Protein and Amino Acids, 1999
Pp. 243-253. Washington, D.C.
National Academy Press
11
Physical Exertion, Amino Acid and Protein Metabolism, and Protein Requirements
Michael J. Rennie1
INTRODUCTION
A whiff of vitalism, the 19th century philosophical doctrine that there is some spiritual essence associated with biological processes, is still discernible in relation to the question of how contractile activity affects protein and amino acid metabolism. The working machine of muscles is, after all, made up of proteins so the idea comes naturally that where machinery works there must be wear and tear, presumably with a greater requirement for maintenance. It is also known that during starvation, i.e. a situation of substantial energy deficit, the lean body mass is the source of gluconeogenic carbon which fuels the central nervous system, erythrocytes and ion pumping in the kidney. Since physical activity requires extra energy it is plausible to imagine that in circumstances of marginal energy intake the lean body mass would also be at risk of diminution.
Over the past 25 years our understanding of the tidal flows of amine acids between the gut, viscera and the peripheral musculature has improved markedly. So has our understanding of the phenomenology and control mechanisms of protein turnover, the coordination of which with intermediary amine acid metabolism is now rather well understood. This increasing sophistication has led us to understand that the old distinctions between, for example, essential and non-essential amine acids is much less clear cut than it was. Since, by definition, conditionally essential amine acids are those which may become required in greater than normal amounts in special circumstances, it is obvious to ask whether or not muscular activity causes some amine acids to become conditionally essential.
There has been a substantial recent upswing in interest in the investigation of the relationship between contractile activity and amine acid and protein metabolism but much that we require to provide definitive answers is still missing, requiring more research. The gaps will become obvious in the following paper. I propose to discuss the known effects of increased contractile activity in skeletal muscle amine acid oxidation, on protein turnover and interactions with the state of energy balance, to provide a background for discussion of protein requirements.
EXERCISE AND AMINO ACID CATABOLISM
Although this kind of exercise probably only contributes a small fraction to the total daily energy expenditure of soldiers in the field, it may be that repeated bouts of exercise have cumulative effects on protein and amine acid metabolism; we may get some clues as to what these are by investigating exercise under laboratory conditions. There is now a substantial body of work which allows us to make some reasonably firm statements about the relative importance of amine acid metabolism in skeletal muscle during increased contractile activity.
The main fuels for sustained moderate to high intensity exercise are, of course, carbohydrate and fat and the most efficient means of converting these into ATP is via the Krebs cycle and oxidative phosphorylation. Theoretically the rate at which acetyl units may be catabolised in the Krebs cycle (and reducing equivalents fed into oxidative phosphorylation) will be limited by the availability of oxaloacetate since without this citrate cannot be formed nor can the two carbons of acetate be (eventually) transformed to CO2 as the cycle turns. One of the most pronounced features of amine acid metabolism in muscle (at least in human muscle), i.e. the increase in alanine production as a result of muscular contraction, may be part of a mechanism to ensure the appropriate expansion of the catalytic pool of Krebs cycle intermediates as the drive to increase ATP production switches on.
It is now well established that glutamate concentrations fall in muscle during exercise at 70-80 percent of VO2max (Katz et al., 1986; Sahlin et al.,
1995). The obvious routes by which this occurs are either transamination with pyruvate (both in the cytosol and in the mitochondria via alanine aminotransferase, to form alanine and α-ketoglutarate) or the glutamate dehydrogenase reaction, which has recently been discovered (Wibom et al., 1990) to have a somewhat greater capacity in human skeletal muscle than hitherto suspected. There is evidence that in the absence of any other mechanism to increase Krebs cycle intermediates (as in patients with McArdle's disease who are unable to generate oxaloacetate from endogenous glycogen stores via the malic enzyme, pyruvic carboxylase, and PEP carboxykinase), muscle glutamate concentration is reduced at rest, and work capacity appears to be limited when glutamate catabolism bottoms out (Sahlin et al., 1995).
The fall in muscle glutamate is puzzling. Branched chain amino acids are transaminated in the cytoplasmic space to form the branched chain ketoacids which are decarboxylated in mitochondria (see below). The carbon from valine and half of that from isoleucine may enter the Krebs cycle as succinyl CoA, processes which are therefore anaplerotic. The puzzling thing is that branched chain amino acids are transaminated at the expense of α-ketoglutarate to produce glutamate, which ought to protect muscle glutamate concentrations and to deplete α-ketoglutarate. The depletion of α-ketoglutarate would be catapleurotic if it extended to the mitochondria. We currently have no information on this.
What other ways can amino acids contribute to the anaplerotic process? Glutamine crosses the inner mitochondrial membrane with much greater ease than glutamic acid and the mitochondrial phosphate-dependent glutaminase would ensure a plentiful supply of glutamate without the necessity of exchanging glutamate for aspartate across the inner mitochondrial membrane. However, one would then expect to see a fall in muscle glutamine concentration, which is, in fact, usually only seen after long term exercise at moderate intensity at least. However, given the very high background of glutamine and the relative imprecision of measurement of glutamine, it is difficult to be sure about the size of the fall which occurs during heavy exercise. The other possible route of glutamine utilisation and production of α-ketoglutarate would be through the action of glutamine transaminase and to produce α-ketoglutaramide which spontaneously deaminates to produce α-ketoglutarate and ammonia. Unfortunately there is no good data to strengthen or weaken this suggestion. More research is needed on this topic.
The branched-chain amino acids are oxidised in muscle during exercise (Figure 11-1) at a rate which appears to be directly proportional to the overall rate of mitochondrial oxidation, and thus the muscle oxygen uptake possibly because oxygen uptake is blood flow dependent; so is catabolism of the
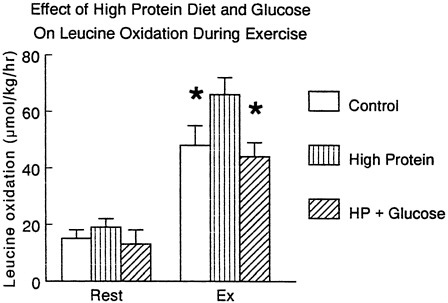
FIGURE 11-1 Effect of exercise in subjects taking a normal diet (control) or a high protein · diet (1.5 g protein/kg/day) on leucine oxidation during exercise studied in the absence and presence of exogenous glucose given orally. Source: J. Bowtell and M.J. Rennie (unpublished work).
branched chain amine acids by the transaminase, which has a high Km (Rennie, 1996). However, the total amount of energy supplied by this process, even at high rates of oxidation, is relatively low, ruling out a major contribution from protein as a metabolic fuel during contractile activity (Millward et al., 1994; Rennie, 1996).
The possible anaplerotic role of the purine nucleotide cycle, which excited a fair amount of interest in the 1970s as a means of generating fumarate, is now thought less likely to be important. The purine nucleotide cycle may not operate fully in contracting muscle and its total capacity is much less than, for example, alanine aminotransferase (Hood et al., 1990; Van Hall et al., 1995b). Ammonia production during exercise is most likely the result of branched chain amine acid catabolism (MacLean et al., 1996; Van Hall et al., 1995b).
The question arises, would repeated muscular exercise lead to a diminution of amine acids from the intramuscular compartment thus possibly limiting any anaplerotic role? If so the corollary is, would exogenous dietary supplementation make sense? These are difficult questions to answer since we do not know what is the lower limit of glutamate concentration before anaplerotic generation would cease to sustain a large enough increase in Krebs cycle intermediates although in long term exercise the pool size of these does fall (Sahlin et al., 1990). It is theoretically possible that repeated bouts of exercise at high intensity might, under circumstances of limited nutrient pro-
vision chronically depress muscle glutamate and glutamine concentrations and thereby limit the ability of the Krebs cycle to accelerate rapidly enough. However, this is, at the moment, speculation. More research is needed.
EFFECTS OF CONTRACTILE ACTIVITY ON MUSCLE PROTEIN TURNOVER
It is well accepted that muscular activity has a marked effect on muscle composition and/or muscle bulk, depending upon the type of contractile activity involved. Moderate, repeated, long-term exercise causes increases in mitochondrial mass and alterations in myosin ATPase to isoforms appropriate to long-term aerobic exercise; and high resistance exercise causes increases in expression of actin and myosin and increased mixed muscle protein synthesis (Rennie, 1996) (Figure 11-2). The anabolic phase occurs in the post-exercise period and can be maximised by the provision of exogenous amino acids.
It has been difficult to make measurements of protein synthesis during exercise in human beings because of the difficulty of detecting Sufficient change over a short period of time but it seems likely that muscle protein synthesis is depressed (Rennie, 1996; Dohm et al., 1980); thus, the rebound observed post-exercise (Chesley et al., 1992; Biolo et al., 1995) appears to fulfill a homeostatic function as much as anything. The extent of this elevation is limited, with a return to baseline 36 hr after a stimulatory bout of exercise (MacDougall et al., 1995). This ability of contractile activity to stimulate anabolism probably
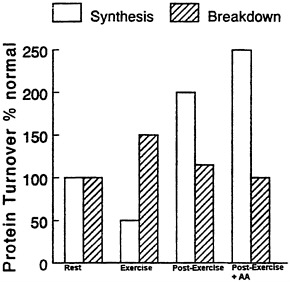
FIGURE 11-2 Notional scheme of changes in muscle protein synthesis and breakdown taking into account most of the current observations in the literature obtained from studies of human muscle.
explains why accustomed exercise increased the efficiency of nitrogen storage in young healthy men (Butterfield and Calloway, 1984).
Protein breakdown during exercise is definitely elevated (Biolo et al., 1995; MacLean et al., 1994) but the increase appears to be confined to the soluble or membrane proteins which are degraded by lysosomal proteases (Kasperek and Snider, 1989; Kasperek et al., 1992). The evidence that myofibrillar protein breakdown occurs during normal exercise is extremely sparse. Nevertheless, there is some evidence that myofibrillar protein breakdown increases as a result of eccentric exercise, i.e. exercise in which muscle is forced to contract as it is stretched, as in walking downhill (Fielding et al., 1991). In the post-exercise period muscle protein breakdown will be elevated to the extent that there is remodeling of muscle (Figure 11-2).
So far as net loss of protein is concerned this can only occur when synthesis occurs at a rate lower than breakdown and the net release of amine acids from muscle in the postabsorptive state and during exercise is a good example of this. However, feeding rapidly reverses the net nitrogen balance (Rennie, 1996) and given the relatively small contribution of amine acids to the fuel economy during exercise, it seems unlikely that the alterations in muscle protein turnover occurring acutely would contribute to increased dietary amine acid requirements. One of the problems for the theory that exercise should increase dietary requirements is of course the fact that eating more protein stimulates the catabolic capacity of the body to oxidise it. This is seen clearly in the results of Figure 11-1.
INTERACTIONS BETWEEN ENERGY SUPPLY AND PROTEIN AND AMINE ACID METABOLISM IN THE CONTEXT OF INCREASED PHYSICAL ACTIVITY
At rest provision of carbohydrate inhibits net protein catabolism, probably mainly by increasing insulin which has inhibitory effects on protein breakdown and stimulatory effects on protein synthesis; in addition the simple provision of carbohydrate inhibits gluconeogenesis from amine acids, diminishing the "pull" from the liver upon the peripheral lean body mass protein. During exercise provision of carbohydrate markedly suppresses leucine oxidation. (Figure 11-1).
There is insufficient evidence available that increased availability of triglycerides and medium chain fatty acids has any effect on the oxidation of amine acids during exercise to make any definitive statements.
What happens when chronic energy expenditure rises to such an extent that a subject is in negative energy balance? Under those circumstances, is muscle mass, for example, at risk? This is a difficult question to answer because the appropriate studies have not actually been done. It would be a reasonable hypothesis, however, that the lean body mass would tend to be preserved as a result of chronic daily exercise, with stores of body fat being used preferentially once the gluconeogenic needs were satisfied by protein breakdown. One study
which does throw some light on the situation, although it gives by no means a complete answer, is that carried out on Stroud and Fiennes during their epic, unaided walk across the Antarctic (Stroud et al., 1996). Energy expenditure was measured by the doubly labeled water method and whole body protein turnover was measured using the [15N]glycine/ammonia + urea end-product method. In one subject, whole body protein turnover increased slightly and in the other it decreased slightly but the remarkable thing was that although both subjects were in marked negative energy balance (with total energy expenditures in the range of 50 MJ/day) they did not show the diminution of whole body protein synthesis, which is characteristic of starvation, suggesting that their physical activity actually maintained protein turnover and possibly helped preserve lean body mass. There is no doubt, however, that both men did lose lean body mass and the definitive answer to whether or not this would have been faster or slower without their daily treck in sub-zero temperatures is currently unanswerable.
POSSIBLE BENEFICIAL EFFECTS OF BRANCHED CHAIN AMINO ACID SUPPLEMENTATION
Theoretically, branched chain amino acid supplementation could provide benefits in one of four ways there are to supply anaplerotic intermediates, to decrease the use of other fuels including glycogen, inhibit muscle protein breakdown, and inhibit the transport of tryptophan into the brain, thus limiting the increase in serotonin synthesis which is implicated in the central fatigue hypothesis (Hassmén et al., 1994).
There is, in fact, some evidence that branched chain amino acid supplementation does, to some extent, spare muscle glycogen (MacLean et al., 1996; Blomstrand and Newsholme, 1996) and limit proteolysis (MacLean et al., 1994). There is also said to be a decreased perceived exertion during exercise (Blomstrand et al., 1997). The serotonin central fatigue hypothesis is not, however, supported by the results of studies using serotonin receptor antagonists which ought to be the case if the central fatigue hypothesis were true (Pannier et al., 1995). In any case, whatever the mental state of subjects during exercise with or without branched chain amino acid supplementation, there is no evidence that there is any beneficial effect on physical performance (Van Hall et al., 1995a; Pannier et al., 1995; Struder et al., 1996; Madsen et al., 1996) of making branched chain amino acids available or of inhibiting serotonin uptake.
GLUTAMINE, THE OVERTRAINING SYNDROME AND IMMUNE FUNCTION
It has been known for some years that white cells have a specific requirement for glutamine. It has been suggested that the low blood and muscle
glutamine observed in athletes in training who suffer the so-called overtraining syndrome is the result of the progressive exhaustion by repeated exercise of the homeostatic system for preserving glutamine (Rowbottom et al., 1995, 1996). This diminishes the supply of glutamine available to white cells and could diminish their capacity for fighting infection. There is some evidence in favour of this proposition (Rohde et al., 1996) but it appears to me that, for the purposes of the present discussion, we need to ask ourselves: are the levels of physical activity which are engaged in by top athletes during training and which lead to the overtraining syndrome similar to those experienced by military personnel on active duty? Personally I doubt this, although it is certainly possible during specific training early after recruitment. There has been a single report of a beneficial effect of glutamine in reducing infection rate of amateur athletes after long distance runs (Castell et al., 1996). We now need to repeat such studies and we need know what the dose-response relationship is between exogenous glutamine and adequate immune function. We also need to understand whether or not small doses of glutamine given at regular intervals would be as efficacious as irregular large doses. The whole area is one which requires further research.
THE CRUCIAL QUESTIONS
Does what we know about physical activity and metabolism suggest to us that protein requirements are increased as a result of exercise? My answer to this would be that in fact there is no evidence that there are increased requirements for protein per se. Most foods contain 10-15 percent of protein on an energy basis and the likelihood is that an adequate supply of energy from mixed rations will inevitably supply sufficient protein.
Is there an optimum of protein to carbohydrate + fat ratio and should the composition of rations be altered to this? So far as I can tell, most of the evidence suggests that exercise actually increases the efficiency of protein utilisation and therefore, if anything, the amount of protein in the diet could be reduced without deleterious effects. I do not think we know the lower limit at the present time, and more work is needed to answer the question.
We do know that increasing the protein content of the diet simply increases the activity of amine acid catabolizing enzymes and the capacity for branched chain amine acid oxidation during exercise is substantially increased by increasing total dietary protein (Figure 11-1). In studies we carried out on subjects habituated to a high protein diet, amine acid oxidation was higher than normal during exercise suggesting no net benefit would accrue. In any case it can be calculated that even with an increase of muscle mass of 15 kg over 3 years a 70 kg man would need less than 5 percent of the protein recommended nutritional intake (RNI) to supply the growth. Thus, it is likely that increasing dietary protein over the current US military ration of 100 g/day would simply lead to increased oxidation without any particular benefit. The classic results of
Chittenden, a physiologist at Yale in the early part of this century (Chittenden, 1907), should not be forgotten. Chittenden persuaded a number of young, all-American athletes to eat substantially less protein than they were accustomed to and recorded their performance over the next year. It actually increased rather than decreased!
Is there any evidence for a differential response between the sexes to changes in protein and amino acid metabolism as a result of exercise? Personally I think that there is insufficient data to answer this question definitively but I would be very surprised if there were any major differences.
Is there a likelihood that particular amino acids will be beneficial in terms of performance, or the preservation of the lean body mass, etc? Glutamine is the most promising of the amino acids from this point of view. Nevertheless, there is no clear indication in the literature that glutamine or any other amino acid has a marked effect in stimulating performance per se in the short-term nor in maintaining lean body mass in the longer term.
AUTHOR'S CONCLUSIONS AND RECOMMENDATIONS
-
Exercise stimulates amino acid catabolism but the extent of the stimulation is too little to have a major effect in contributing to a negative nitrogen balance.
-
Most food contains sufficient protein such that so long as energy balance is maintained sufficient protein is delivered to meet the requirements for amino acid oxidation and also probably for preservation and even growth of the lean body mass.
-
There is no evidence that supplementation with individual amino acids is of benefit to physical performance or to maintenance or growth of lean body mass, especially muscle.
In summary, therefore, rations for military personnel engaged in a high rate of physical activity should have the following characteristics:
-
Will be sufficient in delivery of energy.
-
Contain protein in the range of 0.8 g/kg body weight/day.
-
Need contain no extra amino acid supplements.
ACKNOWLEDGMENTS
The work described in this article was supported by the Medical Research Council, Ministry of Agriculture, Fisheries and Food, The Wellcome Trust and the University of Dundee.
REFERENCES
Biolo, G., S.P. Maggi, B.D. Williams, K.D. Tipton, and R.R. Wolfe. 1995b. Increased rates of muscle protein turnover and amine acid transport after resistance exercise in humans. Am. J. Physiol. 268:E514-E520.
Blomstrand, E., and E. A. Newsholme. 1996. Influence of ingesting a solution of branched-chain amine-acids on plasma and muscle concentrations of amine-acids during prolonged submaximal exercise. Nutrition 12:485-490.
Bltastrand, E., P. Hassmén, S. Ek, B. Ekblom, and E.A. Newsholme. 1997. Influence of ingesting a solution of branched-chain amine acids on perceived exertion during exercise. Acta Physiol. Scand. 159:41-49.
Butterfield, G.E., and D.H. Calloway. 1984. Physical activity improves protein utilization in young men. Br. J. Nutr. 11:171-184.
Castell, L.M., J.R. Poortmans, and E.A. Newsholme. 1996. Does glutamine have a role in reducing infection in animals. Eur. J. Appl. Physiol. 13:488-490.
Chesley, A., J.D. MacDougall, M.A. Tarnopolsky, S.A. Atkinson, and K. Smith. 1992. Changes in human muscle protein synthesis following resistance exercise. J. Appl. Physiol. 73:1383-1388.
Chittenden, R.H. 1907. The Nutrition of Man. London: Heinemann.
Dohm, G.L., G.J. Kasperek, E.B. Tapscott, and G.R. Beecher. 1980. Effect of exercise on synthesis and degradation of muscle protein. Biochem. J. 188:255-262.
Fielding, R.A., C.N. Meredith, K.P. O'Reilly, W.R. Fontera, J.G. Cannon and N.J. Evans. 1991. Enhanced protein breakdown after eccentric exercise in young and older men. J. Appl. Physiol. 11:674-679.
Hassmén, P., E. Blomstrand, B. Ekblom, and E.A. Newsholme. 1994. Branched-chain amine acid supplementation during 10 km competitive run: Mood and cognitive performance. Nutrition 10:405-410.
Hood, D.A., and R.L. Terjung. 1990. Amine acid metabolism during exercise and following endurance training. Sports Med. 1:23-35.
Kasperek, G.J., and R.D. Snider. 1989. Total and myofibrillar protein degradation in isolated soleus muscles after exercise. Am. J. Physiol. 157:E1-E5.
Kasperek, G.J., G.R. Conway, D.S. Krayeski, and J.J. Lohne. 1992. A reexamination of the effect of exercise on rate of muscle protein degradation. Am. J. Physiol. 163:E1144-E1150.
Katz, A., S. Broberg, K. Sahlin, and J. Wahren. 1986. Muscle ammonia and amine acid metabolism during dynamic exercise in man. Clin. Physiol. 6:365-379.
MacDougall, J.D., M.J. Gibala, M.A. Tarnopolsky, J.R. MacDonald, S.A. Interisano, and K.E. Yarasheski. 1995. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can. J. Appl. Physiol. 10:480-486.
MacLean, D.A., T.E. Graham, and B. Saltin. 1994. Branched-chain amine acids augment ammonia metabolism while attenuating protein breakdown during exercise. Am. L Physiol. 167:E1010-1022.
MacLean, D.A., T. E. Graham, and B. Saltin. 1996. Stimulation of muscle ammonia production during exercise following branched-chain amino-acid supplementation in humans. J. Physiol. Lend. 193:909-922.
Madsen, K., D.A. MacLean, B. Kiens, and D. Christensen. 1996. Effects of glucose, glucose plus branched-chain amine acids, or placebo on bike performance over 100 km. J. Appl. Physiol. 11:2644-2650.
Millward, D.J., J.L. Bowtell, P. Pacy, and M.J. Rennie. 1994. Physical activity, protein metabolism and protein requirements. Proc. Nutr. Soc 13:123-240.
Pannier, J.L., J.J. Bouckaert, and R.A. Lefebvre. 1995. The antiserotonin agent pizotifen does not increase endurance performance in humans. Eur. J. Appl. Physiol. 12:175-178.
Rennie, M.J. 1996. Influence of exercise on protein and amino acid metabolism. Pp. 995-1035 in American Physiological Society Handbook of Physiology on Exercise, Chapter 12, Section 12, Control of Energy Metabolism During Exercise , R. L. Terjung, assoc. ed. Bethesda: American Physiological Society.
Rohde, T., D.A. MacLean, A. Hartkopp, and B.K. Pedersen. 1996. The immune-system and serum glutamine during a triathlon. Eur. J. Appl. Physiol. Occupat. Physiol. 14:428-434.
Rowbottom, D.G., D. Keast, C. Goodman, and A.R. Morton. 1995. The hematological, biochemical and immunological profile of athletes. Eur. J. Appl. Physiol. Occupat. Physiol. 10:502-509.
Rowbottom, D.G., D. Keast, and A.R. Morton. 1996. The emerging role of glutamine as an indicator of exercise stress and overtaining. Sports Med. 11:80-97.
Sahlin, K., A. Katz, and S. Broberg. 1990. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am. J. Physiol. 159:C834-C841.
Sahlin, K., L. Jorfeldt, and K.G. Henriksson. 1995. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle's disease. Clin. Sci. 19:687-693.
Stroud, M.A., A.A. Jackson, and J.C. Waterlow. 1996. Protein turnover rates of two human subjects during an unassisted crossing of Antarctica. Br. J. Nutr. 16:165-174.
Struder, H.K., W. Hollmann, P. Platen, J. Duperly, H.G. Fischer, and K. Weber. 1996. Alterations in plasma-free tryptophan and large neutral amino acids. Int. J. Sports Med. 17:73-79.
Van Hall, G., J.S.H. Raaymakers, W.H.M. Saris, and A.J.M. Wagenmakers. 1995a. Ingestion of branched chain amino acids and tryptophan during sustained exercise in man: failure to affect performance. J. Physiol. Lond. 186:789-794.
Van Hall, G., G.J. Van Der Vusse, K. Soderlund, and A.J. Wagenmakers. 1995b. Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J. Physiol. Lond. 189:151-261.
Wibom, R., and E. Hultman. 1990. ATP production rate in mitochondria isolated from microsamples of human muscle. Am. J. Physiol. 159:E204-E209.












