4
Novel Approaches to Treatment of Shock
To stop his wounds, lest he do bleed to death
— Shakespeare, The Merchant of Venice, Act IV, Scene I
Approximately 80 percent of battlefield casualties experience substantial blood loss, and hypovolemic shock is a major contributor to early mortality from trauma, reportedly the leading cause of death in Americans under the age of 45 years. Improved resuscitation fluids and fluid protocols might reduce the rates of morbidity and mortality both in the far-front battlefield ''stay and play'' setting and in the civilian setting, where the "scoop and run" strategy might be logistically easier to institute. The different requirements for differing scenarios of trauma seem to argue for a formulary of boutique fluids, perhaps broadly divided into supportive fluids and therapeutic fluids designed to take into account the pathophysiology of the particular injury. The committee recognizes, however, that military staff training and logistical considerations favor simplicity and a single resuscitation fluid with a single resuscitation strategy wherever possible.
The ideal resuscitation fluid would have to have the properties of an elixir of life: a small-volume cocktail that among its virtues improves perfusion, enhances oxygen (O2) delivery and diffusion, provides adequate metabolic substrates, neutralizes toxic molecules released as a result of tissue injury, provides antimicrobial activity, renders the recipient globally less vulnerable to the effects of hemorrhagic shock, and has prolonged beneficial effects. The solution should further be stable for lengthy periods at a variety of temperatures, be easy to prepare and administer, and, if not inexpensive, be at least affordable. Such a solution is not on the near horizon. However, a variety of research observations and potential novel therapeutics hold promise as additives to some basic resuscitation solutions. Those novel therapeutics that emerge from preclinical testing might be tested as additives to the basic resuscitation fluid in a stepwise, controlled fashion in appropriate animal models and subsequent human studies. Strategic planning for the trauma arena should include a translational research program with goals of rational fluid design and improvement.
Hypovolemic shock results from an acute disturbance in the circulation that leads to an imbalance between O2 supply and demand in the tissues. The shock syndrome is a final common pathway through which a variety of pathologic processes lead to cellular ischemia and ultimately cell death. Shock syndrome may predispose the individual to other major comorbidities such as acute respiratory distress syndrome, sepsis, systemic inflammatory response syndrome, and multiple-organ dysfunction syndrome (MODS). The complex events that result from the initial insult, tissue injury, and resuscitative attempts provide numerous potential therapeutic targets for novel interventions. The novel therapeutics and research strategies might be conveniently, if somewhat artificially, categorized as those that prevent the early complications of the shock syndrome (prevention), those that treat the complications of shock syndrome and reperfusion injury (intervention), and those that render the subject less vulnerable to hypoxia and its consequences (tolerance). The military should maintain a research interest, if not research support, in each of these areas, recognizing that some are approaching sufficient maturity for clinical trials, whereas others are still at a basic science stage.
Prevention
Optimal resuscitation requires a strategy that prevents or limits the course of the complex sequence of molecular events that contribute to the shock syndrome. In theory, such a strategy probably involves use of a solution that (1) optimizes O2 delivery, (2) supports basic cell function, (3) provides a useful energy source, and (4) prevents oxidative reperfusion injury. The development and testing of components and combinations of such a solution are achievable research goals.
Oxygen Therapeutics
Although oxygen delivery is a critical factor in the pathophysiology of hemorrhagic shock, the role of the oxygen-carrying potential of blood and the point at which transfusion becomes essential is less well documented. The hemoglobin concentration at which a healthy resting human develops tissue hypoxia as a result of inadequate critical oxygen delivery is not known. It likely differs for a young, well-conditioned soldier and an elderly civilian with cardiovascular or pulmonary disease, and certainly differs by organ and by the nature of the acute traumatic injury. Acute isovolemic reduction of the blood hemoglobin concentration to 50 grams per liter (g/liter) in conscious, resting humans does not produce evidence of inadequate systemic critical O2 delivery, as assessed by O2 consumption, plasma lactate, and ST changes on an electrocardiogram (Weiskopf et al., 1998). Compensatory mechanisms included a 58 percent increase in systemic vascular resistance and expected increases in heart rate, stroke volume index, and cardiac index. This is consistent with observations
with conscious nonhuman primates (baboons) (Levene et al., 1990) and from clinical experience with Jehovah's Witnesses (Viele and Weiskopf, 1994). Healthy, resting patients tolerate anemia (<<10 milliliters [ml] of O2/kilogram [kg] of body weight/minute), a level reached in healthy humans at a hemoglobin concentration of less than 50 g/liter without systemic acidosis or other apparent adverse metabolic sequelae. Although such studies may underestimate the O2 requirements of severely wounded soldiers (Cerra, 1987), they do suggest that resuscitation solutions with small enhancements in O2-carrying capacity might reduce rates of morbidity and mortality.
Several different oxygen therapeutics (formerly referred to as blood substitutes or red blood cell substitutes) are in various stages of preclinical and clinical trials (Gould et al., 1998; Hughes et al., 1996; Keipert, 1998). It is important to consider each of these formulations as different drugs or biologics with different physical characteristics, biologic activities, and adverse reaction profiles. Among the hemoglobin-based molecules, some are derived from human blood, some are derived from bovine red blood cells, and one is from a recombinant Escherichia coli source. Tetramers have been chemically modified, polymerized, oligomerized, conjugated, and encapsulated in liposomes. Some formulations are pyridoxylated, glutaraldehyde cross-linked, diaspirin cross-linked, raffinose cross-linked, and polyethylene glycol modified. Sizes range from tetramers to distributions of relatively large polymers. Furthermore, it is possible to customize cell-free hemoglobin with a specified O2 affinity, molecular size, heme stability, viscosity, and intravascular retention time depending upon the proposed use and the postulated requirements, if they are understood. The optimal physiologic characteristics of the molecule and of its carrying solution are not agreed upon. It is more difficult still to design appropriate clinical trials to test the specific application and define both efficacy and toxicity for hemorrhagic shock. As important as O2 consumption is to cellular well-being, it is unlikely that these additives will yield Lazarus solutions that raise the dead; rather, they might provide incremental improvements in morbidity and mortality rates among gravely injured subjects.
For military use as a component of a resuscitation fluid, desirable characteristics of the oxygen therapeutics include the ability to deliver O2 effectively, a lack of toxicity, freedom from infectious disease transmission, stability, low immunogenicity, ease of storage and use, and universal compatibility. Enthusiasm for these drugs as components of a resuscitation fluid has oscillated dramatically in the last year alone. This was due in part to contradictory results in clinical trials and in part to an incomplete understanding about how these drugs deliver O2, the mechanisms of their side effects, and the fundamental mechanisms in shock, hemorrhage, and the control of blood flow. Currently the only formulation licensed as a red blood cell replacement is the veterinary product Oxyglobin (Biopure Corp., Cambridge, Mass.), which was licensed in January 1998 for the treatment of acute anemia in dogs. A similar bovine hemoglobin-derived oxygen therapeutic for human use is currently in phase III clinical trials.
Small molecules dissolved in plasma likely deliver O2 in a fundamentally different fashion than red blood cells (erythrocytes) do. Erythrocytes are 7 to 8 micrometers (µm) in diameter, traverse the microcirculation differently than plasma, and release O2 across a protein membrane (Homer et al., 1981). Erythrocyte O2 transport is directly related to hemoglobin concentration. A great deal less is known about how different hemoglobin-based O2 carriers deliver O2 and about how they are distributed, metabolized, and excreted. The mechanisms of O2 delivery and the ideal viscosity and concentration in a resuscitation fluid are fertile areas for research.
First-, Second-, and Third-Generation Therapeutics
Recently, resuscitation trials with one first-generation molecule, diaspirin-cross-linked hemoglobin (HemAssist; Baxter International), were halted in the United States and Europe. A full report of these trials has not yet been published. Clinical studies with a first-generation recombinant hemoglobin (Optro; Somatogen, Inc.) were suspended as well. The side effects of these preparations have been attributed by some to the inherent vasoactive properties of these molecules. Although the mechanism of this effect is unclear, it may relate to nitric oxide binding (Abassi et al., 1997), which is well recognized in cell-free hemoglobin solutions. It has not been proved that this property was responsible for excess morbidity or mortality in the clinical studies. In fact, a polyoxyethlene hemoglobin (Apex Bioscience) that acts as a nitric oxide scavenger rather than as an oxygen therapeutic has been proposed as a drug to effect vasoconstriction in shock. A second theory attributes the vasoreactivity to some auto-regulatory reflex induced by increased O2 available from cell-free hemoglobin (Vandegriff and Winslow, 1995). Whatever the mechanism, larger, polymerized molecules seem to have minimal vasoactivity, and at least one such compound is being studied in a resuscitation trial (Gould et al., 1998). This observation underscores the importance both of considering each of these drugs individually and of continuing studies on later-generation formulations.
Several second- and third-generation oxygen therapeutics are in preclinical studies. Some novel concepts that are being entertained include inclusion of antioxidants such as superoxide dismutase (SOD) and catalase to reduce the effects of ischemia-reperfusion injury resulting from superoxide formation and oxygen radical liberation after reoxygenation. If cell-free heme iron and the lack in these solutions of the antioxidant activity that is found in intact erythrocytes does in fact exacerbate pro-oxidant activity in vivo, it might be advantageous to combine these products with molecules that chelate iron or block the oxidant-mediated injury cascade. Surprisingly few experimental data concerning iron chelation during resuscitation are published, considering that recognition of a connection between iron, free radicals, and tissue damage in hemorrhagic shock goes back almost 30 years (Hedlund and Hallaway, 1991). Polynitroxylation of biomacromolecules (albumin, starch, or hemoglobin) is one example of an approach to scavenging of reactive species to block the oxidant-mediated injury
cascade (Zhang et al., 1998). There are certainly numerous other biochemical possibilities if this concept of prevention proves clinically important.
Perfluorochemicals
Different formulations of perfluorochemicals have been used to dissolve O2 since Clarke's original report in 1966. Perfluorocarbons are chemically synthetic molecules consisting primarily of carbon and fluorine atoms. Perfluorocarbons are formulated to dissolve large quantities of gases, including oxygen, carbon dioxide, and nitrogen, and they do so in a linear fashion. Because they are hydrophobic, they must be emulsified with a surfactant such as phospholipid before they are suitable for intravenous use. The ability of perfluorocarbons to replace red blood cells as temporary O2 carriers was demonstrated more than 30 years ago (Geyer et al., 1968). Perfluorocarbons exchange O2 by simple diffusion; O2-carrying capacity is directly proportional to the partial pressure of O2 in arterial blood. There has been limited preclinical experience with the use of these substances used in hemorrhagic shock (Goodin et al., 1994; Stem et al., 1995). Using tissue oxygenation as an endpoint and 100 percent inspired O2, these studies demonstrate improved survival in dogs compared with that for controls given lactated Ringer's solution. Although these compounds meet many of the requirements of a candidate oxygen carrier, their requirement for supplemental (100 percent) O2 to work optimally and issues with transport and reconstitution, even with the newer emulsions (e.g., perfluorodichlorooctane and perfluorooctyl bromide emulsions), may make them more suitable candidates for such applications as interoperative hemodilution than as additives to early resuscitation fluids to be used in the field. These emulsions may prove to be more useful in ameliorating cerebral, myocardial, and acute limb ischemia when O2 is available after transport to a hospital setting.
Liposomes
Liposomes are self-assembling amphiphilic lipids and can be fabricated with a wide variety of compositions appropriate to the planned application. The composition determines the biological response to the infused component. Liposome formulations with extended intravascular persistence have been formulated for the delivery of pharmaceuticals (Parr et al., 1993). Similar success has eluded formulations of liposome-encapsulated hemoglobin. Hemoglobin encapsulation in lipid vesicles (liposomes) has been tested with primates; such a formulation should attenuate vasoconstriction and prolong the intravascular circulation of an O2 carrier. Given the particle size, recent investigation has concentrated on the interaction of liposomes with the reticuloendothelial system and on the inflammatory cytokine response to infusion. Studies of hemorrhagic shock have been carried out with a rat model. A related approach used encap-
sulation of hemoglobin, together with a variety of red blood cell enzymes including SOD, into biodegradable polymer nanocapsules. The physiology and toxicity profiles, including reports of thrombocytopenia, leukocytosis, and complement activation, of such formulations are now being studied. Although these approaches are promising and an encapsulated hemoglobin molecule comes closest to representing a universal red blood cell, the obstacles to clinical use and practical production are formidable.
Other Novel Approaches
Other novel approaches to the delivery of O2 in the earliest phase of hemorrhagic shock might also qualify as prevention. For example, crocetin, a carotenoid compound derived from the fruit of Gardenia jaminoides Ellis, a Chinese medicinal herb, has been investigated for its protective action in quenching free radicals. However, the compound has also been demonstrated to increase O2 diffusivity, and it has been observed to increase the level of O2 consumption and the rate of survival in a rat hemorrhagic model (Gainer et al., 1993). A similar molecule, trans-sodium crocetinate, persists longer in the circulation (half-life = 25 minutes), improves diffusivity even after it is cleared, and improves the rate of survival of rats in a hemorrhagic model. These data are as yet preliminary and unpublished.
Intervention
During the past decade progressive understanding of the complex patho-physiology of shock and of reperfusion injury after reoxygenation has provided numerous candidate molecular and biochemical targets that might interrupt cellular injury, stabilize the patient's clinical status, and reduce long-term morbidity and mortality. However, recognition of numerous candidate targets has often become an invitation to investigate blindly any and all potential inhibitors of substances whose levels are elevated and phenomena that are observed in the presence of shock. This approach is akin to reversing a waterfall by targeting individual droplets. Worse yet, such targeting may prove harmful because components of these complex responses have undoubtedly been selected through the process of evolution because they have a protective effect. A systematic approach to studying the processes in hopes of reversing or ameliorating those processes that result in deteriorating cellular function should prove more profitable.
Targets for Intervention
It is not possible to describe all of the potential targets for intervention; these are limited only by the imagination. Several examples may be instructive. For instance, investigations might address issues such as mitochondrial function,
which is critical to both energy homeostasis and the regulation of apoptotic cell death. One approach to reducing hypoxic brain injury might concentrate on the elevated calcium and nitric oxide levels that can damage intracellular organelles by reducing excessive synaptic glutamate levels or blocking neuronal glutamate receptors. Adenosine and γ-aminobutyric acid are two such candidate agents. Approaches to blocking nitric-oxide synthase bicalcium, which may protect the central nervous system (CNS) from lipid peroxidation and protein oxidation, might focus on the inhibition of poly-adenosine diphosphate (ADP) ribo-polymerase, overactivation of which during cell injury depletes the cell's normal nicotinamide adenine dinucleotide (NAD) supply. Agents like cyclosporin and FK506 inhibit opening of a high-conductance pore in the mitochondrial inner membrane, preventing necrotic cell death after restoration of the pH following reperfusion, an injury that is not mediated through oxygen free radicals. Similar agents that do not have immunosuppressive effects may likewise inhibit the mitochondrial permeability transition and prevent apoptotic cell death (Lemasters, 1998).
Although the early events in hemorrhagic shock include an increase in circulating as well as tissue concentrations of proinflammatory cytokines (tumor necrosis factor [TNF, interleukin-1 [IL-1], IL-6), the precise mechanism of the cytokine cascade remains elusive. If the cytokine cascade is, as has been hypothesized, a major factor in the development of MODS, which occurs in up to 15 percent of patients who survive the first 48 hours following trauma, a better understanding of the cytokine events and associated neurohormonal alterations should provide novel therapeutic strategies (Molina et al., 1997). Inhibition of individual inflammatory molecules has proved to be less promising in acute inflammation than in such chronic inflammatory conditions as rheumatoid arthritis. Inhibition of certain of the leukotrienes and thromboxanes that are associated with the aggregation of leukocytes and platelets and lipids that contain large amounts of omega-3 polyunsaturated fatty acids may reduce the production of eicosanoids as well as alter IL-1 and TNF release. However, despite promising preclinical data, clinical trials of antagonists of IL-1 (Dinarello, 1997) and TNF-α (Abraham, 1998) with critically ill patients have been disappointing. The multiple and often conflicting signaling pathways of these and other cytokines suggest a number of novel approaches (Ksontini et al., 1998), but better understanding of the process will likely be necessary before rational clinical trials can be contemplated for trauma.
A great deal of attention has been given to preventing the generation of free radicals and the activation of inflammatory cascades at the time of resuscitation. Treatment or reversal of reperfusion injury is a related priority. One mechanistic approach is to investigate control of the inducible inflammatory nitric oxide synthase (iNOS) which is upregulated in human trauma and which is involved in the activation of a variety of stress transcriptional factors. Upregulation appears to be associated with cytokine production and neutrophil accumulation in the lung and liver, at least in some animal models (Hierholzer et al., 1998). Agents such as mercaptoethylguanidine (MEG) that inhibit iNOS and that have a scavenging effect on peroxynitrite, a toxic oxidant formed from nitric oxide
and superoxide, are reported to have beneficial effects in rat and porcine hemorrhage models (Zingarelli et al., 1997). Such agents have broad inhibitory actions and will likely have some unexpected and undesirable consequences. However, the availability of scavengers of excess nitric oxide or inhibitors of iNOS provides opportunities for experimental approaches that may modulate the inflammatory response and ameliorate end-organ damage in hemorrhagic shock.
Therapies for Reperfusion-Mediated Free-Radical Damage
Although free radicals remain difficult to measure in living tissue because of their short half-lives, multiple strategies to the scavenging or neutralization of radicals have provided indirect evidence of increased free-radical production after resuscitation from shock.
Antioxidant or Scavenging Strategies
A plethora of studies have indicated an imbalance in oxidant-antioxidant mechanisms in trauma with hemorrhagic shock. Early studies examined the protective effects of SOD or catalase alone or in combination on both survival and organ injury in the shock state. These scavenging therapies provided modest protection, likely due to the short half-lives of the scavengers as well as their inability to access the intracellular space, the major site of free-radical production. Subsequent studies examined conjugation of either SOD or catalase with polyethylene glycol, a measure that increased the scavenger half-life, significantly improved the survival rate, and reduced the level of tissue injury (Tan et al., 1993; Tominaga et al., 1995).
More recent strategies have focused on the use of SOD and catalase with polyhemoglobin as a new blood substitute for the treatment of hemorrhagic shock. This combined strategy resulted in only a minimal increase in oxygen-radical generation, as measured by the hydroxylation of 4-hydroxybenzoate to 3,4-dihydroxybenzoate (Chang, 1998). Additional modifications to this hemoglobin- and scavenger-containing blood substitute have included the encapsulation of hemoglobin, SOD, and catalase in liposomes or biodegradable nanocapsules. This combined strategy would increase the oxygen carrying capacity of the blood as well as provide significant scavenger activity (see Figure 4-1). Although various degrees of organ protection have been achieved with SOD and catalase, the most promising strategy would appear to be the incorporation of these compounds in a polyhemoglobin compound that would also serve to increase the oxygen-carrying capacity of the blood while scavenging the deleterious by-products of reperfusion.
Another pharmacologic approach to the scavenging of free radicals has been the administration of N-acetylcysteine (40 milligrams [mg]/kg/day). Suter and colleagues (1994) described the ability of this scavenger to enhance recov-
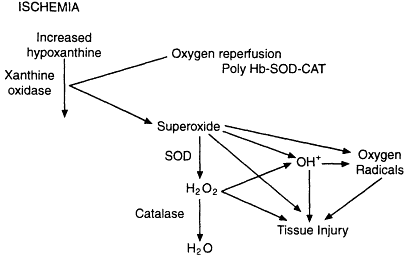
Figure 4-1
Schematic diagram showing the relationship between ischemia and oxygen reperfusion. Source: Reprinted, with permission, from Chang (1998).
ery from acute lung injury in a university hospital intensive care unit. N-Acetylcysteine improved the survival rate, reduced the time of ventilatory support, and improved the oxygenation index (partial arterial pressure O2/fraction of inspired O2); in addition, no adverse effects were observed during N-acetylcysteine treatment. N-Acetylcysteine has also been shown to provide significant protection in a number of models, including ischemia reperfusion and hemorrhagic shock (Demir and Inal-Erden, 1998; Fan et al., 1998).
In addition to neutralizing or scavenging the free radicals produced during resuscitation from shock, antioxidant therapy has been examined in several experimental models. Trolox, a water-soluble vitamin E antilog, and ascorbic acid (vitamin C) have been studied in hemorrhagic shock in rats (Daughters et al., 1996). The addition of vitamin E but not the addition of vitamin C to fluid used for resuscitation from shock significantly improved the 72-hour survival rate (75 percent compared to 40 percent for vitamin E-treated and vehicle-treated animals). The improved survival rate was not related to differences in either blood pressure or neutrophil adhesion and activation. Zollei and colleagues (1989) showed that the synthetic antioxidant of the dihydroquinoline type provided significant protection against hemorrhagic shock-induced gastric lesions, whereas Ekman and colleagues (1994) showed that low-dose ascorbate protected the gastric mucosa if it was given 15 minutes prior to hemorrhage. Anti-oxidant strategies have also been shown to reduce total fluid requirement in trauma, to improve hemodynamic variables, and to provide significant cardioprotection (Horton et al., 1996; Matsuda et al., 1991, 1995).
Iron Chelation
One biochemical consequence of ischemia followed by reperfusion is the release of iron from intracellular storage sites; although the quantities of iron released during the ischemic process are minute, reintroduction of molecular oxygen with fluid resuscitation promotes iron-oxygen interaction, setting the stage for free-radical formation. Earlier experimental studies of ischemia and reoxygenation have shown that deferoxamine (DFO) conjugates reduced micro-vascular injury after reperfusion, suggesting that chelation of iron during volume expansion provides significant vascular protection. Increased levels of iron in plasma after global ischemia were described as early as the 1950s (Mazur et al., 1955, 1958). Those investigators showed that a rise in plasma iron levels was capable of saturating the available transferrin-binding capacity. However, the contribution of ferrous iron and its role as a catalyst in the decomposition hydrogen peroxide to produce the highly reactive hydroxyl radical were not clearly recognized until 1985 (Thomas et al., 1985).
The role of free catalytically active iron in shock resuscitation-mediated tissue injury has been best defined by the use of iron chelators, which have been shown to reduce injury and provide significant long-term protection. The protective effects of DFO have been attributed to inhibition of iron-dependent free-radical reactions (Halliwell, 1989). Although the number of publications implicating iron chelation in shock resuscitation-related injury is extensive, the concept of adding an iron chelator at the time of fluid resuscitation to reduce injury and to attenuate numerous aspects of the postshock inflammatory response has proven to be exceptionally attractive. DFO administered with fluid for resuscitation from hemorrhagic shock significantly improved cellular function (Sanan et al., 1989) whereas the addition of DFO to cardioplegic solutions provided significant cardioprotective effects in models of ischemia (Illes et al., 1989; Menasché et al., 1988). Yet, the short half-life and the hypotensive effects of intravenously administered DFO have limited the clinical application of this compound. Similarly, the normally occurring iron-binding proteins (transferrin or ferroxidase) provide significant antioxidant effects but have not been studied in models of hemorrhagic shock. Instead, the development of a synthetic transferrin (produced by covalent binding of DFO to colloids) reduced the systemic toxicity of DFO (Hallaway et al., 1989), reduced micro-circulatory disturbances and improved organ function in models of hemorrhage, shock and smoke inhalation (Bauer et al., 1995; Demling et al., 1996)
Restriction of the chelator to the intravascular compartment has not been shown to reduce the efficacy of a colloid-deferoxamine conjugate (Hedlund and Hallaway, 1991). Starch-chelator conjugates developed by Biomedical Frontiers (Hedlund, 1998) have been used in two clinical trials and their safety, suitability for freeze-drying, and suitability for incorporation into resuscitation fluids currently in use in civilian and military settings have been confirmed. Those studies have further shown that this resuscitation fluid is well tolerated, even in patients with iron overload. The suitability of this compound for field use is related to
reconstitute it under field conditions. Currently, preclinical trials are needed to determine the optimal volume expansion, rate of excretion, and optimal chelator concentration for continued use of these compounds.
Other compounds described to inhibit iron chelation include tirilazad mesylate, a 21-aminosteroid. Infusion of these aminosteroids prior to fluid resuscitation from hemorrhagic shock have been shown to preserve endothelial structural integrity and to improve outcome, despite no change in neutrophil influx into tissue (Eversole, 1993; Fleckenstein et al., 1991).
Inhibition of Polymorphonuclear Neutrophil Adherence and Activation
Indirect evidence for the role of neutrophils as a source of free radicals and deleterious mediators in trauma with hemorrhagic shock has been provided by studies that use monoclonal antibodies to inhibit specific adhesion molecules involved in the tethering, adherence, and activation of this cell population. Several studies have shown improved hemodynamic performance, reduced cellular injury, a downregulation of the overall inflammatory response, and improved survival in animals resuscitated from hemorrhagic shock with lactated Ringer's solution plus the R6.5 antibody, a specific inhibitor of intracellular adhesion molecule 1 (Mileski et al., 1990, 1991). This antibody strategy reduced the level of adherence of polymorphonuclear neutrophils in the microcirculation of several organs and prevented the transmigration of activated leukocytes into peripheral tissues (Mileski et al., 1990). The application of these monoclonal antibodies with fluid resuscitation in several other types of trauma confirmed the hemodynamic and cardioprotective effects (Horton et al., 1996).
Nitric Oxide Inhibition
Hemorrhagic shock and resuscitation activate the inducible isoform of nitric oxide synthase (iNOS), and selective inhibition of iNOS provides beneficial effects in several types of circulatory shock. Of particular concern is generation of the highly toxic compound peroxynitrite, which is formed by the interaction of nitric oxide and the superoxide radical. Both pharmacologic approaches and iNOS-knockout animals have confirmed that nitric oxide and peroxynitrite play significant roles in cellular injury and organ dysfunction after fluid resuscitation from hemorrhagic shock. Recent strategies have been directed toward specific scavenging of peroxynitrite in an effort to delay vascular decompensation and to reduce cellular energetic failure in severe hemorrhagic shock. In this regard, MEG is a potent inhibitor of iNOS and effectively scavenges peroxynitrite. MEG added to fluid used for resuscitation from shock diminished the shock-related increase in plasma nitrite/nitrate and 6-keto-prostaglandin F 1-α levels, improved arterial blood pressure, and ablated the vascular hyporeactivity associated with crystalloid resuscitation from shock. Lactated Ringer's solution re-
suscitation from shock has been associated with a significant rise in the levels of nitrotyrosine, an indicator of peroxynitrite formation; nitrotyrosine formation was ablated by MEG treatment of shock (Salzman, 1998). The use of MEG in porcine hemorrhagic models provided similar hemodynamic improvement, reduced lipid peroxidation, improved the mean arterial blood pressure and cardiac index, and significantly improved survival.
A host of other studies that have used a number of iNOS inhibitors have been described. Billiar (1998) has described the use of both the iNOS inhibitor N6-(iminoethyl)-L-lysine in rats and iNOS-knockout mice for studies of hemorrhagic shock. An iNOS deficiency was associated with decreased nuclear factor κB (NFκB) activation and decreased signal transducer and activator of transcription (STAT)-3 activation, reduced IL-6 and granulocyte colony-stimulating factor (GCSF) messenger RNA levels, a reduced level of neutrophil accumulation, and a significantly decreased level of lung and liver cell injury. Similarly, a compound described as NOX (Medidox, San Diego, Calif.) has been shown to efficiently scavenge nitric oxide, reduce the level of lung injury, and decrease the level of neutrophil influx into the lungs with minimal effects on endothelial nitric oxide synthase (eNOS) (Harbrecht, 1998). Although compelling evidence implicates iNOS in the proinflammatory response initiated by fluid resuscitation from hemorrhagic shock, a primary concern regarding nitric oxide blockade is secondary alterations in vascular compensatory mechanisms. In addition, constitutive eNOS is essential to limiting neutrophil adhesion and accumulation in tissue, and preservation of this isoform is essential.
Novel Strategies for Scavenging Free Radicals
Current techniques for confirming increased free-oxygen-radical production in circulatory shock have relied on strategies that inhibited free-radical formation, scavenged excess free radicals, or used transgenic models that were deficient in some component of the oxygen-radical-cellular injury cascade. Recently, spin-trapping nitrones have been used to further define the role of free radicals in experimental shock. This group of compounds inactivates free radicals by forming stable adducts and includes N-tert-phenylbutyl-nitrone, α-4-pyridyl—oxide-N-tert-butyl-nitrone, and 5,5-dimethyl-l-pyrroline-N-oxide. These nitrones have been administered intraperitoneally to adult rats subjected to several types of traumatic or septic shock (Novelli, 1992), and spin probes and electron spin resonance were applied to measure cell membrane stiffness, a well-accepted indicator of peroxidative damage. Membranes obtained from animals subjected to trauma or shock had increased cell membrane rigidity compared to membranes from control animals. However, the administration of the nitrones reduced cell membrane stiffness, producing mitochondrial and microsomal membranes that were typical of those observed in control animals (Novelli, 1992). These studies have confirmed the role of toxic oxygen radicals in shock and
have provided evidence that spin-trapping nitrones may be applicable as a therapeutic tool in models of shock.
Another novel strategy designed to reduce oxygen-radical injury in shock models is melatonin therapy (Shelby, 1998). Melatonin is a neurohormone that plays an important role in several physiologic systems, including regulation of circadian rhythms; however, recent work suggested that melatonin has potent antioxidant activity. The effectiveness of melatonin-mediated detoxification of hydroxyl radicals and reduced lipid peroxidation after shock has been confirmed (Bubenik et al., 1998). Other studies have described that intragastric melatonin administration in shock reduces the number of gastric lesions, attenuates the fall in gastric blood flow, and upregulates other antioxidant systems in the body. Most recently, studies have shown that melatonin prevents activation of the transcription factor NFκB, a factor well recognized to play a pivotal role in the transcription of numerous inflammatory cytokines (Chaudry et al., in press). The administration of melatonin with fluid resuscitation from hemorrhagic shock reduced the total volume of crystalloid required to maintain a stabilized blood pressure, suggesting that this neurohormone may have considerable cytoprotective and antioxidant properties (Shelby, 1998).
Other novel technologies for limiting free-radical-mediated injury after fluid resuscitation from shock have included the development of nitroxides, a group of small, synthetic, metal-free molecules that have been shown to have significant antioxidant and radical-scavenging activity (Krishna and Samuni, 1994; Zhang et al., 1998). Nitroxides act to scavenge the superoxide radical, limiting superoxide-mediated injury to cells and inhibiting the interaction of superoxide with nitric oxide. In addition, nitroxides prevent generation of the hydroxyl radical by their interaction with transition-metal ions (Mohsen et al., 1995), provide significant protection against gut mucosal injury induced by oxidants (Karmeli et al., 1995), and inhibit apoptosis (Slater et al., 1995). The nitroxides provide vascular, interstitial, and intercellular protection, preserve the integrity of the vascular endothelium, inhibit leukocyte-endothelial cell adhesion and leukocyte emigration through the epithelium, and improve survival. Enhanced therapeutic activity of the nitroxides has been accomplished by linking these molecules to a biomacromolecule, providing stable antioxidant compounds. Despite the potential advantage of these antioxidant molecules, the type of macromolecules (for example, albumin, hemoglobin, or starch) has not been resolved; in addition, questions regarding oxygen delivery by the resuscitation regimen and dose-response relationships have not been examined. The polynitroxyl human serum albumin produced by covalently labeling human serum albumin with 40 molar equivalents of the nitroxide 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxyl has been studied extensively with animal models of ischemic reperfusion, stroke, and hemorrhagic shock, and clinical trials are scheduled for 2000 (C.J. Hsia, Synzyme, personal communication, September 13, 1999).
Hormonal Influences
Reports of the apparent preponderance of morbidity and mortality for males compared to those for females for a variety of diseases including sepsis pique one's interest in hormonal influences during hemorrhagic shock. There is a burgeoning literature, primarily from studies with rodents, concerning the importance of the hypothalamic, pituitary, and adrenal axis, especially regarding the immune response to sepsis, and those studies indicate a survival advantage for female animals. There are reports of adverse effects of testosterone and the potential therapeutic effects of compounds with estrogenic effects (in males), such as estradiol, on the heart and immune system after trauma-hemorrhage and resuscitation. Those studies have illuminated the understanding of some of the pathways that influence hormonal changes following trauma, such as prolactin release. These alterations seem to have important effects in stressed animals and possibly in clinical hemorrhagic shock. Less convincing is the suggestion that compounds such as metatropamide or fluoramide might prove helpful in combating the immunosuppression of shock.
Diagnostic Instrumentation
Because 59 million episodes of injuries were reported in the United States in 1995 and unintentional injury and violence account for 30 percent of all lost years of productive life before age 65 (Institute of Medicine, 1999), research into effective immediate treatment is warranted. Although diagnosis historically precedes treatment, research into portable, front-line diagnostic instrumentation capable of being operated by the far-forward line soldier who will then determine therapy is probably unrealistic. Such technology is far more applicable to the early-care facility. Research into protective equipment would be far more applicable to the far-forward situation. On the other hand, research into the mechanisms of neuropeptide, endogenous opioid, and endocrine activation and into the concept of ''internal armor'' is more appropriate. Such interventions as cocktails for free-radical scavenging might be given orally immediately prior to battle (i.e., as a prophylaxis) or as part of a resuscitation fluid (i.e., as a therapeutic) to be infused after injury has occurred. Perhaps antioxidants or nitric oxide scavengers that are given prophylactically or as a component of a first resuscitation fluid might lead to an oral medication or fluid additive to ameliorate neural cell injury after trauma.
Tolerance
Perhaps the most interesting, possibly the most promising, and certainly the most challenging approach might be to render the soldier globally less vulnerable to the effects of hemorrhagic shock, either prophylactically or at some point
after an injury has occurred. The classic examples might involve reducing metabolic demand through induced hypothermia or induction of a hibernation state. Although study of the former might provide insights that lead to therapeutic strategies, it is unlikely that induction of hypothermia per se will be practical in any battlefield setting. The possibility of a pharmacologically induced state of "hibernation" in which the organism might be less susceptible to the effects of hypoxia and cell injury is an appealing concept. It is unclear whether naturally occurring animal models of hibernation do afford this protection and, if so, whether it is induced by some circulating factor or by a complex of events.
Recommendations
The committee found that much of the research on hemorrhagic shock has remained focused on hemodynamics or has been directed toward correcting a single biochemical abnormality that accompanies hemorrhage. Such strategies are unlikely to be successful. Rather, novel therapies should be aimed at the metabolic and cellular derangements that accompany traumatic shock. These approaches should take advantage of advances in other related fields (such as ischemia-reperfusion research in specific organs) and should be approached in a systematic manner that involves prophylaxis, immediate intervention, or the development of tolerance to global ischemia. Combinations of novel therapies should be explored, because multiple pathways lead to cell death. Prevention of any component of the shock syndrome's cascading pathologic processes is preferable to treating or attempting to reverse the effects of the syndrome. Correcting the imbalance between O2 supply and tissue demand is highly desirable. Providing a resuscitation fluid with increased O2-carrying capacity represents a potential target to achieve this goal. Numerous oxygen therapeutic agents have been developed, and several are in different stages of clinical trials. Whether a resuscitation fluid that enhances O2-carrying capacity or facilitates O2 delivery would reduce the rate of morbidity or mortality from hemorrhagic shock remains to be demonstrated. Because organ toxicity following hemorrhagic shock results from a complex of interrelated mechanisms that lead to death, it is unlikely that a single drug, vitamin, electrolyte, or other agent would be able to alter organ toxicity significantly. Some markedly altered physiologic states offer protection to cells and organs. Strategies that induce tolerance to hypoxia might improve the survival of patients with shock syndrome.
Recommendation 4.1 Evaluate the applicability of small-volume, stable oxygen (O2)-carrying and O2-facilitating agents that improve and sustain O2 delivery in the wounded subject for 24 to 48 hours.
Recommendation 4.2 Therapeutic agents that target the toxic effects of hypoxic injury (e.g., antioxidants, chelating agents, hormones, and nitric oxide inhibitors) should be studied with animal
models and subsequently in clinical trials. Combinations of several therapeutic agents should also be investigated.
Recommendation 4.3 Mechanisms that may induce tolerance to ischemia and cellular hypoxia (e.g., hibernation, ischemic preconditioning, and hypothermia) should be explored with appropriate preclinical models.
| This page in the original is blank. |



















