3
Experience With and Complications of Fluid Resuscitation
Controversy regarding the use of salt-containing solutions in surgery and trauma has continued for most of the 20th century. In 1911, Evans wrote:
The therapeutic value of a physiologic saline solution administered in large amounts either intravenously, hypodermically, or by the intestinal tract in certain pathologic conditions characterized by changes, quantitative or qualitative, in the blood plasma, has been so abundantly demonstrated by clinical experience that it requires no emphasis here. That under certain circumstances saline solutions are productive of great harm to the tissues of the body, and are even capable of producing death, is as true as it is of many other valuable therapeutic procedures. (Evans, 1911)
The use of large-volume isotonic salt solutions has become routine both in postsurgical settings and in the immediate postinjury phase; the use of fluid and electrolyte therapy has been lifesaving. (The electrolyte characteristics of selected resuscitation fluids are listed in Table 3-1.) Despite the impact of salt solutions on survival in shock, numerous questions and concerns persist regarding the composition, rate, and quantity of fluid resuscitation. This chapter gives an overview of colloid and crystalloid resuscitation, examines the complications of fluid resuscitation in general, and then describes the complications of crystalloid and colloid resuscitation specifically.
Overview of Colloid and Crystalloid Resuscitation
Fogelman and Wilson (1960) attributed hypovolemia after trauma to a reduction in the extracellular volume. In their studies, the mortality rate in dogs subjected to 2 hours of hemorrhagic hypotension was 80 percent if the animals were resuscitated with reinfusion of shed blood; however, addition of lactated Ringer's
solution to the return of autologous blood decreased the mortality rate to 40 percent. Shires and colleagues (1960b) extended this work, measuring intraoperative losses in the effective extracellular fluid volume, plasma volume, and red blood cell mass by the use of radioisotopes. Those studies described a 28 percent decrease in extracellular fluid volume that correlated directly with the degree of operative trauma. Shires and colleagues (1961) subsequently extended this work to trauma patients and described similar shock-mediated losses in extracellular fluid volume. Furthermore, resuscitation of hemorrhagic shock with blood or fresh frozen plasma plus blood failed to correct shock-related reductions in extracellular fluid volume, whereas the addition of lactated Ringer's solution to the return of shed blood ablated extracellular fluid volume deficits and improved the survival rate (Middleton et al., 1969; Shires, 1966; Shires et al., 1964).
The phenomenon of fluid redistribution after major trauma involving blood loss was termed ''third spacing'' and described the translocation of intravascular volume into the peritoneum, bowel, burned tissue, or crush injury sites. Subsequent studies showed that hemorrhagic shock promoted a significant loss of fluid from the extracellular space into the cell, exacerbating third-space losses (Middleton et al., 1969). Fluid was then directed to replace lost intravascular as well as extravascular fluid. Considerable controversy arose, however, regarding the formula or the clinical criteria used to determine the adequacy of fluid resuscitation; in addition, questions arose regarding the type of fluid that was most appropriate for volume replacement. Several studies suggested that normal saline and lactated Ringer's solution were equally effective in maintaining intravascular volume after hemorrhage (Cervera and Moss, 1975; Siegel et al., 1973; Wright, 1974), but complications such as hyponatremia or hypernatremia were reported with the use of 5 percent sodium chloride or molar sodium lactate solutions, respectively. Dillon and colleagues (1966) showed that lactated Ringer's solution (given in a volume that was two to three times the shed blood volume) was as efficacious as 6 percent dextran in saline (given in a volume equal to the shed blood volume) and confirmed that a sodium-containing, colloid-lacking solution could be used effectively to treat blood loss.
Questions arose regarding the effects of large-volume expansion on sodium distribution after hemorrhagic shock as well as the need to correct potassium deficits; although hemorrhage was shown to produce a functional sodium deficit, neither the clinical significance nor the magnitudes of the deficit were determined (Dillon et al., 1966). Large-volume resuscitation with salt-containing solutions gained in popularity because this regimen was consistently associated with improved survival in both clinical and experimental studies of hemorrhagic shock, and few side effects of lactated Ringer's solution were demonstrated. Thereafter research compared the hemodynamic responses to resuscitation with whole blood, plasma expanders, fresh frozen plasma, and saline versus lactated Ringer's solution. In this search for an ideal fluid for adequate restoration of intravascular and extravascular volumes, most studies found no differences in mortality rate or pulmonary function if volume expansion was adequate (Lucas et al., 1986, 1978; Moss et al., 1981).
TABLE 3-1
Electrolyte Characteristics of Selected Resuscitation Fluids and Research Formulations
|
Fluid |
Fluid Compartment |
Osmolarity (mOsM/1) |
pH |
Na+ (meq/1) |
Cl (meq/1) |
K+ (meq/l) |
Mg2+ (meq/l) |
Ca2+ (meq/l) |
Dextrose (g/l) |
Buffer |
|
Blooda |
||||||||||
|
Normal serum |
Intravascular |
308 |
7.4 |
140 |
100 |
4 |
0 |
0 |
0-4 |
Protein, bicarbonate |
|
Crystalloid |
||||||||||
|
0 9% Saline |
Extracellular |
308 |
5.0 |
154 |
154 |
0 |
0 |
0 |
0 |
None |
|
Lactated Ringer's solution |
Extracellular |
275 |
6.5 |
130 |
109 |
4 |
0 |
3 |
0 |
Lactate |
|
Plasmalyte-A, pH 7.4 |
Extracellular |
294 |
7.4 |
140 |
98 |
5 |
3 |
0 |
0 |
Acetate, gluconate |
|
Normosol-R |
Extracellular |
295 |
55-7.0 |
140 |
98 |
5 |
3 |
0 |
0 |
Acetate, gluconate |
|
7.0% Sahne |
Extracellular |
2,396 |
|
1,197 |
1,197 |
0 |
0 |
0 |
0 |
None |
|
5% Dextrose m water |
Extracellular |
252 |
4.0 |
0 |
0 |
0 |
0 |
0 |
50 |
None |
|
Colloid |
||||||||||
|
Natural |
|
|
|
|
|
|
|
|
|
|
|
5% Albumin |
Intravascular |
309 |
6.4-7.4 |
130-160 |
130-160 |
|
0 |
0 |
|
Sodium bicarbonate, sodium hydroxide, or acetic acid |
|
25% Albumin |
Intravascular |
312 |
6.4-7.4 |
130-160 |
130-160 |
|
0 |
0 |
|
Sodium bicarbonate, sodium hydroxide, or acetic acid |
|
Frozen plasma |
Intravascular |
300 |
Variable |
140 |
110 |
4 |
0 |
0 |
0-4 |
Protein, bicarbonate |
|
Synthetic |
|
|
|
|
|
|
|
|
|
|
|
6% Hetastarch |
Intravascular |
310 |
5.5 |
154 |
154 |
0 |
0 |
0 |
0 |
None |
|
10% Pentastarch |
Intravascular |
326 |
5.0 |
154 |
154 |
0 |
0 |
0 |
0 |
None |
|
Dextran 40 |
Intravascular |
311 |
3.5-7.0 |
154 |
154 |
0 |
0 |
0 |
0 |
None |
|
Dextran 70 |
Intravascular |
310 |
3.0-7.0 |
154 |
154 |
0 |
0 |
0 |
0 |
None |
|
Oxypolygelatin |
Intravascular |
200 |
7.4 |
155 |
100 |
0 |
0 |
1 |
0 |
None |
|
Research Formulations |
||||||||||
|
Carolina Rinseb |
|
290-305 |
6.5 |
115 |
122 |
6 |
1.2 |
1.3 |
1.8 |
MOPS |
|
Wisconsin Solutionc |
Intravascular |
320 |
7.4 |
25 |
0 |
125 |
5 |
0 |
0 |
Potassium phosphate |
|
Veech's Fluid |
|
294.3 |
7 |
136 |
106 |
4 |
1 |
2 |
5 |
Bicarbonate |
|
a Included as a point of comparison b Contains hydroxyethyl starch (50 grams/liter), allopurinol (1 millimolar [mM]), desferrioxamine (1 mM), glutathione (3 mM), fructose (10 mM), glucose (10 mM), adenosme (200 micromolar [µM]), nicardipine (2 µM), insulin (100 U/liter), 3-[N]-morpholinopropanesulfonic acid (20 mM), and glycine (5 mM) c Contains hydroxyethyl starch (50 grams/liter), benisine (5 mM), glutathione (3 mM), allopurinol (1 mM), insulin, penicillin, and dexamethesone SOURCE Adapted from Lemasters et al. (1995), Physicians' Desk Reference (1999), Rudloff and Kirby (1998), and Veech (1986) |
||||||||||
Beecher (1955), in the Surgeon General of the Army's Surgery in War Worm II, warned that crystalloids should be used primarily to replace body fluid lost through dehydration and stated that "As blood substitutes, these solutions were not effective, and they could be dangerous" (p. 32). Despite this concern, massive transfusion with crystalloid was routine during the Vietnam conflict. Additionally, studies undertaken during and immediately following the Vietnam conflict raised the concern that a large volume of salt-containing solution increased the incidence of acute respiratory distress syndrome (ARDS) and promoted multiple-organ dysfunction syndrome (MODS; see Figure 3-1). A loss of endothelial integrity and capillary leak coupled with the infusion of protein-free fluid, which in turn diluted plasma proteins, could contribute to pulmonary edema. A significant emphasis was subsequently placed on acute respiratory failure as well as ventilatory management in the shock patient with massive blood losses. Despite the lessons learned in Vietnam (increased incidence of pulmonary failure and ARDS with aggressive fluid resuscitation from shock), crystalloid solutions gained increasing acceptance in both clinical and military areas for fluid resuscitation from trauma. Studies with baboons and sheep confirmed that the hypoproteinemia that occurs after resuscitation with salt-containing solutions did not promote water movement into the lung interstitium (Moss et al., 1981). These studies contributed to increased acceptance of crystalloid volume replacement.
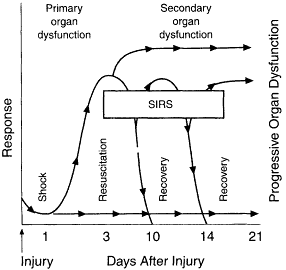
Figure 3-1
Inflammatory and organ dysfunction responses to injury. Normal response to an injury or insult may decrease after 3 to 5 days or be reactivated by a complication. A continuous inflammatory response is seen with systemic inflammatory response syndrome (SIRS) and can eventually progress to organ dysfunction. SOURCE: Reprinted, with permission, from Beal and Cerra (1994). Copyright 1994 by the American Medical Association.
Shoemaker and colleagues (1973, 1976, 1981) examined the effects of fluid resuscitation on tissue oxygenation and suggested that albumin improved hemodynamic and oxygen transport variables more than lactated Ringer's solution, attributing these results to the greater increases in plasma volume expansion and cardiac output with albumin. Similarly, Hauser and colleagues (1980) showed that infusion of 1 liter of lactated Ringer's solution in critically ill surgical patients with shock expanded the plasma volume 194 ± 18 milliliters (ml) whereas 25 grams of 25 percent albumin (100 ml) increased the plasma volume 465 ± 47 ml. Although hemodilution-related falls in the intravascular protein concentration have continued to raise concerns about crystalloid infusion in shock, the adverse effects of albumin administration on extravascular flux of protein and cardiopulmonary function contributed to the continued use of aggressive crystalloid resuscitation (Carey, 1971; Carey et al., 1970; Cloutier et al., 1969; Cochrane Injuries Group Albumin Reviewers, 1998; Lowe et al., 1979; Lowery et al., 1971; Lucas et al., 1980; Metildi et al., 1984; Moss et al., 1969; Poole et al., 1982; Virgilio et al., 1979).
Although the controversy regarding crystalloid versus colloid resuscitation of the shock patient with hemorrhage continues, most investigators agree that acute hemorrhage-induced changes in plasma volume require replacement with crystalloid solutions at volumes at least three times the volume of the shed blood. A major concern with regard to resuscitation of hemorrhage in a military setting is the considerable weight and volume of crystalloid solutions that must be transported in the field. The large bulk of the lactated Ringer's solution that must be transported compromises the resuscitation phase in forward areas of deployment. In addition, patients with hemorrhagic shock in a combat area are frequently dehydrated, presenting an additional problem for successful resuscitation.
Complications of Resuscitation in General
Although fluid resuscitation is necessary to assist a patient to recover from a loss of blood, there are complications that occur in the administration of the fluid. Fluid resuscitation can have an adverse effect on coagulation, and cause oxygen toxicity or reperfusion-mediated injury. Additionally, there are further complications associated with late resuscitation.
Effects of Fluid Resuscitation on Coagulation
Prolonged bleeding time has been described in patients with severe anemia (Hellem et al., 1961). A decrease in hematocrit as a consequence of large-volume crystalloid resuscitation produces anemia, thrombocytopenia, reduced plasma, and oncotic, clotting, and opsonic proteins. In addition to altered oxygen and CO2 transport, hemodilution and a fall in the circulating red blood cell volume reportedly alter several aspects of coagulation, including bleeding time and platelet adhesiveness and have detrimental effects related to excess, unscav-
enged nitric oxide production. Previous studies (Valeri et al., 1998) have suggested that the nitric oxide-mediated platelet dysfunction occurs as a result of hemodilution and the lack of availability of adequate red blood cells to scavenge nitric oxide by oxidation or by binding of nitric oxide to the hemoglobin molecule. In addition, red blood cell scavenging of nitric oxide activates platelets to produce thromboxane A2 and serotonin and stimulates endothelium-derived endothelin production in an effort to restore and maintain microcirculatory hemostasis (C. R. Valeri, personal communication). Anemia-related platelet dysfunction has been described by several laboratories (Blajchman et al., 1994; Duke, 1910; Hellem et al., 1961; Marcus, 1990), and altered bleeding times have been correlated with peripheral hemoglobin and hematocrit contents. The altered bleeding associated with a reduced circulating red blood cell content has been attributed, in part, to the decrease in blood viscosity and increased sheer stress at the levels of the endothelium.
More recently, studies from the Naval Blood Research Laboratory have shown that the hematocrit level and the oxygen state of red blood cells have a greater effect on bleeding time than does the concentration of either platelets or clotting proteins. In correcting the effects of hemodilution on bleeding time, the transfusion of platelets produces only a transient rise in total platelet count related, in part, to the short half-life of this cell type. Transfusion and an increase in the level of circulating red blood cells after aggressive fluid resuscitation from hemorrhagic shock have been shown to have a beneficial effect on platelet function. Red blood cells disperse platelets from the center of the blood vessel, concentrating this cell population near endothelial cells of the vessel walls (Turitto and Weiss, 1980). The ability of red blood cells to stimulate platelet synthesis of thromboxane A2 has important effects on vasoconstriction and platelet aggregation in the presence of continuing blood loss. Anemia and subsequent platelet dysfunction diminish the level of production of thromboxane A2, contributing to continued blood loss. These data raise additional concerns regarding large-volume lactated Ringer's solution resuscitation in a subject with continuing blood loss. Dilution of clotting proteins and increased bleeding time would exacerbate hemorrhage-related blood loss as well as microcapillary oozing. Aggressive fluid resuscitation from hemorrhage and the resulting anemia increase the blood flow but also increase the shear stress on vascular endothelial cells, promoting the release of endothelium-derived nitric oxide (Duke and Abelmann, 1969; Griffith, 1987; Ignarro, 1987; Loscalzo, 1995; Palmer et al., 1987). Shear stress on the endothelium is related to both shear rate and whole-blood viscosity. With hemodilution, blood viscosity falls, but the shear rate increases and the net result is increased shear stress. Finally, hemodilution-related increases in shear stress can promote the release of adenosine diphosphate (ADP) from red blood cells, potentiating shear-related platelet aggregation (Alkhamis, 1988; Alkhamis et al., 1990; Bell et al., 1990; Luthje, 1989; Saniabadi et al., 1987; Santos et al., 1991; Valles et al., 1991). Although increased shear stress on endothelial cells enhances nitric oxide production, the subsequent rise in platelet cyclic guanosine monophosphate (cGMP) levels further inhibits platelet function (Azuma et
al., 1986; Cooke et al., 1990; Mendelsohn et al., 1990; Michelson et al., 1996; Radomski et al., 1987).
Since it was recognized that human immunodeficiency virus (HIV) could be transmitted through blood transfusion, blood products have been used conservatively in patients with hemorrhagic shock, and a measure of hemodilution after aggressive crystalloid resuscitation has been accepted in clinical practice. However, the death rate as a result of HIV-related transfusion is extraordinarily low (Valeri et al., 1998). It is clear that the hemodilutional effects of aggressive crystalloid resuscitation extend far beyond the reduced oxygen-transport capacity and include altered endothelial function. These resuscitation-related changes in endothelial cell function may, in turn, exacerbate the capillary leak syndrome recognized to occur with hemorrhagic shock.
Oxygen Toxicity Associated with Resuscitation
Oxygen therapy is frequently used to treat critically ill patients who have pulmonary insufficiency; such patient populations frequently receive supraphysiologic concentrations of O2 (that is, more than 21 percent O2). The adverse effects of supranormal oxygen concentrations have been well described, and the lungs of patients who receive oxygen therapy are frequently damaged due to the high level of O2 exposure (Kazzaz et al., 1996; Morris, 1994; Stogner and Payne, 1992). Similarly, exposure of cells in culture to hyperoxia produces chromosomal breakage, oxidative damage, and cell cycle arrest at the G2 state (Clement et al., 1992). Hyperoxia-related cell injury has been attributed to the accumulation of oxygen-derived free radicals, which overwhelm cellular antioxidant mechanisms. A number of free radical scavengers including 21 aminosteroids have been shown to provide protection against free-radical-mediated injury and have been confirmed to have cytoprotective effects in hyperoxic insults (Frank and McLaughlin, 1993; Richards et al., 1993). Hyperoxia-mediated lung injury in humans, similar to that seen in ARDS, has been associated with activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and increased levels of hydrogen peroxide release by alveolar macrophages (Kinnula et al., 1992; Vacchiano et al., 1998). These studies suggest that prolonged exposure to a high partial pressure of oxygen produces direct pulmonary injury and exacerbates preexisting lung dysfunction.
Although trauma patients with hemorrhagic shock may receive oxygen therapy during transport and in the emergency department, these patients seldom require supraphysiologic concentrations of oxygen. A primary concern in the treatment of the trauma patient with hemorrhagic shock is aggressive fluid resuscitation and the potential for reperfusion injury related to (1) the return of molecular oxygen to previous ischemic tissues and (2) the toxic cellular effects of oxygen-derived free radicals. An increase in blood oxygen tension after resuscitation regimens that increase the oxygen-transport capabilities of the blood
may promote the increased intracellular production of superoxide, subjecting the endothelial cell to a barrage of deleterious oxygen-derived free radicals. Resuscitation with whole blood produces a burst of superoxide radical release, which may be exacerbated by platelet adherence to the surface of the endothelium and subsequently may promote adherence and activation of leukocytes. This activated cell population, in turn, produces additional toxic O2 radicals, contributing to endothelial damage. The lung is particularly susceptible to free-radical-mediated injury; in the scenario of endothelial damage from adherent and activated leukocytes, free radicals may migrate to the alveolar space, contributing to the progression of pulmonary oxygen toxicity (Sacks et al., 1978; Shinomiya et al., 1998; Steinberg et al., 1979). The toxic effects of oxygen in animals and humans has been well recognized, but oxygen toxicity has been best characterized with regard to reperfusion injury, in which the return of molecular oxygen to previously ischemic tissues contributes to oxygen free-radical generation via xanthine oxidase activation.
Reperfusion-Mediated Injury
A major concern with regard to fluid resuscitation, that is, the reintroduction of molecular oxygen into previously ischemic tissues, the production of excess oxygen-derived free radicals, and subsequent tissue damage, has been investigated extensively. Most molecular species have pairs of electrons within their outer orbitals; each molecule is stabilized by the opposite spin of each of these electrons. A free radical is a molecule with an unpaired electron which is highly reactive and tends to react with other molecules in an effort to pair its lone electron with another electron. This interaction renders the molecule reactive and unstable, contributing to the extremely short half-lives and difficulty in quantitating the radicals in a biological setting. The recent development of electron paramagnetic resonance (EPR) spectroscopy, alone or in combination with spin trapping, has allowed these radicals to be observed. EPR directly measures the amount of energy absorbed by an unpaired electron in a magnetic field at the ultralow temperatures; free radicals are far too unstable to measure at room temperature since the half-life is approximately 6 to 10 seconds (Fantone and Ward, 1982; Grisham and McCord, 1986; Reilly et al., 1991).
Free radicals are the normal by-products of cellular metabolism, and the most common radicals include superoxide, hydrogen peroxide, the hydroxyl radical, and nitric oxide. During oxidative phosphorylation, molecular oxygen is reduced to water within the mitochondria. However, as the conversion of oxygen to water occurs, between 1 and 5 percent of this oxygen escapes the pathway, producing several toxic intermediates. Other endogenous means or sources of free-radical production include the oxidation of purines including hypoxanthine oxidation through xanthine to urate, the metabolism of arachidonic acid to produce prostaglandins and leukotrienes, and the NADPH-dependent oxidase system on neutrophil membrane surfaces. Endogenous antioxidant systems
serve to scavenge or neutralize free radicals, maintaining effective balance between free-radical production and removal (Babbs, 1988).
Oxygen-Derived Free Radicals
The role of free radicals in hemorrhage and shock arises from the fact that volume replacement or reperfusion of previously ischemic tissues has been recognized to produce significant tissue injury and dysfunction. This phenomenon has been described as the "oxygen paradox" and describes the fact that although the restoration of oxygen delivery to ischemic tissue is essential to the maintenance of function and survival, this oxygen may initiate a cascade of deleterious events, producing tissue injury. The free radicals produced during reperfusion or fluid resuscitation from hemorrhagic shock attack multiple components of the cell, including lipids, nucleic acids, and proteins. Therefore, although hypoperfusion itself will produce cellular death with time, the very act of correcting the perfusion deficits introduces significant and greater injury. McCord and Fridovich (1968) proposed that the major source of free radicals during reperfusion was the enzyme xanthine oxidase, an enzyme that is present in the liver and gut.
A decrease in blood flow limits the available oxygen required for adenosine triphosphate (ATP) production. ATP depletion produces a subsequent rise in the level of adenosine monophosphate (AMP), which, in turn, is catabolized to hypoxanthine. With fluid resuscitation and the return of molecular oxygen to previously hypoperfused tissues, hypoxanthine serves as a substrate for xanthine oxidase. A complicated series of reactions converts hypoxanthine to xanthine and finally to uric acid and in the process generates hydrogen peroxide and superoxide, both of which are powerful oxidizing agents. The resulting increased production of superoxide and hydrogen peroxide overwhelms the capacity of endogenous scavengers. Hemorrhagic shock produces "whole-body" ischemia with inadequate perfusion of most tissues. As ATP levels fall dramatically in several tissues, the levels of hypoxanthine in plasma rise. The role of hypoxanthine in hemorrhagic shock was first suggested by Crowell and associates (1969) and was confirmed by others; those studies found that allopurinol provides significant benefits if it is given during blood loss (Bulkley, 1983; Hess et al., 1982; Parks, 1982; Powell and Tortolani, 1992; Rao et al., 1983). Numerous subsequent studies have confirmed the hemodynamic and cardioprotective effects of free-radical scavengers given during either ischemia or shock with hemorrhage (Bernier et al., 1986; Crowell et al., 1969; Cunningham and Keaveny, 1978; Granger et al., 1986; Lee et al., 1987).
Although a major source of oxygen-derived free radicals in hemorrhagic shock is xanthine oxidase, others have shown that adherent and activated neutrophils produce free radicals. Although this serves an important and necessary role in the scavenging of invading bacteria, a burst of neutrophil-produced free radicals may exacerbate the xanthine oxidase activity, producing significant
tissue damage. In addition to free-radical production, hemorrhagic shock clearly impairs endogenous antioxidant defense mechanisms, rendering the subject more susceptible to the damage caused by toxic oxygen metabolites. Hemorrhagic shock alters nonenzymatic defense mechanisms, decreasing α-tocopherol, β-carotene, ascorbic acid, and vitamin E reserves. These normal antioxidants protect membranes against lipid peroxidation, dissipating free-radical energy and scavenging toxic radicals. In addition, hemorrhage impairs endogenous enzymatic defense mechanisms including those involving superoxide dismutase, catalase, and reduced glutathione, which catalyze the breakdown of superoxide free radicals and hydrogen peroxide, respectively. Shock-mediated downregulation of these defense mechanisms predisposes the cell to injury from an unopposed storm of toxic radicals.
Evidence for oxygen-derived free-radical-mediated injury in hemorrhagic shock has been provided by a host of studies. Hemorrhagic shock reduces the activities of copper, zinc-superoxide dismutase, and glutathione peroxidase in several organs, whereas the activity of glutathione reductase remains unchanged (Makarewicz-Plonska et al., 1998). Other studies have described an oxidative-antioxidative imbalance in experimental hemorrhagic shock as reflected by morphologic changes in peripheral organs accompanied by increased malondialdehyde levels, indicating oxidative tissue injury. A fall in the antioxidative potential has been confirmed by altered sulfhydryl compounds and reduced superoxide dismutase activity (Debek et al., 1998). Kapoor and colleagues (1997) provided evidence that oxygen radicals contribute to the deterioration of cardiovascular function and cellular injury during hemorrhagic shock and resuscitation. Those investigators described the increased oxygen radical-producing activity of polymorphonuclear leukocytes, decreased antioxidant enzyme activities (superoxide dismutase, catalase, and glutathione peroxidase), and a rise in cardiac malondialdehyde concentrations.
In addition, a fall in glutathione, α-tocopherol, and plasma glutathione per-oxidase levels paralleled by a rise in the levels of lipid peroxides (expressed as thiobarbituric acid substances) have been described in patients resuscitated from trauma or shock (Kretzschmar, 1998). A progressive loss of plasma sulfhydryl groups and α-tocopherol and a significant increase in the plasma-reduced glutathione level paralleled the development of a multiple-organ failure in this patient population. These data suggest that traumatic injury with hemorrhage produces significant oxidative stress paralleled by a loss of endogenous antioxidants. These factors are major contributing factors to the development of multiple-organ failure after traumatic injury with shock, despite aggressive fluid resuscitation. Other studies have described no significant changes in either superoxide dismutase or catalase levels during hemorrhage alone (Uzuner et al., 1995); however, in those studies, a reduced catalase level after resuscitation suggested reperfusion-mediated injury via the generation of free radicals as well as a fall in antioxidant capacity.
Nitric Oxide
In addition to oxygen-derived free radicals, nitric oxide generated via the nitric oxide synthases contributes to membrane injury and cellular dysfunction during hemorrhagic shock and fluid resuscitation. Numerous studies have shown that hemorrhagic shock upregulates the inflammatory or inducible nitric oxide synthase (NOS2 or iNOS) (Kelly et al., 1997; Thiemermann et al., 1993), suggesting that increased iNOS expression in shock contributes to the proinflammatory response. Nitric oxide mediates cell injury via either direct effects on several intracellular processes or the indirect effects by interaction with superoxide (Szabó, 1996). Attempts to define the contribution of iNOS and nitric oxide to postshock-mediated cell injury have used two approaches: (1) pharmacologic probes (selective iNOS inhibitors as well as inhibitors that block both iNOS and endothelial nitric oxide synthase [eNOS]) and (2) transgenic animals, specifically, animals deficient in iNOS. These studies have suggested that nitric oxide is "a final common mediator" in hemorrhagic shock and a primary mediator of the inflammatory response shown to occur after resuscitation from hemorrhagic shock. However, the role of nitric oxide in shock has remained controversial. The finding that arginine administration provided organ protection in resuscitated hemorrhagic shock suggested that low levels of nitric oxide (likely occurring as a result of eNOS) exert protective effects on organs; decreased arginine availability after resuscitation from hemorrhagic shock could impair basal levels of nitric oxide. Arginine added to fluid used for resuscitation may restore these basal levels of nitric oxide, contributing to the observed organ protection (Chaudry et al., in press).
In contrast, enhanced nitric oxide production during shock via increased iNOS expression produces significant cellular injury including vascular decompensation and nitric oxide-mediated activation of the transcription factor nuclear factor κB (NFκB), providing one mechanism by which nitric oxide modulates oxidant signaling in hemorrhagic shock (see Figure 3-2). Despite this paradigm proposed by Szabó and Billiar (in press), there is abundant evidence that nitric oxide interaction with superoxide radical yields peroxynitrite, exacerbating the injury produced by either nitric oxide or superoxide alone. Peroxynitrite generation occurs particularly during fluid resuscitation from hemorrhagic shock in which the level of production of the superoxide radical exceeds the scavenging capacity of endogenous enzymes. Peroxynitrite contributes to cell injury and cellular death by producing DNA single strand breakage (Szabó and Hoshima, 1997) and activation of the nuclear enzyme poly(ADP ribose) synthetase (PARS), which, in turn, contributes to energy depletion and cellular necrosis. The detrimental effects of peroxynitrite in hemorrhage and resuscitation have been attributed to oxidation of sulfhydryl groups, and nitration of hydroxylation of tyrosine, tryptophan, and guanine, and inhibition of several enzyme systems including membrane sodium potassium adenosine triphosphatase. PARS activation depletes nicotinamide adenine dinucleotide (NAD), altering glycolysis, electron transport, and ATP synthesis; in addition, PARS activates the caspase
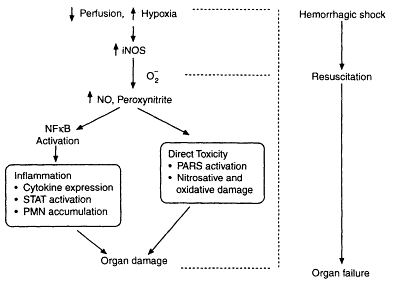
Figure 3-2
Roles of induced NO in hemorrhagic shock. The figure depicts the dual roles of inducible NO synthase-generated NO in hemorrhagic shock. The NO generated during resuscitation combines with the superoxide, forming peroxynitrite. Peroxynitrite generated during resuscitation can exert direct cellular toxicity through oxidative and nitrosative damage and PARS activation. NO and perhaps peroxynitrite can activate inflammatory cascades through the upregulation of NFκB. This results in inflammation manifested by cytokine expression, STAT3 activation, and neutrophil accumulation. Combined, these actions result in organ damage and organ failure. Source: Reprinted, with permission, from Szabó and Billiar (in press). Copyright 1999 by BioMedical Press.
cascade, which triggers DNA fragmentation and cellular apoptosis (Endres, 1998; Szabó, 1998; Virág et al., 1998; Yaoita et al., 1998).
Clearly, hemorrhagic shock produces local and whole-body ischemia; and fluid resuscitation, regardless of the type of fluid administered, increases perfusion of previously ischemic or hypoperfused tissues, triggering the production of numerous free radicals and likely contributing to cellular injury.
Activated Neutrophils
In addition to the generation of free radicals by reperfusion of hemorrhage-induced ischemia, recent attention has focused on the role of activated neutrophils in resuscitation-mediated cellular injury. Intracellular adhesion molecules 1 and 2 (ICAM-1 and ICAM-2, respectively) have been shown to be upregulated by lactated Ringer's fluid resuscitation from hemorrhagic shock (Rhee, 1998). These adhesion molecules are instrumental in binding leukocytes to the
vascular endothelium, and neutrophils are the predominant population of cells that adhere to the vascular endothelium and subsequently migrate into the tissues. Neutrophil-derived free radicals have been shown to play a significant role in tissue injury after resuscitation from hemorrhagic shock, as indicated by organ protection and increased survival in animals treated with monoclonal antibodies specifically directed against ICAM-1 (Mileski et al., 1990, 1991). Similarly, Flynn and colleagues (1996) demonstrated that sialyl Lewis oligosaccharides that block the low affinity selectin ligands (including P, L, and E) were effective in models of trauma and hemorrhagic shock. Pharmacologic strategies designed to inhibit specific adhesion molecules recognized to play a role in the tethering, adherence, and activation of leukocytes provide significant organ protection after hemorrhagic shock. The use of ICAM-1- and P-selectin-knockout mice have provided alternative strategies to confirming the roles of these adhesion molecules and neutrophils in shock-mediated injury. Such transgenic and pharmacologic approaches have been shown to decrease free radical-mediated injury, as indicated by decreased lipid peroxides and malondialdehyde levels and have confirmed that the activated neutrophil is one source of free radicals in resuscitated hemorrhagic shock.
Complications of Late Resuscitation of Shock
In the late phase of shock resuscitation, emphasis is placed on continued volume replacement as well as nutritional support delivered via the enteral or the parenteral route. Metabolic derangements after shock include altered energy production and utilization and altered carbohydrate, lipid, and protein metabolism; in addition, acute-phase protein synthesis, impaired wound healing, impaired glucose oxidation, and accelerated protein catabolism are hallmarks of stress-related injury. Finally, shock alters macrophage and lymphocyte glutamine requirements after burn shock, and several therapies have been directed toward the restoration of shock-mediated changes in tissue glutamine levels. Late treatment of the trauma patient with hemorrhagic shock has been directed toward determination of the patient's metabolic needs. Dietary manipulation after shock has been shown to increase survival in rodent stress models and to improve nitrogen balance and protein synthesis (Manson et al., 1988; Wolfe et al., 1989), to improve cell-mediated immunity in burned guinea pigs (Alexander et al., 1986), and to improve hemodynamic and metabolic function and outcome (Muakkassa, 1991). Nutritional support, however, is considered during the late phase of shock therapy and is not to be confused with front-line therapy in disaster or combat situations, in which the primary concern is restoration of the circulating volume and hematocrit.
Complications of Colloid Resuscitation
Numerous difficulties with current regimens of colloid fluid replacement in patients with shock have been described. The lack of availability of whole blood in the field, the requirements for typing and cross matching, the danger of transmitting numerous infectious agents (including hepatitis B and hepatitis C viruses, and HIV), reduced concentrations of coagulation factors, and the absence of functioning platelets all complicate the use of whole blood for adequate resuscitation of hemorrhage. The limitations of liquid plasma for resuscitation from shock in the field include the relatively short shelf life, cost (currently $126/200 milliliters of plasma), and the risk of transmitting infectious agents. Although detergent treatment of plasma has been shown to reduce the risk of transmission of lipid-encapsulated viruses such as HIV, the persistent problem of possible contamination of large plasma pools with other infectious agents, for example, nonenveloped viruses, and requirements for storage in a frozen state have dampened enthusiasm for this approach to field resuscitation from hemorrhage (Klein et al., 1998). Finally, numerous studies have confirmed shock-mediated alterations in endothelial cell barrier integrity (Maier and Bolger, 1996).
Administration of albumin-containing solutions in the presence of a persistent capillary leak has been proposed to increase interstitial protein concentrations, promoting the movement of fluid from the intravascular compartment to the interstitium and aggravating hemorrhage-induced hypotension. These factors have resulted in an increased interest in synthetic colloids, agents that can be successfully retained in the intravascular compartment to increase circulating plasma volume. With these synthetic agents (the dextrans, hydroxyethyl starch [HES], and several gelatin preparations), the rare occurrence of anaphylactic reactions, mild inhibition of normal hemostasis, and the long-term retention of these agents within the body have tempered enthusiasm for their clinical use (Adelson et al., 1955; Bergqvist, 1982; Cronberg et al., 1966; Dahn et al., 1979; Leibold et al., 1983; Lucas et al., 1980; Macintyre et al., 1985; Metildi et al., 1984; Mishler, 1982; Stump et al., 1983). However, these agents are cheap to manufacture, are stable with storage at room temperature, and eliminate the infectious risks associated with blood products. Recent studies have shown that artificial colloids increased the levels of expression of adhesion molecules, increased the levels of synthesis of several proinflammatory cytokines, and promoted cellular apoptosis (Coimbra et al., 1996; Junger et al., 1997a,b, Rhee, 1998). Newer colloids that carry oxygen and that can be formulated for a variety of oncotic activities have considerable promise, but considerable research is required before their clinical application as resuscitation fluids.
Glucose-containing solutions have been avoided for resuscitation from hemorrhagic shock in patients with head trauma. The stress-related rise in circulating epinephrine levels decreases the level of insulin released by the pancreas, complicating metabolism of the glucose load. Furthermore, increased glucose levels have been shown to alter potassium-adenosine triphosphate (K-ATP) channels, thus excerbating shock-related ion redistribution across mem-
branes. Patients with injury have adrenergic-system-mediated insulin resistance so that the glucose space is expanded. The implications are that with increased gluconeogenesis, glucose-containing fluids are unnecessary and probably harmful.
The debate between colloid and crystalloid resuscitation has recently taken on some new features that merit consideration. A systematic review of 30 randomized trials found no evidence that albumin administration reduced the rate of mortality among critically ill patients with hypovolemia, bums, or hypoalbuminemia and suggested that albumin may in fact increase the rate of mortality (Cochrane Injuries Group Albumin Reviewers, 1998). That analysis has been criticized for its reliance on studies with small numbers of patients and with patients with different diseases and for the use of death as its single, if objective, endpoint. There is also the inherent problem of publication bias in analyses of this kind, and that review should not end the debate. Therapeutic policies determined by such consensus-building mechanisms as the Delphi method, a systematic literature-based consensus process for making complex clinical practice guidelines (Vermeulen et al., 1995), have a place in medical decision making, but they are not a substitute for high-quality clinical trials with large numbers of subjects.
The suggestion that one colloid solution, albumin, has the potential to cause harm further underlines the need for adequate controlled trials. There are several reasons why the use of albumin might become a moot point, including its limited availability and the reluctance of clinicians to use a blood fractionation product. The same may be said for freeze-dried plasma, which cannot yet be treated to eliminate the risk of viral transmission and would require reconstitution under battlefield conditions. Albumin synthesis by recombinant technology is being pursued, but it is not yet an alternative and will likely be expensive. Cardiac decompensation may occur after the rapid infusion of large volumes of a highly concentrated (25 percent) albumin solution or some other colloid, since this leads to a substantial increase in volume retention. Increased filtration of fluids together with proteins into the interstitial space may exceed the ability of the lymphatic system to return extravascular fluids to the circulation (Roberts and Bratton, 1998). However, arguments against the use of colloids rely heavily on older studies with non-human primates that show, for example, that interstitial pulmonary edema develops after albumin administration in hemorrhagic shock (Moss et al., 1979). These studies use conventional colloid formulations. Similarly, studies suggest that conventional albumin resuscitation may impair sodium and water excretion and worsen renal failure (Moon et al., 1989). Furthermore, the timing of resuscitation, in terms of the progression of the shock syndrome, may be as important as the nature and volume of the resuscitation fluid.
Resuscitation with a minimal volume of hypertonic saline has been discussed, but it is important to note that hard evidence of the effectiveness of this strategy exists primarily from the early resuscitation of patients with ruptured aortic abdominal aneurysms and penetrating truncal trauma. Alternative synthetic colloids like hydroxyethyl starch (and other high-molecular-weight starches), long used in Europe, may not leak as readily into the extravascular
space and appear to improve hemodynamics and reduce pulmonary edema compared with saline solutions infused into patients with capillary leak syndrome (Boldt et al., 1998). The mild anticoagulant properties of polydisperse colloids such as starches and dextrans, in part as a result of interference with platelet function, have not been associated with significant bleeding problems. This defect might even prove to be advantageous by reducing the activation and adhesion of platelets and granulocytes to one another and to the vascular endothelium. Similar effects have been reported with the lower-molecular-weight plasma expanders, such as modified gelatin-based colloids, which have also long been used in Europe as resuscitation solutions (Evans et al., 1998). Combinations of colloids, electrolytes, and an energy source in a hypertonic solution merit further clinical study.
Complications of Crystalloid Resuscitation
Effects of Crystalloid Resuscitation on Immune Function
It is well recognized that trauma with hemorrhagic shock produces significant stimulation of the immune system and that neutrophil activation, adherence, and emigration into tissues contribute to the systemic inflammatory response, which frequently culminates in ARDS and MODS. Although the use of crystalloids and artificial colloids has been routine for the resuscitation of patients with acute blood loss, recent studies have raised questions regarding the effects of resuscitation regimens on several aspects of the immune response to hemorrhagic shock. Rhee and colleagues (1998) examined the effects of lactated Ringer's solution on neutrophil function. Neutrophil activation and adherence have been described in trauma patients (Tanaka et al., 1991), and excess superoxide production by activated neutrophils has been implicated in trauma-related capillary endothelial injury and dysfunction (Chen and Christou, 1996; Maier and Bolger, 1996). Rhee and colleagues (1998) used a model with awake swine given a 40 percent volume hemorrhage over 1 hour, followed by resuscitation with either lactated Ringer's solution, shed blood, or 7.5 percent hypertonic saline. A control group included swine given a sham hemorrhage and a lactated Ringer's solution infusion. Flow cytometry was used to detect superoxide burst activity as a measure of neutrophil activation. Hemorrhage-mediated neutrophil activation was exacerbated by lactated Ringer's solution resuscitation, whereas shed blood or hypertonic saline infusion returned neutrophil activity to baseline values. It was of interest that lactated Ringer's solution administration to sham-hemorrhaged swine increased neutrophil activation similar to that seen in the resuscitated shock subjects (see Figure 3-3), emphasizing the need for further research in this totally undefined area.
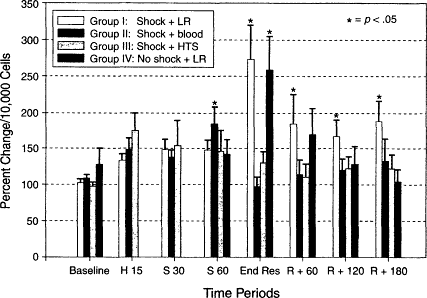
Figure 3-3
Neutrophil fluorescence, percent change from venous baseline values per 10,000 cells ± SEM, (*) = p < 0.05, compared with baseline values, ANOVA with Tu-key's b multiple comparison test. Baseline, before hemorrhage; H 15, end of 15-minute hemorrhage; S 30, 30 minutes into shock period; S 60, 60 minutes into shock period; End Res, end of 60-minute resuscitation period; R + 120, 120 minutes after end of resuscitation; R + 180, 180 minutes after end of resuscitation. Source: Reprinted, with permission, from Rhee et al. (1998). Copyright 1998 by Lippincott Williams & Wilkins.
In a subsequent study, Rhee and colleagues (in press) diluted whole blood from healthy volunteers with various resuscitation fluids to examine the hypothesis that neutrophil adherence and activation correlates with the type of fluid resuscitation used as well as with the degree of hemodilution. Oxidative burst activity was examined with dichlorofluorescein diacetate staining as well as neutrophil adhesion (cell surface receptor expression of CD 18 cells) measured by flow cytometry (flourescence-activated cell sorter analysis). Blood was diluted with several fluids to achieve 10, 25, 50, or 75 percent dilution (see Figures 3-4 and 3-5). The fluids examined included buffered saline, normal saline, lactated Ringer's solution, dextran 40, hespan (6 percent), albumin (25 percent), albumin (5 percent), hypertonic saline (3.5 and 7.5 percent). Blood samples diluted with hypertonic saline were controlled for sodium content so that it was equal to that measured in normal blood samples. These studies showed that crystalloid solutions produced neutrophil adherence, activation, and adhesion in a dose-dependent fashion. Artificial colloid solutions produced neutrophil activation and neutrophil adherence that were similiar to those with crystalloid solutions, whereas 5 percent albumin produced modest activation and 25 percent albumin produced none. It was of interest that
hypertonic saline caused neither neutrophil activation nor neutrophil adherence. The investigators indicated that this cellular activation and adherence was not associated with changes in pH, electrolyte content, hemoglobin, hemodilution, or osmolality. Although many trauma patients with hemorrhagic shock have received larger volumes than necessary for adequate resuscitation with no evidence of inflammatory activation, the data suggest that fluid resuscitation from hemorrhagic shock is not innocuous and that the type of resuscitation fluid may contribute to the inflammatory syndrome.
Subsequent studies (Sun et al., in press) showed that lactated Ringer's solution resuscitation from hemorrhagic shock increased the levels of E-selectin and intracellular adhesion molecule 1 (ICAM-1) expression on isolated neutrophils, raising further serious concerns about the immunologic consequences of crystalloid resuscitation. However, many trauma patients with hemorrhagic shock receive large volumes of resuscitation fluids without evidence of an inflammatory cascade, suggesting that fluids alone are not sufficient to trigger serious immunologic consequences. However, patients who develop systemic inflammatory responses are most frequently those who receive the large volumes of resuscitation fluids, suggesting that the cautious use of fluids should be considered.
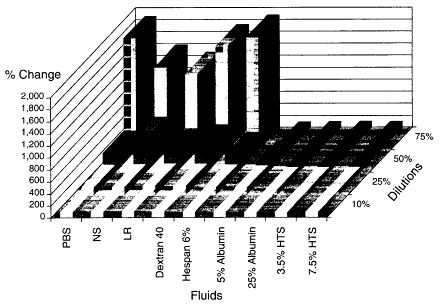
Figure 3-4
Neutrophil fluorescence (activation). Source: Reprinted, with permission, from Rhee et al. (in press). Copyright 1999 by the Society of Critical Care Medicine.
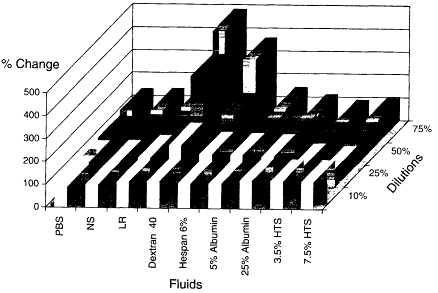
Figure 3-5
Neutrophil CD18 expression (adhesion). Source: Reprinted, with permission, from Rhee et al. (in press). Copyright 1999 by the Society of Critical Care Medicine.
Hemorrhage-induced apoptosis is well recognized (see Chapter 2), and the mechanism of intestinal mucosal immune dysfunction after trauma with hemorrhage has been attributed to apoptosis in Peyer's patches and impaired intestinal mucosal immunity (Xu et al., 1997). Similarly, trauma with hemorrhage has been shown to increase thymic apoptosis, whereas it decreases the level of interleukin-3 (IL-3) release (Xu et al., 1997). Further support for the deleterious effects of lactated Ringer's solution resuscitation from hemorrhage was provided by studies that examined the effects of resuscitation on cellular apoptosis (Deb et al., 1999b). Deb and colleagues examined lactated Ringer's solution resuscitation from hemorrhage in Sprague-Dawley rats (which received a volume of lactated Ringer's solution equivalent to three times the volume of blood that was lost); sham-hemorrhaged rats were given an equal volume of lactated Ringer's solution, and an additional group included hemorrhage rats resuscitated with 7.5 percent hypertonic saline. Lactated Ringer's solution increased the number of apoptotic cells in the mucosa and smooth muscle of the bowel wall and increased the rate of apoptosis in the liver compared to the values for sham-hemorrhaged animals. In contrast, resuscitation with neither shed blood nor hypertonic saline produced significant changes in apoptotic staining in either the mucosa, the bowel wall, or the liver (see Figure 3-6). Furthermore, the mucosa walls and bowel walls of sham-hemorrhaged rats given lactated Ringer's solution produced high levels of apoptotic staining, confirming that large-volume lactated Ringer's solution infusion, even in the absence of hemorrhage, pro-
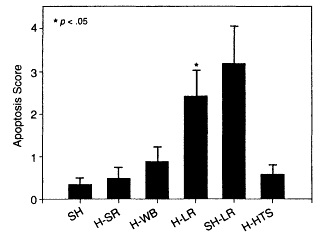
Figure 3-6
Resuscitation with lactated Ringer's solution increased the number of apoptotic cells in the mucosa and smooth muscle of the bowel wall and increased the rate of apoptosis in the liver compared to the values for sham-hemorrhaged rats. Source: reprinted, with permission, from Deb et al. (1999b). Copyright 1999 by the Society of Critical Care Medicine.
motes apoptosis in highly vulnerable tissues. The lower rate of programmed cell death in rats given whole blood resuscitation may be related to the presence of free-radical scavengers (red blood cells); however, the mechanisms by which hypertonic saline provided cellular protection remain unclear.
In subsequent studies, Deb and colleagues (1999a) examined the effects of various fluid resuscitation regimens on the upregulation of the Bax protein, a member of the Bcl-2 protein family and a potent inducer of apoptosis (see Chapter 2). In their study, Sprague-Dawley rats were hemorrhaged and maintained through a 75-minute hemorrhagic shock phase; fluid resuscitation consisted of either 5 percent albumin, whole blood, 6 percent hetastarch, dextran 40, or lactated Ringer's solution. The resuscitation volume was equivalent to the hemorrhage volume except in the groups given lactated Ringer's solution, which received lactated Ringer's solution at three times the shed blood volume. Lactated Ringer's solution and hetastarch administration to hemorrhaged rats significantly increased the levels of expression of Bax in the lung compared with those for the sham-hemorrhaged group; in contrast, there was no statistically significant increase in the level of Bax protein expression in the sham-hemorrhaged or albumin-, blood-, or dextran-treated groups (see Figure 3-7). These studies confirm that increased programmed cell death and apoptosis are modulated by the type of fluid resuscitation from shock.
In summary, several studies have provided definitive evidence that distinct immunologic complications including cellular apoptosis are related to the type of fluid resuscitation. Although those studies confirm the deleterious effects of
infusions of large volumes of lactated Ringer's solution, even in the absence of hemorrhage and hypotension, the consequences of initiating fluid resuscitation with a small bolus of hypertonic saline followed by lactated Ringer's solution to maintain mean arterial pressure and cardiac output have not been addressed. Similarly, no studies, to date, have examined the immune consequences of resuscitation with a reduced volume of lactated Ringer's solution rather than the large and aggressive volumes that have become routine for the treatment of the trauma patients with hemorrhagic shock. The absence of evidence-based research in this regard suggests that additional studies are needed to examine both the immune consequence of small-volume lactated Ringer's resuscitation and the immune consequences of small-volume hypertonic saline resuscitation followed by lactated Ringer's resuscitation.
Effects of Crystalloid Resuscitation on Cytokine Response
Recently Hierholzer and colleagues (1998) showed a marked increase in the levels of IL-6 gene expression and peptide secretion after hemorrhage but only in animals that had received aggressive crystalloid resuscitation. Several studies have shown that hemorrhagic shock increases the levels of IL-1, IL-6, and tumor necrosis factor (TNF) production by Kupffer cells (Ayala et al., 1992; Yamashita, 1998; Zhu et al., 1995). Molina and colleagues (1997), using a con-
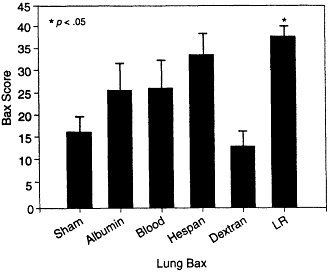
Figure 3-7
Lactated Ringer's solution administered to hemorrhaged rats significantly increased the levels of expression of Bax in the lung compared with those for the sham-hemorrhaged group. Source: Reprinted, with permission, from Rhee (1998).
scious rat model in which mean arterial blood pressure was reduced to 40 mm Hg over 25 minutes, showed significant increases in circulating TNF levels; circulating cytokine levels remained elevated after resuscitation with lactated Ringer's solution (with four times the shed blood volume), despite significant hemodilution. Unresuscitated hemorrhage produced a detectable rise in tissue TNF content (spleen and lung), and cytokine production was exacerbated by crystalloid resuscitation. The cytokine response to hemorrhage resuscitation correlated with sympathetic upregulation. Abraham (1998) confirmed the sympathetic modulation of tissue cytokine response to hemorrhage and fluid resuscitation; the marked elevation in lung IL-1 and TNF messenger RNA (mRNA) levels in pulmonary polymorphonuclear neutrophils persisted after fluid resuscitation from hemorrhage shock but was attenuated by phentolamine and adrenergic blockade. However, studies examining the effects of colloid resuscitation, reduced-volume lactated Ringer's solution resuscitation, or hypertonic saline resuscitation from hemorrhagic shock on cytokine response are lacking.
Adverse Effects of Large-Volume Crystalloid Resuscitation
Although Shires' work proposed that volume resuscitation after hemorrhagic shock should consider extracellular fluid deficits (Shires et al., 1960a, 1961, 1964), others proposed that altered extracellular fluid volume was an inadequate basis for salt and water infusion in critically ill individuals (Bishop et al., 1991; Gattinoni et al., 1986; Lowell, 1990; Ray, 1974; Roth et al., 1969; Simmons et al., 1987). More recently, Lyons (1996) described a significant increase in surgical mortality that was attributed to crystalloid overresuscitation in instances in which resuscitation began during surgery and continued uninterrupted throughout the postoperative period. A review of 37 deaths on the surgical service between January and June 1990 showed that over half of the deaths were related to respiratory failure; a significant portion of these deaths were related to fluid overload on the basis of criteria of continuous intravenous infusions, significant acute weight gain, clinical evidence of abnormal extracellular fluid volume, and ''cumulative crystalloid balance far in excess of any physiologic need'' (Lyons, 1996, p. 42). Although this was a small clinical study, excess crystalloid administration after hemorrhage or during the perioperative period posed a significant clinical risk of ARDS.
To further address the question of optimal volume of crystalloid for the restoration of intravascular deficits after hemorrhage, Lilly and colleagues (1992) used a large-animal model to confirm that the infusion of normal saline in a volume that was 1.8 times the hemorrhage volume provided significant benefit, even though the mean arterial pressure was unchanged from the levels during the hemorrhage. These investigators proposed that early small-volume resuscitation could provide significant benefit, particularly in patients with uncontrolled hemorrhage, in whom arterial pressure and thus bleeding would be unaffected by this type of resuscitation. The consistent finding of an acute
weight gain of 10 to 20 percent in patients after trauma or surgery suggests a significant risk for pulmonary edema (Lowell, 1990). As described by Lyons (1997), a 10 to 20 percent weight gain in a 70-kilogram (kg) man represents "a 2-3 gallon increase in the intracellular and extracellular fluid compartments." These studies collectively suggest that reassessment of both the composition and the rates of fluid resuscitation in the patient with hemorrhagic shock is warranted.
Adverse Effects of Lactated Ringer's Solution
Although Ringer's solution (Ringer, 1883) has been widely used for the treatment of hemorrhagic shock, bums, and sepsis, the negative effects of Ringer's solution were recognized as early as 1901, when Cushing described "the poisonous effects of both Ringer's and normal saline on nerve muscle preps" (Cushing, 1901). Subsequently, lactated Ringer's solution was described as superior to normal saline for treatment of infant diarrhea since the lactate could be metabolized to carbon dioxide (CO2) and water (H2O), providing a safe and acceptable substitute for bicarbonate (Hartmann, 1934). The deleterious effects of normal saline were also recognized in that the kidneys are unable to excrete the excess chloride resulting from the transfusion of large volumes of normal saline, producing hyperchloremic acidosis.
Current research has focused on the electrolyte compositions of fluids and whether current regimens of volume replacement aggravate shock-mediated plasma, extracellular electrolytes, and intracellular electrolyte imbalances. The well-characterized loss of intracellular potassium ion (K+) concentrations and increases in intracellular sodium ion (Na+) and calcium ion (Ca2+) concentrations in shock can be exacerbated by sodium chloride (isotonic or hypertonic), producing hyperchloremic acidosis; additionally, Ringer's acetate solution can promote selective sequestration of calcium in the mitochondria, depleting the calcium that is essential for cellular function.
Although lactated Ringer's solution has been used to treat several types of trauma and hemorrhage, concerns regarding the side effects of lactated Ringer's solution in the injured subject with blood loss have been raised (Dronen et al., 1992). Lactated Ringer's solution is a racemic mixture containing two stereo-isomers of lactate: D(-)-lactate and L(+)-lactate. L-lactate is a product of glycolysis, and concentrations in serum (0.5 to 0.6 millimolar) directly correlate with food intake and physical activity levels; D-lactate is produced either from ketone bodies or by microorganisms (Anderson et al., 1997). Metabolism of D-and L-lactate occurs via different pathways and produces distinct metabolic consequences. A rise in serum D-lactate levels alters neurologic function, producing encephalopathy (Thurn et al., 1985). Although the toxicity of D-lactate is well recognized, several limitations of L-lactate alone in solution have been proposed. The L-lactate-pyruvate balance is tightly controlled in normovolemia; however, resuscitation with lactated Ringer's solution after traumatic injury alters the lactate-pyruvate balance, reducing the ratio of the concentration of oxy-
dized nicotinamide adenine dinucleotide (NAD+) to the concentration of reduced nicotinamide adenine dinucleotide (NADH), reducing the cytosolic phosphorylation potential, and depleting cellular energy stores. An altered cellular redox state may alter hormonal function and diminish cardiac filling. In addition, resuscitation with existing regimens of lactated Ringer's solution and normal saline has been shown to lower the cellular phosphorylation potential (Veech et al., 1986) and exacerbate shock-induced intra- and extracellular calcium shifts, worsening shock-mediated cell injury and dysfunction.
The significant advantage of the currently available lactated Ringer's solution is that it provides a source of bicarbonate as a result of the metabolism of lactate to CO2 and H2O; and unlike bicarbonate, lactated Ringer's solution does not precipitate calcium when it is added to intravenous fluids. D-Lactate is oxidized to pyruvate via an enzyme that has not been identified in human tissues. Previous studies have shown that D-lactate is metabolized at a slower rate than L-lactate and that D-lactate is excreted in urine (Cori and Cori, 1929). The toxicity of D-lactate has been described in patients undergoing peritoneal dialysis with racemic lactate mixtures (Chan et al., 1994), indicating the limitations and dangers of this solution for select patient populations. However, no incidences of either neurologic impairment or encephalopathy have been attributed directly to lactated Ringer's solution resuscitation from hemorrhagic shock.
However, the cardiotoxicity of lactated Ringer's solution resuscitation from hemorrhagic shock has been examined in adult Sprague-Dawley rats. Ringer's solutions containing L-lactate, D-lactate, or the racemate were compared in conscious unrestrained rats after hemorrhage to a mean arterial blood pressure of 40 millimeters of mercury (mm Hg) over a 10-minute period. The volume of lactated Ringer's solution equaled four times the maximal bleed-out volume. Electrocardiograms were monitored for changes in rhythm, ectopy, ventricular tachycardia, sinus bradycardia, heart block, and asystole. Rats resuscitated with either the racemate- or L-lactated Ringer's solution had no changes in cardiac function, whereas D-lactated Ringer's solution produced various degrees of cardiac arrhythmogenicity, premature ventricular contractions, ventricular tachycardia and bradycardia as well as ventricular fibrillation, third-degree heart block, and asystole (Delman et al., 1996). The absence of cardiotoxicity with the use of either racemate- or L-lactated Ringer's solution is consistent with clinical reports of successful resuscitation of trauma patients with hemorrhage and an absence of serious side effects. For example, the clinical use of lactated Ringer's solution (particularly in burn patient populations who may require 15 to 20 liters of lactated Ringer's solutions during the first 24 hours postburn) has not been associated with notable cardiac or neurologic deficits. These data emphasize the safety of current regimens of lactated Ringer's solution and suggest that the form of lactate in lactated Ringer's solution is not important clinically as long as there is a racemic mixture.
Safety and Efficacy of Hypertonic Saline Solutions
The use of hypertonic saline for resuscitation from hemorrhage was first described in 1980, when Velasco and colleagues and DeFelippe and colleagues reported in separate studies that hyperosmotic sodium chloride rapidly expands plasma volume after major blood loss (DeFelippe et al. 1980; Velasco et al., 1980). Subsequent clinical studies examined the role of concentrated salt solutions in the resuscitation of patients with severe burn injuries (Caldwell and Bowser, 1979; Monafo et al., 1984). Current interest in hypertonic saline solutions for clinical application arose from studies by Kramer and colleagues (1986), and Holcroft and colleagues (1987), who described the use of a small bolus of hypertonic saline-dextran (HSD; 4 ml/kg) to initiate fluid resuscitation from hemorrhagic shock in a sheep model. In their study, the infusion of HSD was followed by the infusion of lactated Ringer's solution to maintain hemodynamic function and urine output at prehemorrhage levels. This small-volume resuscitation was shown to restore blood pressure and cardiac output within 2 minutes of infusion, to maintain both oxygen consumption and urine output above baseline levels during a 30-minute simulated transport period, and to decrease the total volume of lactated Ringer's solution used for resuscitation.
These studies in the early 1980s produced a storm of experimental and clinical studies examining the use of HSD as a pharmacologic intervention for early restoration of blood pressure and cardiac output in the field. Over 300 papers have appeared in the last 10 years, and these studies from several independent laboratories with several animal models of hemorrhagic shock have confirmed the consistent physiologic benefits of small-volume hypertonic saline resuscitation (Coimbra et al., 1997; Dubick and Wade, 1994; Greene et al., 1998; Horton et al., 1995; Kramer et al., 1986; Lilly et al., 1992; Maningas et al., 1986a, b; Nakayama et al., 1985; Pascual et al., 1992; Velasco et al., 1980; Velasco et al., 1989; Wade et al., 1990). These studies have confirmed the safety of HSD, the early volume-sparing benefits, as well as the significant improvements in hemodynamic function. Differences in the composition of hypertonic solutions were studied by a group at the University of California at Davis (Smith et al., 1985); the benefits of hypertonic sodium acetate, sodium chloridemannitol and sodium chloride-6 percent dextran 70, and glucose were compared by using an ovine shock model. These solutions were used as a bolus of 4 ml/kg, and although all solutions were initially successful, the beneficial effects were transient with all solutions except the 7.5 percent saline-dextran 70. This optimal hypertonic solution provided posttrauma cardioprotection, reduced the oxygen debt, and improved the microcirculation.
Since 1986, several clinical trials of HSD for initial resuscitation from hemorrhagic shock have been performed (Holcroft et al., 1987). The initial trial included injured adults who required helicopter transport and who were randomized to receive either HSD or normal saline during transport; HSD significantly improved the overall survival rate, but most importantly, this initial study confirmed that rapid HSD infusion has no adverse effects in the hypovolemic pa-
tient. Subsequent prospective studies compared HSD with either hypertonic saline alone or lactated Ringer's solution. Despite the improved survival rate when HSD was used in the field, the application of this resuscitation regimen in the emergency department failed to affect the survival rate (Vassar et al., 1990). Other clinical trials included a multicenter trial at Ben Taub General Hospital, the Denver General Hospital, and the Milwaukee County Medical Complex (Mattox et al., 1991), and included a total of 422 patients enrolled over a 13-month period. Patients were randomized in a blinded fashion to receive an initial bolus of HSD or crystalloid administered during transport by ambulance. Blood pressure was significantly improved in the HSD group upon arrival at the emergency department, and HSD significantly affected the survival rate in that sub-population of patients requiring emergency surgery. This prospective clinical study confirmed the safety of small-volume HSD administration in injured patients, and the major benefit of resuscitation with HSD appeared in the population of patients with craniocerebral trauma, in whom resuscitation with large-volume crystalloid tended to increase intracranial pressure.
To date, eight double-blind randomized trials have evaluated the use of small-bolus HSD for prehospital or emergency department treatment of patients with traumatic hypotension (Holcroft et al., 1987; Shackford et al., 1992; Vassar et al., 1990, 1993a,b; Wade et al., 1997a). Improved rates of survival after discharge were reported with HSD in seven of eight trials, although statistically significant improvement in overall survival was seen in only one trial. Metaanalysis for the evaluation of HSD as the initial treatment for hypovolemic shock included the original records from six trials and included 604 subjects. Overall discharge survival rates were improved with HSD resuscitation, and HSD resuscitation was particularly effective for a subpopulation of subjects with head injury with a discharge survival rate of 38 percent compared with a rate of 27 percent for the control group receiving saline (Wade et al., 1997b). The striking benefits of the HSD solutions have been described recently. Hypertonic saline solutions were shown to provide immunologic protection, whereas hypo-osmolar lactated Ringer's solution as well as artificial colloids upregulated adhesion molecule expression, increased proinflammatory cytokine synthesis, and promoted cellular apoptosis (Coimbra et al., 1996; Junger et al., 1997a,b; Rhee, 1998).
The limits of hypertonic saline resuscitation include the fact that its efficacy is lessened in patients who receive an initial bolus of isotonic lactated Ringer's solution. In addition, rapid administration of HSD may produce profound vasodilation and hypotension. Hypertonic saline-dextran for resuscitating hemorrhagic shock in the presence of head injury has received considerable attention. Despite significant concerns regarding the use of concentrated salt solutions for resuscitation from hemorrhagic shock, current regimens of hypertonic resuscitation produce a transient rise in serum osmolality that remains less than 350 milliosmolar (mosM)/liter. The serum sodium level has been shown to remain less than 160 milliequivalents (meq); the 15-gram dextran load in HSD resuscitation has been associated with no morbidity.
However, Gross and colleagues (1988) raised the question as to whether early resuscitation of the injury patient with HSD increases blood loss in the presence of uncontrolled hemorrhage. With several rodent models, including rats with transected tails or ileocolic artery injury, deleterious effects of HSD in the presence of continuing blood loss were described (Gross et al., 1989). Despite these data, there has been a remarkable absence of deleterious effects with HSD administration in more than 1,000 trauma and surgical patients who have been treated with HSD (Kramer et al., 1995). No incidence of hypernatremic seizures, increased bleeding or blood needs, coagulopathies, renal failure, cardiac arrhythmias, or central pontine myelinolysis have been described in trauma patients. However, HSD administration in patients with significant cardiac dysfunction has been shown to produce hypertension and signs of acute fluid overload. In patients with underlying cardiac dysfunction, titration with smaller volumes such as 1 mg/kg have been recommended (Kien et al., 1997; Welte et al., 1997, 1995).
Although results vary with individual studies, evidence from human trials suggests that both the safety and efficacy of hypertonic (7.5%) saline alone are similar to those for the hypertonic saline-6% Dextran 70 solution. Clinical trials in civilian trauma patients (Vassar et al., 1990, 1993a,b) demonstrated no adverse clinical effects of infusing either solution, and the addition of the colloid did not appear to offer any additional benefit.
Given the information presented above, the safety and efficacy of small-volume hypertonic resuscitation has been confirmed. In light of the continuing concerns of transportation of large crystalloid volumes required for resuscitation, the ability to recover mean arterial pressure and cardiac output in the field with small-volume HSD resuscitation confirms the value of this regimen as the initial treatment for hemorrhagic shock in the field. The added benefits of HSD-related reductions in the total volume of fluid used for resuscitation may contribute to a decrease in the incidence of ARDS, MODS, and systemic inflammatory response syndrome currently associated with crystalloid resuscitation. Finally, the finding that HSD resuscitation did not enhance the cytokine response or alter any aspect of the immune response to hemorrhage are added benefits.
Alternative Resuscitation Approaches
Carolina Rinse Solution, Initially Applied As A Rinse And Storage Solution For Transplanted Organs, Significantly Increased Organ Survival Above That Described With Lactated Ringer's Solution. The Solution Contains Antioxidants, Allopurinol, Desferrioxamine, Nicardipine, Glutathione, Insulin, Low-Concentration Adenosine To Inhibit Kupffer-Cell Activation, And The Cytoprotective Amino Acid Glycine (Lemasters Et Al., 1995). Carolina Rinse Solution Has Been Shown To Provide Considerable Protection Of The Small Bowel And Liver Before Transplantation In Both Humans And Animals (Abdennebi Et Al., 1998; Massberg Et Al., 1998; Gao Et Al., 1991). Compared To Lactated Ringer's Solution, The Carolina Rinse Solution
improved the excretion of indocyanine green which is a known marker of parenchymal and nonparenchymal cell integrity, from bile, improved nutritive perfusion after small-bowel transplantation, attenuated the leukocyte-endothelial cell interaction, and almost completely prevented mucosal ischemia reperfusion injury. Additional studies showed that Ringer's solution containing adenosine (0.1 mM) increased the survival time when it was used to rinse cold-stored liver grafts. Despite the improved survival following liver transplantation, however, Ringer's solution with adenosine did not prevent parenchymal cell injury, suggesting that the adenosine in the Carolina Rinse solution may work via non-hepatic mechanisms (Gao et al., 1991).
In a human clinical trial, the use of the Carolina Rinse solution improved the transaminase release by transplanted human livers. Recently, a modified Carolina Rinse solution has been examined in a cardiac preparation; coronary artery occlusion in open-chest pigs produced myocardial infarction, as expected. Modified Carolina Rinse solution plus cyclosporine, used as the initial solution for reperfusion of the cardiac tissue, decreased creatine kinase release, confirming the significant rescue of ischemic and hypoxic tissue (J. Lemasters, 1998, School of Medicine, University of North Carolina at Chapel Hill, personal communication). The inclusion of an immunosuppressant such as cyclosporine likely modulated the reperfusion injury via several mechanisms, including blockade of the mitochondrial transition pore and altered translation of protein synthesis. Despite the advantages of the Carolina Rinse solution in attenuating postischemic microvascular injury after small-bowel or liver transplantation, the value of this solution for volume replacement after trauma or hemorrhage has not been evaluated. However, the value of adding antioxidants, cytoprotective amino acids, or adenosine to resuscitation fluids warrants further study.
Summary
Numerous recent studies have raised questions about the type, volume, and rate of fluid resuscitation from shock. The incidence of ARDS and MODS in patients who have received large-volume crystalloid resuscitation is a significant concern. The finding that lactated Ringer's solution and artificial colloids increase the levels of expression of adhesion molecules, promote the release of proinflammatory cytokines, and promote cellular apoptosis suggests that fluid resuscitation has significant immunologic consequences. It was of interest that hypertonic saline solutions, shown to stabilize arterial blood pressure and cardiac output with small-volume infusion, have been shown to have no deleterious effects on immune function or to promote programmed cell death. Valid concerns have been raised regarding the composition of lactated Ringer's solution, and the omission of D-lactate with a concomitant reduction in the total L-lactate load appears feasible. The increasing availability of solutions with improved oxygen transport capabilities coupled with therapeutic strategies that limit reperfusion injury (antioxidants, iNOS inhibitors) have great potential. However,
current research and clinical experience caution against the indiscriminate use of crystalloids for resuscitation. As Evans cautioned in 1911, "under certain circumstances saline solutions are productive of great harm to the tissues of the body" (Evans, 1911).
Conclusions and Recommendations
The committee found that although the existing resuscitation fluids are rarely questioned in clinical practice, there are at least theoretical disadvantages to these fluids, and many have not been modified for several decades. In contrast, there have been significant advances in organ preservation resulting from new fluid formulations. New resuscitation fluids should be developed and tested as described in the committee's recommendations below. Such fluids should address the metabolic and cellular consequences of traumatic shock and the potential disadvantages of existing fluid formulations.
An ideal fluid for intravenous use should provide adequate control of pH, partial CO2 pressure/bicarbonate ratio, the phosphorylate potential, the redox state, and osmotic pressure; adequate control of sodium chloride, calcium, and potassium levels; and adequate control of the lactate and pyruvate ratio. Veech and colleagues (1986) proposed modification of existing regimens of fluid resuscitation to more nearly mimic the composition of intravascular fluid. For example, the use of parenteral normochloremic carbonate (HCO3+)/CO2 saline would have a more physiologic ratio of sodium chloride, thus avoiding the risk of hyperchloremia. The stability of such electrolyte solutions could contribute to a long shelf life in both combat situations and civil disasters. Concerns regarding the toxicity of lactated Ringer's solution (which contains 14 mM D-lactate) (Chan et al., 1994) have prompted the recommendation that D-lactate be excluded from current preparations of lactated Ringer's solution and that more physiologic levels of L-lactate be used. Veech (1986) further proposed replacing D- and L-lactate in lactated Ringer's solution with "physiological ratios of L-lactate-pyruvate, HCO3-:CO2, and d-β-hydroxybutyrate:aceloacetate" (p. 547). Inclusion of such compounds would allow tighter control of the phosphorylation state as well as better control of the redistribution of water and electrolytes in shock. The rapid reversal of such shock-mediated shifts in sodium, potassium, and chloride levels should reduce the level of tissue injury. However, evidence-based research demonstrating (1) the ability of such solutions to effectively restore blood volume, (2) the hemodynamic, hematologic, cellular, and immunologic advantages, and (3) an absence of side effects, is lacking. Other concerns, which exist with proposed modifications of existing regimens with saline, include the concern that the unphysiologic concentrations of bicarbonate may produce alkalosis (Veech, 1986, 1991). To guide the development of an ideal fluid, the committee makes the following recommendations.
Recommendation 3.1 Research involving modifications of existing lactated Ringer's solutions could include:
|
1. |
elimination of D-lactate, |
|
2. |
reduction of total L-lactate load, |
|
3. |
addition of ketones as an energy source, and |
|
4. |
addition of free-radical scavengers and antioxidants (vitamins E and C, glutathione, or iron chelators). |
Recommendation 3.2 Studies examining modifications of the existing lactated Ringer's solution formula must include examining the effects of the modified solution on:
|
1. |
immunologic-related function, |
|
2. |
cellular apoptosis, |
|
3. |
intravascular retention, and |
|
4. |
specific end-organ function such as pulmonary, renal, and cardiac function (i.e., the presence or absence of arrhythmias). |
Recommendation 3.3 Previous concerns regarding the detrimental effects of aggressive fluid resuscitation with large volumes of crystalloids suggested the need to examine both the immunologic as well as hemodynamic consequences of small-volume lactated Ringer's resuscitation. Studies examining reduced volume of lactated Ringer's solution should examine the effects of this volume on:
|
1. |
hemodynamic function, |
|
2. |
immunologic-related function, |
|
3. |
cellular apoptosis, and |
|
4. |
specific end-organ function, such as pulmonary, renal, and cardiac function. |
| This page in the original is blank. |

































