3
Metals and Radionuclides: Technologies for Characterization, Remediation, and Containment
Many different types of inorganic contaminants are present in groundwater and soil at Department of Energy (DOE) facilities. Table 1-3 presents the results of several studies that ranked metal and radionuclide contaminants according to frequency of occurrence. In many instances, contaminants occur as mixtures of metals and radionuclides, organic complexing agents, and organic solvents. These lists of frequency of occurrence indicate the types of contaminants encountered but do not provide information about toxicity, risk, and cost-benefit of cleanup. DOE's Subsurface Contaminants Focus Area (SCFA) singled out several inorganic contaminants of concern in an informal ranking procedure based on prevalence in the weapons complex, mobility, and toxicity, as shown in Table 3-1. This chapter focuses on key contaminants from this list (U, Pu, 137Cs, 90Sr, Ra, Th, Tc, and Cr). The chapter does not review remediation techniques for 3H because this element is generally treated using containment and natural attenuation. The chapter also does not assess remediation technologies for mercury because it is not prevalent throughout the DOE complex; it is found at Oak Ridge, primarily in sediment and surface water, not in groundwater.
FACTORS AFFECTING RISKS OF METAL AND RADIONUCLIDE CONTAMINATION
Persistence is one of the key factors considered in assessing the risk associated with a chemical in the environment. Many organic
Table 3-1 Inorganic Contaminants of Particular Concern at DOE Sites
|
Element |
Mediuma |
Priorityb |
Site |
|
Tc |
GW |
High |
Portsmouth |
|
Cr(VI) |
GW |
Medium-High |
Hanford |
|
|
Soil |
Medium |
Hanford, Sandia National Laboratories, White Sands |
|
U |
GW |
Medium-High |
Fernald, Oak Ridge, Rocky Flats |
|
|
Soil |
Medium |
Rocky Flats, Fernald, Oak Ridge |
|
137Cs |
Soil |
Medium-High |
Hanford, Savannah River Site |
|
90Sr |
GW |
Medium-High |
Hanford |
|
|
Soil |
Medium |
Hanford |
|
Pu |
Soil |
Medium |
Mound, Rocky Flats, Nevada Test Site |
|
Ra |
Soil |
Medium |
Uranium mill tailings sites |
|
3H |
GW |
Medium-Low |
Savannah River Site, Hanford, Brookhaven |
|
Hg |
GW |
Low |
Oak Ridge |
|
|
Soil |
Low |
Oak Ridge |
|
Th |
Soil |
Low |
Uranium mill tailings sites |
|
a GW groundwater b Priority based on prevalence in DOE complex, mobility, and toxicity (according to a survey by the SCFA). |
|||
compounds biodegrade, reducing the potential for human and ecological exposure over the long term. Metals, on the other hand, are infinitely persistent. Radionuclides undergo natural radioactive decay that, for some compounds (such as tritium), may significantly reduce risks over relatively short time periods. However, for other radionuclides (including various isotopes of Tc, U, Pu, and Th), half-lives are very long, meaning that risks posed by the presence of these compounds will persist for a very long time. As shown in Table 3-2, half-lives for radionuclides vary quite significantly depending on the isotopes present.
The potential for humans or sensitive ecosystems to be exposed to metals and long-lived radioactive materials is strongly affected by a number of factors that must be considered in assessing these contaminants. Some metals and radioactive contaminants have more than one oxidation state, which differ in mobility and toxicity (see Box 3-1). Like organic compounds, metals and radioactive contaminants can partition into organic matter present in soils. They also can be sorbed by other soil components, including cation exchange sites and metal oxides, and they can precipitate. Because of the mul-
Table 3-2 Half-Lives of Radioactive Compounds Common at DOE Installations
|
Radionuclide |
Isotope |
Half-Life (years) |
|
Tc |
99 |
2.12 × 105 |
|
U |
234 |
2.47 × 105 |
|
|
235 |
7.1 × 108 |
|
|
238 |
4.51 × 109 |
|
Cs |
137 |
30 |
|
Sr |
90 |
28 |
|
Pu |
238 |
86 |
|
|
239 |
24.4 × 103 |
|
|
240 |
6.58 × 103 |
|
3H |
|
12 |
|
Th |
228 |
1.91 |
|
|
230 |
8.0 × 104 |
|
|
232 |
1.41 × 1010 |
|
SOURCE: Weast, 1980. |
||
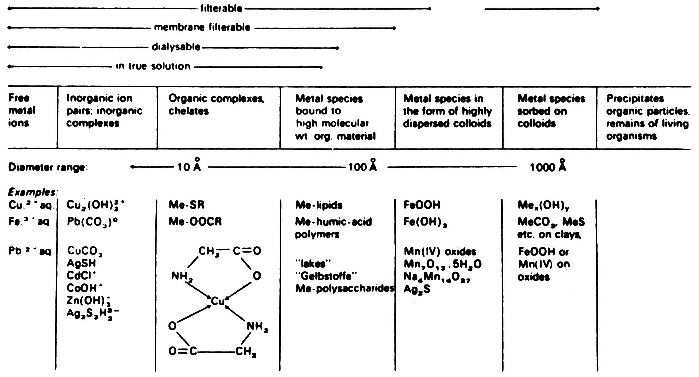
tiple possible associations of a metal or radioactive contaminant with a soil, determination of the total contaminant concentration is unlikely to provide sufficient information to allow valid assessments of potential risk or amenability to remediation by specific processes. Figure 3-1 summarizes the types of species in which metals may be present in the environment.
|
BOX 3-1 Oxidation States Many metal and radionuclide contaminants exist in the environment in multiple forms. For example, chromium is found as Cr(VI) (+6 oxidation state) under environmental conditions known as oxidizing conditions and as Cr(III) (+3 oxidation state) under reducing conditions. Oxidizing conditions generally prevail in the absence of biodegradable organic matter and in near-surface environments. Reducing conditions generally prevail when an excess of biodegradable organic matter is present and the oxygen supply is limited. The different oxidation states of metals and radionuclides may exhibit greatly different chemical behavior. For example, in simple, dilute, neutral aqueous solutions, the predominant form of Cr(VI) is the highly soluble, mobile oxyanion CrO42-, while the predominant form of Cr(III) is the highly insoluble solid Cr(OH)3. Metals in different oxidation states also have different risks. For example, Cr(VI) is highly toxic, whereas Cr(III) is relatively harmless to humans. |
Geochemical Characteristics of Metal and Radionuclide Contaminants: Effects on Treatment Options
Because metal and radionuclide contaminants are generally non-degradable except by radioactive decay, treatment technologies must involve some form of mobilization or immobilization for removal or containment, respectively. Thus, solubility and the propensity for complexation in solution or sorption to surfaces are key properties to consider when evaluating treatment options. Table 3-3 summarizes these properties for the contaminants that are the focus of this study.
Table 3-3 Speciation of Inorganic Contaminants
|
A. Elements with Multiple Oxidation States |
||
|
Element |
Oxidizing Conditions |
Reducing Conditions |
|
Tc |
Tc(VII): TcO4-, high solubility, very weak adsorption |
Tc(IV): TcO2nH2O(s); low solubility |
|
Cr |
Cr(VI): CrO42-, HCrO4-, Cr2O72- depending on total Cr concentration and pH value; high solubility, weak adsorption |
Cr(III): Cr(OH)3(s); low solubility |
|
U |
U(VI): UO22+ high solubility, moderate sorption; highly soluble, weakly sorbing anionic U(VI) carbonate complexes may predominate in waters with high carbonate concentrations |
U(IV): UO2(s), low solubility |
|
Pu |
Pu(VI), PU(V), Pu(IV): Pu4+, PuO2+, PuO2+ complex, redox-active aqueous chemistry with moderate solubility and moderately sorbing species |
Pu(IV): PuO2(s), moderately low solubility |
|
B. Elements with Single Oxidation States |
||
|
Element |
Speciation in Water |
|
|
Cs |
Cs(I): Cs+, essentially no hydrolysis, moderate adsorption, no oxidation-reduction activity |
|
|
Sr |
Sr(II): Sr2+, essentially no hydrolysis, moderate to weak adsorption, no oxidation-reduction activity |
|
|
Ra |
Ra(II): Ra2+, essentially no hydrolysis, moderate to strong adsorption, no oxidation-reduction activity |
|
|
Th |
Th(IV): Th(OH)n(4-n)+, strong hydrolysis, moderate solubility, very strong adsorption |
|
As shown in Table 3-3, Tc, Cr, U, and Pu exhibit multiple oxidation states, of which the reduced forms are quite insoluble in water. The oxidized forms Tc(VII) and Cr(VI) are both anions in water and generally sorb weakly to the negatively charged surfaces typically encountered in nature. The alkali and alkaline earth ions Cs+, Sr+2, and Ra+2 do not exhibit redox activity and are hard cations, which do not hydrolyze strongly, are not expected to sorb strongly to oxide surfaces, and are subject to competition in any complexation reaction by other alkali and alkaline earth ions present at much higher concentrations (e.g., Na+, Ca+2). Thorium(IV) does hydrolyze strongly in water and adsorbs rather strongly to oxide surfaces.
Table 3-4 summarizes treatment technologies for different classes of inorganic contaminants, and Table 3-5 summarizes technologies for different media. Box 3-2 provides a glossary of remediation technology terms. These technology options are discussed in more detail later in the chapter.
CHARACTERIZATION OF METAL AND RADIONUCLIDE CONTAMINATION
Because speciation controls the environmental transport and risks of metals and radionuclides, it is as important to characterize as the total amount (or total concentration) of the contaminant. Traditionally, concentrations of metals and radionuclides have been determined by taking samples of groundwater from monitoring wells or soil from borings to the laboratory for analysis. A variety of techniques, from computer models, to spectroscopic and electrochemical analyses, to sequential extraction methods, are available to determine speciation of metals and radionuclides in samples in a laboratory. More recently, techniques have been developed for measuring metal and radionuclide concentrations in situ, without bringing samples to the laboratory. The advantages of in situ analysis include reduction in time and cost of site characterization as well as reduction of exposure of personnel to hazardous contaminants. The following brief descriptions of laboratory and in situ techniques for characterizing metals and radionuclides are intended only as an introduction to this complex subject; the references cited with the descriptions provide technical details on carrying out these analyses.
Ex Situ Analysis for Speciation
Speciation of metals and radionuclides based on analysis of laboratory samples can be determined computationally or experimentally.
Table 3-4 Treatment Technology Options for Different Classes of Inorganic Contaminants
Table 3-5 Technology Types Applicable to Different Contaminated Media
|
Context |
Solidification, Stabilization |
Containment |
Biological and Chemical Reactions |
Separation, Mobilization, and Extraction |
|
Surface soils, sediments, and sludge |
Pozzolanic agents Cement Vitrification |
Capping |
Phytoremediation Chemical reduction |
Solvent extraction Soil washing • Acids, bases, and chelating agents • Surfactants and cosolvents |
|
Unsaturated zone |
Pozzolanic agents Cement Vitrification |
|
Chemical reduction Microbial reduction |
Electrokinetic systems Soil flushing • Acids, bases, and chelating agents • Surfactants and cosolvents |
|
Saturated zone |
|
Grout walls Slurry walls Sheet pile walls |
Chemical reduction Permeable-reactive barriers • Fe0 • Microbiological • Enhanced sorption • Ion exchange |
Electrokinetic systems Soil flushing • Acids, bases, and chelating agents • Surfactants and cosolvents |
|
High-concentration source areas in the saturated zone |
|
Grout walls Slurry walls Sheet pile walls Pump-and-treat systems |
Chemical reduction |
Electrokinetic systems Soil flushing • Acids, bases, and chelating agents • Surfactants and cosolvents |
|
BOX 3-2 Remediation Technologies for Inorganic Contaminants* Stabilization-Solidification and Containment Technologies In Situ Precipitation or Coprecipitation. A permeable reactive barrier (see definition below) that causes the precipitation of a solid (usually carbonate, hydroxide, or sulfide mineral) to maintain a toxic metal in an immobile form. Formation of solid phases is controlled primarily by pH, redox potential, and concentrations of other ions. Pozzolanic Agents. Cement-like materials that form chemical bonds between soil particles and can form chemical bonds with inorganic contaminants, decrease permeability, and prevent access to contaminants. The most common pozzolanic agents are portland cement, fly ash, ground blast furnace stag, and cement kiln dust. Vitrification. Melting of contaminated soil to form a glass matrix from the soil, either in place (in situ vitrification) or in a treatment unit. Nonvolatile metals and radioactive contaminants become part of the resulting glass block after cooling. Organic contaminants are either destroyed or volatilized by the extremely high temperatures. The method is generally expensive due to the large energy requirements. Biological Reaction Technologies Phytoremediation. Removal of contaminants from surface soil through plant uptake. Subsequent treatment of the plant biomass may be necessary. Chemical Reaction Technologies Enhanced Sorption. A passive-reactive barrier (see definition below) that creates zones that cause contaminant sorption, either microbiologically (biosorption) or chemically (through materials with surface complexation, ion exchange, or hydrophobic partitioning properties). In Situ Redox Manipulation. The injection of chemical reductants into the ground |
Experimental methods can be further subdivided into those that provide characteristics of the contaminant and those that provide information on specific chemical contaminants.
Computational Methods
Chemical equilibrium computer programs are useful for computing the distribution of species in samples for which total concentrations of metals and ligands (ions or molecules that can attach to metals) have been measured, provided appropriate stability constants are available (Nordstrom et al., 1979). Commonly used programs include MINTEQA2 (Allison et al., 1991) and MINEQL+ (Schecher
|
to create reducing conditions in an aquifer, which will then lead to reduction and immobilization of certain contaminants in groundwater. Permeable-Reactive Barriers. Permeable containment barriers that intercept contaminant plumes and remove contaminants from groundwater solution through chemical and/or biological reactions within the barrier. Zero-Valent Iron Barrier. A passive-reactive barrier (see definition above) that creates strongly reducing conditions, resulting in hydrogen generation. Dissolved chlorinated solvents (chlorinated ethanes, ethenes, and methanes) are chemically degraded at relatively rapid rates. Some metals form relatively insoluble solids at low redox potential and can be treated with this method. Separation, Mobilization, and Extraction Technologies Electrokinetics. The movement of water and/or solutes through a porous medium under the influence of an applied electric field. Electromigration is the migration of ionic species through a soil matrix. The process can function in both saturated and unsaturated environments. Electroosmosis is the movement of pore water through a fine-grained matrix. This technique has long been understood as a means to control water movement in fine-grained media and is currently being investigated to remove contaminants at waste sites. Soil Flushing. An in situ process that uses chemical amendments and fluid pumping to mobilize and recover contaminants (see also cosolvent flushing and surfactant flushing). Soil Washing. An ex situ process in which contaminated soils are segregated and then washed with a water-based solution. Generally, soil fines have a high concentration of contaminants, while coarse materials may be sufficiently clean that contaminant concentrations are below action levels, allowing coarse materials to be disposed of separately. Once fines are separated from coarse soils, the fines may be disposed of directly or extracted. |
and McAvoy, 1992). Systems in which the principal ligands are inorganic and that contain relatively uncomplicated solid surfaces are most amenable to modeling with computations.
Considerable research has been conducted to describe the binding of metal ions to oxide surfaces. Applicable surface complexation models that can be used to describe this binding based on electrical double-layer models are discussed by Dzombak and Morel (1990) and Stumm (1992).
The description of metal complexation with natural organic matter (NOM; for example, humic substances) is much more complicated. NOM is an unresolvable mixture of a very large number of compounds varying in their properties, including their ability to bind
metal ions. Several approaches have been proposed for modeling the way in which metals form complexes with NOM and humic substances. These include gaussian distribution models (Perdue and Lytle, 1983) and multiple discrete site models (Fish et al., 1986). Tipping (1994) has presented a model, using multiple classes of organic matter reaction sites, that is able to predict relatively accurately metal cation and proton binding to naturally occurring organic matter.
Because of the heterogeneity of soils, metals can be associated with many types of surfaces in a single soil. Recently, Radovanovic and Koelmans (1998) presented a model to predict the binding of a series of cationic metals to suspended particles in natural waters as a function of the characteristics of the aqueous and solid phases. Because similar properties control the speciation and partitioning of metals between soil and pore water, this or similar models could be applied to soil samples.
Experimental Methods
Perhaps the most fundamental physical means for determining the speciation of metals is physical separation of the dissolved and particulate phases. A number of procedures are available for this separation (Bufflap and Allen, 1995). The separation processes are subject to significant error, particularly as a result of incomplete separation of particulate and dissolved phases.
Analysis of chemical species is possible by chromatographic, spectroscopic, and electrochemical methods. The separation and quantitation of ethylenediaminetetraacetic acid (EDTA) and other complexes of metals has been achieved by ion chromatography (Hajós et al., 1996) and by capillary electrophoresis (Buergisser and Stone, 1997). Several electrochemical methods are widely used for the analysis of trace metals in natural waters and in soil solution. Among these are selective ion electrodes, anodic stripping voltammetry, and cathodic stripping voltammetry (Florence, 1989; Van den Berg, 1984). All are capable of determining submicrogram-per-liter concentrations of metals and can be used in titrations to determine the concentration of available binding sites for a metal and the strength of the complexation reaction.
Sequential extraction procedures, most commonly that of Tessier et al. (1979), frequently are used to correlate the presence of metal species in samples with observed effects, including toxicity, bioavailability (availability for uptake by living organisms), and mobility. These procedures use increasingly strong extractants to release trace metals
associated with (1) exchangeable, (2) carbonate, (3) metal oxide or reducible, (4) organic and sulfide, and (5) residual mineral phases. The procedures have been criticized as being unable to provide accurate information about the associations of trace metals (Martin et al., 1987; Kheboian and Bauer, 1987; Rapin et al., 1986; Rendall et al., 1980; Sheppard and Stephenson, 1997; Tipping et al., 1985). Criticisms have focused mainly on the application of the procedures to the assessment of associations of cationic metals with specific solid phases. Sheppard and Thibault (1992) reported mixed success in using the Tessier extraction scheme for the soil litter layer, a sandy soil, and a clay subsoil that had been contaminated with Cr, Cs, Mo, Np, Pb, Tc, Th, and U. They found that the selective extraction procedure did not work well either for organic-rich soils or for anions such as TcO4-.
Single-extractant procedures are also widely used to estimate metal availability for uptake by plants. Among the extractants that have been used are diethylenetriaminepentaacetic acid (DTPA), EDTA, acetic acid, and the mineral acids HNO3 and HCl (Adriano, 1986). A linear relationship between the logarithm of the concentration of the metal taken up by the plant and that extracted from soil has frequently been reported (Browne et al., 1984). The quality of predictions decreases as the soil chemistry becomes diverse. Allen and Yin (1998) suggest that the correlation failure occurs because these procedures relate to the binding phases for the metal rather than the strength of metal binding.
In Situ Chemical Analysis
One method of in situ site characterization that is increasingly being used is the incorporation of contaminant sensors into penetrometers, which are rods that are pressed into the ground. Traditionally, penetrometers were fitted with sensors to measure tip and sleeve resistance for the determination of soil stratigraphy. More recently, sensors have been incorporated into penetrometers to measure concentrations of metals, radionuclides, and other substances. These sensors include those for laser-induced breakdown spectroscopy, x-ray fluorescence (for elemental analysis), and a gamma-ray spectrometer for gamma-emitting contaminants. Each of these methods has been successfully field-tested (Ballard and Cullinane, 1997).
DOE has developed another promising technology for radiological characterization of soil surfaces, particularly for use in association with excavation activities. The technology, known as the dig-face sensor, depends on an appropriate sensor, a precise x-y-z positioning
system, a method to move the sensor systematically over the area to be investigated, and a data reduction and display device (Josten et al., 1995). Advantages include quality real-time data for better decision making, a potential to reduce the amount of material requiring excavation, reduction in hazard to personnel, and reduction in the risk that contaminants will inadvertently be left in place. The system has been successfully applied for 232Th, 227Ac, 137Cs, and 231Pu contamination in field tests. (See Chapter 5 for more information on this technology.)
PHYSICAL BARRIERS FOR CONTAINING CONTAMINANTS
Barrier systems are among the most widely used technologies for managing contaminated sites. A wide variety of designs has been developed to meet particular needs. Physical barriers can be grouped into three categories: (1) vertical barriers, (2) surface caps, and (3) emplaced horizontal barriers (bottoms).
Vertical barriers can provide rapid and significant risk reduction by isolating the contaminant source from the flowing groundwater. They also can provide opportunities for enhanced remediation by controlling groundwater hydraulics and/or allowing chemical treatment of the aquifer that would not be possible without physical containment. In the context of many DOE-related groundwater problems, another important characteristic of vertical barriers is their potential to stabilize contamination over periods of years to decades. Such contaminant stabilization allows time for chemical degradation, radioactive decay, or the development of improved remediation technologies.
Surface caps, such as those used on modern landfills, are also widely used at many DOE facilities to control infiltration and water movement through contaminated soils. Because of their location at the surface, caps generally have a more sophisticated layered structure than vertical barriers and can be instrumented much more easily with water collection systems and sensors. Their role in groundwater contaminant transport is limited to reducing leaching from the vadose zone to the groundwater. As a consequence, surface caps are not discussed further in this chapter.
Like surface caps, horizontal barriers are widely used beneath modern municipal, hazardous waste, and DOE landfills. Emplacement of horizontal barriers beneath existing uncontained sources of groundwater contamination is likely to become more common. These constructed ''bottoms'' are potentially quite important in the context of DOE's dense nonaqueous-phase liquid (DNAPL) and metal con-
tamination problems because they may be able to minimize downward migration of the contaminants.
For additional detail on vertical and bottom barriers beyond that presented below, see Rumer and Mitchell (1995) and DOE (1997).
Vertical Barriers
In conceptually simple form, a vertical barrier can be used to completely surround a source of groundwater contamination with an essentially impermeable wall. Ideally, this wall can be physically connected to a naturally occurring horizontal barrier (for example, an aquifer confining unit) and result in isolation of the contamination source from the groundwater. In practice, all barriers and confining units have some level of permeability, and as a consequence, water can move into or out of the contained aquifer. This fact, and the desire to eliminate any contaminant movement from the source, frequently means that a small-scale pump-and-treat system is coupled with the barrier to maintain a constant inward hydraulic gradient across all faces of the barrier. If containment is not complete (for example, if there is no confining unit), pumping at significantly higher rates may be necessary to maintain inward gradients.
Another hydraulic aspect of vertical barriers is that the presence of the barrier may affect the surrounding groundwater flow. For example, if a site is completely surrounded by a vertical barrier, ground-water will "mound up" at the upgradient edge of the barrier. As a consequence, groundwater hydraulically upgradient of the site will be deflected around the site. Although this may not affect the site directly, it could affect adjacent sites.
Partial barriers that do not completely surround the contaminant source area also can be used for containment. Partial barriers are used primarily for plume capture to prevent off-site migration. As with fully contained systems, the presence of the barrier will cause mounding of the water table and lateral and potentially downward diversion of the plume. As a consequence, barriers constructed perpendicular to groundwater flow will have to extend upgradient for some distance to ensure containment. In addition, changes in regional flow direction can cause the contaminant plume to shift and miss the barrier. For these reasons, extending the upgradient portions of the barrier (the "wings") to the point where they are cross-gradient from the source may be necessary. Thus, modeling ground-water flow and contaminant transport in relation to barriers is a critical component of barrier design (Rabideau et al., 1996; Russel and Rabideau, 1997; Smyth et al., 1997a).
Geologic Conditions for Barrier Emplacement
Geologic conditions must be suitable for emplacement of vertical barriers, or conversely, the installation method must be compatible with geologic conditions at the site. From a geologic perspective, the important parameters will include aquifer permeability, heterogeneity, the presence of bedrock or large cobbles, the presence of an aquitard, and the depth to the bottom of the contaminated zone.
All techniques used for emplacement of vertical barriers have overall depth limitations, even in geologically favorable conditions. For example, excavators have an operational depth limit of approximately 15 m (50 ft), and sheet pile can generally be driven only to depths of 30 to 45 m (100–150 ft). For other techniques such as deep-soil mixing or jet grouting, the necessity to interlock barrier panels at depth may ultimately limit the depth to which they can be applied. At many sites, aquifer characteristics may limit the applicability of many or all barrier techniques. For example, fractured bedrock aquifers are not well suited for most barrier technologies. Similarly, installing barriers in aquifers consisting of large cobbles may be difficult.
Effects of Contaminant Properties and Site Conditions
In addition to hydrologic and geologic conditions, the success of vertical barriers may depend on a number of other processes related to contaminated properties and site conditions. These can include physical processes, such as molecular diffusion, and chemical processes, such as sorption, ion exchange, dissolution-precipitation, and oxidation-reduction. In some cases, the presence of the waste may cause geochemical changes that affect barrier integrity (for example, shrinking and cracking of the barrier due to geochemical weathering or the presence of solvents or destruction of the barrier caused by the presence of strong acids, bases, or solvents). As a consequence, understanding possible interactions among the barrier, the geochemistry of the subsurface, and the contaminants is essential.
Installation Methods
The choice of installation methods for vertical, low-permeability barriers depends on a number of factors. Table 3-6 summarizes five different categories of installation procedures.
Trenching Trenching is the excavation of native materials and their replacement with lower-permeability media such as clayey soils (see Figure 3-2). This procedure is usually accomplished with exca-
Table 3-6 Installation Procedures for Vertical Barriers
|
Wall Type |
Width, m (ft) |
Depth, m (ft) |
Unit Cost, $/m2 ($/ft2) |
Production Rate, m2/10 h (ft2/10h) |
|
Soil bentonite trench |
0.6–0.9 |
(2–3) |
24 (80) |
22–86 (2–8) 230–1,400 (2,500–15,000) |
|
Cement bentonite trench |
0.6–0.9 (2–3) |
24 (80) |
54–190 (5–18) |
93–740 (1,000–8,000) |
|
Deep soil mixing (DSM) |
0.76(2.5) |
27 (90) |
65–160 (6–15) |
93–280 (1,000–8,000) |
|
DSM structural |
0.76(2.5) |
27(90) |
160–320 (15–30) |
93–280 (1,000–3,000) |
|
Jet grouting |
0.46–0.91 (1.5–3) |
61 (200) |
320–860 (30–80) |
28–230 (300–2,500) |
|
Cryogenica |
0.30–3. |
(1–10) |
61+ (200+) |
650(60)— |
|
Sheet pile |
<0.03 (<0.1) |
30 (100) |
160–430 (15–40) |
93–280 (1,000–3,000) |
|
a From Peterson et al., 1996. SOURCE: Adapted from Filz et al., 1996. |
||||
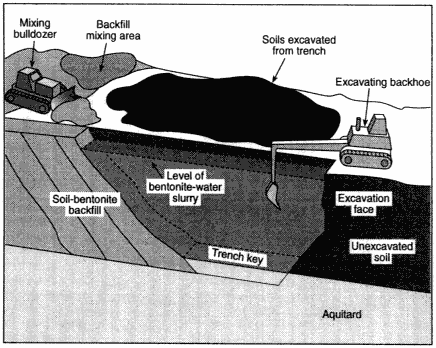
Figure 3-2
Installation of a soil-bentonite cutoff wall. Source: Rumer and Ryan, 1995.
vators or shovels, depending on the depth and materials. Below the water table the trenches generally have to be kept open until back-filled. In some applications, excavation and backfilling are carried out almost concurrently. In others, the trench is held open by mechanical supports or by viscous fluids such as guar gum.
Trenching is best suited to large sites in which the contaminant zone can be surrounded while a minimum amount of contaminated soil is excavated and at which complete treatment of the contaminated materials would be prohibitively expensive. Like other physical containment methods, trenched barriers have limited application at depths greater than 30 m (100 ft). Unfortunately, this depth limitation restricts the use of trenched barriers as a treatment option at a number of DOE facilities.
Pressurized Injection Pressurized injection involves injecting grout into the subsurface under pressure. In general, pressurized injection
barriers are constructed by intersecting short sections of grout wall (e.g., columns or sheets). The final composition of the barrier is a mixture of the native materials and the injected material. Injection can occur at very high pressure, in which the soil structure is disrupted and the soil is mixed with the injected material (jet grouting). Injection can also occur at lower pressure using low-viscosity materials that move into the existing soil structure (permeation grouting). Pressurized injection is applicable when conventional trenching is not practical, either because of space constraints or because excavation of contaminated soils cannot be accomplished. An advantage of pressurized injection relative to trenches is that in general, a substantially smaller volume of soil has to be excavated. However, constructing an intact pressurized injection barrier is generally much more difficult than constructing a conventional trench. To date, there have been relatively few thorough examinations of the permeability of pressure-injected barriers. Based on work to date it appears that the permeabilities of pressurized injection barriers are not as low as those of conventional trenched barriers because of the difficulty in ensuring complete connection of the barrier sections at depth.1
Driven Rigid Barriers (e.g., sheet pile) To date, most environmental applications of driven sheet pile have occurred in research settings, where these barriers have proven useful for controlled field studies (Smyth et al., 1997b). More recently, the use of driven sheet pile is increasing in "funnel-and-gate" applications of permeable barriers, in which the sheet pile directs contaminated groundwater into a wall section containing media designed to react with the contaminants. Sheet pile used for these purposes is specifically designed and includes grout or gaskets to seal joints and minimize leaks.
A number of potentially significant limitations to the use of driven sheet piles remain. Their use is limited to locations at which the sheets can be driven (e.g., areas with no cobbles or boulders) and to depths of approximately 30 m (100 ft). In some cases driving sheet piles has caused land subsidence and foundation damage. The process of driving may also mobilize contaminants and provide pathways for vertical contaminant transport. In addition, there is some concern that corrosion could limit the lifetime of steel sheet pile,
although corrosion is generally significant only under oxidizing conditions and subsurface contamination frequently creates reducing conditions in the aquifer. Finally, sheet pile is a relatively expensive barrier technology at present. Nonetheless, it has a number of potentially important advantages. First, installation is rapid, and soil excavation is not required. Perhaps equally as important, sheet pile can be removed when it is no longer needed. In this context, sheet pile couples well with aggressive in situ treatment technologies, such as chemical flooding systems (discussed later in this chapter).
Deep-Soil Mixing Deep-soil mixing involves mixing contaminated soil with chemical agents to treat contaminants directly and to improve treatment by homogenization (i.e., removal of heterogeneities) (Siegrist et al., 1995; Korte et al., 1997) (see Figure 3-3). Its use for reduction of the overall permeability of the soil by the addition of cements or grouts is uncommon (Filz et al., 1996).
Cryogenic Barriers Cryogenic barriers are formed by freezing the soil to prevent transport of water and contaminants. As with driven sheet pile, these barriers were initially used in the construction industry, and their use in environmental applications is more recent. Cryogenic barriers have a number of desirable traits, which include the following: (1) they are self-healing; (2) their removal can be accomplished by letting the barriers thaw; and (3) they couple well with directional drilling techniques, which can allow barriers to be installed as "bottoms." However, cryogenic barriers are not without shortcomings. Primary among these is that they require ongoing operation and maintenance. In addition, they can be used only in water-saturated soils (Dash et al., 1997; Lesmes et al., 1997; Peters, 1994; Peterson et al., 1996; Williams et al., 1997).
Installation Materials
A wide variety of materials are currently being used for vertical barriers. Their applicability depends on installation techniques, hydrogeologic conditions (including soil type and especially depth), compatibility with relevant contaminants, the time frame over which the material is to be used as a barrier, and cost. Barrier materials and some of their characteristics are listed in Table 3-7, along with selected references.
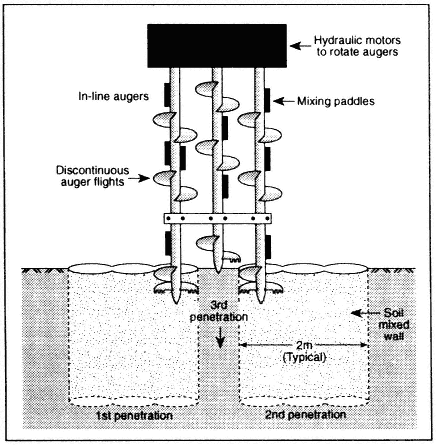
Figure 3-3
Deep-soil mixing for installation of a vertical barrier. Source: Filz et al., 1996.
Bottom Barriers
Bottom barriers, emplaced beneath existing in situ contaminants, have a number of features in common with vertical barriers, including the fact that (1) they can be used either in the vadose zone or beneath the water table, (2) their primary function is to minimize groundwater flow using low-permeability materials, (3) they can be constructed of similar materials, and (4) they can be emplaced using some similar technologies. However, the range of options for constructing bottom barriers is significantly less than that for vertical barriers (Peterson et al., 1996).
Table 3-7 Barrier Materials
|
Material |
Referencea |
Metal Compatibility |
DNAPL Compatibility |
Lifetime (years) |
Hydraulic Conductivity (cm/sec) |
Cost, $/m2 ($/ft2) |
|
Cement |
1 |
Good |
Good |
>25 |
10-8–10-9 |
54–190 (5–18) |
|
Bentonite |
1 |
Good |
Good |
>25 |
<10-7 |
22–86 (2–8) |
|
Cement replacement materials |
1 |
Good |
Good |
>25 |
10-6–10-9 |
22–160 (2–15) |
|
Sheet piles |
2 |
Good |
Good |
>25 |
10-9 |
160–430 (15–40) |
|
Geomembranes |
3 |
Good |
Good |
>25 |
10-9 |
54–270 (5–25) |
|
Sodium silicate |
4 |
Good |
Fair |
10–20 |
10-5 |
130 (12) |
|
Acrylate gel |
5,6 |
Good |
Fair |
10–20 |
10-7 10-9 |
230 (21) |
|
Colloidal silica |
6 |
Good |
Good |
>25 |
10-8 |
54–320 (5–30) |
|
Iron hydroxides |
6 |
Good |
Good |
>25 |
10-7 |
54–160 (5–15) |
|
Mantan wax |
6 |
Good |
Fair |
>25 |
10-4–10-7 |
320 (30) |
|
Sulfur polymer cement |
6 |
Good |
Fair |
>25 |
10-10 |
180 (17) |
|
Epoxy |
6 |
Good |
Good |
>25 |
10-10 |
480 (45) |
|
Polysiloxane |
6 |
Good |
Good |
>25 |
10-10 |
54–1,600 (30–150) |
|
Furan |
6 |
Good |
Good |
>25 |
10-8–10-10 |
800 (75) |
|
Polyester styrene |
6 |
Good |
Fair |
>25 |
10-10 |
970 (90) |
|
Vinylester styrene |
6 |
Good |
Good |
>25 |
10-10 |
1,100 (100) |
|
Acrylic |
6 |
Good |
Good |
>25 |
10-9–10-11 |
1,900 (180) |
|
Cryogenic |
7 |
Good |
Good |
>25 |
10-5–10-9 |
160–430 (15–40) |
|
a 1 = Evans et al., 1996; 2 = McMahon et al., 1996; 3 Koerner et al., 1996; 4 Whang et al., 1996; 5 = Peterson et al., 1996; 6 = Persoff et al., 1994. |
||||||
Installation Methods
The most straightforward approach to installing bottom barriers is angled drilling. One such method involves drilling a set of angled holes down either side of a contaminated site to form a V-shaped trough beneath it. The angled holes can be used to introduce low-permeability materials (e.g., using jet grouting) or as access holes for a cryogenic barrier. Directional drilling can also be used, in which case a continuous hole under the contaminated zone (and potentially up to the ground surface on the other side) could be emplaced (see Figure 3-4).
Materials
The installation method generally determines which materials can be used to construct bottom barriers. For example, materials suitable for jet grouting will be appropriate for installations using angled drilling. However, at this time there is little experience either within DOE or in the private sector in constructing bottom barriers by using directional drilling. Given the trend in recent years toward risk reduction by containment, significant advances likely will be made in bottom barrier installation by directional drilling in the next few years. Installation of cryogenic bottom barriers, using either angled or directional drilling, is also likely to increase in the next five years. Nonetheless, all of the bottom barrier technologies are likely to remain expensive for the foreseeable future. In addition, verification of bottom barrier performance is likely to remain a challenging task (Peterson et al., 1996).

Figure 3-4
Creation of a bottom barrier using directional drilling. Source: Peterson et al., 1996.
Verifying Barrier Performance
Performance verification is a critical component in the use of barriers, and planning for verification should be an integral part of decisions regarding barrier design and materials. The method(s) used for verification will depend on the hydrogeologic setting (e.g., above or below the water table) and the risk posed by the contaminants. Four approaches to barrier verification are (1) hydraulic tests, (2) tracer tests, (3) emplaced sensors, and (4) geophysical methods.
Hydraulic Tests
Hydraulic tests are useful for characterizing the large-scale features of a barrier (Snyder et al., 1997). For example, if a barrier completely encloses a portion of an aquifer and is closed at the bottom, the presence of relatively small defects in the barrier can be deduced from pumping tests. However, if the barrier only partially encloses a site, then hydraulic tests are relatively insensitive to small leaks. Hydraulic tests also frequently provide very little detail about the locations of leaks.
Tracer Tests
Tracer tests can pinpoint leaks in barriers, but they require installation of numerous monitoring points around the barrier (Williams et al., 1997). Using tracer tests for barrier verification has proven very feasible in the vadose zone, where gas-phase tracers can be used and diffusion coefficients are relatively high and isotropic. The ability of detection systems to identify accurately the locations and extents of leaks requires a clear understanding of gas-phase diffusion within the soils surrounding the barrier. To date, such tests have been applied primarily in relatively dry soils, where the diffusion coefficients are large. They have not yet been demonstrated in complex geologic systems where the diffusion coefficient may vary spatially and/or temporally by several orders of magnitude.
Tracers are less effective in the groundwater zone because diffusion is much slower than in the gas phase and because advection frequently dominates, so the probability of detecting the tracer with a monitoring network is much lower. In groundwater, the contaminants themselves may be the best tracers. The detection of contaminants in monitoring wells indicates that the barrier has leaked.
Emplaced Sensors
A wide range of physical and chemical sensors has been developed in the last 10 years (Borns, 1997; Inyang et al., 1996). These sensors can be deployed in a variety of ways, either within a barrier or adjacent to it. Depending on the hydrogeologic and chemical setting, these sensors may directly indicate the presence of water (e.g., in the vadose zone), or they may be selective for specific chemical contaminants. Once again, in the vadose zone, sensors to detect gas-phase transport are likely to be quite successful. In the groundwater zone, the difficulty is that given very limited dispersion, effectively covering potentially hundreds of square meters of barrier would require hundreds if not thousands of sensors. At present this is not a practical alternative.
Geophysical Methods
Geophysical methods have great potential for verifying barriers. However, although these methods have been applied effectively in some cases, a significant number of less successful applications also have occurred. In some cases, problems were due to inadequate resolution of the instruments. In others, the hydrogeologic conditions were inappropriate for a given geophysical technique. A number of potentially useful geophysical techniques for barrier verification are listed in Table 3-8, along with general comments about their application. In general, application of these techniques for barrier verification will
Table 3-8 Geophysical Methods for Barrier Verification
|
Geophysical Method |
Comments |
References |
|
Ground-penetrating radar |
Resolution 0.5–1 m with cross-borehole, tomographic method |
Pellerin, 1997 Davis and Annan, 1989 Lesmes et al., 1997 |
|
Electromagnetics |
Resolution >0.5 m in vicinity of borehole |
Pellerin, 1997 |
|
Electrical resistivity |
Resolution depends on electrode spacing, typically 0.3–2 m; tomographic method |
Pellerin, 1997 Daily and Ramirez, 1997 |
|
Seismic |
Tomographic method |
Pellerin, 1997 Steeples and Miller, 1993 |
require the collection of detailed, three-dimensional data sets and the tomographic imaging of these data using numerical methods.
TECHNOLOGIES FOR IMMOBILIZING METALS AND RADIONUCLIDES
Immobilization of metal and radionuclide contaminants by physical, chemical, or biologically mediated binding of the contaminant in some way within the soil matrix is becoming an increasingly common approach to site remediation. The broad categories of immobilization technologies discussed in this section include in situ vitrification, solidification and stabilization, permeable reactive barriers, in situ redox manipulation, and bioremediation.
In Situ Vitrification
Description
In situ vitrification (ISV) is an immobilization and destruction technology designed to treat soils and other similar media contaminated with heavy metals, organic compounds, and radionuclides. Soils are heated and melted by applying an alternating electrical current between electrodes placed in the ground (see Figure 3-5). Paths of graphite and glass frit are placed in the soil between the electrodes to aid in the start-up of the ISV process. The temperature of the molten soil may exceed 1700°C. At these temperatures, organic compounds either volatilize and are captured in a hood or are destroyed. Upon
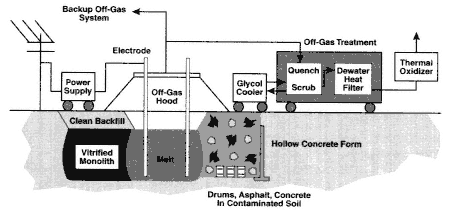
Figure 3-5
Schematic of an ISV System. Source: GeoSafe, Inc.
cooling, the molten soils become an impermeable glass or crystalline solid. The glass or crystalline solid is very leach resistant compared to the original untreated soil; metals and radionuclides may be chemically bound to the matrix or physically entrapped. As a result, the vitrified material can remain without significant risk to human health or the environment. Secondary residuals generated by ISV, which typically require treatment, include air emissions, scrubber liquids, carbon filters, and used hood panels.
Physical and Chemical Principles
The development of in situ vitrification was based on principles of ex situ vitrification processes, which are well established. Soils containing sufficient concentrations of conductive cations are slightly conductive to electricity, so electric currents can pass through. The relatively high resistivity of soil requires high voltages to achieve flow of the electrical current. Ultimately, passing a current through the soil creates large amounts of heat, which melts the soil.
When raised to temperatures exceeding its melting point, soil forms a liquid, or melt, that upon cooling forms either a glass or a crystalline material. Glasses and crystalline materials are highly impermeable and have extremely small surface areas compared to untreated soils. As a result, vitrified soils leach poorly, and metals and/or radionuclides contained within the vitrified soil are very immobile. The heating process may also cause some metals and/or radionuclides to bond chemically to the vitrified soil matrix, further reducing mobility.
The high temperatures achieved during vitrification either pyrolize or volatilize the organic compounds that may be present along with metals or radionuclides (see Chapter 4). The increased vapor pressures caused by the heating and the creation of a low-pressure zone in the overlying hood cause the organic vapors to migrate to the hood, where they are captured and treated.
Application
In the vitrification system developed by Battelle Pacific Northwest Laboratories and licensed to Geosafe, Inc., four graphite electrodes are inserted to a shallow depth in a square pattern in the soil to be treated. A pattern of electrically conductive graphite and glass frit is placed on the soil to complete an electrical circuit between the electrodes. A large electrical source (usually trailer mounted) is applied to the electrodes. The electrical resistance results in heating of the glass frit and graphite path and the soils near the path. As heating
continues, the melting of soils continues outward from the graphite and glass frit path because molten soils are electrically conductive and also transfer heat to adjacent soils. As the melting continues downward, the electrodes are moved deeper into the soil. The melting is continued until the targeted depth is reached.
Contaminant vapors formed during ISV can be captured by placing a hood over the melt area and applying a partial vacuum to the hood. Off-gas treatment is required. Monitoring of off-gases (stack emissions) is conducted for organic compounds present in the untreated matrix: oxygen, carbon dioxide, carbon monoxide, particles, metals, and hydrogen chloride. These analyses are compared to discharge permit requirements and system performance criteria. The quenched and scrubbed water is analyzed for organics, pH, and metals to determine if discharge requirements are being met.
Once the targeted soils have melted, the electric current is turned off, and the soils are allowed to cool. The electrodes are cut off at the surface and allowed to become part of the melt. Over an extended period the soil solidifies into a glass and/or crystalline monolith. Following adequate cooling of the surface, typically 24 hours or longer, clean soil is placed on the surface of the treated area to make up for the subsidence created by the soil consolidation that results from melting. Once cooling occurs, the treated matrix is sampled for constituents of concern. Analyses are made for total constituents, and the toxicity characteristic leaching procedure (TCLP) is carried out, if required, to determine the toxicity of leachate from the monolith.
Modifications of ISV include various staged in situ alternatives in which excavated material is placed in a subsurface zone for treatment, either in a single layer or in multiple layers. In the latter, material can be treated below grade, and additional material can be added for further treatment. The reverse can be accomplished by treating the upper layer of material, removing the glassified matrix, and then treating the next layer. Methods also have been developed to address soils with insufficient cation content.
Prior to implementing ISV, treatability tests are conducted on 45-to 90-kg (100-to 200-lb) soil samples to determine heat requirements and vitrified product properties. Design considerations include the lateral dimensions, depth, and composition of the matrix to be treated. Moisture is also an important consideration.
Current technology can be implemented to a maximum depth of 6 m (20 ft). If intact steel drums containing organic liquids are present, pretreatment is required to rupture the drums. If the amounts of organic compounds are excessive, heat may damage the equipment. Appropriate modifications for soils with high organic content include lower melt rates or dilution of the matrix.
ISV is applicable to sludges, sediments, and soils. ISV has been demonstrated to immobilize heavy metals and radionuclides and to remove and/or destroy volatile and semivolatile organic compounds, polychlorinated biphenyls (PCBs), dioxins or furans, pesticides, and munitions (discussed in more detail in Chapter 4). ISV can treat soils contaminated with large amounts of metals, although molten metal may sink to the bottom of the melt. Buried steel drums are not a problem unless they contain liquids and have maintained their structural and sealing integrity. These drums will eventually fail, releasing vapors to the melt in a potentially disruptive fashion. Pretreatment is required for intact steel drums containing organic liquids. ISV can tolerate waste and debris within the treatment zone. Organic debris is destroyed primarily by pyrolysis. Inorganic debris is typically incorporated into the melt and vitrified product. Examples of debris that have been present in ISV-processed soils include wood, vegetation, plastic, rubber, cardboard, asphalt, oils, and construction materials.
Performance
Table 3-9 lists several sites at which ISV has been used to treat radionuclides and metals.
Table 3-9 Applications of In Situ Vitrification
|
Site, Location |
Constituents |
Comments |
|
Parsons Chemicals Works, Grand Ledge, Mich. |
Pesticides heavy metals |
Excavated materials 14,800 Met all cleanup standards |
|
Oak Ridge National Laboratory |
Cesium-137 |
Melt exploded; test terminated |
|
Maralinga, Australia |
Radionuclides heavy metals |
Demonstration program Inorganic debris ~50% volume reduction >99.9998% retention of U and P |
|
Hanford Site |
9 radionuclides, 13 metals |
20 pilot-scale tests; 6 large-scale tests |
|
Ube City, Japan |
Organics, PCBs, heavy metals |
Tests with soil, mortar, asphalt, and drums |
|
SOURCE: Geosafe, Inc., personal communication, 1998. |
||
Limitations
ISV has several limitations. One limitation is that the soil organic content must be less than about 7 to 10 percent under normal operations. ISV can accommodate somewhat higher organic content with a reduced power level, but this slows the rate of treatment. Limitations on organic content are a result of the release of organic vapors, which occurs primarily through the dry soils along the edge of the melt. If, as a result of high organic content, organic vapors pass through the melt, the heat removal capacity may be exceeded. Sputtering and splattering of molten material may result, damaging the vapor collection hood.
The presence of rapidly recharging water within the treatment matrix, such as would occur within permeable aquifers, can also cause problems with ISV. Prior to melting of the matrix, water vaporizes and escapes upward along the outside of the melt. Condensation of moisture a short distance (typically one-third of a meter or so) outside the melt can create a saturated soil barrier and temporarily trap organic vapors. Sudden releases of steam under pressure can occur, causing overpressurization of the above-ground system. This may have been the cause of an accident that occurred during ISV implementation at the Oak Ridge, Tennessee, DOE facility, in which 20 tons of molten product erupted from the subsurface and damaged the off-gas hood (Spalding et al., 1997). No personnel were injured, but the project was terminated as a result of the accident.
For ISV to be effective, the matrix to be vitrified must contain sufficient conductive cations (sodium, lithium, magnesium, etc.) for the molten mass to be adequately conductive. Additionally, the matrix should contain adequate amounts of glass-forming elements such as silicon and aluminum (seldom a problem in soils).
ISV is not appropriate without some form of pretreatment or alternative approach (such as excavation of the upper several feet of soil or addition of alumina or silicate) under the following conditions:
-
depth greater than about 6 m (20 ft);
-
excessive moisture levels or high moisture recharge rates, especially if volatile organic compounds are present;
-
presence of operational utility trenches within the treatment zone;
-
presence of intact steel drums containing organic liquids;
-
a matrix composition that results in an excessively high melting temperature or that will not form a glass and/or crystalline product upon melting and cooling;
-
a soil organic content exceeding safe levels (7 to 10 percent by weight);
-
a metal content exceeding 15 percent by weight (which requires an electrode feeding procedure);
-
inorganic debris exceeding 20 percent by weight; or
-
inadequate surface area to set up above-ground components, including a crane for placing the vapor recovery hood.
Advantages
Although it has a number of limitations, ISV also has some unique advantages. A significant advantage of ISV is that it can treat complex matrices containing mixtures of contaminant types in a single step, a capability that few technologies share. Another significant advantage for DOE sites is that treatment can occur without bringing radioactive materials to the surface, which can help reduce exposure risks and potential transportation problems. An additional possible advantage of ISV is that the cooled vitrified mass can serve as a foundation for various types of construction, allowing for a wide range of uses of the area where treatment occurred.
Solidification and Stabilization
Description
Solidification and stabilization processes are designed to reduce the mobility of contaminants by reducing the contaminant solubility or the permeability of the medium (NRC, 1997). Solidification is the formation of a stabilized mass in which the contaminants are physically bound or contained. In stabilization, chemical reactions are induced between the stabilizing agent and the contaminant to reduce mobility. Both ex situ and in situ methods are available. Ex situ processes are among the most mature technologies, and excavated soils are frequently treated prior to disposal. Solidification and stabilization procedures have been described by Smith et al. (1995) and EPA (1994, 1997c).
Physical and Chemical Principles and Application
Principal stabilization materials are portland-type cements, pozzolanic materials, and polymers:
-
Portland cements typically consist of calcium silicates, alumino-silicates, aluminoferrites, and sulfates. Metals are immobilized in cement-type binders as hydroxides or other stable solids.
-
Pozzolans are very small spherical fly ash particles formed in the combustion of coal, in lime and cement kilns, and in other combustion processes. Those that are high in silica content have cement-like properties when mixed with water.
-
Polymeric compounds can be used to bind metal and radionuclides by microencapsulation. Materials that have been investigated for this purpose include bitumen, which is the least expensive, as well as polyethylene and other polyolefins, paraffins, waxes, and sulfur cement. DOE has used polyethylene encapsulation to treat a number of radionuclides (including cesium, strontium, and cobalt) and toxic metals (including chromium, lead, and cadmium).
Introduction of chemical reagents in stabilization processes can cause in situ chemical modification. For example, hydrogen sulfide has been used to precipitate metals (IAEA, 1997). However, such procedures lack good operational control, and the process efficacy is not known. A significant effort is currently being directed toward the application of low-cost amendments to soils to immobilize lead. Many of these processes involve the formation of secondary minerals, such as metal phosphates. The monolith is left onsite or landfilled. The stability of these materials has not been subjected to long-term testing, but it is expected to be significantly better than that of hydroxide precipitates.
Performance
Geo-Con, Inc., reportedly has used in situ solidification and stabilization at dozens of sites in the United States (EPA, 1995a). Projects have included construction of a 20-m-deep soil-bentoite wall to contain groundwater contamination in a former waste pond and shallow soil mixing and stabilization of 82,000 yd3 of contaminated soil at a former manufactured gas plant site. In a demonstration of the process conducted under the SITE (Superfund Innovative Technology Evaluation) program, the permeability of the treated soil decreased from 10-2 cm/sec to between 10-6 and 10-7 cm/sec. Polychlorinated biphenyl immobilization appeared likely as a result of mostly undetectable PCB concentrations in leaching tests on the treated soil, although this conclusion could not be confirmed because of low PCB concentrations in the untreated soil. However, data collected during this demonstration were insufficient to evaluate the effectiveness of the process in immobilizing metals.
Limitations
The success of in situ solidification or stabilization depends on the ability to mix the stabilizing agent with the soil. However, ensuring that sufficient mixing has occurred is difficult. Soils with high clay content or with large amounts of debris are not suitable for this treatment method. The process is not effective for anionic species such as As(III), As(V), and Cr(VI) because these remain mobile after treatment (Evanko and Dzombak, 1998). Ex situ mixing may cause the release of organic vapors.
Advantages
Solidification or stabilization processes are broadly applicable to a wide variety of metals and to wastes that contain mixtures of metals and some types of organic compounds (EPA, 1997c).
Permeable Reactive Barriers
Description
A permeable reactive barrier is a passive in situ treatment zone of reactive material that immobilizes metal or radionuclide contaminants as groundwater flows through it (Vidic and Pohland, 1996; EPA, 1998; Schultz and Landis, 1998). In this type of system, a permeable treatment wall is installed or created across the flow path of a contaminant plume (see Figure 3-6). Sorption or precipitation reactions occurring within the barrier remove metals and radionuclides from the groundwater, immobilizing the contaminants.
Physical and Chemical Principles
Sorption and precipitation reactions for treating metals and radionuclides in reactive barriers can be brought about through various physical, chemical, and biological processes. For example, inorganic contaminants can be sorbed to zeolites, hydrous ferric oxide, peat, silica, and polymer gels; reduced and precipitated by Fe0, ferrous hydroxide, H2S, or dithionite; or precipitated by lime or limestone. Biologically active zones can reduce and precipitate, as well as sorb, inorganic contaminants.
Because metals do not degrade and because most radioactive contaminants do not decay on the time scale of interest, the reversibility of the immobilization reaction must be closely scrutinized. If the immobilization reaction is reversible on the time scale of interest, the
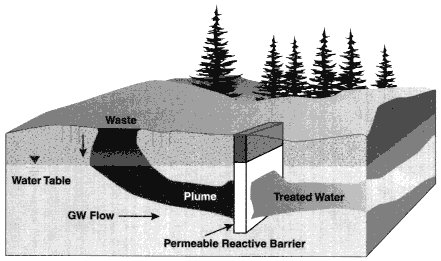
Figure 3-6
Schematic of a permeable reactive barrier. Note: GW = ground-water.
Source: EPA, 1998.
''immobilization'' is actually only a retardation, and the conditions that were responsible for immobilization must be maintained. For example, if Cr and U are immobilized by reduction, and oxygen is subsequently reintroduced to the barrier, reoxidation would be slow for Cr but more rapid for U.
Application
Several approaches for installing the reactive treatment zone are possible. One approach, limited to shallow depths, is to excavate and backfill a trench with the reactive material, often in one pass. A second approach is to use slurry wall construction technology to create a larger and deeper permeable curtain. In this approach, a polymer mixed with reactive material replaces subsurface materials as excavation proceeds. When excavation is complete, the polymer is removed by pumping and biodegradation, leaving a permeable wall that contains the reactive material. A third approach is to install temporary sealable sheet piling to allow dewatering and installation of a reactive zone. A fourth approach is to inject the reactive material directly with a jet. For the first three approaches, costs are likely to be high if a continuous zone is installed across zones of contaminated water. A promising alternative is to use sealable piling to
funnel the natural groundwater flow through narrow zones that contain the reactive material. This method allows greater control of the treatment zone and facilitates removal or replacement of the reactive material (NRC, 1994).
Performance
Table 3-10 provides examples of permeable reactive barrier installations with various reactive media. One of the most carefully studied reactive barriers is at Elizabeth City, North Carolina. A full-scale field demonstration of an Fe0 reactive barrier to intercept a Cr(VI)
Table 3-10 Summary of Selected Permeable Reactive Barrier Installations for Treating Metals and Radionuclides
|
Reactive Medium |
Contaminant |
Study Type |
Site |
Reference |
|
Fe0 |
Cr |
Commercial |
Elizabeth City, N.C. |
EPA, 1995b; RTDF, 1999; Puls et al., 1998 |
|
|
Cr |
Field |
Elizabeth City, N.C. |
EPA, 1995b; Sabatini et al., 1997; Puls et al., 1998 |
|
|
U |
Field |
Durango, Colo. |
Dwyer et al., 1996 |
|
|
U |
Field |
Fry Canyon, Utah |
RTDF, 1999; Naftz, 1997 |
|
Lime or limestone |
Acid mine drainage |
Commercial |
Various sites |
Kleinmann et al., 1983 |
|
|
Pb, Cd, As, Zn, Cu |
Commercial |
Nesquehoning, Pa. |
RTDF, 1999 |
|
Fe (OH)3 |
U |
Lab |
Monticello, Utah |
Morrison and Spangler, 1993; Morrison et al., 1995 |
|
|
U |
Pilot |
Monticello, Utah |
|
|
|
U |
Field |
Fry Canyon, Utah |
RTDF, 1999; Naftz, 1997 |
|
Zeolites |
Sr |
Lab |
|
Fuhrmann et al., 1995 |
|
Modified zeolites |
Cr |
Lab |
|
Haggerty and Bowman, 1994; RTDF, 1999 |
|
Bentonite |
Cs |
Lab |
|
Oscarson et al., 1994 |
|
Peat |
Cr |
Lab |
|
Ho et al., 1995 |
|
|
U |
Lab |
|
Morrison and Spangler, 1992 |
|
PO4 |
U |
Field |
Fry Canyon, Utah |
RTDF, 1999 |
|
Organic carbon |
Ni, Fe |
Commercial |
Sudbury, Ontario |
RTDF, 1999 |
|
Source: Adapted from Vidic and Pohland, 1996; RTDF, 1999 |
||||
and trichloroethylene (TCE) plume is operating at a Coast Guard air station at this site. The mixed waste contaminant plume is between 4.3 and 6.1 m (14 and 20 ft) below ground surface, and the water table ranges from 1.5 to 1.8 m (5 to 6 ft) below ground surface. Chromium(VI) concentrations range as high as 28 mg/liter near the contaminant source. A pilot-scale demonstration began at this site in September 1994, and the full-scale field test began in June 1996. For the pilot test, 21 20-cm (8-in.) holes were installed in a staggered three-row array over a 5.6 m2 (60 ft2) area. A mixture of 50 percent iron filings, 25 percent clean coarse sand, and 25 percent aquifer material (by volume) was poured down the hollow stem augers to a depth of 3 to 6.7 m (10 to 22 ft) below ground surface (EPA, 1995b). The full-scale test involves a trench that was excavated and simultaneously backfilled with the reactive medium. The barrier is 0.6 m thick, 46 m long, and 7.3 m deep.
Chromium(VI) concentrations in the effluent from the full-scale barrier have been decreased to below detection (<0.01 mg/liter). Under the highly reducing conditions that prevail within the wall, the reduction of Cr(VI) to Cr(III) and the formation of an insoluble precipitate constitute the likely mechanism causing the contamination decrease.
Limitations
Because metals and radionuclides are nondegradable, treatment by sorption or precipitation within a reactive barrier must be regarded as a retardation of contaminant migration rather than as a permanent solution to the problem. If retardation is accomplished through reduction to an immobile form, either the reducing conditions must be maintained to prevent remobilization or the reduction reaction must be effectively irreversible. If sorption is responsible for the retardation, the degree of reversibility of the reaction must be considered.
Another limitation is that because of the difficulties of emplacing the barrier, barriers are generally limited to near-surface contamination. In addition, the long-term performance of reactive barriers remains an open question. Principal concerns are reduction of permeability of the barrier due to buildup of reaction products or passivation of the reactive surface of the iron.
Advantages
Permeable barriers require little or no energy input once installed and thus can result in lower overall treatment costs. Because the
reaction zone is limited in area, it may be easier to design, monitor, maintain, and control than in systems operating over larger areas. Another strong advantage is the ability of this technology to treat contaminant mixtures (see Chapter 4).
In Situ Redox Manipulation
Description
In situ redox manipulation is the injection of chemical reductants into the ground or the stimulation of naturally occurring iron-reducing bacteria with nutrients in order to create reducing conditions in the subsurface, leading to reduction and immobilization of certain contaminants in groundwater (see Figure 3-7) (Amonette et al., 1994; Fruchter et al., 1997). This type of technology can be viewed as a special type of permeable reactive barrier, in which a part of the subsurface is transformed to a containment treatment zone.
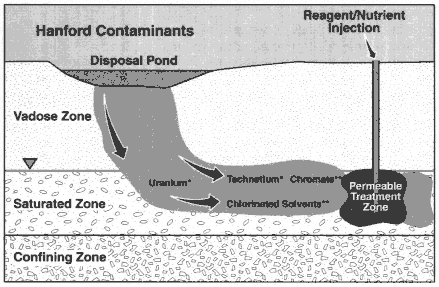
* Potential Candidate for Redox Treatment
** Favorable Candidate for Redox Treatment
Figure 3-7
Schematic of an in situ redox manipulation system. Source: Fruchter et al., 1997.
Application
This technology was conceived primarily for selected priority metal and radionuclide contaminants that are mobile in their oxidized form but immobile in the reduced form (Cr, U, Tc, Pu). Certain halogenated organic contaminants, including TCE and other chlorinated solvents, in theory can be treated at the same time (Betts, 1998).
Physical and Chemical Principles
The primary redox buffer in the reducing zone created by in situ redox manipulation systems is generally structural iron—that is, iron bound in clay interlayers of the aquifer material. This reduced iron forms a large reducing buffer region that is not reoxidized easily by oxygen, yet reacts readily enough with the contaminants to retard their transport. The elements Cr, Tc, U, and Pu can be reduced by mineral-bound Fe(II) to form very insoluble oxides. Various reductants have been tested, including N2H4, NH2OH, SO32-, S2-, S2 O42-, colloidal Fe(II) in clays, and Fe0. In tests at the Hanford Site, dithionite (S2O42-) was most effective (Amonette et al., 1994). Lactate injection has been proposed as a method for stimulating iron-reducing bacteria (Fruchter et al., 1997), but field tests to date have focused on chemical reductants.
The reaction kinetics are critical. First, it is necessary for the reductant to persist long enough to reduce the structural iron in the clays but not long enough to become a contaminant of concern itself. Second, the immobile structural iron must be sufficiently reactive with the contaminants that they are in fact reduced and immobilized, but it must not be so reactive with other oxidants, such as dissolved oxygen, that the iron is reoxidized before it reacts with the contaminants. Third, the reoxidation of the entire system must be slow enough that the contaminants are not remobilized after having been reduced.
Application
The technology is appropriate for contaminants that are widely dispersed in the unsaturated zone or in groundwater that is not readily accessible from the surface—that is, more than 15 m (50 ft) below ground surface. Liquid or gaseous chemical reductants, or substrate and nutrients to stimulate microbial growth, are injected to create reducing zones. The reductant must be easy to inject to the desired treatment depth via an injection well and must be acceptable to regulatory agencies.
Performance
Three field tests related to the use of in situ redox manipulation for Cr(VI) treatment have been conducted at the Hanford 100-H site: a bromide tracer test, a mini injection-withdrawal test, and a full-scale injection-withdrawal test (PNNL, 1996; Fruchter et al., 1997) (see Table 3-11). A large-scale treatability test is now under way at the Hanford 100-D site (Betts, 1998).
In the full-scale injection-withdrawal test, approximately 77,000 liters of 0.1 M sodium dithionite in a 0.44 M potassium bicarbonate-carbonate buffer at pH 11.2 were injected into an unconfined sandy-gravel aquifer of the Hanford formation. After the 18-hour injection period and 83-hour reaction period, approximately five injection volumes were withdrawn to recover unused reagent; 87 percent of the injected dithionite was recovered (Fruchter et al., 1997). The objectives were to create a reducing zone approximately 15 m in diameter and to monitor the removal of Cr(VI) and the lifetime of the reduced zone. The depth of the test was approximately 15 m (50 ft). The test was conducted with a single injection well and 15 monitoring wells. The target contaminant was chromate.
Monitoring data indicated that a year after the test, chromium levels in groundwater had decreased from an initial value of 60 µg/liter to below the detection limit (Fruchter et al., 1997). From 60 to 100 percent of the iron in the sediments was reduced by dithionite. No significant plugging of the aquifer formation (a potential problem
Table 3-11 Summary of In Situ Redox Manipulation Tests
|
Reactive Medium |
Contaminants |
Study Type |
Site |
Reference |
|
Dithionite injection to reduce structural iron |
Cr |
Field |
Hanford 100-Hf full scale |
PNNL, 1996; Fruchter et al., 1997 |
|
|
|
Field |
Hanford 100-Hf push-pulla |
Vermeul et al., 1995; Fruchter et al., 1996 |
|
|
|
Intermediate |
Physical model of 7-m-radius; 10-degree wedge of contaminated aquifer |
Fruchter et al., 1996 |
|
a A push-pull test uses a single well for both injection of reactive agents and withdrawal of water samples |
||||
due to precipitation reactions) occurred. Data from after the test injection indicated that the aquifer remains reducing and chromate remains below the 8-µg/liter detection limit (Fruchter et al., 1998). The lifetime of the reducing zone has been estimated at 10 years.
Because of the success of this field test, a large-scale demonstration of in situ redox manipulation is now under way at Hanford. In this demonstration, a treatment zone approximately 50 m (150 ft) long is being created by overlapping cylindrical reduced zones created with five injection wells (Fruchter et al., 1998). Bench-scale tests are also under way at Hanford and elsewhere to test the performance of this method for treating chlorinated solvents.
Limitations
For application in relatively deep aquifers, verifying that the manipulated zone intercepts the contaminant plume will remain an uncertainty. Furthermore, the kinetics of all the reactions must be appropriate, as outlined above. Finally, this containment technology must be maintained far into the future to avoid reoxidation and mobilization of the contaminant. Reoxidization may not be a problem for Cr(VI) that has been reduced to Cr(III), because the reaction may be irreversible (or have very slow kinetics). However, reoxidation is a concern for other contaminants, such as U, for which the reaction is reversible.
Advantages
A prime advantage of in situ redox manipulation is the ability to treat contamination at depths that are inaccessible by excavation of any sort. The technology also is relatively inexpensive to install and operate. It allows management of large quantities of water in situ, avoiding the safety and regulatory issues associated with bringing water to the surface.
Bioremediation
Description
Bioremediation is usually associated with the microbiological degradation of organic contaminants to more benign forms. As applied to inorganic contaminants, bioremediation refers to processes through which contaminants are mobilized or immobilized as a direct result of microbiological activity. Mobilization can occur through complexation of an inorganic contaminant by soluble biologically produced
complexing agents such cyclodextrans and exopolysaccharides or by reductive dissolution of metal oxides ("microbial leaching"); these mobilization processes are discussed later in this chapter. Immobilization, discussed here, can occur through reduction to an insoluble form, for example, Cr(VI) to Cr(III), U(VI) to U(IV), Pu(V, VI) to Pu(III, IV), and Tc(VII) to Tc(IV); immobilization may be enhanced by sorption to biomass (Ahmann, 1997).
Physical and Chemical Principles
Respiratory microorganisms obtain energy from the enzymatically mediated oxidation of a substrate (e.g., acetate, glucose, H2) coupled to the reduction of a terminal electron acceptor (e.g., O2, NO3-, Fe(III), SO42-). Several toxic metals and radionuclides (including U, Pu, and Cr) have been shown to be reduced during this process, either directly as the terminal electron acceptor in the metabolic process or indirectly. If reducing conditions can be maintained by the addition of substrate and suitable nutrients, inorganic contaminants will remain in their highly insoluble, immobile forms.
Application
No definitive field studies have been reported specifically on manipulating the subsurface environment to cause microbiological reduction and immobilization of metals and radionuclides. Presumably, a field test could be designed similar to a field test for microbial treatment of chlorinated solvents by reductive dehalogenation, as described in Chapter 4. Examples of laboratory tests are given in Table 3-12.
Performance
Laboratory tests (see Table 3-10 and Tucker, 1996) have indicated that the immobilization of metals and radionuclides by bioremediation could be very effective, with removal of contaminants from the mobile aqueous phase to below critical values.
Limitations
Contaminant immobilization caused by bioremediation must be regarded as the retardation of contaminant migration rather than as a permanent solution to the problem. Reducing conditions that favor the immobilization reactions may have to be maintained to prevent remobilization.
Table 3-12 Selected Laboratory Tests of Microbiological Reduction and Immobilization of Metals and Radionuclides
|
Contaminant |
Reference |
|
Cr |
Cifuentes et al., 1996 Turick et al., 1996 Losi et al., 1994a |
|
U |
Lovley et al., 1991 Lovley and Phillips, 1992 Sheppard and Evenden, 1992 |
|
Pu |
Rusin et al., 1994 Zorpette, 1996 Macaskie, 1991 |
|
SOURCE: Adapted from Ahmann, 1997. |
|
Advantages
Biological immobilization of metals and radionuclides could be performed simultaneously with bioremediation of organic compounds. The treatment occurs in situ, which decreases exposure risks and disposal problems. Costs are moderate.
TECHNOLOGIES FOR MOBILIZING AND EXTRACTING METALS AND RADIONUCLIDES
In addition to being treated by immobilization, some metals and radionuclides in groundwater and soil can be mobilized and extracted from the subsurface for treatment or disposal at the surface. Electrokinetic, soil flushing, soil washing, and phytoremediation processes, discussed below, are the primary technologies being developed for this purpose.
Electrokinetic Processes
Description
The application of an electric field to soil to remove chemical contaminants is called electrokinetic remediation. This process is particularly attractive for application to low-permeability soils that are difficult to flush. The reactions can be used to stabilize the contaminants in situ, or contaminants concentrated near the electrodes can be removed and treated ex situ.
In this process, a series of electrodes are placed into the contaminated area, and a 50- to 150-V direct current potential is applied between the electrodes (EPA, 1997b). The potential field causes movement of water and migration of the contaminants toward the electrode of opposite charge. Four processes are responsible for contaminant movement (Acar et al. 1995; EPA, 1997b):
-
electromigration (transport of charged chemical species in the electric gradient);
-
electroosmosis (transport of water or added pore fluid in the electric gradient);
-
electrophoresis (transport of charged particles in the electric gradient); and
-
electrolysis (chemical reactions at the electrodes resulting from the applied electrical potential).
Both electromigration to desorb and move anions and cations from the soil and transport them to the electrodes, and electroosmosis to drive a flushing fluid between the anode and cathode, have been used as the basis for electrokinetic remediation (EPA, 1997b). The contaminants removed with water or processing fluid can be treated ex situ by conventional processes.
Application
Electrokinetic processes have been used for the remediation of soils containing a number of inorganic contaminants (EPA 1997b,c). Removal of many contaminants, including cadmium, cesium, chromium, copper, lead, mercury, nickel, strontium, uranium, and zinc, has been demonstrated. Formation of complex thorium species may allow thorium removal.
An electrokinetic system at the Savannah River Site removed mercury and uranium. Ions were trapped in ion exchange polymer matrices in the electrode compartments (EPA, 1997b). In a bench-scale electrokinetic test conducted under the EPA SITE program, uranium, but not radium or thorium, was removed from kaolinite. Uranium precipitated as the hydroxide. The introduction of acetic acid into the cathode compartment prevented its precipitation near the cathode.
Limitations
In water, electrolysis produces acid at the anode and hydroxide at the cathode. The pH can drop to less than 2 at the anode and
increase to more than 12 at the cathode. Unless movement of the acid front is retarded, the transport of hydrogen ion will predominate (Acar and Alshawabkeh, 1993). Transport of the acid front can be limited by the cation exchange capacity of the soil and by reaction with organic materials (e.g. humic acid) and inorganic compounds (e.g. calcium carbonate). Alternatively, water from one electrode can be extracted and reinjected at the other. The electrodes can also be placed in ceramic casings, which are kept filled with processing fluids chosen to maintain pH balance and assist in the solubilization and movement of contaminants (EPA, 1997b). The processing fluids can be pumped and the contaminants removed from them by precipitation or other treatment means.
Advantages
Electrokinetic systems can mobilize both metals and organic compounds as a result of the several processes that are responsible for contaminant movement.
Soil Flushing and Washing
Description
Soil flushing is an in situ process and soil washing is an ex situ process in which contaminants are removed from the soil by using a suitable extracting solution. Commonly used mobilizing agents are acids and chelating agents. Soil washing has been widely applied. Soil flushing has been used to recover metals in the mining industry, and considerable research has been conducted on the use of soil flushing for organic contaminants (see Chapter 4). However, this method has not been developed for the treatment of metals and radionuclides.
Physical and Chemical Principles and Application
Soil washing is generally applied after segregation of smaller-size (<63 µm) soil particles (NRC, 1997; Evanko and Dzombak, 1998). In general, the contaminant concentration is greater in smaller soil particles than in larger-size particles. Smaller particles preferentially bind contaminants as a consequence of their greater surface area and physicochemical reactivity.
A number of physical processes are available for pretreatment of the soil in soil washing systems (EPA, 1988; Smith et al., 1995; Evanko and Dzombak, 1998). Processes often employed include screen siz-
ing, classification by settling velocity in air or water, gravity separation, flotation, and magnetic separation.
For soil washing, mobilizing chemicals are added to the separated soil in a reactor. For soil flushing, extractant chemicals are applied to the contaminated soil by surface flooding, sprinklers, leach fields, vertical or horizontal injection wells, basin infiltration systems, or trench infiltration systems (Evanko and Dzombak, 1998). After contact with the contaminated soil, the extractant is recovered for disposal or treatment and reuse.
Commonly used extractants include acids and chelating agents (Ehrenfeld and Bass, 1984; Rulkens and Assink, 1984; Smith et al., 1995). The most commonly used acids are sulfuric (H2SO4), hydrochloric (HCl), and nitric (HNO3), and the most commonly used chelating agents are EDTA, citric acid, and DTPA (Smith et al., 1995). Although highly effective in metal mobilization, chelating agents such as EDTA are expensive and difficult to recover; however, Allen and Chen (1993) have reported that both EDTA and the contaminant metal can be recovered electrochemically. Because many metals are redox sensitive, oxidants and reductants have also been employed in mobilizing solutions. Soil flushing using water alone is often effective in removing hexavalent chromium because of its high solubility and mobility.
Ion exchange can be used to concentrate contaminants after recovery of mobilizing solutions. Other treatments for recovered solutions include evaporation and solidification, precipitation, coprecipitation, and sorption onto clay (IAEA, 1997). DOE has studied a number of conventional and advanced methods for the recovery of metals and radionuclides from water. Among these are ion exchangers attached to magnetic particles, semipermeable membranes, selective solid-phase extraction, and concentration with chelators.
Performance
A number of commercial vendors offer soil washing technology (EPA, 1995a). The technology has been demonstrated numerous times as an effective ex situ method for treating soil contaminated with both metals and organic contaminants. For example, in a SITE demonstration of a soil washing and chemical treatment process at the Twin Cities Army Ammunition Plant Site F in Minnesota, the process reduced lead concentrations from initial levels of 3,000 to 10,000 ppm (parts per million) to treated levels of less than 300 ppm.
In a recent field test of an in situ soil flushing system in the Province of Utrecht, The Netherlands, remediation was conducted by
infiltrating acidified water (0.001 M HCl) into the subsurface (Otten et al., 1997). The treated area was 6,000 m2 and 4–5 m deep and was contaminated with an estimated 725 kg of cadmium at concentrations ranging from 5 to 20 mg/kg. Treatment reduced the Cd concentration to less than 2.5 mg/kg in most of the treated area, except in a small zone where the initial Cd concentration was too high.
Limitations
Soils with high concentrations of clay and silt may be difficult to treat because of the difficulty in removing more tightly bound contaminants. Mineralized metals and metal particles (i.e., nonionic) are not easily treated. Suitable washing solutions may be difficult to find for complex mixtures of contaminants. Soil flushing may mobilize chemicals that are difficult to recover. Also, soil flushing can be applied only in geologic formations with sufficient permeability to allow circulation and recovery of the flushing solution.
Advantages
Transfer of the contaminant to a liquid stream often facilitates its treatment. There are a large number of methods for the treatment of liquid waste streams, for example, those employed in industrial waste treatment. Soil flushing has the added advantage of requiring no excavation.
Phytoremediation
Description
Phytoremediation refers to the use of plants to extract metals and metalloids from contaminated soils as shown in Figure 3-8. In phytoremediation, contaminated soil is seeded with special plants known as ''hyperaccumulators'' that can take up large quantities of metals or radionuclides through their root systems. The plants are then grown and harvested. Multiple harvests generally are required to clean up the site.
Phytoremediation is the direct and/or indirect use of green plants for remediation of contaminated groundwater or soil. The method has been of interest for several years because of the many observed mechanisms by which plants can remove, degrade, or immobilize a wide variety of contaminants. Phytoremediation has been applied to organic compounds, as well as metals and radionuclides.
Figure 3-8
Phytoremediation of metals.
Phytoremediation can involve uptake of contaminants within the root zone and transport or accumulation within the plant. It also can involve enhancement of microbial processes within the soil for contaminant immobilization or degradation. In some cases it may involve degradation directly within the plant. The various types of processes have been described by the following somewhat overlapping terms (Schnoor, 1997; Dupont et al., 1998;):
-
Phytoextraction involves the uptake of metals and radionuclides by plant roots, and their accumulation in the above-ground parts of the plant. The plant is harvested and processed to concentrate the contaminants.
-
Phytostabilization refers to the use of vegetation to prevent the erosion of contaminanted soil, immobilize contaminants in the soil, or control groundwater movement through transpiration.
-
Rhizodegradation involves stimulating microorganisms around the root zone, resulting in enhanced microbial degradation of the contaminants.
-
Phytodegradation refers to the transformation of organic contaminants to less toxic compounds through their adsorption, uptake, or degradation by either the plant itself or plant-associated microflora.
-
Phytovolatilization is a process by which contaminants can be taken up by the plant and then volatilized.
In general, phytoextraction and phytostabilization are the most
important phytoremediation methods for treating contaminants in groundwater and soil (although phytovolatilization could be used to remove Hg). Phytostabilization is a special form of containment technology, whereas phytoextraction can be used to remove metals and radionuclides from the subsurface; thus, this discussion focuses on phytoextraction.
Physical and Chemical Principles
Many plants exude chelating agents from the roots to complex essential micronutrients and make them available to the plant. Phytoextraction causes plants to take up contaminant metals in the same way they extract micronutrients. For this method to be effective, some mechanism must exist for the plant to select a contaminant over other similar but more abundant metals (e.g., selection of Ra or Sr rather than Ca).
Application
Phytoextraction is most applicable to large areas of surface soils with low to moderate levels of contamination. Plants that accumulate at least 0.1 percent by weight of Co, Cu, Cr, Pb, or Ni or 1 percent by weight of Mn or Zn are defined as hyperaccumulators and are suitable for phytoextraction. Although many plants have been tested, plants of the genera Brassica, Thlaspi, Cardaminopsis, and Alyssum appear to be the most promising (Ahmann, 1997, after Kumar et al., 1995). For metals that are bound extremely tightly to soils, the addition of chelating agents, such as EDTA promotes their accumulation in the plant. In such cases, care must be exercised that adding the chelating agent does not cause leaching of the contaminant from the surface soil zone. Ultimately the plants are harvested, dried, and combusted or composted to reduce their mass prior to disposal. Multiple harvests are generally required to achieve cleanup goals.
Performance
Commercialization of phytoextraction and other phytoremediation methods has been relatively slow but appears to be occurring. Table 3-13 lists several field demonstrations of phytoextraction for the treatment of metals and radionuclides.
Table 3-13 Field Demonstrations of Phytoextraction of Metals and Radionuclides
Limitations
Phytoextraction is applicable only to the rooting zone of soils Although the goal of phytoextraction is mobilization of contaminants from soils to plant biomass for subsequent recovery or disposal, care must be exercised to prevent mobilization into the biosphere (for example, from animals eating the plants) where recovery is no longer possible. Furthermore, care must be exercised that natural or synthetic chelating agents associated with the uptake of contaminants do not mobilize the contaminant into groundwater. The plants must be able to grow vigorously at the site. Disposal of plant biomass can be a problem when plants are contaminated with heavy metals and radionuclides.
Advantages
Phytoextraction is a low-cost method to remove contaminants from large areas of the surface zone of contaminated soils.
Conclusions
Treatment technologies for cleaning up metal and radionuclide contaminants in groundwater and soil act either by immobilizing contaminants in place to prevent transport to humans and sensitive ecosystems or by mobilizing contaminants for extraction and treatment at the surface. Because metals and radionuclides, unlike organic contaminants, are nondegradable except by radioactive decay and because the risk posed by these compounds is highly sensitive to geochemical conditions, managing these contaminants is very different from managing the types of organic contaminants discussed in Chapter 4. Few well-established technologies are available for treating metals and radionuclides in the subsurface, but a number of new technologies are being developed. Available technologies for treating metals and radionuclides are summarized in Tables 3-4 and 3-5 and include the following:
-
Impermeable barriers are among the least expensive and most widely used methods for preventing the spread of metal and radionuclide contaminants in groundwater. Vertical barriers are well developed and widely available; methods are being developed for the installation of horizontal barriers beneath existing waste.
-
In situ vitrification for immobilization of metal and radionuclide contaminants is an emerging technology that is particularly suitable for sites with high concentrations of long-lived radioisotopes within 6 to 9 m
-
of the soil surface (depending on water table depth and soil moisture). However, it is among the most expensive treatment technologies.
-
Solidification and stabilization are mature technologies for use ex situ but are considered emerging technologies for use in situ. Ensuring sufficient mixing is difficult when this technology is used in situ. Improved mixing methods are being tested. The longevity of the solidified or stabilized material is another concern that must be addressed before these methods can be considered established technologies.
-
Permeable reactive barriers are among the most promising and rapidly developing emerging treatment technologies for metal and radionuclide contaminants. A variety of reactive media has been tested for a variety of contaminants, including organics. Because the technology is relatively new, the longevity of the barrier is a major uncertainty.
-
In situ redox manipulation is an emerging method that is appropriate at both shallow depths and depths at which trenches are impractical. It is an excellent technology for elements (e.g., Cr) that can be reduced to solids that are resistant to reoxidation by ambient oxygen, but it is less suitable for elements (e.g., Tc) that are susceptible to reoxidation.
-
Bioremediation is in the early stages of development for the treatment of metals and radionuclides. If better developed, it could be a relatively low-cost alternative and could be used to treat mixtures of organic and inorganic contaminants.
-
Electrokinetics may be advantageous for extracting metals and radionuclides from media with very low hydraulic conductivity. The consequences of generating large amounts of acid and base must be considered. Extensive field tests of electrokinetics for the remediation of metal and radionuclide contamination have yet to be conducted in the United States.
-
Soil washing has been well developed for ex situ treatment of contaminated, coarse-grained, near-surface soils but requires excavation of the soil prior to treatment.
-
Soil flushing can potentially flush metals and radionuclides from soil in situ. Although used in the mining industry, this technology has not seen widespread application in the remediation of metals and radionuclides.
-
Phytoremediation is primarily advantageous for extracting metals from large areas of contaminated surface soils. Its advantages are low cost and ease of implementation. Potential disadvantages are the excessive mobilization of metals from chelators that often must be added and the need to dispose of plant biomass.
In general, only containment and ex situ technologies are well developed for treating metal and radionuclide contaminants. Additional development work is needed to increase the range of options
for treating metals and radionuclides in situ and for extracting them for ex situ treatment.
REFERENCES
Acar, Y. B., and A. N. Alshawabkeh. 1993. Principles of electrokinetic remediation. Environmental Science and Technology 27:2638–2647.
Acar, Y. B., R. J. Gale, A. N. Alshawabkeh, R. E. Marks, S. Puppala, M. Bricka, and R. Parker. 1995. Electrokinetic remediation: Basics and technology status. Journal of Hazardous Materials 40:117–137.
Adriano, D. C. 1986. Trace Elements in the Terrestrial Environment. New York: Springer-Verlag
Ahmann, D. 1997. Bioremedation of metal-contaminated soil. Society for Industrial Microbiology News 47:218–233.
Allen, H. E., and P-H. Chen. 1993. Remediation of metal contaminated soil by EDTA incorporating electrochemical recovery of metal and EDTA. Environmental Progress 12:284–293.
Allen, H. E., and Y. Yin. 1998. Combining chemistry and biology to derive soil quality criteria for pollutants. Presented at Sixth World Congress of Soil Science, Montpellier, France, August 25.
Allison, J. D., D. S. Brown, and K. J. Novo-Gradac. 1991. MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: Version 3.0 users manual. EPA/600/3-91/021. Washington, D.C.: U.S. Environmental Protection Agency.
Amonette, J. E., J. Szecsody, J. C. Templeton, Y. A. Gorby, and J. S. Fruchter. 1994. Abiotic reduction of aquifer materials by dithionite: A promising in situ remediation technology. In In-Situ Remediation: Scientific Basis for Current and Future Technologies, G. W. Gee and N. R. Wing, Eds. Richland, Wash.: Battelle Press.
Ballard, J. H., and M. J. Cullinane, Jr. 1997. Tri-Service Site Characterization and Analysis Penetrometer System (SCAPS) Technology Verification and Transition. Vicksburg, Miss.: U.S. Army Corps of Engineers Waterways Experiment Station.
Betts, K. S. 1998. Novel barrier remediates chlorinated solvents. Environmental Science and Technology 3(2):495A.
Borns, D. J. 1997. Geomembranes with incorporated optical fiber sensors for geotechnical and environmental applications. Pp. 1067–1073 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Browne, C. L., Y. M. Wong, and D. R. Buhler. 1984. A predictive model for the accumulation of cadmium by container-grown plants. Journal of Environmental Quality 13:184.
Buergisser, C. S., and A. T. Stone. 1997. Determination of EDTA, NTA, and other amino carboxylic acids and their Co(II) and Co(III) complexes by capillary electrophoresis. Environmental Science and Technology. 31:2656–2664.
Bufflap, S. E., and H. E. Allen. 1995. Sediment pore water collection methods: A review. Water Research 29:165–177.
Chappell, J. 1998. Phytoremediation of TCE in ground water using populus. Washington, D.C.: EPA.
Cifuentes, F. R., W. C. Lindemann, and L. L. Barton. 1996. Chromium sorption and reduction in soil with implications to bioremediation. Soil Science 161:233–241.
Daily, W., and A. Ramirez. 1997. A new geophysical method for monitoring emplacement of subsurface barriers, Pp. 1053–1059 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Dash, J. G., H. Y. Fu, and R. Leger. 1997. Frozen soil barriers for hazardous waste confinement. Pp. 607–613 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Davis, J. L., and A. P. Annan. 1989. Ground penetrating radar for high resolution mapping of soil and rock stratigraphy. Geophysical Prospecting, 37:531–551.
Dupont, R. R., C. J. Bruel, D. C. Downey, S. C. Huling, M. C. Marley, R. D. Norris, and B. Pivetz. 1998. Innovative site remediation technology: Design and application: Bioremediation. Annapolis, Md.: American Academy of Environmental Engineers.
Dwyer, B. D., D. C. Marozas, K. Cantrell, and W. Stewart. 1996. Laboratory and field demonstration of reactive barrier systems. In Proceedings of the 1996 Spectrum Conference, Seattle, Wash., August 18–23.
Dzombak, D. A., and F. M. M. Morel. 1990. Surface Complexation Modeling: Hydrous Ferric Oxide. New York: Wiley.
Ehrenfeld, J., and J. Bass. 1984. Evaluation of Remedial Action Unit Operations at Hazardous Waste Disposal Sites. Park Ridge, N.J.: Noyes Publications.
EPA (Environmental Protection Agency). 1988. Technological Approaches to the Cleanup of Radiologically Contaminated Superfund Sites. EPA/540/2-88/002. Washington, D.C.
EPA. 1992. Vitrification Technologies for Treatment of Hazardous and Radioactive Waste. EPA/625/R-92/002. Washington, D.C.: EPA, Office of Research and Development.
EPA. 1994. Innovative Site Remediation Technology: Solidification/Stabilization, Vol. 4 EPA 542/B-94/001. Washington, D.C.
EPA. 1995a. Contaminants and Remedial Options at Selected Metal-Contaminated Sites. EPA 540/R-95/512. Washington, D.C.
EPA. 1995b. In Situ Remediation Technology Status Report: Treatment Walls. EPA 542-K-94-004. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1997a. Cleaning Up the Nation's Waste Sites: Markets and Technology Trends. 1996 Edition. EPA 542-R-96-005. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1997b. Electrokinetic Laboratory and Field Processes Applicable to Radioactive and Hazardous Mixed Waste in Soil and Groundwater. EPA 402/R-97/006. Washington, D.C.
EPA. 1997c. Recent Developments for In Situ Treatment of Metal-Contaminated Soils. EPA 542/R-97/004. Washington, D.C.
EPA. 1997d. Best Management Practices (BMPS) for Soil Treatment Technologies: Suggested Guidelines to Prevent Cross-Media Transfer of Contaminants During Clean-Up Activities. EPA 530/R-97/007. Washington, D.C.
EPA. 1997e. Engineering Bulletin. Technology Alternatives for the Remediation of Soils Contaminated with Arsenic, Cadmium, Chromium, Mercury, and Lead. EPA 540/S-97/500. Washington, D.C.
EPA. 1998. Permeable Reactive Barrier Technologies for Containment Remediation. EPA/600/R-98/125. Washington, D.C.: EPA.
Evanko, C. R., and D. A. Dzombak. 1998. Remediation of Metals-Contaminated Soils and Ground-Water. Technology Evaluation Report. Pittsburgh, Pa.: Ground-Water Remediation Technologies Analysis Center.
Evans, J. C., M. Allan, S. R. Day, G. M. Filz, H. I. Inyang, S. Jefferis, L. E. Kukacka, L. Martinenghi, R. Mitchell, and K. Potter 1996. Soil-and cement-based vertical barriers with focus on materials. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. PB96-180583. Springfield, Va.: National Technical Information Service .
Filz, G. M., J. K. Mitchell., L. R. Anderson, J. D. Betsill, E. E. Carter, R. R. Davidson, S. R. Day, A. Esnault, J. C. Evans, S. Jefferis, M. Manassero, L. Martinenghi, R. L. Stammes, G. J. Tamaro, G. A. M. van Meurs, and D. S. Yang. 1996. Design, construction and performance of soil-and cement-based vertical barriers. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. #PB96-180583. Springfield, Va.: National Technical Information Service.
Fish, W., D. A. Dzombak and F. M. M. Morel. 1986. Metal-humate interactions. 2. Application and comparison of models. Environmental Science and Technology 20: 676–683.
Florence, T. M. 1989. Electrochemical techniques for trace element speciation in waters. In Trace Element Speciation: Analytical Methods and Problems, G. E. Batley, Ed. Boca Raton, Fla.: CRC Press.
Fruchter, J. S., J. E. Amonette, C. R. Cole, Y. A. Gorby, M. D. Humphrey, J. D. Istok, F. A. Spane, J. E. Szecsody, S. S. Teel, V. R. Vermeul, M. D. Williams, and S. B. Yabusaki. 1996. In Situ Redox Manipulation Field Injection Test Report-Hanford 100H Area. PNNL-11372. Richland, Wash.: Pacific Northwest National Laboratory.
Fruchter, J. S., C. R. Cole, M. D. Williams, V. R. Vermeul, S. S. Teel, J. E. Amonette, J. E. Szecsody, and S. B. Yabusaki. 1997. Creation of a subsurface permeable treatment barrier using in situ redox manipulation. Pp. 704–710 in Proceedings, International Containment Technology Conference, February 9–12. Washington, D.C.: Department of Energy.
Fruchter, J. S., C. R. Cole, M. D. Williams, V. R. Vermeul, J. E. Szecsody, and J. C. Evans. 1998. Recent progress on in situ redox manipulation barriers for chromate and trichloroethylene. Presented at Fifth Meeting of the NRC Committee on Technologies for Cleanup of Subsurface Contamination in the DOE Weapons Complex, Richland, Wash., May 14–15.
Fuhrmann, M., D. Aloysius, and H. Zhou. 1995. Permeable, subsurface sorbent barrier for 90Sr: Laboratory studies of natural and synthetic materials. In Proceedings of Waste Management '95, Tucson, Az., February 16–March 2.
Haggerty, G. M., and R. S. Bowman. 1994. Sorption of chromate and other inorganic anions by organo-zeolite. Environmental Science and Technology 28:452–458.
Hajós, P., G. Révész, and O. Horváth. 1996. The simultaneous analysis of metal-EDTA complexes and inorganic anions by suppressed ion chromatography. Journal of Chromatographic Science 34:291–299.
Ho, Y. S., D. A. Wase, and C. G. Forster. 1995. Batch nickel removal from aqueous solution by sphagnum moss peat. Water Research 29:1327–1332.
IAEA (International Atomic Energy Agency). 1997. Technical Options for the Remediation of Radioactively Contaminated Groundwater. Vienna: IAEA.
Inyang, H. I., J. D. Betsill, R. Breeden, G. H. Chamberlain, S. Dutta, L. Everett, R. Fuentes, J. Hendrickson, J. Koutsandreas, D. Lesmes, G. Loomis, S. M. Mangion, C. Pfeifer, R. W. Puls, R. L. Stamnes, T. D. Vandel, and C. Williams. 1996. Performance monitoring and evaluation. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. PB96-180583. Springfield, Va.: National Technical Information Service.
Josten, N. E., R. J. Gehrke, and M. V. Carpenter. 1995. Dig-Face Monitoring During Excavation of a Radioactive Plume at Mound Laboratory, Ohio. INEL-9510633. Idaho Falls: Idaho National Engineering and Environmental Laboratory.
Kheboian, C., and C. F. Bauer. 1987. Accuracy of selective extraction procedures for metal speciation in model aquatic sediments. Analytical Chemistry 59:1417–1423.
Kleinmann, R. L. P., T. O. Tiernan, J. G. Solch, and R. L. Harris. 1983. A low-cost, low-maintenance treatment system for acid mine drainage using sphagnum moss and limestone. In National Symposium on Surface Mining, Hydrology, Sedimentology and Reclamation. Lexington, Kentucky: University of Kentucky.
Koerner, R. M., J. L. Guglielmetti, R. C. Bachus, P. T. Burnette, N. Cortlever, J. M. Cramer, R. E. Landreth, S. M. Mangion, M. Phifer, and W. M. Walling. 1996. Vertical barriers: Geomembranes. In Assessment of Barrier Containment Technologies , R. E. Rumer and J. K. Mitchell, Eds. PB96-180583. Springfield, Va.: National Technical Information Service.
Korte, N., O. R. West, F. G. Gardner, S. R. Cline, J. Strong-Gunderson, R. L. Giegrist, and J. Baker. 1997. Deep soil mixing for reagent delivery and contaminant treatment. Pp. 525–530 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Kumar, P. B., V. Dushenkov, H. Motto., and I. Raskin. 1995. Phytoextraction: The use of plants to remove heavy metals from soils. Environmental Science and Technology 29:1232–1238.
Lesmes, D., D. Cist, and D. Morgan. 1997. Ground penetrating radar investigation of a frozen earth barrier. Pp. 1074–1080 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Losi, M. E., C. Amrhein, and W. T. Frankenberger, Jr. 1994. Factors affecting chemical and biological reduction of hexavalent chromium in soil. Environmental Toxicology and Chemistry 13:1727–1735.
Lovley, D. R., and E. J. P. Phillips. 1992. Bioremediation of uranium contamination with enzymatic uranium reduction. Environmental Science and Technology 26:2228–2234.
Lovley, D. R., E. J. Phillips, Y. A. Gorby, and E. R. Landa. 1991. Microbial reduction of uranium. Nature 350:413–416.
Macaskie, L. E. 1991. The application of biotechnology to the treatment of wastes produced from the nuclear fuel cycle: Biodegradation and bioaccumulation as a means of treating radionuclide containing streams. Critical Reviews in Biotechnology 11:41–112.
Martin, J. P., P. Nire, and A. J. Thomas. 1987. Sequential extraction techniques: Promises and problems. Marine Chemistry 22:313–342.
McMahon, D. R., R. Fuentes, E. W. Gleason, B. M. LaRue, P. C. Repetto, D. J. A. Smyth, and A. Street. 1996. Vertical barriers: Sheet piles. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. PB96-180583. Springfield, Va.: National Technical Information Service.
Morrison, S. J., and R. R. Spangler. 1992. Extraction of uranium and molybdenum from aqueous solution: A survey of industrial materials for use in chemical barriers for uranium mill tailings remediation. Environmental Science and Technology 26:1922–1931.
Morrison, S. J., and R. R. Spangler. 1993. Chemical barriers for controlling ground water contamination. Environmental Progress 12:175–181.
Morrison, S. J., V. S. Tripathi, and R. R. Spangler. 1995. Coupled reaction transport modeling of a chemical barrier for controlling uranium(VI) contamination in groundwater. Journal of Contaminant Hydrology 17:347–363.
Naftz, D. L. 1997. Field demonstration of reactive chemical barriers to control radionuclide and trace-element contamination in ground water, Fry Canyon, Utah. Presented at 1997 GSA Annual Meeting, Salt Lake City, October 20–23.
Nordstrom, D. K., L. N. Plummer, T. M. L. Wigley, T. J. Wolery, J. W. Ball, E. A. Jenne, R. L. Bassett, D. A. Crerar, T. M. Florence, B. Fritz, M. Hoffman, G. R. Holdren, Jr., G. M. Lafon, S. V. Mattigod, R. E. McDuff, F. Morel, M. M. Reddy, G. Sposito, and J. Thraikill. 1979. Comparison of computerized chemical models for equilibrium calculations in aqueous systems. In Chemical Modeling in Aqueous Systems: Speciation, Sorption, Solubility and Kinetics, E. A. Jenne, Ed. Symp. Ser. 93. Washington, D.C.: American Chemical Society.
NRC (National Research Council). 1994. Alternatives for Ground Water Cleanup. Washington, D.C.: National Academy Press.
NRC. 1997. Innovations in Ground Water and Soil Cleanup. Washington, D.C.: National Academy Press.
Oscarson, D. W., H. B. Hume, and F. King. 1994. Sorption of cesium on compacted bentonite. Clays and Clay Minerals 42:731–736.
Otten, A., A. Alphenaar, C. Pijls, F. Spuij, and H. de Wit. 1997. In Situ Soil Remediation. Boston: Kluwer Academic Publishers.
Pellerin, L. 1997. Geophysical verification of the thin diaphragm wall barriers at the Dover National Test Site. Final report submitted by LBNL to the DOE-EM SCFA. Berkeley, Calif.: Lawrence Berkeley National Laboratory.
Perdue, E. M., and C. R. Lytle. 1983. Distribution model for binding of protons and metal ions by humic substances. Environmental Science and Technology 17:654–660.
Persoff, P, G., J. Moridis, J. Apps, K. Pruess, and S. J. Muller. 1994. Designing injectable colloidal silica barriers for waste isolation at the Hanford Site. Pp. 87–102 in In-Situ Remediation: Scientific Basis for Current and Future Technologies, G. W. Gee and N. R. Wing, Eds. Richland, Wash.: Battelle Press.
Peters, R. 1994. Demonstration of ground freezing for radioactive/hazardous waste disposal. Pp. 103–112 in In-Situ Remediation: Scientific Basis for Current and Future Technologies, G.W. Gee and N.R. Wing, Eds. Richland, Wash.: Battelle Press.
Peterson, M. E., R. C. Landis, G. Burke, M. Cherrington, B. Dwyer, B. Gemmi, A. Iskandar, G. Loomis, R. Peters, R. Waters, and P. Yen. 1996. Artificially Emplaced Floors and Bottom Barriers. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. PB96-180583.
PNNL (Pacific Northwest National Laboratory). 1996. In Situ Redox Manipulation Field Injection Test Report—Hanford 100 H Area. PNNL-11372. Richland, Wash.: Battelle Pacific Northwest National Laboratory.
Puls, R. W., D. W. Blowes, and R. W. Gillham. 1998. Emplacement verification and long-term performance monitoring for permeable reactive barrier at the USCG support center, Elizabeth City, North Carolina. Pp. 459–466 in Groundwater Quality: Remediation and Proctection. PB98-151285. Springfield, Va.: National Technical Information Service.
Rabideau, A. J., C. B. Andrews, C. Chiang, P. Grathwol, J. S. Hayworth, P. Culligan-Hensley, M. Manassero, J. W. Mercer, H. Mott, P. R. Schroeder, C. Shackelford, G. Teutsch, G. A. M. van Meurs, R. Wilhelm, and C. Zheng. 1996. Containment transport modeling. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. PB96-180583. Springfield, Va.: National Technical Information Service.
Radovanovic, H., and A. A. Koelmans. 1998. Prediction of in situ trace metal distribution coefficients for suspended solids in natural waters. Environmental Science and Technology 32:753–759.
Rapin, F., A. Tessier, P. G. C. Campbell, and R. Carignan. 1986. Potential artifacts in the determination of metal partitioning in sediments by a sequential extraction procedure. Environmental Science and Technology 20:836–840.
Rendall, P. S., G. E. Batley, and A. J. Cameron. 1980. Adsorption as a control of metal concentrations in sediment extracts. Environmental Science and Technology 14:314–318.
Riley, R. G., J. M. Zachara, and F. J. Wobber. 1992. Chemical Contaminants on DOE Lands and Selection of Contaminating Mixtures for Subsurface Science Research. DOE/ER-0547T. Washington, D.C.: DOE, Office of Energy Research.
RTDF (Remediation Technologies Development Forum). 1999. Permeable Reactive Barrier Installation Profiles. Washington, D.C.: EPA. http://www.rtdf.org/public/permbarr/barrdocs.htm.
Rulkens, W. H., and J. W. Assink. 1984. Extraction as a method for cleaning contaminated soil: Possibilities, problems and research. Pp. 576–583 in Proceedings of the 5th National Conference on Management of Uncontrolled Hazardous Waste Sites, Washington, D.C.
Rumer, R. R., and J. K. Mitchell. 1995. Assessment of Barrier-Containment Technologies: A Comprehensive Treatment for Environmental Remeditaion Applications. Springfield, Va.: National Technical Information Service.
Rumer, R. R., and M. E. Ryan. 1995. Barrier Containment Technologies for Environmental Remediation Applications . New York: Wiley.
Rusin, P., L. Quintana, J. Brainard, B. Streitlemeier, C. Tait, S. Ekberg, P. Palmer, T. Newton, and D. Clark. 1994. Solubilization of plutonium hydrous oxide by iron reducing bacteria. Environmental Science and Technology 28:1686–1690.
Russel, K., and A. Rabideau. 1997. Impact of vertical barriers on performance of pump-and-treat systems. Pp. 902–909 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12..
Sabatini, D. A., R. C. Knox, E. E. Tucker, and R. W. Puls. 1997. Environmental Research Brief: Innovative Measures for Subsurface Chromium Remediation: Source Zone, Concentrated Plume, and Dilute Plume. EPA/600/5-971005. Washington, D.C.: EPA.
Schecher, W. D., and D. C. McAvoy. 1992. MINEQL+: A software environment for chemical equilibrium modeling. Computers, Environment and Urban Systems 16:65.
Schnoor, J. L. 1997. Phytoremediation. Pittsburgh, Pa.: Ground-Water Remediation Technologies Analysis Center .
Schultz, D. S., and R. C. Landis. 1998. Design and cost estimation of permeable reactive barriers. Remediation 9(1):57–68.
Sheppard, M. I., and M. Stephenson. 1997. Critical evaluation of selective extraction methods for soils and sediments. Pp. 69–97 in Contaminated Soils: Proceedings of 3rd International Conference on the Biogeochemistry of Trace Elements. R. Prost, Ed. Paris: INRA.
Sheppard, M. I. and D. H. Thibault. 1992. Desorption and extraction of selected heavy metals from soils. Journal of the Science Society of America 56:514–523.
Sheppard, S. C., and W. G. Evenden. 1992. Bioavailability indices for uranium: Effect of concentration in eleven soils. Archives of Environmental Contamination and Toxicology 23:117–124.
Siegrist, R. L., O. R. West, M. I. Morris, D. A. Pickering, D. W. Greene, C. A. Muhr, D. D. Davenport, and J. S. Gierke. 1995. In situ mixed region vapor stripping in low-permeability media. 2. Full-scale field experiments. Environmental Science and Technology 29(9):2198–2207.
Smith, L. A., J. L. Mearis, A. Chen, B. Alleman, C. C. Chapman, J. S. Tixier, Jr., E. Brauning, A. R. Gavaskar, and M. D. Royet. 1995. Remedial Options for Metals-Contaminated Sites. Boca Raton, Fla.: Lewis Publishers.
Smyth, D. J. A., S. G. Shikaze, and J. A. Cherry. 1997a. Hydraulic performance of permeable barriers for in situ treatment of contaminated groundwater. Pp. 881–887 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Smyth, D., R. Joewtt, and M. Gamble. 1997b. Sealable joint steel sheet piling for ground-water control and remediation: Case histories. Pp. 206–214 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Snyder, G., G. Mergia, and S. Cook. 1997. Containment performance assessment through hydraulic testing-Baltimore works site with comparison. Pp. 1046–1052 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Spalding, B. P., J. S. Tixier, and C. L. Timmerman. 1997. In situ vitrification field-scale treatability study at ORNL seepage pit 1. Poster presented at Ninth National Technology Information Exchange Workshop, Shilo Inn, Idaho Falls, Idaho, August 26–28.
Steeples, D. W., and R. D. Miller. 1993. Basic principles and concepts of practical shallow seismic reflection profiling. Mining Engineering October: 1297–1302.
Stumm, W. 1992. Chemistry of the Solid-Water Interface. New York: Wiley.
Stumm, W., and J. J. Morgan. 1981. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters. (Second Edition). New York: Wiley.
Subsurface Contaminants Focus Area (SCFA). 1996. Subsurface Contaminants Focus Area Technology Summary. DOE/EM-0296. Oak Ridge, Tenn.: Office of Scientific and Technical Information.
Tessier, A., P. G. C. Campbell, and M. Bisson. 1979. Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry 51:844–851.
Tipping, E. 1994. WHAM-A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Computers and Geoscience 20:973–1023.
Tipping, E., N. B. Hethering, J. Hilton, D. W. Thompson, E. Bowels, and J. Hamilton-Taylor. 1985. Artifacts in the use of selective chemical extraction to determine distributions of metals between oxides of manganese and iron. Analytical Chemistry 57:1944–1946.
Tucker, M. D. 1996. Technical Considerations for the Implementation of Subsurface Microbial Barriers for Restoration of Ground Water at UMTRCA Sites. SAND96-1459. Albuquerque, N.M.: Sandia National Laboratories.
Turick, C. E., W. A. Apel, and N. S. Carmiol,. 1996. Isolation of hexavalent chromium reducing anaerobes from hexavalent chromium-contaminated and noncontaminated environments. Applied Microbiology and Biotechnology 44:683–688.
Van den Berg, C. M. G. 1984. Determination of the complexing capacity and conditional stability constants of complexes of copper(II) with natural organic ligands in seawater by cathodic stripping voltammetry of copper-catechol complex ions. Marine Chemistry 15:1–18.
Vermeul, V. R., S. S. Teel, J. E. Amonette, C. R. Cole, J. S. Fruchter, Y. A. Gorby, F. A. Spane, J. E. Szecsody, M. D. Williams, and S. B. Yabusaki. 1995. Geologic, Geochemical, Microbiologic, and Hydrologic Characterization at the In Situ Redox Manipulation Test Site. PNL-10633. Richland, Wash.: Pacific Northwest Laboratory.
Vidic, R. D., and F. G. Pohland. 1996. Treatment Walls. Technical Evaluation Report TE-96-01. Pittsburgh, Pa.: Ground-Water Remediation Technologies Analysis Center.
Weast, R. C., Ed. 1980. CRC Handbook of Chemistry and Physics. Boca Raton, Fla.: CRC Press, Inc.
Whang, J. M. 1996. Chemical-based barrier materials. In Assessment of Barrier Containment Technologies, R. E. Rumer and J. K. Mitchell, Eds. PB96-180583. Springfield, Va.: National Technical Information Service.
Williams, C. V., S. Dalvit Dunn, and W. E. Lowry. 1997. Tracer verification and monitoring of containment systems. Pp. 1039–1045 in Proceedings of the International Containment Technology Conference, St. Petersburg, Fla., February 9–12.
Williams, M. D., S. B. Yabusaki, C. R. Cole, and V. R. Vermeul. 1994. In situ redox manipulation field experiment: Design analysis. In In-Situ Remediation: Scientific Basis for Current and Future Technologies, G. W. Gee and N. R. Wing, Eds. Richland, Wash.: Battelle Press.
Williams, M. D., S. B. Yabusaki, C. R. Cole, and V. R. Vermeul. 1994. In-situ redox manipulation field experiment: Design analysis. In Thirty-Third Hanford Symposium on Health and the Environment: In-Situ Remediation: Scientific Basis for Current and Future Technologies, Part 1, G. W. and N. R. Wing, Eds. Columbus, Ohio: Battelle Press.
Zorpette, G. 1996. Confronting the nuclear legacy: Hanford's nuclear wasteland. Scientific American (May):88–97.