4
Past and Future Strategies for Sorting and Ranking Chemicals: Applications to the 1998 Drinking Water Contaminant Candidate List Chemicals
John D. Walker, D. Anthony Gray, and Michelle K. Pepling
The 1998 Drinking Water Contaminant Candidate List (CCL) includes chemicals, chemical classes, and microorganisms. This paper discusses only chemicals and chemical classes. However, the strategies for sorting and ranking drinking water contaminant chemicals and chemical classes discussed here are probably applicable to and should be considered for sorting and ranking other types of chemicals as well as drinking water contaminant microorganisms.
EPA'S Responsibilities
Under the August 1996 Amendments to the Safe Drinking Water Act (SDWA), the U.S. Environmental Protection Agency (EPA) must publish the CCL in February 1998 and every five years thereafter, develop the National Drinking Water Contaminant Occurrence Database (NCOD) in August 1999, publish the Unregulated Contaminant Monitoring Regulation List (UCMR) in August 1999 and every five years thereafter, and identify five drinking water contaminants for potential regulation by August 2001 and every five years thereafter.
The EPA published the Draft CCL on October 6, 1997 (EPA, 1997a). After soliciting public comment on the proposed list as mandated under Section 1412(b)(1) of the SDWA, the EPA published the 1998 CCL on March 2, 1998
Under the 1996 Amendments to the SDWA, the EPA must also consider whether drinking water contaminant chemicals should be screened for endocrine disruption potential. As a result, the 206 chemicals with Chemical Abstract Service (CAS) Registry numbers that were on the Draft CCL were included in the Endocrine Disruptor Priority Setting Database (Walker et al., 1999). During development of the Endocrine Disruptor Priority Setting Database, the strengths and weaknesses of the Draft CCL were proposed strength—prepared by screening many sources of chemicals that were likely to contaminate public drinking water systems; weaknesses-concentrations, frequencies of occurrence, and locations of contaminants were not provided, and there were uncertainties concerning the probabilities that proposed contaminants could persist in drinking water. These weaknesses are likely to be addressed as EPA develops the NCOD in August 1999 and publishes the UCMR in August 1999 and every five years
thereafter. In the interim the EPA asked the National Research Council to establish a Committee on Drinking Water Contaminants.
Committee on Drinking Water Contaminants
The Committee on Drinking Water Contaminants was asked to assist the EPA with three tasks: (1) developing a scientifically sound approach for deciding whether or not to regulate contaminants on the current and future CCL(s), (2) convening a workshop that will focus on emerging drinking water contaminants and the database that should be created to support future decision making on such contaminants, and (3) creating a scientifically sound approach for developing future CCLs.
As part of its effort to assist the EPA with these tasks, the Committee on Drinking Water Contaminants issued a report entitled, Setting Priorities for Drinking Water Contaminants (NRC, 1999). That report described the committee's review of 10 existing prioritization schemes, review of methods for assessing microbial pathogens, approaches used to develop the 1998 CCL, and suggestions for selecting candidates on the CCL for future action. The committee concluded that processes that rank contaminants are not appropriate for selecting regulatory candidates, contaminants occurring at frequencies and concentrations that cause health effects should be regulated, and processes that rank contaminants may be useful to sort and select future contaminants.
In their discussion of processes for ranking contaminants, the committee recommended that professional judgments be used to select regulatory candidates. In addition, the Committee on Drinking Water Contaminants suggested that drinking water contaminants could be organized into four categories: Category 1, Ready for Rule-Making; Category 2, Ready for Guidance Development (e.g., health advisories); Category 3, Needing Additional Occurrence Data; and Category 4, Needing Additional Research (e.g., health effects).
The committee recognized that, as expected, the quantity and quality of information would be different for each drinking water contaminant and based its categorization criteria on that premise. The committee also recommended a staged process for assessing drinking water contaminants: Stage 1, review existing data (health effects, exposure, treatment, analytical methods); Stage 2, conduct preliminary risk assessment; Stage 3, prepare decision document (regulation, drop, additional research); and Stage 4, prepare data development plan to meet five-year SDWA Requirements.
Stages 1 through 3 of the committee's process for assessing drinking water contaminants could be used to assign contaminants to one of the four categories listed above. The review of existing data is more extensive than ordinarily required to categorize chemicals because options for treatment must be considered as well as analytical methods for measuring contaminants in drinking water. The preliminary risk assessment recommended by the committee is analogous to that conducted by the EPA for the premanufacture of new chemicals under the Toxic Substances Control Act (i.e., a 90-day process that uses models to estimate exposure potential and structure activity relationships (SARs) and quantitative structure activity relationships (QSARs) to predict toxicity). The
decision document is necessary to assure a consistent decision-making process. The need to prepare a data development plan is critical to publishing the CCL, publishing the UCMR List, and identifying five drinking water contaminants for potential regulation every five years to meet the statutory deadlines of the 1996 amendments to the SDWA.
Past Techniques for Prioritizing Chemicals
With very few exceptions, there are two major differences between past techniques for prioritizing chemicals and those currently being developed for future use: (1) past techniques did not have distinct sorting and ranking phases and (2) past techniques were not peer-reviewed or published in peer reviewed journals or books. The exception to those that did not have distinct sorting and ranking phases and have not been published in peer reviewed journals or books were those developed by and for the Toxic Substances Control Act (TSCA) Interagency Testing Committee (ITC) (Welsh and Ross, 1982; Walker, 1993a). The exception to those that have not been peer-reviewed are those that have been proposed for public comment (e.g., those developed for the ITC, the Agency for Toxic Substances and Disease Registry, the EPA's Office of Solid Waste, and California's Office of Environmental Health Hazard Assessment). The techniques developed by and for the ITC were the first techniques used by the U.S. government to sort and rank chemicals (Davis et al., 1997). Sequential sorting and ranking are critical because they promote simultaneous allocation of resources to highest-ranking chemicals within classes that have been sorted based on chemical structure or categories that have been sorted based on uses, human exposures, environmental releases, environmental fate parameters, health or ecological effects, and so forth. Peer review is significant because it provides credibility to the chemical sorting and ranking process.
Techniques Reviewed by the Committee on Drinking Water Contaminants
The Committee on Drinking Water Contaminants reviewed 10 existing chemical prioritization schemes and considered their relevance for developing a prioritization scheme for drinking water contaminants (NRC, 1999). Three prioritization schemes were developed by private organizations for drinking water contaminants. Three schemes were developed by federal and state organizations to prioritize all contaminants. Four schemes were developed by federal organizations to prioritize contaminants for specific media.
Schemes to Prioritize Drinking Water Contaminants
The first prioritization scheme for drinking water contaminants was initiated by compiling a list of chemicals from a few of the sources proposed by the EPA (1997a). This scheme provided a risk index score based on four weighted criteria: production quantity, exposure quantity, occurrence in water, and human health effects. If one criterion was missing, the chemical was not scored. The second scheme provided a sequential prioritization process for health effects, exposure potential, controlling release and treatment technology. No chemicals were evaluated using this scheme, which apparently does not exclude a chemical if all data were not available. The third scheme for drinking water contaminants was not used to evaluate any chemicals but would exclude a chemical if all data were not available (NRC, 1999).
Schemes to Prioritize All Contaminants
The first prioritization scheme for all contaminants was used to screen a number of chemicals and provided a chemical score based on toxicity (human health or ecological effects), persistence, bioaccumulation, and mass of the contaminant in waste streams. The second prioritization scheme was used to sort chemicals by chemical substructure and associated health or ecological effects as provided by expert opinions; exposure and toxicity could be scored by using empirical data or predictions, including SARs or QSARs, and a need for data by a U.S. government organization(s) could be factored into the weighting criteria. The third scheme uses toxicity information and expert opinion to assign priorities and exposure information to determine the order in which priority toxic chemicals are assessed (NRC, 1999).
Schemes to Prioritize Contaminants for Specific Media
The first scheme to prioritize contaminants for specific media ranks hazardous waste sites and is relevant to setting priorities for drinking water contaminants, because the contaminants at the hazardous waste sites determine the potential to cause adverse effects to human health or the environment. The second scheme ranks hazardous waste sites and the contaminants at hazardous waste sites for potential to cause adverse human health effects. The third scheme to prioritize contaminants for specific media is used to indicate sediment contamination potential. The fourth scheme is used to estimate the potential for pesticides applied to apples and potatoes to contaminate groundwater (NRC, 1999).
Other Techniques
There are two other techniques for ranking chemicals that may be relevant to developing a prioritization scheme for drinking water contaminants. The Michigan Critical Materials Register ranks chemicals that may threaten water quality in
Michigan. The Ontario Ministry of the Environment Scoring System ranks chemicals that may threaten surface water quality in Ontario. Both of these techniques have been described by Davis et al. (1997).
Future Procedures for Sorting and Ranking Chemicals
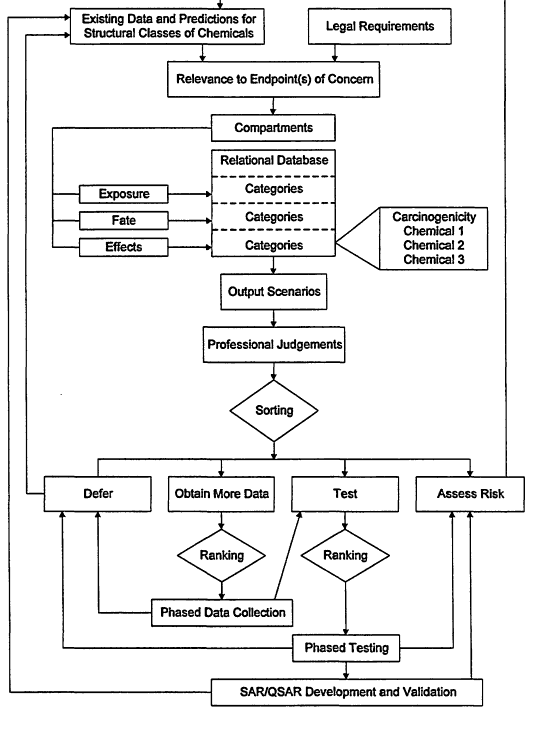
Future procedures for sorting and ranking chemicals must consider the continued resource and testing facility limitations for assessing the risks of chemicals, promoting pollution prevention and so forth. Strategic planning will be required to assure that these resources are effectively allocated. Formulation of strategic plan should involve development of a scheme that considers vital information and facilitates sorting and ranking of chemicals into phased programs that can be accomplished with the given resources. A scheme that considers vital information and facilitates sorting and ranking of chemicals into phased data collection and testing programs is illustrated in Figure 4-1. This scheme to sort and rank structural classes of chemicals for data collection, testing, and risk assessment includes:
- a process to organize chemicals into structural classes;
- a review of existing data and predictions for structural classes of chemicals;
- consideration of legal (e.g., statutory-mandated) requirements;
- development of a relational database, consisting of exposure, effects, fate and other compartments, chemicals sorted into categories within each compartment (e.g., within the effects compartment, there may be chemicals sorted into carcinogenicity, acute toxicity, aquatic toxicity categories, etc.), and chemicals ranked (when possible) within each category (e.g., within the aquatic toxicity category, chemicals could be ranked on fish LC50 values, aquatic invertebrate EC50 values, etc.);
- use of the relational database to produce output scenarios (e.g., chemicals with annual production volumes exceeding a certain threshold that have been measured in surface waters (the concentration of which is given) and that are toxic to fish (the LC50 values are given) that can be used with professional judgments to sort chemicals into groups for which (1) no additional data are needed at that time (defer), (2) more data need to be obtained, (3) testing (including screening tests) should be conducted or, (4) risk should be assessed);
- a phased short- and long-term program to which highest-ranking chemicals (from database categories of ranked chemicals and professional judgments) within groups needing data or testing are assigned to the first phases of data collection or testing;
- processes that assure that results from phased data collections and testing are reviewed and decisions to defer, test, or assess risk are made; and
- feedback loops that provide pathways for phased testing data to be (a) used for development and validation of SAPs and QSARs, (b) incorporated into risk assessments and (c) included in future data assessments of structurally related chemicals.
1998 Drinking Water Contaminant Candidate List
The 1998 Drinking Water Contaminant Candidate List consists of 48 chemicals with unique Chemical Abstract Service Registry numbers and chemical structures (See Table 4-1). There are 20 industrial organic chemicals, 22 pesticides, and six inorganic chemicals on the list.
TSCA Interagency Testing Committee (ITC)
The ITC is an independent advisory committee to the EPA administrator that was created in 1976 under Section 4(e) of the TSCA. Sixteen U.S. government organizations are ITC members: the Agency for Toxic Substances and Disease Registry (ATSDR), the Council on Environmental Quality (CEQ), the Consumer Product Safety Commission (CPSC), the U.S. Department of Agriculture (USDA), the U.S. Department of Commerce (DOC), the U.S. Department of Defense (DOD), Food and Drug Administration (FDA), the U.S. Department of the Interior (DOI), the U.S. Environmental Protection Agency (EPA), the National Cancer Institute (NCI), the National Institute of Environmental Health Sciences (NIEHS), the National Institute for Occupational Safety and Health (NIOSH), the National Library of Medicine (NLM), the National Science Foundation (NSF), the National Toxicology Program (NTP) and the Occupational Safety and Health Administration (OSHA). Members from these U.S. government organizations nominate industrial chemicals to the ITC when their organizations need data that can be obtained through the ITC. These data include unpublished production volume, use, exposure, monitoring, environmental fate, ecological effects, and health effects data. The ITC coordinates data needs for the nominated chemicals with those of other member organizations and determines if these chemicals should be (1) added to the Priority Testing List and recommended or designated for testing, (2) deferred for testing and not added to the list or (3) removed from the list. The ITC meets monthly to identify and coordinate federal data needs for industrial chemicals, recommends these chemicals in Federal Register Reports to the administrator every May and November, and establishes partnerships with manufacturers, importers, processors, and users of recommended chemicals to discuss data needed. By coordinating federal data needs and establishing partnerships, the ITC provides an infrastructure to obtain information on industrial chemicals (http://www.epa.gov/opptintr/itc/).
ITC Decisions for the 1998 CCL Chemicals
The ITC has made testing decisions on about 40,000 chemicals (Walker, 1993a). The ITC has deferred testing on 1998 CCL chemicals that are only used as pesticides because chemicals that are registered active pesticide ingredients are regulated under the Federal Insecticide Fungicide Rodenticide Act, not TSCA (See Table 4-2). However, the ITC does review data on pesticides to facilitate
toxicity and persistence predictions for structurally related industrial chemicals and to develop SARs and QSARs. Eleven of the 1998 CCL chemicals were recommended for testing by the ITC in Federal Register reports to the EPA administrator (Table 4-2). Eight of the recommended 1998 CCL chemicals have been removed from the Priority Testing List because the EPA implemented the ITC's testing recommendations (Table 4-2).
As result of the ITC's recommendations and EPA's implementation of the ITC's testing recommendations, many of the 1998 CCL chemicals recommended for testing by the ITC are well-characterized industrial chemicals. The most well-known 1998 CCL chemical recommended by the ITC is probably methyl-tert butyl ether (MTBE), because it was recommended by the ITC before it was commercially significant and at a time in the commercial life cycle of the chemical when it was easiest to request voluntary development of test data (Walker, 1993b).
TSCA Section 4 and 8(d) Studies Indexed in the TSCA Test Submissions (TSCATS) Database
As a result of ITC recommendations and related EPA actions, over 1,300 unpublished studies have been submitted to the EPA for the 1998 CCL chemicals (See Table 4-3). These studies are indexed in the TSCATS database. For each study there is a reference in TSCATS, and the reference may be a document that contains more than one study, explaining why the number of studies in Table 4-3 is equal to or greater than the number of references. Procedures for retrieving studies indexed in TSCATS were recently published (Walker and Smock, 1995). TSCATS is a pointer system—that is, it is a database that points to unpublished studies that have been submitted to EPA under TSCA. It can be accessed through the World Wide Web from the following universal resource locators: (url) http://www.rtk.net or http://igm.nlm.nih.gov/. From the Right-To-Know (RTK) page the user must search on databases. From the NLM page the user must select TOXLINE and then, under the "Apply Limits" section, choose "Toxic Substances Control Act Test Submissions."
When the EPA publishes a Federal Register notice under TSCA Section 4(a), manufacturers and processors of chemicals mentioned in that notice can provide TSCA Section 4(a) studies to reduce the possibility of having to conduct tests under a TSCA Section 4(a) rule or they can provide TSCA Section 4(d) studies that were conducted as a result of testing to meet the requirements of a TSCA Section 4(a) notice. The total number of TSCA Section 4 studies listed in Table 4-3 includes both TSCA Section 4(a) and 4(d) studies.
When the ITC adds a chemical to the Priority Testing List, the EPA automatically promulgates a TSCA Section 8(d) Health and Safety Data Rule. This rule requires manufacturers and processors of chemicals recommended by the ITC to submit unpublished health and safety studies (health effects, environmental fate, ecological effects, environmental and occupational monitoring, industrial hygiene, etc.) to the EPA. By comparing the chemicals recommended or designated by the ITC (Table 4-2) with the number of TSCA
Section 8(d) studies for 1998 CCL chemicals (Table 4-3), it is obvious that there are some chemicals that were deferred by the ITC for which a number of TSCA Section 8(d) studies have been submitted to the EPA. There are two explanations for this: (1) EPA published a TSCA Section 8(d) rule for chemicals other than those recommended by the ITC or (2) when manufacturers and processors submitted documents (references) in response to a TSCA Section 8(d) rule, the documents contained studies on other chemicals that were not subject to the rule. The ITC encourages manufacturers and processors of ITC-recommended chemicals to submit these and other studies electronically through the Voluntary Information Submission Innovative Online Network (VISION; http://www.epa.gov/opptintr/itc/vision/).
Uses and Substructure-Based Computerized Chemical Selection Expert System (SuCCSES) Chemical Classes for the 1998 CCL Chemicals
Uses were identified for the 1998 CCL chemicals; most are used as herbicides or insecticides (See Table 4-4). Identifying uses is important because it promotes pollution prevention through the recognition that less toxic and less persistent chemicals can be substituted for more toxic and more persistent chemicals with similar uses.
SuCCSES is the first computerized system that uses expert opinions to predict potential environmental-human health interactions of individual chemicals and chemical classes that share common substructures (Walker, 1995a; Walker and Gray, 1999). For chemical categories in SuCCSES, expert opinions were offered on the potential of chemicals containing specific substructures to cause adverse human health effects or to cause effects on the environment by adversely affecting ecologically diverse classes of organisms. SuCCSES contains over 100 chemical substructures associated with about 10,000 chemicals. Effects on human health indicated potential for chemicals containing one or more of these substructures to cause acute, chronic, mutagenic, oncogenic, developmental, reproductive, or neurotoxic effects or membrane irritation (Walker, 1991, 1995a). Effects on the environment included predictions on chemicals containing one or more of these substructures to potentially cause adverse effects to algae, aquatic invertebrates, birds, fish, mammals, microorganisms, plants, or terrestrial invertebrates (Walker and Bink, 1989; Walker, 1991).
Using SuCCSES it was possible to assign the 1998 CCL chemicals to one of 17 SuCCSES classes (Table 4-4). Organizing chemicals into SuCCSES classes is critical for estimating potential health or ecological effects of structurally related chemicals and developing or validating SARs and QSARs.
Log Octanol Water Partition Coefficient (log Kow) Values, Soil or Sediment Sorption Coefficient (Koc) Values, and Henry's Law Constant for 1998 CCL Chemicals
The log Kow values, Koc values and Henry's Law constants for the 1998 CCL chemicals (arranged by SuCCSES class) are listed in Table 4-5. These three environmental fate parameters were selected to estimate the potential of chemicals to remain in drinking water. These parameters can be used when reservoirs (containing aquatic species that can bioconcentrate chemicals and sediment to which chemicals can sorb) are the source of drinking water. For other drinking water sources, different parameters may have to be used. Using previously described criteria for log Kow values, Koc values, and Henry's Law constants it was possible to estimate the potential of chemicals to bioconcentrate, sorb to sediment or soil, and evaporate from water, respectively (Walker, 1995b). These criteria are listed below:
|
log Kow |
Bioconcentration Potential |
Koc |
Sorption Potential |
Henry's Law Constant (atm m3/mole) |
Evaporation Potential |
|
<3 |
Low |
<2,700 |
Low |
>10-2 |
High |
|
3-8 |
Moderate to High |
>2,700 |
High |
10-2- 10-7 |
Moderate |
|
>8 |
Low |
|
|
<10-7 |
Low |
By organizing the 1998 CCL chemicals into SuCCSES classes (number of chemicals in each class in parentheses), it was possible to estimate the potential of chemicals within these classes to remain in reservoirs of drinking water:
- High potential to remain in reservoir water (low bioconcentration, sorption, and evaporation potential): acetanilides (2), phenols (2), triazines (7), and ureas (2).
- Moderate potential to remain in reservoir water (moderate bioconcentration, sorption, or evaporation potential): aliphatic halides (8) (aldrin, dieldrin, and hexachlorobutadiene may bioconcentrate or sorb to soil or sediment); aromatic halides (4) (DDE may bioconcentrate or sorb to soil or sediment); aromatic hydrocarbons (2); carbamic acid esters (1); ethers (1); halophenols (2); hydrazines (1); nitroaromatics (3); phosphonothioates (1); phosphorodithioates (2); and phosphorothioates (1).
- Low potential to remain in reservoir water (low bioconcentration and sorption potential but high evaporation potential): aliphatic halides (2) (1,1-dichloropropene and 2,2-dichloropropane); aromatic hydrocarbons (1) (p-isopropyltoluene).
Log Kow, Koc, and Henry's Law constants may be used to estimate the potential of chemicals and chemical classes to remain in drinking water. However, some professional judgment should be used before these chemicals or chemical classes are sorted or ranked (Figure 4-1). Professional judgment would consider other environmental fate parameters that could affect the ability of some chemicals or chemical classes to remain in drinking water (e.g., hydrolysis of carbamic acid esters, hydrazines).
Application Of Past Chemical Sorting Techniques To The 1998 Ccl Chemicals
As noted earlier, almost all past techniques did not have distinct sorting and ranking phases. However, a few techniques developed in the recent past do promote sorting (e.g., the Use Cluster Scoring System, SuCCSES, and the Endocrine Disruption Priority Setting Database (EDPSD).
The Use Cluster Scoring System can be used to sort chemicals into use categories (Davis et al., 1997). SuCCSES was described earlier. The EDPSD can be used to sort chemicals into structural classes and categories based on uses, production volumes, environmental fate parameters, occurrences in fish and wildlife tissues, reproductive effects, estrogen receptor binding affinity potentials, and so on (Walker et al., 1999). Past sorting techniques (uses and environmental fate parameters) were applied to the 1998 CCL chemicals; the results are summarized in Table 4-5 and discussed earlier.
Application Of Past Chemical Ranking Techniques To The 1998 Ccl Chemicals
To illustrate the application of past ranking techniques, the aliphatic halides from Table 4-5 were ranked based on exposure and effects scores developed by and for the ITC (Walker, 1993b, 1995a).
Exposure Scores
Exposure scores and criteria for assigning scores to exposure factors that were relevant to ranking aliphatic halides are production volume, environmental persistence, and bioaccnmulation potential (See Table 4-6). These scores and criteria were applied to the aliphatic halides from the 1998 CCL (See Table 4-7). Ranking on production volume indicated that there were three groups of aliphatic halides: those with annual production volumes greater than 10 million pounds, greater than 1 million pounds, or less than 1 million pounds (Table 4-7). Ranking on environmental persistence indicated that there were two groups of aliphatic halides: those that could persist for years and those that could persist for months (Table 4-7). Ranking on bioaccumulation potential indicated that there were three groups of aliphatic halides based on based on log Kow (Table 4-7). The three aliphatic halides that could persist for years (aldrin, dieldrin, and
hexachlorobutadiene) were the same three that could bioconcentrate or sorb to soil or sediment (Table 4-5).
Effects Scores
Four biological effects that were relevant to ranking the 1998 CCL chemicals were acute toxicity, mutagenicity, carcinogenicity, and ecotoxicity (See Table 4-8). The scores and criteria for these biological effects were applied to the aliphatic halides from the 1998 CCL (See Table 4-9). Positive scores (based on empirical data) and negative scores (based on predictions) for biological effects were evaluated separately. Based on acute toxicity and ecotoxicity scores, aldrin and dieldrin would rank the highest and 1,1-dichloropropene and 2,2-dichloropropane the lowest. Based on mutagenicity and carcinogenicity scores, 1,3-dichloropropane would rank the highest and 2,2-dichloropropane the lowest.
Application Of Future Chemical Sorting Procedures To The 1998 CCL Chemicals
Procedures related to chemical properties, waste management, and resource productivity have been suggested for scoring and ranking chemicals in the future (Jensen et al., 1997). While only the procedures related to chemical properties were described in sufficient detail to warrant immediate consideration, there are merits to waste management and resource productivity approaches as evidenced by EPA's development of the Waste Minimization Prioritization Tool, which was proposed for public comment on June 23, 1997 (EPA, 1997b), substantially revised in response to public comments, and published with the Draft Resource Conservation Recovery Act Persistent Bioaccumulator Toxics list on November 9, 1998 (EPA, 1998b). Procedures related to chemical properties that might be considered in the future include flammability, ignitability, explosivity, oxidizability, reactivity, corrosivity, chemical-environmental interactions, global warming potential, ozone depletion potential, photochemical oxidant creation potential, odor threshold values, eutrophication potential, and acidification potential (Jensen et al., 1997). While criteria have been proposed to score all these chemical properties, it was not possible to use them to sort the 1998 CCL chemicals. However, two new procedures not considered by Jensen et al. (1997) could be used to sort the 1998 CCL chemicals.
The first procedure, based on a chemical's mode of toxic action, was used in conjunction with SuCCSES classes to sort the 1998 CCL chemicals (See Table 4-10). Results from testing the acute toxicity of about 600 chemicals to fathead minnows were used to develop a computer-based expert system that predicts mode of toxic action based on chemical structure (Russom et al., 1997). The models and substructure search methods were designed for the ASsessment Tools for the Evaluation of Risk (ASTER) expert system and database. ASTER is an integration of the AQUatic toxicity Information REtrieval (AQUIRE)
database and the QSAR system for use in ecological risk assessments (Russom et al., 1991). Using substructure rules based on mode of toxic action, ASTER provides high-quality data for discrete organic chemicals, when available in the associated databases, and QSAR-based estimates when there is a dearth of information. QSARs are based on the mode of toxic action of the chemical of concern. For chemicals with substructures associated with multiple modes of action, the equation that results in the highest level of hazard is selected.
Sorting the 1998 CCL chemicals based on modes of toxic action in conjunction with SuCCSES classes makes it is possible to sort groups of chemicals within and between SuCCSES classes. Within the SuCCSES class of aliphatic halides, modes of toxic action can be used to sort aliphatic halides into three groups of chemicals: those with nonpolar narcosis, reactivity; alkylation or arylation reaction and neurotoxicant; cyclodiene-type modes of action (Table 4-10). These differences are important because, while all the aliphatic halides have a common substructure that was the basis of assigning them to a particular SuCCSES class, the modes of toxic action help identify important subclasses that could be used in the future to develop more well-defined SuCCSES classes. These structural differences and subclasses are clearly illustrated in Table 4-1 (e.g., dieldrin is the epoxide of aldrin, and both are cyclodiene-type neurotoxicants, and 1,3-dichloropropene and hexachlorobutadiene are both β-chlorinated olefins, and both have alkylation reactivity). The other six aliphatic halides are all chlorinated alkanes, except 1,1-dichloropropene, an β-chlorinated olefin, but all have a nonpolar narcosis mode of toxic action. Based on modes of toxic action, the aliphatic halides could be sorted into three subclasses or three new SuCCSES classes. In contrast, consider the three SuCCSES classes—phosphonothioates, phosphorodithioates, and phosphorothioates—which all have an organophosphate-mediated acetylcholinesterase inhibition mode of toxic action and could be classified in one mode of toxic action category because of their structural similarities (Table 4-1).
The second procedure, based on a chemical's carcinogenicity concern level, was used in conjunction with SuCCSES classes to sort the 1998 CCL chemicals (Table 4-10). The carcinogenicity concern level was estimated using expert judgment and EPA's Cancer Expert System (Woo et al., 1995, 1993; Lai et al., 1996). The Cancer Expert System is a knowledge rule-based artificial intelligence system that evaluates the carcinogenic potential of chemicals based on SAR analysis and available short-term predictive data. While the system provides six semiquantitative carcinogenicity concern levels, the six levels were merged into three for this paper: high, moderate, and low. Chemicals with high concern level either have known human carcinogenicity or high probability of being potent multispecies carcinogens. For chemicals with moderate concern level, there is either evidence or reasonably high probability of being moderately active animal carcinogens or possible human carcinogens. Chemicals with low concern levels either lack evidence of carcinogenicity, are marginally active, or are considered unlikely to be of significant concern as possible human carcinogens.
Sorting the 1998 CCL chemicals based carcinogenicity concern levels in conjunction with SuCCSES classes makes it is possible to sort groups of chemicals within and between SuCCSES classes. Within the SuCCSES class of aliphatic halides, carcinogenicity concern levels can be used to sort aliphatic
halides into three groups of chemicals: those with high, moderate, and low levels of concern (See Table 4-11). While there may be some connection between modes of toxic action (Table 4-10) and carcinogenicity concern levels (Table 4-11), it would be difficult to make that connection without additional data on species tested, routes of administration, target organs and so forth. However, as with modes of action (Table 4-10), the three SuCCSES classes—phosphonothioates, phosphorodithioates, and phosphorothioates—could be sorted into a low carcinogenicity concern level category because of their structural similarities (Table 4-1).
Application Of Future Chemical Ranking Procedures To The 1998 CCL Chemicals
As noted earlier, while Jensen et al. (1997) suggested procedures for scoring and ranking chemicals in the future, it is not possible to use any of those procedures to rank the 1998 CCL chemicals. However, two new procedures not considered by Jensen et al. (1997) could be used to do so.
The first procedure to rank the 1998 CCL chemicals uses the expert opinions generated during development of SuCCSES. The use of expert opinions to identify potential effects of chemicals is not new. However, the use of expert opinions to predict the potential of chemicals containing specific substructures to cause adverse effects to human health or the environment is new and was developed as part of SuCCSES (Walker and Gray, 1999). To illustrate how SuCCSES might be used to rank the 1998 CCL chemicals, three SuCCSES classes and their potential health effects were selected as examples:
|
SuCCSES Class |
Potential Health Effects |
|
Nitroaromatics |
Carcinogenicity Mutagenicity Other chronic effects |
|
Halophenols |
Carcinogenicity Mutagenicity Other chronic effects |
|
Hydrazines |
Acute toxicity Membrane irritation Oncogenicity |
There are three nitroaromatics, two halophenols, and one hydrazine on the 1998 CCL (Table 4-4). For carcinogenicity, mutagenicity, and acute toxicity the chemicals can be ranked based on the scores presented in Table 4-8 (note that data or predictions can be used to generate scores). In addition, they could be ranked for carcinogenicity concern levels using the estimates in Table 4-11.
The second procedure to rank the 1998 CCL chemicals uses QSARs. There are at least three QSARs that are sufficiently robust and well validated to rank the 1998 CCL chemicals: (1) fish LC50, (2) rat oral LD50, and (3) hologram QSAR (HQSAR) for estrogen receptor binding affinity. HQSAR describes
molecules in terms of molecular fingerprints that encode two-dimensional compositional and topological information and provides estimates of estrogen-receptor binding affinity. Estimates are generated by using a simplified 3D-QSAR model and reported as the negative log of the LC50 in mM (Walker et al., 1999). The HQSAR is used to illustrate how a QSAR could be used to rank the 1998 CCL chemicals. HQSAR values were available in the EDPSD for 35 of the 1998 CCL chemicals. The HQSAR values ranged from -0.61 (dieldrin) to -3.46 (DDE) and might be used to sort and rank classes of the 1998 CCL chemicals (e.g., HQSAR values for all aliphatic halides, except aldrin and dieldrin ranged from -2.32 to-2.48; HQSAR values for all triazines ranged from -2.34 to -2.97).
Identification Of Future CCL Chemicals
There are many methods by which future CCL chemicals could be identified: (1) structural relationships to known drinking water contaminants, (2) uses, (3) environmental release patterns that are similar to existing drinking water contaminants, (4) environmental fate parameters and models that indicate potential to enter and persist in water, (5) frequency of occurrence in drinking water, (6) ability to persist in drinking water and so forth. Two examples illustrate how future CCL chemicals might be identified.
The first example compared environmental fate parameters used to estimate the potential of the 1998 CCL chemicals to remain in water with wildlife tissue concentrations of these chemicals or the occurrence frequency of these chemicals in wildlife tissues. The environmental fate parameters that were selected to estimate the potential of the 1998 CCL chemicals to remain in drinking water were discussed earlier and are summarized in Table 4-5. Data on occurrence or tissue concentration data for the 1998 CCL chemicals in wildlife were extracted from the Contaminant Exposure and Effects—Terrestrial Vertebrates database (Rattner et al., 1999). These data are summarized in Table 4-12. By comparing the environmental fate parameters for 1998 CCL chemicals that remain in drinking water (low bioconcentration, sorption, and evaporation potential) with the 1998 CCL chemicals in wildlife it should be possible to: (1) identify other structurally related chemicals with low bioconcentration, sorption, and evaporation potential that would contaminate drinking water and wildlife species and (2) identify wildlife species that are likely to be contaminated by structurally related chemicals with low bioconcentration, sorption, and evaporation potential that could serve as sentinel species to evaluate potential effects and contribute to the understanding of environmental-human health effects interactions.
The second example analyzed use data for structurally related chemicals. One of the aliphatic halides on the 1998 CCL (1,1,2,2-tetrachloroethane) was selected to exemplify how this procedure would work. First, consider the uses for 1,1,2,2-tetrachloroethane: a solvent in rubber manufacture, a solvent in styrene-butadiene rubber manufacture, a solvent in polystyrene production, a solvent used in machinery manufacture and repair, and a solvent for varnishes.
Second, consider similarly used aliphatic halides (See Table 4-13). There are at least three aliphatic halides (not on the 1998 CCL) with uses similar
to those of 1,1,2,2-tetrachloroethane; all have at least three uses that are similar to 1,1,2,2-tetrachloroethane, and some have more uses (Table 4-13). Third, consider the production, use, and environmental release volumes for aliphatic halides (not on the 1998 CCL) that have similar uses to 1,1,2,2-tetrachloroethane (See Table 4-14).
Fourth, consider the log Kow values, Koc values, and Henry's Law constants (environmental fate parameters that can be used to estimate if chemicals will remain in drinking water) for aliphatic halides (not on the 1998 CCL) that have similar uses to 1,1,2,2-tetrachloroethane (See Table 4-15).
The information in Tables 4-13, 4-14, and 4-15 suggests that 1,1,1-trichloroethane, carbon tetrachloride, and 1,1,2-trichloroethane could be future CCL chemicals. Data that could be used to decide if they should be added to the 2003 CCL include uses that could result in environmental releases and potential to persist in drinking water. While 1,1,2-trichloroethane has the fewest uses related to 1,1,2,2-tetrachloroethane and a low-use volume, the Toxics Release Inventory (TRI) volume is higher than 1,1,2,2-tetrachloroethane and the Henry's Law constant is lower than 1,1,2,2-tetrachloroethane, suggesting that it could be a potential addition to the 2003 CCL. Carbon tetrachloride has three uses related to 1,1,2,2-tetrachloroethane, more total uses, a low-to-medium use volume, and a higher TRI volume than 1,1,2,2-tetrachloroethane, but the Henry's Law constant is high enough that it could evaporate from drinking water. 1,1,1-Trichloroethane has all uses related to 1,1,2,2-tetrachloroethane, more total uses, a medium to high use volume and a much higher TRI volume than 1,1,2,2-tetrachloroethane, but the Henry's Law Constant is high enough that it could evaporate from drinking water. However, considering the magnitude of the TRI volume, if the release rate is higher than the evaporation rate, 1,1,1-trichloroethane could be a potential addition to the 2003 CCL.
Algorithms, Weighting, And Scaling Factors
An adequate discussion of algorithms, weighting, and scaling factors and application to the 1998 CCL chemicals is beyond the scope of this paper. However, the authors want to make readers aware of algorithms, weighting, and scaling factors that could be used to rank the 1998 CCL of chemicals. There are previously used exposure algorithms (Walker and Brink, 1989) and weighting and scaling factors (Walker, 1993a).
There is an algorithm developed for and by the ITC for producing health effects (HE) scores by summing scores for health effects endpoints (H), monitoring (M), and occupational exposures (X) and adding to that a number that is equal to three times the log of the annual production or importation volume:
Also, there is an algorithm developed for and by the ITC for producing ecological effects (EE) scores by summing scores for ecological effects endpoints
(E) and monitoring (M) and adding to that a number that is equal to three times the log of the annual production or importation volume:
Finally, there is an algorithm developed for and used by the EPA's Office of Solid Waste for the Waste Minimization Prioritization Tool (W (EPA, 1997b, 1998b). This algorithm considers human toxicity (HT), mass of the substance that is produced (M), persistence (P), and bioaccumulation (B) plus ecotoxicity (ET), mass of the substance that is produced (M), persistence (P), and bioaccumulation (B):
WMPT Score=(HT + M + P+ B) + (ET + M + P + B)
Acknowledgments
The authors are very grateful to the following individuals for unselfishly providing information that allowed us to complete this paper: Heather Printup and Michael Heal from the Environmental Sciences Center, Syracuse Research Corporation, for providing the data to support the positive biological effects scores in Table 4-9; Chris Russom from the EPA's Duluth, Minnesota, laboratory for providing mode of action data (Table 4-10); David Lai and Yin-Tak Woo from EPA's Office of Pollution Prevention and Toxics for providing carcinogenicity concern levels (Table 4-11); and Barnett Rattner for providing data on occurrence and tissue concentrations of the 1998 CCL chemicals in wildlife (Table 4-12).
References
Davis, G., D. Fort, B. Hansen, F. Irwin, B. Jones, S. Jones, A. Socha, R. Wilson, B. Haaf, G. Gray, and B. Hoffman. 1997. Framework for chemical ranking and scoring systems. Pp. 1-29 in Chemical Ranking and Scoring: Guidelines for Relative Assessments of Chemicals, M. B. Swanson and A. C. Socha, eds. Pensacola, Fla.: SETAC Press.
EPA (U.S. Environmental Protection Agency). 1997a. Announcement of the Draft Drinking Water Contaminant Candidate List; Notice. Federal Register 62:52194-52219.
EPA. 1997b. Availability of Waste Minimization Software and Documents; Notice. Federal Register 62:33868-33870.
EPA. 1998a. Announcement of the Drinking Water Contaminant Candidate List; Notice. Federal Register 63:10274-10287.
EPA. 1998b. Availability of Draft RCRA Waste Minimization PBT Chemical List; Notice. Federal Register 63:60332-60343.
Jensen, A. A., J. D. Walker, F. Fiala, K. Ahmed, M. Ralston, R. Ross, F. Schmidt-Bleek and D. Tolle. 1997. Other chemical characteristics. Pp. 113-129 in Chemical Ranking and Scoring: Guidelines for Relative Assessments of Chemicals, M. B. Swanson and A. C. Socha, eds. Pensacola, Fla.: SETAC Press.
Lai, D. Y., Y. T. Woo, M. F. Argus, and J. C. Arcos. 1996. Cancer risk reduction through mechanism-based molecular design of chemicals. Pp. 62-73 in
Designing Safer Chemicals, S.C. DeVito and R.L. Garrett, eds. ACS Symposium Series No. 640, Washington, D.C.: American Chemical Society.
NRC (National Research Council). 1999. Setting Priorities for Drinking Water Contaminants. Washington, D.C.: National Academy Press.
Rattner, B. A., J. L. Pearson, N. H. Golden, J. B. Cohen, R. M. Erwin, and M. A. Ottinger. 1999. Contaminant Exposure and Effects—Terrestrial Vertebrates Database: Trends and Data Gaps for Atlantic Coast Estuaries. Environmental Monitoring and Assessment 12 pp.
Russom, C. L., E. B. Anderson, B. E. Greenwood, and A. Pilli. 1991. ASTER: An integration of the AQUIRE data base and the QSAR system for use in ecological risk assessments. The Science of the Total Environment. 109/110:667-670.
Russom, C. L., S. P. Bradbury, S. J. Broderius, D. E. Hammermeister, and R. A. Drummond. 1997. Predicting modes of toxic action from chemical structure: Acute toxicity in the fathead minnow (Pimephales promelas ). Environmental Toxicological and Chemistry 16(5):948-967.
Walker, J. D. 1991. Chemical selection by the TSCA Interagency Testing Committee: Use of computerized substructure searching to identify chemical groups for health effects, chemical fate and ecological effects testing. The Science of the Total Environment 109/110:691-700.
Walker, J. D. 1993a. The TSCA Interagency Testing Committee's approaches to screening and scoring chemicals and chemical groups: 1977-1983. Pp. 77-93 in Access and Use of Information Resources in Assessing Health Risks from Chemical Exposure, P. Y. Lu, ed. Oak Ridge, Tenn.: National Laboratories.
Walker, J. D. 1993b. The TSCA Interagency Testing Committee, 1977 to 1992: Creation, structure, functions and contributions. Pp. 451-509 in Environmental Toxicology and Risk Assessment: Second Volume, J. W. Gorsuch et al., eds. ASTM STP 1216. Philadelphia: ASTM.
Walker, J. D. 1995a. Estimation methods used by the TSCA Interagency Testing Committee to prioritize chemicals for testing: Exposure and biological effects scoring and structure activity relationships. Toxicology Modeling 1:123-141.
Walker, J. D. 1995b. Predictive Methods for Chemical Fate: Screening and Testing Chemicals in Commerce. Washington, D.C.: Office of Technology Assessment.
Walker, J. D., and R. H. Brink. 1989. New cost-effective, computerized approaches to selecting chemicals for priority testing consideration. Pp. 507-536 in Aquatic Toxicology and Environmental Fate, 11th Vol., G. W. Suter and M. A. Lewis, eds. ASTM STP 1007. Philadelphia: ASTM.
Walker, S. D., and D. A. Gray. 1999. The Substructure-based Computerized Chemical Selection Expert System (SuCCSES): Providing chemical right-to-know information on potential actions of structurally-related chemical classes on the environment and human health. In Quantitative Structure Activity Relationships (QSARs) to Predict Environmental-Human Health Interactions, J. D. Walker, ed. Pensacola, Fla.: SETAC Press.
Walker, J. D., and W. H. Smock. 1995. Chemicals recommended for testing by the TSCA Interagency Testing Committee: A case study with octamethylcyclotetrasiloxane. Environmental Toxicological and Chemistry 14:1631-1634.
Walker, J. D., C. Waller, and S. Kane. 1999. The Endocrine Disruptor Priority Setting Database (EDPSD): A Tool to Assist Rapid Sorting and Priority Setting of Chemicals for Endocrine Disruption Screening and Testing. In Quantitative Structure Activity Relationships (QSARs) to Predict Endocrine Disruption, J. D. Walker, ed. Pensacola, Fla.: SETAC Press.
Welsh, S. L., and R. H. Ross. 1982. An approach to scoring of toxic chemicals for environmental effects. Environmental Toxicological and Chemistry 1:95-102.
Woo, Y. T., D. Y. Lai, M. F. Argus, and J. C. Arcos. 1995. Development of structure-activity relationship rules for predicting carcinogenic potential of chemicals . Toxicology Letters 79:219-228.
Woo, Y. T., D. Y. Lai, M. F. Argus, and J. C. Arcos. 1998. An integrative approach of combining mechanistically complementary short-term predictive tests as a basis for assessing the carcinogenic potential of chemicals. Environmental Carcinogen Ecotoxicology Reviews (Journal of Environmental Science and Health, Part C) C16:101-122.
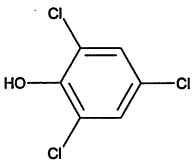
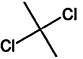
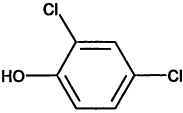
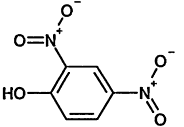
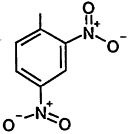
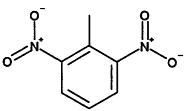
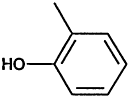
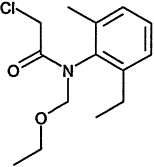
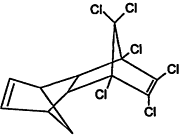
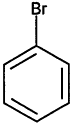
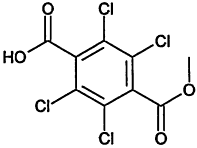
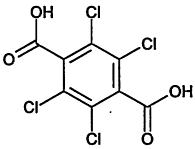
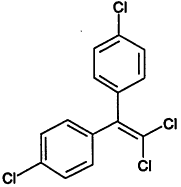
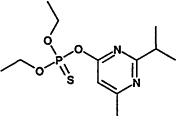
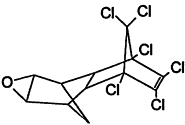
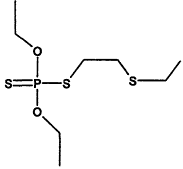
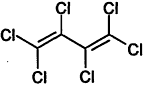
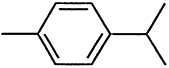
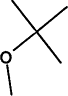
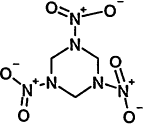
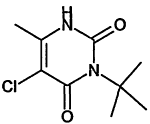
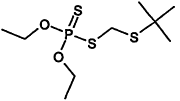
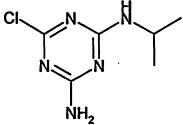
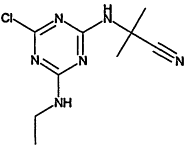
TABLE 4-1
Chemical Abstracts Service (CAS) Registry Numbers. Names and Structures for the 1998 CCL Chemicals
|
CAS No. |
Chemical Name |
Structure |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
 |
|
000095-63-6 |
1,2,4-Trimethylbenzene |
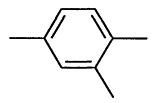 |
|
000075-34-3 |
1,1-Dichloroethane |
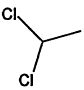 |
|
000563-58-6 |
1.1-Dichloropropene |
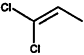 |
|
000122-66-7 |
1.2-Diphenylhydrazine |
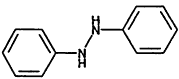 |
|
000142-28-9 |
1.3-Dichloropropane |
|
|
000542-75-6 |
1,3-Dichloropropene |
|
TABLE 4-2
ITC Decisions for the 1998 CCL Chemicals
|
CAS No. |
Chemical Name |
Decision |
ITC Report No. |
Federal Register Citation |
Date |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
Deferred |
|
|
|
|
000095-63-6 |
1,2,4-Trimethylbenzene |
Designated |
10 |
47FR22585 |
5/25/82 |
|
|
|
Removed |
13 |
48FR55674 |
12/14/83 |
|
000075-34-3 |
1,1-Dichloroethane |
Designated |
32 |
58FR38490 |
7/16/93 |
|
000563-55-6 |
1,1-Dichloropropene |
Deferred |
|
|
|
|
000122-66-7 |
1,2-Diphenylhydrazine |
Recommended |
28 |
56FR41212 |
8/19/91 |
|
|
|
Removed |
32 |
58FR38490 |
7/16/93 |
|
000142-28-9 |
1,3-Dichloropropane |
Deferred |
|
|
|
|
000542-75-6 |
1,3-Dichloropropene |
Deferred |
|
|
|
|
000088-06-2 |
2,4,6-Trichlorophenol |
Deferred |
|
|
|
|
000594-20-7 |
2,2-Dichloropropane |
Deferred |
|
|
|
|
000120-83-2 |
2,4-Dichlorophenol |
Recommended |
28 |
56FR41212 |
8/19/91 |
|
|
|
Removed |
32 |
58FR38490 |
7/16/93 |
|
000051-28-5 |
2,4-Dinitrophenol |
Recommended |
27 |
56FR9534 |
3/06/91 |
|
|
|
Removed |
33 |
59FR3764 |
1/26/94 |
|
000121-14-2 |
2,4-Dinitrotoluene |
Designated |
32 |
58FR38490 |
7/16/93 |
|
000606-20-2 |
2,6-Dinitrotoluene |
Deferred |
|
|
|
|
000095-45-7 |
2-Methylphenol |
Designated |
1 |
42FR55026 |
10/12/77 |
|
|
(o-cresol) |
Removed |
13 |
48FR55674 |
12/14/83 |
|
034256-82-1 |
Acetochlor |
Deferred |
|
|
|
|
000309-00-2 |
Aidrin |
Deferred |
|
|
|
|
007429-90-5 |
Aluminum |
Deferred |
|
|
|
|
007440-42-8 |
Boron |
Deferred |
|
|
|
|
000108-86-1 |
Bromobenzene |
Deferred |
|
|
|
|
000857-54-7 |
DCPA mono-acid |
Deferred degradate |
|
|
|
|
002136-79-0 |
DCPA di-acid degradate |
Deferred |
|
|
|
|
000072-55-9 |
DDE |
Deferred |
|
|
|
|
CAS No. |
Chemical Name |
Decision |
ITC Federal No. |
Report Register Citation |
Date |
|
000333-41-5 |
Diazinon |
Deferred |
|
|
|
|
000060-57-1 |
Dieldrin |
Deferred |
|
|
|
|
000298-04-4 |
Disulfoton |
Deferred |
|
|
|
|
000330-54-1 |
Diuron |
Deferred |
|
|
|
|
000759-94-4 |
EPTC (s-ethyl-dipropylthiocarbamate) |
Deferred |
|
|
|
|
000944-22-9 |
Fonofos |
Deferred |
|
|
|
|
000087-68-3 |
Hexachlorobutadiene |
Designated |
1 |
42FR55026 |
10/12/77 |
|
|
|
Removed |
12 |
48FR24443 |
6/01/83 |
|
000099-87-6 |
p-Isopropyltoluene (p-cymene) |
Deferred |
|
|
|
|
000330-55-2 |
Linuron |
Deferred |
|
|
|
|
007439-96-5 |
Manganese |
Deferred |
|
|
|
|
000074-83-9 |
Methyl bromide |
Deferred |
|
|
|
|
001634-04-4 |
Methyl-t-butyl ether |
Recommended |
19 |
51FR41417 |
11/14/86 |
|
|
(MTBE) |
Designated |
20 |
52FR19020 |
5/20/87 |
|
|
|
Removed |
22 |
53FR18196 |
5/20/88 |
|
051218-45-2 |
Metolachlor |
Deferred |
|
|
|
|
021087-64-9 |
Metribuzin |
Deferred |
|
|
|
|
002212-67-1 |
Molinate |
Deferred |
|
|
|
|
000091-20-3 |
Naphthalene |
Designated |
35 |
59FR67596 |
12/29/94 |
|
000098-95-3 |
Nitrobenzene |
Designated |
I |
42FR55026 |
10/12/77 |
|
|
|
Removed |
9 |
47FR5456 |
2/05/82 |
|
001610-18-0 |
Prometon |
Deferred |
|
|
|
|
000121-82-4 |
RDX |
Deferred |
|
|
|
|
007440-23-5 |
Sodium |
Deferred |
|
|
|
|
014808-79-8 |
Sulfate |
Deferred |
|
|
|
|
005902-51-2 |
Terbacil |
Deferred |
|
|
|
|
013071-79-9 |
Terbufos |
Deferred |
|
|
|
TABLE 4-3
TSCA Section 4 and 8(d) References and Studies Indexed in the TSCA Test Submissions (TSCATS) Database for 1998 CCL Chemicals
|
CAS No. |
Chemical Name |
No. of TSCATS Entries |
|||
|
|
|
TSCA Section 8(d) |
TSCA Section 4 |
||
|
|
|
References |
Studies |
References |
Studies |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
75 |
100 |
8 |
9 |
|
000095-63-6 |
1,2,4-Trimethylbenzene |
24 |
36 |
4 |
4 |
|
000075-34-3 |
1,1-Dichloroethane |
110 |
134 |
8 |
8 |
|
000563-58-6 |
1,1-Dichloropropene |
1 |
3 |
0 |
0 |
|
000122-66-7 |
1,2-Diphenylhydrazine |
8 |
10 |
1 |
1 |
|
000142-28-9 |
1,3-Dichloropropane |
12 |
22 |
2 |
3 |
|
000542-75-6 |
1,3-Dichloropropene |
98 |
137 |
1 |
1 |
|
000088-06-2 |
2,4,6-Trichlorophenol |
24 |
47 |
4 |
4 |
|
000594-20-7 |
2,2-Dichloropropane |
0 |
0 |
0 |
0 |
|
000120-83-2 |
2,4-Dichlorophenol |
31 |
54 |
5 |
5 |
|
000051-28-5 |
2,4-Dinitrophenol |
12 |
17 |
4 |
6 |
|
000121-14-2 |
2,4-Dinitrotoluene |
44 |
71 |
3 |
4 |
|
000606-20-2 |
2,6-Dinitrotoluene |
19 |
23 |
2 |
2 |
|
000095-48-7 |
2-Methylphenol (o-cresol) |
59 |
90 |
19 |
30 |
|
034256-82-1 |
Acetochlor |
0 |
0 |
0 |
0 |
|
000309-00-2 |
Aldrin |
12 |
12 |
3 |
3 |
|
007429-90-5 |
Aluminum |
25 |
29 |
1 |
1 |
|
007440-42-8 |
Boron |
6 |
6 |
1 |
1 |
|
000108-86-1 |
Bromobenzene |
7 |
9 |
3 |
3 |
|
000887-54-7 |
DCPA mono-acid. |
0 |
0 |
0 |
0 |
|
002136-79-0 |
DCPA di-acid degradate |
0 |
0 |
0 |
0 |
|
000072-55-9 |
DDE |
10 |
10 |
3 |
3 |
|
000333-41-5 |
Diazinon |
1 |
1 |
3 |
3 |
|
000060-57-1 |
Dieldrin |
13 |
13 |
3 |
3 |
|
000298-04-4 |
Disulfoton |
1 |
1 |
1 |
1 |
|
CAS No. |
Chemical Name |
No. of TSCATS Entries |
|||
|
|
|
TSCA Section 8(d) |
TSCA Section 4 |
||
|
|
|
References |
Studies |
References |
Studies |
|
000330-54-1 |
Diuron |
0 |
0 |
0 |
0 |
|
000759-94-4 |
EPTC (s-ethyl-dipropyl thiocarbamate) |
0 |
0 |
0 |
0 |
|
000944-22-9 |
Fonofos |
0 |
0 |
0 |
0 |
|
000087-68-3 |
Hexachlorobutadiene |
61 |
87 |
16 |
20 |
|
000099-87-6 |
p-Isopropyltoluene (p-cymene) |
5 |
5 |
1 |
1 |
|
000330-55-2 |
Linuron |
1 |
2 |
0 |
0 |
|
007439-96-5 |
Manganese |
43 |
63 |
3 |
3 |
|
000074-83-9 |
Methyl bromide |
88 |
107 |
4 |
4 |
|
001634-04-4 |
Methyl-t-butyl ether |
92 |
216 |
50 |
78 |
|
051218-45-2 |
Metolachlor |
0 |
0 |
0 |
0 |
|
021087-64-9 |
Metribuzin |
0 |
0 |
0 |
0 |
|
002212-67-1 |
Molinate |
0 |
0 |
0 |
0 |
|
000091-20-3 |
Naphthalene |
161 |
234 |
12 |
12 |
|
000098-95-3 |
Nitrobenzene |
33 |
49 |
6 |
9 |
|
001610-18-0 |
Prometon |
0 |
0 |
0 |
0 |
|
000121-82-4 |
RDX |
0 |
0 |
1 |
1 |
|
007440-23-5 |
Sodium |
27 |
32 |
1 |
1 |
|
014808-79-8 |
Sulfate |
17 |
20 |
1 |
1 |
|
005902-51-2 |
Terbacil |
0 |
0 |
0 |
0 |
|
013071-79-9 |
Terbufos |
0 |
0 |
0 |
0 |
|
006190-65-4 |
Triazines (atrazine-desethyl) |
0 |
0 |
0 |
0 |
|
021725-46-2 |
Triazines (cyanazine) |
1 |
1 |
1 |
I |
|
007440-62-2 |
Vanadium |
17 |
20 |
2 |
2 |
TABLE 4-4
Substructure-Based Computerized Chemical Selection Expert System (SuCCSES) Classes and Uses for 1998 CCL Chemicals
|
CAS No. |
Chemical Name |
SuCCSES Class |
Use |
|
034256-82-1 |
Acetochlor |
Acetanilides |
Herbicide |
|
051218-45-2 |
Metohchlor |
Acetanilides |
Herbicide |
|
000060-57-1 |
Dieldrin |
Aliphatic halides |
Insecticide |
|
000074-83-9 |
Methyl bromide |
Aliphatic halides |
Organic synthesis, fumigant |
|
000075-34-3 |
1,1-Dichloroethane |
Aliphatic halides |
Chemical intermediate |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
Aliphatic halides |
Solvent |
|
000087-68-3 |
Hexachlorobutadiene |
Aliphatic halides |
Chemical intermediate (no longer produced in U.S.) |
|
000142-28-9 |
1,3-Dichloropropane |
Aliphatic halides |
Unknown |
|
000309-00-2 |
Aidrin |
Aliphatic halides |
Insecticide |
|
000542-75-6 |
1,3-Dichloropropene |
Aliphatic halides |
Organic synthesis, soil fumigant |
|
000563-58-6 |
1,1-Dichloropropene |
Aliphatic halides |
Unknown |
|
000594-20-7 |
2,2-Dichloropropane |
Aliphatic halides |
Unknown |
|
000072-55-9 |
DDE |
Aromatic halides |
Degradation product of DDT |
|
000108-86-1 |
Bromobenzene |
Aromatic halides |
Solvent, organic synthesis |
|
000887-54-7 |
DCPA mono-acid degradate |
Aromatic halides |
Degradation product of DCPA |
|
002136-79-0 |
DCPA di-acid degradate |
Aromatic halides |
Degradation product of DCPA |
|
000091-20-3 |
Naphthalene |
Aromatic hydrocarbons |
Moth repellant, fungicide |
|
000095-63-6 |
1,2,4-Trimethylbenzene |
Aromatic hydrocarbons |
Dyes, pharmaceuticals |
|
CAS No. |
Chemical Name |
SuCCSES Class |
Use |
|
000099-87-6 |
p-Isopropyitoluene (p-cymene) |
Aromatic hydrocarbons |
Heat-transferring agent |
|
000759-94-4 |
EPTC (s-ethyl-dipropylthiocarbamate) |
Carbamic acid esters |
Herbicide |
|
007429-90-5 |
Aluminum |
Elements |
Numerous consumer/industrial applications |
|
007439-96-5 |
Manganese |
Elements |
Numerous industrial applications |
|
007440-23-5 |
Sodium |
Elements |
Numerous applications |
|
007440-42-8 |
Boron |
Elements |
Numerous consumer/industrial applications |
|
007440-62-2 |
Vanadium |
Elements |
Numerous industrial applications |
|
001634-04.4 |
Methyl-t-butyl ether (MTBE) |
Ethers |
Octane booster for unleaded gasoline |
|
000088-06-2 |
2,4,6-Trichlorophenol |
Halophenols |
Mfg. of 2,4,5-T (no longer produced in U.S.) |
|
000120-83-2 |
2,4-Dichlorophenol |
Halophenols |
Mfg. of 2,4-D, organic synthesis |
|
000122-66-7 |
1,2-Diphenylhydrazine |
Hydrazines |
Mfg. of benzidine (no longer produced in U.S.) |
|
014808-79-8 |
Surfate |
Inorganics |
Numerous applications |
|
000098-95-3 |
Nitrobenzene |
Nitroaromatics |
Solvent for cellulose ethers, mfg. of aniline |
|
CAS No. |
Chemical Name |
SuCCSES Class |
Use |
|
000121-14-2 |
2,4-Dinitrotoluene |
Nitroaromatics |
Explosives, dyes, organic synthesis |
|
000606-20-2 |
2,6-Dinitrotoluene |
Nitroaromatics |
Explosives, dyes, organic synthesis |
|
000051-28-5 |
2,4-Dinitrophenol |
Phenols |
Mfg. of explosives |
|
000095-48-7 |
2-Methylphenol (o-cresol) |
Phenols |
Chemical intermediate |
|
000944-22-9 |
Fonofos |
Phosphonodithioates |
Insecticide |
|
000298-04-4 |
Disulfoton |
Phosphorodithioates |
Insecticide |
|
013071-79-9 |
Terbufos |
Phosphorodithioates |
Herbicide (soil) |
|
000333-41-5 |
Diazinon |
Phosphorothioates |
Insecticide |
|
000121-82-4 |
RDX |
Triazines |
Explosives |
|
001610-18-0 |
Prometon |
Triazines |
Herbicide |
|
002212-67-1 |
Molinate |
Triazines |
Herbicide |
|
005902-51-2 |
Terbacil |
Triazines |
Herbicide |
|
006190-65-4 |
Triazines (atrazine-desethyl) |
Triazines |
Herbicide |
|
021087-64-9 |
Metribuzin |
Triazines |
Herbicide |
|
021725-46-2 |
Triazines (cyanazine) |
Triazines |
Herbicide |
|
000330-54-1 |
Diuron |
Ureas |
Herbicide |
|
000330-55-2 |
Linuron |
Ureas |
Herbicide |
TABLE 4-5
Log Octanol-Water Partition Coefficient (Log Kow) Values, Soil or Sediment Sorption Coefficient (Koc) Values and Henry's Law Constants for the 1998 CCL Chemicals Arranged by SuCCSES Classes
|
CAS No. |
Chemical Name |
Log Kow |
Koc |
Henry's Law Constant (atm m3/mole) |
|
|
Acetanilides |
|
|
|
|
034256-82-1 |
Acetochlor |
3.03 |
176 |
2.23E-08 |
|
051218-45-2 |
Metolachlor |
3.13 |
292 |
9.00E-09 |
|
|
Aliphatic halides |
|
|
|
|
000060-57-1 |
Dieldrin |
5.40 |
10,600 |
5.80E-05 |
|
000074-83-9 |
Methyl bromide |
1.19 |
14 |
6.24E-03 |
|
000075-34-3 |
1,1-Dichlowethane |
1.79 |
35 |
5.62E-03 |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
2.39 |
107 |
3.67E-04 |
|
000087-68-3 |
Hexachlorobutadiene |
4.78 |
994 |
1.03E-02 |
|
000142-28-9 |
1,3-Dichloropropane |
2.00 |
81 |
9.76E-04 |
|
000309-00-2 |
Aidrin |
6.50 |
105,600 |
4.93E-04 |
|
000542-75-6 |
1,3-Dichloropropene |
2.29 |
81 |
3.55E-03 |
|
000563-58-6 |
1,1-Dichloropropene |
2.53 |
68 |
5.00E-02 |
|
000594-20-7 |
2,2-Dichloropropane |
2.92 |
49 |
1.61E-02 |
|
|
Aromatic halides |
|
|
|
|
000072-55-9 |
DDE |
6.51 |
152,500 |
3.52E-5 |
|
000108-86-1 |
Bromobenzene |
2.99 |
268 |
2.08E-03 |
|
000887-54-7 |
DCPA mono-acid degradate |
3.19 |
53 |
2.11E-10 |
|
002136-79-0 |
DCPA di-acid degradate |
2.16 |
557 |
6.58E-13 |
|
|
Aromatic hydrocarbons |
|
|
|
|
000091-20-3 |
Naphthalene |
3.30 |
1,837 |
4.83E-04 |
|
000095-63-6 |
1,2,4-Trimethylbenzene |
3.63 |
718 |
6.16E-03 |
|
000099-87-6 |
p-Isopropyltoluene (p-cymene) |
4.10 |
1,324 |
1.10E-02 |
|
CAS No. |
Chemical Name |
Log Kow |
Koc |
Henry's Law Constant (atm m3/mole) |
|
|
Carbamic acid esters |
|
|
|
|
000759-94-4 |
EPTC (s-ethyl-dipropylthiocarbamate) |
3.21 |
258 |
2.26E-05 |
|
|
Elements |
|
|
|
|
007429-90-5 |
Aluminum |
ND* |
bid |
ND |
|
007439-96-5 |
Manganese |
ND |
ND |
ND |
|
007440-23-5 |
Sodium |
ND |
ND |
ND |
|
007440-42-8 |
Boron |
ND |
ND |
ND |
|
007440-62-2 |
Vanadium |
ND |
ND |
ND |
|
|
Ethers |
|
|
|
|
001634-04-4 |
Methyl-t-butyl ether (MTBE) |
0.94 |
5 |
5.87E-04 |
|
|
Halophenols |
|
|
|
|
000088-06-2 |
2,4,6-Trichlorophenol |
3.69 |
1,186 |
2.60E-06 |
|
000120-83-2 |
2,4-Dichlorophenol |
3.06 |
718 |
2.19E-06 |
|
|
Hydrazines |
|
|
|
|
000122-66-7 |
1,2-Diphenylhydrazine |
2.94 |
3,481 |
4.39E-09 |
|
|
Inorganics |
|
|
|
|
014808-79-8 |
Sulfate |
ND |
ND |
HD |
|
|
Nitroaromatics |
|
|
|
|
000098-95-3 |
Nitrobenzene |
1.85 |
191 |
2.40E-05 |
|
000121-14-2 |
2,4-Dinitrotoluene |
1.98 |
364 |
130E-07 |
|
000606-20-2 |
2,6-Dinitrotoluene |
2.10 |
371 |
7.47E-07 |
|
|
Phenols |
|
|
|
|
000051-28-5 |
2,4-Dinitrophenol |
1.67 |
364 |
7.94E-10 |
|
000095-48-7 |
2-Methylphenol (o-cresol) |
1.95 |
443 |
1.20E-06 |
|
|
Phosphonothioates |
|
|
|
|
000944-22-9 |
Fonofos |
3.94 |
836 |
1.12E-04 |
|
|
Phosphorodithioates |
|
|
|
|
000298-04-4 |
Disulfoton |
4.02 |
819 |
3.99E-06 |
|
013071-79-9 |
Terbufos |
4.48 |
979 |
2.40E-05 |
TABLE 4-6
Scores and Criteria for Assigning Exposure Scores to Exposure Factors for Which Data Were Available and that are Relevant to the 1998 CCL Chemicals
|
Exposure Factor |
Scores and Criteria for Assigning Exposure Scores |
|||
|
|
+3 |
+2 |
+1 |
0 |
|
Annual production volume (lbs.) |
100,000,000 |
10,000,000 |
1,000,000 |
1,000,000 |
|
Environmental persistence |
Years |
Months |
Days |
Hours |
|
Bioaccumulation potential (log Kow) |
>5 |
3 to 5 |
1 to 3 |
<1 |
TABLE 4-7
Exposure Scores for Aliphatic Halides from the 1998 CCL Chemicals
|
CAS No. |
Chemical Name |
Annual Production Volume (lbs.) |
Environmental Persistence |
Bioaccumulation Potential (log Kow) |
|
000060-57-1 |
Dieldrin |
0 |
3 |
3 |
|
000074-83-9 |
Methyl bromide |
2 |
2 |
1 |
|
000075-34-3 |
1,1-Dichloroethane |
0 |
2 |
1 |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
I |
2 |
1 |
|
000087-68-3 |
Hexachlorobutadiene |
0 |
3 |
2 |
|
000142-28-9 |
1,3-Dichloropropane |
I |
2 |
1 |
|
000309-00-2 |
Aldrin |
0 |
3 |
3 |
|
000542-75-6 |
1,3-Dichloropropene |
1 |
2 |
1 |
|
000563-58-6 |
1,1-Dichloropropene |
1 |
2 |
1 |
|
000594-20-7 |
2,2-Dichloropropane |
1 |
2 |
1 |
TABLE 4-8
Scores nod Criteria for Assigning Effects Scores to Biological Effects for Which Data Were Available and that Are Relevant to the CCL Chemicals
|
|
1998 Scores and Criteria for Assigning Biological Effects Scores |
||||||
|
Biological |
+3 |
+2 |
+1 |
0 |
-1 |
-2 |
-3 |
|
Effects |
|
Tested |
Tested |
Tested |
Predicted |
Predicted |
Predicted |
|
Acute toxicity |
LD50 < 50 mg/kg oral LD50 < 5 mg/L inhal. LD50 < 1 mg/kg derm. |
LD50 = 50-500 mg/kg oral LC50 = 5-50 mg/L inhal. LD50 =1-50 mg/kg derm. |
LD50=500-5,000 mg/kg oral LC50=50-500 mg/L inhal. LD5050-500 mg/kg derm. |
LD50>5,000 mg/kg oral LD50<500 mg/L inhal. LD50<500 mg/kg derm. |
Suspected to be slightly to moderately toxic |
Suspected to be very toxic |
Suspected to be extremely toxic |
|
Mutagenicity |
Positive in two or more whole mammalian test systems |
Positive in vitro and interacts with germinal-cell DNA in vivo |
Positive in one test system |
Negative in more than one system |
Slight suspicion based on structure |
Suspicion based on SARs to known mutagens or carcinogens |
Strong suspicion based on SARs |
|
Carcinogenicity |
Established carcinogen in humans or two animal species |
Established carcinogen in one animal species |
Insufficient data but some suspicion of carcinogenicity |
Negative results in two animal species |
Suspect carcinogen, potent organ-specific toxin, or enzyme inducer |
Structural relationship to known carcinogen |
Structural relationship to a known carcinogen or strong suspicion based on SARs |
|
Ecotoxicity |
Effects at low concentrations (g/L) |
Effects at moderate concentrations (mg/L) |
Effects at high concentrations (g/L) |
Negative results |
Likely to cause effects at high concentrations |
Likely to cause effects at moderate concentrations |
Likely to cause effects at low concentrations based on SARs |
TABLE 4-9
Biological Effects Scores for Aliphatic Halides from the 1998 CCL Chemicals
|
CAS No. |
Chemical Name |
Acute Toxicity |
Mutagenicity |
Carcinogenicity |
Ecotoxicity |
|
000060-57-1 |
Dieldrin |
+3 |
+2 |
0 |
+3 |
|
000074-83-9 |
Methyl bromide |
+2 |
+2 |
0 |
+2 |
|
000075-34.3 |
1,1-Dichloroethane |
+1 |
+1 |
+1 |
+2 |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
+2 |
+2 |
+2 |
+2 |
|
000087-68-3 |
Hexachlorobutadiene |
+2 |
+2 |
+1 |
+3 |
|
000142-28-9 |
1,3-Dichloropropane |
+1 |
+3 |
-2 |
+2 |
|
000309-00-2 |
Aldrin |
+3 |
+2 |
+2 |
+3 |
|
000542-75-6 |
1,3-Dichloropropene |
+2 |
+2 |
+3 |
+2 |
|
000563-58-6 |
1,1-Dichloropropene |
-1 |
+1 |
-1 |
-2 |
|
000594-20-7 |
2,2-Dichloropropane |
-1 |
-1 |
-1 |
-2 |
TABLE 4-10
Modes of Toxic Action for the 1998 CCL Chemicals Arranged by SuCCSES Classes
|
CAS No. |
Chemical Name |
Mode of Action |
|
|
Acetanilides |
|
|
034256-82-1 |
Acetochlor |
Nonpolar narcosis |
|
051218-45-2 |
Metolachlor |
Nonpolar narcosis |
|
|
Aliphatic halides |
|
|
000060-57-1 |
Dieldrin |
Neurotoxicant: Cyclodiene-type |
|
000074-83-9 |
Methyl bromide |
Nonpolar narcosis |
|
000075-34-3 |
1,1-Dichloroethane |
Nonpolar narcosis |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
Nonpolar narcosis |
|
000087-68-3 |
Hexachlorobutadiene |
Reactivity: Alkylation or arylation reaction |
|
000142-28-9 |
1,3-Dichloropropane |
Nonpolar narcosis |
|
000309-00-2 |
Aldrin |
Neurotoxicant: Cyclodiene-type |
|
000542-75-6 |
1,3-Dichloropropene |
Reactivity: Alkylation or arylation reaction |
|
000563-58-6 |
1,1-Dichloropropene |
Nonpolar narcosis |
|
000594-20-7 |
2,2-Dichloropropane |
Nonpolar narcosis |
|
|
Aromatic halides |
|
|
000072-55-9 |
DDE |
Nonpolar narcosis |
|
000108-86-1 |
Bromobenzene |
Nonpolar narcosis |
|
000887-54-7 |
DCPA mono-acid degradate |
Nonpolar narcosis |
|
002136-79-0 |
DCPA di-acid degradate |
Nonpolar narcosis |
|
|
Aromatic hydrocarbons |
|
|
000091-20-3 |
Naphthalene |
Nonpolar narcosis |
|
000095-63-6 |
1,2,4-Trimethylbenzene |
Nonpolar narcosis |
|
000099-87-6 |
p-Isopropyltoluene |
Nonpolar narcosis |
|
|
(p-cymene) |
|
|
|
Carbamic acid esters |
|
|
000759-94-4 |
EPTC (s-ethyl-dipropylthio-carbamate) |
Nonpolar narcosis |
|
CAS No. |
Chemical Name |
Mode of Action |
|
|
Elements |
|
|
007429-90-5 |
Aluminum |
N.A.* |
|
007439-96-5 |
Manganese |
N.A. |
|
007440-23-5 |
Sodium |
N.A. |
|
007440-42-8 |
Boron |
N.A. |
|
007440-62-2 |
Vanadium |
N.A. |
|
|
Ethers |
|
|
001634-04-4 |
Methyl-t-butyl ether (MTBE) |
Nonpolar narcosis |
|
|
Halophenols |
|
|
000088-06-2 |
2,4,6-Trichlorophenol |
Polar narcosis |
|
000120-83-2 |
2,4-Dichlorophenol |
Polar narcosis |
|
|
Hydrazines |
|
|
000122-66-7 |
1,2-Diphenylhydrazine |
Reactivity: hydrazines |
|
|
Inorganics |
|
|
014808-79-8 |
Sulfate |
N.A. |
|
|
Nitroaromaties |
|
|
000098-95-3 |
Nitrobenzene |
Nonpolar narcosis |
|
000121-14-2 |
2,4-Dinitrotoluene |
Reactivity: dinitroaromatic group |
|
000606-20-2 |
2,6-Dinitrotoluene |
Reactivity: dinitroaromatic group |
|
|
Phenols |
|
|
000051-28-5 |
2,4-Dinitrophenol |
Uncoupler of oxidative phosphorylation |
|
000095-48-7 |
2-Methylphenol (o-cresol) |
Polar narcosis |
|
|
Phosphonothioates |
|
|
000944-22-9 |
Fonofos |
Organophosphate mediated acetylcholinesterase inhibition |
|
|
Phosphorodithioates |
|
|
000298-04-4 |
Disulfoton |
Organophosphate mediated acetylcholinesterase inhibition |
|
013071-79-9 |
Terbufos |
Organophosphate mediated acetylcholinesterase inhibition |
TABLE 4-11
Carcinogenicity Concern Levels for the 1998 CCL Chemicals Arranged by SuCCSES Classes
|
CAS No. |
Chemical Name |
Carcinogenicity Concern Levels |
Comments |
|
|
Acetanilides |
|
|
|
034256-82-1 |
Acetochlor |
Moderate |
+Data |
|
05121845-2 |
Metolachlor |
Moderate |
|
|
|
Aliphatic halides |
|
|
|
000060-57-1 |
Dieldrin |
Moderate |
+Data |
|
000074-83-9 |
Methyl bromide |
Moderate |
|
|
000075-34-3 |
1,1-Dichloroethane |
Low |
- Data |
|
000079-34-5 |
1,1,2,2-Tetrachloroethane |
Moderate |
+ Data |
|
000087-68-3 |
Hexachlorobutadiene |
Moderate |
+ Data |
|
000142-28-9 |
1,3-Dichloropropane |
Moderate |
|
|
000309-002 |
Aldrin |
Moderate |
+ Data |
|
000542-75-6 |
1,3-Dichloropropene |
High |
+ Data |
|
000563-58-6 |
1,1-Dichloropropene |
Moderate |
|
|
000594-20-7 |
2,2-Dichloropropane |
Low |
|
|
|
Aromatic halides |
|
|
|
000072-55-9 |
DDE |
Moderate |
+ Data |
|
000108-86-1 |
Bromobenzene |
Moderate |
|
|
000887-54-7 |
DCPA mono-acid degradate |
Low |
|
|
002136-79-0 |
DCPA di-acid degradate |
Low |
|
|
|
Aromatic hydrocarbons |
|
|
|
000091-20-3 |
Naphthalene |
Moderate |
+ Data |
|
000095-63-6 |
1,2,4-Trimethylbenzene |
Low |
|
|
000099-87-6 |
p-Isopropyltoluene (p-cymene) |
Low |
|
|
|
Carbamic acid esters |
|
|
|
000759-94-4 |
EPTC (s-ethyl-dipropylthiocarbamate) |
Moderate |
|
|
|
Elements |
|
|
|
007429-90-5 |
Aluminum |
Low |
|
|
007439-96-5 |
Manganese |
Low |
|
|
007440-23-5 |
Sodium |
Low |
|
|
007440-42-8 |
Boron |
Low |
|
|
007440-62-2 |
Vanadium |
Low to moderate |
|
|
CAS No. |
Chemical Name |
Carcinogenicity Concern Levels |
Comments |
|
|
Ethers |
|
|
|
001634-04-4 |
Methyl-t-butyl ether |
Moderate |
+ Data |
|
|
Halophenols |
|
|
|
000088-06-2 |
2,4,6-Trichlorophenol |
Moderate |
+ Data |
|
000120-83-2 |
2,4-Dichlorophenol |
Low |
- Data |
|
|
Hydrazines |
|
|
|
000122-66-7 |
1,2-Diphenylhydrazine |
Moderate |
|
|
|
Inorganics |
|
|
|
014808-79-8 |
Sulfate |
Low |
|
|
|
Nitroaromatics |
|
|
|
000098-95-3 |
Nitrobenzene |
Moderate |
|
|
000121-14-2 |
2,4-Dinitrotoluene |
High |
+ Data |
|
000606-20-2 |
2,6-Dinitrotoluene |
Moderate |
+ Data |
|
|
Phenols |
|
|
|
000051-28-5 |
2,4-Dinitrophenol |
Moderate |
|
|
000095-48-7 |
2-Methylphenol (o-cresol) |
Low |
|
|
|
Phosphonothioates |
|
|
|
000944-22-9 |
Fonofos |
Low |
|
|
|
Phosphorodithioates |
|
|
|
000298-04-4 |
Disulfoton |
Low |
- Data |
|
013071-79-9 |
Terbufos |
Low |
- Data |
|
|
Phosphorothioates |
|
|
|
000333-41-5 |
Diazinon |
Low |
|
|
|
Triazines |
|
|
|
000121-82-4 |
RDX |
Moderate |
|
|
001610-18-0 |
Prometon |
Low |
Equivocal data |
|
002212-67-1 |
Molinate |
Moderate |
+ Data |
|
005902-51-2 |
Terbacil |
Low |
- Data |
|
006190-65-4 |
Triazines (atrazine-desethyl) |
Moderate |
+ Data for parent compound |
TABLE 4-12
Wildlife Species Contaminated with the 1998 CCL Chemicals
|
Chemical |
Species |
Records |
Individuals |
Matrix |
Range (µg/g) |
|
Aldrin |
Blue jay |
1 |
1 |
Brain |
0.04 |
|
|
Fulvous whistling duck |
2 |
30 |
Liver |
0.0021, 0.0094 |
|
|
Sharp-shinned hawk |
1 |
1 |
Brain |
0.04 |
|
Aluminum |
American alligator |
3 |
13 |
Egg |
1.3-2.0 |
|
|
American |
2 |
12 |
Eggshell |
52.36 |
|
|
crocodile |
|
|
Egg |
10.86 |
|
|
Black-crowned night-heron |
1 |
7 |
Egg |
9.55 |
|
|
Muskrat |
3 |
76 |
Kidney |
3.45-13.19 |
|
|
Peregrine falcon |
10 |
10 |
Egg |
6.74-17.4 |
|
|
Short-tailed shrew |
5 |
5 |
Carcass |
130.0-561.0 |
|
|
Snapping turtle |
2 |
19 |
Liver |
15.97, 78.83 |
|
|
White-footed mouse |
4 |
4 |
Carcass |
45.0-180.0 |
|
Boron |
Common tern |
10 |
10 |
Egg |
22.4-36.8 |
|
|
Peregrine falcon |
3 |
3 |
Egg |
0.75-1.29 |
|
|
Snapping turtle |
1 |
12 |
Liver |
3 |
|
|
Tree swallow |
3 |
9 |
Egg |
1.52-3.55 |
|
Diazinon |
American brant |
2 |
21 |
Liver |
0.003 |
|
|
|
|
|
Small intestine |
0.002-3.2 |
|
|
|
|
|
Gizzard |
0.12-0.77 |
|
|
American robin |
2 |
3 |
Gizzard |
1.5 |
|
|
Blackbird |
1 |
60 |
|
blot given |
|
|
Blue jay |
2 |
3 |
Alimentary canal |
0.09-3.48 |
|
|
Boat-tailed grackle |
1 |
3 |
Gizzard |
12 |
|
|
Bobwhite quail |
1 |
160 |
Wing |
2.93 |
|
|
Canada goose |
12 |
112 |
Gizzard |
0.34-9.13 |
|
|
|
|
|
Liver |
0.014-0.05 |
|
|
Common grackle |
4 |
72 |
Gizzard |
16 |
|
|
Mallard |
9 |
74 |
Brain |
161 |
|
|
|
|
|
Gizzard |
0.32-3000 |
TABLE 4-13
Aliphatic Halides (not on the 1998 CCL) with Uses Similar to Those of 1,1,2,2-Tetrachloroethane (on the 1998 CCL)
|
|
|
Uses |
|||||
|
CAS No. |
Chemical |
Machinery |
Polystyrene |
SBR Rubber |
Rubber |
Varnish |
Total Uses |
|
79-34-5 |
1,1,2,2- Tetrachloroethane |
X |
X |
X |
X |
X |
5 |
|
71-55-6 |
1,1,1- Trichloroethane |
X |
X |
X |
X |
X |
15 |
|
56-23-5 |
Carbon tetrachloride |
X |
|
|
X |
X |
9 |
|
79-00-5 |
1,1,2-Trichloroethane |
|
X |
X |
|
X |
3 |
TABLE 4-14
Production, Use, and Environmental Release Volumes for Aliphatic Halides (not on the 1998 CCL) that Have Similar Uses to 1,1,2,2-Tetrachloroethane (on the 1998 CCL)
|
Chemical Name |
Production Volume |
Use Volume |
Toxics Release Inventory Volume (lbs.) |
|
1,1,2,2-Tetrachloroethane |
High |
Low |
16,000 |
|
1,1,1-Trichloroethane |
Extremely high |
Medium to high |
8,800,000 |
|
Carbon tetrachloride |
Extremely high |
Low to medium |
400,000 |
|
1,1,2-Trichloroethane |
Very high |
Low |
340,000 |
TABLE 4-15
Log Octanol-Water Partition Coefficient (log Kow) Values, Soil, or Sediment Sorption Coefficient (Koc) Values, and Henry's Law Constants for Aliphatic Halides (not on the 1998 CCL) that Have Similar Uses to 1,1,2,2-Tetrachloroethane (on the 1998 CCL)
|
Chemical Name |
Log Kow |
Koc |
Henry's Law Constant (atm m3/mol) |
|
1,1,2,2-Tetrachloroethane |
2.39 |
107 |
3.67E-04 |
|
1,1,1-Trichloroethane |
2.49 |
85 |
1.72E-02 |
|
Carbon tetrachloride |
2.83 |
71 |
2.76E-02 |
|
1,1,2-Trichloroethane |
1.89 |
97 |
8.24E-04 |