Protein and Amino Acids, 1999
Pp. 19-75. Washington, D.C.
National Academy Press
1
Committee Overview
INTRODUCTION
Proteins form the major constituents of muscle, catalyze virtually all chemical reactions in the body, regulate gene expression, and comprise the major structural elements of all cells. Individual amino acids, the components of proteins, also serve as neurotransmitters, hormones, and modulators of various physiological processes. Every aspect of physiology involves proteins. According to Bier (see Chapter 5), credit for the name "protein" is given to the Dutch chemist Gerardus Johannes Mulder, who wrote an article in French that was published in a Dutch journal on July 30, 1838. In this article, he asserted that this material was the essential general principle of all animal body constituents and defined it by the Greek word proteus (which he translated to the Latin, primarius, meaning primary). Mulder appears to have taken this word directly from a letter sent to him by the Swedish chemist Jacques Bursailleus on July 10, 1838, in which the name protein had been suggested. Aside from the amazing fact of a Dutch chemist borrowing n Latin word from a Swedish
chemist, which he defined in Greek in an article written in French for a Dutch journal, the entire sequence of events appears to have occurred in a period of 20 days, demonstrating the efficiency of both mail service and scientific publication in those days.
The relationship between dietary protein and bodily protein metabolism is a major focus of research today. Many questions remain regarding the validity of methods for assessing protein balance; thus, the question of how best to assess dietary protein requirements remains unanswered. In addition, the influence of genetics, hormones, physical activity, infectious processes, and environmental stresses on protein metabolism and protein requirements continues to be explored. Another major focus of research that is of great interest is the role of dietary protein and amine acids in modulating physiological function, as measured for example by physical and mental performance. The possibility that protein or individual amine acids in quantities that exceed those required to maintain protein balance may have the potential to contribute to performance optimization is of great interest.
THE ARMY'S INTEREST IN DIETARY PROTEIN AND PROTEIN BALANCE
Because of the unique demands placed on soldiers in combat, the military is particularly concerned about the role that dietary protein may play in controlling muscle mass and strength; response to injury, infection, and environmental stress; and cognitive performance. As described in Chapter 3 by Karl Friedl, the longer, more isolated deployments and maneuvers that are becoming more commonplace may limit access to rations. The nutritional studies of Ranger trainees conducted by the U.S. Army Research Institute of Environmental Medicine (USARIEM) (IOM, 1992, 1993b) identified losses of up to 30 percent in lean body mass (including organs, such as the liver, plasma, and proteins) after 3 weeks of limited food intake and high energy expenditure. Although increased energy intake offset these losses somewhat, they were still significant, suggesting the need for additional energy, protein, or both. In these studies, the observed decrease in lean body mass was accompanied by changes in serum levels of several hormones including testosterone, insulin-like growth factor I (IGF-I), and triiodothyronine (T3) (Friedl, 1997; Nindl et al., 1997), the significance of which is unclear. Because the administration of these hormones is known to stimulate protein synthesis under some conditions, the Army maintains considerable interest in exploring their potential both to ameliorate the losses in lean body mass sustained by troops under conditions of extreme negative energy balance and to stimulate an increase in muscle mass and physical performance. In contrast to the limited intakes of protein and energy measured in the Ranger studies, a more recent study, in which soldiers subsisted on the field ration known as Meals, Ready to Eat (MREs) for 30 days, showed
average energy intakes of 2500 kcal/d and protein intakes of 103 g/d (Thomas et al., 1995), raising questions about the optimum protein-to-energy ratio for performance and health.
As the typical battlefield scenario becomes more automated, soldiers must attend to increasing numbers of signals in the face of increasing amounts and sources of noise, with increasingly dangerous consequences for failure. Thus, the possibility that cognitive performance may depend on diet and that performance optimization may be achievable through dietary modifications such as amino acid supplements is of considerable interest to the military. A 1994 report by the Committee on Military Nutrition Research (CMNR), entitled Food Components to Enhance Performance, briefly considered the influence of protein and amino acids (and all other dietary components) on physical and cognitive performance and response to stress (IOM, 1994). Data were presented on the effect of protein-to-carbohydrate ratio on mental alertness, the effect of physical activity on protein requirements, and the influence of branched-chain amino acids, tyrosine, and tryptophan in pharmacological amounts on cognitive function. The report concluded that the potential ability of tyrosine supplements to sustain alertness and cognitive performance in the face of environmental stress merited further investigation.
Finally, the risk of injury and infection faced by soldiers in the field is extremely high. At the same time, conditions of sleep and food deprivation, environmental extremes, and heightened emotional stress all exert a negative impact on the immune system. The CMNR report Military Strategies for Sustainment of Nutrition and Immune Function in the Field (IOM, 1999), considered the effects of diet, including protein and individual amino acids such as glutamine, on immune response and concluded that although the role of energy intake in immune function is probably more significant than that of protein, individual amino acids such as glutamine and arginine appear to play crucial roles in modulating immune function. The effects of these amino acids are considered in greater detail in this report.
ESTIMATION OF PROTEIN REQUIREMENTS
Current estimates of protein requirements for mature humans and the methods used to assess these requirements are being scrutinized by the research community and are a source of considerable disagreement.
Protein Metabolism
The requirement for protein arises from growth, from the need to replace obligatory losses, and from the need to respond to environmental stimuli. The
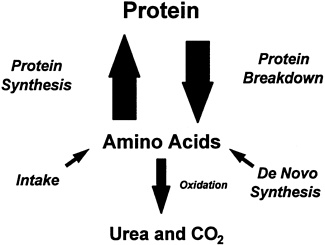
FIGURE 1-1 Pathways of protein turnover. Source: Young and Marchini, 1990.
breakdown products of protein—amino acids—enter the free amino acid pool of the body (distributed among body fluids and tissues) from four sources: (1) dietary proteins; (2) so-called dispensable (nonessential) amino acids, which can be synthesized in the body; (3) breakdown products of body protein (particularly skeletal muscle, the largest tissue in the body and the site of protein storage); and (4) the products of recycling by intestinal microbes (Figure 1-1). In mature humans, a homeostatic mechanism maintains the balance between tissue protein synthesis and breakdown by drawing on the free amino acid pool.
Methods for Assessment of Protein Requirements
Because the majority of nitrogen in the body is associated with protein and amino acids, nitrogen has been used as a marker for assessing whole-body and tissue protein flux and status. The traditional method for assessing whole-body protein metabolism is nitrogen balance, where nitrogen (N) intake and output (in feces, sweat, and urine, as well as other miscellaneous sources) are measured and the difference [Nbal=(Nin = Nout)] is expressed in grams of nitrogen per day (g N/d). Total body protein loss or retention is then calculated using the conversion factor of 6.25 g N/g protein (Munro and Crim, 1994).
A state of positive nitrogen balance exists when the total nitrogen output is less than the total nitrogen ingested. Positive nitrogen balance requires adequate protein and energy intake plus a stimulus for synthesis. A state of positive nitrogen balance (an anabolic state) exists for the synthesis of new tissues during the growth observed in childhood, adolescence, and pregnancy. When dietary protein or energy intake is inadequate or an individual experiences an acute-
phase response, nitrogen excretion exceeds nitrogen intake and a state of negative nitrogen balance exists (net protein catabolism). When dietary protein is adequate or more than adequate and energy intake matches energy output, a state of nitrogen equilibrium exists (Nin = Nout for any intake above the required level). A state of nitrogen equilibrium is required to maintain total body protein mass. Altering protein or energy intake or physical activity may alter nitrogen balance.
Despite the usefulness of nitrogen balance assessment in estimating the adequacy of protein intake, there are significant limitations to its use, including overestimation of nitrogen intake and incomplete collections of urine, feces, or sweat, which result in an underestimation of nitrogen output. The net outcome is an overestimate of nitrogen retention and of the body's ability to adapt to inadequate protein intakes; these overestimates limit the ability of the nitrogen balance technique to assess nitrogen requirement. The limitations of nitrogen balance assessment are discussed further below and by Millward and Young in Chapters 9 and 10, respectively.
In the mid-1940s, stable isotopes of hydrogen (2H) and nitrogen (15 N), were made available for use in biomedical research. However, the mass spectrometry technology that would use these isotopes for rapid analysis of biological specimens was not widely available until the late 1970s. With the improvement of this technology and the widespread availability of stable isotope-labeled metabolites, amino acid kinetic studies have come to augment nitrogen balance in examining the effects of dietary protein, energy, and physical activity on overall protein metabolism.
Amino acids labeled with stable isotopes of hydrogen (2H), nitrogen (15N), and carbon (13C) have been administered orally and intravenously. With the use of the primed continuous infusion technique, amino acid turnover can be studied in subjects of all ages under many physiological conditions (Munro and Crim, 1994). The calculation of amino acid flux (Q) is based on the following assumptions: (1) the body's flee amino acid pool is a homogeneous mixture that can be sampled from the plasma pool; (2) the only sources of the target amino acids entering the body pool are dietary protein (I) and intracellular protein breakdown (B); and (3) amino acid removal from this pool occurs by irreversible oxidation (E) or synthesis into protein (Z). In reality, a large quantity of recycled amino acids is derived daily from the breakdown of body proteins. In addition to the sizable turnover of blood cells, mucosal cells of gastric and intestinal villi are continuously moved toward villus tips where they slough off and undergo digestion; released free amino acids are then reabsorbed into the plasma pool (Munro and Crim, 1994). Thus, the equation Q = B + I = E + Z describes the steady-state relationship in which the total entry of amino acids into the free amino acid pool (B + I) is equal to the total exit of amino acids from the free amino acid pool (E + Z). Rates of protein synthesis and protein breakdown can be calculated from this equation (Picou and Taylor-
Roberts, 1969). At isotopic steady state, total amine acid turnover (Q) is measured, and the rate of protein breakdown can be calculated knowing the rate of amine acid intake. Likewise, the rate of protein synthesis can be calculated when the rate of amine acid disappearance is known. If a 13C-labeled amine acid is used, oxidation can be measured from 13CO2 excretion rates.
Because of its unique role as an amine acid that is oxidized in skeletal muscle and not converted to a tricarboxylic acid (TCA) cycle intermediate, leucine (in the 13C form) has been the amine acid of choice for many amine acid kinetic studies. However, due to its unique metabolism, it may not be representative of the entire pool of amine acids. Glycine labeled with 15N has also been used extensively to study protein synthesis and breakdown because it has the advantage of ubiquitous utilization.
FAO/WHO/UNU Requirements and RDAs: Current Estimates of Average Protein Intake
Estimations of protein and amine acid requirements are currently based on nitrogen balance studies. The 1985 report of the Food and Agriculture Organization (FAO), World Health Organization (WHO), and United Nations University (UNU) proposed a protein requirement of 0.625 g per kilogram of body weight per day (g/kg BW/d) for egg or beef protein and a ''safe" level of 0.75 g/kg BW/d for mixed protein if the protein is as digestible as egg or beef (FAO/WHO/UNU, 1985). The current recommended dietary allowance (RDA) for protein in the U.S. diet (which is derived by adding two standard deviations to the estimated requirement) is 0.8 g/kg BW/d for adult men and women (Table 1-1) (NRC, 1989).
Also based on nitrogen balance data, the recommendation for total essential or indispensable amine acids (IAAs) as a percentage of protein intake is 43 percent for infants and 11 percent for adults (FAO/WHO/UNU, 1985). Essential (indispensable) and nonessential (dispensable) amine acids are traditionally distinguished on a nutritional basis because essential amine acids cannot be synthesized by the body and must be part of the diet to permit growth or to maintain nitrogen balance, whereas nonessential amine acids can be synthesized by the body. Metabolically, however, the distinctions are less clear because a number of essential amine acids can be formed by transamination (at least in laboratory animals). By this criterion, only the amine acids lysine and threonine appear not to be synthesized by transamination and are therefore indispensable (as discussed further below, the concentrations of these two amine acids in cereal proteins are so low as to limit their ability to sustain growth). By this same argument, glutamic acid and serine are the only truly dispensable amine acids because they can be synthesized by reductive amination of ketoacids. A
TABLE 1-1 Recommended Dietary Allowances for Protein
|
Age (years) or Condition |
Weight (kg) |
RDA g/d |
RDAg/kg BW/d |
|
Males |
|||
|
19-24 |
72 |
58 |
0.8 |
|
25-50 |
79 |
63 |
0.8 |
|
51+ |
77 |
63 |
0.8 |
|
Females |
|||
|
19-24 |
58 |
46 |
0.8 |
|
25-50 |
63 |
50 |
0.8 |
|
51+ |
65 |
50 |
0.8 |
|
Pregnant |
|
60 |
|
|
Lactating (first 6 months) |
|
65 |
|
|
Lactating (second 6 months) |
|
62 |
|
|
SOURCE: Adapted from NRC (1989). |
|||
third class—the conditionally essential amino acids—is synthesized from other amino acids. However, this synthesis is confined to particular organs and may be limited by certain physiological factors such as age or disease state (Reeds and Becket, 1996). As knowledge increases and techniques improve, the distinction between essential and nonessential amino acids becomes less clear. Adding to this lack of clarity are observations such as the one by Stucky and Harper (1962), who found that if rats were fed a diet adequate in nitrogen but lacking in nonessential amino acids, the growth rate of the animals was significantly decreased.
Importance of the Debate over Indispensable Amino Acid Requirements
Although consensus exists at present for the adult protein requirement this is not the case for the adult requirement of indispensable amino acids. Since the 1985 FAO/WHO/UNU report, Young and coworkers have presented data that contradict the findings of the report; based on these data, Young suggests that the adult requirement for total IAAs is 31 percent of the protein requirement, or about three times the FAO/WHO/UNU estimate (McLarney et al., 1996; Young, 1987, 1994; Young and El-Khoury, 1995a; Young and Marchini, 1990; Young et al., 1989; see also Chapter 10). This contention of the group at Massachusetts Institute of Technology (MIT) for higher indispensable amino acid needs has been countered by Millward and colleagues (Millward, 1994; Millward and Rivers, 1988, 1989; see also Chapter 9), who find significant methodological problems in the studies of Young and coworkers. This debate is important, because it influences whether or not protein quality is an issue to be considered
in setting protein requirements. Protein quality, a measure of the efficiency with which dietary protein is utilized, can be assessed by comparison of the amine acid profile of a given protein to various amine acid scoring patterns such those developed by the FAO/WHO for various age groups. If the requirement for IAAs is low (as proposed by FAO/WHO and Millward), the pattern is easily matched by most proteins, and protein quality ceases to be an issue in setting protein requirements for adults. However, if the FAO estimates are incorrect and indispensable amine acids are required in the higher amounts proposed by Young, individual protein sources may duplicate the scoring patterns poorly, and protein quality may then become a significant determinant of protein requirements.
Argument for Higher Indispensable Amine Acid Requirements
Young has based his argument for higher indispensable amine acid requirements on two related measures: the obligatory oxidative losses of these amine acids and the calculated obligatory losses based on daily nitrogen loss. In the latter calculation, Young assumes that the efficiency of dietary protein use is about 70 percent and that the lost protein has the composition of mixed body protein. Indispensable amine acid requirements calculated in these two ways (the MIT pattern) are approximately the same. In 1991, an expert panel of FAO/WHO also agreed that the IAA needs for adults are greater than those in the 1985 report and proposed that the amine acid pattern for preschool children (FAO/WHO, 1991), a pattern similar to the MIT pattern, be recommended for adults. Young argues that protein and indispensable amine acid intakes have to be high enough to provide sufficient flux for optimum "metabolic control." This concept proposes that a high flux rate of amine acids or other substrates provides a kinetic basis for a sensitive control mechanism to ensure adequate provision of metabolic intermediates. In the case of protein, these important intermediates would be amine acids such as glutamine, tyrosine, and tryptophan, which have important physiological roles to play independent of their incorporation into protein.
To prove their point, Young and colleagues carried out a long-term study to compare the effects of the FAO (FAO/WHO/UNU, 1985), MIT, and egg patterns of indispensable amine acids on amine acid balance in healthy young adults (Marchini et al., 1993). After a week on the egg pattern (high in IAAs), 20 young men were placed on diets resembling either the FAO, the MIT, or the egg pattern for three weeks. Based on a negative leucine balance while the subjects were on the FAO (compared with the MIT) pattern and changes in serum amine acid profiles, Marchini et al. (1993) concluded that the FAO pattern is not capable of maintaining amine acid homeostasis.
Since the 1991 FAO/WHO meeting, several groups have reevaluated the existing data and concluded that the original FAO recommendations were likely
to be underestimates but stopped short of endorsing the MIT pattern (Fuller and Garlick, 1994; Waterlow, 1996). In 1994, an expert panel met to consider the issue. After the meeting, the panel recommended that the entire question of how amino acid requirements are determined be reexamined but that, in the interim, the MIT pattern be accepted (Clugston et al., 1996). However, as subsequently pointed out by Millward and Waterlow (1996), this recommendation was not the consensus of the attendees but was inserted during postmeeting editing.
Argument Against Higher Indispensable Amino Acid Requirements
Millward and colleagues have challenged Young's data point by point (Millward, 1994; Millward and Rivers, 1988, 1989; see also Chapter 9). They suggest first that Young's stable isotope amino acid oxidation data, derived from stable isotope-labeled amino acid infusion studies, are flawed for two reasons. First, the amount of tracer used in the infusion studies is itself high enough to influence the oxidation of the amino acid and thus the balance determined. Second, the enrichment of the amino acid precursors being oxidized is not accurately measured, a critical issue in the interpretation of stable isotope research.
Next, Millward argues that there is no valid basis for assuming that the obligatory amino acid losses (as calculated from obligatory nitrogen loss) resemble the pattern of body protein, because some of the amino acids released during normal turnover are known to be preferentially recycled (lysine and threonine). In addition, he believes that the metabolic demand for IAAs is determined not by the need for high flux rates, but by the obligatory losses and the relative ability of the body to adapt on a diurnal basis to varying levels of these amino acids in the diet (he notes that digestive enzymes secreted in response to a meal can, over the short run, assist in meeting the indispensable amino acid needs by breaking down themselves). Finally, Millward points out that in the longer-term study mentioned above (Marchini et al., 1993), nitrogen balance did not differ significantly between the MIT and the FAO patterns; this finding suggests that both patterns support overall body protein economy.
The Rebuttal
Young agrees with Millward that there are inherent difficulties in defining requirements for indispensable amino acids. The two most serious and difficult-to-resolve problems are (1) accounting for the mass of stable isotope infused, which is large enough to affect nitrogen balance, and (2) determining the true precursor enrichment rate of the amino acid being infused and under study. On the first point, the agreement between IAA requirements calculated from oxidation rates and from nitrogen balance leads Young to conclude that the
mass of stable isotope infused does not "profoundly" affect the calculation of amine acid oxidation rates. He agrees, however, that this issue deserves more attention.
On the question of true precursor amine acid enrichments in the stable isotope experiments, Young points out that this is a problem primarily for lysine since measurements in experiments with branched-chain amine acids are made from keto acids derived intracellularly from the infused amine acid. Studies using L-[l-13C]phenylalanine as an indicator amine acid for determining the lysine requirement have yielded a requirement of 40 mg/kg BW/d (Duncan et al.; 1996, Zello et al., 1993), an estimate close to Young's own tentative new requirement for lysine (50 mg/kg BW/d). In this technique, the indicator amine acid (labeled phenylalanine) is infused at graded levels of lysine intake, and the "breakpoint" in 13CO2 excretion is measured, under the assumption that the uptake of phenylalanine into protein will be sharply decreased and its oxidation sharply increased at the point where lysine intake becomes inadequate.
Young's definition of the maintenance amine acid pattern for adults is generally similar to the amine acid pattern in body protein, except for lysine, threonine, and methionine, whose patterns were derived more from the results of his tracer studies. Young agrees that the body has significant ability to conserve lysine under conditions of inadequate intake. His calculations suggest that the lysine requirement is 30 percent lower than that found in mixed body protein, due to lysine conservation that results from diurnal cycling.
Resolution of the Debate
The practical implications of the debate between Young and Millward revolve primarily around lysine: the lysine content of cereal proteins is limiting for growth. If Millward is correct, then all dietary proteins, whether plant or animal, contain enough lysine and other amine acids to support adequate protein nutriture of adults if consumed in amounts that meet the protein requirement (although some military personnel in the 18-22-year age group are still growing, a factor that might influence the requirement for some amine acids). Millward has shown that wheat protein, a protein that is particularly low in lysine, is well utilized in adults in the postprandial period, even when net protein synthesis occurs. He suggests that the low level of lysine in this protein is supplemented by the tissue free amine acid pools. However, older data from Longenecker (Longenecker, 1961, 1963; Longenecker and Hause, 1959, 1961) show that the ingestion of wheat protein by dogs or humans may result in decreased plasma lysine levels accompanied by increased levels of other indispensable amine acids. Such data support the contention that a postprandial breakdown of body protein may supply the indispensable amine acids necessary for synthesis. However, under such circumstances, other IAAs may be used less efficiently for protein synthesis when lysine is limiting in the protein consumed,
which supports Young's belief that the indispensable amino acid requirement is higher than currently recommended. Thus, the controversy over requirements for IAAs is still unresolved.
The implications of this debate for the current state of knowledge of protein and amino acid requirements for the military depend in part on the current intake of dietary protein and amino acids by military personnel and in part on other factors influencing protein requirements in these individuals, as discussed below.
STRESSORS THAT INFLUENCE PROTEIN REQUIREMENTS
As discussed by Friedl in Chapter 3, the stressors encountered most frequently by military personnel are high levels of physical activity with Or without energy restriction; illness, injury, and infection; and environmental extremes. Although each of these stressors may somehow influence protein metabolism and protein requirements directly, they also produce changes in hormonal status that can influence protein metabolism as well. The impact of each of these factors on protein metabolism and requirements has been the subject of intense investigation in the civilian research community. A brief summary of relevant findings is presented here.
Physical Activity and Energy Restriction
The question of whether individuals who routinely engage in intensely physical occupational or athletic activities have increased requirements for dietary protein appears to have arisen from the observations that during exercise, muscle protein is utilized for fuel and that exercise can lead to an increase in muscle mass. However, whether protein requirements are in fact increased by physical activity is unclear and a subject of intense controversy. In Chapter 11, Rennie reviews the role of protein and its breakdown products, amino acids, in exercising muscle and discusses changes in protein metabolism induced by energy deficit.
Exercise and Amino Acid Catabolism
A major function of amino acid breakdown in muscle during periods of exercise is to supply tricarboxylic acid intermediates (anaplerosis) so that the oxidation of acetyl coenzyme A (CoA) can proceed at rates appropriate to the energy needs of the contractile apparatus. The exercise-induced increase in muscle alanine production may be a marker for this process. Specifically, glutamate can react with pyruvate, via the action of alanine-aminotransferase, to produce alanine and α-ketoglutarate. The latter then feeds into the TCA cycle,
and the former provides a mechanism for shuttling nonacidic gluconeogenic precursors to the liver. In addition, valine and isoleucine can be deaminated and enter the TCA cycle via succinyl CoA. Leucine, in contrast, may deplete TCA intermediates (cataplerosis) by promoting the transamination of α-ketoglutarate to glutamate. Rennie proposes that a major function of circulating glutamine, an amine acid that decreases in the circulation under circumstances of severe stress or trauma, may be to augment glutamate stores. Glutamine crosses the cell membrane easily and can be converted by the enzyme glutaminase to glutamate or by transaminase to α-ketoglutaramide, which can then be converted to α-ketoglutarate and ammonia. According to Rennie, these proposed mechanisms await validation, and the magnitude of the conversions has not been shown to limit energy availability. In addition, a threshold glutamate concentration for TCA cycle intermediate generation has not been established.
A different but possibly related question is why obese individuals placed on starvation diets experience potentially fatal muscle wasting despite adequate stores of energy. Owen and coworkers (1998) recently conducted a series of experiments to address this question and to test the hypothesis that in spite of adequate energy stores, muscle must be broken down to supply TCA cycle intermediates, so that energy can be used, and to supply precursors for hepatic gluconeogenesis. The administration of phenylacetate, which binds to plasma glutamine resulting in its excretion as phenylacetylglutamine, to obese subjects for the last 3 days of a 3-week fast resulted in no change in urinary creatinine, urea, uric acid, ammonium, or ketone body excretion and no decrease in hepatic or renal gluconeogenesis. These observations are consistent with a continuous demand for amine acid oxidation, presumably to supply TCA cycle intermediates and gluconeogenic precursors.
The extent of any increase in amine acid oxidation during exercise depends on several variables. These include exercise intensity, availability of energy from glucose and fat, nutritional status, training status (trained athletes experience less amine acid oxidation in muscles), and gender (Millward et al., 1994). In Chapter 11, Rennie concludes that although the oxidation of amine acids in muscle supplies energy, the magnitude of this oxidation does not appear to be sufficient to allow protein to be considered a major metabolic fuel during periods of exercise. Although it is theoretically possible that repeated bouts of intense physical activity in the face of limited nutrient intake might deplete glutamate and glutamine to an extent that would affect the TCA cycle, this possibility has not been tested.
Contractile Activity and Muscle Protein Turnover
Muscle protein synthesis appears to decrease during exercise and to rebound after exercise; thus, any net change in nitrogen balance or muscle protein is observed only over a period of several days. Muscle protein
breakdown during normal exercise is limited to soluble or membrane proteins degraded via lysosomal proteases. The degradation of myofibrillar protein appears to increase only with eccentric exercise (exercise such as walking downhill, in which muscle is forced to contract as it is stretched) (Fielding et al., 1991); the consequences of this damage to muscle tissue are discussed later. Little evidence exists to suggest that exercise leads to significant accumulation of muscle protein in the absence of steroid-induced growth (Forbes, 1985, cited in Millward et al., 1994).
According to Rennie (1996), any negative balance of protein synthesis and breakdown created during exercise is rapidly reversed by protein intake. Attempts to investigate the possibility that protein requirements are increased by physical activity are confounded by the observation, discussed by Millward (Millward et al., 1994; see also Chapter 9), that increases in protein intake result in increases in the catabolic processes leading to the oxidation of amino acids. A potentially significant implication of this adaptation is that individuals who habitually consume high-protein diets may face the risk of significant losses of protein stores if suddenly forced to curtail protein intake.
Energy Balance and Protein Requirements
The direct influence of energy intake on nitrogen balance has been recognized for many years (Cuthbertson and Munro, 1937). In an attempt to quantify this effect, Calloway (1975) fed groups of men diets in which first the levels of protein were varied while energy was held constant and then the levels of protein were held to the level nearest individual need (determined by N balance) while energy was varied. When 85 percent of maintenance energy was provided, nitrogen balance fell to -0.61 g/d. In contrast, when 115 percent of maintenance energy was provided, nitrogen balance rose to 0.59 g/d, with the greater increase occurring when energy intake rose from 85 to 100 percent of maintenance. Marginal protein intake appeared to have less effect than marginal energy intake. In a similar set of experiments, Kishi and coworkers (1978) fed diets of increasing energy content to groups of men for 3-week periods and found that at energy intakes of 40, 45, 48.2, and 57 kcal/kg, the estimated protein requirements to maintain nitrogen balance were 0.78, 0.56, 0.51, and 0.42 g/kg BW per day, respectively. This effect of energy intake on apparent maintenance protein requirements is observed only when protein intake is not limiting. Moreover, although it is clear that experimentally determined nitrogen balance is the result of both protein and energy intake, energy intake has not been considered in determining the protein requirements of various groups. If the results of Kishi and coworkers were applied to a 70 kg man, the protein requirement would be 54.6 g/d at an energy intake of 2800 kcal/d (the equivalent of two MREs). It is not known whether the relationship between energy intake and protein intake can be extrapolated to lower energy intakes
(such as those of Ranger trainees, who in past studies have been observed to consume approximately 1500-1700 kcal/d, including approximately 50 g protein). Furthermore, because the studies of Kishi and coworkers and Calloway relied on the technique of nitrogen balance, more reliable and precise results would be expected if the studies were repeated using more up-to-date techniques. Finally, it is possible that the estimation of resting energy expenditure and energy requirements could be improved by the use of newer techniques such as magnetic resonance imaging (MRI) derived organ-tissue mass measurement (Gallagher et al., 1998).
The mechanism by which energy intake exerts its effect on protein balance is not completely understood. In the resting state, carbohydrate intake inhibits protein catabolism. Two possible mechanisms have been proposed. One possibility is that the carbohydrate-stimulated increase in insulin secretion increases synthesis and decreases breakdown of protein. An alternative possibility is that the provision of carbohydrate simply decreases liver gluconeogenesis, so that amine acids are no longer drawn away from muscle. The provision of carbohydrates prior to and during exercise inhibits protein breakdown and oxidation of amine acids. Although it is known that carbohydrate and fatty acids are the primary fuels during exercise, the effect of triglycerides and medium-chain fatty acids on protein turnover is less well understood.
The issue of whether or not physical activity increases protein requirements is complicated. Results of studies depend in part on the energy intake of the participants, the kind and intensity of the exercise studied, and the length of time given for adaptation to varying protein intakes (Butterfield, 1987). R has long been held that individuals experiencing an energy deficit—whether it is the result of a decrease in energy intake, an increase in energy expenditure, or both—exhibit an increase in protein breakdown and protein requirements (Calloway and Spector, 1954; Calloway, 1975). However, the effect of chronic increases in energy expenditure and negative energy balance on muscle mass of exercising individuals is unclear. Several groups of investigators have observed that athletes undergoing strength and endurance training require greater protein intake to maintain nitrogen balance than do sedentary individuals (Lemon et al., 1992; Meredith et al., 1989; Phillips et al., 1993; Tarnopolsky et al., 1988, 1990a). However, the results of these studies have been questioned, in part because no differences were observed in performance or lean tissue mass between athletes consuming high-protein diets and those on lower-protein diets. In addition, many of these studies were too short to allow for adaptations to changes in protein intake (about 10 days) or exercise regimen (about 2 weeks) (Gontzea et al., 1975), which makes their interpretation difficult.
The possibility that moderate (approximately 50-60 percent of maximum capacity) exercise may have a protective effect on muscle protein in the negative energy balance situation has been suggested by a small number of
studies (Carraro et al., 1990; Stein et al., 1959; Stroud et al., 1996; Todd et al., 1994); however insufficient research has been conducted to confirm this observation. The classic study of college athletes and soldiers by Chittenden (1907) showed that a gradual 50 percent reduction in protein intake (to 0.75 g/kg BW/d) over 5 months did not decrease and in fact increased strength while decreasing fatigue. A study by Butterfield and Calloway (1984) also showed that moderate physical activity optimized protein utilization by previously untrained individuals who consumed lower-than-average amounts of protein. Buskirk (1996) has suggested that although controversy remains with regard to protein requirements and optimal protein intakes for athletes, a protein intake of 1.5 g/kg BW/d (the usual protein intake for most Americans) should be sufficient to preserve muscle mass and nitrogen balance if energy intake is sufficient.
Although the protein requirements of women have been studied inadequately, the small amount of available data suggests that the needs of nonpregnant women may be similar to those of men. Kurzer and Calloway (1986) showed variations in urea excretion in sedentary women, corresponding to the phases of the menstrual cycle, but also showed that nitrogen balance could be maintained on a protein intake approximating 0.8 g/kg BW/d. As mentioned above, protein requirements are related to energy intake. Because energy intake in active women is often lower than predicted from activity pattern (Mulligan and Butterfield, 1990), these individuals may be in negative energy balance and, therefore, would have increased requirements for protein. The reported tendency of military women to restrict energy intake intentionally because of concerns about weight and body mass (IOM, 1998) may further interfere with their ability to meet protein requirements during field operations.
In summary, the evidence to date suggests that sustained physical activity does not result in increased protein requirements for appropriately trained (and possibly even sedentary) individuals who are in energy balance. Data on physically active women are insufficient to determine whether their protein requirements are higher than those of sedentary women.
Infection, Injury, and Illness
Systemic infections and severe injuries trigger complex but predictable alterations in body protein metabolism that lead to increases in protein requirements. These effects, which are a component of acute-phase reactions now known to be initiated by the proinflammatory cytokines, include a rapid catabolic destruction of skeletal muscle proteins and, simultaneously, an equally rapid synthesis of many other body proteins.
These shifts in protein metabolism appear to be purposeful. The responses to infection and injury provide the body with newly formed proteins and the
immune system elements needed to mount a successful and effective defense, but at considerable nutritional costs.
In the response of protein metabolism to injury and infection, the contractile proteins of skeletal muscle serve as an amine acid ''bank." These muscle proteins undergo rapid catabolic breakdown, and the free amine acids liberated are released into plasma and used in visceral organs for a number of purposes. Some amine acids (especially the branched-chain group) are metabolized in situ within the muscle cell to provide energy, and their nitrogen components are immediately reutilized to create new amine acids (for example, glutamine and alanine) that are released into the circulation. Both glutamine and alanine are metabolized in visceral tissues, as sources of the additional energy needed to sustain the body-wide hypermetabolic state characteristic of acute-phase reactions.
Under the influence of proinflammatory cytokines, the liver takes up large quantities of plasma free amine acids. Hepatic uptake of amine acids is so large that their plasma concentrations may decline despite the massive quantities of amine acids being made available by muscle protein catabolism (Beisel, 1992; Kinney and Elwyn, 1995; Wilmore, 1991).
In addition to their metabolic degradation as sources of fuel, the resulting amine acids are used by the liver to manufacture numerous enzymes, metallothioneins, lipoproteins, and the large array of acute-phase reactant plasma proteins (α1-antitrypsin, α1-acid glycoprotein, haptoglobin, C-reactive protein, fibrinogen, the third component of complement, ceruloplasmin, amyloid, and orosomucoid). Protein synthesis is also enhanced in lymphocytes and immunologic tissues (Beisel, 1992).
In the liver, excess phenylalanine is converted to tyrosine, and excess tryptophan is metabolized via the kynurenine pathway, resulting in the creation of diazo reactants that are excreted in the urine. The hepatic enzymes needed to metabolize excess phenylalanine and tryptophan are induced rapidly as part of the body's cytokine-induced acute-phase response (Beisel, 1992). Nitrogen components of other degraded amine acids are converted into urea and excreted in the urine (Beisel, 1992; Wilmore, 1991). Losses of body nitrogen are thus markedly increased with infection or trauma.
Changes in dietary protein and energy requirements to sustain muscle protein synthesis during recovery from infections and severe injury are of considerable importance to the military situation (Wolfe, Chapter 13). A full discussion of the impact of illness and injury on protein and energy requirements is beyond the scope of this report; the reader is referred to a comprehensive review such as that by Souba and Wilmore (1994). In Chapter 8, Wilmore notes that protein requirements are approximately 1.5 g/kg BW/d in almost all patients recovering from serious injury or systemic infection, with the exception of burn patients, whose requirements are elevated to 2-2.5 g/kg BW/d. In addition, the postinjury energy requirements of bum victims are
among the highest known (Sakurai et al., 1995). However, the evolution of care for the injured, including those with thermal injuries, has greatly decreased the duration of hypermetabolic states and attenuated the peak response (D.W. Wilmore, Los Angeles, personal communication). The provision of intravenous energy sources and large quantities of amino acids to severely burned patients is unable to induce effective production of muscle protein (Sakurai et al., 1995). As discussed later, hormonal therapies have been used in combination with nutritional support in an attempt to improve this situation. In badly burned children, growth hormone has demonstrated positive effects on muscle protein synthesis (Gore et al., 1991). Despite the insulin resistance that develops during infection and trauma, insulin (along with adequate glucose) markedly increases the synthesis of muscle protein (Sakurai et al., 1995); however, protein breakdown increases as well, which negates the effect. Wolfe (see Chapter 13) speculates that testosterone, which stimulates protein synthesis under normal circumstances and is known to be suppressed in male burn patients, might have a synergistic effect with insulin on muscle protein synthesis.
Other Stressors
There is no question that deployed soldiers in field operations experience the simultaneous effects of multiple stressors. In addition to an interest in the effects of physical activity, injury, and infection, the military maintains considerable interest in the effects of temperature extremes and high altitude on protein requirements. Therefore, several previous reports of the CMNR have reviewed this topic.
Heat
As reviewed by Buskirk (1993), Mitchell and Edman (1949, 1951) postulated that protein requirements would increase slightly in hot environments as the result of increased sweat losses of nitrogen or tissue catabolism secondary to hyperthermia. Studies by Consolazio and coworkers (Consolazio and Shapiro, 1964) found that the increased protein intake of soldiers in the heat appeared to be due to the increase in energy intake, rather than to some innate need for more protein in hot environments. Calloway and colleagues (1971) estimated dermal losses of protein due to exercise-induced swearing and found that such losses could add approximately 0.5 g/d to previously calculated protein losses in active individuals, but they proposed that sweat-induced losses would likely be less in sedentary individuals. Paul (1989) observed that because protein contributes to energy needs during prolonged exercise and because sweat losses of nitrogen increase during intense prolonged exercise in hot weather, protein requirements might be increased under these circumstances (the effects of acclimatization are
not known). Nevertheless, Buskirk (1993) concluded that no evidence exists for an increase in the apparent protein requirement of soldiers (beyond the Military Recommended Dietary Allowance [MRDA] for protein) in hot environments. He further cautioned that an increase in dietary protein would raise fluid requirements (see discussion of protein and renal function later in this chapter) in individuals whose fluid requirements were already elevated by the effects of exercise and heat and whose intake might already be restricted by logistical factors.
Cold and High Altitude
In 1996, the CMNR reported on the effects of cold and high-altitude conditions on nutrient requirements (IOM, 1996). According to studies summarized by Jones and Lee (1996) and by LeBlanc (1996), there is no conclusive evidence that protein needs are increased by brief or prolonged exposure to cold temperatures. In a classic study cited by Rennie in Chapter 11, Stroud and coworkers (1996) showed that among two subjects who trekked across the Antarctic while in profound negative energy balance, whole-body protein turnover was slightly increased in one and slightly decreased in the other.
The MRDAs do not include a higher protein allowance for cold weather; however, they do include a higher energy allowance. Thus, the recommended contribution of protein to total energy intake in the cold (and the ratio of protein to total energy in the Ration, Cold Weather) is actually lower than that in moderate temperatures (and in the MRE). Because the metabolism of extra dietary protein results in increased urea excretion and, therefore, an increased fluid requirement, this lowered protein-to-energy ratio may be advantageous in cold environments where the availability of drinking water may be limited (Askew, 1989).
Butterfield (1996) and Hoyt and Honig (1996) reviewed the influence of high altitude on metabolism and on macronutrient requirements, and reported significant losses of lean tissue mass, body weight, and fat mass among most subjects. However, as demonstrated by Butterfield and coworkers (1992), if subjects consumed sufficient energy to maintain energy balance, weight losses were corrected and nitrogen balance was maintained. These findings suggest that the loss of lean tissue mass and the negative nitrogen balance often experienced at high altitude are due entirely to the negative energy balance caused by altitude-induced anorexia and increased metabolic rate, rather than to an increase in protein requirements. The CMNR concluded that there appeared to be no rationale for increasing the MRDA for protein for individuals working at high altitudes; however, the committee also suggested the need for further research on the effects of intense physical exertion on protein requirements at high altitudes.
Combined Stressors
It is generally recognized that at any given time, deployed soldiers and those in field training face a combination of stressors that may include energy imbalance (secondary to undereating, intense physical activity, or both), severe injury, systemic infection, climatic extremes, and changes in altitude. As described above, attempts to identify the effects of such stressors, alone or in combination, on protein requirements have thus far been inconclusive. Additional stressors, such as exposure to unidentified environmental contaminants and the emotional consequences of the battlefield and of separation from a familiar environment, have also been recognized, but far less is understood about their impact on physiology and nutritional status and how this might influence protein requirements (Friedl, 1997; IOM, 1995).
THE MRDA FOR PROTEIN
The protein (and energy) content of military operational rations was formulated during World War II on the basis of Food and Nutrition Board recommendations (NRC, 1941). Based on data for energy consumption and expenditure of soldiers during the war, the initial standard of 70 g protein per 3000 kcal for the 70 kg reference man was increased in 1947 to 100 g protein per day (based on 3600-kcal total energy intake) for physically active military men in temperate climates. The protein MRDA for women is 80 g/d based on a daily energy intake of 2000-2800 kcal (AR40-250, 1947). This amount of protein is equivalent to 11 percent of total recommended energy intake (results of recent national nutritional surveys, including the National Health and Nutrition Examination Survey (NHANES) III, have shown that protein intake in the U.S. population averages 14 to 16 percent of total food energy for both males and females).
Since the 1940s, the mean weight of male soldiers has increased from 68 to 78 kg; similarly, the mean weight of female soldiers has increased from 61 to 63 kg. Thus, the MRDA for protein expressed on a g/kg BW basis is 1.3 for both men and women. Based on observations suggesting that protein requirements may be increased for individuals engaged in specific types of exercise, the question arises whether recommendations established for protein intake by soldiers during World War II are still appropriate for military personnel today.
Protein Intake Studies of Military Personnel
Studies conducted in the 1940s and over the past decade have shown that military personnel maintain relatively high protein intakes during field operations as well as in garrison. Table 1-2 summarizes the average and range
of intakes measured in studies reviewed by Cline and others (Baker-Fulco et al., 1995; King et al., 1993; see also Cline, Chapter 4). Estimated protein intakes in the field and in garrison for both men and women met or exceeded the MRDA of 100 g/day and 80 g/day, respectively. Relative to body weight, the average daily protein intake for men in the field was 1.3 g/kg BW and for women, 1.2 g/kg BW, exceeding the estimated protein requirement of 0.8 g/kg BW and even the increased requirement suggested by Tarnopolsky and coworkers (1990b). Both energy and protein intake appear to have decreased between the 1940s and more recent times, whereas average body weight has increased over this period. The significance of this apparent decrease is not known; the observation may be attributable to changes in the method of dietary intake assessment or may be related to an observation by Cline and Warber (see Chapter 4) that approximately one-fourth of soldiers report trying to lose weight during field training exercises. Nevertheless, protein consumption by soldiers is still high relative to the RDA.
Operational rations include the individual combat rations such as the Meal, Combat, Individual (MCI); the Meal, Ready-To-Eat; and other rations (including A, B, K, T, and unitized group ration [UGR]; see Appendix B) used to support operations in the field. The energy and protein contents of operational rations are set at a level of 3600 kcal and 100 g/d, respectively. Because of their high energy content, operational rations theoretically contain 2.8 g protein per 100 kcal. According to Cline and Warber (see Chapter 4), the actual energy content of operational rations varies from 2794 kcal in C rations to 4300 kcal in B rations. The protein content varies from 79 g in K rations to 142 g in the UGR. Therefore, the protein-to-energy ratio varies from 2.8 to 4.3 g protein per 100 kcal (11-17 percent of energy), depending on the type of operational ration.
In comparing macronutrient intake in the field and in garrison, it appears that protein and energy intakes in the field did not exceed and may have been lower than intakes in garrison, even though energy expenditure would be expected to be higher in the field than in garrison. In the small number of studies in which energy expenditure was estimated, soldiers in the field tended to be in negative energy balance (as evidenced by weight loss), although their protein intake met or exceeded the MRDA (none of these studies included women). Dietary surveys indicate that soldiers preferentially eat the high-protein entrees of the rations (Baker-Fulco, 1995). As a result, mean protein intakes of soldiers in the field as a percentage of total dietary energy are higher than suggested by the ration contents.
Pregnancy, Lactation, and the MRDA for Protein
At the present time, there are no MRDAs for pregnancy or lactation. As shown in Table 1-1, the RDA for protein is increased by 10 g/d for pregnant women and 15 g/d for lactating women (NRC, 1989). Thus, the recommended
protein intakes for women in the weight range of 46-63 kg would be 44-57 g/d during pregnancy and 60-72 g/d during lactation. Some studies have suggested that the RDA for protein during lactation may be insufficient to meet the requirements of lactating women and have shown that actual protein requirements may be as high as 1.5 g/kg BW/d or 69-94 g/d (Motil et al., 1990, 1996). The MRDA of 80 g/d would therefore be sufficient to meet the apparent protein requirements of most pregnant or lactating women.
Summary
In summary, the protein intake of soldiers in both garrison and field situations appears adequate relative to the current MRDA. Because energy expenditures have not been measured for average soldiers in the field, it is not possible to determine their risk for negative energy balance, which could increase requirements for protein. MRDAs for women appear to be adequate to support pregnancy and lactation.
PERFORMANCE BENEFITS AND HEALTH RISKS OF SUPPLEMENTAL PROTEIN, AMINO ACIDS, AND PLANT PROTEINS
Supplement Use Among Army Personnel
The use of various protein and amino acid supplements by military personnel reflects the current public interest in strength training and body building. Such products are readily available at military commissaries, exchanges, and fitness centers, according to Cline and Warber (see Chapter 4). In a recent Army survey of protein supplement use, 36 percent of Army personnel less than 30 years old reported having used amino acids, and 30 percent had used protein powders. Supplement use was lower in persons more than 30 years of age: 26 percent reported having used amino acids, and 19 percent reported using protein powders. The highest use was reported among combat arms personnel (this category includes infantry, armor, field artillery, air defense, and Special Forces), followed by combat service support personnel (includes ordnance, quartermaster, and transportation), and combat support personnel (includes engineer, chemical, military intelligence, military police, signal, aviation, and civil affairs). Supplement use among men was approximately double that among women, and individuals required to eat in military dining facilities reported a greater likelihood of supplement use than those who received food allowances to eat off-site. Reasons given for supplement use included the desire to increase energy, improve athletic performance, increase muscle mass, engage in strength training, and gain weight (Warber et al., 1996).
TABLE 1-2 Dietary Protein Intake of Military Personnel (g/d)
|
Reference |
Location |
Protein Intake (% of total energy) |
N |
Duration and Type |
|
Kretsch et al., 1986 |
USMA |
84 (13.7) |
54 women |
5 d training |
|
|
|
125 (13.4) |
13 men |
|
|
Rose and Carlson, 1986 |
Ft. Sill |
129 |
31 men |
8 d field |
|
USACDEC/USARIEM, 1986 |
Hawaii |
67 (14.6) |
40 women |
44 d field |
|
|
|
98-113a |
33-38 men |
16, 48 d field |
|
Askew et al., 1987 |
Camp E Allenb |
112 |
17 men |
30 d training |
|
Carlson et al., 1987 |
NCO Academy |
124 (15.5) |
43 men |
8 d training |
|
Szeto et al., 1987 |
Ft. Lewis, WA |
75 (16.4) |
12 women |
6 d garrison |
|
|
|
103 (15.1) |
31 men |
|
|
Szeto et al., 1988 |
Ft. Devens |
114 (14.0) |
54 men |
7 d garrison |
|
Szeto et al., 1989 |
Ft. Devens |
126 (16.1) |
51 men |
8 d garrison |
|
Rose RW et al., 1989 |
Ft. Jackson, SC (BCT) |
96 (15.6) |
40 women |
7 d training |
|
|
|
125 (15.5) |
41 men |
|
|
Rose MS et al., 1989 |
Ft. Hood |
82 (14.0) |
N/A women |
8 d field |
|
|
|
113c |
N/A men |
|
|
Edwards et al., 1991 |
Bolivia |
68 (9.0) |
13 women |
15 d field |
|
|
|
97d |
35 men |
|
|
|
|
100d |
32 men |
|
|
Baker-Fulco et al., 1992 |
Marine OCS |
160 (14.5) |
12 men |
5 d training |
|
Klicka et al., 1993 |
USMA |
79 (13.7) |
86 women |
S d training |
|
|
|
130 (14.6) |
11 men |
|
|
Baker Fulco et al., 1994 |
Marine OCS |
169 (14.5) |
16 men |
5 d training |
|
King et al., 1994 |
Ft. Jackson |
82 (12.7) |
49 women |
7 d training |
|
Reference |
Location |
Protein Intake (% of total energy) |
N |
Duration and Type |
|
Thomas et al., 1995 |
Ft. Chaffeee |
125 (17.2) |
32 men |
30 d garrison and training |
|
|
|
103 (16.7) |
34 men |
|
|
Cline et al., 1998 |
Ft. Sam Houston |
75 (14.7) |
50 women |
56 d basic training |
|
Baker-Fulco, 1997 |
Camp Mackall |
43.7 (9.8)f |
34 women |
7 d training |
|
personal communication |
|
59.4 (14.1)g |
33 women |
|
|
|
|
65.6 (10.3)f |
35 men |
|
|
|
|
83.5 (14.9)g |
95 men |
|
|
Hirsch et al., in press |
Camp Parks |
103.5 (19.9)h |
19 women |
4 d |
|
|
|
81.8 (14.8)g |
18 women |
|
|
|
|
57.1 (9.8)i |
13 women |
|
|
|
|
135.4 (20.1)h |
13 men |
|
|
|
|
103.7 (14.9)g |
16 men |
|
|
|
|
69.6 (10.3)i |
11 men |
|
|
NOTES: BCT, basic combat training; N/A, not available; NCO, noncommissioned officer training; OCS, officer candidate school; USMA, U.S. Military Academy, West Point. a Wt. loss 1.3-2.1%. b 2 A rations + 1 MRE or 3 MRE: wt loss 4.8%, c Wt. loss 1.0%. d Wt. loss 2.0%. e Wt. loss 2.2%, f Concept ration. g MRE. h High-Protein MRE. i High-carbohydrate MRE. |
||||
No data are available on the voluntary use of specific amine acids by military personnel or on the effects of protein supplement use on performance of military personnel. The effects of tyrosine and tryptophan supplementation on soldier performance have been examined by Lieberman, Askew, and coworkers and are reviewed in the next section.
Protein and Amine Acid Supplements and Cognitive Performance
Although it is well known that several amine acids are precursors to neurotransmitters or neurotransmitters themselves, the brain's precise need for these amine acids is not well known. The concentrations of these precursor amine acids in blood influence their availability to brain neurons and, as a result, the ability of neurons to synthesize neurotransmitter products (because the rate of production of some transmitters is directly influenced by local concentrations of their precursors). The accessibility of amino acids to the brain is controlled by specific transport carriers located at the blood-brain barrier, the physical site of which is the endothelial cell of the brain capillaries (Pardridge, 1977). These sites can have a major influence on whether or not the brain meets its amine acid needs. It is thus essential to understand how this transport carrier operates. In Chapter 14, Lieberman notes that these carriers are selective for groups of amine acids based on size and charge. Of particular relevance to neurotransmitter production in brain is the large neutral amine acid (LNAA) transporter, which is responsible for the uptake of tryptophan (the precursor of serotonin) and of tyrosine and phenylalanine (precursors of the catecholamines). Several other amine acids are also transported into brain by this carrier. The LNAA transporter has been found to be competitive at normal plasma levels of these amine acids; consequently, phenomena that change plasma levels of one or more LNAAs indirectly modify their uptake into the brain. According to Lieberman (see Chapter 14), when the availability of these transmitter precursors to the brain changes, brain functions are modified, including cognitive performance and affective state (mood). Since the availability of one or more of these amine acids to brain becomes compromised during periods of undernutrition or stress, he argues that the resulting reduction in transmitter production could diminish brain function. This issue is highly relevant to the performance of soldiers in the field, who often operate under conditions of food restriction and increased exposure to multiple stressors (Lieberman, 1994).
Tryptophan
The rate of serotonin synthesis and release by brain neurons is directly influenced by the tryptophan concentration in brain (Fernstrom, 1990; Sharp et al., 1992) and, thus, by the uptake of tryptophan from the circulation. The
consumption of a protein-free meal increases the concentration of tryptophan in blood relative to that of the other LNAAs. The mechanism of action of this influence involves the stimulation of insulin release by dietary carbohydrate. Insulin stimulates the release of nonesterified fatty acid (NEFA) molecules from the serum carrier protein, albumin, and their subsequent uptake by adipocytes. The amino acid tryptophan, which has an affinity for albumin, binds in place of the NEFA. As a result, the total serum tryptophan concentration remains constant (unlike the concentrations of other amino aids, which decrease in response to insulin), and the bound tryptophan is able to enter the brain. In contrast, consumption of a high-protein meal results in a decrease in brain tryptophan and serotonin levels because the other LNAAs, which compete with tryptophan for binding to the LNAA transporter and thus for transport to the brain, are present in higher concentrations in dietary protein than is tryptophan (Fernstrom, 1990). As a result, serotonin synthesis is modified in several brain regions, including the hypothalamus and cerebral cortex. Thus, brain serotonin synthesis may be modulated by the protein-to-carbohydrate ratio of the overall diet or a recent meal.
To date, it is not known whether meal-related changes in serotonin production influence brain functions, but a number of studies have linked the changes in serotonin synthesis that follow the administration of tryptophan or its LNAA competitors to functional effects. In laboratory animals, serotonin neurons are most active when animals are awake and physically active (Jacobs and Fornel, 1993) and play an important role in channeling sensory information to the brain (Messing and Lytle, 1977; Walters et al., 1979).
Of particular relevance to the military, Lieberman notes, is the observation that tryptophan administration produces "mental fatigue" and has been used to promote sleep. Administration of tryptophan is also reported to reduce pain sensitivity (Lieberman et al., 1983; Seltzer et al., 1983). A decade ago, significant toxicity was attributed to tryptophan supplements, apparently due to a contaminant that survived the purification process for the amino acid (Hartmann and Greenwald, 1984; Lieberman et al., 1985).
Relatively little is known about how a reduction in serotonin synthesis might influence performance. The administration of an amino acid mixture that should reduce brain tryptophan levels and serotonin synthesis has been found to promote aggressiveness (Cleare and Bond, 1994) and depression (Delgado et al., 1990) in human subjects. These findings suggest that the changes in brain serotonin synthesis that accompany the ingestion of normal foods may produce similar, though less remarkable, effects on these behaviors (because meals cause smaller changes in serotonin than those produced by amino acid treatments). At present, no data exist that evaluate the magnitude of such effects.
Chronic, substantial reductions in protein intake can reduce brain tryptophan levels and serotonin production in laboratory animals (Fernstrom and Wurtman, 1971), with the ingestion of proteins naturally low in tryptophan
having the most pronounced effects on serotonin (Fernstrom and Hirsch, 1975) and behavior (Fernstrom and Lytle, 1976). These data are insufficient to allow an estimate to be made of a brain tryptophan ''requirement" in humans in relation to habitual dietary protein intake. Some human populations (for example, those that subsist on a corn-based diet) may experience chronic tryptophan deficiency (Wurtman and Fernstrom, 1979). However, although such groups might be expected to experience the behavioral effects predicted from animal studies (insomnia, increased pain sensitivity, depression), no data currently exist to support such a possibility. At least several weeks would be required for such a deficiency state to develop in humans. Nevertheless, the combat situation is typically associated with increased physical activity and stress, which stimulate serotonin turnover (Chaouloff, 1989; IOM, 1994). Thus, serotonin production may be impeded at a time when the requirement for this neurotransmitter is increased to support crucial brain functions. It is possible that aspects of sensory and motor function might become diminished under field conditions in which food intake (protein intake, in particular) is reduced for an extended period. Under such conditions, protein and/or tryptophan supplementation could have potential benefit. However, no research has been conducted to evaluate this possibility in animals or humans under the conditions of intended use.
Tyrosine
The only other amine acid studied in any detail in relation to its conversion to a brain neurotransmitter is tyrosine, another LNAA and a catecholamine precursor. Lieberman notes that when catecholamine neurons are actively firing in brain, the rate at which they synthesize catecholamines (primarily dopamine or norepinephrine) increases and becomes responsive to the local tyrosine concentration (Fernstrom, 1990; Lieberman, 1994; Wurtman et al., 1981).
Catecholamine neurons typically increase their activity under a variety of stressful conditions (Fernstrom, 1994; Stone, 1975), and appear to be important regulators of such behavioral parameters as attention, state of arousal, and mood (Lieberman and Shukitt-Hale, 1996). Stress-induced increases in catecholamine production can be magnified in animals by the administration of tyrosine, which may result in beneficial effects on spatial reference and working memory of laboratory animals under stressful conditions (Lehnert et al., 1984a; Shukitt-Hale et al., 1996; Shurtleff et al., 1993). Tyrosine administration also appears to improve memory and learning in humans under adverse environmental circumstances (Banderet and Lieberman, 1989; Deijen and Orlebeke, 1994). For example, Marine Corps sharpshooters who received tyrosine supplements experienced a partial reversal of the cold-induced deterioration in their marksmanship performance (Shurtleff et al., 1994). In another study, tyrosine administration was associated with an amelioration of the sleep deprivation-
induced decline in psychomotor function and vigilance (Neri et al., 1995). However, such effects are less evident under normal circumstances, and no recent studies have updated or reevaluated these findings.
Finally, in studies of soldiers examined under field conditions and consuming military rations, deficits in energy intake as well as in mental performance often develop along with reductions in plasma levels of tyrosine and tryptophan. A study by Askew and coworkers (1987) showed that the development of behavioral changes under these circumstances correlated most closely with decreases in plasma tryptophan, suggesting that plasma tryptophan levels may be most closely predictive of the development of a state of tryptophan deficiency in brain and changes in cognitive function.
In summary, very little is known regarding the influence of stressful conditions on brain requirements for the amino acids that are neurotransmitter precursors, an issue of particular relevance to the military. In addition, data on tyrosine supplementation are insufficient to demonstrate conclusive effects on cognitive performance.
Protein, Amino Acids, Muscle Mass, and Physical Performance
Measurement of Muscle Mass
Although skeletal muscle (SM) contains more than half of the body's protein, methods that quantitate the amount and quality of skeletal muscle remain underinvestigated and inadequately validated. The methods, which range from atomic to whole-body levels of body composition, are reviewed by Heymsfield in Chapter 12.
Since methods for determination of human body composition are by necessity indirect, all methodologies are dependent on mathematical models. Heymsfield suggests that it is possible to subdivide these body composition methods into (1) descriptive methods and (2) model-based methods involving the type of mathematical models used in the quantitation of SM.
Descriptive Methods. Empirical methods include anthropometry, bioelectric impedance analysis (BIA), ultrasound, and urinary biochemical markers (3-methylhistidine, creatinine) and are based on limited theory. These methods rely on the use of linear regression models based on a criterion method for the quantitation of SM. Each descriptive method would measure a quantity related to skeletal muscle, and this measurable quantity could be used in a linear regression model to predict SM: SM = a x (measurable quantity) + b. Based on a brief review of these descriptive methods, Heymsfield noted that anthropometry is perhaps the most limited in accurately predicting SM.
Segmental BIA can be used to quantitate specific skeletal muscle groups. In addition to the conventional placement of electrodes on the hands and feet, the electrodes can be placed leg to leg for an estimation of lean tissue in the legs.
Likewise, placement of electrodes in an arm-to-arm pattern (or some segment of the arm) results in an estimation of arm lean tissue. Further developments in BIA methodology may increase its usefulness for quantitating muscle mass (Baumgartner et al., 1989; Heymsfield et al., 1995; Nuñez et al., 1995, 1997; Wang et al., 1996). However, the use of BIA in this way will require determination of the actual composition of various body parts from which to interpret the BIA data.
Methods cited by Heymsfield based on the quantitation of urinary metabolites include the use of urinary creatinine and 3-methylhistidine. Measurement of 24-h urinary creatinine excretion has been used as a surrogate for measurement of muscle mass, based on a well-known association between muscle mass and creatinine metabolism. The large majority of creatinine is produced from creatine within skeletal muscle (and, thus, in proportion to muscle mass), which is followed by quantitative urinary excretion of the creatinine. Creatinine excretion in humans varies by gender, due to gender differences in muscle mass, and is affected by total dietary protein and the amount of muscle-containing foods (red meat) consumed. Thus, its use as a measure of muscle mass requires some dietary control.
A second urinary metabolite that has been used as a marker of muscle metabolism is the post-translationally modified amine acid 3-methylhistidine (3-MeHis), which is released as a breakdown product from myofibrillar muscle protein. While urinary 3-MeHis excretion is an indicator of muscle mass, 3-MeHis is also produced in the gut as a by-product of meat digestion and therefore must also be measured on a meat-free diet. High correlations were found when computerized tomography (CT) measurements of total body skeletal muscle were compared with urinary 3-methylhistidine excretion or muscle mass predicted from 3-methylhistidine (Lukaski et al., 1981; Wang et al., 1998).
The development of prediction equations for muscle mass would benefit from additional research into the use of urinary metabolites as biomarkers to predict muscle mass.
Model-Based Methods. Model-based methods, which include (1) the imaging techniques of computerized tomography and magnetic resonance imaging, (2) dual-energy x-ray absorptiometry, and (3) in vive neutron activation and whole-body counting, are based on sound and well-developed theory. These methods rely on the use of a biological model based on in vive quantitation of SM using phantoms or cadavers. Each model-based method would measure a quantity related to skeletal muscle and this measurable quantity could be used in a mathematical model to predict SM:
SM=a×(measurable quantity).
The most significant advance in the field of estimating muscle mass has been in the use of imaging methods; CT was introduced in the late 1970s and MRI within the past 15 years. The advance is based in part on the ability to perform accurate calibrations of these methods using phantoms and human cadavers, resulting in accurate estimates of muscle areas (CT) or volumes (MRI). The coefficient of variation associated with muscle mass determination by CT or MRI is 2-3 percent when the instruments are calibrated by comparing the results obtained with excised cadaver tissue or filled balloons to the actual weights of these standards (Heymsfield et al., 1997).
CT methodology in vivo relies on measurement of the CT number. Each type of tissue has a particular CT number, with the number for adipose tissue being lower than that for muscle tissue, which in turn is lower than that for bone. By using the CT number and algorithms, it is possible to separate in each slice the pixels that belong to adipose tissue and bone, while the remaining pixels, which belong to muscle, are determined by difference.
The CT number is also directly related to the composition of muscle and adipose tissue and enables the researcher and clinician to distinguish between anatomic muscle and nonadipose muscle tissue. The nonadipose skeletal muscle would contain the actual myofibrillar protein, while the anatomic muscle contains both intramuscular adipose tissue and nonadipose tissue skeletal muscle. Thus, within an anatomic muscle, the actual mass of functional contractile protein can be distinguished from the noncontractile fat tissue.
The advantage of MRI over CT is the absence of radiation exposure. The disadvantage of MRI is the time involved in its use. Although it is currently possible to scan the entire body from head to toe over 40 to 50 slices, in 20 minutes, subsequent analysis is time-consuming, requiring at least one full day per person to analyze each slice using sophisticated software programs for adipose tissue and lean tissue.
With the advent of stronger magnets, it has become possible to use magnetic resonance spectroscopy for analysis of carbon, hydrogen (protons), phosphorus, and sodium in vivo; from these measurements, tissue compartments can be calculated. This type of spectroscopy not only measures the amount of tissue present but also monitors in real time in vivo metabolic processes such as glycogenolysis, lipogenesis, and water balance. This powerful tool, magnetic resonance spectroscopy, is a major resource for future research.
Dual-energy x-ray absorptiometry (DXA) is a method originally designed for the quantitation of bone and bone density and therefore has been most useful in studying diseases such as osteoporosis. More recently, algorithms have been developed to use in interpreting total body scans for other body components. The differential attenuation of the energies from the two x-rays by soft tissue and bone is used to quantitate the amount of these tissues. Further partitioning of the soft tissue into lean and fat is similarly accomplished. DXA software then allows the separation of the body into discrete regions. Research in
Heymsfield's laboratory has estimated that 75 percent of total body muscle mass is found in the arms and legs. Other regions of interest such as the lower leg also can be identified and isolated using the software. This approach has been validated by comparing total body muscle mass determined by CT to appendicular skeletal muscle estimated by DXA. The correlation coefficient between the two is 0.95, which indicates a strong association and the feasibility of using DXA for the quantitation of muscle mass in humans in vive.
The primary limitations on the accuracy of using imaging techniques to estimate muscle mass are those imposed by the technology employed. As mentioned above, even the most accurate methods of CT and MRI have a minimal error of measurement of 2 to 3 percent. Thus, for estimating changes in muscle mass, the expected change would have to be greater than 6 percent to make the measurement feasible. Such a change in muscle mass could be seen only under drastic circumstances, such as a change in total body weight of more than 10 percent. Current research includes the further development of BIA and imaging techniques, improvement of the urinary creatinine method, and perhaps most importantly, developments in the measurement of dynamic in vive changes in muscle metabolism, composition, and subsequent muscle function.
Control of Muscle Mass and Function
The function and regulation of skeletal muscle mass have been reviewed by Nair in Chapter 6. Muscle mass constitutes 40 to 45 percent of body weight and accounts for approximately 70 percent of body cell mass. Nair presents a model that links muscle mass and function (in the form of contractility) with metabolic processes. Skeletal muscle has important locomotive and metabolic functions. Many of the metabolic functions that occur in skeletal muscle depend on muscle mass as well as interrelated factors such as circulating hormone levels, training status, and age. Thus, indicators of muscle function such as strength and endurance are influenced by these factors as well.
Strength. Although muscle mass and strength are significantly correlated, muscle strength may be regulated independently from muscle mass. Strength is a functional indicator of overall muscle "quality." Sarcopenia syndrome, which refers to the age-related decline in muscle mass, is characterized by loss of strength, power, speed, and endurance, as well as by poor balance, resulting in an increased potential for bone injury due to falls. According to a model presented by Nair, adjustment for the age-related decline in muscle mass reveals an apparent functional impairment, which points to a disturbance in the quality of muscle with age and inactivity.
Endurance. Evaluation of endurance with measures such as maximal oxygen consumption reveals gender differences; however these differences
essentially disappear after appropriate adjustment for differences in body composition. In contrast, the age-related decline in endurance persists even after such adjustments for body composition are made, supporting the observation of an apparent change in muscle quality with aging. Endurance is compromised by myocardial problems such as decreased cardiac output, reduction in maximal exercise heart rate and stroke volume, and increased peripheral resistance. Each of these factors influences the delivery of oxygen to working muscle.
Muscle Metabolism. According to Nair, muscle protein quality is maintained by a remodeling process involving the replacement of old and damaged protein with new protein. Both mitochondrial and sarcoplasmic proteins undergo this process, which declines with age. The decline in the fractional synthetic rate of myosin heavy chain (a sarcoplasmic protein) is correlated with a decline in leg curl strength. The decline in mitochondrial protein synthesis is associated with a decrease in the activity of the mitochondrial enzyme cytochrome C oxidase, which is involved in energy utilization and storage. This decline may explain both a decrease in the efficiency of substrate metabolism and an increase in the fatigability of aging muscle. These changes in mitochondrial and sarcoplasmic proteins taken together are associated with an age-related decline in endurance capacity. The functional significance of these age-related changes is unknown for younger muscle.
Hormonal Interactions
Anabolic Hormones. Muscle is metabolically sensitive to insulin action; insulin administration decreases protein breakdown but does not appear to increase muscle protein synthesis in humans except when preceded by a large infusion of amino acids. In insulin-dependent diabetes, muscle protein breakdown is increased, with little effect on muscle protein synthesis. As a result, insulin deprivation is associated with increased net muscle protein loss. Insulin deprivation also is associated with increased glucagon levels, which have been shown to increase oxidation of the essential amino acid leucine. The insulin resistance observed in severely injured or postoperative patients and those with systemic infections may contribute to the muscle catabolism observed in these individuals (Black et al., 1982). Administration of insulin is associated with an increase in muscle protein synthesis in catabolic patients given amino acid-containing formulas (Pearlstone et al., 1994; Sakurai et al., 1995).
Growth hormone (GH) and IGF-I also stimulate muscle protein synthesis over the short term. Growth Hormone replacement in GH-deficient children and adults results in increased lean body mass or muscle mass (Collipp et al., 1973; Cuneo et al., 1991) and improvement in muscle function (endurance and
strength) (Jorgensen et al., 1989). GH supplementation of elderly subjects, who normally have low GH concentrations, also increases lean body mass and muscle mass (Schwartz, 1995). The effects of GH administration on skeletal muscle protein synthesis appear to depend on experimental factors such as study duration and route of administration. For example, local infusion of growth hormone resulted in an increase in forearm protein synthesis with no change in protein breakdown; these effects may be mediated by a stimulation of IGF-I synthesis (Fryburg et al., 1991). Systemic infusion of GH for 3 days resulted in increased synthesis and decreased breakdown of forearm protein (Fryburg and Barrett, 1993; Wolf et al., 1992). Butterfield and coworkers (1997) observed increases in skeletal muscle protein synthesis, nitrogen balance, whole-body protein synthesis, and net protein synthesis after I month of daily injections of GH given to postmenopausal women. However, systemic infusion of men with GH to achieve the same range of serum concentrations, for varying lengths of time, resulted in no change in protein synthesis or degradation in the vastus lateralis muscle of the leg, as measured by the methods of continuous infusion or balance (Copeland and Nair, 1994; Welle et al., 1996; Yarasheski et al., 1995).
Administration of IGF-I, a paracrine hormone that is believed to mediate the effects of GH, also has demonstrated contradictory effects on muscle protein synthesis. Systemic infusion of IGF-I produced no effect on whole-body protein synthesis or breakdown (Elahi et al., 1993), but local infusion increased forearm protein synthesis and, at higher doses, decreased breakdown (Fryburg 1994, 1996; Fryburg et al. 1995). Injection of IGF-I twice daily for I month in postmenopausal women resulted in increased nitrogen balance, whole-body protein synthesis, skeletal muscle protein synthesis, and net protein synthesis and in decreased protein breakdown (Butterfield et al., 1997). The question of whether IGF-I mediates all or some of the effects of GH on muscle protein synthesis is unresolved, in part because the effects of IGF-I are influenced not only by its binding to IGF-I receptors but also by the secretion of its binding proteins, the control of which is not fully understood. Because of the influence of IGF-binding proteins and the apparent localization of at least some IGF-I effects, determining an effective dose of IGF-I is difficult. In addition, measurement of plasma levels of IGF-I may indicate trends but has limited functional significance.
Efforts to determine the effects on skeletal muscle protein synthesis of exogenous GH or IGF-I combined with resistance training have shown no additional effects of GH beyond those of the training itself in young and elderly sedentary men and weight lifters (Yarasheski et al., 1995), although GH increased fat-free mass and whole-body protein synthesis in young men and fat-free mass in elderly men (suggesting the possibility that some body tissues may be more sensitive to the effects of GH than skeletal muscle) (Rooyackers and Nair, 1997).
Administration of GH and IGF-I to critically ill patients has demonstrated positive effects on nitrogen balance and preservation of lean body mass in some studies but not in others. In badly burned children, GH has demonstrated positive effects on muscle protein synthesis (Gore et al., 1991). IGF-I administered to HIV patients improves nitrogen balance and protein turnover transiently (Lieberman et al., 1994). In contrast, Wolf and coworkers (1992) found no effect of GH on skeletal muscle protein synthesis in catabolic cancer patients. Sandstrom and coworkers reported no effect of IGF-I administration on nitrogen balance or protein breakdown in postoperative patients receiving dextrose with no added amino acid source. Nevertheless, it is believed that the combination of GH and IGF-I may increase nitrogen balance and prevent skeletal muscle protein breakdown in critically ill patients if the GH can counteract the hypoglycemic effects of high doses of IGF-I and if amino acid concentrations are adequate (Rooyackers and Nair, 1997). Recently, however, two large clinical trials of recombinant human GH with intensive care patients (recovering from any of several types of surgery or trauma) were terminated prematurely due to an unexpected and as yet unexplained increase in the mortality rate among the GH-treated groups (B. Lippe, Los Angeles, personal communication, 1998). Thus, further study is needed before GH can be considered for the treatment of critically ill patients.
Testosterone is undoubtedly the anabolic hormone most closely associated with building muscle mass. Levels of free testosterone in the blood decline with age and are associated with the rate of synthesis of myosin heavy chain. Testosterone administration to hypogonadal men substantially increases muscle mass, muscle strength, and muscle protein synthesis. Administration of testosterone in supraphysiological doses has recently been shown to increase muscle mass and muscle strength (Bhasin et al., 1996); however the effects of moderate doses on athletes in training have been inconsistent. Nair (see Chapter 6) indicates that the mechanism by which testosterone increases muscle mass and muscle strength remains unknown. Wolfe (see Chapter 13) speculates that testosterone, which stimulates protein synthesis under normal circumstances and is known to be suppressed in male bum patients, might have a synergistic effect with insulin on muscle protein synthesis.
Catabolic Hormones
Catabolic hormones, such as the glucocorticoids and glucagon, increase muscle protein breakdown and net catabolism of amino acids. The catabolic effect of glucocorticoids on skeletal muscle is evident in individuals suffering from Cushing's disease, which is characterized by an excess production of corticosterone. Glucocorticoid-induced protein breakdown has been shown to be inhibited by GH alone and completely reversed by coadministration of GH and
IGF-I (Berneis et al., 1997). The catabolic effect of glucagon is observed primarily in patients with Type-I (insulin-dependent) diabetes.
Effects of Protein, Energy, and Amine Acid Supplementation on Physical Performance
Protein and Energy Intake in Long-Distance Cyclists
Recent metabolic studies of cyclists engaged in the Tour de France bicycle race may provide some insight into nutritional factors that can contribute to the ability of an individual to perform sustained strenuous endurance activity. Saris and coworkers (1997) studied four cyclists during the race, which involves covering a distance of approximately 2640 miles (4000 kin) over a period of 22 days, with 1 day of rest. During this race, mean daily energy expenditure ranged from 12.9 to 32.7 MJ/d (3071-7786 kcal/d), with an overall mean of 25.4 (6048 kcal/d); the duration of work was as much as 8-9 h/d, and altitudes frequently reached more than 2 kin; however, the cyclists lost no weight or body fat. Mean daily energy intake matched or slightly exceeded energy expenditure, with protein representing 15.4 percent, carbohydrates 60.6 percent, and fat 22.4 percent of energy consumed (a high proportion of the energy consumed was in the form of carbohydrate-rich drinks). Further analysis revealed that intake closely followed expenditure, so that energy balance was maintained on a day-to-day basis with a maximum lag of 2 days (glycogen repletion was found to be complete within 16 hours). Although the contribution of protein to total energy was normal, total protein intake was very high compared with the RDA. Resting total plasma amine acid concentrations did not change during the race, although a large number of amine acids decreased or increased over the course of the race. This finding suggests that the daily recovery periods were too short to restore amine acid balance, but it was not possible to interpret the significance of individual changes or to draw conclusions about the effect of strenuous exercise on amine acid metabolism. Nevertheless, the results of this study demonstrate that individuals can sustain high levels of endurance activity over an extended period with no loss of lean body mass when energy intake is sufficient to match output.
The Fatigue Theory
Branched-chain amino acid (BCAA) supplements could theoretically benefit physical performance in several ways. They could supply TCA cycle intermediates, decrease the use of other energy sources (sparing glycogen), inhibit muscle protein breakdown, or limit the transport of tryptophan into the brain. A major mechanism by which the BCAAs are hypothesized to affect
performance positively is via the last mechanism, known as the "central fatigue theory" of Newsholme (see Wagenmakers, Chapter 14; see also Blomstrand et al., 1991). This hypothesis states that exercise fatigue is in part a result of the amount of serotonin produced in the brain and that it is the availability of the precursor tryptophan that regulates the synthesis of serotonin. As discussed above, tryptophan levels in the brain are dependent on the activity of the large neutral amino acid transporter (see Lieberman, Chapter 14), which responds to the relative concentration(s) of free tryptophan and the BCAAs. If the ratio of tryptophan to BCAAs increases, the transporter will carry more tryptophan and serotonin synthesis in the brain will similarly increase.
In the exercising individual, the free tryptophan concentration in the blood increases due to competition with free fatty acids (FFAs) for binding sites on circulating albumin. The FFAs liberated from fat stores by the hormonal milieu generated by exercise essentially displace tryptophan from the albumin molecule. At the same time, BCAA concentrations in the blood are decreasing due to their use as a metabolic fuel by muscle. Thus, the theory proposes that exercise increases free tryptophan availability to the transporter and thus the brain, and the increase in brain serotonin shortens the time to fatigue. According to this theory, manipulation of the tryptophan-to-BCAA ratio by increasing BCAA intake during exercise should theoretically decrease serotonin synthesis, lengthen the time to fatigue, and improve performance.
However, studies have shown that the administration of BCAA during strenuous exercise results in virtually no change in time to fatigue or improvement in performance (Blomstrand et al., 1991; Davis et al., 1992; Kreider et al., 1993), and according to Wagenmakers (see Chapter 15), BCAA administration may actually decrease performance in circumstances of intense, long-duration exercise where glycogen stores are depleted. Wagenmakers and Rennie describe the importance of TCA cycle intermediates in the provision of energy substrates to the mitochondria and point out that, in fact, high levels of circulating leucine may deplete α-ketoglutarate, one of these intermediates. When α-ketoglutarate is converted to glutamate by the enzyme branched-chain α-keto acid dehyrogenase, the leucine carbon "skeleton" is left to be burned for energy. This hypothesis is borne out by work in individuals with McArdle's disease, who cannot release glucose from glycogen and who respond to BCAA administration with a significant decrease in maximum oxygen consumption (Sahlin et al., 1995). Similarly, research in healthy individuals infused with α-keto acids of the BCAAs, who showed an improvement in performance that was theoretically due to augmentation of the TCA intermediate pool, actually experienced a depletion in the TCA cycle intermediate pool (Katz et al., 1986; Sahlin et al., 1990). Thus, Wagenmakers cautions against the use of BCAA supplements as ergogenic aids for military troops, suggesting that these supplements may deplete TCA cycle intermediates when exercise is intense and of long duration and could thus hasten the time to fatigue.
Based on the observation that protein and carbohydrate combined together in a postexercise feeding results in a high insulin response and high rates of glycogen synthesis (Zawadzki et al., 1992), Wagenmakers conducted work on the effect of glutamine supplementation on glycogen synthesis. To date he has found no positive effect of glutamine but has confirmed the synergistic effect of carbohydrate and protein, and concludes that implementation of a postexercise feeding regimen including these two nutrients may be useful for promotion of the continued endurance of troops performing strenuous exercise over several days.
Thus, there appears to be no evidence at the present time to suggest that amine acid supplementation would optimize physical performance in healthy individuals who consume the MRDA for protein. There may, however, be situations in which such supplementation would theoretically be beneficial. For example, with exercise during moderate protein restriction, exogenous glutamine may enhance skeletal muscle protein synthesis and aid general maintenance of acid-base homeostasis. However, it should be emphasized that no studies are available to confirm these hypotheses. What data are available confirm that when adequate energy and protein are consumed, supplements will not be beneficial.
Amine Acids and Immune Function
The role of protein and specific amine acids in immune function has been reviewed in detail in a recent CMNR report (IOM, 1999). It is fully evident that every aspect of immune function and all innate host defensive mechanisms are entirely dependent upon the body's ability to synthesize new proteins. It is also evident that immunologically related proteins comprise a vast array of unique, highly specific individual molecules, each with its own purpose and function. Such proteins include antibodies, cytokines, thymic hormones, acute-phase reactant proteins, metal-binding proteins, the numerous proteins involved in complement, kinin and coagulation systems, lipoproteins, cell surface protein receptors and other components of newly synthesized lymphocytes and phagocytic cells, as well as numerous enzymes and structural proteins distributed throughout the body.
The synthesis of all these immunologically important proteins depends upon a complete and balanced array of free amine acids in body cells, and a lack of adequate dietary protein, as seen worldwide in subjects with protein-energy malnutrition (PEM), is the most common cause of nutritionally acquired immune dysfunction syndromes (NAIDS) and cachexia-related deaths. Young children, aged individuals, and patients with severe debilitating illness or trauma are the most frequent victims of NAIDS. Although healthy, well-fed military personnel should not experience protein-related immune dysfunction, the stress of severe Ranger training, with its associated dietary deprivation and extreme
loss of body weight and muscle mass (IOM, 1992), did produce early evidence of both PEM and NAIDS.
Although, as noted above, the structure and function of immunologically important proteins is dependent upon the balanced availability of amino acids, there is growing evidence that the supplemental administration of certain amino acids can produce immunological benefits. Glutamine is the best example of this, as noted in an earlier CMNR report (IOM, 1999). Plasma glutamine concentrations decline with strenuous exercise and in ''overtrained" individuals (Rowbottom et al., 1995), who are at an increased risk for infection. Wagenmakers (see Chapter 15) discusses the role of the conditionally essential amino acid glutamine in immune function. This amino acid is made in large amounts (20-25 g/d) in muscle and liver; it provides fuel for cells lining the intestine (VanderHulst et al., 1993) and may be important for the maintenance of function in immune cells (mucosal effects of glutamine may play a role in reducing the incidence or severity of intestinal infection).
Under circumstances of extreme stress and trauma, as well as intense exercise, the circulating level of glutamine diminishes, reaching a minimum attained 2 hours after the cessation of an exercise bout. Under normal conditions, skeletal muscle produces more glutamine than any other amino acid. Preliminary studies by Castell and colleagues (1996) of infection rate in very active athletes suggest that glutamine supplementation may decrease the rate of infection (that is, "overtraining effects"). However, Wagenmakers points out design flaws in the experiment that make this conclusion suspect. Some evidence suggests that the proliferation of lymphocytes and other parameters of immune function are boosted by glutamine (Jacobi et al., 1997; O'Riordain et al., 1994; Ziegler et al., 1994). In studies of hospitalized, critically ill patients, glutamine supplementation of total parenteral solutions resulted in increased survival and decreased length of hospital stay (Griffiths et al., 1997; Wilmore, 1997a; Ziegler et al., 1992).
The amino acid arginine also may serve as an immunostimulant and as an immune system modulator (Barbul et al., 1990; Wilmore, 1991). These effects may not be as great as those seen with glutamine; however, arginine is the sole substrate for the nitric oxide synthase-catalyzed formation of nitric oxide (NO), with citrulline as the other product.
Growing evidence now indicates that NO is highly important as a microbicidal and tumoricidal molecule, with an effectiveness that may surpass that of better known-oxidative mechanisms. Nitric oxide appears to have many other actions throughout the body (Albino and Mateo, 1995), some of which support mechanisms of host defense. However, more evidence is needed to determine if arginine supplements might reduce the incidence of infectious illness in healthy subjects.
Issues of Protein Quality and Timing of Consumption
Diets consumed by people worldwide typically supply protein at 9 to 14 percent of energy. The greatest differences in the protein value of human diets are in protein quality, not quantity. Diets in the developing world are based largely on cereals, with small contributions from legumes and little if any animal protein; thus, these diets are typically of low quality. Protein quality is a measure of the efficiency with which dietary protein is converted to body protein. Proteins of higher quality are needed in lesser amounts, and proteins of lower quality in greater amounts to synthesize body proteins. Protein quality is assessed in relation to a reference amine acid pattern with adjustment made for digestibility. All cereals contain less of the amine acid lysine than suggested by the reference pattern limitations, and corn is also lower than the reference in the amine acid tryptophan. Legumes and animal proteins supply excess lysine (with respect to the reference pattern) but are limiting in methionine. Thus, cereals, on the one hand, and legumes, meat, or milk, on the other, are known as complementary proteins since their amine acid compositions "complement" each other when they are consumed together. Since the cyclical nature of protein metabolism makes it possible for an amine acid deficiency in a meal to be made up from body stores, it is not necessary that complementary proteins be consumed in the same meal, but consuming them during the same day is highly desirable (Young et al., 1989). Thus, timing the consumption of complementary proteins is not an issue for operational rations employed by the military as these rations are currently formulated.
Considerable debate surrounds the need for high-quality protein in adults. If the estimated indispensable amine acid requirements were as low as indicated by classic nitrogen balance experiments—that is, about IS percent of the total protein requirement—even poor-quality cereal diets would meet this requirement. On the other hand, if the need for IAAs is as high as indicated by recent stable isotope experiments (that is, about 50 percent), then protein quality is as important a consideration in diets consumed by adults as it is in those of infants and preschool children.
In considering this issue with respect to operational rations, it is desirable that the protein supplied be of high quality. During recovery from infection or trauma, including but not limited to blood loss, lost body protein must be replaced and this protein requirement is high for indispensable amine acids, especially lysine. Moreover, higher-quality proteins are used with greater efficiency, resulting in the excretion of less urea and a decreased renal solute lead. For military women who become pregnant or who are lactating, the proteins synthesized by the body (breast milk and the products of conception) are high-quality animal proteins that are high in IAAs. The 1991 FAO/WHO working group on protein and amine acid requirements suggested that protein
quality be assessed using amino acid requirements appropriate for the preschool child as the scoring pattern (Clugston et al., 1996).
Potential Benefits of Plant and Legume Proteins
Plant proteins are generally associated with less total fat, less saturated fat, and more polyunsaturated fat compared to most animal proteins. In addition, plants do not contain cholesterol, whereas red meats, poultry, and some shellfish do. The consumption of plant proteins such as soy-derived protein or a combination of plant proteins in place of part or all of the animal protein in the diet would therefore decrease the level of total fat, saturated fatty acids, and cholesterol in the diet—changes that are known to lower serum cholesterol and saturated fat. In addition, some plant protein sources contain other substances not found to be associated with animal proteins, such as soluble fiber, which also decrease the levels of serum cholesterol and saturated fatty acids. Plant proteins, such as the cereal proteins, are low in one or more of the indispensable amino acids. If Young were correct in his assessment of the IAA needs of adults, substitution of these proteins for animal proteins in military rations would decrease their nutritional value. However, among the plant proteins, soy has a better balance of the essential amino acids necessary for maintenance of lean mass (thus, it is higher-quality protein). A meta-analysis of the effects of soy-based diets (Anderson et al., 1995) supports the beneficial influence of these diets on blood lipids, although these data require confirmation and the responsible factor(s) must be determined. Substitution of soy protein for animal proteins in whole or in part would not decrease the nutritional value of military rations. The use of soy has the potential to improve the long-term health benefits of the diet. However to achieve these benefits, it might be desirable for foods made with soy to have the organoleptic (sensory) qualities of traditional animal protein sources. In addition, although soy-based foods have found acceptance in civilian markets, testing must be accomplished to ensure acceptance of these foods in military rations.
Effects of Timing of Protein Intake
The effect of tuning of protein intake on exercise and cognitive performance has not been investigated extensively. However, available data suggest that postexercise feeding may be beneficial to maintenance of glycogen and protein stores. Early work by Cuthbertson and Munro (1937) showed that feeding immediately after an exercise bout decreased nitrogen loss in the urine over the subsequent 24 h. These authors suggested that the provision of protein immediately after exercise conserved body protein as an energy source. More recent work by Zawadzki and coworkers (1992) and others has shown that
feeding protein in conjunction with carbohydrate (CHO) immediately after exercise resulted in an increase in circulating insulin, in comparison to feeding CHO alone or feeding nothing. This increase in circulating insulin translates into an improvement in glycogen storage, a factor shown by some to be important in the ability to sustain long bouts of strenuous exercise. Tipton and Wolfe (1998) have shown an improvement in protein synthesis when amine acids are provided immediately after exercise, compared to no feeding. Borchers and Butterfield (1992) showed that feeding either CHO, protein and CHO, or protein alone in equicaloric amounts immediately after exercise resulted in a diminution of urinary urea in the 24 h after the exercise bout, compared with no meal at all (reflecting improved protein utilization), even when total energy intake over the day was constant.
Thus, postexercise feeding, especially of CHO and protein, may result in an improvement in glycogen storage and maintenance of lean mass, thereby potentially increasing the ability to continue strenuous activity on a subsequent day. It should be noted, however, that all these data were derived from men, and the results may be different from women. Tarnopolsky and coworkers (1995) have shown that active women respond differently to glycogen loading than do men.
Risks Associated with High-Protein Diets and Supplements
Data from national surveys on food consumption demonstrate that protein intake is highly variable among both male and female adults. Variation also is evident for the intake of some individuals on a day-to-day basis. Chronic high levels of protein intake may increase amine acid catabolism and foster the body's adaptation to higher protein intakes. As noted by Bier in Chapter 5, this adaptation may be detrimental in situations where stress increases need or intake is diminished. The individual who has adapted to an increased amine acid catabolism may show a greater deficit in protein balance when a more moderate or a low intake of protein occurs. Further, high-protein diets lead to the generation of excess nitrogenous end products, which in turn require a greater intake of fluids to permit their excretion. This extra fluid requirement may be an added stress in hot environments.
Protein and Renal Function
Evidence from several studies has suggested that chronic high protein intake may contribute to the deterioration of renal function that is observed with aging. However, few studies have included considerations of energy intake or physical activity, and even fewer studies have included women specifically.
Nevertheless, available data do not suggest that dietary protein per se is a causative factor in the age-related deterioration of renal function in humans. Tobin and Spector (1986) measured renal function on two occasions separated by 10 to 18 years in 198 healthy men who were participants in a longitudinal study on aging. No correlation could be found between the observed changes in renal function and the levels of protein intake. Moreover, no relationship was identified between the decline in creatinine clearance with age and the level of protein intake. Other studies in humans have led to similar conclusions (Kerr et al., 1982). Animal studies also fail to support the hypothesis that high-protein diets compromise renal function; chronic high-protein diets fed to rodents for 2 years had no effect on glomerular filtration rate (GFR) or renal pathology (Collins et al., 1990).
In Chapter 7, Walser reviews the well-known adverse effects of a high protein intake in patients with impaired renal function that had reached end stage (Walser, 1992) and the therapeutic value of reducing protein intake of such patients, but these concerns do not seem applicable to healthy young adults. Similarly, the potentially deleterious effects of high protein intakes in aged individuals (who are losing renal function in association with senescence) are not applicable to individuals of military age. In fact, as noted by Walser, the renal clearances of both inulin (a chemical used to measure clearance) and creatinine in healthy subjects were increased by higher-protein diets.
The one potential renal danger of high protein intakes in healthy individuals, as cited by Walser, is nephrolithiasis (Robertson et al., 1979b). Renal stone formation has a highly complex pathogenesis that involves the excretion of calcium, sodium, sulfate, oxalate, and purines, all of which are likely to be increased in subjects consuming a high-protein diet (Tschope and Ritz, 1955). A major consequence of high-protein diets is increased excretion of urea, which results from the amino groups of oxidized amino acids. A restricted intake of water may increase the work required of the kidney to excrete these by-products in a concentrated urine, resulting in a compromise to the kidney and predisposition to nephrolithiasis. Adverse effects on renal function were not mentioned among the potential dangers cited by Maher (FASEB/LSRO, 1992; see also Chapter 16) from high intakes of individual amino acids, however he cited the need for additional studies.
Thus, although exact comparison studies are not available, existing data suggest that such studies, if performed using age, gender, and fitness-matched individuals, would find no correlation between protein intake and intrinsic renal disease. However, indirect effects may be observed in the form of renal stone formation.
Protein and Calcium Status
The calciuretic effect of high-protein diets is well established and has been demonstrated in both men (Allen et al., 1979; Linkswiler et al., 1974) and women (Hegsted and Linkswiler, 1981), although fewer studies have been done in women. Based on their review of 16 separate human studies, Kerstetter and Allen (1990) concluded that there is a linear relationship between dietary protein and urinary calcium such that for each 50-g increment of dietary protein, an extra 60 mg of urinary calcium is lost. This loss appears to be related to a direct effect of protein on renal function. An increase in glomerular filtration rate in response to high protein increases the filtered lead of calcium. In addition, there is a decrease in fractional tubular reabsorption, which is thought to be related to the sulfur and acid lead from protein (Zemel, 1988).
Body retention of calcium in response to high-protein diets is influenced by the dietary content of other nutrients, particularly calcium, phosphorus, and sodium. Thus, high intakes of various dietary sources of protein have differing effects on the magnitude of urinary calcium losses and calcium retention, depending on the minerals that they provide (Zemel, 1988). Spencer and coworkers (1988) failed to show increased calcium loss in response to high protein intake provided by red meat and other complex proteins, and attributed this difference in results to the phosphorus provided by meat. Although phosphorus is known to decrease urinary calcium losses, negative calcium retention in response to high protein intakes is not necessarily prevented by increasing the phosphorus content of the diet (Hegsted et al., 1981). In contrast to the results of Spencer and coworkers, the addition of meat to the diets of young men, which resulted in an increase in protein intake from 55 to 146 g and phosphorus from 890 to 1660 mg, led to increased urinary calcium losses and negative calcium balance (Schuette and Linkswiler, 1982). When the added protein was in the form of a mixture of meat and dairy products that provided calcium in addition to similar amounts of protein and phosphorus, an increase in urinary calcium also was observed, but calcium retention was positive.
Although it has been postulated that high protein intakes may represent a risk factor in osteoporosis because of their calciuretic effect, evidence to support or dispute such a relationship is limited. A recent study in young adults (seven men, eight women) showed that short-term intake of a high-protein (2.71 g/kg BW), high-calcium (1589 mg) diet had no effect on urinary pyridinium cross-link excretion, a sensitive indicator of bone resorption, compared to the effects of a low-protein diet (0.44 g/kg BW) with similar calcium content. A low-protein (0.49 g/kg BW), low-calcium (429 mg) diet, however, resulted in higher urinary pyridinium cross-link excretion, suggesting increased bone resorption (Shapses et al., 1995). Based on food frequency data and self-reports of bone fractures, a 12-year prospective study of participants in the Nurses' Health Study found that total dietary protein was associated with an increased risk of
forearm fracture for women who consumed more than 95 g protein per day compared with those who consumed less than 68 g/d per day. An increase in risk of forearm fracture was also observed for animal protein but not vegetable protein. Women who consumed five or more servings of red meat per week also had a significantly increased risk of forearm fracture compared to women who ate red meat less than once a week. The incidence of hip fractures was not associated with protein intake (Feskanich et al., 1996).
The relatively high protein intakes of male soldiers both in garrison (98-132 g/d) and in field settings (105 g/d) would be expected to be associated with higher urinary calcium excretion than lower-protein diets. However, these protein intakes would not necessarily have a negative impact on calcium retention depending on other dietary factors such as the intake of phosphorus and calcium. Calcium intakes by soldiers in a variety of settings have been reported by Baker-Fulco (1995). Mean calcium intakes of male soldiers in field studies reached approximately 1000 mg/d (the dietary reference intake [DRI] for men; IOM, 1997) or more in five of the nine field studies reported, while calcium intake of men in garrison and at the U.S. Military Academy exceeded 1000 mg/d in all studies reported. In five of seven studies that included women, mean calcium intakes approached or exceeded 1000 mg, the DRI for women 19 to 50, while in the other two studies, intakes averaged around 750-800 mg (the MRDA for calcium).
Kerstetter and Allen (1990) suggested that calcium balance is close to equilibrium with daily protein intakes up to about 74 g and calcium intakes in the range of 500-1400 mg/d. Based on this statement, serious problems with calcium status in relation to protein intake would seem unlikely in military women. Although protein availability from operational rations is high, actual consumption is considerably lower. In addition, these diets are intended for short-term consumption, and although research is limited, some evidence suggests that regular weight-bearing physical activity contributes to bone strength. For these reasons, it would appear that operational rations would not likely be associated with a significantly increased risk of stress fracture or osteoporosis for women in the military.
Toxicity of Amino Acid Supplements
In 1994, the CMNR reviewed scientific information related to the use of selected supplements for enhancing performance (IOM, 1994) and concluded that the supplementation of certain amino acids at levels that were in the range of those found in a typical diet might have a positive impact on performance. The committee stated that the addition of amino acids at these levels is not likely to cause harm to healthy adults with short-term use.
The efficacy and safety of protein and amino acid supplements consumed by individuals in the hope of enhancing performance is revisited by Maher (see
Chapter 16). Maher points out that there is no credible scientific evidence to suggest that normal, healthy persons consuming diets adequate in protein would benefit nutritionally in any way from supplementation with any single amine acid. Further, he asserts that indiscriminate supplementation has the potential for real harm to people who eat less-than-ideal diets, because of the possible antinutritional (growth-inhibiting) effects of amine acid-imbalanced diets. In contrast, supplementation of diets containing poor-quality proteins with the amine acids that are limiting in the protein could be beneficial.
Maher also expresses concern regarding the consumption of amine acids at higher levels to achieve hypothetical pharmacological rather than nutritional benefits. He summarizes the findings of an expert panel (FASEB/LSRO, 1992) that reviewed the safety of consumption of amine acid supplements. Substantial potential exists for deleterious interactions of amine acids with a number of over-the-counter and prescription medicines. Maher points out that supplemental use of D-amine acids is especially risky since the D enantiomers not only have no nutritional value but also are likely to be more toxic at high doses (Friedman, 1991). According to Maher, lack of safety data regarding the consumption of high intakes of individual amine acids (D or L) suggests that recommendations should be conservative with regard to their use as supplements.
Maher further points out that the purity of individual amine acids is a very important consideration in their use as supplements. Experience with the consumption of L-tryptophan, which contained a suspected low-level contaminant, has shown that extreme harm can result (Hertzman et al., 1990).
High-Protein Diets, Amine Acid Supplements, and Pregnancy. The results of a controlled clinical trial in New York City that enrolled low-income, pregnant, African-American women suggest that the consumption of high-protein supplements during pregnancy may be detrimental to the fetus. Three dietary treatments were allocated randomly to evaluate fetal outcome: supplement, which consisted of two 8-ounce cans of a high-protein beverage; complement, which consisted of two 8-ounce cans of a balanced protein-energy beverage; and control, which consisted only of routine vitamin-mineral tablets (Rush et al., 1980). The protein content of the supplement was 8.5 g per 100 kcal, which provided 34 percent of calories compared with 1.9 g per 100 kcal, or S percent of calories, in the complement. With balanced protein-energy supplementation, gestational duration was increased, the proportion of low-birthweight infants was reduced, and mean birthweight was increased by 41 g (not statistically significant). With high-protein supplementation, a tendency toward increased incidence of very early premature births and associated neonatal deaths (at 20-35 weeks, not statistically significant) and significant growth retardation in the preterm infants were observed. Because the women in the supplement group who delivered prematurely consumed more of the
supplement but fewer total calories, interpretation of the study findings is confounded; nevertheless, this historic study strongly warns against the use of high-protein supplements in pregnant women. It should be noted that there is no evidence that a high-protein diet from food is detrimental to pregnancy outcome.
There is no evidence that supplementation of the diet with a single amino acid during pregnancy would be of benefit nutritionally to normal, healthy individuals. On the contrary, supplementation of the diet with a single amino acid may be potentially dangerous to the developing fetus. Several studies in laboratory animals demonstrate antinutritional effects (that is, depressed growth and other adverse effects) associated with the intake of imbalanced amino acid diets. High doses of single amino acids given to rat dams elevated their plasma amino acid concentrations and resulted in offspring with lower birthweight, decreased brain weight, and altered behavior. Significant effects have been reported for such amino acids as leucine, isoleucine, valine, histidine, threonine, tryptophan, and tyrosine (Bums and Kacser, 1987; Frieder and Grimm, 1984; Funk et al., 1991; Huether, et al., 1992; Matsueda and Niiyama, 1982). The use of amino acid supplements in pregnant women, although not examined, might therefore also be expected to elevate maternal plasma amino acid levels and possibly lead to similar, negative effects in their offspring (as observed in rats). Based on the results of these studies, pregnant and lactating women might be at greater risk of adverse effects from ingestion of particular amino acids.
In summary, the results of human and animal studies show that consumption of single protein or amino acid supplements in excess of recommended intakes during pregnancy may have detrimental effects on fetal growth and development. Such effects must be considered in light of the increasing representation of women among deployed forces and the fact that, at any given time, approximately 10 percent of female active-duty personnel are pregnant.
REFERENCES
Albino, J.E., and R.B. Mateo. 1995. Nitric oxide. Pp. 99-123 in Amino Acid Metabolism and Therapy in Health and Nutritional Disease, L.A. Cynober, ed. Boca Raton, Fla.: CRC Press.
Allen, L.H., E.A. Oddoye, and S. Margen. 1979. Protein-induced hypercalciuria: A longer term study. Am. J. Clin. Nutr. 32:741-749.
AR (Army Regulation) 40-250. 1947. See U.S. Department of the Army, 1947.
Anderson, J.W., B.M. Johnstone, and M.E. Cooke-Newell. 1995. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 333(5): 276-282.
Askew, E.W. 1989. Nutrition for a cold environment. Phys. Sportsmed. 17:77-89.
Askew, E.W., I. Munro, M.A. Sharp, S. Siegel, R. Popper, M.S. Rose, R.W. Hoyt, K. Reynolds, H.R. Lieberman, D. Engell, and C.P. Shaw. 1987. Nutritional status and physical and mental performance of soldiers consuming the Ration, Lightweight or the Meal, Ready-to-Eat military field ration during a 30 day field training exercise (RLW-
30). Technical Report No. T7-87. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Baker-Fulco, C.J. 1995. Overview of dietary intakes during military exercises. Pp. 121-149 in Not Eating Enough, Overcoming Underconsumption of Military Operational Rations, B.M. Marriott, ed. Institute of Medicine. Washington, D.C.: National Academy Press.
Baker-Fulco, C.J., J.C. Buchbinder, S.A. Torri, and E.W. Askew. 1992. Dietary Status of Marine Corps officer candidates. Fed. Am. Soc. Exp. Biol. J. [FASEB J] 6(4):A1682.
Baker-Fulco, C.J., S.A. Torri, I.E. Arsenault, and J.C. Buchbinder. 1994. Impact of menu changes designed to promote a training diet [abstract]. J. Am. Diet. Assn. 94:A9.
Banderet, L.E., and H.R. Lieberman. 1989. Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain Res. Bull. 22:759-762.
Barbul, A., S.A. Lazarou, D.T. Efron, H.L. Wasserkrug, and G. Efron. 1990. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 108(2):331-336.
Baumgartner, R.N., W.C. Chumlea, and A.F. Roche. 1989. Estimation of body composition from bioelectric impedance of body segments. Am. J. Clin. Nutr. 50(2):221-226.
Beisel, W.R. 1992. Metabolic responses of the host to infections. Pp. 1-13 in Textbook of Pediatric Infectious Diseases, Vol. I, 3rd ed., R.D. Feigin and J.D. Cherry, eds. Philadelphia: W.B. Saunders Co.
Berneis, K., R. Ninnis, J. Girard, B.M. Frey, and U. Keller. 1997. Effects of insulin-like growth factor I combined with growth hormone on glucocorticoid-induced whole-body protein catabolism in man. Clin. Endocrinol. Metab. 82:2528-2534.
Bhasin, S., T. Storer, N. Berman, C. Callegari, B. Clevenger, J. Phillips, T.J. Bunell, R. Tricker, A. Shirari, and R. Casaburi. 1996. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 335:1-7.
Black, P.R., D.C. Brooks, P.Q. Bessey, R.R. Wolfe, and D.W. Wilmore. 1982. Mechanisms of insulin resistance following surgery. Ann. Surg. 196:420-435.
Blomstrand, E., P. Hassmén, B. Ekblom, and E.A. Newsholme. 1991. Administration of branched-chain amine acids during sustained exercise—Effects on performance and on plasma concentration of some amine acids. Eur. J. Appl. Physiol. 63:83-88.
Borchers, J., and G.E. Butterfield. 1992. The effect of meal composition on protein utilization following an exercise bout. Med. Sci. Sports. Exer. 24:S51.
Burns, J.E., and H. Kacser. 1987. Genetic effects on susceptibility to histidine induced teratogenesis in the mouse . Genet. Res. 50(2):147-153.
Buskirk, E.R. 1993. Energetics and climate with emphasis on heat: A historical perspective. Pp. 97-116 in Nutritional Needs in Hot Environments: Applications for Military Personnel in Field Operations. B.M. Marriott, ed. Institute of Medicine. Washington, D.C.: National Academy Press.
Buskirk, E.R. 1996. Exercise. Pp. 420-429, Chapter 41 in Present Knowledge in Nutrition, E.E. Ziegler and L.J. Filer, Jr., eds. Washington, D.C.: ILSI Press.
Butterfield, G.E. 1987. Whole-body protein utilization in humans. Med. Sci. Sports. Exerc. 19:S157-165.
Butterfield, G.E. 1996. Maintenance of body weight at high altitudes: In search of 500 kcal/day. Pp. 357-378 in Nutritional Needs in Cold and in High-Altitude Environments, B.M. Marriott and S.J. Carlson, eds. Institute of Medicine. Washington, D.C.: National Academy Press.
Butterfield, G.E., and D.H. Calloway. 1984. Physical activity improves protein utilization in young men. Br. J. Nutr. 11:171-184.
Butterfield, G.E., J. Gates, S. Fleming, G.A. Brooks, J.R. Sutton, and J.T. Reeves. 1992. Increased energy intake minimizes weight loss in men at high altitude. J. Appl. Physiol. 72:1741-1748.
Butterfield, G.E., J. Thompson, M.J. Rennie, R. Marcus, R.L. Hintz and A.R. Hoffman. 1997. Effect of rhGH and IGF-1 treatment on protein utilization in elderly women. Am. J. Physiol. 272:E94-E99.
Calloway, D.H. 1975. Nitrogen balance of men with marginal intakes of protein and energy. J. Nutr. 105:914-923.
Calloway, D.H., and H. Spector. 1954. Nitrogen balance as related to caloric and protein intake in active young men. Am. J. Clin. Nutr. 2:405-412.
Calloway, D.H., A.C.F. Odell, and S.J. Marten. 1971. Sweat and miscellaneous nitrogen losses in human balance studies. J. Nutr. 101:775-786.
Carlson, D.E., T.B. Dugan, J.C. Buchbinder, J.D. Allegretto, and D.D. Schnakenberg. 1987. Nutritional assessment of the Ft. Riley Non-commissioned Officer Academy dining facility. Technical Report T14-87. Natick, Mass.: U.S. Array Research Institute of Environmental Medicine.
Carraro, F., W.H. Hartl, C.A. Smart, D.K. Layman, F. Jahoor, and R.R. Wolfe. 1990. Whole body and plasma protein synthesis in exercise and recovery in human subjects. J. Appl. Physiol. 258:E821-E831.
Castell, L.M., J.R. Poortmans, and E.A. Newsholme. 1996. Does glutamine have a role in reducing infection in animals. Eur. J. Appl. Physiol. 13:488-490.
Chaouloff, F. 1989. Physical exercise and brain monoamines: a review. Acta. Physiol. Scand. 137:1-113.
Chittenden, R.H. 1907. The Nutrition of Man. London: Heinemann.
Cleare, A.J., and A.J. Bond. 1994. Effects of alterations in plasma tryptophan levels on aggressive feelings. Arch. Gen. Psychiatry 51(12): 1004-1005.
Cline, A.D., J.F. Patton, W.J. Tharion, S.R. Strowman, C.M. Champagne, J. Arsenault, K.L. Reynolds, J.P. Warber, C. Baker-Fulco, J. Rood, R.T. Tulley, and H.R. Lieberman. 1998. Assessment of the relationship between iron status, dietary intake, performance, and mood state of female Army officers in a basic training population. Technical Report No. T98-24. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Clugston, G., K.G. Dewey, C. Fjeld, J. Millward, P. Reeds, N.S. Scrimshaw, K. Tontisirin, J.C. Waterlow, and V.R. Young. 1996. Report of the working group on protein and amino acid requirements. Europ. J. Clin. Nutr. 50:S193-S195.
Collins, D.M., C.T. Rezzo, J.B. Kopp, P. Ruiz, T.M. Coffman, and P.E. Klotman. 1990. Chronic high protein feeding does not produce glomerulonephrosis or renal insufficiency in the normal rat. J. Am. Soc. Nephrol. 1:624.
Collipp, P.J., J. Thomas, V. Curti, R.K. Sharma, V.T. Maddaiah, S.E. Cohn. 1973. Body composition changes in children receiving human growth hormone. Metabolism 22:589-595.
Consolazio, C.F.: and R. Shapiro. 1964. Energy requirements of men in extreme heat. Pp. 121-124 in Environmental Physiology and Psychology in Add Conditions: Proceedings of the Lucknow Symposium. Liege, Belgium: United Nations, UNESCO.
Copeland, K.C., and K.S. Nair. 1994. Acute growth hormone effects on amino acid and lipid. J. Clin. Endocrinol. Metab. 78:1040-1047.
Cuneo, R.C., F. Salomon, C.M. Wiles, R. Hesp, P.H. Sonksen. 1991. Growth hormone treatment in growth hormone-deficient adults. II. Effects on exercise performance. J. Appl. Physiol. 70:695-700.
Cuthbertson, D. and H.N. Munro. 1937. A study of the effect of over-feeding on the protein metabolism of man. III. The protein-saving effect of carbohydrate and fat when superimposed on a diet adequate for maintenance. Biochem. J. 31:694-705.
Davis, J.M., S.P. Bailey, J.A. Woods, F.J. Galiano, M.T. Hamilton, and W.P. Bartoli. 1992. Effects of carbohydrate feedings on plasma free tryptophan and branched chain amine acids during prolonged cycling. Eur. J. Appl. Physiol. 65:513-519.
Deijen, J.B., and J.F. Orlebeke. 1994. Effect of tyrosine on cognitive function and blood pressure under stress. Brain Res. Bull. 33:319-323.
Delgado, P.L., D.S. Charney, L.H. Price, G.K. Aghajanian, H. Landis, and G.R. Heninger. 1990. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan.. Arch. Gen. Psychiatry 47:411-418.
Duncan, A.M., R.O. Ball, and P.B. Pencharz. 1996. Lysine requirement of adult males is not affected by decreasing dietary protein intake. Am. J. Clin. Nutr. 64:718-725.
Edwards, J.S.A., E.W. Askew, N. King, C.S. Fulco, R.W. Hoyt, and J.P. DeLany. 1991. An assessment of the nutritional intake and energy expenditure of unacclimatized U.S. Army soldiers living and working at high altitude. Technical Report No. T10-91. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Elahi, D., M. McAloon-Dyke, N.K. Fukagawa, A.L. Sclater, G.A. Wong, R.P. Shannon, K.L. Minaker, J.M. Miles, A.H. Rubenstein, and C.J. Vandepol. 1993. Effects of recombinant human IGF-I on glucose and leucine kinetics in men. Am. J. Physiol. 265:E831-E838.
FASEB/LSRO. 1992. Safety of amine acids used as dietary supplements. Center for Food Safety and Applied Nutrition. FDA Contract No. 223-88-2124, Task No. 8.
FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). 1991. Protein Quality Evaluation. Report of a Joint FAO/WHO Expert Consultation. FAO Food and Nutrition Paper 51. Rome: FAO.
FAO/WHO/UNU ((Food and Agriculture Organization of the United Nations/World Health Organization)/United Nations University). 1985. Energy and protein requirements. Report of a joint expert consultation. World Health Organization Technical Report Series No. 724. Geneva: World Health Organization.
Fernstrom, J.D. 1990. Aromatic amine acids and monoamine synthesis in the central nervous system: Influence of the diet. J. Nutr. Biochem. 1:508-517.
Fernstrom, J.D. 1994. Stress and monoamine neurons in the brain. Pp. 161-175 in Food Components to Enhance Performance, B.M. Marriott, ed. Institute of Medicine. Washington, D.C.: National Academy Press.
Fernstrom, J.D., and M.J. Hirsch. 1975. Rapid repletion of brain serotonin in malnourished, corn-fed rats following L-tryptophan injection. Life Sciences 17:455-464.
Fernstrom, J.D., and L.D. Lytle. 1976. Corn malnutrition, brain serotonin, and behavior. Nutr. Rev. 34:257-262.
Fernstrom, J.D., and R.J. Wurtman. 1971. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science 173:149-152.
Feskanich, D., W.C. Willett, M.J. Stampfer, and G.A. Colditz. 1996. Protein consumption and bone fractures in women. Am. J. Epidemiol. 143:472-479.
Fielding, R.A., C.N. Meredith, K.P. O'Reilly, W.R. Fontera, J.G. Cannon and N.J. Evans. 1991. Enhanced protein breakdown after eccentric exercise in young and older men. J. Appl. Physiol. 11:674-679.
Frieder, B., and V.E. Grimm. 1984. Prenatal monosodium glutamate (MSG) treatment given through the mother's diet causes behavioral deficits in rat offspring. Int. J. Neurosci. 23(2):117-126.
Friedl, K.E. 1997. Variability of fat and lean tissue loss during physical exertion with energy deficit . Pp. 431-450 in Physiology, Stress, and Malnutrition: Functional Correlates, Nutritional Intervention, J.M. Kinney and H.N. Tucker, eds. Philadelphia: Lippincott-Raven Publishers.
Friedman, M. 1991. Formation, nutritional value, and safety of d-amino acids. Pp. 447-492 in Nutritional and Toxicological Consequences of Food Processing. New York: Plenum Press.
Fryburg, D.A. 1994. Insulin-like growth factor I exerts growth hormone and insulin-like actions on human muscle protein metabolism. Am. J. Physiol. 267:E331-E336.
Fryburg, D.A. 1996. NG-monomethyl-L-arginine inhibits the blood flow but not the insulin-like response of forearm muscle to IGF- I: possible role of nitric oxide in muscle protein synthesis. J. Clin. Invest. 97:1319-1328.
Fryburg, D.A., and E.J. Barrett. 1993. Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism 42:1223-1227.
Fryburg, D.A., R.A. Gelfand, and E.J. Barrett. 1991. Growth hormone acutely stimulates forearm protein synthesis in normal subjects. Am. J. Physiol. 260:E499-E504
Fryburg, D.A., L.A. Jahn, S.A. Hill, D.M. Oliveras, and E.J. Barrett. 1995. Insulin and insulin-like growth factor I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J. Clin. Invest. 96:1722-1729.
Fuller, M.F., and P.J. Garlick. 1994. Human amino acid requirements: Can the controversy be resolved? Ann. Rev. Nutr. 14:217-241.
Funk, D.N., B. Worthington-Roberts, and A. Fantel. 1991. Impact of supplemental lysine or tryptophan on pregnancy course and outcomes in rats. Nutr. Res. 11:501-512.
Gallagher, D., D. Belmonte, P. Deurenberg, Z. Wang, N. Krasnow, F.X. Pi-Sunyer, S.B. Heymsfield. 1998. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am. J. Physiol. 275:E249-E258.
Gontzea, I., P. Sutzescu, and S. Dumitrache. 1975. The influence of adaptation to physical effort on nitrogen balance in man. Nutrition Reports International 22:231-236.
Gore, D.C., D. Honeycutt, F. Jahoor, R.R. Wolfe, and D.N. Herndon. 1991. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch. Surg. 126:38-43.
Griffiths, R.D., C. Jones, and T.E.A. Palmer. 1997. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 13(4):295-302.
Hartmann, E., and D. Greenwald. 1984. Tryptophan and human sleep: An analysis of 43 studies. Pp. 297-304 in Progress in Tryptophan and Serotonin Research, H.G. Schlossberger, W. Kochen, B. Linzen, and H. Steinhart, eds. Berlin: Walter de Gruyter.
Hegsted, M., and H.M. Linkswiler. 1981. Long-term effects of level of protein intake on calcium metabolism in young adult women. J. Nutr. 111:244-251.
Hegsted, M., S.A. Schuette, M.B. Zemel, and H.M. Linkswiler. 1981. Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake. J. Nutr. 111:553-562.
Hertzman, P.A., W.L. Blevins, J. Mayer, B. Greenfield, M. Ting, and G.J. Gleich. 1990. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N. Engl. J. Med. 322:869-873.
Heymsfield, S.B., D. Gallagher, M. Visser, C. Nuñez, and Z-M. Wang. 1995. Measurement of skeletal muscle: Laboratory and epidemiological methods. J. Gerontol. 50A:23-29.
Heymsfield, S.B., R. Ross, Z. Wang, D. Frager. 1997. Imaging Techniques of Body Composition: Advantages of Measurement and New Uses. Pp. 127-150 in Emerging Technologies for Nutrition Research, S.J. Carlson-Newberry and R.B. Costello, eds. Institute of Medicine. Washington, DC: National Academy Press.
Hirsch, E., W. Johnson, P. Dunne, C. Shaw, N. Hotson, W. Tharion, H. Lieberman, R. Hoyt, and D. Dacumos. In press. The effects of diet composition on food intake, food selection, and water balance in a hot environment. Technical Report. Natick, Mass.: Natick Research, Development and Engineering Center.
Hoyt, R.W., and A. Honig. 1996. Body fluid and energy metabolism at high altitude. Pp. 1277-1289 in Handbook of Physiology, Section 4: Environmental Physiology, C.M. Blatteis and M.J. Fregly, eds. New York: Oxford University Press for the American Physiological Society.
Huether, G., F. Thomke, and L. Adler. 1992. Administration of tryptophan-enriched diets to pregnant rats retards the development of the serotonergic system in their offspring. Brain Res. Dev. Brain Res. 68(2):175-181.
IOM (Institute of Medicine). 1992. A Nutritional Assessment of U.S. Army Ranger Training Class 11/91. March 23. Washington, D.C.
IOM. 1993b. Review of the Results of Nutritional Intervention, U.S. Army Ranger Training Class 11/92 (Ranger II), B.M. Marriott, ed. Washington, D.C.: National Academy Press.
IOM. 1994. Food Components to Enhance Performance, An Evaluation of Potential Peformance-Enhancing Food Components for Operational Rations, B.M. Marriott, ed. Washington, D.C.: National Academy Press.
IOM. 1995. Not Eating Enough, Overcoming Underconsumption of Military Operational Rations, B.M. Marriott, ed. Washington, D.C.: National Academy Press.
IOM. 1996. Nutritional Needs in Cold and in High-Altitude Environments, Applications for Military Personnel in Field Operations, B.M. Marriott and S.J. Carlson, eds. Washington, D.C.: National Academy Press.
IOM. 1997. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington D.C.: National Academy Press.
IOM. 1998. Assessing Readiness in Military Women: The Relationship of Body Composition, Nutrition, and Health. Washington, D.C.: National Academy Press.
IOM. 1999. Military Strategies for Sustainment of Nutrition and Immune Function in the Field. Washington, D.C.: National Academy Press.
Jacobi, C.A., J. Ordemann, F. Wenger, K. Zuckerman, H.D. Volk, and J.M. Muller. 1997. The influence of glutamine substitution in postoperative parenteral nutrition on immunologic function. First results of a prospective randomized trial (abstract). Shock 7(S):605.
Jacobs, B.L. and C.A. Fornel. 1993.5-Hydroxytryptamine and motor control: a hypothesis. Trends in Neurosciences. 16:346-352.
Jones, P.J.H., and I.K.K. Lee. 1996. Macronutrient requirements for work in cold environments. Pp. 189-202 in Nutritional Needs in Cold and in High-Altitude Environments: Applications for Military Personnel in Field Operations, B.M. Marriott and S.J. Carlson, eds. Institute of Medicine. Washington, D.C. : National Academy Press.
Jorgensen, J.O.L., L. Thuesen, T. Ingemann-Hansen, S.A. Pedersen, J. Jorgensen, N.E. Skakkebaek, and J.S. Christiansen. 1989. Beneficial effects of growth hormone treatment in GH deficient adults. Lancet 1:1221-1225.
Katz, A., S. Broberg, K. Sahlin, and J. Wahren. 1986. Muscle ammonia and amino acid metabolism during dynamic exercise in man. Clin. Physiol. 6:365-379.
Kerr, G.R., E.S. Lee, M.M. Lan, R.J. Lorimor, E. Randall, R.N. Forthofer, M.A. Davis, and S.M. Magnetti. 1982. Relationships between dietary and biochemical measures of nutritional status in NHANES I data. Am. J. Clin. Nutr. 35:294-308.
Kerstetter, J.E., and L.H. Allen. 1990. Dietary protein increases urinary calcium. J. Nutr. 120:134-136.
King, N., K.E. Fridlund, and E.W. Askew. 1993. Nutrition issues of military women. J. Am. Coll. Nutr. 12:344-348.
Kretsch, M.J., P.M. Confetti, and H.E. Sauberlich. 1986. Nutrient intake evaluation of male and female cadets at the United States Military Academy, West Point, New York, Report No. 218. Presidio of San Francisco, Calif. Letterman Army Institute of Research.
Kishi, K., S. Miyatani, and G. Inoue. 1978. Requirements and utilization of egg protein by Japanese young men with marginal intakes of energy. J. Nutr. 108:658-669.
King, N., J.E. Arsenault, S.H. Mutter, C.M. Champagne, T.C. Murphy, K.A. Westphal, and E.W. Askew. 1994. Nutritional intake of female soldiers during the U.S. Army basic combat training. Technical Report No. T94-17. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Kinney, J.M., and D.H. Elwyn. 1995. Amino acid metabolism in health and nutritional disease. Pp. 1-12 in Amino Acid Metabolism in Health and Nutritional Disease, L.A. Cynober, ed. Boca Raton, Fla.: CRC Press.
Klicka, M.V., D.E. Sherman, N. King, K.E. Friedl, and E.W. Askew. 1993. Nutritional assessment of cadets at the U.S. Military Academy: Part 2. Assessment of nutritional intake. Technical Report T94-1. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Kreider, R.B., V. Miriel, and E. Bertum. 1993. Amino acid supplementation and exercise performance—Analysis of the proposed ergogenic value. Sports Med. 16:190-209.
Kurzer, M.S. and D.H. Calloway. 1986. Effects of energy deprivation on sex hormone patterns in healthy menstruating women. Am. J. Physiol. 251:E483-E488.
LeBlanc, J.A. 1996. Cold exposure, appetite, and energy balance. Pp. 203-214 in Nutritional Needs in Cold and in High-Altitude Environments: Applications for Military Personnel in Field Operations, B.M. Marriott and S.J. Carlson, eds. Institute of Medicine. Washington, D.C.: National Academy Press.
Lehnert, H.R., D.K. Reinstein, and R.J. Wurtman. 1984a. Tyrosine reverses the depletion of brain norepinephrine and the behavioral deficits caused by tail-shock stress in rats. Pp. 81-91 in Stress: The Role of Catecholamines and Other Neurotransmitters, E. Usdin and R. Kvetnansky, eds. New York: Gordon and Beach.
Lemon, P.R., M.A. Tarnopolsky, J.D. MacDougall, and S.A. Atkinson. 1992. Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders. J. Appl. Physiol. 73:767-775.
Lieberman, H.R., 1994. Tyrosine and stress: Human and animal studies. Pp. 277-299 in Food Components to Enhance Performance, An Evaluation of Potential Performance—Enhancing Food Components for Operational Rations, B.M. Marriott, ed. Washington, D.C.: National Academy Press.
Lieberman, H.R. and B. Shukitt-Hale. 1996. Food components and other treatments that may enhance performance at high altitude and in the cold. Pp. 453-465 in Nutritional Needs in Cold and in High Altitude Environments, B. Marriott and S. Newberry, eds. Washington, D.C.: National Academy Press.
Lieberman, H.R., S. Corkin, B.J. Spring, J.H. Growdin, and R.J. Wurtman. 1983. Mood, performance, and pain sensitivity: Changes induced by food constituents. J. Psychiatr. Res. 17(2):135-145.
Lieberman, H.R., S. Corkin, B.J. Spring, R.J. Wurtman, and J.H. Growdon. 1985. The effects of dietary neurotransmitter precursors on human behavior. Am. J. Clin. Nutr. 42:366-370.
Lieberman, S.A., G.E. Butterfield, D. Harrison, and A.R. Hoffman. 1994. Anabolic effects of recombinant insulin-like growth factor-I in cachectic patients with the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 78:404-410.
Linkswiler, H.M., C.L. Joyce, and R. Anand. 1974. Calcium retention of young adult males as affected by level of protein and of calcium intake. Proc. N.Y. Acad. Sci. 36:333-340.
Longenecker, J.B. 1961. Relationship between plasma amino acids and clinical chemistry of dogs. Pp. 469-485 in Progress in Meeting Protein Needs of Infants and Ire-school Children. Publ. 843. Washington, D.C.: National Academy of Sciences.
Longenecker, J.B. 1963. Utilization of dietary protein. Pp. 113-144, Chapter 2, in Newer
Methods of Nutritional Biochemistry, A.A. Albanese, ed. New York: Academic Press.
Longenecker, J.B., and N.L. Hause. 1959. Relationship between plasma amino acids and composition of the ingested protein. Arch. Biochem. Biophys. 84:46-60.
Longenecker, J.B., and N.L. Hause. 1961. Relationship between plasma amino acids and composition of the ingested protein. II. A shortened procedure to determine plasma amino acid (PAA) ratios. Am. J. Clin. Nutr. 9:356-363.
Lukaski, H.C., J. Mendez, E.R. Buskirk, and S.H. Cohn. 1981. Relationship between endogenous 3-methylhistidine excretion and body composition. Am. J. Physiol. 240: E302-E307.
Marchini, J.S., J. Cortiella, T. Hiramatsu, T.E. Chapman, and V.R. Young. 1993. Requirements for indispensable amino acids in adult humans: Longer term amino acid kinetic study with support for the adequacy of the Massachusetts Institute of Technology amino acid requirement pattern. Am. J. Clin. Nutr. 58:670-683.
Matsueda, S., and Y. Niiyama. 1982. The effects of excess amino acids on maintenance of pregnancy and fetal growth in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 28:557-573.
McLarney, M.J., P.L. Pellett, and V.R. Young. 1996. Pattern of amino acid requirements in humans: An interspecies comparison using published amino acid requirements recommendations. J. Nutr. 126:1871-1882.
Meredith, C.N., M.J. Zackin, W.R. Frontera, and W.J. Evans. 1989. Dietary protein requirements and body protein metabolism in endurance-trained men. J. Appl. Physiol. 66:2850-2856.
Messing, R.B., and L.D. Lytle. 1977. Serotonin-containing neurons: their possible role in pain and analgesia. Pain 4:1-21.
Millward, D.J. 1994. Can we define indispensable amino acid requirements and assess protein quality in adults? J. Nutr. 124:1509S-1516S.
Millward, D.J., and J.P. Rivers. 1988. The nutritional role of indispensable amino acids and the metabolic basis for their requirements. Eur. J. Clin. Nutr. 42:367-393.
Millward, D.J., and J.P. Rivers. 1989. The need for indispensable amino acids: The concept of the anabolic drive. Diab. Metab. Rev. 5(2):191-211.
Millward, D.J., and J.C. Waterlow. 1996. Letter to the editor. Eur. J. Clin. Nutr. 50:832-833. Millward, D.J., J.L. Bowtell, P. Pacy, and M.J. Rennie. 1994. Physical activity, protein metabolism and protein requirements. Proc. Nutr. Soc. 53(1):223-240.
Mitchell, H.H., and M. Edman. 1949. Nutrition and Resistance to Climatic Stress, with Reference to Man. Chicago, Ill.: Quartermaster Food and Container Institute for the Armed Forces.
Mitchell, H.H., and M. Edman. 1951. Nutrition and Resistance to Climatic Stress, with Particular Reference to Man. Springfield, Ill.: Charles C. Thomas.
Motil, K.J., C.M. Montandon, M. Thotathuchery, and C. Garza. 1990. Dietary protein and nitrogen balance in lactating and nonlactating women. Am. J. Clin. Nutr. 51:378-384.
Motil, K.J., T.A. Davis, C.M. Montandon, W.W. Wong, and P.D. Klein. 1996. Whole-body protein turnover in the fed state is reduced in response to dietary protein restriction in lactating women. Am. J. Clin. Nutr. 64:32-39.
Mulligan, K., and G.E. Butterfield. 1990. Discrepancies between energy intake and expenditure in physically active women. Br. J. Nutr. 64(1):23-36.
Munro, H.N., and M.C. Crim. 1994. Protein and amino acids. In Modern Nutrition in Health and Disease, M.E. Shils, J.A. Olson, and M. Shike, eds. Philadelphia: Lea and Febiger.
Neri, D.F., D. Wiegmann, R.R. Stanny, S.A. Shappell, A. McCardie, and D.L. McKay. 1995. The effects of tyrosine on cognitive performance during extended wakefulness. Aviat. Space Environ. Med. 66:313-319.
Nindl, B.C., K.E. Friedl, P.N. Frykman, L.J. Marchitelli, R.L. Shippee, and J.F. Patton. 1997. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int. J. Sports Med. 18:1-8.
NRC (National Research Council). 1941. Recommended Dietary Allowances. Food and Nutrition Board. Washington, D.C.: National Academy Press.
NRC. 1989. Recommended Dietary Allowances, 10th ed. Institute of Medicine. Washington, D.C.: National Academy Press.
Nuñez, C., D. Gallagher, and S.B. Heymsfield. 1995. Appendicular skeletal muscle mass: Measurement with single frequency bioimpedance analysis. FASEB J. 9(4):A1012.
Nuñez, C., D. Gallagher, M. Visser, F.X. Pi-Sunyer, Z. Wang, and S.B. Heymsfield. 1997. Bioimpedance analysis: Evaluation of leg-to-leg system based on pressure contact footpad electrodes. Med. Sci. Sports Exerc. 29:524-31.
O'Riordain, M., K.C. Fearon, J.A. Ross, P. Rogers, J.S. Falconer, D.C. Bartolo, O.J. Garden, and D.C. Carter. 1994. Glutamine-supplemented parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann Surg. 220:212-221.
Owen, O.E., K.J. Smalley, D.A. D'Alessio, M.A. Mozzoli, E.K. Dawson. 1998. Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis. Am. J. Clin. Nutr. 68:12-34.
Pardridge, W.M. 1977. Regulation of amino acid availability to the brain. Pp. 141-190 in Nutrition and the Brain, Vol. 1, R.J. Wurtman and J.J. Wurtman, eds. New York: Raven Press.
Paul, G.L. 1989. Dietary protein requirements of physically active individuals. Sports Mad. 8:154-176.
Pearlstone, D.B., R.F. Wolf, R.S. Berman, M. Burt, M.F. Brennan. 1994. Effect of systemic insulin on protein kinetics in postoperative cancer patients. Ann. Surg. Oncol. 1(4):321-332.
Phillips, S.M., S.A. Atkinson, M.A. Tarnopolsky, and J.D. MacDougall. 1993. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J. Appl. Physiol. 75:2134-2141.
Picou, D., and T. Taylor-Roberts. 1969. The measurement of total protein synthesis and catabolism and nitrogen turnover in infants in different nutritional states and receiving different amounts of dietary protein.. Clin. Sci. 36:283-296
Reeds, P.J., and P.R. Becket. 1996. Protein and amino acids. Pp. 67-86 in Present Knowledge in Nutrition, 7th ed., E.E. Ziegler and L.J. Filer, eds. Washington, D.C.: ILSI Press.
Rennie, M.J. 1996. Influence of exercise on protein and amino acid metabolism. Pp. 995-1035 in American Physiological Society Handbook of Physiology on Exercise, Chapter 12, Section 12, Control of Energy Metabolism During Exercise, R. L. Terjung, ed. Bethesda, Md.: American Physiological Society.
Robertson, W.G., P.J. Heyburn, M. Peacock, F.A. Hanes, and R. Swaminathan. 1979b. The effect of high animal protein intake on the risk of calcium-stone-formation in the urinary tract. Clin. Sci. 57:285-288.
Rooyackers, O., and K.S. Nair. 1997. Hormonal regulation of human muscle protein metabolism. Ann. Rev. Nutr. 17:457-485.
Rose, M.S. and D.E. Carlson. 1986. Effects of A Ration meals on body weight during sustained field operations. Technical Report T2-87. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Rose, M.S., P.C. Szlyk, R.P. Francesconi, L.S. Lester, L. Armstrong, W. Matthew, A.V. Cardello, R.D. Popper, I. Sils, G. Thomas, D. Schilling, and R. Whang. 1989. Effectiveness and acceptability of nutrient solutions in enhancing fluid intake in the
heat. Technical Report No. T10-89. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Rose, R.W., C.J. Baker, W. Wisnaskas, J.S.A. Edwards, and M.S. Rose. 1989. Dietary assessment of U.S. Army basic trainees at Fort Jackson, South Carolina. Technical Report No. T6-89. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Rowbottom, D.G., D. Keast, C. Goodman, and A.R. Morton 1995. The haernatological, biochemical and immunological profile of athletes suffering from the overtraining syndrome. Eur. J. Appl. Physiol. 70:502-509.
Rush, D., Z. Stein, and M.A. Susser. 1980. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 65:653-697.
Sahlin, K., A. Katz, and S. Broberg. 1990. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am. J. Physiol. 159:C834-C841.
Sahlin, K., L. Jorfeldt, and K.G. Henriksson. 1995. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle's disease. Clin. Sci. 19:687-693.
Sakurai, Y., A. Aarsland, D.N. Herndon, D. L. Chinkes, E. Pierre, T.T. Nguyen, B.W. Patterson, and R.R. Wolfe. 1995. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann. Surg. 222(3):283-294.
Schuette, S.A., and H.M. Linkswiler. 1982. Effects on Ca and P metabolism in humans by adding meat, meat plus milk, or purified proteins plus Ca and P to a low protein diet. J. Nutr. 112:338-349.
Schwartz, R.S. 1995. Trophic factor supplementation: effect on the age-associated changes in body composition. J. Gerontol. A. Biol. Sci. Med. Sci. 50:151-156.
Seltzer, S., D. Dewart, R. L. Pollack, and E. Jackson. 1983. The effects of dietary tryptophan on chronic maxillofacial pain and experimental pain tolerance. J. Psychiat. Res. 17(2):181-186.
Shapses, S.A., S.P. Robins, E.I. Schwartz, and H. Chowdhury. 1995. Short-term changes in calcium but not protein intake alter the rate of bone resorption in healthy subjects as assessed by urinary pyridinium cross-link excretion. J. Nutr. 125:2814-2821.
Sharp, T., S.R. Bramwell, and D.G. Grahame-Smith. 1992. Effect of acute administration of L-tryptophan on the release of 5-HT in rat hippocampus in relation to serotoninergic neuronal activity: An in vivo microdialysis study. Life Sci. 50:1215-1223.
Shukitt-Hale, B., M.J. Stillman, and H.R. Lieberman. 1996. Tyrosine administration prevents hypoxia-induced decrements in learning and memory. Physiol. Behav. 59:867-871.
Shurtleff, D., J.R. Thomas, S.T. Ahlers, and J. Schrot. 1993. Tyrosine ameliorates a cold-induced delayed matching-to-sample performance decrement in rats. Psychopharmacol. 112:228-232.
Shurtleff, D., J.R. Thomas, J. Schrot, K. Kowalski, and R. Harford. 1994. Tyrosine reverses a cold-induced working memory deficit in humans. Pharmacol. Biochem. Behav. 47(4):935-941.
Souba, W.W., and D.W. Wilmore. 1994. Diet and nutrition in the care of the patient with surgery, trauma, and sepsis. Pp. 1207-1240 in Modem Nutrition in Health and Disease, 8th e., M.E. Shils, J.A. Olson, and M. Shike, eds. Philadelphia: Lea and Febiger.
Spencer, H., L. Kramer, and D. Osis. 1988. Do protein and phosphorus cause calcium loss? J. Nutr. 118:657-660.
Stein, T.P., R.W. Hoyt, M.O. Toole, M.J. Leskiw, and M.D. Schluter. 1989. Protein and energy metabolism during prolonged exercise in trained athletes. Int. J. Sports Med. 10:311-316.
Stone, E. A. 1975. Stress and catecholamines. Pp. 31-71 in Catecholamines and Behavior, A.J. Freidhoff, ed. New York: Plenum Press.
Stroud, M.A., A.A. Jackson, and J.C. Waterlow. 1996. Protein turnover rates of two human subjects during an unassisted crossing of Antarctica. Br. J. Nutr. 16:165-174.
Stucky, W.P., and A.E. Harper. 1962. Effects of altering indispensable to dispensable amino acids in diets for rats. J. Nutr. 78:278-286.
Szeto, E.G., D.E. Carlson, T.B. Dugan, and J.C. Buchbinder. 1987. A comparison of nutrient intakes between a Ft. Riley contractor-operated and a Ft. Lewis military-operated garrison dining facility. Technical Report No. T2-88. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Szeto, E.G., T.B. Dugan, and J.A. Gallo. 1988. Assessment of habitual diners' nutrient intakes in a military-operated garrison dining facility, Ft. Devens I. Technical Report No. T3-89. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Szeto, E.G., J.A. Gallo, and K.W. Samonds. 1989. Passive nutrition intervention in a military-operated garrison dining facility, Ft. Devens II. Technical Report No. T7-89. Natick, Mass: U.S. Army Research Institute of Environmental Medicine.
Tarnopolsky, M.A., J.D. Mac Dougal, and S.A. Atkinson. 1988. Influence of protein intake and training status on nitrogen balance and lean body mass. J. Appl. Physiol. 64:187-193.
Tarnopolsky, M.A., P.W.R. Lemon, J.D. MacDougall, and J.A. Atkinson. 1990a. Effect of body building exercise on protein requirements. Can. J. Sport Sci. 15:225-226. Tarnopolsky, L.J., J.D. MacDougall, S.A. Atkinson, M.A. Tarnopolsky, and J.R. Sutton. 1990b. Gender differences in substrate for endurance exercise. J. Appl. Physiol. 68:302-308.
Tarnopolsky, M.A., S.A. Atkinson, S.M. Phillips, and J.D. Mac Dougal. 1995. Carbohydrate loading and metabolism during exercise in men and women. J. Appl. Physiol. 78:1360-1368.
Thomas, C.D., K.E. Friedl, M.Z. Mays, S.H. Mutter, and R.J. Moore. 1995. Nutrient intakes and nutritional status of soldiers consuming the Meal, Ready-to-Eat (MRE XII) during a 30-day field training exercise. Technical Report T95-6. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Tipton, K.D., and R.R. Wolfe. 1998. Exercise-induced changes in protein metabolism. Acta Physiol. Scand. 162(3): 377-387.
Tobin, J., and D. Spector. 1986. Dietary protein has no effect on future creatinine clearance. Gerontologist 25:59A.
Todd, K.S., G.E. Butterfield, and D.H. Calloway. 1984. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J. Nutr. 114:2107-2118.
Tschope, W., and E. Ritz. 1985. Sulfur-containing amino acids are the major determinant of urinary calcium. Mineral Electrolyte Metab. 11:137-139.
USACDEC/USARIEM (U.S. Army Combat Developments and Experimentation Center and U.S. Army Research Institute of Environmental Medicine). 1986. Combat Field Feeding System-Force Development Test and Experimentation (CFFS-FDTE) Technical Report CDEC-TR-85-006A . Vol. 1, Basic Report; vol. 2, Appendix A; vol. 3, Appendixes B through L. Fort Ord, Calif.: U.S. Army Combat Developments and Experimentation Center.
U.S. Department of the Army. 1947. Army Regulation 40-250. Nutrition. Washington, D.C.
Van der Hulst, R.R., B.K. van Kreel, M.F. von Meyenfeldt, R.J. Brummer, J.W. Arends, N.E. Deutz, and P.B. Soeters. 1993. Glutamine and the preservation of gut integrity. Lancet 341 (8957): 1363-1365.
Walser, M. 1992. Dietary proteins and their relationship to kidney disease. Pp. 168-178 in Dietary Proteins in Health and Disease, G.U. Liepa, ed. Champaign, Ill.: American Oil Chemists' Society.
Wang, Z., M. Visser, R. Ma, R. Baumgartner, D. Kotler, D. Gallagher, and S.B. Heymsfield.
1996. Skeletal muscle mass: Evaluation of neutron activation and dual-energy x-ray absorptiometry methods. J. Appl. Physiol. 80(3):824-831.
Wang, Z., P. Deurenberg, D.E. Matthews, and S.B. Heymsfield. 1998. Urinary 3-methylhistidine excretion: Association with total body skeletal muscle mass by computerized axial tomography. J. Parenter. Enteral Nutr. 22(2): 82-86.
Warber, J.P., F.M. Kramer, S.M. McGraw, L.L. Lesher, W. Johnson, and A.D. Cline. 1996. The Army Food and Nutrition Survey, 1995-97. Technical Report. Natick, Mass.: U.S. Army Research Institute of Environmental Medicine.
Walters, J.K., M. Davis, M.H. Sheard. 1979. Tryptophan-free diet: effects on the acoustic startle reflex in rats. Psychopharmacology (Berl) 62(2):103-109.
Waterlow, J.C. 1996. The requirements of adult man for indispensable amine acids. Eur. J. Clin. Nutr. 50:S151-176.
Welle, S., C. Thornton, M. Statt, and B. McHenry. 1996. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J. Clin. Endocrinol. Metab. 81:3239-3243.
Wilmore, D.W. 1991. Catabolic illness: Strategies for enhancing recovery. N. Engl. J. Med. 325(10):695-702.
Wilmore, D.W. 1997a. Glutamine saves lives! What does it mean? Nutrition 13(4):375-376.
Wolf, R.F., D.B. Pearlstone, E. Newman, M.J. Heslin, A. Gonenne, M.E. Burt, and M.F. Brennan. 1992. Growth hormone and insulin reverse net whole body and skeletal muscle protein catabolism in cancer patients. Ann. Surg. 216:280-258.
Wurtman, J.J., and J.D. Fernstrom. 1979. Free amine acid, protein and fat contents of breast milk from Guatemalan mothers consuming a corn-based diet. Early Human Development 3:67-77.
Wurtman, R.J., F. Hefti, and E. Melamed. 1981. Precursor control of neurotransmitter synthesis. Pharmacol. Rev. 32:315-335.
Yarasheski, K.E., J.J. Zachwieja, J.A. Campell, and D.M. Bier. 1995. Effect of growth hormone and resistance training on muscle growth and strength in older men. Am. J. Physiol. 268:E268-E276.
Young, V.R. 1987. McCollum Award Lecture: Kinetics of human amine acid metabolism: Nutritional implications and some lessons. Am. J. Clin. Nutr. 46:709-725.
Young, V.R. 1994. Adult amine acid requirement: The case for a major revision in current recommendations. J. Nutr. 124:1517S-1523S.
Young, V.R., and A. E. El-Khoury. 1995a. Can amine acid requirements for nutritional maintenance in adult humans be approximated from the amine acid composition of body mixed proteins? Proc. Natl. Acad. Sci. 921:300-304.
Young, V.R., and J.S. Marchini. 1990. Mechanisms and nutritional significance of metabolic responses to altered intakes of protein and amine acids, with reference to nutritional adaptation in humans. Am. J. Clin. Nutr. 51:270-289.
Young, V.R., D.M. Bier, and P.L. Pellet. 1989. A theoretical basis for increasing current estimates of the amine acid requirements in adult man with experimental support. Am. J. Clin. Nutr. 50:80-92.
Zawadzki, K.M., B.B. Yaspelkis, and J.L. Ivy. 1992. Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. J. Appl. Physiol. 72:1854-1859.
Zello, G.A., P.B. Pencharz, and R.O. Ball. 1993. Dietary lysine requirement of young adult males determined by oxidation of l-[l-13C]phenylalanine. Am. J. Physiol. 264:E677-E685.
Zemel, M.B. 1988. Calcium utilization: Effect of varying level and source of dietary protein. Am. J. Clin Nutr. 48:880-883.
Ziegler, T.R., L.S. Young, K. Benfell, M. Scheltinga, K. Hortos, R. Bye, F.D. Morrow, D.O.
Jacobs, R.J. Smith, J.H. Antin, and D.W. Wilmore. 1992. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann. Intern. Med. 116(10):821-828.
Ziegler, T.R., R.L. Bye, R.L. Persinger, L.S. Young, J.H. Antin, and D.W. Wilmore. 1994. Glutamine-enriched parenteral nutrition increases circulating lymphocytes after bone marrow transplantation. J. Parenter. Enteral Nutr. 18:17S.


























































