4
Guidelines for Sulfur Mustard Agents
INTRODUCTION
The various forms of sulfur mustard that have been used as chemical warfare agents are vesicants that produce blisters on exposed skin. They can damage the eyes as well as the respiratory and gastrointestinal tracts and are lethal at high doses. Sulfur mustard (bis(2-chloroethyl)sulfide, C4H8Cl2S) is a cellular poison, a mutagen, and a recognized human carcinogen (Saracci, 1981; NTP, 1989; BNA, 1990; IOM, 1993). Chemical and physical properties are more fully described in Veterans at Risk (IOM, 1993) and in the “Material Safety Data Sheets” prepared by the Edgewood Research Development and Engineering Center (ERDEC, 1990, 1992).
Modern chemical warfare agents might include any of the following three sulfur mustard formulations: Agent HD (distilled sulfur mustard), Agent THD (HD “thickened” with the addition of an acryloid copolymer to increase viscosity and, thus, persistence), and Agent HT (a plant-run mixture of about 60% HD and 40% T (bis-2-(2-chloroethylthioethyl)-ether, C8H16Cl2OS2) and some impurities). The addition of T lowers the freezing point and expands the effective temperature range over which HT might be used in chemical warfare.
The literature on the toxicity of sulfur mustard agents primarily contains information on the toxicity of HD. The subcommittee assumed
that the toxicity of the other sulfur mustard agents—THD and HT—is similar to that of HD.
The field drinking-water standard for sulfur mustard that is used by the armed services (triservices) is 200 µg/L of water for periods of consumption that do not exceed 7 consecutive days (short-term consumption) (U.S. Army, 1986, 1990c). That standard assumes that field drinking water contains no other toxic materials. The proposed triservice standard, as documented in Dacre and Burrows (1988) and approved by the Tri-Service Steering Committee in 1991, is 140 µg/L and 47 µg/L, assuming a water consumption of 5 and 15 L/day, respectively. The standard was based on a no-observed-effect level (NOEL) of 100 µg/kg in a 90-day exposure study of rats (Sasser et al., 1988a,b). The adverse effect observed was epithelial hyperplasia of the rat forestomach. It should be noted that field drinking-water disinfection (i.e., excess chlorination) can degrade sulfur mustard agents and might eliminate the threat of ingestion exposure; chlorine solutions are considered decontaminants for sulfur mustard agents (Sidell, 1992).
The Army Field Manual 10-52-1 (U.S. Army, 1991) indicates that HD is not considered a water contaminant because of its density and water insolubility and that blister agents (mustard and lewisite) are less of a threat than nerve agents because of their low solubility. These determinations are supported by physical and chemical characteristics known for sulfur mustard—i.e., that HD is sparingly soluble (0.68-0.92 g/L at 25°C) in water, and HT is considered practically insoluble; that sulfur mustard freezes at 13-15°C and might become a semisolid at temperatures near the freezing point (such as those found at the bottom of water pools); and that hydrolysis occurs slowly, forming a thin “monolayer,” after which reaction rates for the entire volume of agent droplet or mass are negligible (Dacre and Burrows, 1988; Somani, 1992; IOM, 1993). The subcommittee agrees with observations made by Dacre and Burrows (1988) that any sulfur mustard agent in drinking water is most likely undissolved.
TOXICITY
A review of the toxicity data on sulfur mustard agents reveals that
there are no controlled oral studies in humans, thus necessitating extrapolation from animal data (Dacre and Burrows, 1988; Papirmeister et al., 1991; Watson and Griffin, 1992; IOM, 1993). Two recent gavage studies involving exposure of Sprague-Dawley rats to HD have been completed (Sasser et al., 1989a,b). These investigations provide data that can be used to support a determination of a field drinking-water standard for HD.
In one of the studies (Sasser et al., 1989a), HD dissolved in sesame oil was administered by gavage 5 days per week for 13 weeks to Sprague-Dawley rats. Doses administered were 0, 0.003, 0.01, 0.03, 0.1, and 0.3 mg/kg of body weight. The authors reported the following:
No dose-related mortality was observed. A significant decrease in body weight was observed in both sexes in the 0.3 mg/kg [300 µg/kg/day] dose group. . . . The only treatment-related lesion associated with the gavage exposure upon histological evaluation was epithelial hyperplasia of the forestomach of both sexes at 0.3 mg/kg. The forestomach of one 0.1 mg/kg male was also ulcerated. The hyperplastic change was minimal and was characterized by cellular disorganization of the basilar layer. . . . The estimated NOEL [no-observed-effect level] for HD in this 90-day study is 0.1 mg/kg/day when administered orally [Sasser et al., 1989a, p. 4].
The incidence of forestomach ulcers (1/24) in the exposed animals did not differ from that observed in the controls. However, the study does not state whether pair-feeding was performed. Thus, it is unclear whether the observed weight loss in rats is due to a toxic response or to reduced ingestion of food.
The subcommittee concluded that the findings of Sasser et al. (1989a) and the analysis of Dacre and Burrows (1988, p. 14) on the drinking-water criteria do not support consideration of 300 µg/kg/day (0.3 mg/kg/ day) as the acute lowest-observed-effect level (LOEL). The subcommittee concluded that the findings of Sasser et al. do support consideration of 100 µg/kg/day (0.1 mg/kg/day) as the NOEL, as used in the analysis of Dacre and Burrows (1988). Note that the procedure used by Dacre and Burrows to estimate a NOEL from the data of a draft report of Sasser et al. (1989a) (i.e., LOEL/rating-effect value (RVe)) is an unsubstantiated procedure and not endorsed by the U.S. Environmental Protection Agency (EPA). The RVe is used to calculate composite scores for
determining reportable-quantity (RQ) estimates (EPA, 1984). NOEL and LOEL values are to be based on animal or human data from the literature.
Based on a NOEL of 100 µg/kg/day (0.1 mg/kg/day) in rats and an uncertainty factor of 10 to account for interspecies differences, the calculated average allowable daily intake (ADI) in humans is 100/10 = 10 µg/kg/day. No uncertainty factor for intraspecies differences in toxic response was applied because military personnel are assumed to be healthy. Therefore, the guidelines recommended by the subcommittee for sulfur mustard in field drinking water, assuming a water consumption of 5 and 15 L/day, are calculated as follows:
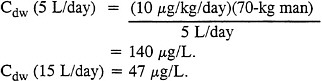
The majority of the subcommittee recommends use of a single uncertainty factor of 10 for the following reasons:
-
The experimental NOEL identified from the study of Sasser et al. (1989a) was based on forestomach hyperplasia in rats. Humans have no organ comparable to the rat forestomach, so the relevance of these rat data to humans is debatable. Also, the use of sesame oil as a carrier likely enhanced the potential for cellular damage from exposure to sulfur mustard agents because sulfur mustard is freely soluble in oils and fats.
-
The acute toxic effects observed in rats are not considered to be those that would incapacitate military personnel during a short (7 days) exposure.
A companion two-generation reproductive study of HD was performed in the same laboratory in 1989 (Sasser et al., 1989b). In this study, Sprague-Dawley rats were administered HD by gavage at doses of 0, 0.03, 0.1, 0.4 mg/kg before mating and throughout gestation, parturition, and lactation for 42 weeks. “No adverse effect on reproductive performance, fertility or reproductive organ weights of male or female rats” was observed (Sasser et al., 1989b). At the highest dose,
growth of F1 rats of both sexes was reduced, and the growth of F1 and F2 offspring was depressed during lactation. A dose-related lesion of the forestomach squamous epithelial mucosa was observed in both sexes. The investigators concluded that the NOEL “in this study was <0.03 mg/kg for toxicity and >0.4 mg/kg for reproductive effects” (Sasser et al., 1989b).
An additional ingestion study considered by the subcommittee is the dose-range study performed by Hackett et al. (1987a); the NOEL identified from this study was 0.2 mg/kg/day. However, the small sample size and single-gender (pregnant females) population precluded its use in the present analysis.
CANCER-RISK ESTIMATE
Sulfur mustard is a classic alkylating agent and readily reacts with components of DNA, RNA, and proteins. These characteristics make sulfur mustard a potent cell poison that is particularly toxic to mitotic cells; cytostasis, mutation, and cell death can also occur. A number of cell systems exhibit chromosomal aberrations following experimental exposure to sulfur mustard. In addition, mutagenic responses have been observed in Drosophila, mouse lymphoma cells, Neurospora crassa, and Salmonella. The cytogenetic and mutagenic response of sulfur mustard exposure is considered similar to that of x-rays (Watson et al., 1989; Watson and Griffin, 1992; IOM, 1993).
Retrospective studies of military veterans exposed to battlefield concentrations of sulfur mustard during World War I as well as British and Japanese chemical-weapons factory workers exposed during production of sulfur mustard and sulfur mustard munitions during World War II have been sufficiently compelling to have sulfur mustard classified as a Class 1 human carcinogen by the International Agency for Research on Cancer (IARC) (Saracci, 1981; Watson et al., 1989; IOM, 1993). Malignancies were found primarily in the upper respiratory tracts of humans after inhalation exposure. Thus, animal and human data are considered sufficient to support a casual relationship between exposure to sulfur mustard and subsequent cancer induction in humans.
By deriving an ingestion dose-response slope Q*, by the procedure
outlined by Watson et al. (1989, 1992) (Q*HD = 14.95 [(mg/kg)/day]-1), an estimate of lifetime cancer risk at the proposed and recommended ingestion guidelines can be calculated. Estimates are presented with adjustment for a single 7-day exposure in a lifetime (70 years) (i.e., 2.7 × 10-4) and Risk = (Q*)(D), where Risk is the additional lifetime risk of developing cancer from ingestion of field drinking water containing sulfur mustard agents, Q* is the risk per milligrams per kilograms per day and D is the dose (Anderson and the Carcinogen Assessment Group, 1983).
For a total intake of 700 µg/kg/day for 7 days (140 µg/L × 5 L/day), the estimated cancer risk is 4.1 × 10-5. However, because of the limited water solubility of HD, the actual dose might be much less than computed, making this cancer-risk estimate too high. The Code of Maryland Regulations (Title 26.11.15, Part .01 A(8)) (BNA, 1990) has defined an acceptable cancer risk for inhalation exposure to sulfur mustard as “not more than 1 in 100,000 (1 × 10-5).” Exposures that generate lifetime cancer risks less than 10-6 are rarely regulated by EPA or the Food and Drug Administration. Consumption of drinking water containing sulfur mustard agents at the concentrations proposed here theoretically carries a potential increased lifetime risk of cancer on the order of 10-5.
Field commanders and their troops need to be aware of the potential for increased lifetime risk of developing malignancy from exposure to sulfur mustard agents, as well as the magnitude of that potential risk. However, no acute effects (nausea, gastrointestinal upset, etc.) are expected to occur following consumption (for a period of 7 days or less) of field drinking water contaminated with sulfur mustard agents at or below the concentrations proposed here.
EXTRAPOLATION DIFFICULTIES
In the studies described above, rats exposed to sulfur mustard exhibited forestomach ulceration or hyperplasia. Since humans do not have an organ that is homologous to the rat forestomach, there is some debate as to the relevance of these lesions in estimating potential adverse health effects in humans exposed to sulfur mustard agents.
Other animal-to-human extrapolation difficulties stem from the nature
of the vehicle used to administer sulfur mustard agents to the rats and the route of exposure. The use of sesame oil as a carrier for a lipid-soluble compound, such as sulfur mustard, artificially enhances the potential for cellular damage in tissues (e.g., rat forestomach) coming in contact with the mustard-sesame oil solution. With respect to the route of exposure, the agent was administered by gavage, resulting in portal entry effects, not systemic effects. The gavage route of exposure is not directly comparable to drinking-water ingestion in humans.
Based on these extrapolation difficulties, it can be assumed that any estimates of field drinking-water guidelines derived from these data will result in conservative estimates considered protective for humans.
CONCLUSIONS AND RECOMMENDATIONS
The field drinking-water guidelines for sulfur mustard recommended by the subcommittee are 140 µg/L and 47 µg/L, assuming a water consumption of 5 L/day and 15 L/day, respectively. Those guidelines represent a 30% reduction from the existing short-term standard of 200 µg/L for 5 L/day water consumption.
Field commanders and their troops should understand the following with respect to the proposed guidelines:
-
Providing that no other toxic compounds are present in the water supply, acute effects (e.g., nausea or gastrointestinal upset) are not expected to occur following consumption of field drinking water at the recommended guideline concentrations.
-
These field drinking-water guidelines are based on extrapolations from limited studies in laboratory animals administered sulfur mustard by gavage.
-
Sulfur mustard is a known human carcinogen. Consumption of drinking water containing sulfur mustard for 7 days at guideline concentrations theoretically increases the lifetime risk of developing cancer by approximately 10-5. This risk should be weighed against the soldiers' daily needs for adequate hydration.
-
It is not known the extent to which chlorination or iodination will degrade sulfur mustard present at various concentrations in field drinking
-
water. It is possible that current military training and disinfectant 1 materials that are used for field drinking-water treatment will substantially reduce concentrations of sulfur mustard in water. It is recommended that this approach for mitigating exposure to sulfur mustard agents be investigated further.
-
Some available data characterize the efficacy of a new technique for removing HD from experimental waters. This technique involves a purification tablet that would replace the Globaline iodine tablet now in use (fielded since 1952) (Geomet, 1991; Powers, 1993). Use of the new water-purification tablets (Chlor-Floc) enhanced mustard agent degradation in EPA No. 2 standard water by approximately 10% at 5°C and by approximately 30% at 10°C (Geomet, 1991; Powers, 1993). Unfortunately, these investigations do not provide a comparison of degradation of HD in experimental waters treated with the new tablets and degradation of HD in waters treated with the currently used iodine tablets or excess chlorination. The subcommittee recommends that such studies be conducted.
|
1 |
“Any oxidant, including but not limited to chlorine, chlorine dioxide, chloramines and ozone added to water in any part of the treatment or distribution process, that is intended to kill or inactivate pathogenic organisms” (U.S. Army, 1991, p. G-3). Military doctrine and guidance for disinfectant use are provided in Field Manuals 10-52 and 10-52-1 (U.S. Army, 1990c, 1991). |








