |
Trajectory |
SAPRC-90 |
Calculation of the contributions of 18 compounds to ozone concentrations in the Lower Fraser Valley. |
|
Derwent et al. 1996 |
Harwell trajectory model |
Harwell mechanism |
Updated calculation of VOC POCPs. |
|
Reference |
Model Type |
Mechanism |
Application |
|
Bergin et al. 1998a |
Three-dimensional (CIT) |
SAPRC-90 |
Rate constant uncertainty calculations for the scales and compounds in the Bergin et al. (1995) study above. |
|
Derwent et al. 1998 |
Harwell trajectory model |
Master Chemical Mechanism |
Calculation of VOC POCPs using a large, detailed mechanism. |
|
Khan et al. 1999 |
Trajectory and three-dimensional |
SAPRC-90 |
Calculation of eight compound reactivities in three domains using both grid and box modeling. |
chemistry is VOC-limited (see Figure 3-1). Mathematically, it is approximately expressed as
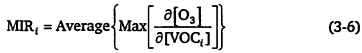
where MIRi is the MIR of species i, and [VOCi] is the input amount of species i. In practice, Carter fixed the VOC concentrations and adjusted the NOx to maximize the reactivity for the specific model run (or trajectory). The MOIR scale is the incremental reactivity computed for conditions that maximize the ozone concentration (see Figure 3-1), and thus tends to represent conditions in which the VOC to NOx ratio is moderate and the chemistry is approaching, or in, the transitional region between VOC limitation and NOx limitation (see Chapter 2). Mathematically, it is
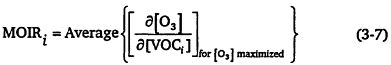
In this case, the NOx levels in the trajectories are typically set to give the maximum ozone levels, and then the sensitivity of the ozone to the
TABLE 3-6 Summary of Major Characteristics of the Primary Carter Reactivity Scales
|
Scale |
Type of Scenarios Used |
Derivation of Scale from Individual Scenario Reactivities |
Ozone Quantification |
Reflects Effect of VOC on |
|
Maximum incremental reactivity (MIR) |
Low VOC-to-NOx ratio conditions in which ozone is most sensitive to VOC changes |
Averages of incremental reactivities in the MIR scenarios |
Maximum ozone |
Ozone formation rates |
|
Maximum ozone incremental reactivity (MOIR)a |
Moderate VOC-to-NOx ratio conditions in which highest ozone yields are formed |
Averages of incremental reactivities in the MOIR scenarios |
Maximum ozone |
Ultimate ozone yield |
|
Equal benefit incremental reactivity (EBIR) |
Higher VOC-to-NOx ratio conditions in which VOC and NOx control are equally effective in reducing ozone |
Averages of incremental reactivities in the EBIR scenarios |
Maximum ozone |
Ultimate ozone yield |
|
a The MOIR scale is also referred to as the maximum ozone reactivity (MOR) scale. |
||||
individual VOCs is assessed. EBIR is the incremental reactivity for the conditions in which the sensitivity of ozone to VOC is equal to that of NOx. Thus, the EBIR scale is calculated for conditions that lie midway between VOC limitation and NOx limitation (i.e., the transitional regime).
CARB (1990) proposed using the MIR scale for regulatory applications, because the MIR scale reflects reactivities under environmental conditions that are most sensitive to the effects of VOC controls. Although the MIR scale might not be accurate for lower NOx conditions, State of California officials reasoned that, because of the lower sensitivity of ozone to VOC under these conditions, the impact of these inaccuracies would not be as critical (i.e., the scale would be most accurate for VOC-limited conditions, the conditions for which VOC controls would be most effective). The MIR scale was also found to correlate well to scales based on integrated ozone yields, even in lower NO x scenarios. Perhaps for
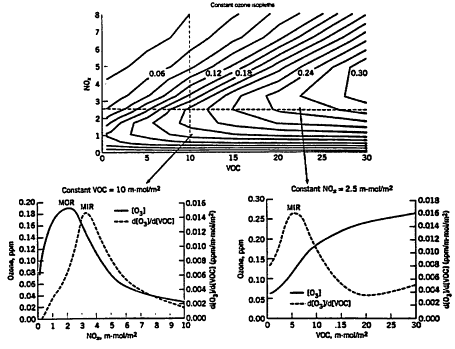
Figure 3-1
Dependencies of peak ozone concentrations and the peak ozone sensitivities ![]() with respect to initial VOC and NOx concentrations. The top graph illustrates peak ozone concentrations (as isopleths) as a function of both VOC and NOx. The bottom left hand graph shows how peak ozone levels vary when NOx is increased at a constant VOC input, and the right hand graph shows how ozone changes as VOC is varied at constant NOx input. Also shown is how the sensitivity
with respect to initial VOC and NOx concentrations. The top graph illustrates peak ozone concentrations (as isopleths) as a function of both VOC and NOx. The bottom left hand graph shows how peak ozone levels vary when NOx is increased at a constant VOC input, and the right hand graph shows how ozone changes as VOC is varied at constant NOx input. Also shown is how the sensitivity ![]() varies. The peak in the ozone sensitivity
varies. The peak in the ozone sensitivity ![]() plot corresponds to MIR conditions (in essence, maximum sensitivity), and the peak in the ozone concentration plot corresponds to MOIR (i.e., maximum ozone) conditions. The maximum ozone concentrations were calculated using a 1-day box-model simulation using the averaged conditions scenario of Carter (1994) and the SAPRC-93 mechanism.
plot corresponds to MIR conditions (in essence, maximum sensitivity), and the peak in the ozone concentration plot corresponds to MOIR (i.e., maximum ozone) conditions. The maximum ozone concentrations were calculated using a 1-day box-model simulation using the averaged conditions scenario of Carter (1994) and the SAPRC-93 mechanism.
Source: Bergin et al. 1998a. Reprinted with permission from Encyclopedia of Environmental Analysis and Remediation, copyright 1998, John Wiley & Sons, New York.
these reasons, MIR has been the reactivity scale used most extensively for policy-making in the United States, For example, in California, the MIR
scale is used as a basis for deriving reactivity adjustment factors (RAFs)4 in California's LEV/CF regulations (CARB 1991). The MIR scale was also used to compare reactivities of vehicle emissions during various driving cycles as well as with the use of various reformulated gasolines in the Auto/Oil Study sponsored by the petroleum and automobile manufacturing industries (AQIRP 1993). Thus, the analyses presented later in this report are also largely based on the MIR scale.
Nevertheless, it should be noted that the MOIR and EBIR scales have advantages. For example, MOIR is representative of conditions for the worst case scenario in which ozone concentrations would be highest. Both MOIR and EBIR are more representative of lower NOx conditions that are typically found in the eastern United States. Moreover, the MIR scale tends to predict lower reactivities for slowly reacting compounds than might be appropriate, because the higher NOx concentrations used for MIR scenarios tend to suppress radical levels and thus also suppress the kinetic reactivity of slower-reacting compounds.
Other trajectory-model investigations of VOC reactivity have included Andersson-Skold et al. (1992), Derwent and Jenkin (1991), and Derwent et al. (1996, 1998). Those researchers derived a comparable set of VOC reactivities, termed photochemical ozone creation potentials (POCPs). POCP is defined as the reactivity normalized to ethene calculated using a two-layer trajectory model covering an idealized 5-day trajectory across Europe. The second layer contains reacted material from previous days. The POCPs are calculated from the change in mid-afternoon ozone concentration due to each species in the trajectory that results from removing the test VOC from the emissions, divided by the integrated emissions of the test VOC up to the time of the ozone observation.
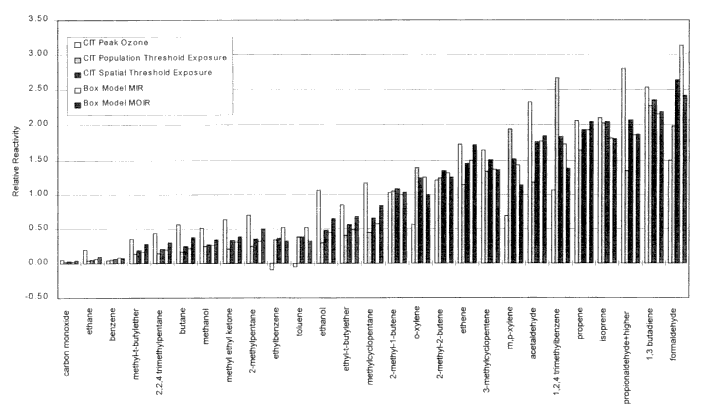
A comparison of MIR, MOIR, and POCP for selected VOCs is shown in Figure 3-2. The MIR and MOIR scales usually give similar relative reactivities for most compounds, and are consistent in their predictions of which compounds are highly reactive and which are not. However, for reasons indicated above, the MOIR scale gives lower relative reactivities for aromatics, and also predicts lower relative reactivities for radical
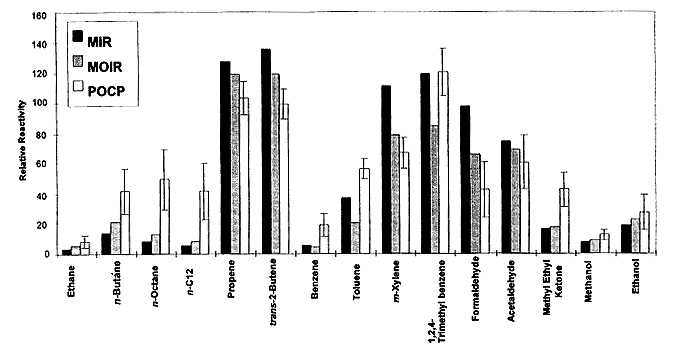
Figure 3-2
Comparison of MIR, MOIR, and POCP for selected VOCs. (Incremented reactivities (MIR and MOIR) are normalized relative to ethane = 100. POCP reactivities are averages for various trajectories. Error bias represent standard deviation of averages.
Source: Bergin et al. 1998a. Reprinted with permission from Encyclopedia of Environmental Analysis and Remediation; copyright 1998, John Wiley & Sons, New York.
initiators, such as formaldehyde, which have larger effects on rates of ozone formation than on total ozone formation over longer periods. Effects of differences and uncertainties in chemical mechanisms on calculated incremental-reactivity scales are discussed in more detail later in this chapter.
Eulerian-Model Reactivity Assessments
A serious concern about the regulatory application of scales, such as MIR and MOIR, is chat they are based on a box-model or trajectory-model simulation of a single-day air-pollution episode. For example, although MIRs are often developed from 10-hr simulations, some organic compounds can remain in an urban airshed for 2 to 3 days if stagnation is sufficiently severe or there is significant recirculation. Thus, MIRs might underestimate the relative reactivity of the slower-reacting compounds. Moreover, trajectory models lack the physical detail, the spatial and temporal detail of emissions and resulting pollutants, and the multi-day pollution effects that can be represented in Eulerian models. For that reason, reactivities derived using box and trajectory models should ideally be evaluated using more detailed Eulerian models. On the other hand, such an evaluation is not without its own inherent challenges. One of the most crucial is establishing a protocol for comparing model results; that is, what aspect of the spatially and temporally detailed Eulerian-model predictions are most appropriate to compare with a relatively simple MIR or MOIR predicted by a trajectory model? Perhaps somewhat arbitrarily, investigators have typically used either the Eulerian-model predicted values for the peak ozone concentration in the airshed or the population-weighted exposures to ozone.
Thus far, the most comprehensive comparison of reactivities calculated using trajectory models with those derived from an Eulerian model have been carried out using the Carnegie/California Institute of Technology (CIT) model (Harley et al. 1992) applied to a 3-day air-pollution episode in the Los Angeles air basin (McNair et al. 1992; Bergin et al. 1995, 1998b; Khan et al. 1999). McNair et al. (1992) used the CIT model with a highly lumped chemical mechanism (the Lurmann et al. (1987) mechanism (LCC)) to quantify the reactivity of 11 individual and lumped VOCs. This study allowed comparison with single-cell-model reactivity studies by others; it also allowed comparison of the different
metrics used to derive reactivities. The results showed that MIRs derived from trajectory models did not perform well in predicting peak ozone sensitivities to specific VOC species, but performed reasonably well in predicting the effects of VOC species on the integrated exposure to ozone over the air-quality standard. The MOIR scale did not compare as well as the MIR scale with results derived from airshed model for either the peak ozone concentration or ozone exposure concentrations greater than the air-quality standard.
Subsequent to the study of McNair et al. (1992), the SAPRC-90 mechanism was implemented in the CIT model by Bergin et al. (1995, 1998a) for more direct comparison with the MIR and MOIR scales. Reactivities were normalized to a mixture of VOCs representative of exhaust emissions, as in the reactivity studies of Carter (1994) and Yang et al. (1996). Again, the results for the exposure metrics compared well with the MIR scale (e.g., regression gave a slope of 0.98 and r2 = 0.97). To a lesser extent, the MOIR scale compared reasonably well with the peak ozone metric from three-dimensional modeling (slope = 0.95, r2 = 0.74), which occurs in a region that is less NOx-rich. These results suggest that the MIR scale is most appropriate in areas rich in NOx, though is less well suited to areas that are more NOx poor. This is examined further in the discussion on variabilities.
Uncertainties in Species' Reactivities due to Chemical-Mechanism Uncertainty
A concern often raised with regard to the use of reactivities in policy-making is their dependence on model-derived quantities that might be significantly distorted by uncertainties in knowledge of atmospheric chemistry and its representation through chemical mechanisms. Measurement errors in laboratory kinetic and product studies contribute to uncertainty in the chemical mechanisms used to calculate incremental reactivities. Moreover, as discussed above, the products of the initial OH radical, NO3 radical and/or ozone reactions, and their subsequent products, of many of the organic compounds emitted into urban atmospheres are not well characterized. Their representation in chemical mechanisms is based on analogy to compounds of similar structure, creating added uncertainty. At issue is whether the uncertainties in the chemistry, not only of the target species but others present in the atmosphere as well,
significantly limit the reliability of model-derived reactivities for organic compounds. The impact of uncertainties in chemical mechanisms on the reliability of reactivities derived from models should be discussed at two levels. First, how uncertainties affect the reactivity of individual VOCs is addressed in this section. Second, how they affect the reactivity of a source of emissions whose composition is made up of a large number of VOCs is addressed later in this chapter with particular emphasis on light-duty vehicular (LDV) emissions.
One way to assess the effects of chemical-mechanism uncertainty is to compare reactivity predictions using different state-of-the-art mechanisms that incorporate differing assumptions concerning unknown areas of the chemistry and differing lumping approaches. As discussed above, the SAPRC-90 mechanism was used for calculation of the MIR, MOIR, and other reactivity scales because of the large number of VOCs it can explicitly represent. The RADM-2 and LCC mechanisms employ assumptions similar to SAPRC-90 concerning uncertain portions of the aromatics and other mechanisms, and would be expected to give similar reactivities for the species that the condensed mechanisms are designed to represent. However, this might not be the case for the CB4 mechanism, which employs different assumptions concerning some of the uncertainties in the aromatics mechanisms, and uses different methods for treating alkane and alkene reactions (Gery et al. 1988). In addition, since the CB4 mechanism and SAPRC-90 mechanism were developed, there have been significant changes in the understanding of alkene and ozone reactions, new data on aromatics mechanisms, new laboratory data concerning a number of potentially important reactions, and a large database of new smog-chamber experiments designed explicitly to test VOC-reactivity scales (Carter et al. 1993; Jeffries and Sexton 1995; Carter et al. 1995a,b,c).
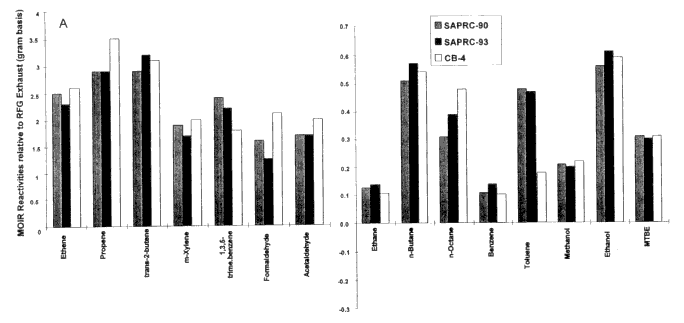
Figure 3-3 shows a comparison of MOIRs and MIPs for vehicular exhaust emissions (relative to standard exhaust) calculated with the SAPRC-90, CB4, and the updated SAPRC-93 mechanisms. Other than the mechanism, the scenarios and the calculation methodology were the same (Carter 1994). Differences of about 20% or more are not uncommon. However, for ethanol and MTBE, the agreement among the mechanisms is remarkable. The most conspicuous difference is for toluene.
More systematic studies of the effects of mechanism uncertainties were carried out by researchers (Derwent and Hov 1988; Russell et al. 1995; Yang et al. 1995, 1996; Bergin et al. 1996, 1998a; Yang and
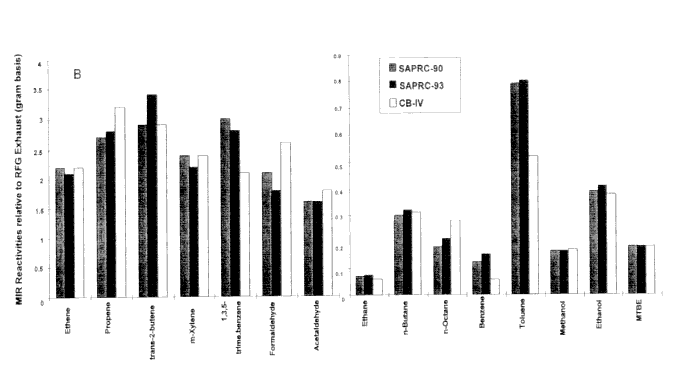
Figure 3-3
(A and B) Comparison of incremental reactivities of representative VOCs, relative to standard exhaust calculated using the SAPRC-90, SAPRC-93, and Carbon Bond IV mechanisms.
Source: Bergin et al. 1998a. Reprinted with permission from Encyclopedia of Environmental Analysis and Remediation; copyright 1998, John Wiley & Sons, New York.
Milford 1996) using airshed models and box models to explore to what degree uncertainties in chemical-rate parameters affect the calculated compound reactivities. Yang et al. (1995, 1996) used Monte Carlo analysis with Latin Hypercube Sampling to calculate reactivity uncertainties derived from a trajectory model. Bergin et al. (1998a) extended this analysis to a three-dimensional model by focusing on only those uncertainties in the chemical mechanism identified by Yang et al. to be most critical. Generally, these studies suggest that the uncertainty5 in the mean MIR value calculated for most individual VOCs generally is in the range of 20% to 60%. The estimated uncertainty in the predicted peak ozone concentration for the average MIR simulation conditions was about 30%, relative to a mean prediction of ~0.15 ppm. For predicted ozone and MIRs, the most influential uncertainties are those in rate parameters that control the availability of NOx and radicals (Yang et al. 1995). For Mills, uncertainties in the rate parameters of primary oxidation reactions, or reactions of relatively stable intermediates, are also influential. However, because uncertainties in the rate constants and parameterizations used in the chemical mechanisms apply to the calculations for all VOC reactivities, the effects of these uncertainties on the reactivities of individual VOCs are strongly correlated between VOCs. For example, an increase in the photolysis rate for NO2 increases the reactivity of most species by about the same proportion. Thus, the relative reactivities tend to have significantly smaller uncertainties than those of the absolute reactivities (Yang et al. 1995, 1996). Generally, through the use of three-dimensional modeling, the uncertainties in the relative reactivities of individual VOCs have been found to range from about 15% to 40% (Bergin et al. 1998a).
Variability of Ozone-Forming Potential With Environmental Conditions
Another concern about the use of reactivities within a regulatory context
|
5 |
In this and subsequent sections, uncertainty denotes two times the standard error of the mean. Such confidence intervals will contain the actual value 95% of the time. A more detailed discussion of uncertainty and its implications for policy-makers is presented in Chapter 7. |
relates to the fact that the ozone-forming potential of any given VOC can be heavily dependent upon local ambient conditions. In the extreme, a compound can go from being an efficient generator of ozone under one set of conditions w having a negative impact on ozone production under another set of conditions. This is due, in part, to the formation of an organic nitrate that ties up both a photochemically active oxidized nitrogen molecule and a reactive organic radical. While some compounds (e.g., toluene) do appear to have this property, a variety of studies indicate that such compounds represent exceptions rather than the rule, and that, as in the case of mechanistic uncertainty, the impact of environmental variability can be minimized by using relative reactivities instead of absolute reactivities. A few studies that have addressed those complications are discussed below.
In order to assess the magnitude of reactivity variability from one dry to another, Russell et al. (1995) derived absolute and relative reactivities along 39 trajectories using the box model of Carter (1994). Mean absolute reactivities and mean relative reactivities, along with their respective standard deviations of the mean, were then calculated. The magnitude of those standard deviations thus provides an indication of how different environmental conditions affect reactivities. Inspection of Table 3-7, in which some of the standard deviations calculated by Russell et al. are listed, indicates that environmental variability does in fact introduce significant variability into reactivities for many of the ubiquitous VOCs. However, such variability can be reduced by almost of factor of 2, from about 25-60% to 15-40% through the use of relative reactivities instead of absolute reactivities.
TABLE 3-7 Uncertainty in the Mean Absolute and Relative MIRs from 39 Separate Trajectory Simulations Representing Different Environmental Conditions
|
|
95% Confidence Interval (% of Mean Value) |
|
|
Compound |
Absolute Reactivity |
Relative Reactivity |
|
Formaldehyde |
28 |
16 |
|
Methanol |
39 |
23 |
|
Ethane |
56 |
38 |
|
Toluene |
38 |
21 |
|
Pentene |
39 |
21 |
|
Source: Derived from Russell et al. 1995. |
||
An alternate approach, adopted by Bergin et al. (1995, 1998a), assessed the impact of environmental variability by comparing reactivities calculated using a three-dimensional, grid-based model for the Los Angeles area with those derived from box-model simulations for 30 cities. Because of their large spatial domain, three-dimensional models cover domains with a wide range of environmental conditions and the reactivities derived from these models represent composite averages over this domain. Reactivities from box models, on the other hand, focus on a single set of environment conditions corresponding to a specific air mass following a specific trajectory. In Figure 3-4 the relative reactivities for a variety of VOCs calculated on the basis of peak ozone concentrations, population-weighted ozone exposures, and spatially weighted ozone exposures from a three-dimensional-model simulation are compared along with box-model-derived results. Here again, while significant variability is seen (and in the cases of toluene and ethylbenzene a change in the sign of the relative reactivity), the general trend in the reactivities from one species to another tends to be reasonably consistent.
Another relevant study is that of Khan et al. (1999), who conducted a reactivity study on eight VOC solvents having a wide range of reactivities in three different airsheds: Los Angeles, the Swiss Plateau, and Mexico City. Although the relative reactivities for the eight compounds were found to be similar between the Los Angeles and Switzerland domains, the very high VOC loadings found in Mexico City led to more substantial differences, one being that the aromatic species could have negative reactivities. The reactivity of the aromatics being greatly reduced in regions with lower NOx and higher VOCs was discussed earlier.
Uncertainties in Relative Reactivities of Motor-Vehicle Emissions
The previous discussion pertains to the reactivities of individual compounds and their attendant uncertainties. However, the charge to this committee (see Chapter 1) is to look at the use of relative reactivities as applied to motor-vehicle emissions, which are composed of hundreds of compounds. This complexity introduces some extra issues, in particular emission-composition uncertainty. A variety of modeling studies, listed in Table 3-8, have examined the reactivity of source emissions. In large
TABLE 3-8 Summary of Source Emissions Reactivity Modeling Studies
|
Reference |
Model Type |
Mechanism |
Application |
|
Trijonis and Arledge (1976) |
Calculated (not modeled) Trajectory |
EPA smog chamber data |
Estimated major source reactivities for metropolitan Los Angeles. |
|
Chang et al. (1989) |
Trajectory |
LCC |
Methanol-fueled vehicle impacts with respect to conventionally fueled vehicles. |
|
Russell et al. (1990) |
Three-dimensional (CIT) |
LCC |
Potential methanol-fueled vehicle impacts for the SCAQS episode (compared with equal mass emissions from conventional vehicles). |
|
McNair et al. (1994) |
Three-dimensional (CIT) |
LCC |
Calculations of RAFs for four fuels. Simulations were performed for the SCAQS episode. |
|
Yang et al. (1996) |
Trajectory |
SAPRC-90 |
Rate constant and exhaust composition uncertainty calculations for the RAFs from reformulated gasolines and methanol. |
|
Bergin et al. (1996) |
Trajectory and three-dimensional (CIT) |
SAPRC-90 |
Report on box model study described above and a three-dimensional study of the effects of rate constant and product yield uncertainties on predicted ozone impacts of five alternative fuel RAFs. |
|
Russell et al. (1995) |
Trajectory and three-dimensional (CIT) |
SAPRC-90 |
Evaluation of combined results of most previous studies. An economic analysis was also performed. |
|
Dunker et al. (1996) |
Three-dimensional (UAM) |
CB4 |
Extensive evaluation of how reformulated and alternative fuels would affect ozone formation in Los Angeles, New York, and Dallas. Tied directly to program to assess how fuel blends affect both emissions composition and emissions rates. |
|
Guthrie et al. (1996) |
Three-dimensional (UAM) |
CB4 |
Modeling of potential impacts of the use of three alternative fuels (CNG, M85, and RFG) in two urban areas. |
part, because of the alternative fuel regulations promulgated in California (see Chapter 5), this issue has been explored in most detail for motor-vehicle exhaust emissions.
When CARB implemented regulations for the LEV/CF6 program, it introduced the concept of reactivity adjustment factors (RAFs) to provide a mechanism for manufacturers who build vehicles powered by alternative fuels (including reformulated gasoline) to take advantage of the lower ozone-forming potential of the emissions from these vehicles. An RAF is defined as the ratio of the specific exhaust reactivities of two fuels (per gram of emission of an alternatively-fueled vehicle to that of a conventionally fueled vehicle). The specific reactivity of fuel i (SRi) is

where FAi is the fraction of species i in fuel A and Ri is the MIR of species i. The RAF for fuel A is defined as the ratio of the exhaust reactivities:
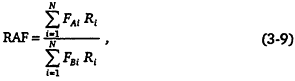
where FBi, is the fraction of species i in the base (reference) fuel. If the alternative fuel's RAF is less than 1, then a proportionally greater amount of VOCs can be emitted, such that the RAF times the mass of emissions meets some total emissions standard. In practice, the appropriateness of RAF—calculated using MIR values—was tested using a grid model and adjustments were made as necessary.
The sources and magnitude of the uncertainties in RAFs have been investigated by a variety of investigators, including Yang et al. (1996), McBride et al. (1997), and AQIRP (discussed later). The studies of Yang
et al. (1995) and McBride et al. (1997) revealed that although the 2-σ uncertainty in the relative reactivity of individual species due to uncertainties in chemical mechanisms generally range from about 20% to 40%, that range grossly overstates the uncertainty in the composite relative reactivity of a specific emissions source. An example would be reactivities from a fleet of motor vehicles using one type of fuel versus another. In this case, much of the chemical uncertainty tends to cancel out (provided one is using relative reactivities instead of absolute reactivities), leaving an uncertainty of only a few percent. A much larger uncertainty arises from the variability and difficulty in characterizing how different vehicles respond to fuel composition changes. This is largely due to the limited amount of test data and the limited knowledge of how well a vehicle fleet is characterized by the data. This leads to substantial uncertainties in the composition of the emissions, which feed directly into the calculation of the source reactivity. The result is an uncertainty (95% confidence level) in relative reactivities for source categories such as motor-vehicle emissions of about 15-30% (Yang and Milford 1996; Bergin et al. 1998a).
Reactivity for 1-HR Peak and 8-HR Averaged ozone Concentrations
Another specific question under consideration is whether reactivity scales developed for a peak 1-hr ozone concentration (i.e., in accordance with the current form of the National Ambient Air Quality Standards (NAAQS)) would be significantly different from a similar scale developed for a peak 8-hr ozone concentration (i.e., the new form of NAAQS). At present there is little information to assess this issue. of relevance is a study of Khan et al. (1999) in which the authors compared the reactivities of several compounds based on their impact on the peak 1-hr and the average 8-hr ozone concentrations. The comparison is shown in Figure 3-5. Major differences were only found in the halogenated aromatics that had very small reactivities to begin with. The relative reactivities of the other species did not change appreciably. This result, albeit limited, appears to suggest that reactivity scales derived for peaks of 1-hr averaged ozone concentration should largely apply to peaks of 8-hr averaged ozone concentrations in urban areas.
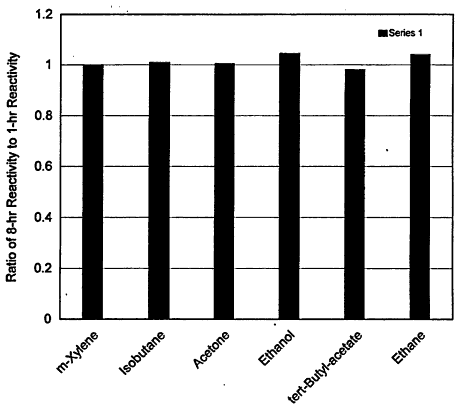
Figure 3-5
Ratio of 8-hr average peak ozone relative reactivity to 1-hr average peak ozone relative reactivity for six solvents. Results are for a 3-clay simulation in Los Angeles.
Source: Adapted from Khan et al. 1999.
On the other hand, a number of caveats should be borne in mind before this result is used to justify the application of trajectory-model-derived reactivity scales based on VOC impact on peak 8-hr averaged ozone concentration. In the first place, recall that Eulerian-model-derived reactivities based on the model's predicted peak ozone concentration did not compare well with the trajectory-model-derived MIPs. Second, reactivities derived from trajectory models are typically based on very limited simulation times, and thus the use of those models to derive a peak 8-hr averaged ozone-reactivity scale is questionable. Finally, the promulgation of the new 8-hr NAAQS for ozone is likely to extend nonattainment into larger geographical regions that include rural as well
as urban and suburban areas (Chameides et al. 1997). Thus far, little work has been done to assess reactivities at these large, regional scales. Moreover, ozone chemistry at the regional scale and in rural areas has generally been found to be NOx-limited (OTAG 1997), where implementation of VOC emission controls and using a VOC-reactivity scale might prove to be less effective.
Outstanding Technical Issues in Quantifying Reactivity
The scientific and policy-making communities have made significant advances in understanding and implementing methodologies for quantifying VOC ozone-forming potential using the concept of incremental reactivity. Nevertheless, key issues remain. Among these are the uncertainties in the understanding of the atmospheric chemistry of specific VOCs, and thus in the ability to quantify their ozone-forming potential, and the variability in reactivity between different environments. It was earlier stated that ozone sensitivity to VOC can, in general, vary from place to place within a given airshed and from episode to episode. Thus, environmental variability is not limited solely to one city versus another, but also to different locations within a city and also from one time to another. Further, it is not apparent that a reactivity scale developed for high-ozone episodes will be the same as one developed for more typical conditions. Also, as was found in Los Angeles, the impact on the peak ozone concentration is not likely to be the same as the impact on ozone exposure surrogates.
Another important issue relates to the role of NOx. VOC reactivity, and its use in control strategies, is of much less relevance in a system and in locations that are strongly NOx-limited. Thus, VOC reactivity should be viewed as a way of providing extra benefits to a strategy based on the implementation of VOC emissions controls. A major complication can arise, however, when a given control measure affects NOx emissions as well as VOC emissions, especially if the emission changes for the two sets of precursors are directionally different, which might be the case for reformulated gasoline using ethanol versus MTBE. Under these circumstances, one can, in principle, derive reactivities for NOx as well as VOCs to assess the net impact of the control measure on ozone. However, little
research has been undertaken on the derivation and application of NOx reactivities. Moreover, as implied earlier, NOx reactivities would likely be even more dependent upon location and episodic conditions than VOC reactivities. Application of NOx reactivities for a national ozone mitigation program would therefore be problematic.
Finally, consideration should be given to the future use of reactivity scales for particulate matter (PM) and ozone formation. Similar to ozone, different VOCs can lead to a substantial variation in the formation of secondary particulate matter; many VOCs will form no extra secondary organic particles, but others can lead to a substantial amount. In some cases, the compounds that lead to little ozone formation lead to little PM formation, and those that have a high ozone-forming potential also can form a large amount of particles. In other cases, the opposite is true. Models for simultaneously assessing PM reactivity and ozone reactivity are still under development.
Summary
Ozone atmospheric chemistry involves many thousands of reactions and a similar number of compounds. The two primary precursors to ozone formation are VOCs (and Co) and NOx, although this, alone, is an oversimplification. There are hundreds of different VOCs emitted into the atmosphere, and no two have the same chemistry; thus, they each have a different impact on ozone. Further complexity comes from the fact that the atmosphere is highly variable, both in its physical and chemical make-up. Thus, not only does ozone formation respond differently to different VOC species, but it will often respond differently to the same compound in different locations or during different episodes at the same location.
A variety of metrics or scales have been proposed to quantify the ozone-forming potential of an individual VOC or a mixture of VOCs arising from a specific source or type of emission. The reactivity paradigm is but one of a number of approaches that have been developed for this purpose. It is based on scientifically sound concepts and can provide a useful approach for policy-makers attempting to decide which VOCs or types of emissions to regulate and to what degree. Indeed, the state of California has already applied the reactivity paradigm to its regulation
of motor-vehicle emissions and the fuels used to power those vehicles. Exactly what metric should be chosen is, in part, a question of policy reflecting a set of priorities of the relevant stakeholders.
Within the reactivity paradigm, a number of different scales can be used. Each one provides a measure of the ozone-forming potential of a VOC or mixture of VOCs under a specific set of conditions. In this report, the maximum incremental reactivity (MIR) scale is used as the primary quantitative measure of ozone-forming potential. That scale reflects the ozone-forming potential of VOCs under conditions where ozone control is most sensitive to decreases in VOCs and is also the scale that the state of California has proposed using for its regulatory applications. For simplicity and in the interest of brevity, the term "reactivity" is used to denote the MIR, unless stated otherwise. Moreover, reactivity is expressed in a variety of ways. The specific reactivity, derived from box modeling, is the reactivity normalized to the change of mass of VOC emissions and has units of grams of ozone formed per grams change of VOC emitted or grams of ozone per grams change of VOC. The total reactivity is obtained by multiplying the specific reactivity by the mass of VOC emissions per mile driven and has units of grams of ozone per mile. The relative reactivity is a unitless quantity which is derived by dividing the (specific or total) reactivity of a compound or class of compounds by the (specific or total) reactivity of some reference VOC, standard VOC, or VOC mixture. Sometimes the term absolute reactivity is used in this report to denote either the specific or total reactivity as a way of distinguishing them from the relative reactivity. Each of these terms is listed in Table 3-9.
There are a number of limitations to the reactivity approach that should be borne in mind. Because the ozone-forming potential of VOCs can vary from locale to locale, it should not, in principle, be uniformly applied to the entire nation, except to facilitate regulatory application. Ideally, its use as a certification tool on a nationwide basis would allow for regionally-specific applications and, potentially, the development of regionally-tailored control strategies. Assessing the economic viability of implementing regionally-specific rules for certifying RFGs is beyond the scope of this report.
In its current state of development, a limitation of the use of a reactivity approach beyond full certification is that it only considers the ozone-forming potential of VOCs and CO. Thus it is of less use for
TABLE 3-9 Terms Used in the Report to Denote Reactivitya
developing VOC-based control strategies in areas where only NOx emission controls are needed. The reactivity approach is also of limited utility in assessing the impacts of control strategies that increase (decrease) VOCs emissions, while decreasing (increasing) NOx emissions. As it turns out, this might occur in the case of motor-vehicle emissions using specific types of RFG blends.
It is also important to note that the determination of reactivities for VOCs is a computational process that requires the application of a numerical model. The types of models that can be used for this purpose range from rather simplistic trajectory or box models to very complex, three-dimensional grid-based or Eulerian models. All of those models rely on a chemical mechanism for simulating the ozone-forming process, and a variety of algorithms for representing this chemistry have been adopted. Although differences between model results do occur (for example, in the case of the reactivities of the aromatics), in general, the relative reactivity of VOCs derived from different models and models using different chemical mechanisms tend to be reasonably consistent. For this reason, it is believed that the uncertainties (or potential errors) in reactivities can be minimized by focusing on relative as opposed to absolute reactivities.
In general, the 2-σ (or 95% confidence level) uncertainty in the relative reactivities in most of ubiquitous VOCs (that have been studied extensively) is about 20-40%. The relative reactivity of a composite set of VOCs arising from a single source, such as motor vehicles, tends to be somewhat smaller (i.e., about 15-30%). Much of the uncertainty in this later case arises from potential errors in defining the speciation of the emissions as opposed to those associated with the chemistry of the species. For this reason, the use of relative reactivity to assess the ozone-forming potential of different sources is best suited to situations where the reactivity of the emissions is quite different. As will become apparent in later chapters, this tends to not be the case for emissions from motor vehicles using slightly different RFGs. That will, in turn, limit the ability to use reactivity to distinguish robustly between the air-quality benefits of various RFG blends.