A
Data Sources and Methods
In an effort to be comprehensive in addressing the task of reviewing the current policies of the Organ Procurement and Transplantation Network (OPTN) and the potential impact of the Final Rule, the committee explored various data sources in a concerted effort to cast a broad net for the collection and assessment of information. These sources included public input and testimony from federal agencies, professional societies, organizations, and individuals; a review of recent scientific literature; and statistical analyses of over 68,000 records of patient listings for liver transplantation.
In addition to these fairly traditional sources of data, expert liaisons were assembled for the committee to consult with throughout the project (see Box A1). The expert liaisons are people with recognized experience and expertise on the issues before the committee. They provided technical advice and guidance in framing the issues, identifying important sources of information, and ensuring a comprehensive analysis. A summary description of the committee's evidence-gathering method follows.
TESTIMONY AND PUBLIC INPUT
Over the course of the study, the committee requested and received written responses and presentations from organizations and individuals representing many perspectives of organ procurement and transplantation. The committee felt it was important to receive as much input as possible from public groups involved with or seeking involvement in the organ allocation process, as well as from health professional and other organizations. To accomplish this, the committee convened public meetings on March 11 and April 16, 1999, to gather information and hear from groups and individuals. The committee made every effort to include as many groups as possible, given the short time available.
Committee members heard presentations and asked questions to explore the particular issues and unique perspectives that each organization represented. In particular, the committee was interested in hearing of the potential impact of the Final Rule on these respective parties. The organizations and individuals that addressed the committee are listed in Box A-2.
|
BOX A-1 Expert Liaisons Patients and Donor Families Vicki Crosier, National Kidney Foundation Donor Family Council Charlie Fiske, National Transplant Action Committee Pushkal Gang, Johns Hopkins University Robert J. Kelly, Recipient Family Member George Walton, Donor Family Member Bruce Weir, Transplant Recipient International Organization Transplantation Ronald W. Busuttil, University of California at Los Angeles Clive Callender, Howard University Hospital Anthony D'Alessandro, University of Wisconsin Hospital and Clinics Arnold Diethelm, University of Alabama, Birmingham Ronald M. Ferguson, Ohio State University John Fung, University of Pittsburgh William E. Harmon, Children's Hospital, Boston John F. Neylan, Emory University Procurement Carol Beasley, Partnership for Organ Donation James Childress, University of Virginia Rudolph C. Morgan, Organ and Tissue Acquisition Center, San Diego, Calif. Howard Nathan, Gift of Life Transplant Program Robert M. Sade, Medical University of South Carolina Rodney Taylor, National Minority Organ Tissue and Transplant Education Program Charles Thomas, Samaritan Transplant Services, Phoenix, Ariz. Kathy Witmer, University of Washington |
|
BOX A-2 Organizations and Individuals Appearing Before the Committee March 11, 1999 Milton Benjamin, American Society of Transplant Surgeons Vicki Crosier, National Kidney Foundation Donor Family Council Marcia Crosse, U.S. General Accounting Office Beverly Dennis, U.S. Department of Health and Human Services Mike Hall, American Liver Foundation William Harmon, American Society of Transplantation Craig Irwin, National Transplant Action Committee Richard Luskin, Association of Organ Procurement Organizations Robert Merion, Patient Access to Transplantation Coalition William W. Pfaff, United Network for Organ Sharing Bruce Weir, Transplant Recipient International Organization Andrea Zachary, American Society of Histocompatibility and Immunogenetics April 16, 1999 Ronald W. Busuttil, University of California at Los Angeles Clive Callender, Howard University Hospital Ronald M. Ferguson, Ohio State University Jameson Forster, University of Kansas Doug Hanto, University of Cincinnati Robert Higgins, Henry Ford Hospital Mark Joensen, CONSAD Research Corporation Goran Bo Gustaf Klintmalm, Baylor University Medical Center Patrick McCarthy, Kaufman Center for Heart Failure, Cleveland Robert Metzger, Translife, Orlando, Fla. William Minogue, Suburban Hospital, Bethesda, Md. Paulita Narag, Hendrick Medical Center, Abilene, Texas Howard Nathan, Delaware Valley Transplant Program Mary Ann Palumbi, North American Transplant Coordinators Organization William W. Pfaff, United Network for Organ Sharing Timothy L. Pruett, University of Virginia Byers Shaw, University of Nebraska Medical Center Kevin Stump, Mississippi Organ Recover Agency Carlton Young, University of Alabama, Birmingham |
In addition to the participants listed in Box A-2, many other individuals attended and participated in the public meetings, and/or provided written information to the committee. These individuals are listed below:
Other Participants and Contributors
Patricia Adams Bowman Gray School of Medicine
Mike Adcock Patient Access to Transplantation Coalition
Jason Altmire UPMC Health Systems
Denise Alveranga Lifelink Transplant Institute
Bill Applegate American Society of Transplantation
David Benor Department of Health and Human Services
Audrey Bohnengel Ohio Solid Organ Transplantation Consortium
Jodi Chappell American Society of Transplantation
Dolph Chianchiano National Kidney Foundation
Karen Chiccehitto United Network for Organ Sharing
Coralyn Colladay Department of Health and Human Services
Pat Daily United Network for Organ Sharing
Todd Dickerson University of Cincinnati
Isabel Dunst Hogan and Hartson Washington, D.C.
Gail Durant American Society of Transplant Surgeons
Erick Edwards United Network for Organ Sharing
Jon Eiche The Living Bank International
Mary Ellison United Network for Organ Sharing
Lorraine Fishback Department of Health and Human Services
John Ford U.S. House of Representatives Committee on Commerce
Walton Francis Department of Health and Human Services
Robert Goldstein Juvenile Diabetes Foundation
Walter Graham United Network for Organ Sharing
Carol Green U.S. Senate Committee on Health Education, Labor, and Pensions
Pamela Guarrera Transplantation Institute
Ann Harper United Network for Organ Sharing
Baxter Harrington American Society of Minority Health and Transplant Professionals
Russell Hereford Office of Evaluation and Inspections
Roy Hogberg General Accounting Office
Lesly Hollman Bureau of National Affairs
A. J. Hostetler Richmond Times-Dispatch
Melody Hughson Hoffman-LaRoche
Kent Jenkins United Network for Organ Sharing
Linda Jones Lifeline of Ohio
Karen Kennedy Transplant Resource Center of Maryland
Jerry Klepner United Network for Organ Sharing
Lisa Kory Transplant Recipient International Organization
Evan Krisely Patient Access to Transplantation Coalition
Eugene Laska Nathan Kline Institute
Judy LaSov Maryland Patient Advocacy Group
William Lawrence United Network for Organ Sharing
Sue Leffell American Society of Histocompatibility and Immunogenetics
Becky Levin Renal Physicians Association
Pearl Lewis Maryland Patient Advocacy Group
Chris Lu U.S. House of Representatives Government Reform Committee
Michael Manley Alaska Regional Organ Recovery Agency
Mark Marin University of Cincinnati
Mary Mazanec Senator William Frist's Office
Patrick McCarthy Kaufman Center for Heart Failure
Eileen Meier North American Transplant Coordinators Organization
Laura Melkler Associated Press
Behn Miller General Accounting Office
Joshua Miller American Society of Transplant Surgeons
Marlene Mitman American Society of Transplant Surgeons
Joseph Morton Maryland Patient Advocacy Group
Elizabeth Neus Gannett News Service
Jill Nusbaum National Kidney Foundation
Joseph O'Donnell Transplant Resource Center of Maryland
Lazar Palnick University of Pittsburgh
Matthew Piron Transplant Recipient International Organization
Dave Ress Richmond Times-Dispatch
Lisa Rossi University of Pittsburgh
Paul Schwab Association of Organ Procurement Organizations
Timothy Shaver INOVA Fairfax Hospital
Haimi Shiferaw The Blue Sheet
Bernice Steinhardt General Accounting Office
S. John Swanson, III Organ Transplant Service and Consultant to Army Surgeon General for Transplantation
Alice Thurston American Association of Kidney Patients
Sibyl Tilson Congressional Research Service
Jennifer Van Horn U.S. Senate Committee on Health, Education, Labor, and Pensions Subcommittee on Public Health
Cliff VanMeter United Network of Organ Sharing
Angela Vincent National Medical Association
Jim Warren Journal of Transplant News
Lynn Wegman Department of Health and Human Services
Marc Wheat U.S. House of Representatives Committee on Commerce
J. White Department of Health and Human Services
Marlene Whiteman Strategic Alliance Management
Donna Henry Wright United Network for Organ Sharing
Elaine Young Juvenile Diabetes Foundation
Troy Zimmerman National Kidney Foundation
To gain the perspective of people who could not attend the public meetings, a notice was mailed to more than 1,000 professional societies, organizations, and interest groups. The mailing included a one-page description of the study, the committee roster, and a cover letter explaining the committee's purpose for requesting the information. The letter asked those interested to send or fax comments pertinent to the committee's five tasks. The information submitted supplemented the materials obtained by the committee through the literature review, public meetings, and data analyses.
All written materials presented to the committee were reviewed and considered with respect to the five tasks. This material can be examined by the public. The public access files are maintained by the National Research Council Library at 2001 Wisconsin Avenue, N.W., Harris Building, Room HA 152, Washington, DC 20007; tel: (202) 334-3543.
LITERATURE REVIEW
The committee conducted numerous literature searches as part of its effort to be comprehensive. Search terms used included organ donation policy, ethics, organ donation, organ procurement, organ preservation, ischemic time, costs of transplantation, and secondary analyses of existing databases. In addition, many transplant professionals and the expert liaisons provided literature to the committee for review and consideration.
STATISTICAL ANALYSIS
At the committee's request, the United Network for Organ Sharing (UNOS) provided a large amount of data regarding organ-specific allocation policies; waiting list mortality rates; waiting lists from multiple organ procurement organizations (OPOs); citizenship of patients recently added to the waiting lists; survival rates and transplant rates by OPO population size; OPO death rates on the liver waiting list by initial status and status at death; algorithms; and audits regarding classification of recipients.
Analysis of Waiting Time
The statistical development of the model used in this analysis is described by Hedeker and Gibbons (1994). Note that as previously described, the unit of analysis is the patient-day and not the patient. Following Efron (1988) we assume that days within patients are conditionally independent on the prior days as long as the competing risk outcomes of interest (i.e., death or mortality) can only occur on the final day for each subject. Using the terminology of multilevel analysis (Goldstein, 1995) let i denote the level-2 units (OPOs) and let j denote the level-1 units (patient-days within OPOs). Assume that there are i = 1, . . . , N level-2 units (i.e., OPOs) and j = 1, . . . , ni level-1 patient-days nested within each OPO. The ni patient-day measurements include the set of all available measurement days for all patients in OPO i (i.e., ni is the total number of daily measurements in OPO i). Let yij be the value of the nominal variable associated with level-2 unit i and level-1 unit j. In our case, these represent transplant, death, and other and we code the K + 1 response categories as 0, 1, 2.
Adding random effects to the multinomial logistic regression model of Bock (1970), Nerlove and Press (1973), and others, we get that the probability, for a given OPO i, and patient-day j, Yij = k (a response occurs in category k), conditional on β and α, is:
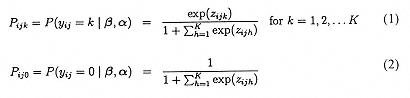
where zijk = χ′ ijβik + w′ ijαk. Here, wij is the p × 1 covariate vector and χij is the design vector for the r random effects, both vectors being for the jth patient-day nested within OPO i. Correspondingly, αk is a p × 1 vector of unknown fixed regression parameters, and βik is a r × 1 vector of unknown random effects for OPO i. The distribution of the random effects is assumed to be multivariate normal with mean vector μk and covariance matrix Σk. Notice that the regression coefficient vectors β and α carry the k subscript. Thus, for each of the p covariates and r random effects, there will be K parameters to be estimated. Additionally, the random effect variance-covariance matrix Σk is allowed to vary with k.
It is convenient to standardize the random effects by letting βik = Τkθi + μk, where ΤkΤk=Σk is the Cholesky decomposition of Σk. The model is now given as
In this form, it is clear that this generalizes Bock's (1972) model for educational test data by including covariates wij, and by allowing a general random-effects design vector xij including the possibility of multiple random effects θi.
Parameter Estimation
Let yi denote the vector of nominal responses from OPO i all ni patient-day measurements nested within. Then the probability of any yi, conditional on the random effects θ and given αk, μk, and Τk, is equal to the product of the probabilities of the patient-day responses:
where dijk = 1 if yij = k, and 0 otherwise. Thus, associated with the response from a particular patient-day, dijk = 1 for only one of the K + 1 categories and zero for all others. The marginal density of the response vector yi in the population is expressed as the following integral of the likelihood, l(·), weighted by the prior density g(·):
where g(θ) represents the population distribution of the random effects.
For parameter estimation, the marginal log-likelihood from the N OPOs can be written as: log L = ΣiN log h(yi). Then, using ηk to represent an arbitrary parameter vector,
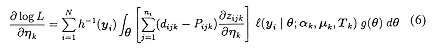
where
Jr is a transformation matrix eliminating elements above the main diagonal (see Magnus, 1988), and ν(Τk) is the vector containing the unique elements of the Cholesky factor Τk. If Τk is a r x 1 vector of independent random effect variance terms, then ![]() zijk /
zijk /![]() Τk = xijθ in the equation above.
Τk = xijθ in the equation above.
Fisher's method of scoring can be used to provide the solution to these likelihood equations. For this, provisional estimates for the vector of parameters Θ, on iteration ι are improved by
where the empirical information matrix is given by:
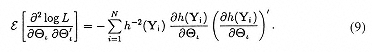
In general, the total number of parameters equals the K x p fixed regression coefficients (αk; k = 1, . . . , K), plus the K x r means of the random effects (μk; k = 1, . . ., K), and the K x r x (r -1)/2 random effect variance-covariance terms (v[Τk]; k = 1, . . ., K). Notice that the parameter vector v(Τk), which indicates the degree of OPO population variance, is what distinguishes the mixed-effects model from the ordinary fixed-effects multinomial logistic regression model.
At convergence, the MML estimates and their accompanying standard errors can be used to construct asymptotic z-statistics by dividing the parameter estimate by its standard error (Wald, 1943). The computed z-statistic can then be compared with the standard normal table to test whether the parameter is significantly different from zero. While this use of the standard errors to perform hypothesis tests (and construct confidence intervals) for the fixed effects μk and αk is generally reasonable, for the variance and covariance components v(Τk) this practice is problematic (see Bryk and Raudenbush, 1992, p. 55).
Numerical Quadrature
In order to solve the above likelihood equations, numerical integration on the transformed θ space can be performed. If the assumed random-effect distribution is normal, Gauss-Hermite quadrature can be used to approximate the above integrals to any practical degree of accuracy. In Gauss-Hermite quadrature, the integration is approximated by a summation on a specified number of quadrature points Q for each dimension of the integration; thus, for the transformed θ space, the summation goes over Qr points. For the standard normal univariate density, optimal points and weights (denoted Bq and A(Bq), respectively) are given in Stroud and Sechrest (1966). For the multivariate density, the r-dimensional vector of quadrature points is denoted by Bq´ = (Bq1, Bq2, . . ., Bqr), with its associated (scalar) weight given by the product of the corresponding univariate weights,
If another distribution is assumed, other points may be chosen and density weights substituted for A(Bq) or A(Bqh) above (note, the weights must be normalized to sum to unity). For example, if a rectangular or uniform distribution is assumed, then Q points may be set at equal intervals over an appropriate range (for each dimension) and the quadrature weights are then set equal to 1/Q. Other distributions are possible; Bock and Aitkin (1981) discussed the possibility of empirically estimating the random-effect distribution.
For models with few random effects the quadrature solution is relatively fast and computationally tractable. In particular, if there is only one random effect in the model (as in the present case), there is only one additional summation over Q points relative to the fixed effects solution. As the number of random effects r is increased, the terms in the summation (Qr) increase exponentially in the quadrature solution. Fortunately, as is noted by Bock, et al., (1988) in the context of a dichotomous factor analysis model, the number of points in each dimension can be reduced as the dimensionality is increased without impairing the accuracy of the approximations; they indicated that for a five-dimensional solution as few as three points per dimension were sufficient to obtain adequate accuracy. In general, specifying between 10 to 20 quadrature points for a unidimensional solution and 7 to 10 points for a two-dimensional solution is usually reasonable.
Hazard Rates and Cumulative Survival
For a model with one random-effect and three categories, we can estimate the probability of each outcome conditional on a particular covariate vector as
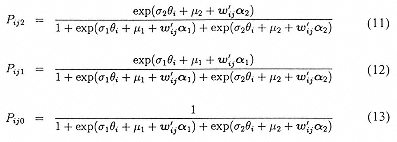
These are referred to as ''subject-specific" probabilities because they indicate response probabilities for particular values of the random subject effect θi (Neuhaus et al., 1991, Zeger et al., 1988). Replacing the parameters with their estimates and denoting the resulting subject-specific probabilities as ![]() , marginal probabilities
, marginal probabilities ![]() are then obtained by integrating over the random-effect distribution, namely
are then obtained by integrating over the random-effect distribution, namely ![]() . Numerical quadrature can be used for this integration as well. These marginal probabilities represent the hazard rate for a particular competing risk of interest (i.e., transplant, mortality or other)
. Numerical quadrature can be used for this integration as well. These marginal probabilities represent the hazard rate for a particular competing risk of interest (i.e., transplant, mortality or other)
expressed as a daily rate for status 1 or monthly rate for status 2B and 3 patients. The cumulative survival rate is then computed by summing the daily risk for status 1, or monthly risk in the case of status 2B and 3, over time adjusting for the number of subjects remaining on the list at that time point (i.e., adjusted for the competing risk).
All computations were performed using the MIXNO program developed under a grant from the National Institute of Mental Health and available at no charge at http://www.uic.edu/labs/biostat/.
ANALYSIS OF COSTS
The General Accounting Office (GAO) provided the committee with data that were instrumental in analyzing the potential effects of the Final Rule on transplantation costs. These included data on costs of solid organ transplantation, transportation costs, and costs of assembling a transplantation team. Roger Evans assisted Institute of Medicine staff and the committee in the analysis of these cost issues.












