5
Guidelines for T-2 Toxin
INTRODUCTION
T-2 toxin is a mycotoxin, a compound that belongs to the trichothecene class. It is formed as a secondary metabolite by some species of Fusarium molds. The oral LD50 of T-2 toxin in animals ranges from 3 to 5 mg/kg, and the dose-response curve is very steep. Because of the lipophilic nature of trichothecenes, they are rapidly and completely absorbed from the gastrointestinal tract and quickly distributed to all major organs. The mechanism by which T-2 toxin causes cell death is through the inhibition of protein synthesis at the 80S ribosome. T-2 toxin is strongly emetic at doses of 0.1-1 mg/kg of body weight in some animal species (such as swine and monkeys), making it difficult to estimate LD50s accurately in these species. The mechanism of action of emesis is not known.
Although T-2 toxin has been implicated as a chemical warfare (CW) agent in Southeast Asia and Afghanistan, no accurate human-exposure data are available. T-2 toxin purportedly was dispersed aerially as a CW agent in combat zones in Southeast Asia and Afghanistan, where inhabitants described the yellow substance as “granules or mists that fell like rain”; this substance later became known as “yellow rain” (Haig, 1982; NRC, 1983). T-2 toxin was claimed to be the lethal ingredient in “yellow rain” that was dispersed in Laos and Kampuchea in 1981 and to
appear as yellow spots on some rocks and leaves and in water samples taken from locations near battlefields.
Although there is little reliable information on the adverse health effects of T-2 toxins in humans, the most common toxic effect is thought to be nausea and vomiting. This finding is based on the considerable data compiled on the toxicology of another structurally similar trichothecene mycotoxin, diacetoxyscirpenol (DAS). DAS was used as an experimental antineoplastic drug in the late 1970s. The LD50 values and emetic dose for DAS in laboratory animals are similar to those for T-2 toxin. DAS was used in several Phase I and Phase II clinical trials for the treatment of cancer patients who had not responded to approved therapies. According to these studies, nausea and vomiting were the most common side effects of treatment. Other effects included hypotension, CNS disturbances, diarrhea, headaches, fever, and chills. Myelosuppression was also observed with prolonged exposure. Some of these effects were observed at doses as low as 0.2 mg/m2 (NRC, 1983). Based on toxic episodes related to Fusarium-contaminated grain products, there is circumstantial evidence linking T-2 toxin to alimentary toxic aleukia (ALA). In outbreaks such as these, there is usually more than one mycotoxin involved, so it is difficult to determine the contribution of T-2 toxin alone to the etiology of ALA (NRC, 1983). The use of the data from the clinical trials is limited because the trials involved a small number of people with an existing disease (i.e., cancer).
The Army conducted experiments to study the toxicity of T-2 toxin in monkeys (Wannemacher et al., 1991). The experiment included LD 50 studies via the intravenous and dermal routes as well as pharmacokinetic studies. The results of the LD50 studies showed that the toxic effects of T-2 toxin are similar to the chemotherapeutic drug DAS. Both T-2 toxin and DAS produce severe gastrointestinal toxic effects, such as diarrhea and vomiting. The pharmacokinetic studies in monkeys identified metabolites of T-2 toxin; the T-2 metabolites can be used to identify T-2-exposed persons.
FIELD DRINKING-WATER STANDARDS
The Army's proposed field drinking-water standards for T-2 toxin are
26 µg/L and 8.7 µg/L, assuming a water consumption of 5 L/day and 15 L/day, respectively (Daniels, 1990b). These values are based on data derived from Phase I and Phase II clinical trials in which DAS was administered to cancer patients. Toxicological studies in animals indicate that T-2 toxin and DAS are approximately equally potent. Therefore, the use of DAS dose-response data was considered appropriate when estimating the toxicity of T-2 toxin in humans.
To determine the concentrations of CW agents in field drinking water, a 7-day exposure and a water consumption of either 5 L/day or 15 L/day for arid climates were assumed. Based on a consideration of the circumstances under which these water standards would be invoked (war time) and the population that would be exposed (presumably healthy soldiers), the use of conservative safety factors, such as those used for setting standards affecting general populations, was deemed inappropriate. Specifically, safety factors were not used when deriving the concentrations for the proposed guidelines because of the following:
-
The acceptable concentrations of T-2 toxin in water were based on data from 5-day clinical trials in which DAS, an equipotent structural analog, was administered to patients with cancer. Patients with cancer are assumed to represent a more sensitive population, due to their disease and age, in comparison to a military population. The major performance-limiting effect that a soldier would exhibit following exposure to T-2 toxin would be nausea and emesis. It is not clear that the performance decrement caused by nausea and emesis in soldiers would be as incapacitating as nausea and emesis in patients who are already weakened by advanced disease and side-effects from previous exposure to chemotherapeutic agents.
-
Given that T-2 toxin and DAS are strongly emetic at doses approximately one-tenth the LD50, the highest dose of DAS observed not to cause nausea and emesis in the 5-day clinical trials was identified as the NOEL (Murphy et al., 1978). That NOEL was then adjusted to account for an exposure period of 7 days.
-
In the clinical trials, DAS was administered to patients by rapid intravenous (i.v.) infusion. An increase in the time of total-dose administration led to an increase in the tolerated dose (Goodwin et al., 1978). Therefore, it is reasonable to assume that the peak blood concentration
-
of T-2 toxin expected from drinking water would be below the concentration achieved by rapid i.v. infusion. However, it should be borne in mind that an i.v. dose is not necessarily more potent at causing nausea and emesis than an oral dose (Goodwin et al., 1978). In fact, the opposite might be true if the toxin acts directly on the stomach. In support of that possibility, Goodwin et al. (1978) reported a 5- to 6-fold increase in the potency of oral versus i.v. administration in swine and monkeys given a minimum emetic dose of T-2 toxin.
Thus, these three extrapolations or approximations (cancer patients to soldiers; 5-day exposure adjusted for 7-day exposure; and exposure by i.v. administration vs. gavage) might together provide a small margin of safety for battlefield exposures. In fact, the proposed concentration is comparable to values extrapolated from animal data in which an emetic dose was administered in a long-term feeding trial and adjusted using a 10- and 100-fold safety factor.
Data from two of several clinical trials (Goodwin et al., 1978; Murphy et al., 1978) in which DAS was administered to patients with cancer were used to compute the acceptable exposure concentrations. Data from four other clinical studies were also examined (Diggs et al., 1978; Yap et al., 1979; Thigpen et al., 1981; Bukowski et al., 1982). This comprehensive analysis supported the Army's proposed standards for field drinking water. Short of a study in which humans are exposed to T-2 toxin or a study in which doses of CW agents used in combat are accurately reconstructed, the available data appear to be adequate. Figure 5-1 shows a summary of the data used to develop the standards.
The current field test kit for detecting T-2 toxin in water has a detection limit of 470 µg/L. That limit is not useful when a standard of 8.7-26 µg/L is being proposed. The subcommittee recommends that a field-test kit capable of detecting T-2 toxin at or below the concentrations of the proposed standards be developed and made available to soldiers.
CONCLUSIONS AND RECOMMENDATIONS
The subcommittee concludes that the Army's extrapolations or approximations concerning exposure and toxicity are reasonable, appropri-
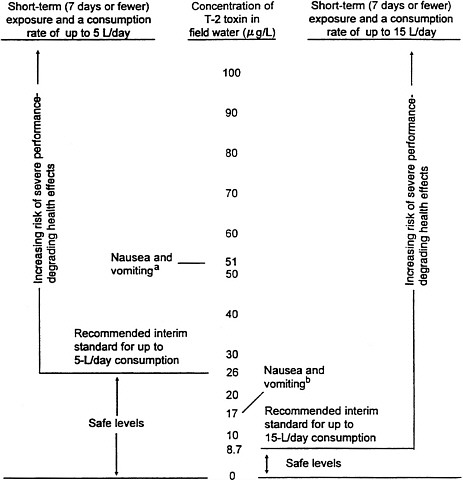
FIGURE 5-1 Health-effects summary for T-2 toxin in field drinking water (based on administration of DAS in clinical trials to cancer patients).
aPotentially performance-degrading health effects might include nausea, vomiting, diarrhea, generalized burning erythema, and mental confusion according to studies of DAS in clinical trials.
bBased on the lowest daily i.v. dose of DAS reported by Goodwin et al. (1978) to produce nausea and vomiting in cancer patients. Most severe health effects, including gastrointestinal problems, were reported in cancer patients administered a daily dose of DAS by rapid i.v. infusion for 5 days, a dose about 30 times greater than that used to calculate the standards. Therefore, concentrations of T-2 toxin that are 30 times greater than the recommended interim field drinking-water standards are expected to produce the most severe toxic symptoms.
Source: Daniels, 1990b.
ate, and conservative. The similarity in results obtained from the DAS clinical trials and the animal-data extrapolations provides some confidence in the appropriateness of the proposed field drinking-water standards of 26 µg/L for 5 L/day water consumption and 8.7 µg/L for 15 L/day water consumption. The subcommittee concludes that the Army's proposed field drinking-water standards for T-2 toxin are appropriate. Thus, the field drinking-water guidelines recommended by the subcommittee are the same as the Army's proposed standards.






