10
PHARMACEUTICALS FROM MARIJUANA
When the U.S. Food and Drug Administration approves the sale of a new medicine, its decision typically marks the conclusion of several years of expensive and labor-intensive development. Few compounds that appear promising in the early stages of research actually complete the journey from discovery to approval, and even fewer repay the cost of their development.
This is the path that novel medicines from marijuana compounds and their synthetic analogs must travel before reaching the pharmacy. But unless marijuana-based products can be made profitable as well as safe and effective, pharmaceutical firms are unlikely to pursue the research and development needed to produce them. This chapter examines the development of marijuana-based medicines from the perspective of their potential manufacturers. Along with the requirements for FDA approval, this chapter describes how the U.S. Drug Enforcement Administration (DEA) regulates marijuana and how marijuana's status as a controlled substance represents a potential barrier to developing cannabinoid medications.
Despite rather daunting odds, one cannabinoid product has been on the market for more than a decade: dronabinol, or synthetic THC. Currently sold as an oral medication under the brand name Marinol, dronabinol is being investigated for use in a vari-
ety of new applications. Its continuing story is recounted here—a story that offers insights into the development process—and a description of additional cannabinoids under development is provided. Finally this chapter assesses the outlook for new marijuana-based drugs as well as prospects for marketing whole marijuana as medicine.
THE DRUG-APPROVAL PROCESS
Under the federal Food, Drug, and Cosmetic Act, the FDA decides whether a drug is sufficiently safe and effective to enter the marketplace. The agency bases its decision on evidence assembled from clinical trials conducted by the drug's sponsor. Pharmaceutical companies sponsor the majority of clinical trials, but academic and government laboratories also participate in drug development. For example, the National Institutes of Health funds collaborative programs to promote the commercial development of drugs for conditions such as AIDS, cancer, addiction, and epilepsy. Such programs supported most of the research that brought dronabinol to market.
Drug development begins with a compound that has either been synthesized in a chemical laboratory or purified from a natural source. If scientists find it has a useful biological activity, they will proceed to test the compound in animals in order to determine its effects on whole organisms. For example, after discovering that a compound extracted from a plant binds to receptors on nerve cells involved in appetite stimulation, researchers might perform tests to see if the compound could actually cause mice to increase their food consumption and gain weight. Such early experiments, which occur before human testing of an experimental medicine, are known as the preclinical phase of drug development.
When evidence from animal research suggests that a drug should be safe and effective in humans, the manufacturer submits an Investigational New Drug (IND) application to the FDA. The IND submission contains a plan for human clinical trials and documents the results of preclinical testing. If the FDA does not contest the IND within 30 days, the manufacturer may proceed to conduct clinical tests of the new drug in humans.
Clinical trials generally consist of three phases (see Figure 10.1). During Phase I, healthy volunteers take the drug to confirm that it is safe for human use and to determine dosage. In Phase II a small group of patients who have the condition intended for treatment with the experimental drug test the compound to evaluate its safety and its potential for causing side effects. If the drug passes the first two phases successfully, it proceeds to Phase III trials in larger groups of patients. These tests are designed to confirm that the drug is effective and to monitor any adverse reactions that might occur during long-term use.
Progress through all three phases takes an average of five years to complete, but a variety of factors can impede this process. Most important, researchers must be able to find enough volunteers to participate in trials; this is often difficult for the second and third phases since the pool of available patients may be small. In addition, several factors related to the specific compound can slow its passage through clinical trials. Generally, the more complex the experimental medicine or the disease or symptom being treated, the lengthier the clinical trial process. Drugs that produce multiple effects and those intended for long-term use—for example, to treat symptoms of chronic conditions such as AIDS or glaucoma—demand extra time in the clinic. But even a relatively straightforward passage through clinical testing consumes most of the $200 million to $600 million spent to develop the average drug.* And since only about one in five drugs that begin Phase I eventually secures FDA approval, clinical trials represent a significant financial risk.
Once a compound has passed all three phases of clinical testing, its manufacturer submits a request to market the drug, called a New Drug Application (NDA), to the FDA. An NDA is a massive document that includes not only the results of clinical testing but also of experiments designed to characterize the drug's chemistry and physiological activity as well as a detailed description of the process that will be used to manufacture it. In the case of a cannabinoid drug the NDA would probably also include the re-
|
* |
How best to estimate the cost of developing a drug is subject to debate. Different methods produce different estimates, but the general range is from $200 million to $600 million. |
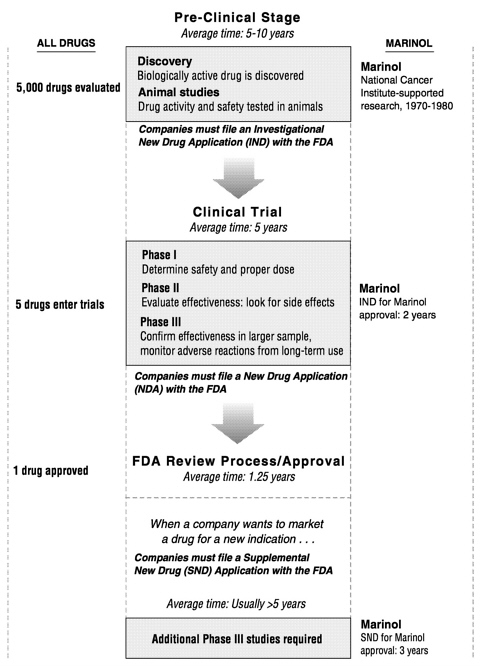
FIGURE 10.1 The drug development process consists of a series of stages. The process begins with the discovery of a biologically active drug and ends with the granting of approval for its sale by the Food and Drug Administration. Approximately one in five drugs that begin Phase I trials secure FDA approval.
sults of a series of studies designed to assess the drug's potential for abuse. In 1996 it took the FDA an average of 15 months to review and approve a successful NDA; in 1990 it took about two years. Federal legislation passed in 1992 that allowed the FDA to charge user fees to industry—and thereby hire additional reviewers —has greatly expedited this process.
The approval of an NDA by the FDA permits the drug's manufacturer to market it for the treatment of a single specific condition or symptom. Physicians are free to prescribe the drug for additional indications, a practice known as off-label use. To obtain permission to market an approved drug for additional indications, the manufacturer must submit a supplemental application to the FDA. This is usually a far simpler and less expensive process than the initial NDA, since the drug in question has already been proven safe and its side effects have been well characterized. Generally, the pharmaceutical company must conduct one or two new Phase III clinical studies to demonstrate an approved drug's efficacy in treating an additional indication, a process estimated to cost $10 million to $40 million.
Receiving FDA approval to market a drug for a new indication also takes time. Because the agency assigns lower priority to reviewing these so-called efficacy supplements than to evaluating NDAs, approval for a supplement has in some instances taken even longer than the original NDA. This process may eventually be reformed according to the Food and Drug Modernization Act of 1997, which allows manufacturers to provide information about off-label uses for drugs without prior FDA approval. However, since the new rules specified in this act are likely to be modified and refined in the courts, its ultimate impact remains uncertain.
Pharmaceutical companies must also get approval from the FDA before marketing an approved drug in a new dosage form. For example, if Unimed Pharmaceuticals, the manufacturer of Marinol, wanted to produce an inhaled version of the medicine, it would first have to conduct research to prove that the new delivery method is safe and effective. Moreover, in this particular case the company would probably also have to document the abuse potential of inhaled dronabinol.
While FDA approval represents a considerable hurdle in the
path toward developing novel cannabinoid drugs, the agency also sponsors two programs that may encourage progress in this area. One program, authorized under the Orphan Drug Act of 1983, provides incentives to manufacturers to develop drugs to treat rare so-called orphan disorders. Such diseases, by definition, affect no more than 200,000 people in the United States. Since drug companies are unlikely to recoup the costs of developing medicines for so few patients, the FDA offers a variety of economic incentives that allow firms to make a profit from orphan drugs, including the right to an exclusive market for their product for seven years. Some of the medical conditions for which cannabinoids show promise, such as spasticity or Huntington 's disease, may meet the definition of an orphan disease.
In addition, disorders affecting more than 200,000 people in the United States may contain subgroups that qualify as orphan populations. For example, while Parkinson's disease affects approximately 1 million Americans, a small number of these patients may share a specific symptom, such as early-morning motor dysfunction, that can be relieved by an orphan drug. The FDA can also grant orphan drug privileges to a medicine intended for more than 200,000 patients if it will cost more to develop than the manufacturer can recover in profits.
The second FDA program that might expedite cannabinoid drug development is known as the treatment IND. It allows patients with life-threatening diseases, such as AIDS or cancer, access to experimental medications before they have received approval for marketing. Once a drug enters Phase III clinical trials, treatment INDs may be issued to allow patients who are not part of the trials to use the drug as long as no comparable approved medication exists. This program might thus permit some patients to try a promising novel cannabinoid much sooner than if it followed standard FDA procedure. Success at this early stage could boost sales of the drug once it was approved for widespread marketing.
SCHEDULING OF CONTROLLED SUBSTANCES
The federal Controlled Substances Act of 1970 requires that every drug with a potential for abuse, including marijuana and
compounds derived from it, be classified and regulated according to whether there is a currently accepted medical use for the drug as well as the likelihood that the drug will be abused. The DEA conducts the classification process, assigning each controlled substance into one of five categories called schedules (see Box 10.1). Each schedule invokes a specific set of regulatory controls on research, manufacturing, distribution, prescription, sale, and use of the drug. For example, patients who take morphine, a Schedule II medication, must appear in person to have their prescriptions filled, rather than phone in their requests to the pharmacy.
The possibility that a drug might be scheduled under the Controlled Substances Act represents a major deterrent to its development by a pharmaceutical company because scheduling can limit its profits in two main ways. First, the company must document the drug's abuse potential, which adds to research costs as well as the time it takes to bring the drug to market. Testing for abuse liability may require the manufacturer to conduct studies on both animals and humans to gauge the likelihood that people will want take the drug for nonmedical purposes. These studies attempt to predict whether the drug might be sold on the black market or otherwise pose a threat to public health. The second drawback of scheduling for pharmaceutical companies is the tendency of physicians to avoid prescribing scheduled drugs, which further reduces potential sales.
The scheduling of a controlled substance may be initiated by any of several parties, including the DEA, the U.S. Department of Health and Human Services (DHHS), the drug's manufacturer, or by public petition. Final rulings on scheduling rest with the DEA, in consultation with the secretary of the DHHS, who makes his or her recommendation (which the DEA usually follows) after reviewing the relevant scientific evidence. Once the DEA receives a DHHS scheduling recommendation for a particular drug, it usually takes weeks to months for the agency to announce its decision. In addition to scheduling at the federal level by the DEA, several states also impose scheduling laws of their own on the manufacture and distribution of controlled substances.
Currently, tetrahydrocannabinols, including THC and all other chemically related compounds derived from the marijuana
|
Box 10.1 Scheduling Definitions for Controlled Substances as Established by the Controlled Substances Act of 1970 Schedule I (includes heroin, LSD, and marijuana)
Schedule II (includes methadone, morphine, methamphetamine, and cocaine)
Schedule III (includes Marinol, anabolic steroids)
Schedule IV (includes Valium and other tranquilizers)
Schedule V (includes codeine-containing analgesics)
SOURCES: 21 U.S.C. §812 and 21 C.F.R. 1308, April 1, 2000. |
plant, are assigned to the most restrictive category (Schedule I) because they have no currently accepted medical use and have a high potential for abuse. Synthetic cannabinoids with activity similar to THC would also be automatically assigned to Schedule I, according to DEA regulations. An exception to this is dronabinol (Marinol); initially a Schedule II drug, it was reassigned to Schedule III in July 1999 as a result of a petition filed by its manufacturer.
Both the FDA and the DEA tightly regulate research on Schedule I substances, even when it does not involve human trials. For example, scientists studying cannabinoids found in marijuana plants must first receive DEA approval of both their experimental plans and their research facilities (see Chapter 11). Because com-
pliance with these regulations is both costly and time consuming, few pharmaceutical companies are likely to undertake such studies. By contrast, cannabinoids that are not found in the marijuana plant and that are chemically distinct from THC, including anandamide and several synthetic cannabinoids currently being evaluated in preclinical studies, were not classified as controlled substances at the time of writing. These compounds therefore represent more attractive candidates for drug development than their marijuana-derived counterparts. Nevertheless, since a cannabinoid from a source other than marijuana has yet to be tested in clinical trials in the United States, it remains to be seen whether such compounds will continue to remain unscheduled.
It is too soon to tell to what extent scheduling will affect the
overall development of cannabinoid drugs. On an individual basis, cannabinoids are likely to be scheduled if they exist naturally in the marijuana plant, if their chemical structure or pharmacological activity resembles that of THC, or if they otherwise show potential for abuse. If a cannabinoid is actually derived from marijuana, it automatically falls under Schedule I. To market such a cannabinoid as a medicine, the manufacturer would first have to petition the DEA to have it rescheduled—a strong disincentive to developing such drugs.
Of course, the rescheduling of marijuana to a less restrictive category would drastically change the outlook for cannabinoid drug development. The National Organization for the Reform of Marijuana Laws (NORML) and others continue to petition the DEA to remove marijuana and THC from Schedule I; so far these efforts have been unsuccessful. If marijuana were to be rescheduled, that decision would result in the rescheduling of any cannabinoid found in the plant.
THE MARINOL STORY
Marinol is the brand name for an oral form of dronabinol, the only marijuana-based prescription medicine currently available in the United States. Each gelatin-coated Marinol capsule contains 2.5, 5, or 10 milligrams of dronabinol—a synthetic compound identical to natural THC—dissolved in sesame oil. The only difference between THC and dronabinol is their origins. Both are the products of a series of chemical reactions; those that produce THC occur in plants, while those that produce dronabinol take place in a laboratory or chemical factory. Rather than perform an expensive extraction to purify THC from marijuana plants, which are illegal to grow in the United States, Unimed Pharmaceuticals manufactures Marinol from pure dronabinol.
To date, Marinol has received FDA approval for two applications: to control nausea and vomiting associated with cancer chemotherapy and to counteract AIDS wasting. However, as will be discussed later in this section, dronabinol appears promising for additional indications and may also be adaptable to several new delivery methods, so it is likely to be marketed more widely in the future. Another synthetic cannabinoid, nabilone (Cesamet),
has been approved for use in the United Kingdom. A close relative of THC, nabilone is also prescribed for chemotherapy-induced nausea and vomiting.
Dronabinol's greasy consistency presents several problems to a drug manufacturer. First, it makes the compound difficult and expensive to purify. Second, because dronabinol does not dissolve readily in water, only a fraction of the orally ingested compound reaches the patient's circulation. That amount is further reduced by the action of the liver, which recognizes dronabinol as a contaminant and removes it from the bloodstream. As a result, researchers have estimated that only 10 to 20 percent of the dronabinol in each capsule actually reaches its target in the body: cells bearing cannabinoid receptors (see Chapter 2).
Compared with other oral medications, dronabinol takes effect quite slowly. Absorption through the gastrointestinal tract is inherently slow; however, a typical over-the-counter pain reliever achieves results within 30 minutes, while dronabinol's peak activity does not occur until two to four hours after ingestion. This is not the case when dronabinol is injected or inhaled. Delivered by these methods, the drug reaches its maximum level in the body nearly instantaneously because it enters the bloodstream immediately (inhaled dronabinol is absorbed directly into capillaries in the lungs). While peak dronabinol concentrations may vary greatly among patients who take it orally, inhalation and injection produce more consistent levels of the drug.
Another drawback of dronabinol use is the frequency of side effects, most of which involve the nervous system. These include anxiety, confusion, dizziness, mood changes, sleepiness, and thinking abnormalities. In two recent clinical trials about one-third of patients who received dronabinol reported having such symptoms, although only a small number of these patients actually discontinued their use of the drug.1 Reducing the dose of dronabinol appears to minimize most of its adverse side effects, particularly feelings of disquiet or malaise.
Given the considerable challenges of bringing Marinol to market, it may seem remarkable that Unimed accomplished that task. But the company had plenty of help in the form of government-sponsored research on THC and incentives for drug development. Most of the preclinical and clinical studies on THC that culmi-
nated in the initial FDA approval of Marinol in 1985 were conducted or funded by the National Cancer Institute beginning in the 1970s. Unimed estimates that it contributed only about onequarter of the total research effort that secured Marinol's entry into the U.S. market. Its development also proceeded more quickly than usual, moving from IND to approval in two years, compared with five years for the average drug.
Unimed later applied for FDA approval to market Marinol for a second indication—AIDS wasting. At that time, the agency required Unimed to complete two relatively small Phase III studies, which lasted three years and cost approximately $5 million—again, a relative bargain in terms of both time and money. Under the Orphan Drug Act, the FDA also granted Marinol seven years of exclusive marketing for this application, beginning with its approval in 1992.
After Marinol received FDA approval for AIDS wasting in 1992, its sales grew significantly. This gain was especially welcome, since profits from medication for chemotherapy-induced nausea were beginning to decline as a result of the introduction of more effective antinausea drugs, such as ondansetron, that are also unscheduled.
Since its commercial introduction in 1985, Marinol had been listed in the most restrictive schedule for medically useful controlled substances along with morphine, cocaine, and other prescription medications with a “high potential for abuse.” While such a distinction clearly limited Marinol's availability, it did not delay the drug's initial entry into the market because the scheduling decision was made by the DEA prior to FDA approval; nor did any delays occur as a result of state scheduling laws.
When Unimed later prepared to petition the DEA to reschedule Marinol, the company commissioned a study to determine the extent to which its product was being abused. The study was conducted by researchers at the Haight Ashbury Free Clinic in San Francisco—where significant numbers of marijuana users, as well as people with HIV and AIDS, receive treatment—and it included information gathered from addiction medicine specialists, oncologists, cancer and AIDS researchers, and law enforcement officials.2 The researchers reported that they found no evidence that Marinol was being abused or diverted from medical use.
They attributed the drug's low abuse potential to the fact that it is slow to take effect and also because of the negative mood changes it sometimes produces.
In July 1999 the DEA granted Unimed's petition to reschedule Marinol from Schedule II to Schedule III. This action lifted many of the restrictions that previously limited Marinol's availability. Now physicians who prescribe Marinol in quantity or who specify refills face far less cumbersome paperwork than when the drug was listed in Schedule II. Not surprisingly, Unimed estimates that moving Marinol to Schedule III could produce a 15 to 20 percent increase in the drug's sales, currently estimated at $20 million (a modest figure by industry standards).
Beyond this important gain, Marinol's market could expand even further if the drug were approved for additional indications. Currently, 80 percent of the patients using Marinol take it to relieve AIDS wasting, 10 percent to relieve chemotherapy-induced nausea, and the remaining population for off-label conditions. The latter group is thought to consist mainly of Alzheimer's patients; in a recent study the drug showed promise in treating appetite loss and behavioral disturbances associated with that disease. Unimed cannot, however, market Marinol to treat complications of Alzheimer 's disease without first receiving FDA approval.
The company is currently conducting research in pursuit of approval for this indication and in late 1998 received a use patent for the application of Marinol to improve disturbed behavior in people with various forms of dementia, including Alzheimer's disease. This gives Unimed 20 years of patent protection for dementia treatments based on its product provided that the additional indication gains FDA approval.
Another likely market for Marinol consists of people with AIDS who receive combination antiretroviral therapy (see Chapter 5). For these patients dronabinol offers a double benefit: not only does the drug stimulate appetite, it also appears to relieve nausea and vomiting, common side effects of the standard daily doses of antiretroviral drugs. Unimed is presently conducting a Phase II study in this area; if the results are promising, the company plans to seek FDA approval for the additional indication.
In addition to possible applications for Alzheimer's and AIDS patients, Unimed—along with its marketing partner, Roxane
Laboratories—is exploring the possibility of using dronabinol to treat spasticity in multiple sclerosis as well as intractable pain. The companies are also studying its ability to stimulate appetite in people with cancer and renal disease. Each group of patients would represent a significant new market for the drug.
While expanding the number of approved indications for dronabinol represents an important route to increasing its sales, making a more effective version of the drug could potentially give it an even bigger boost. As described earlier, dronabinol is slowly and poorly absorbed. It is also difficult to find the right dose for each patient because it takes several hours for the drug's effects to reach their peak. Moreover, as with any oral drug, much of the initial dose is lost due to inefficient absorption or is destroyed in the liver. Thus, it makes sense that Unimed and Roxane are also pursuing new ways to deliver dronabinol that would avoid these pitfalls.
In 1998 Unimed filed an IND as a step toward developing four new formulations for dronabinol designed to deliver the drug more rapidly to the bloodstream. These include an inhaler, a method the IOM researchers consider to be particularly promising, and two nasal preparations, a spray and a gel. The company is also exploring oral formulations that would allow dronabinol to be absorbed directly into the blood vessels that lie beneath the tongue, rather than swallowed as a pill.
Other researchers are exploring the use of rectal suppositories to deliver THC or dronabinol, but this method is considerably slower than the previous four; it is also likely to be acceptable to fewer patients. Unfortunately, attempts to deliver THC through the skin, via a transdermal patches like those used to deliver nicotine or hormone therapy, have so far been unsuccessful. However, if chemists were to find a way to synthesize a THC analog that penetrated the skin more effectively, it could be delivered this way.
While more efficient methods of administering dronabinol, particularly the inhaled aerosol and spray routes, promise more rapid relief from symptoms that respond to the cannabinoid, they also carry an increased potential for abuse. As addiction experts have observed, the more rapidly a drug takes effect, the more likely it is to be abused. Unimed anticipates that FDA approval of
more efficient delivery methods may require the company to determine their liability for abuse as well as their efficacy and physiological characteristics. Unimed estimates that each new formulation will cost between $7 million and $10 million to develop.
These additional research costs are likely to affect the price of new dronabinol formulations. In capsule form, dronabinol (Marinol) currently costs about $200 per month for its most common use—to combat AIDS wasting; the cost of treating chemotherapy-induced nausea is lower, since it is not a chronic condition. Several patients who spoke at the IOM's public workshops found Marinol's price to be prohibitive and said that one of the advantages of using marijuana for medical purposes was its relatively low cost. But this is a deceptive comparison, for the indirect costs of marijuana use—criminal penalties (see Chapter 11)—can be prohibitive. Moreover, marijuana users assume the risks of using a substance of uncertain quality and composition.
In fact, it is almost impossible to directly compare the costs of Marinol and medical marijuana use. The cost of Marinol varies, depending on the patient's situation. Public and private health insurance plans generally reimburse for all or part of the cost of Marinol but not, of course, for marijuana. Roxane Laboratories also sponsors an assistance program to provide Marinol for indigent patients. The price of marijuana is also quite variable; at California buyers' clubs, the IOM team learned, patients typically paid $2 to $16 per gram, depending on the grade of marijuana. (An average marijuana cigarette weighs approximately 1 gram.) Street prices are even less consistent, as is the quality of the product. The THC concentration of marijuana can easily vary from 2 to 15 percent. And while home cultivators can produce quality marijuana at low cost they also bear an increased risk of criminal penalty.
Based on the above considerations, Unimed has estimated that Marinol is in fact cheaper than marijuana for patients with health insurance or for those eligible for financial assistance from Roxane. For those who must assume the entire cost of Marinol out of pocket, it may still be cheaper than using whole marijuana if the patient smokes two or more average-sized joints per day. If medical marijuana were to become legally available, these comparisons would no longer hold. But for the moment Unimed be-
lieves that only a small portion of its potential market for Marinol is being lost to competition with marijuana.
While Marinol embodies the promise of cannabinoid medicines for treating multiple indications through a variety of delivery methods, its history also reflects the numerous challenges involved in developing such products. From purification and delivery, to the costs of research and regulatory compliance, to the difficulties of marketing a controlled substance, the barriers to producing cannabinoid drugs are many. It is also important to remember that government support for the research and development of Marinol significantly lowered these hurdles. Thus, while instructive, the exact conditions that produced Marinol are unlikely to be duplicated for another cannabinoid drug.
Turning from this specific case, we now examine the factors that are likely to determine whether new medicines will continue to be developed from marijuana, the outlook for drugs based on individual cannabinoid compounds, and the prospects for developing medicines from the entire marijuana plant.
PROSPECTS FOR NOVEL CANNABINOID MEDICATIONS
The potential therapeutic value of cannabinoids extends far beyond remedies for nausea and weight loss. As detailed in previous chapters, marijuana and THC have already shown some promise in treating pain and muscle spasms and in providing simultaneous relief for several symptoms, particularly in AIDS patients. Although all of these conditions represent opportunities to fulfill unmet patient needs, pharmaceutical companies must weigh additional factors in determining whether to pursue the development of marijuana-based therapies.
Before assuming the financial risk of initiating preclinical research or clinical trials, manufacturers must first determine whether such investments are likely to produce adequate returns. These decisions are typically made on the basis of a market analysis, an attempt to forecast the potential costs and benefits of developing a specific drug. For cannabinoid drugs, the development costs will probably be higher than average because of the additional expense of testing for abuse liability. And if the compound under consideration is classified as either a neuropharmaceutical
or a nonsteroidal antiinflammatory drug—the two therapeutic categories associated with the highest development costs—the price of approval could rise even higher.
To estimate potential returns from a candidate drug, a pharmaceutical company assesses several contributing factors. It calculates the drug's projected market size based on the estimated patient population, sales of existing medications, and the degree to which existing drugs will compete with a new product. The company would also consider whether patients will use the drug for a limited time or whether it will be used to treat chronic condition. Treatments for chronic conditions that emerge early in life are especially valuable from a commercial standpoint.
The ability to patent a candidate drug also affects its potential market since it gives the holder the exclusive right to sell a novel and non-obvious product for 20 years. Other types of market protections, such as orphan drug status, confer similar advantages. As discussed earlier, scheduling under the Controlled Substances Act tends to decrease the market for a drug, as do adverse side effects and interactions with other medications. Finally, markets may be swayed by other less predictable factors, such as social attitudes—an important consideration for marijuana-based medicines—and the likelihood that insurers will reimburse patient costs.
Several companies and individuals have presumably weighed these factors and decided in favor of pursuing cannabinoid drugs since the public record shows that at least three such compounds are currently being developed (see Table 10.1). According to the IOM, all of these compounds, except dronabinol (THC) and marijuana, remain in the preclinical stage.
The list of cannabinoids in Table 10.1 is far from comprehensive. Other firms may be working on related compounds and keeping their progress a secret; since IND information is confidential, it is even possible that clinical trials on additional cannabinoids are under way. Researchers have also produced a wide variety of natural and synthetic cannabinoids for experimental purposes that may have therapeutic applications. In addition, uses may be found for cannabinoids that are currently suspended or withdrawn from pharmaceutical development.
Based on the information in Table 10.1, we can draw three
TABLE 10.1 Cannabinoids Under Development for Human Use as of July 2000
|
Name of Drug |
Possible Indication(s) |
Investigator |
Stage of Development |
FDA Status |
|
HU-211 (Dexanabinol) |
Neuroprotection (Neurotrauma, stroke, multiple sclerosis, Parkinson 's, Alzheimer's) |
Pharmos Corp. |
Phase II trial completed March 2000 in Israel; Contract for Phase III trial awarded June 2000 |
None |
|
CT-3 |
Antiinflammatory Analgesia |
Atlantic Pharmaceuticals |
Phase I trial in France |
IND |
|
THC (Marinol) |
[see text] |
Unimed Roxane Labs |
Phase I trials in U.S. |
IND |
|
Marijuana Plant |
Multiple sclerosis, spinal cord injury, pain |
GW Pharmaceuticals |
Phase I trial completed in England; Phase II trial begun May 2000 |
None |
|
HIV-related appetite stimulation |
Donald Abrams, University of California at San Francisco |
Phase I trial completed May 2000 |
IND |
|
|
Source: Adapted from Institute of Medicine. 1999. Marijuana and Medicine: Assessing the Science Base. Washington, DC: National Academy Press, p.209. |
||||
tentative conclusions about the present state of cannabinoid drug development. First, nearly all of the investigators listed are either small companies or individuals, who are generally willing to assume greater commercial risk than a large pharmaceutical company. Because such small enterprises typically obtain funding from venture capital, stock offerings, or collaborations with larger companies rather than from sales, they can pursue riskier goals.
Second, except for THC and marijuana, no plant-derived cannabinoids appear in Table 10.1. While a number of such compounds have shown promising results in animal and human experiments (see Chapter 2), commercial interest in them appears
to be lacking. This is probably due to several factors. First, under the Controlled Substances Act, such compounds would initially be placed in Schedule I; even if the necessary rescheduling proceeded smoothly, controlled substances carry a stigma that limits their sales potential. An additional deterrent to developing cannabinoids from the marijuana plant is the fact that such natural products are ineligible for product patents. Plant-derived compounds may, however, secure use patents such as those awarded to dronabinol and other orphan drugs. Finally, chemical analogs of plant-derived compounds, which could be patented, may offer improved solubility, fewer side effects, or benefits over their natural counterparts.
The third point apparent from Table 10.1 is that cannabinoids are being considered for even more applications than were discussed in previous chapters. One of the most prominent among these additional uses is neuroprotection, which involves rescuing nerve cells from destruction due to trauma, oxygen deprivation, or neurological disease. Cannabinoids are thought to provide this protection through their association with receptors on nerve cells (see Chapter 2). Also, both THC and cannabidiol can act as potent antioxidants to protect nerves from toxic forms of oxygen that arise when the body is under stress.
Another antioxidant, the synthetic cannabinoid HU-211, also has been shown to protect nerve cells from exposure to excessive amounts of the neurotransmitter glutamate, a byproduct of trauma or disease. The cannabinoid acts by blocking glutamate receptors on the neuron, thereby preventing glutamate and other damaging agents from binding. 3 HU-211 has been tested for safety in humans in the United Kingdom and has progressed to Phase II clinical trials in Israel for the treatment of severe brain injury.4
In addition to these possibilities, several plausible scenarios might encourage pharmaceutical firms to pursue marijuana-based products in the future. If cannabinoids seemed likely to fulfill important unmet medical needs, their development might be worth the financial risk. Pain relief represents such a potential market; in 1997 Americans spent an estimated $4.4 billion on prescription and over-the-counter pain relievers. Yet there is a longstanding need for medicines to treat both acute and chronic pain that are safe, nonhabit forming, and easy for patients to take. A
cannabinoid medication with these attributes would be a welcome addition to existing therapies.
Another group of potentially lucrative compounds act by binding cannabinoid receptors without activating them, thereby producing the opposite effect of molecules that activate the receptor. Similar effects also could be achieved with compounds that interfere with receptor binding by natural cannabinoids. Neither class of drugs would be subject to the same scheduling restrictions as natural cannabinoids or their mimics because abuse of such products is unlikely. And since such compounds are not derived from marijuana, both types of drugs could receive product patents. Similarly, one could envision another related class of compounds capable of increasing the synthesis of natural cannabinoids or interfering with their breakdown. Such drugs would probably be scheduled, though, because they could increase the concentration of cannabinoids in the body, creating conditions that might produce the marijuana “high” and therefore the potential for abuse.
Scientists have already synthesized several chemicals that affect interactions between cannabinoids and their cellular receptors. To date, these compounds have largely been used as tools to probe cannabinoid function, but they produce physiological effects of their own and may thus prove therapeutically useful. For example, since THC reduces short-term memory, a drug that prevents THC from binding cannabinoid receptors might enhance memory. Similar blockers might prevent cannabinoid-induced immune suppression (see Chapter 2 and Chapter 3), while drugs that exert the opposite effects of THC, thereby suppressing the appetite, could potentially promote weight loss.
As marijuana-based medicines currently under development proceed through the regulatory pipeline, pharmaceutical firms will be watching. If this handful of experimental drugs perform well in the clinic, receive FDA approval with relative ease, avoid scheduling restrictions, and above all turn a profit, more companies are likely to pursue similar compounds. Since industry experience to date is limited to the case of Marinol, and most of its developmental costs were borne by the U.S. government, it remains to be seen whether other cannabinoid drugs can survive the rigors of development. For the present, both the apparent
dearth of compounds and the small size of the companies involved attest to the high risk of such a venture.
PROSPECTS FOR MEDICAL MARIJUANA USE
The possibility that marijuana itself will emerge as a new medicine is far more remote than the prospects for drugs based on individual chemicals derived from the plant. Along with many of the downside risks associated with the approval and scheduling of cannabinoid drugs, medicinal marijuana faces several additional barriers to development. Thus, it is not surprising that FDA approval has never been sought for the medical use of marijuana.
The first hurdle presents itself early in the development process: conducting research on marijuana. As discussed in greater detail in the next chapter, obtaining research-grade marijuana and the legal permission to study it are difficult and time-consuming endeavors. At present the only existing IND for medical marijuana authorizes a Phase I (safety) study on the treatment of AIDS wasting with smoked marijuana.
Before marijuana could be marketed legally in the United States, it would have to satisfy a long list of regulatory requirements. As a botanical product, marijuana could, in theory, be classified as dietary supplement. Most herbal medicines fall into this category, which exempts them from FDA review. But since marijuana is also a controlled substance, it is unlikely that it would ever win approval for sale without restriction and probably not without obtaining FDA approval in the form of an NDA.
While the FDA is currently developing standards to review botanicals as drugs, no such preparation on the market today has received the agency's approval. Indeed, appraising whole-plant medicines is problematic since it is difficult to assure that such products will remain consistently stable, potent, and free of contamination over time. A medical marijuana preparation would, presumably, need to meet all of these standards. An even greater obstacle to development lies in the fact that marijuana is smoked, which represents a significant safety risk (see Chapter 2). Marijuana delivered by a vaporizing device that permits inhalation of cannabinoids while filtering out carcinogens would still require
FDA approval but would allay many smoking-related safety concerns.
Moreover, marijuana could only be brought to market if it were rescheduled to acknowledge its “accepted medical use,” according to DEA standards. To meet that requirement, a compound must have a known and reproducible chemical structure; its safety and efficacy must be proven; its use must be approved by qualified experts; and scientific evidence for its medical use must be widely accepted. Yet even if all of these criteria were satisfied and marijuana were rescheduled, international treaties may prevent it from being classified in a less restrictive category than Schedule II.
Finally, because marijuana is a natural product, it cannot be patented under U.S. law. Only new marijuana strains not found in nature might be eligible for a product patent, which prohibits anyone else from selling an identical strain for 20 years. A Dutch company, HortaPharm B.V., has developed several unique marijuana strains and has registered them in Europe but has not yet applied for a patent in this country. In fact, marijuana from HortaPharm cannot presently enter the United States because Dutch authorities have refused to issue the necessary export permit (the DEA has approved the importation of HortaPharm plants for research purposes, however).
The path to developing the whole marijuana plant as a medication is so crowded with scientific, regulatory, and commercial roadblocks that it seems highly unlikely to be taken. The appearance of new cannabinoid pharmaceuticals, while more promising, is still years away. Meanwhile, patients, caregivers, policymakers, and voters are weighing the legal consequences of using marijuana for medical purposes. The next chapter explores the legal landscape surrounding the medical use of marijuana, which both influences and is influenced by scientific knowledge of the drug's effects.
NOTES
1. Beal JE, Olson RLL, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. 1995. “Dronabinol as a treatment of aneorexia associ-
ated with weight loss in patients with AIDS.” Journal of Pain and Symptom Management 10:89-97; Beal, JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, Murphy R, Powderly W, Plasse TF, Mosdell KW, Shepard KV. 1997. “Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia.” Journal of Pain and Symptom Management 14:7-14.
2. Calhoun SR, Galloway GP, Smith DE. 1998. “Abuse potential of dronabinol (Marinol).” Journal of Psychoactive Drugs 10(2):187-196.
3. Shohami E, Weidenfeld J, Ovadia H, Vogel Z, Hanus L, Fride E, Breuer A, Ben-Shabat S, Sheskin T, Mechoulam R. 1996. “Endogenous and synthetic cannabinoids: Recent advances.” CNS Drug Reviews 2:429-451; Striem S, Bar-Joseph A, Berkovitch Y, Biegon A. 1997. “Interaction of dexanabinol (HU-211), a novel NMDA receptor antagonist, with the dopaminergic system.” European Journal of Pharmacology 388:205-213.
4. Knoller N, Levi L, Israel Z, Razon N, Reichental E, Rappaport Z, Ehrenfreund N, Fiegon A. 1998. “Safety and outcome in a Phase II clinical trial of dexanabinol in severe head trauma.” Congress of Neurological Surgeons Annual Meeting, Seattle, October 7.























