2
Scientific Discussion
In this chapter, recent improvements in understanding supersonic aircraft emissions, the transport of these emissions, and their chemical and radiative impacts are reviewed. The contributions that AESA has made to these developments are discussed, as are the areas where significant uncertainties remain and where more research is needed. Special focus is given to two components of AESA's research program—global models and field campaigns—because these constitute the dominant part of the overall program. However, it should be noted that other "elements" of the research program (namely, laboratory studies, operational scenarios, near-field interactions, and emissions) have all been important components of the overall assessment of this issue.
Gas-Phase Aircraft Emissions
An assessment of the stratospheric impact of HSCT operations requires an accurate inventory of the emissions from such future aircraft. It has been suspected since the early 1970s that emissions, particularly of nitrogen oxides, from stratospheric aircraft could affect the level and distribution of stratospheric ozone. More recently, concern has been raised over the effect of particles and water vapor emissions on the ozone budget and on regional and global climate. Table 1, adapted from a 1997 European assessment (Brasseur et al., 1997), lists the aircraft emissions that are thought to have the greatest potential atmospheric effects and summarizes what those effects might be.
There are many ways to describe the emissions from aircraft, but the most useful in terms of assessing effects is the "emission index" (EI), defined as the
TABLE 1 Major potential impact of chemical compounds released by aircraft (after Brasseur et al., 1997)
|
CO2 |
|
|
H2O |
|
|
NOx |
|
|
SOx |
|
|
Soot* |
|
|
CO |
|
|
Hydrocarbons |
|
|
* defined as carbonaceous particulate matter |
|
number of grams of the emitted species per kilogram of fuel consumed. In assessing the atmospheric effects of high-speed aircraft, the emission indices of relevant species provide critical input information. However, because future high-speed aircraft have not yet been designed or built, the emission indices of important species such as NOx are not known with certainty. As a consequence, the assessments typically make use of a range of likely emission indices for the species of interest. For example, the EI (Nox)3 of the only current HSCT, the
Concorde, was measured recently in situ at 23 g/kg fuel burned (Fahey et al., 1995). A long-term goal of NASA's High Speed Research (HSR) program has been development of an HSCT with an EI (NOx) of 5. Because this goal will require new engine designs, and success is not guaranteed, the current AESA assessment studies employed EI (NOx) values of 5, 10, and 15 to capture the range of NOx emissions that might result from the new HSCT aircraft.
The goal of lower NOx emissions is driving the design of completely new combustors. Two concepts, the Rich-burn, Quick-quench, Lean-burn (RQL) and the Lean-Premixed-Prevaporized (LPP) both have the potential to provide EI (NOx) at the levels desired, with combustion efficiency greater than 99.9 percent. NASA has recently decided to emphasize the LPP concept, which has demonstrated low NOx emissions (EI [NOx ]) in the range of 3–7 for supersonic cruise simulations) in flame tube and combustor rig tests at NASA's Lewis Research Center (NASA/HSR, unpublished information). However, substantial challenges remain, as the emissions of NOx and other species from a full-scale engine have not yet been tested. Thus, the use of a range of likely emission indices for assessment will continue to be necessary.
Although combustor design has been driven strongly by the desire to reduce NOx emissions, other emissions also can have an impact on the stratosphere, and progress has been made in characterizing the emissions of several of these other species. NASA has projected emission indices for CO2 and water vapor of 3155 and 1237 g/kg fuel burned, respectively, for the year 2015, the same values used for current generation aircraft turbine engines. Carbon monoxide and hydrocarbons are emitted by aircraft engines even at the high combustion efficiency projected for the HSCT. NASA's estimates for EI (CO) and EI (total hydrocarbons) are 2.9 and 0.3 g/kg fuel burned, respectively, for supersonic cruise operation, also in the range of EIs for current generation aircraft turbine engines.
Emissions of sulfur oxides depend on the amount of sulfur in the fuel and are expected to decline from 0.8 to 0.4 g/kg fuel burned over the period from 1990 to 2015, due to a projected decline in fuel sulfur from 0.04 to 0.02 percent over that period. Using a current generation engine, Wey et al. (1998) found that EI (SO2) was independent of altitude and combustor inlet temperature and pressure, and that 85–100 percent of the fuel sulfur was emitted as gaseous SO2. This percentage was independent of fuel type, power level of the engine, or altitude. Sulfur particle emissions are discussed in more detail in the following section.
As noted above, NOx emissions have been a focus of the HSCT design, and considerable effort has gone into characterizing EI (NOx) for current engines and projecting EI (NOx) for the HSCT. However, the distribution of the NOx components is also important, and both test stand measurements and in-flight measurements with current generation engines yield NO/NOx ratios of 0.85-0.9. A similar ratio is anticipated for the HSCT.
Other trace-level oxidized nitrogen compounds are emitted by aircraft engines, and recent studies have improved our understanding of two such species:
nitric acid (HNO3) and nitrous acid (HONO). Wey et al. (1998) have compiled the first extensive set of HNO3 emission indices for a current generation aircraft engine. They found that El (HNO3) is largely independent of fuel type and altitude, but strongly dependent on combustor inlet temperature. As an example, El (HNO3) was about 0.15 g/kg fuel burned at a simulated altitude of 15.2 km and a combustor inlet temperature of 620K. HONO was observed in the plume of a DC-9 at 9.5 km altitude by Arnold et al. (1992), and inferred in the Concorde plume based on the hydroxyl radical profile (Fahey et al., 1995). More recently, Ristori and Baudoin (1996) reported high concentrations of HONO at the exit of an engine combustor; however, an emission index for HONO was not reported.
Progress has been made in characterizing and understanding gas-phase emissions from supersonic aircraft and in the development of low NOx engines for the future HSCT, but because the HSCT is still a concept, rather than a real airplane, its actual emissions cannot be known at this time. NASA has thus used ranges of emission indices in the assessment of atmospheric effects. PAEAN agrees with the strategy NASA employed in the modeling component of the assessment of using a range of EIs for emissions of critical species such as NOx. This approach should be continued in the future for all critical species whose EI is uncertain.
Other chemicals have been identified in turbine engine emissions, such as HONO and HNO3, but the effect of the LPP combustor on emissions of these chemicals is unknown. PAEAN recommends that the effects of trace species such as these be included in future assessments. Fuel-bound nitrogen may also be worth some additional investigation. While the vast majority of oxidized nitrogen in the exhaust of current generation combustors is due to fixation of atmospheric nitrogen, advanced combustors (e.g. LPP) are designed for greatly reduced production of NOx by this mechanism, so that fuel-bound nitrogen is likely to produce a more significant fraction of exhaust NOx.
It is also recommended that additional emphasis be placed on sulfur emissions. Improved understanding is needed of the chemistry and kinetics of fuel sulfur combustion in the engine, and sulfur particle formation in the near-field exhaust plume. Also, if fuel sulfur levels decrease over the next few years, as predicted, then some type of lubricant may need to be added to the fuel to offset the lost sulfur. The nature of any replacement lubricant and its impact on the atmosphere must be assessed.
The new combustor concepts, such as LPP, are designed for optimum operation in the stratosphere. But for significant portions of HSCT flights (i.e., over land), they will be operating subsonically, in both the stratosphere and troposphere. It is not known how HSCT emissions will be affected by subsonic operation. In the same vein, for the current fleet of commercial aircraft, a large fraction of the organic emissions and products of incomplete combustion occur during idle and taxi operation around airports. Some consideration should be given to the emissions from the new combustors under these conditions, to be certain that exposure of the population around airports to toxic chemicals will not be exacerbated by HSCT operations.
Aircraft Particle Formation and Emissions
Aircraft flying in the stratosphere produce a large number of small volatile particles. An experimental measurement of aerosol particles in aircraft wakes was made in 1995, when an ER-2 aircraft was able to sample the exhaust plume of a Concorde aircraft (Fahey et al., 1995). A large number of aerosol particles was found in the plume with peak values ranging up to 15,000 cm-3, while the background concentration was approximately 6-18 cm-3. A large fraction of these submicron particles volatilized upon heating to 192°C, and their composition was consistent with that of sulfuric acid-water solution. Fahey et al. (1995) calculated the volatile particle number emission indices (Number EI) to be in the range of 1.7-6.5 x 1017 particles/kg fuel burned. They also measured non-volatile particle emissions (which are thought to be dominated by soot), with a Number EI in the range of 4.3-8.7 x 1016 particles/kg fuel burned.
The AESA assessment report (Kawa et al., 1999) discusses a series of more recent measurements that provide detailed information on particle emissions and their variability for different aircraft and measurement techniques (Table 2). Several measurements report large numbers of volatile particles below 20 nm diameter which are thought to be composed of sulfate (Anderson et al., 1998a; Kärcher et al., 1998b). Their results also suggest that particle number increases with fuel sulfur content, in contrast to previous findings (Durlak, 1997). Recent studies of soot carbon emissions show the presence of these species in widely varying quantities but at magnitudes significantly below the number and mass contributions represented by inorganic constituents (Pueschel et al., 1998; Petzold and Schröder, 1998; Anderson et al., 1998ab; Kärcher et al., 1998b). While indirect evidence from the variation in particle number with fuel sulfur content suggests that the particles originate from sulfate, there is no direct evidence precluding a significant organic contribution (Kärcher et al., 1998b; Miake-Lye et al., 1998; Arnold et al., 1998; Curtius et al., 1998).
There is strong evidence that the volatile aerosols are formed through the conversion of SO2 emitted by the engines and then subsequently oxidized to sulfuric acid. The Fahey et al. (1995) measurements yielded a conversion rate of SO2 to sulfuric acid of 12–45 percent, which is significantly higher than the one percent conversion rate predicted by previous studies. This finding has led to a large number of modeling studies and subsequent measurements, but for subsonic aircraft only (SUCCESS campaign, SULFUR-5 and 6 campaigns4. Modeling studies also have indicated that the conversion rates should decrease when the
fuel sulfur content increases, a finding that has been verified in the SULFUR-5 flights (Schröder et al., 1998). However, data collected during the SUCCESS campaign show an increase of the conversion rate with fuel sulfur content (Miake-Lye et al., 1998). Resolving this fundamental relationship between the production of particles and the fuel sulfur content, as well as the rate of conversion of fuel sulfur to sulfuric acid, is central to understanding particle production in jet engines.
The magnitude and mechanisms of particle formation processes remain very unclear. Ground-based engine test measurements suggest that the conversion rate is on the order of a few percent; modeling studies lead to values between 1 and 8 percent; and there is some evidence that the conversion efficiency depends upon the sulfur content in the fuel. The most direct approach to resolving this uncertainty would be to measure directly and accurately SO3 and H2SO4, in addition to SO2, but this measurement is presently not technologically feasible. To date, measurements of ultrafine particles have been made only with physical characterization techniques such as condensation nuclei (CN) counters, such that we have no direct measurements of the composition of newly formed particles (which may contain metals and hydrocarbons in addition to inorganic ions). Hence, the calculation of the conversion rates must rely on the assumption that the volatile aerosols are composed only of sulfuric acid-water solutions of a prescribed size (or size distribution). Variations in these embedded assumptions can probably explain the large differences among the reported conversion rates (although real variations in engine operations could also play a role). Miake-Lye et al. (1998) and Arnold et al. (1998) have recently employed an advanced, species-specific technique, namely, CIMS (Chemical Ionization Mass Spectrometry), to measure SO2 and H2SO4 in situ in the aircraft plume and also show significant variations in the reported conversion rates of SO2 to SO3 and H2SO4.
The formation and evolution of the particles formed in the engine plume is also not well-represented by existing models or theory. Until very recently, the formation of aerosols was explained in terms of classical (homogeneous heteromolecular) nucleation theory, a process that has been shown to produce large numbers of new particles on short time scales (Doyle, 1961; Mirabel and Katz, 1974). This efficiency stems from the fact that sulfuric acid has a very low equilibrium vapor pressure and a very large Gibbs free energy of mixing with water. However, binary nucleation theory is also known to break down in several conditions, including at the low temperatures found in the upper atmosphere. The evolution of the aerosol is controlled by "condensational" growth and self-coagulation (only the latter is effective on the time scales of seconds in aircraft plumes). However, the simulations based on these models have been unable to reproduce the observations made behind the Concorde (for which the calculated particle sizes remained smaller than the observed ones) or Attas (SULFUR-5 experiment), at least for the assumed conversion factor of SO2 (which is taken to be adjustable up to 45%).
TABLE 2 Measurements of volatile particle number EI and fraction of fuel S converted to S(VI), η, measured in the exhaust of aircraft in flight in the absence of contrails. Volatile particles are presumed to be H2SO4/H2O. (Adapted from Kawa et al., 1999)
|
Number EI (/kgfuel) |
η, fraction of fuel S converted to S(VI) |
Technique |
Aircraft |
|
8(±3) x 1016 |
|
CNC |
MD80-2 |
|
1.±0.2 x 1015 |
|
|
ATTAS |
|
5-20 x 1015 |
0.55 |
CNC/model |
ATTAS |
|
~ 2 x 1015 |
>0.08(±0.03) |
CNC |
NASA 757 |
|
|
0.06(0.0-0.34) |
CIMS |
NASA 757 |
|
|
0.37 |
Impactor/electron microscopy |
NASA 757 |
|
2.1(±0.3) x 1014 |
.11 |
DMA |
NASA 757 |
|
1.7-6.5 x 1017 |
>0.12 |
CNC |
Concorde |
|
~8 x 1016 |
>0.15(±0.07) |
CNC |
NASA 757 |
|
|
0.31(0.16-0.52) |
CIMS |
NASA 757 |
|
2.5(±0.4) x 1015 |
.022 |
DMA |
NASA 757 |
|
|
0.10-0.26 |
Impactor/electron microscopy |
NASA 757 |
|
1.0(±0.3) x 1017 |
|
CNC |
NASA DC-8 |
|
1.3(±0.4) x 1017 |
|
CNC |
NASA 757 |
|
~ 1-2 x 1017 |
0.018 |
CNC |
ATTAS |
To overcome these difficulties, it has been postulated that formation and growth is promoted by the presence of ''chemi-ions'' generated by combustion (Yu and Turco, 1998). The main effect of these ions is to increase the efficiency of the collision in the coagulation process, leading to larger aerosol sizes (in the case of neutral particles). However, the number densities of ions needed to reproduce the observations (on the order of 3 X 109 cm-3 at the exit of the engines) have not been substantiated by measured emissions. In particular, Arnold et al. (1998) reported number densities in the range of 10 7_108 cm-3. If these number densities are used, the model of Yu and Turco (1998) cannot reproduce the observations, regardless of the conversion factor of SO2 used. In addition, even with the ions, the models still have difficulties reproducing the measured size distributions, because the particles are still smaller than the observed ones. Another hypothesis
|
Engines |
Flight Conditions |
Fuel S Content (ppmm) |
Reference |
|
|
cruise |
unknown |
Anderson et al. [1998a] |
|
|
|
unknown |
Petzold and Schroeder [1998] |
|
|
varied |
20 |
Schröder et al. [1998] |
|
|
|
|
Kärcher et al. [1998a] |
|
RB211 |
varied |
72 |
Miake-Lye et al. [1998] |
|
RB211 |
varied |
72 |
Miake-Lye et al. [1998] |
|
RB211 |
varied |
72 |
Pueschel et al. [1998] |
|
RB211 |
varied |
72 |
Hagen et al. [1998] |
|
Olympus 593 |
Supersonic cruise |
230 |
Fahey et al. [1998] |
|
RB211 |
varied |
676 |
Miake-Lye et al. [1998] |
|
RB211 |
varied |
676 |
Miake-Lye et al. [1998] |
|
RB211 |
varied |
676 |
Hagen et al. [1998] |
|
RB211 |
varied |
676 |
Pueschel et al. [1998] |
|
CFM56-2-C1 |
slow cruise |
700 |
Anderson et al. [1998] |
|
|
|
800 |
Anderson et al. [1998] |
|
|
varied |
2700 |
Schröder et al. [1998] |
|
|
|
|
Kächer et al. [1998a] |
suggests that organic material may also condense on the newly formed particles thereby changing their size distribution (Yu and Turco, 1999).
Rapid conversion of fuel sulfur to small particles in the plume leads to greater enhancement in lower stratospheric aerosol surface area than does dispersion of sulfur gases followed by oxidation and condensation on pre-existing particles (Fahey et al., 1995; Weisenstein et al., 1996). It is important to improve understanding of these different particle formation pathways, to assure that the scenarios used to simulate the impacts of aircraft emissions are as realistic as possible.
The panel believes that the recent progress of AESA in measuring particulate emissions from aircraft in the SUCCESS missions resolves some of the questions posed in the 1998 AESA assessment. The measurements to date characterize the emissions from ER-2 stratospheric flights and from the best available model-
HSCT, the Concorde. Their findings, however, do present some contradictory results and do not show a clear trend with fuel or altitude. Important contradictions in measurements of variability with fuel sulfur content merit further investigation. The results clearly demonstrate the need for a mechanistic understanding of the particle formation processes in engines, which warrants detailed laboratory studies of sulfur oxidation chemistry. In addition, although much work has been done to characterize the particles formed in engine exhaust, the composition and rate of their formation remains unclear. To resolve this issue, accurate measurements of SO2 (with less than 20 percent uncertainty), S(VI) or SO3 or H2SO4, condensable organic species, chemi-ions, particle size distribution, and particle composition are needed, as well as better models to predict particle number densities and size distribution.
Atmospheric Transport
The evolution of HSCT emissions and subsequent reaction products will be controlled by both atmospheric transport and chemical processes. Cruise altitudes for HSCTs lie in the lower stratosphere, so a thorough understanding of advection and mixing in the stratosphere, and exchange of mass and constituents between the troposphere and stratosphere are needed to assess potential impacts of an HSCT fleet.
The basic model for transport in the lower and middle stratosphere consists of a single meridional cell in each hemisphere. Air rises in the tropics, drifts poleward, then sinks at middle and high latitudes. This mean meridional transport, now known as the Brewer-Dobson circulation, was originally postulated based on stratospheric water vapor and ozone measurements. The extreme dryness of the middle and high latitude stratosphere can be explained by a "freeze drying" of the air by passage through the cold tropical tropopause and subsequent poleward transport (Brewer, 1949). The observed high concentrations of ozone in the lower high latitude stratosphere, far from the tropical region of maximum photochemical production, can also be explained by air drifting poleward out of the topics and sinking at high latitudes (Dobson, 1956). The observed distribution of other trace species in the stratosphere also fits the Brewer-Dobson model for transport.
This general description of stratospheric transport is well accepted. Recent work suggests the overall driving force for the Brewer-Dobson circulation to be momentum deposition by planetary waves at mid-latitudes in the stratosphere (Haynes et al., 1991). The momentum deposition then acts as an "extratropical pump" that draws air up and out of the tropical lower stratosphere (Holton et al., 1995). The rate of upwelling, both on an annual average and considering seasonal variations, is reasonably well modeled by current radiative heating algorithms, as demonstrated in Mote et al. (1995, 1996). Current qualitative understanding of the large-scale transport seems sound.
Holton et al. (1995) divide the stratosphere into two regimes. These are the "overworld," where lines of constant potential temperature (isentropic surfaces) do not cross the tropopause, and the "lowermost stratosphere," where isentropes do cross the tropopause. The exchange of air and constituents between these two regions requires radiative heating or cooling, while adiabatic exchange can occur between the lowermost stratosphere and troposphere. Vertical motions in the stratosphere will be small, due to the stable lapse rate, while the troposphere is a region of strong vertical mixing. However, the stable lapse rate in the stratosphere does not inhibit isentropic (horizontal) mixing and transport. There is evidence from aircraft and satellite constituent measurements that middle latitude air is entrained into the tropics at precisely the levels HSCTs are projected to fly. The rate of entrainment into the tropics is larger lower in the stratosphere, with transport barriers stronger in the middle stratosphere (Herman et al., 1998; Mote et al., 1998; Schoeberl et al., 1997; Hitchman et al., 1994; Tuck et al., 1997a).
Uncertainties are large in regards to understanding how much mixing occurs between high and low latitudes in the stratosphere. Specifically, how much isentropic mixing occurs will impact the stratospheric residence time of HSCT effluent. Significant transport of HSCT effluent from the middle latitude emission region to the ascending branch of the Brewer-Dobson circulation in the tropics would increase the residence time of HSCT effluent and likely increase its impact on ozone chemistry.
A means of determining how well models simulate the potential residence time of HSCT effluent is to compare modeled "mean age" of stratospheric air with that derived from long-lived tracer measurements. The "age" of an air parcel is defined as the average of the transit times from first entering the stratosphere for the ensemble making up the air parcel (Hall and Plumb, 1994). If the tropospheric concentration of a chemically inert species is increasing linearly with time, the age can be estimated by taking the time lag between the observation time and when the tropospheric mixing ratio was equivalent to the observed parcel mixing ratio. The age has been determined from several measurements of tracers with approximately linear trends. These include SF6 (Elkins et al., 1996; Harnisch et al., 1996; Patra et al., 1997; Waugh et al., 1997a), CO2 (Bischof et al., 1985; Andrews et al., 1999; Boering et al., 1996; Nakazawa et al., 1995; Schmidt and Khedim, 1991), CFC-115 (Daniel et al., 1996; Pollock et al., 1992), and HF (Russell et al., 1996). Results show that at 20 km, mean age varies from approximately 1 year in the tropics to approximately 5.5 years at high latitudes, while at 30 km it varies from 4 years in the tropics to 5–8 years at high latitudes.
The residence time of HSCT exhaust should be related to mean age, as both mean age and HSCT residence time depend on the strength of the mean meridional circulation and the isentropic mixing in the stratosphere between the tropics and middle latitudes. Because the stratosphere is not well mixed, and the HSCT emissions will be highly localized at middle latitudes, the mean age and HSCT residence time will not be identical. However, they should scale with one an-
other. Hence, if a specific model does not reproduce atmospheric mean age estimates well, it is unlikely to accurately model the HSCT effluent residence time. Models used for the assessment report tend to have younger mean ages than those deduced from measurements (Figure 1).
The major transport uncertainty in regards to assessing HSCT effluent is determining how much mixing occurs between the tropics and middle latitudes in the lower stratosphere. The amount of isentropic mixing affects the age of stratospheric air and the stratospheric residence time of HSCT exhaust. Progress, both in estimating the amount of tropical-middle latitude mixing and estimating the age of stratospheric air, has resulted from tracer measurements made during AESA-sponsored high altitude aircraft campaigns. PAEAN's interim review of AESA recommended that the program emphasize the analysis and interpretation of data from aircraft and satellites to better quantify the meridional and vertical transport in the stratosphere between 20 and 30 km. Since then, AESA-sponsored research has improved understanding of both meridional mixing and tropical upwelling in the lower stratosphere, but there are still significant variations between different estimates, and questions regarding the seasonal cycle of vertical transport and isentropic mixing. More tracer measurements on either side of the subtropical jet stream in the lower stratosphere, either from aircraft or a balloon platform, covering the seasonal cycle at a variety of longitudes around the globe, are needed to better quantify the amount of isentropic mixing between the tropics and middle latitudes. Additionally, differences in transport characteristics depending on the phase of the quasi-biennial oscillation (QBO) may require extensive measurements covering at least a 2-year period.
Impacts on Ozone
Gas-Phase Chemistry
An earlier report of this panel (NRC, 1998a) reviewed the chemistry of stratospheric ozone from a historical perspective and noted several outstanding issues relevant to the impact of aviation that the research program needed to address. In general, over the history of the AESA program, the relative importance of NOx chemistry has diminished due to recognition of the significance of aerosol reactions at mid-latitude that convert NOx to less reactive N compounds. Accordingly, the relative importance of HOx chemistry (which may be influenced by H2O emissions from an HSCT fleet) has increased. Significant progress has been made on several of the outstanding problems noted in the earlier report, but other problems remain unresolved. Trace gas data from POLARIS, the latest in a sequence of missions sponsored in part by AESA, have helped investigators improve the representation of gas phase kinetics in chemical models. New laboratory kinetic studies continue to provide improvements in rate constants applied in these models.
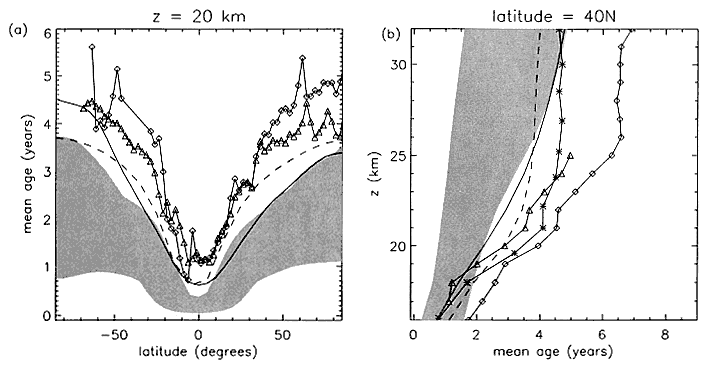
FIGURE 1
Comparison of mean ages from observations and models. (a) Latitudinal profile of in situ aircraft measurements at 20km, and (b) in situ SF6 and CO2 mean ages from an OMS balloon flight (September 1996, at 35N) and from SF6 whole air samples (September 1993, at 44N). The shaded region indicates the range of mean ages from a majority of models in the M&M II intercomparison (Park et al., in press), while the curves correspond to mean age profiles from the GSFC (dashed) and Monash1 (solid) models. The symbols correspond to mean age inferred from observations: in situ CO2 (triangles), in situ SF6 (diamonds), and whole-air samples of SF6 (asterisks). Adapted from Kawa et al., 1999.
Views on the accuracy of the representation of photochemistry used in the AESA assessment models have evolved over time. In some respects, model predictions were judged in good agreement with observations, e.g., for NOx/NOy determined in a stratosphere strongly perturbed by aerosol from the Mt. Pinatubo eruption. More recent measurements, long after the eruption, indicate important discrepancies that were not apparent earlier. On the other hand, the factor-of-two discrepancy between early observations and modeled ClO/Cly is now seen as a likely artifact of the measurements, because it is not apparent in more recent observations (Kawa et al., 1999). Discrepancies between observed and predicted daytime concentrations of HOx have been resolved to some extent by inclusion of heterogeneous hydrolysis reactions of chlorine and bromine nitrate. However, HOx observations at high solar zenith angle made during the POLARIS mission imply the existence of an additional unknown HOx source (Wennberg et al., 1999).
It should be recalled that each component of the photochemical model is dependent on the others so that the NOx/NOy discrepancy may reveal a problem in the underlying assumptions that allow good agreement for other components. An encouraging recent development is the interaction between studies of measurements of summer polar ozone chemistry during POLARIS and re-interpretation of the laboratory studies of the kinetics of OH + NO2—>HNO3 (De More et al., 1997). It is likely that revision of the relevant rate constant will resolve the NOx/NOy issue (Gao et al., 1999).
As a consequence of the developments described above, there is now improved confidence in the representation of chemistry of the nonpolar regions in the assessment models, i.e., earlier concerns about "missing chemistry" have abated, particularly for the region below ~22 km. However, in the tropics there is the potential for rapid upward transport of HSCT emissions to above 22 km. This is the region, about 22 to 26 km in most models, where HSCT NOx emissions are projected to switch from a net sink to a source of ozone (because, at lower altitudes, added NOx interferes with the destruction of ozone by hydrogen and halogen radicals). There remains a relative lack of complete in situ measurements of ozone chemistry precursors and products in this region, due to the difficulty of reaching this altitude with large, multi-instrument measuring platforms. Other potentially important issues affecting gas-phase ozone chemistry include discrepancies between the modeled and measured ClNO3/HCl ratio and uncertainties in the rate constant of the ClO + HO2 reaction. However, the modeling analysis of Khosravi et al. (1998) indicates that new reaction rate and observational data may help address some of these uncertainties.
A further problem is revealed by examination of Figure 9 of PAEAN's interim report (NRC, 1998a). The change with latitude of the measured ozone trend (due to halogen chemistry) is not in close accord with the assessment models, and it is unclear whether this discrepancy can be explained by deficien-
cies in the representation of polar processing, or whether some processes at the mid-latitudes are poorly represented or missing. It will be important to re-examine this data in light of the recent revisions in the models discussed above.
Some of the remaining issues pertinent to gas phase kinetics, including possible missing chemistry and final resolution of the NOx/NOy issue, may be addressed in the context of other research programs and may not require an aircraft-focused effort. Other questions, however, related to the sensitivity of particular regions of the atmosphere, and the coupling of chemistry to transport (such as the issue of how emissions are transported to the middle stratosphere in the tropics) are unlikely to be resolved outside of focused measurement programs, such as those specifically designed to examine the impacts of aviation emissions.
Overall, the AESA assessment provides a good guide to current understanding and future research priorities for gas-phase chemistry. One exception is the rather limited discussion of discrepancies that exist between trends in the observed ozone distribution and trends predicted by models. Such discrepancies could reflect important uncertainties in the models' representation of chemistry and dynamics.
Heterogeneous Chemistry
Heterogeneous chemistry and gas phase chemistry in the stratosphere are strongly coupled. For example, a fundamental element of the AESA program was developing an understanding of the significance of formation of nitric acid on background sulfuric acid aerosol at mid-latitude (Fahey et al., 1993). Incorporation of heterogeneous hydrolysis of chlorine and bromine nitrate has improved agreement between modeled and observed HOx concentrations. The possibility that mid-latitude particle distributions would be altered substantially by HSCT exhaust has a significant influence on ozone depletion projected by assessment models.
At the polar latitudes, heterogeneous chemistry contributes to ozone depletion through the conversion of slowly reacting haline compounds (primarily HCl and ClONO2) to more reactive forms, such as Cl2, that catalyze ozone destruction. Quantifying this sink for ozone requires characterizing the surface area and composition of the background and aircraft-emitted particle distributions and predicting the associated reaction rates on these surfaces at ambient conditions. Understanding the formation and microphysical properties of aerosol is a dynamic problem; lifetimes and transport of emissions in both the troposphere and the stratosphere need to be understood to determine the role of heterogeneous chemistry. One outstanding issue—quantifying increases in particle number and mass due to condensible emissions from aircraft—is discussed in an earlier section of the report. Below is a discussion of several other outstanding issues.
Reaction Rates
Reactions on polar stratospheric cloud surfaces have been shown to be responsible for catalyzing reactions leading to the formation of the ozone hole (Molina et al., 1987; Tolbert et al., 1987; Solomon et al., 1990); in particular, conversion of ClONO2 to Cl2 and HOCl, and of BrONO2 to HOBr via heterogeneous reactions are thought to provide catalysts for ozone destruction. Del Negro et al. (1997) have shown that at low temperatures HSCT contributions to particulate mass via condensation of nitrogen oxide emissions provide significant additional surface area on which heterogeneous reactions can occur. However, their calculations illustrate important uncertainties remaining in this mechanism. The first of these uncertainties is the rates of the relevant reactions, which are illustrated by Figure 2.
Recent laboratory studies of these reaction rates have identified kinetics for chlorine formation for some postulated types of polar stratospheric cloud particles. At equilibrium, the cloud conditions in the stratosphere are consistent with the formation of two-phase PSC particles in which a solid core is coated with a supercooled H2O/HNO3 liquid layer. The measured rate coefficients for ClONO2
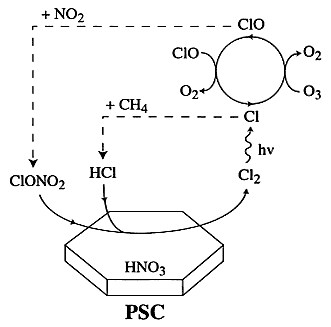
FIGURE 2
Schematic of the heterogeneous reactions occurring on polar stratospheric clouds (Reprinted with the permission from Zondlo et al., 1998. Copyright 1998, American Chemical Society).
hydrolysis (at 185 K) are about 100 times slower on particles with NAT and NAD compositions (type 1a and 1b PSC particles) than on pure water surfaces (Barone et al., 1997; Zondlo et al., 1998). The reaction rates depend strongly, however, on the PSC composition, temperature, and phase.
PAEAN believes that the importance placed on PSC uncertainties in the AESA assessment report is warranted, but the report failed to highlight the continued need for laboratory studies to unravel the fundamental kinetics of the complicated particle compositions that can exist under PSC-forming conditions. Although significant progress has been made in identifying the composition-and temperature-dependence of these processes, detailed laboratory studies in these areas need to continue in order to provide the required process information for modeling studies.
Background Particle Characterization and Processes
The impacts of particle emissions from an HSCT fleet cannot be predicted without having a good understanding of the background atmospheric composition. How large is the perturbation of the atmospheric aerosol that is introduced by aircraft emissions? To what extent will atmospheric processes such as cloud formation be affected by increased aerosol concentrations? Because the distribution of ambient aerosols varies greatly in the atmosphere (both vertically and horizontally), the location and transport of emissions is an important factor to consider. In addition, thermodynamically controlled processes, such as the formation of PSCs, may affect the total number of particles and the reactivity of particle surfaces. In order to understand the atmospheric context for aircraft emissions, it is necessary to identify the spatial distribution and characteristics of the stratospheric and upper tropospheric aerosol and to be able to predict processes such as the nucleation of cloud ice particles from aerosol particles. Currently, both of these issues remain largely unresolved (Kawa et al., 1999).
Recent progress has been made on identifying the composition of particles in the upper troposphere and lower stratosphere. Murphy et al. (1998) found that particles in the stratosphere were composed primarily of sulfuric acid and water, but also contained significant inclusions of iron, sodium, aluminum, potassium, and other elements, consistent with meteoritic material. Upper troposphere particles had more complex compositions, often dominated by organic species. Their results also indicated the presence of condensed mercury compounds directly above the tropopause in over half of the particles. Soot and crustal materials have also been identified in particles in the lower stratosphere by Sheridan et al. (1994).
In the absence of large volcanic eruptions, little is known about the amount of sulfate that crosses the tropopause, and about what controls the aerosol surface area in the lower stratosphere at mid-latitudes. Brock et al. (1995) have shown that the number of particles in this region can be explained by new particle
formation in the upper tropical troposphere, with subsequent transport into the stratosphere. These studies, together with the atmospheric transport processes described in Holton et al. (1995), support the need to study the tropical tropopause if one wants to understand the background stratospheric aerosol loading.
Efforts to characterize global aerosol distributions have benefited greatly from AEAP-supported programs, and some initial stratospheric aerosol climatologies have been developed (for instance, see Hitchman et al., 1994; Thomason et al., 1997). Yet many aspects of the stratospheric aerosol budget remain highly uncertain. The panel supports further assessment of the impact of volcanic eruptions and other sources of stratospheric aerosol on the global aerosol climatology, through aircraft and satellite measurements and field studies to help characterize the concentration and phase of consensible species entering the stratosphere in the tropical tropopause region.
In general, the details of how PSC particles form and grow in even the unperturbed atmosphere are not well understood. Del Negro et al. (1997) have found large fractions of nitric acid in particles collected during winter in polar regions, which is consistent with thermodynamic calculations that both solid nitric acid hydrates and ternary mixtures of water with nitric and sulfuric acid may exist under PSC-forming conditions (Carslaw et al., 1994). David et al. (1998) and Wegner et al. (1998) have observed PSCs composed of supercooled liquid solutions and NAT, whereas additional evidence suggests the presence of other species (such as NAD and amorphous nitric acid solids).
In describing the formation and evolution of PSCs and their role in ozone depletion, the relative importance of different atmospheric processes is unclear, and these gaps in understanding can significantly affect model predictions of ozone concentration. Models are formulated with differing assumptions about the factors controlling PSC formation, distribution, and lifetime, and there is not yet a consensus for including these mechanisms in models. As a result, the models show varying sensitivities to different atmospheric processes. Becker et al. (1998) found that uncertainties in the composition of PSC particles do not significantly impact the predicted ozone loss rate, but other studies show significant sensitivities of column ozone concentration to PSC surface reaction rates and particle size (Considine et al., 1999). Recent work by Carslaw et al. (1998a,b; 1999) has illustrated the potential role of leewaves in PSC formation. Chipperfield and Pyle (1998) have shown that ozone loss in polar regions is sensitive to the rates of denitrification and dehydration. Using a 2-D model that incorporated temperature probability distributions (but omitted denitrification processes), Grooß et al. (1998) predicted ozone depletion due to aircraft of about two percent, an effect 20 times larger than that predicted by Considine et al. (1994) in a study that was based on very similar assumptions. Using a three-dimensional model, Dameris et al. (1998) estimated column ozone depletion to be over four percent in the mid-latitudes and less than two percent in the tropics.
The importance of PSC processes was recognized in the 1998 AESA assess-
ment and has received significant attention in modeling and laboratory studies. In situ characterization of PSCs lags behind these advances, but is an important goal of the planned SAGE III Ozone Loss and Validation Experiment (SOLVE) campaign. The panel recommends that this effort continue to be supported by NASA, in order to resolve the significant uncertainties associated with the formation and processing of aerosol by PSCs and the consequent implications for ozone destruction.
Cirrus-Related Effects
For subsonic aircraft, there is concern that emissions could lead to a considerable increase in cirrus cloud cover. Normally the HSCTs fly sufficiently high that the issue of cirrus formation is not important. However, when HSCTs fly though the cold boreal vortex, their exhaust could lead to the formation of cirrus that could provide surfaces for heterogeneous reactions. AESA investigators have estimated that only about 1–3 percent of HSCT emissions would occur directly inside the polar vortex and thus concluded that this is likely to be an unimportant issue. However, they also recognized that there are periods when the vortex is ''displaced'' to lower latitudes and could thus encompass significantly more emissions. One also should consider that there are still uncertainties about the amount of mid-latitude emissions that are transported into the vortex, and that air masses processed on polar cloud surfaces could ultimately affect the chemistry of a disproportionately large region. Certainly, this issue requires further investigation.
Impacts on Climate
The Earth's climate system is extremely complex and climate models have only limited success simulating the details of this system. This makes it tremendously challenging to accurately model the impacts of an anthropogenic perturbation to this system. Emissions from HSCTs can cause radiative forcing in the stratosphere by modifying the O3 budget and increasing the background levels of sulfate particles and water vapor. The overall contribution this makes to global climate change is extremely difficult to quantify, as aviation's climate "signal" is estimated to be at least an order of magnitude smaller than the total anthropogenic climate signal, which itself is characterized by a substantial uncertainty.
Rind and Lonergan (1995) have made some initial attempts to simulate future climatic changes due to aviation using a general circulation model (Figure 3). The radiative forcing due to HSCTs has been estimated to be up to several tenths of a W/m2, due mainly to water vapor; and as the size of the HSCT fleet approaches 1,000 aircraft (in the year 2050), the contribution to radiative forcing approaches 2–7 percent of total anthropogenic forcing. These estimates, however, are based on many highly uncertain assumptions about the state of the
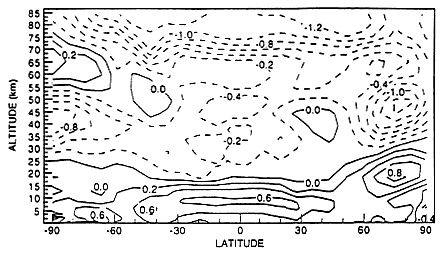
FIGURE 3
Model annual average temperature changes due to an increase in stratospheric water vapor of 0.2 ppm(m), as simulated with the GISS model (Rind and Lonergan, 1995; reprinted with permission from the American Geophysical Union).
future atmosphere and the consequent effects on climate. For instance, the relative importance of radiative forcing from HSCT emissions will depend upon feedbacks associated with stratospheric temperatures, water vapor concentration, and ozone levels (which, in turn, can be affected by anthropogenic halogen emissions, subsonic aviation emissions, and greenhouse gas buildup) (Forster and Shine, 1997; Ponater et al., 1996).
The climate response to HSCT emissions will undoubtedly be influenced by the vertical, latitudinal, and seasonal characteristics of these emissions; thus, the methods used to introduce these possible perturbations into coupled 3-D chemistry/climate models need to be carefully assessed. These models also require sufficient vertical and horizontal resolution and appropriate parameterization of processes such as stratosphere-troposphere exchange and gravity waves.
An additional challenge to consider is that the Earth's climate and atmospheric composition will evolve over the next 50–100 years, so simulating the climate response (of a year 2050 Earth atmosphere) to a fleet of HSCTs will require a modeling approach that accounts for this evolution. Efforts should also be made to include the various feedbacks that may exist within the climate system (e.g., Shindell et al., 1998). The preliminary elucidation of these feedbacks, even if only in a conceptual form, may assist in guiding further climate model development.
Modeling Issues
Atmospheric models can be used as diagnostic tools, to analyze what has happened, and as prognostic tools, to predict what will happen under a given set of circumstances. They can be as simple as box models or as complex as a three-dimensional, time-dependent, coupled ocean-atmosphere climate model that includes detailed chemistry. Models must be able to address atmospheric phenomena occurring over a wide variety of scales, which can make it very difficult to model accurately while still capturing the essence of the physical processes that occur. For instance, individual solar photons are absorbed on the molecular scale, whereas the energy that they deposit is transported by giant Rossby waves in the atmosphere, which can be thousands of kilometers long. Likewise, modeling the impact of a fleet of aircraft in the atmosphere encompasses scales from the sub-micron (describing the particles formed near and within the engine) to hundreds of kilometers in length (describing the mixing of the plume with the background atmosphere).
Overview of Aesa Modeling Efforts
The principal modeling tools used within the AESA program have been box models, 2D models and most recently, 3D models. Although the atmosphere is inherently 3D, if time and spatial scales for chemical species are carefully chosen, then box models can be used as powerful analytical tools. For instance, by analyzing radical species with short time constants over small spatial scales, and assuming that the species with longer time constants can be accurately prescribed using field data, box models have been used very successfully. Thus the AESA box models, combined with the powerful observational data set obtained by the measurement program and up-to-date photolytic and homogeneous gas phase kinetic data, have helped pinpoint many problem areas and, in turn, have pointed the way towards possible resolutions to these problems. They remain important diagnostic tools for many applications.
The principal modeling tool for assessment of the impacts of fleets of HSCTs has been the 2D model that represents a zonal average of the atmosphere. For many applications in the stratosphere, this provides a meaningful approximation to the real atmosphere. (For the troposphere, however, zonal averages are less useful, due to the heterogeneous nature of this region of the atmosphere.) In addition, 2D models are less computationally intensive than 3D models, and thus model simulations representing scenarios of future atmospheres and requiring 50–100 (model) year runs become feasible while still including detailed chemistry.
One of the important processes in the stratosphere for which longitudinal (zonal) information is necessary is the formation of polar stratospheric clouds. Most 2D models attempt to include this phenomenon by introducing parameterization schemes that approximate the very low temperatures that can develop in
the polar lower stratosphere locally (but would not be represented adequately by a zonally averaged temperature field). However, the various models yield very discrepant results, and thus it would seem that these parameterizations require more stringent testing if they are to be useful within the 2D context.
A related problem with the 2D models is a general lack of ability to adequately represent various dynamical barriers that occur in the stratosphere. Tropical air should remain more isolated than is the case in most of these models; also the polar vortex in each hemisphere does not remain isolated, so that highly processed polar air can be mixed to mid-latitudes. Some representation of these barriers can be (and has been) added empirically but does not allow for either the variability of the atmosphere or for changing atmospheric conditions associated with a variable climate.
For the most part, the 2D assessment models are run in a mode where climatological fields have been used, and this does not allow issues concerning the impact of natural variability to be addressed. Because of the limitations of 2D models, AEAP developed the Global Modeling Initiative (GMI), a 3D chemical transport model with modules for transport, gas phase chemistry, heterogeneous chemistry (as yet with no microphysics), and the inclusion of emissions from aircraft. Although the GMI domain extends from the surface into the mesosphere (determined by the vertical extent of available wind data), it has been built to focus on the stratosphere, so that tropospheric processes such as convection, deposition in the planetary boundary layer, rainout, and tropospheric gas sources are either very much simplified or missing altogether. However, the basic structure of the GMI would readily allow the inclusion (or improved treatment) of these processes where we have adequate understanding of them. This would be important for taking a more comprehensive approach to investigating the effects of aircraft on the atmosphere. The GMI is able to use prescribed winds from a variety of sources such as general circulation models (climate models), weather forecast winds, or assimilated winds. Thus, it is able to address many of the concerns that arise due to the limitations of 2D models such as natural variability and the impact of zonal structure.
Recent Progress
An essential aspect of any program to assess the impacts of an HSCT fleet is a careful analysis of the uncertainties involved. One of the important exercises that AESA has undertaken has been to seriously address this question, by looking at both the various modules of the 2D models (such as the photochemistry and transport) and at model properties through studies of the mean age of stratospheric air and the correlation of species.
On the chemistry front, there have been strenuous efforts to standardize and update the gas phase photochemistry in the 2D models, so that there is now little difference between the chemical schemes in the different models. Also, despite
improvements in laboratory measurements of rate coefficients (De More et al., 1997), their uncertainty still cannot be neglected in the context of uncertainty in the ozone perturbation due to aircraft. Studies (Stolarski et al., 1995) to directly assess the propagation of errors in the chemical kinetic data in 2D models have shown that it can contribute as much as one percent to the uncertainty in the ozone column change (when the range of ozone change itself is-2.5 to +0.5 percent). Almost all 2D models now include the effects of the sulfate layer on stratospheric chemistry; and one of the models is also capable of calculating the detailed microphysics of the evolution of the sulfate layer and its effects on chemistry.
Perhaps more disconcerting than the chemical differences is the difference in transport between the various models, both 2D and 3D. One study (Danilin et al., 1998) investigated the transport of an inert species within seven 2D and three 3D models (Figure 4). In this study, the distribution of the simulated inert species was quite different from model to model. Although certain characteristics were common, such as general shape and location of the maxima, the size of the maxima were very different.
Stratospheric mean age-of-air and the NOy lower stratospheric distribution have both been used for model-model and model-measurement comparisons. The general inability of models to accurately simulate the age-of-air was discussed earlier. Most models also tend to overestimate NOy in the lower stratosphere by a factor of two or three. This underscores the importance of the limited quantitative understanding of transport processes and does not lend confidence in the model estimates of future HSCT impacts.
One important part of the physics missing from both the 2D and 3D models used in this assessment is chemical-dynamical feedback. The models do account for photochemical feedbacks. For instance, if the ozone layer changes, this will affect photolysis rates, which in turn has consequences for many chemical reactions. However, the models use fixed temperature fields which will produce repeating wind fields and thus no feedback into the transport that redistributes material from the troposphere and from aircraft. Likewise, even if the total column ozone change caused by aircraft emissions is small, there can be significant changes in the ozone vertical profile, which will result in important changes in solar heating and thus in atmospheric dynamics.
This lack of feedback capabilities may be difficult to address quickly because most 3D climate models (or GCMs) have limited credibility in stratospheric dynamics. For example, few GCMs exhibit a quasi-biennial oscillation (QBO) or an isolated tropical upwelling region, and this will impact transport within the model. There is also the question of computing resources necessary to run these models. Stratospheric models with chemical and dynamical feedback have been developed; and in these it is necessary to implement scenarios that last 10–30 years or more in order to develop a climatology against which to measure the small changes expected due to HSCT fleets. This is just beginning to occur
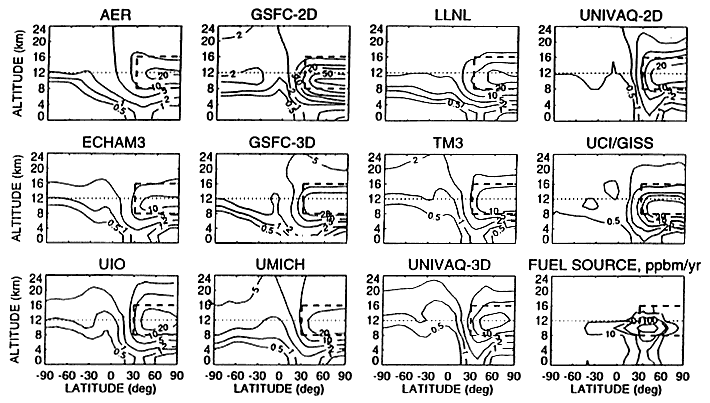
FIGURE 4
Zonally and annually averaged distribution of the fuel tracer in ng (tracer)/g (air) from the participating models. The fuel source is shown in the bottom right panel. The thick dashed line shows the region between 8–16 km and 30N–90N, the thick dashed line depicts the 12 km altitude (from Danilin et al., 1998; reprinted with permission from the American Geophysical Union.)
among various groups worldwide, but it is clearly the way of the future. An applied research program addressing potential environmental impacts to carry out its mandate will require substantial computer resources.
PAEAN suggested in its interim report (NRC, 1998a) that AESA should continue to support development of the GMI model but, in the short term, that they not undertake climate studies. However, at this point, the stage has been reached where a more general approach should be taken with climate/chemical models used to investigate variability and feedback. Diagnosis and analysis of the contemporary atmosphere can be addressed by continuing to use chemical-transport models, such as GMI, driven by objectively analyzed winds. Three other areas of particular concern are discussed below.
Combined Stratosphere and Troposphere Modeling
The IPCC report Aviation and the Global Atmosphere (IPCC, 1999) dealt with potential impacts of both the current subsonic fleet and a possible fleet of HSCTs. One conclusion from this report is that neither tropospheric nor stratospheric modeling can be treated in isolation. Subsonic aircraft fly at about 10-12 km, in a region particularly difficult to model and that involves the exchange of air between the troposphere and stratosphere. General understanding of this region of the atmosphere has improved during the last several years (e.g., Holton et al., 1995), but further clarification is still required. Also, it is unclear what impact the current subsonic fleet is already having on the stratosphere. For example: What fraction of the NOx emissions will end up being transported via the tropics into the stratospheric "overworld" or into the polar vortices? What fraction of the upper tropospheric air will exchange with stratospheric air at midlatitudes (Lelieveld et al., 1997)? Such uncertainties could potentially affect the magnitude of the HSCT stratospheric impact.
Changing Atmosphere Issues
The atmosphere in which a potential fleet of 500 or more HSCTs will fly will not be today's atmosphere. Carbon dioxide will have increased, methane and nitrous oxide are likely to have increased, and CFCs are expected to decrease. There will likely be an increased subsonic fleet perturbing the troposphere. One of the more relevant aspects of this changing atmosphere will be a decrease in stratospheric temperatures due to increased CO2. This will impact the dynamics of the stratosphere and both gas-phase and heterogeneous chemistry. Also, water vapor plays an important role in stratospheric chemistry. A changing atmosphere may change tropospheric conditions, and in particular, may modify the temperature of the tropical tropopause, thus affecting the lower stratospheric water vapor distribution.
Model Scale and Resolution Issues
A limitation of current 3D models is their vertical and horizontal resolution. Some studies appear to indicate that a vertical resolution of about 0.5 km may be necessary to properly resolve the transport in the vicinity of the tropopause (e.g., Austin et al., 1997; Untch et al., 1999). Greater horizontal resolution will be necessary in order to resolve filamentary structures, if it is found that knowledge of these structures is necessary to accurately calculate ozone loss. In addition, small-scale topographic structures (e.g., mountains) can induce gravity waves in the lower stratosphere. These waves can lead to temperature perturbations sufficiently large to induce PSC formation in a region where the synoptic temperatures would appear to preclude their appearance (Carslaw et al., 1998a). These effects must be accounted for in the next generation of models. Perhaps the next generation of models will either have a variable grid (Cote et al., 1998a,b) able to focus high horizontal resolution over areas of interest but remain global in nature, or alternatively, a series of nested models to allow a focus of scale on interesting regions.
Field Campaigns
AESA has contributed funding to a variety of field missions. These include: Stratospheric Photochemistry, Aerosols and Dynamics Expedition (SPADE); Airborne Southern Hemisphere Ozone Experiment/Measurements for Assessing the Effects of Stratospheric Aircraft (ASHOE/MAESA); Stratospheric Tracers of Atmospheric Transport (STRAT); and Photochemistry of Ozone Loss in the Arctic Region in Summer (POLARIS). Some AESA funding also supported the Airborne Arctic Stratospheric Expedition (AASE-II) in 1991. Additionally, AESA funds are committed to the SOLVE campaign, scheduled for winter 1999/2000. Of these, SPADE, MAESA, and STRAT were designed to specifically answer questions related to the effects of HSCTs.
Scientific questions addressed by the measurement campaigns included: (1) What ozone-related chemical processes are important in today's atmosphere and in a future atmosphere perturbed by HSCT emissions? (2) How consistent are observations with the current understanding of the HSCT-related chemistry? (3) What are the predicted atmospheric changes associated with HSCTs and what are the uncertainties in these perturbation predictions? It should be emphasized that even though the focus of these campaigns was to examine the potential impact of HSCTs flying in a future atmosphere, a great deal of basic understanding of present day stratospheric chemistry and transport has come from AESA-sponsored research. Differences in aircraft-measured tracer interrelationships across sharp edges in tracer fields in the subtropics (Murphy et al., 1993) contributed to the development of the "tropical pipe" model of stratospheric transport (Plumb, 1996). Combining the suite of in situ measurements collected during AESA
missions with simple chemical models allowed testing of the understanding of laboratory-determined reaction rates (Wennberg et al., 1994.)
SPADE was the first campaign specifically dedicated to AESA objectives. SPADE science flights using a NASA ER-2 (high altitude aircraft) took place during April and May 1993. Two objectives of the mission were: (1) to study chemical processes potentially affecting ozone at altitudes most strongly influenced by stratospheric aviation by making comprehensive measurements of radicals and reservoir species and (2) to examine distributions of tracers whose concentrations in the lower stratosphere vary on time scales ranging from months to years in order to estimate dispersal and removal of aircraft effluent emitted into the lower stratosphere. An overview of SPADE results is given in Wofsy et al. (1994). The aircraft was based in California, and flights covered the latitude range from 15N to 60N, and altitudes 15–20 km. SPADE measurements allowed empirical determination of rates of ozone recombination (Wennberg et al., 1994). Measurements of CO 2 (Boering et al., 1994) also allowed an estimate of the age-of-air in the lower stratosphere, and an estimate of transport times from the tropics to middle latitudes in the lower stratosphere. Encounters of the instrumentation with the ER-2 plume allowed estimates of emission indices for NOx, CO, and N2O (Fahey et al., 1995).
ASHOE/MAESA science flights took place over four deployments in 1994, with the NASA ER-2 based in Christchurch, New Zealand, with transit flights between California and New Zealand (Tuck et al., 1997b). The mission was designed to address questions about the causes of the year-round, mid-latitude ozone loss observed in the Southern Hemisphere. Specifically, flights were designed to examine the relative roles of vortex air transported to mid-latitudes and in situ loss induced by heterogeneous chemistry on sulfuric acid. Flights were also designed to study the exchange of air between the tropics and middle latitudes of both hemispheres, in order to aid in the assessment of HSCTs. One flight in particular also sampled the exhaust of a Concorde (Fahey et al., 1995). Additionally, measurements allowed the amount of transport between the tropical and mid-latitude lower stratosphere to be quantified (Volk et al., 1996).
STRAT science flights took place in 1995 and 1996. The main objective of STRAT was to make measurements of the morphology of long-lived tracers and dynamical quantities as functions of altitude, latitude, and season in order to help determine rates for global-scale transport and future distributions of HSCT exhaust emitted into the lower stratosphere. Flights covered the latitude range from the equator to 60N. Measurements taken allowed estimates of the age of stratospheric air to be made (Boering et al., 1996) along with estimates of mixing between low and middle to high latitudes in the Northern Hemisphere (Waugh et al., 1997b; Minschwaner et al., 1996; Newman et al., 1996).
The POLARIS campaign took place over three deployments of the ER-2 based in Fairbanks, Alaska, between April and September 1997. The scientific objective of the experiment was to evaluate the reduction of stratospheric ozone
over a range of altitudes and latitudes in the summer season of the Northern Hemisphere. Aircraft measurements of select species within the reactive nitrogen (NOy), halogen (Cly), and hydrogen (HOx) reservoirs, aerosols, and other long-lived species were made at middle to high latitudes in spring and summer in the lower stratosphere. A few tropical flights were also done during the September phase. POLARIS measurements allowed the effectiveness of the respective catalytic loss cycles of ozone to be calculated directly for sampled air parcels. These results, along with computer models of the atmosphere, meteorological data, and satellite and balloon observations were to be used to evaluate summer ozone changes due to chemistry and transport at high latitudes. A special section of the Journal of Geophysical Research, planned for late in 1999, will present results from the POLARIS mission.
The SAGE III Ozone Loss and Validation Experiment (SOLVE) is a measurement campaign that will take place during winter 1999/2000 and will consist of measurements from balloon, the ER-2 and DC-8 aircraft platforms, and ground-based instruments. The mission is designed to examine the processes controlling ozone levels in the Arctic high latitude region. Correlative data will also be acquired to validate the Stratospheric Aerosol and Gas Experiment (SAGE) III satellite measurements, also designed to quantitatively assess high-latitude ozone loss. This campaign should expand knowledge of heterogeneous chemical processes and transport processes during winter at high northern latitudes.
The field measurements of a variety of reactive and trace species in these AESA-sponsored aircraft campaigns have greatly improved understanding of both chemistry and transport in the upper troposphere and lower stratosphere. However, there are still important questions remaining that could be addressed through additional field work. Unraveling questions about the strength of transport barriers in the lower stratosphere (and determining whether there are preferential longitudinal locations for tropical-middle latitude exchange) may require additional field studies, including extensive measurement at a variety of longitudes and seasons on either side of the subtropical jet. There is also a need for focused study of the tropical tropopause, a region that is critical for controlling transport from the troposphere to the stratosphere, but that is largely uncharacterized due to the difficulties of in situ sampling in this region. Some chemistry questions also remain, such as why models underestimate the measured NOx/NOy ratio in the summer lower extra-tropical stratosphere. Additional field measurements (along with laboratory studies) would likely help determine whether there is missing chemistry in the assessment models.



























