Protein and Amino Acids, 1999
Pp. 121-136. Washington, D.C.
National Academy Press
6
Regulation of Muscle Mass and Function: Effects of Aging and Hormones
Niels Moller and K. Sreekumaran Nair1
INTRODUCTION
Comprising close to 50 percent of total body weight in lean individuals, skeletal (or striated) muscle constitutes the largest single component of the body and serves as the major repository of protein (close to 50 percent of total body protein) and free amino acids in the body. Besides its locomotive functions, skeletal muscle is also an important metabolic organ. Metabolic functions are crucial not only for locomotion but also for maintaining homeostasis of substrates in the circulation and providing amino acids for various body functions. Mitochondria in skeletal muscle convert energy from nutrients into adenosine triphosphate (ATP) by oxidative phosphorylation. For mechanical functions involving mainly locomotion, this chemical energy (ATP) is further
converted into mechanical energy by the enzymatic (ATPase) action of myosin and actin in the sarcomere (Figure 6-1). In this process, a major proportion of the energy loss inevitably appears as heat. Under resting conditions, muscle accounts for 20 to 30 percent of total resting energy expenditure, the variability of which to a large extent is determined by differences in muscle metabolism (Zurlo et al., 1990). Under conditions of cold exposure and shivering thermogenesis, the function of muscle as a "heater" for the body and the resultant energy loss become still more conspicuous. In addition, skeletal muscle supplies amine acids for synthesis of proteins in other tissues (crucial during wound healing), for the immune functions, and for gluconeogenesis (alanine and glutamine) under catabolic conditions. Skeletal muscle also oxidizes glucose and fatty acids and stores large amounts of glycogen postprandially.
To fulfill these requirements optimally, muscle tissue function relies on a variety of factors as illustrated in Figure 6-1. First, the contractile myofibrillar apparatus consisting of actin and myosin filaments must exist in sufficient quality and quantity, and nervous activation of the contractile elements must take place in a controlled fashion. Second, the quality and quantity of mitochon-drial proteins (mostly enzymes) must be sufficiently maintained to ensure efficient ATP production. Also critical, that the metabolic demands of the muscle cell be met to ensure a continuous ATP production (i.e., supplies of fuel substrates such as glucose and fatty acids and oxygen must be adequate) and that the metabolic by-products such as carbon dioxide are removed efficiently and in a timely manner. The quality of all proteins, both structural and metabolically active (such as myosin, actin, and mitochondrial enzymes), are maintained by a continuous remodeling process involving protein breakdown and protein
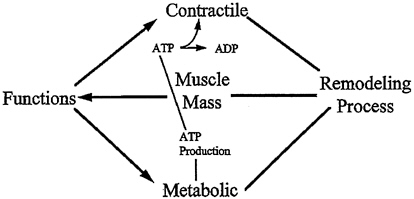
FIGURE 6-1 Muscle mass and quality are determined by a remodeling process. Muscle mass is one determinant of muscle function, which is also dependent on the quality of distinct muscle proteins. Muscle functions include both contractile and metabolic aspects. Two metabolic processes critical for contractility are mitochondrial ATP production and subsequent hydrolysis of ATP in the sarcomere (by myosin and actin) to release chemical energy for contractile function.
synthesis. The tissue concentration of a specific protein is determined by the balance between protein breakdown and protein synthesis. Nutrient supply to the muscle tissue (for ATP production) and the removal of metabolic by-products (e.g., carbon dioxide) are dependent on uninterrupted and dynamic circulatory systems. All of these individual processes are controlled by regulatory mechanisms, which include circulating and local levels of hormones and substrates, which in turn are influenced by the physiological state of the individual in terms of age, gender, nutritional status, exercise, and chronic or acute illness. Using aging as an example, this brief review outlines some of the control mechanisms and other biological factors involved in the regulation of muscle mass and function.
SARCOPENIA OF AGING
Aging can be described as a model in which many of the regulatory mechanisms are disrupted, resulting in functional disabilities involving both locomotive and metabolic aspects.
In spite of vigorous attempts by individuals to avert the physical impact of age, all population-based studies show a relentless loss of muscle mass and strength with aging. In addition, increased muscle fatigability and decline in endurance capacity substantially retard the functional capabilities of the elderly population. Some evidence indicates that this phenomenon is not solely due to a loss of muscle quantity, but also to an impairment of muscle quality (Reed et al., 1991; Rooyackers et al., 1996) (Figures 6-2 and 6-3). The combination of loss
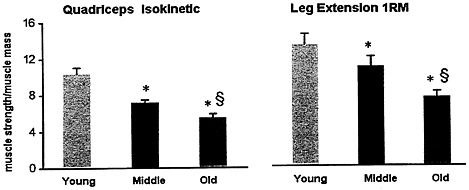
FIGURE 6-2 Muscle strength is not solely determined by muscle mass. With advancing age, a continuous loss of muscle efficiency occurs, indicating that muscle quality is declining. This study normalized muscle strength, quadriceps isokinetic strength, and leg extension for regional muscle mass (measured by Dual Photon X-ray) and showed a progressive decline with aging (P < 0.05-0.01). * Significant difference (P< 0.01) from young age group; § Significant difference (P< 0.05) from middle age group. Source: Adapted from Balagopal et el. (1997).
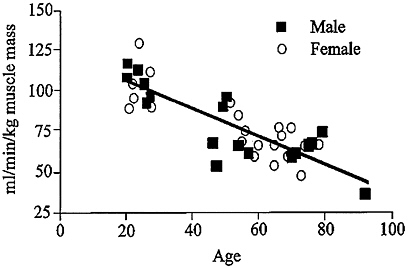
FIGURE 6-3 VO2max declines with aging after normalizing for muscle mass, indicating that the decline in endurance capacity depends not only on muscle mass but also on other factors. Source: Rooyackers, O.E., D.B. Adey, P.A. Ades, and K.S. Nair. 1996. Effect of age on in vive rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA 93:15364-15369. Copyright 1996 National Academy of Sciences, U.S.A.
of muscle mass and increased muscle weakness and fatigability, which results in substantial impairment of muscle function, has been coined sarcopenia of aging and may contribute substantially to morbidity of the elderly by restricting physical activity, increasing the risk of falls and fractures, and causing changes in body metabolism and composition, which results in increased incidence of noninsulin-dependent diabetes mellitus.
It has been reported that in elderly in comparison with young subjects, there is a decline in the synthesis rate of mixed muscle protein—both total and myofibrillar proteins (Welle et al., 1993; Yarasheski et al., 1993). Interestingly, a recent study demonstrated that not only did synthesis of muscle mitochondrial proteins (pivotal to oxidative phosphorylation and ATP generation) decrease in the elderly, but also that this 40-percent decrease in mitochondrial protein synthesis occurred as early as middle age (average age 52 years) (Rooyackers et al., 1996) (Figure 6-4). The decline in mitochondrial protein synthesis was markedly more pronounced than the concomitant 10- to 15-percent decline in synthesis rates of mixed muscle proteins (Rooyackers et al., 1996). These changes were also associated with a decline in cytochrome-c-oxidase activity and endurance capacity (Rooyackers et al., 1996) (Figure 6-5). It is possible that the decline in mitochondrial protein synthesis may cause the impairment of endurance capacity and the more pronounced muscle fatigability in the aging population. In addition, robust ATP production is crucial for synthesis of other muscle proteins. A general decline in synthesis rates of several muscle proteins
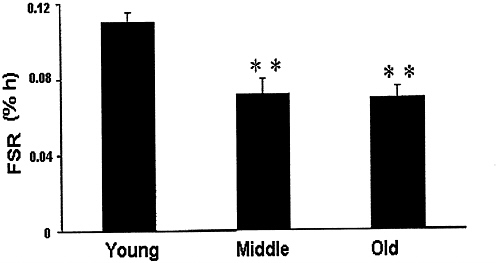
FIGURE 6-4 A decline in fractional muscle mitochondrial protein synthesis occurred with age. Approximately a 40 percent decline occurred by middle age (P < 0.01), but there was no further decline with advancing age. ** Indicates significant difference from young age. Source: Rooyackers, O.E., D.B. Adey, P.A. Ades, and K.S. Nair. 1996. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA 93:15364-15369. Copyright 1996 National Academy of Sciences, U.S.A.
(such as myosin heavy chain and mitochondrial proteins) occurs with age (Balagopal et al., 1997; Rooyackers et al., 1996; Welle et al., 1993; Yarasheski et al., 1993), perhaps reflecting the inability of mitochondria to produce sufficient ATP. Furthermore, recent studies have demonstrated that synthesis rates of myosin heavy chain, a major myofibrillar protein involved in hydrolysis of ATP and conversion of chemical energy from ATP to mechanical energy, also decline by middle age (Balagopal et al., 1997). These results suggest that the aging process selectively affects the ATP-generating machinery of muscle and imply that any intervention should seek to restrict this loss of mitochondrial capacity. In addition, the reduced synthesis rate of myosin heavy chain is compatible with the notion that the ability to maintain adequate muscle protein quality declines with age, thereby potentially compromising the efficiency of the locomotive apparatus to extract mechanical energy from fuel stores. As discussed below, it is likely that age associated decrements in circulating levels of anabolic hormones, such as growth hormone (GH), insulin-like growth factor I (IGF-I), sex steroids, and fading effectiveness of insulin are all involved in the involution of muscle that occurs with aging.
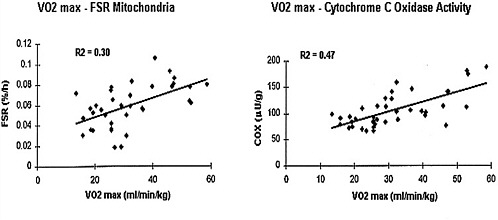
FIGURE 6-5 Relationship between maximal aerobic capacity per kilogram muscle mass and the fractional synthesis rate (FSR) of mitochondrial proteins and the activity of cytochrome c oxidase (COX) in muscle biopsies. Data (derived from Rooyackers et al. [1996]) indicate that cytochrome oxidase activity and fractional muscle protein rate arc significantly correlated to VO2max (P < 0.01).
HORMONAL EFFECTS ON MUSCLE PROTEIN
Insulin
Insulin is an anabolic hormone. After a meal, the ensuing insulin surge suppresses lipolysis and lipid oxidation and stimulates glucose storage and oxidation in skeletal muscle. The effects of insulin on muscle protein metabolism are less certain. Based on the clinical observation that insulin-deprived diabetic patients become cachectic and sarcopenic, it is, however, undisputed that insulin action is fundamental to preservation of functioning muscle mass. This clinical observation was convincingly confirmed when it was shown that insulin treatment increases nitrogen content in diabetic patients and that perfusion of the forearm with insulin in humans decreased amino acid release (Pozefsky et al., 1969). It remained to be resolved, however, whether this anticatabolic effect of insulin was due to stimulation of protein synthesis or inhibition of protein breakdown.
Studies performed in Type I diabetic patients showed that insulin replacement inhibits both protein breakdown and synthesis at the whole body level, but protein conservation occurs because the magnitude of insulin's effect on protein breakdown is more pronounced than on protein synthesis (Nair et al., 1995). It was also observed that insulin replacement has no effect on synthesis rate of muscle protein, indicating that the main effect of insulin on protein synthesis occurs in tissues other than muscle (Nair et al., 1995) (Figure 6-6). In that study, which employed sampling of the femoral artery, femoral vein, and hepatic vein and administration of amino acid tracers in insulin-deprived patients with diabetes, it was demonstrated that insulin replacement inhibited protein breakdown in the leg, with no effect on protein synthesis, while it inhibited protein breakdown and synthesis in the splanchnic bed, indicating that insulin's anticatabolic effect is largely due to its inhibition of muscle protein breakdown (Nair et al., 1995). This concept is supported (Gelfand et al., 1987) by the finding of one study that local forearm perfusion with insulin inhibited protein degradation without any effect on protein synthesis. However, the bulk of in vitro experiments suggest that insulin stimulates protein synthesis (Kimball and Jefferson, 1988), and a recent human in vivo study reported, based on data from arteriovenous differences combined with a muscle biopsy, that insulin augmented protein synthesis in the perfused leg (Biolo et al., 1995).
The reason for these discrepancies is not clear, but it may relate to the possibility that the response to insulin depends on the prevailing concentration of substrate (amino acids), in particular intracellularly, in the immediate precursor pool for protein synthesis (amino acyl tRNA). In addition, hyperinsulinemia is normally prompted by meals, and most human studies were performed in the postabsorptive state because of theoretical problems relating to isotope modeling in a nonsteady state. In the postprandial state, especially
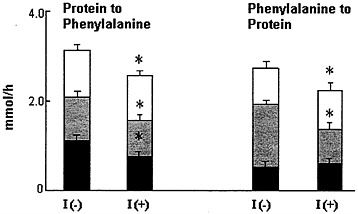
FIGURE 6-6 Phenylalanine kinetics in insulin-dependent diabetic subjects in the insulin deprived [I(-)] and insulin treated [I(+)] state. Splanchnic region ( ) accounted for a similar proportion of approximately 30 percent of whole body breakdown on both occasions, whereas the contribution of skeletal muscle (![]() ) decreased from 37 to 30 percent with insulin treatment. The skeletal muscle component of whole body protein synthesis increased from 19 to 27 percent during insulin treatment, concomitant with a decrease of splanchnic protein synthesis from 50 to 34 percent. The entire decrease in whole body protein synthesis during insulin treatment is due to changes in the splanchnic bed. This study clearly demonstrated that insulin replacement in the diabetic patients achieves protein conservation mainly by decreasing muscle protein breakdown. Source: Data derived from Nair et al. (1995).
) decreased from 37 to 30 percent with insulin treatment. The skeletal muscle component of whole body protein synthesis increased from 19 to 27 percent during insulin treatment, concomitant with a decrease of splanchnic protein synthesis from 50 to 34 percent. The entire decrease in whole body protein synthesis during insulin treatment is due to changes in the splanchnic bed. This study clearly demonstrated that insulin replacement in the diabetic patients achieves protein conservation mainly by decreasing muscle protein breakdown. Source: Data derived from Nair et al. (1995).
after a mixed meal, amine acid concentrations increase, whereas insulin administration in the postabsorptive state decreases amine acid concentration. It is therefore crucial to determine whether insulin's effect on muscle protein synthesis is qualitatively different in the postabsorptive and postprandial states. Thus, the precise mechanism of insulin's anticatabolic effect, in particular on skeletal muscle, remains to be clearly defined.
Another pertinent question is whether insulin differentially affects the individual muscle proteins. Novel techniques that allow assessment of fractional synthesis and breakdown rates of specific proteins, such as mitochondrial proteins, myosin heavy chain, and myosin isoforms, have been, or are being, developed and will soon serve to clarify these issues.
Growth Hormone and IGF-I
Growth hormone (GH) has widespread metabolic actions, including a catabolic lipolytic effect in adipose tissue (Moller et al., 1995). In most other tissues, GH acts anabolically, in part by increasing concentrations of IGF-I in the circulation and locally. It is well established that patients with GH deficiency
have a decreased muscle mass and impaired muscle function (Jorgensen et al., 1989) and that both abnormalities tend to normalize during treatment. When high doses of GH are given for a week to normal volunteers, whole body protein synthesis increases (Horber and Haymond, 1990), but this acute increase seems to occur mostly in tissues other than skeletal muscle (Copeland and Nair, 1994). Studies assessing the specific effects of GH on muscle protein synthesis and breakdown have yielded conflicting results (Butterfield et al., 1997; Copeland and Nair, 1994; Fryburg et al., 1991; Welle et al., 1996; Yarasheski et al., 1995), although infusion of GH directly into an artery has been reported to enhance muscle protein synthesis (Fryburg et al., 1991). Recently, one study was able to detect a 50-percent increase in muscle protein synthesis afar I week of GH treatment in elderly women (Butterfield et al., 1997). Other studies, however, failed to show any effect of GH on muscle protein synthesis in elderly subjects in comparison with placebo (Welle et al., 1996) or in young or elderly subjects undergoing a. resistance training program (Yarasheski et al., 1995). It is intriguing, however, that muscle mass and strength increased on GH administration (Welle et al., 1996) in these subjects without any effect on myofibrillar protein synthesis. It is possible that GH-induced accretion of protein occurs during the postprandial state since all muscle protein synthesis measurements were performed in the postabsorptive state. It is also important to include measurements of synthesis of specific proteins, which may clarify the discrepancies among many previous studies.
When considering the effects of (GH on muscle metabolism, it is important to remember that GH exposure invariably leads to increments in the levels of insulin, IGF-I, and in general, free fatty acids and that all of these compounds have an independent protein anabolic impact.
IGF-I has potent anabolic properties. Dissection of the metabolic effects of IGF-I is entangled by the facts that (1) in the circulation only a minute percentage of IGF-I is free, the remainder being bound to numerous distinct binding proteins with specific kinetic characteristics, and (2) many of the actions of IGF-I may be executed in an autocrine or paracrine manner in body compartments not readily accessible for investigations (LeRoith, 1997).
Studies in which IGF-I has been perfused locally indicate that IGF-I preferentially stimulates protein synthesis in muscle (Fryburg, 1994), whereas systemically administered IGF-I only stimulates muscle protein synthesis when additional amino acids are supplied (Fryburg et al., 1995). The roles of circulating versus local IGF-I and of free versus bound fractions, as well as the importance of each individual binding protein, remain to be determined.
Sex Steroids
Little is known about the possible effects of estrogen and progesterone on muscle metabolism, and it is uncertain whether the age-related decline in muscle mass in postmenopausal women is associated with the loss of ovarian function.
Testosterone has anabolic actions on muscle. Many years ago, testosterone replacement in adult hypogonadal men was shown to enhance nitrogen retention (Kenyon et al., 1940). A recent study concluded that supraphysiological doses of testosterone increased muscle mass and strength in normal men, in particular when combined with a muscle training program (Bhasin et al., 1996). It has also been shown that 6 months of testosterone replacement in five hypogonadal men led to a 20 percent increase in muscle mass and a 50 to 60 percent increase in mixed muscle protein synthesis (Brodsky et al., 1996). There was a nonsignificant increase in the synthesis rate of myosin heavy-chain, a component of the contractile apparatus (Brodsky et al., 1996) (Figure 6-7). Administration of testosterone has also been reported to enhance muscle protein synthesis in elderly men (Urban et al., 1995). Although it has been clearly
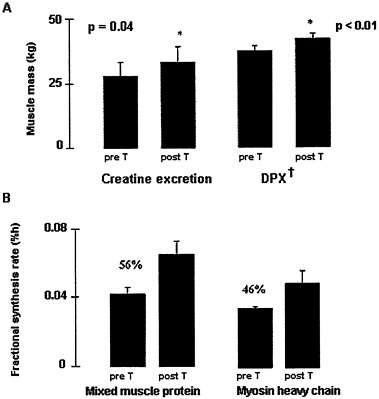
FIGURE 6-7 Six months of testosterone replacement in hypogonadal men increased muscle mass (P < 0.05) and decreased fat mass; an associated increase in fractional synthesis of mixed muscle protein (P < 0.05) and myosin heavy chain (P < 0.09) is likely to explain the increase in muscle mass. † Dual Photon X-ray. Source: Data derived from Brodsky et al. (1996).
established that levels of biologically available testosterone fall with aging, the effects of testosterone on age-related sarcopenia and other problems associated with aging remain to be clearly defined. It is, however, also unclear whether testosterone replacement in elderly men may increase the incidence of prostatic cancer and/or other problems such as lipid disorders.
CATABOLIC HORMONES (GLUCAGON, CORTISOL, EPINEPHRINE, AND THYROID HORMONES)
Glucagon, a catabolic hormone, increases the net loss of protein from the body in the postprandial state due both to an acceleration of amino acid disposal and to inhibition of protein synthesis (Charlton et al., 1996). There is no evidence that glucagon has any direct effect on muscle; rather, it seems likely that the primary effect is a stimulation of hepatic ureagenesis and consumption of glucogenic amino acids for glucose production leading to decreased circulating levels of amino acids. Glucagon has been shown to increase leucine oxidation during insulin deficiency (Nair et al., 1987), but this effect seems to be modest, in particular when insulin is replaced (Hartl et al., 1990).
In patients with Cushing's syndrome due to glucocorticoid excess, muscle wasting is a symptom. On the whole body level, it has been observed that 1 week of prednisone treatment increased protein breakdown and oxidation (Horber and Haymond, 1990). Whether this overall effect is secondary to specific events in muscle or caused by alterations in, for instance, hepatic amino acid metabolism is unclear at present.
Although catecholamines are generally regarded as being catabolic hormones, there is no solid evidence to support the concept that catecholamines (epi- and norepinephrine) have any negative impact on protein balances. As catabolic hormones, they do, however, cause a hypermetabolic state, which in turn may increase overall breakdown of protein.
The clinical observation that both hypo- and hyperthyroid patients have muscle weakness suggests that thyroid hormones may have a diphasic effect on muscle mass and function with an interposed ''euthyroid" optimum. Arteriovenous studies demonstrated that hyperthyroidism is associated with a net increase of muscle protein breakdown, although no changes were observed in hypothyroid patients (Morrison et al., 1988). Direct measurement of muscle protein synthesis is required to determine the effect of thyroxine on muscle protein synthesis.
In addition to the hormones mentioned above, it is possible that cytokines and, probably, prostaglandins may have important actions on muscle protein metabolism. It should be noted that many of these agents have diverse effects when given systemically. For instance, administration of tumor necrosis factor-a to humans leads to a full-blown pyrogenic response with fever, malaise, and release of all major stress hormones together with hyperglycemia and signs of augmented lipolysis and ketogenesis (Starnes et al., 1988).
SUBSTRATES AND NUTRITION
Prevailing levels of substrates in the circulation or, more precisely, in the microenvironment have important implications for muscle function and muscle mass. An adequate fuel supply is crucial to maintain tissue protein turnover and to preserve appropriate tissue balances. There is mounting evidence that the abundance of fuel substrates, per se, including amine acids is a significant regulator of protein metabolism in muscle. After a meal, concentrations of glucose, fatty acids, and amine acids rise together with pancreatic secretion of insulin. Under these conditions, protein accretion in muscle is increased due to both enhanced synthesis and inhibited protein breakdown (Tessari et al., 1996). A number of studies have shown that elevation of plasma amine acid concentrations stimulates protein synthesis and inhibits breakdown (Nair et al., 1992; Svanberg et al., 1996; Tessari et al., 1996). Glucose and fatty acids have protein-sparing effects (Tessari et al., 1986), but to what extent these effects are independent of insulin is currently unresolved. In addition, ketone bodies (specifically 3-hydroxybutyrate, a by-product of fatty acid metabolism) have been shown to stimulate the synthesis rate of muscle protein (Nair et al., 1988).
It thus appears that under the free-living conditions of everyday life, hormones and substrates act in mutual support to restrict protein loss and preserve muscle mass and function.
AUTHORS' CONCLUSIONS
The maintenance of functioning muscle mass is a complex process that involves orchestration of the effects of anabolic and catabolic hormones, nutritional state, and supply of substrates to the site of protein synthesis together with physical activity. As illustrated in Figure 6-8, hormones play a key role, although the precise level of their action and the interaction of hormones with other factors such as substrate supply remains to be clearly defined. It is hoped that newly developed techniques that allow assessment of the contribution of each of these factors to the fractional synthesis of specific muscle proteins will substantially improve our understanding of this field. Regulation of breakdown of specific muscle proteins is beyond current investigative capabilities because of the paucity of technology to quantify breakdown of specific muscle proteins. It is possible, however, to measure the regulation of the enzyme systems responsible for protein breakdown. Further studies that integrate the effects of hormones and substrates on muscle protein turnover with the effects of genetic factors are necessary to fully understand the regulation of muscle mass and functions.
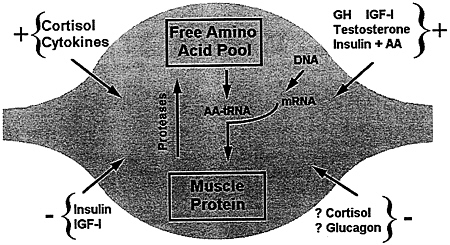
FIGURE 6-8 The quality and quantity of protein in a muscle cell are regulated by an intricate interplay between hormonal, nutritional, genetic, nervous, and physical factors, some of which are outlined here. The effects of any one of these factors on the specific processes of synthesis and breakdown of individual muscle proteins remain to be clearly defined.
REFERENCES
Balagopal, P., O.E. Rooyackers, D.B. Adey, P.A. Ades, and K.S. Nair. 1997. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 273:E790-E800
Bhasin, S., T. Storer, N. Berman, C. Callegari, B. Clevenger, J. Phillips, T.J. Bunell, R. Tricker, A. Shirari, and R. Casaburi. 1996. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men . N. Engl. J. Med. 335:1-7.
Biolo, G., R.Y.D. Fleming, and R.R. Wolfe. 1995a. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Invest. 95:811-819.
Brodsky, I.G., P. Balagopal, and K.S. Nair. 1996. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men. J. Clin. Endocrinol. Metab. 81:3469-3475.
Butterfield, G.E., J. Thompson, M.J. Rennie, R. Marcus, R.L. Hintz and A.R. Hoffman. 1997. Effect of rhGH and IGF-I treatment on protein utilization in elderly women. Am. J. Physiol. 272:E94-E99.
Charlton, M.R., D.B. Adey, and K.S. Nair. 1996. Evidence for a catabolic role of glucagon during an amino acid load. J. Clin. Invest. 98:90-99.
Copeland, K.C., and K.S. Nair. 1994. Acute growth hormone effects on amino acid and lipid. J. Clin. Endocrinol. Metab. 78:1040-1047.
Fryburg, D.A. 1994. Insulin-like growth factor I exerts growth hormone and insulin-like actions on human muscle protein metabolism. Am. J. Physiol. 267:E331-E336.
Fryburg, D.A., R.A. Gelfand, and E.J. Barrett. 1991. Growth hormone acutely stimulates forearm protein synthesis in normal subjects. Am. J. Physiol. 260:F499-E504 metabolism.
Fryburg, D.A., L.A. Jahn, S.A. Hill, D.M. Oliveras, and E.J. Barrett. 1995. Insulin and insulin-like growth factor I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J. Clin. Invest. 96:1722-1729.
Gelfand, R.A., and E.J. Barrett. 1987. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J. Clin. Invest. 80:1-6.
Hartl, W.H., H. Miyoshi, F. Jahoor, S. Klein, D. Elahi, and R.R. Wolfe. 1990. Bradykinin attenuates glucagon-induced leucine oxidation in humans. Am. J. Physiol. 259:E239-E245.
Horber, F.F., and M.W. Haymond. 1990. Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J. Clin. Invest. 86:265-272.
Jorgensen, J.O.L., L. Thuesen, T. Ingemann-Hansen, S.A. Pedersen, J. Jorgensen, N.E. Skakkebaek, and J.S. Christiansen. 1989. Beneficial effects of growth hormone treatment in GH deficient adults. Lancet 1:1221-1225.
Kenyon, A.T., K. Knowlton, I. Sandiford, F.C. Koch, and G. Lotwin. 1940. A comparative study of the metabolic effects of testosterone proprionate in normal men and women and in eunuchoidism. Endocrinology 26:26-45.
Kimball, S.R., and L.S. Jefferson. 1988. Cellular mechanisms involved in the action of insulin on protein synthesis. Diabetes Metab. Rev. 4:773-787.
LeRoith, D. 1997. Insulin-like growth factors. N. Engl. J. Med. 336:633-640.
Moller, N., J.O.L. Jorgensen, J. Moller, P. Ovesen, O. Schmitz, J.S. Christiansen, and H. Orskov. 1995. Metabolic effects of growth hormone in humans. Metabolism 44(suppl.):33-36.
Morrison, W.L., J.N.A. Gibson, R.T. Jung, and M.J. Rennie. 1988. Skeletal muscle and whole body protein turnover in thyroid disease. Eur. J. Clin. Invest. 18:62-68.
Nair, K.S., G.C. Ford, K. Ekberg, E. Fernqvist-Forbes, and J. Wahren. 1995. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J. Clin. Invest. 95:2926-2937.
Nair, K.S., D. Halliday, D.E. Matthews, and S.L. Welle. 1987. Hyperglucagonemia during insulin deficiency accelerates protein catabolism. Am. J. Physiol. 253:E208-E213.
Nair, K.S., R.G. Schwartz, and S. Welle. 1992. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am. S. Physiol. 263:E928-E934.
Nair, K.S., S. Welle, D. Halliday, and R.G. Campell. 1988. Effect of 3-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans . J. Clin. Invest. 82:198-205.
Pozefsky, T., P. Felig, J.D. Tobin, J.S. Soeldner, and G.F. Cahill. 1969. Amine acid balance across tissue of forearm in postabsorptive man. Effect of insulin at two dose levels. J. Clin. Invest. 48:2273-2282.
Reed, R.L., L. Pearlmutter, K. Yochum, E.E. Meredith, and A.D. Mooradian. 1991. The relationship between muscle mass and muscle strength in the elderly. J. Am. Geriatr. Soc. 39:555-561.
Rooyackers, O.E., D.B. Adey, P.A. Ades, and K.S. Nair. 1996. Effect of age on in vive rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. 93:15364-15369.
Starnes, H.F., R.S. Warren, M. Jeevanandam, J.L. Gabrilove, W. Larchian, H.F. Oettgen, and M.F. Brennan. 1988. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J. Clin. Invest. 82:1321-1325.
Svanberg, E., A.C. Moller-Loswick, D.E. Mathews, U. Korner, M. Andersson, and K. Lundholm. 1996. Effects of amine acids on synthesis and degradation of skeletal muscle proteins in humans. Am. J. Physiol. 271:E718-E724.
Tessari, P., S.L. Nissen, J. Miles, and M.W. Haymond. 1986. Inverse relationship of leucine flux and oxidation to free fatty acid availability in vive. J. Clin. Invest. 77:575-581.
Tessari, P., M. Zanetti, R. Barazonni, M. Vettore, and F. Michielan. 1996. Mechanisms of postprandial protein accretion in human skeletal muscle. Insight from leucine and phenylalanine forearm kinetics. S. Clin. Invest. 98:1361-1372.
Urban, R.J., Y.H. Bodenburg, C. Gilkison, J. Foxworth, R.R. Wolfe, and A. Ferrado. 1995. Testosterone administration to healthy elderly men increases muscle strength and protein synthesis. Am. J. Physiol. 296:E820-E826.
Welle, S., C. Thornton, R. Jozefowicz, and M. Statt. 1993. Myofibrillar protein synthesis in young and old men . Am. J. Physiol. 264:E693-E698.
Welle, S., C. Thornton, M. Statt, and B. McHenry. 1996. Crowth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J. Clin. Endocrinol. Metab. 81:3239-3243.
Yarasheski, K.E., J.J. Zachwieja, and D.M. Bier. 1993. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. 265:E210-E214.
Yarasheski, K.E., J.J. Zachwieja, J.A. Campell, and D.M. Bier. 1995. Effect of growth hormone and resistance training on muscle growth and strength in older men. Am. J. Physiol. 268:E268-E276.
Zurlo, F., K. Larson, C. Bogardus, and E. Ravussin. 1990. Skeletal muscle is a major determinant of resting energy expenditure. J. Clin. Invest. 86:1423-1427.
DISCUSSION
ROBERT NESHEIM: Any questions?
JEFF ZACHWIEJA: What is the dose of testosterone that you used in the hypogonadal man?
K. SREEKUMARAN NAIR: Just a replacement dose. We administered, I believe, 3 mg/kg of testosterone every two weeks.
JEFF ZACHWIEJA: You got a similar increase in muscle protein synthesis in men who are not hypogonadal?
K. SREEKUMARAN NAIR: No. Only in hypogonadal men. We did not administer testosterone to normal subjects. In the study that the UCLA group did (Bhasin et al., 1996), they administered testosterone supraphysiological doses and found substantial increase in muscle mass in men who are not hypogonadal. We administered replacement doses. We did not look at the dose effect or what is the effect of testosterone in healthy men on muscle protein synthesis. The UCLA group gave supraphysiological doses and measured the effect on muscle mass and strength.
JEFF ZACHWIEJA: Was it 600 milligrams?
K. SREEKUMARAN NAIR: That is right.
JEFF ZACHWIEJA: Was there any functional improvement in the muscle of those men?
K. SREEKUMARAN NAIR: In this group we did not measure their muscle strength. It was an outpatient study where the patients were administered testosterone for that study. The UCLA group showed increase of muscle strength on supraphysiological testosterone administration.
GAIL BUTTERFIELD: In terms of the military's interest, do you find the kinds of changes that you observed in mitochondrial protein and myosin heavy chain, with a decrease in energy intake or a decrease in protein intake?
K. SREEKUMARAN NAIR: We do not have any data on that. It is only the first study we have done in the aging population. But certainly, from the military point of view, I am sure that you may have the endurance capacity and also the muscle strength decline with aging. It is an open question what impact the changes in nutrition has on this age related changes.
ROBERT NESHEIM: Last question.
PATRICK DUNNE:With the military situation, it is important to consider the interactions of exercise and dietary carbohydrate intake and their impact on hormones. It may be desirable to design diets that are higher or lower in carbohydrate or protein for their effects on muscle function, but it is necessary to consider whether people will eat those diets. That is a factor that must be included in this discussion.
ROBERT NESHEIM: Thank you very much, Dr. Nair. I guess that one ought to stay young if one is going to be in the military. I am kidding.
















