| This page in the original is blank. |
INSTITUTE OF MEDICINE
NATIONAL ACADEMY OF SCIENCES
2101 CONSTITUTION AVENUE, N.W. WASHINGTON, DC 20418
February 12, 1999
Maj. General John Parker
Commander U.S. Army Medical Research and Materiel Command
504 Scott Street Fort Detrick, MD 21702-5012
Dear General Parker:
At the request of MAJ Vicky Thomas, MS, RD, Nutrition Staff Officer, Office of the Surgeon General, and LTC Karl Friedl, Ph.D., Program Director, Army Operational Medicine, U.S. Army Medical Research and Materiel Command (USAMRMC) and Grant Officer Representative of USAMRMC for Grant No. DAMD17-94-J-4046 to the National Academy of Sciences for support of the Food and Nutrition Board's (FNB) Committee on Military Nutrition Research (CMNR), members of CMNR met in Washington, D.C. on July 29–31, 1998.
The Office of the Surgeon General (OSG), through USAMRMC, requested that CMNR provide interim guidance on the potential value of supplemental antioxidants for the health and readiness of service members. The questions posed by the OSG related to the value of specific supplements (Vitamins C, E and β-carotene) administered proactively to protect individuals against hazards in the military environment which may not be typical of exposures in the general U.S. population. This issue was to be addressed with a one day workshop (including presentations from outside experts, as appropriate) and a rapid turnaround letter report conveying the committee's conclusions and recommendations.
Further, the USAMRMC was aware of the ongoing activity of a FNB panel currently working to develop Dietary Reference Intakes (DRIs) for dietary antioxidants and related compounds which has the broader objective of defining nutrient intakes to promote health and decrease risk of chronic disease in the general population. Thus, in the Task Statement, USAMRMC limited its request to concerns for individuals in the military environment, which may not be typical of exposures in the general population. The CMNR was not to address whether supplements of vitamins E, C, and β-carotene may protect service members against chronic disease but was to consider the short-term antioxidant effects that may be only tenuously linked to chronic disease.
The CMNR was particularly pleased to have Lt. Gen. Ronald Blanck, Surgeon General of the U.S. Army attend the workshop, and provide further elaboration of his interest and that of the Medical Command in promoting the health of military personnel and their dependents beyond the traditional role of providing medical treatment services.
The primary concern of the OSG, and thus the focus of the Task Statement, is the evaluation of potential oxidative stress exposures of military personnel which are believed to be high as a consequence of some occupational-specific demands (e.g., high intensity work) and working environments (e.g., exposures to air pollutants during deployments to some parts of the world). The consequences of such exposures may include acute effects on suppression of immune function indices and possibly increased susceptibility to infectious disease and/or reduced capacity to respond to immunizations, as discussed in the CMNR report Military Strategies for Sustainment of Nutrition and Immune Function in the Field (IOM, 1999). Physical capacity may be diminished through oxidative stress effects, at least in the specific case of work at altitude (Nutritional Needs in Cold and High-Altitude Environments , IOM, 1996). Long-term health consequences, including progression of neurodegenerative diseases, may result from low level toxins, radio frequencies, and psychological stress exposures; these have been postulated to operate through oxidative stress mechanisms. Given the many suspected links between oxidative stress and health and performance, the CMNR was asked to determine if it would be prudent to provide supplements of β-carotene, vitamin C, and vitamin E to service members rather than wait for conclusive research results. The decision to supplement would depend, in part, on what risks are known to be associated with high intakes of these substances, and on the strength of the evidence that these may be of any benefit to service members, whether through antioxidant properties or through other effects. Specifically, the CMNR was requested to address the following three key questions:
- What is the strength of the evidence to suggest that oxidative stress is a concern for service members during extremes of physical activity and other stresses encountered in training and operations?
- What is the strength of the evidence that vitamin C, vitamin E, and/or β-carotene are likely to protect health and performance of service members exposed to multiple environmental stresses during military training and operations (e.g. severe air pollution in some urban environments; radiation hazards to crew at altitude; radio frequency radiation hazards on ships and around communications facilities; lung and tissue blast overpressure effects and physical and psychological stresses in extreme training courses such as Ranger training and USMC crucible training)?
- Is there evidence of any health risk associated with supplementing intakes of vitamin C, vitamin E, and β-carotene by service members, with the intention of maintaining health and performance in adverse military training and operational environments?
A workshop developed to assist the CMNR in a review of the state of scientific knowledge to address these issues was held immediately following another FNB sponsored workshop that examined issues related to the selection of appropriate biomarkers for assessing nutritional status of the general population in relation to dietary antioxidant and related compounds, and critical adverse effects that might be used to determine tolerable upper intake levels of vitamins E, C, the carotenoids and selenium. Thus the CMNR was able to obtain much additional information on the antioxidant compounds of specific interest to the Surgeon General.
This report has two parts. Part I is this letter that contains the conclusions and general recommendations of the CMNR. Part II includes two attachments. Attachment A is the answers to the specific questions posed by the Army representatives with pertinent references. Attachment B contains the workshop agenda, list of speakers, and abstracts of the presentations.
This report of the CMNR has been reviewed in accordance with National Research Council guidelines by a separate scientific review panel whose membership is listed in Attachment A. The report is based on executive session discussions by the Committee and is a thoughtfully developed presentation incorporating the scientific opinion of the CMNR and the comments of the peer review panel.
Conclusions and Recommendations
The CMNR commends the Surgeon General for promoting efforts to move military medicine into a preventive mode rather than focusing almost entirely on treatment concerns. There are aspects of military service that may enhance overall health compared with that of the general public. The two most notable aspects are required exercise relating to maintaining physical fitness, and maintenance of desirable weight as specified by the weight [body composition] standards.
It is also quite clear that proactive lifestyle changes have by far the greatest potential for improving the overall health of military personnel and their dependents beyond any benefits that might be identified from supplemental antioxidant use. Estimates of the major health benefits that accompany lifestyle changes include: (1) cessation of smoking: a 50–70% reduced risk of heart disease; (2) the reduction of blood cholesterol from 220 to 198: a 20–30 % reduced risk of heart disease; and (3) pharmocologic therapy for diastolic blood pressures over 90 mm Hg - a 16% reduction in risk of heart disease and a 42 % lower risk of stroke. Moderation of alcohol intake, adequate exercise, maintenance of a desirable weight, and consuming diets consistent with the Dietary Guidelines for Americans (USDA, 1995) have all been shown to provide substantial benefits greater than any potential value of supplemental antioxidants. (Hennekens, 1998; DHHS, 1991).
The CMNR presents the following conclusions and recommendations to the Surgeon General, and the U.S. Army Medical Research and Materiel Command regarding the benefits and risks of supplementing military personnel with vitamins C, E and β-carotene for protection from militarily unique situations of oxidative stress.
Conclusions
Information presented at this meeting, in earlier CMNR reports, and other scientific literature provide evidence that military service leads to exposure to unique oxidative stresses that may have adverse health consequences. Some of these stresses are reasonably well characterized, such as those associated with strenuous exercise, work in extremes of environmental temperatures, and at altitude. Much less is known about other sources of oxidative stress, such as radiofrequency and microwave radiation hazards, exposure to blast overpressure, and psychological stress related to extreme training courses or deployment .
The multiple occupational stresses associated with military tasks often occur together in deployment situations. These stresses may have more severe effects as food intake becomes more limited; thus, those individuals with both diminished food intake and high stress would be the most likely target population. Among them, those exposed to these adverse conditions for extended periods of time might be the most vulnerable.
Military personnel living on military bases and those in deployment situations have access to diets that are formulated according to the Military Recommended Dietary Allowances (MRDA). The MRDAs provide guidance for recommended daily intakes of nutrients, based on the National Research Council's Recommended Dietary Allowances (NRC, 1980). Studies of military personnel living in garrison reveal satisfactory dietary intakes of vitamin C (averaging 2x the MRDA). Vitamin E intakes measured in four studies indicated that in three of the locations evaluated, median intakes were equal to or greater than the MRDA of 10 mg. However, one study of Army Rangers at Fort Stewart indicated a median vitamin E intake of only 6.9 mg/ day (Corey Baker Fulco, U.S. Army Research Institute of Environmental Medicine, personal communication, July 17, 1998).
Military rations formulated in accordance with the MRDAs provide nutrients in amounts consistent with meeting nutrient needs—including the antioxidant nutrients—when these rations are consumed at levels required to maintain body weight in the usual range of physical activity for military task requirements. There is little evidence that supplementation with vitamins C, E or with β-carotene in normal conditions (i.e. in garrison) would enhance overall health.
In contrast to garrison situations, the CMNR recognizes that there are circumstances where the various environmental and emotional stresses of military operations may result in a tendency to reduce ration intake. Studies carried out in field training exercises consistently demonstrate that caloric intake is reduced to approximately 75% of troops' usual intakes. In basic training, negative energy balance and weight loss also occur, probably as a result of both decreased energy intake and greatly increased energy expenditure. Such situations may be associated with intakes of vitamins C, E, and β-carotene (vitamin A) below recommended levels. In the report, Not Eating Enough (IOM, 1995), the CMNR proposed establishing a Food Doctrine with suggestions of how to minimize the underconsumption of rations in the field in order to reduce the impact of these environmental factors on food intake and consequent overall nutrient intake. The committee recognizes there may be some benefit to enhancing body reserves of these nutrients prior to deployment but at current levels of reported consumption, body stores have not been evaluated.
There is little evidence currently available to indicate that supplementation of vitamins C and E and β-carotene would be beneficial in protecting against short term, acute oxidative stress. In addition, the use of antioxidant compounds to minimize this stress is not without risk .
Trials reviewed by the committee in which supplemental β-carotene was given to smokers appeared to increase the risk of lung cancer. These data are especially relevant since a large segment of the military population still engages in smoking. Recent studies from the National Institutes of Health (NIH) indicate that daily intakes of 100 mg of vitamin C saturated circulating immune cells and was reflective of other body tissues. Furthermore, as intake exceeded 100 mg per day, bioavailability decreased and above 500 mg, the entire absorbed dose was excreted. Thus there appears to be little benefit in intakes of vitamin C above 100 mg. In fact, the value of intake levels adequate to provide saturation of tissues has not been clarified. There is also some evidence that high levels of vitamin E (400 to 800 rag/day) inhibit platelet aggregation, and when taken with non-steroidal anti-inflammatory medication can result in excessive bleeding which would be a particular concern for injured or wounded personnel. Data which the committee considered important in making recommendations are detailed in Part II, Attachment A of this report.
Recommendations
Based on the review and discussion of information presented at the July 29–31, 1998 meeting, recent scientific literature, and previous reports prepared by CMNR, the committee recommends that:
- Effective methods of promoting lifestyle changes as outlined in Diet and Health (NRC, 1989), Healthy People 2000 (DHHS, 1991) and Healthy People
- 2010 (in draft) be developed as these have the greatest potential of maintaining health and performance of military personnel and their dependents, particularly in view of the introductory comments of Lt. General Blanck concerning the transition of military medicine to a health promotion emphasis.
- Aggressive educational efforts be directed to military personnel engaged in operations of various intensities and in stressful environments on the importance of striving to maintain food intakes consistent with physical demands and energy requirements to avoid excessive weight loss.
- Emphasis be placed on meeting the recommendations of the Dietary Guidelines for Americans (USDA/DHHS, 1995) rather than supplementing with individual nutrients.
- Supplementation should not be considered except in specific high stress situations where intake is likely to be markedly inadequate. If supplementation is determined to be necessary, however, data on the benefits of doses exceeding 100 mg/day of vitamin C and 50 mg/day of vitamin E as alpha tocopherol are not definitive and need to be confirmed. Supplementation of β-carotene for military personnel is NOT recommended at this time.
- As study results become available in trials of the interrelationships of vitamin E and vitamin C to muscle soreness and to immunological function, these recommendations should be reviewed again.
Future Research Considerations
The CMNR believes that the military services, through their pool of volunteer personnel, have an excellent and often unique opportunity to generate statistics about nutrition, health, and well-being of service personnel that can be directly applied toward improved health of both military personnel and the general U.S. population. Research on the following topics is recommended.
- Research focused on the protective effects of antioxidants against acute oxidative stress is strongly encouraged as information is most lacking in this area.
- Validation of a battery of biomarkers for detecting oxidative tissue damage in human subjects in ambulatory or field situations.
- Evaluation of the extent to which the presence of tissue oxidative damage impacts performance.
- The extent and duration of oxidative stress that might be associated with hyperoxia, prolonged exposure to ionizing radiation, radiofrequency, blast overpressure, and psychological stress.
- Supplementation of vitamins C, E and β-carotene in a controlled, randomized way so that their true efficacy in decreasing oxidative tissue damage based on validated biomarkers can be determined. This research is essential both with respect to optimizing health and performance of personnel, and optimizing cost effectiveness.
The CMNR is pleased to provide this review as part of the Committee's continuing response to the U.S. Army Medical Research and Materiel Command. The Committee always welcomes comments and suggestions from you or your staff regarding how these reports can better serve the Army.
John E. Vanderveen, Ph.D. (Chair)
Lawrence E. Armstrong, Ph.D.
William R. Beisel, M.D
Gail E. Butterfield, Ph.D., R.D.
Wanda Chenoweth, Ph.D., R.D.
Johanna T. Dwyer, D.Sc., R.D.
John D. Fernstrom, Ph.D.
Robin B. Kanarek, Ph.D.
Orville A. Levander, Ph.D.
Esther M. Sternberg, M.D.
Douglas W. Wilmore, M.D.
References
DHHS (Department of Health and Human Services). 1991. Healthy People 2000: National Health Promotion and Disease Prevention Objectives. DHHS Publication No. (PHS) 91-50213. Washington, D.C.: U.S. Government Printing Office.
Hennekens, C.H. 1998. Antioxidant Vitamins: Current and Future Directions. Presented at IOM Workshop: Antioxidants and the Effects of Oxidative Stress in Military Personnel. July 30, 1998. Washington, D.C.
IOM (Institute of Medicine). 1993. Nutritional Needs in Hot Environments. B. Marriott (ed.). Washington, D.C.: National Academy Press.
IOM. 1995. Not Eating Enough. B. Marriott (ed.). Washington D.C.: National Academy Press
IOM. 1996. Nutritional Needs in Cold and High Altitude Environments. B. Marriott and S.J. Carlson (eds.). Washington, D.C. National Academy Press.
IOM. 1999. Military Strategies for Sustainment of Nutrition and Immune Function in the Field. Washington, D.C: National Academy Press (in press)
NRC (National Research Council. 1980. Recommended Dietary Allowances, 9th Edition. Washington D.C.: National Academy Press
NRC (National Research Council). 1989. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, D.C.: National Academy Press.
USDA (U.S. Department of Agriculture) and DHHS. 1995. Nutrition and Your Health: Dietary Guidelines for Americans, Fourth Edition. Home and Garden Bulletin No. 232. Washington, D.C.: U.S. Government Printing Office.
A Answers to the Three Specific Questions Presented to the CMNR by Army Representatives
The responses to the three specific questions posed to the CMNR are:
1. What is the strength of the evidence to suggest that oxidative stress is a concern for service members during extremes of physical activity and other stresses encountered in military training or in the field?
Oxidative stress is a pathophysiological process in which the balance between pro-oxidants and antioxidants is shifted toward pro-oxidants. Strenuous physical exercise is associated with increased production of reactive oxygen species (pro-oxidants) in various tissues as well as a decline in levels of antioxidants (Sen, 1995; Kanter, 1998). The impact of this shift on health and physical performance is not well defined. However, the relatively consistent finding of an increase in antioxidant enzyme activity in the tissues of trained subjects (Alessio and Goldfarb, 1988; Hameren et al, 1993) suggests a protective adaptation to the habitual stress of exercise. Furthermore, it has been demonstrated that one of the most sensitive markers for exercise-induced oxidative stress, blood glutathione oxidation, returns to normal levels within 24 hr post-exercise in adequately nourished individuals (Sen et al, 1994).
Exercise is a daily part of military life. Depending on the intensity of the exercise, oxidative stress may become a factor. Routine conditioning provides general health benefits that may include some enhancement of ability to deal with subsequent oxidative stress. Although military assignments generally do not involve this type of stress, some duty assignments do involve extremes of exercise which can increase oxidative stress. Examples of military scenarios which would likely result in oxidative stresses severe enough to overwhelm the antioxidant defense mechanisms include battle operations, Ranger/Seals training, basic inductee training, and mobilization of reservists into active duty assignments.
To determine the strength of the scientific evidence that oxidative stress is a concern for military personnel during extremes of physical activity, the following issues must be considered: (1) Evidence that prolonged or high-intensity exercise bouts increase muscle-tissue free radicals, muscle tissue nitric oxide (NO, a powerful oxidant), conversion of xanthine dehydrogenase to xanthine oxidase, and conversion of glutathione (GSH) to glutathione disulfide (GS-SG); (2) Evidence that these forms of exercise simultaneously decrease tissue/blood levels of antioxidants, resulting in increased oxidation products and decreased protective biological antioxidants that could potentially lead to a situation of unopposed oxidative damage; (3) Evidence in laboratory animals and in vitro cell culture systems that such pro-oxidants cause lipid peroxidation, protein oxidation, and leukocyte DNA damage; (4) The availability and validity of biomarkers for detecting oxidative tissue damage in human subjects in ambulatory or field situations is not clear. No consensus exists regarding the overall value or accuracy of any of the markers currently used as indicators of total body or individual tissue levels of oxidative stress or damage. Biomarkers in breath, blood, urine, and biopsy specimens have been studied. Biomarkers in breath are easy to collect, especially in field conditions; these include measurements of pentane and aldehydes. Pentane analyses may have some value although standardization under various conditions needs to be improved. Biomarkers in blood include plasma lipid peroxides, glutathione, and xanthine oxidase. Urine biomarkers include malondialdehyde, 4-hydroxynonenal, 8-hydroxydeoxyguanosine and F2-isoprostanes (Seis, 1986; Janero, 1990; Collins et al., 1996; Patrono and Fitzgerald, 1997). Different markers and timing of samples provide different results. For this reason, a battery of markers might be valuable if such markers can be validated.
There is evidence that in addition to strenuous physical activity during basic combat training, special forces training, or field operations, military personnel may also be exposed to other oxidative stresses when carrying out these physical activities in both hot and cold environmental temperature extremes, and at altitude. These additional oxidative stresses include hypoxia and extensive exposure to high intensity UV radiation from sunlight, and light reflection from snow or sand (Askew, 1995; Clarkson, 1993; Simon-Schnass, 1996). Strenuous activity under these conditions is frequently accompanied by reduced food intake, thus further reducing the availability of antioxidant nutrients (IOM, 1995).
A number of studies have been conducted to determine the effects of vitamin E supplementation on endurance exercise performance. In two studies of trained swimmers, swimming speed was not affected by vitamin E supplementation (Lawrence et al., 1975; Sharman et al., 1976). Physical performance of racing cyclists was also not improved by supplementation of 330 mg/day vitamin E for five months although serum creatine kinase and serum levels of malondialdehyde were significantly reduced in the vitamin E supplemented group (Rokitzki et al., 1994). Effects of vitamin E on tissue damage and physical performance has been studied in mountain climbers.
Prolonged exposure to physical exertion at high altitudes resulted in significantly increased breath pentane output and decreased physical performance as measured by a decrease in anaerobic threshold in unsupplemented climbers compared to climbers supplemented with 400 mg/day of vitamin E (Simon-Schnass and Pabst, 1988)
Another study, involving eight adult males, investigated the effect of vitamin E supplementation on DNA damage of peripheral white blood cells following a single bout of exhaustive exercise. Short term vitamin E supplementation (800 mg given at 12 and 2 hours pre- and 22 hr post-exercise) reduced DNA strand breakage associated with strenuous exercise. When subjects were supplemented with 1200 mg vitamin E/day for 14 days prior to exercise, DNA damage was significantly reduced (Hartman et al., 1995)
Several studies indicated some benefit of supplemental vitamin C in acclimation to work in hot environments, but Clarkson (1993) has questioned whether similar results would be found in better-nourished populations than the South African mine workers who were the subjects of those studies. Reynolds (1996), in an interpretive review of the effects of cold and altitude on vitamin and mineral requirements, concluded that there is a lack of research in these situations to establish a need for these nutrients above current recommended dietary allowances.
Musculoskeletal injuries have a greater impact on health and readiness of the U.S. Army than any other medical complaint during peacetime or combat. Reynolds et al. (1994) reported that these types of injuries (i.e., sprains, strains, and musculoskeletal pain) resulted in limited duty periods on average of 16.7 days, 3.0 days and 2.8 days per injury respectively. However, very little research has been done to determine if performance decrements are associated with the observed increases in markers of oxidative damage following strenuous exercise. Jakeman and Maxwell (1993) measured muscle contractile function using maximum voluntary contractions (MVC) and tetanic stimulation before and after eccentric exercise, and for 7 days during recovery. Their data suggested that prior supplementation with vitamin C, but not vitamin E (400mg/d for 21 days prior to exercise), may exert a protective effect against muscle damage induced by eccentric exercise. Francis and Hoobler (1986) also found no benefit of vitamin E in improving muscle soreness, range of motion, or peak muscle torque following exhaustive eccentric exercise. Using biomarkers of oxidative stress, Meydani et al. (1993) found a protective effect of vitamin E (800 mg/day for 48 days prior to exercise) on exercise-induced oxidative damage in both young and older adults, but did not examine any functional measures of muscle damage or performance indicators.
Additional research is needed to determine supplementation effects on performance decrements, timing of supplementation prior to exposure, level of supplementation, and duration of supplementation.
2. What is the strength of the evidence that Vitamin C, Vitamin E and/or β-carotene are likely to protect health and performance of service members exposed to multiple environmental stresses during military operations (e.g. severe air pollution in some urban environments; radiation hazards to air crews at altitude; radiofrequency radiation hazards on ships and around communications facilities; lung and tissue effects of blast overpressure; and physical and psychological stresses in extreme training courses such as Ranger training and USMC crucible training)?
The occupational hazards that military personnel encounter are heterogeneous. Many situations experienced by military personnel may increase oxidative stress. In addition to exercise, military duty includes various forms of stress likely to increase risk of oxidative exposure and potential injury in the following categories:
- Physical exposures: hypoxia, vibration, blast overpressure, lasers, G-forces, hyperoxia, weightlessness, microwaves, UV radiation, nuclear radiation, and continuous physical duty;
- Chemical exposures: cigarette smoking, toxic chemicals, and air/water pollution;
- Physiological conditions: undernutrition, sleep deprivation, immune activation associated with trauma, perfusion/reperfusion injury, wound healing, inflammation, and infectious illness; and
- Psychological: separation from family members, novel environments, fear/anxiety-provoking situations (e.g., dangerous mission assignments), role conflicts, threat of attack, and constant performance evaluation.
Some of these risks can be reduced or prevented by occupational health measures (protection from UV light exposure, from blast overpressure etc.). Others are innately related to military tasks. Moreover, the multiple occupational stresses associated with military tasks often occur together in conflict-related duty, and these stresses may have more severe effects as food becomes more limited. Thus, those individuals with both diminished food intake and high stress are the most likely target population for supplementation. Among them, those who are exposed to these adverse conditions for extended periods of time might be most vulnerable, although the scientific evidence is not as well defined for these other types of stress as for extreme exercise.
Figure 1 presents a theoretical scale describing the various situations which could occur among military personnel with respect to adequacy of food intake and exposure to environmental stresses which may lead to or be associated with acute oxidative stress. The shaded area under the curve represents where the combination of stressors and reduced intake could lead to inadequate antioxidant intake. Military personnel exposed to multiple stresses in situations of restricted food intake (e.g. special operations in a combat theater) would be at greatest
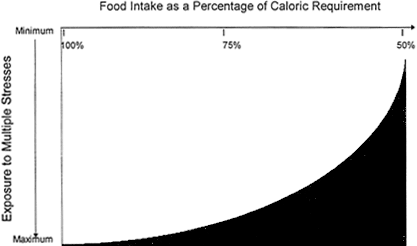
FIGURE 1
Conceptual relationship between food intake, stress exposure, and the potential need for supplemental antioxidants. Individuals with the lowest intakes and greatest stress exposure would be most likely to benefit from supplementation as indicated by the shaded area under the curve.
risk, and thus could potentially benefit from supplementation, while those meeting their caloric requirements in situations of minimum stress (e. g. routine duties in garrison) would be at minimal risk.
Under normal circumstances in garrison the members of the armed services have access to and consume a diet based on the Military Recommended Dietary Allowances (MRDA). Studies of military personnel living on and off base reveal generally satisfactory dietary intakes of vitamins C, but a summary of data from six intake surveys at different military installations indicated that in all but one survey, median vitamin A/β-carotene intakes were below the MRDA. Median vitamin E intakes were equal to or slightly above the MRDA of 10 mg in 3 of 4 studies, but the 4th study which involved Rangers indicated median vitamin E intakes of only 6.9 mg per day (Corey Baker-Fulco, USARIEM, July 17, 1998, personal communication). Little evidence exists that supplementation in non-stress conditions would enhance overall health, however.
In contrast to garrison situations, studies carried out during field training exercises consistently demonstrate that caloric intake is reduced to approximately 75% or less of troops' usual intakes (IOM, 1993; 1995). Several published military studies have documented immune dysregulation and suppression coupled with increased infections in soldiers participating in the Special Forces Assessment Schools (SFAS) and Ranger Training (Moore et al., 1992; Shippee et al., 1994; Bernton et al., 1995). Negative energy balance and
weight loss occurs in these training situations, as well as in basic combat training, probably as a result of both decreased energy intake and greatly increased energy expenditure. Such situations may be associated with intakes of vitamin C, E, and β-carotene (as precursor of vitamin A) below recommended levels. Information about serum or tissue vitamin levels under these training and/or combat conditions is more limited.
In a two week study of Special Forces personnel, a treatment drink providing approximately 15 mg β-carotene, 400 mg α-tocopherol, 500 mg ascorbic acid and 100 mcg of selenium per day was compared to a placebo control. Serum levels of E and C were maintained, whereas on the placebo, serum vitamin E levels were maintained but ascorbic acid levels declined; β-carotene changes were not reported. In that study, antioxidant supplementation minimized immune dysfunction as measured by lymphocyte proliferation, but had little effect on delayed skin test hypersensitivity.
Preliminary unpublished data from a second study by the same investigators using a novel nutritional supplement containing these vitamins, as well as zinc, copper, structured lipid (triglycerides synthesized from a mixture of medium- and long-chain fatty acids) and other nutrients indicated more positive immune effects, including reduced anergy, increased proliferation after mitogen stimulation, and reduced incidence of upper respiratory infections in those receiving the supplement (Wood et al. Ross Laboratories, personal communication, July 30, 1998). Unfortunately, use of such a complex supplement precludes attributing benefit to the individual antioxidant compounds, but underscores the fact that it may be the interactions among nutrients that provide beneficial effects.
High altitude and cold stress (which increases energy output) have been studied in limited circumstances. The CMNR has recently reviewed these studies in depth (IOM, 1996). Although questions have been raised about increased needs for vitamin C and other nutrients in cold and high altitude environments, with the possible exception of vitamin E, there is little scientific basis at this time to indicate that cold or altitude exposures change the nutritional requirements for any vitamins or minerals. The MRDAs supply liberal amounts of these nutrients and should meet these needs. Although preliminary studies of increasing vitamin E intakes to 400 mg α-tocopherol per day showed promise in providing protective effects at high altitude, additional research is needed before questions regarding efficacy and effective doses are fully addressed and before implementing a supplement policy. Preliminary results of studies conducted at the Marine Mountain Warfare Training Center indicate a positive effect of a mixture of antioxidants (C, E, β-carotene, zinc, and selenium) in reducing oxidative stress as measured by breath pentane, whereas vitamins C, E, and β-carotene administered individually had no effect (E. Wayne Askew, Utah State University, personal communication, July 30, 1998).
Another source of stress relatively unique to military personnel is that of blast overpressure. Blast overpressure is the abrupt, rapid rise in atmospheric pressure resulting from explosive detonation, firing of large caliber weapons or
accidental occupational explosions. This shock wave of air causes physical damage, mostly to the hollow organs such as ear, lung, and intestinal tract, but can also damage solid organs. The amount of damage is proportional to the peak pressure generated by the blast. The biochemical tissue damage has been shown to be a result of free radical reactions leading to oxidative stress as evidenced by increases in plasma lipid peroxidation products (thiobarbituric acid reactive substances (TBARS) and conjugated dienes), coupled with declines in blood levels of vitamins C, E, and glutathione (Elsayed et al., 1997, Elsayed, 1997). Significant changes were noted one hour following exposure, with tissue damage continuing to increase up to 24 hr after exposure. In vitro studies of lung and blood tissue removed from animals exposed to blast overpressure demonstrated that tissue levels of tocopherol declined rapidly and immediately, while tissue levels of glutathione and ascorbic acid did not show significant decline until 60 minutes post-exposure. However, addition of ascorbic acid or glutathione to the tissue medium actually increased tissue damage. Thus there is evidence that providing supplemental antioxidants after exposure may be detrimental. Preliminary data were presented to the committee from a follow-up study by these same investigators that provided pharmacological doses of either vitamin E (800 mg as α-tocopherol), vitamin C (1000 mg) or lipoic acid (25 mg) daily to rats for three days preceding blast exposure. The preliminary data showed a protective effect of vitamin E supplementation at this level prior to exposure, but vitamin C was not protective (Nabil Elsayed, Walter Reed Army Institute for Research, personal communication, July 30, 1998).
Microwave and radiofrequency wave exposure is another potential source of oxidative stress for military personnel. Military uses of microwaves include communications, radar and ultra-wide band (UWB) detection, signal jamming, electronic disabling, physical disruption (high power microwave systems) and Extremely Low Frequency (ELF) which includes electrical power main frequencies of 50–60 hertz. In humans, maximum absorption of microwaves occurs between 50 and 150 megahertz, which is the radiofrequency range. There is evidence from a wide variety of studies that link exposure to radiofrequency (electromagnetic fields) to increases in oxidative stress indicated by increased levels of nitric oxide and peroxynitrite, altered calcium metabolism, decreases in melatonin levels, and increased DNA strand breaks in (rat) brain tissue. Several recent epidemiological studies have indicated a link between occupational exposure to electromagnetic fields and increased risk of neurodegenerative disease, both Alzheimer's and ALS (Sobel et al., 1995; 1996; Gunnarsson et al., 1992; Davanipour et al., 1997). A recent study with Air Force personnel demonstrated an increase in risk of brain tumors from occupational exposure to electromagnetic radiation (Grayson, 1996). There is no information available concerning the potential of supplemental antioxidants to alleviate or protect against these effects. This is an area where much additional research is needed.
Military research on occupational exposure to hazardous materials (hydraulic fluid, rocket motor propellants, etc.) has been done as a part of occupational health programs. Risks of exposure to chemical and biological
warfare agents have increased as well as the potential for environmental warfare as was seen in the Persian Gulf. Deployments have become more frequent, and often into heavily polluted environments. Ozone and nitrogen dioxide are present in high concentrations in heavily polluted environments and can initiate free radical reactions that lead to lung damage. Studies in humans have shown protective effects of vitamin E against pollution damage. A study of 12 adult subjects evaluated the effects of daily vitamin E supplementation (600 mg) on red blood cell susceptibility to ozone-related free radical damage. Vitamin E significantly protected red blood cells at the highest levels of exposure, but not at lower levels of ozone exposure (Calabrese et al., 1985). However two other studies of Los Angeles residents exposed to photochemical smog showed no protective effect of vitamin E against short-term ozone exposure (Posin et al., 1979; Hackney et al., 1981). A triservice medical research and development program has begun to address some of these threats through a deployment toxicology research initiative, but no data are currently available in terms of nutritional strategies that would serve as protective mechanisms.
No data were available to assess the role of antioxidants in situations of hyperoxia, increased exposure to ionizing radiation, radiofrequency, UV light, and psychological stress. Studies of military personnel at high risk owing to their occupational duties are needed.
In conclusion, the limited data available suggest that supplementation for individuals exposed to multiple-stress situations associated with diminished food intake (e.g. Lower right hand shaded area of Figure 1) may be beneficial in maintaining intakes of antioxidants at or above the MRDA for those nutrients. An outcome monitoring component should be included as part of the overall health and environmental exposure activities since clear-cut evidence for efficacy of higher doses is not established.
3. Is there evidence of any health risk associated with supplemental intakes of Vitamin C, Vitamin E, or β-carotene by service members, taken with the intention of maintaining health and performance in adverse military training and operational environments?
The occurrence of adverse effects from consumption of large doses of vitamins C, E, and β-carotene appears to be low, however supplementation of these antioxidants is not without risk.
Vitamin C
Vitamin C intake in the range of 250–500 mg is very unlikely to cause adverse effects in the overwhelming majority of the population (Bendich, 1997). Possible exceptions would be individuals who form oxalates, and thus are prone to kidney stone formation (Chalmers et al., 1986).
A number of studies have been conducted on the effect of vitamin C intake on urinary oxalate excretion (Briggs, 1976; Hatch et al., 1980; Hughes et al., 1981; Fituri et al., 1983; Tsao et al., 1984). Daily doses have ranged from 1 to 10 grams. Findings have been conflicting, with some studies reporting no effects and others reporting large increases in urinary oxalate. Conflicting results may be explained in part by methodological problems in oxalate measurement since the presence of vitamin C in the urine interferes with urinary oxalate assays. No evidence clearly linking kidney stone formation to excess vitamin C intake was found (Curhan et al., 1996).
Other individuals who may be at risk include those who have iron storage diseases such as hereditary hemochromatosis or severe thalassemias. However the scientific basis for risk in these latter groups has not been firmly established (Bendich and Cohen, 1990). In the case of hereditary hemochromatosis it seems logical that increased consumption of vitamin C might increase the absorption of iron, but data are lacking on what the extent and impact of such an increase would be. Unfortunately the clinical manifestations of the disease are often not detected until middle age. In the case of severe thalassemias such as β-Thalassemia Major most of the increase in body iron is derived from repeated transfusions over a number of years and thus increased iron absorption is not considered to be the main cause of the iron overload. In vitro studies have shown that Vitamin C reduces unbound ferric iron, which in turn generates hydroxyl free radicals (Elsayed, 1997). However, virtually all iron in biological systems is in a bound state and not susceptible to this type of reaction.
There may be other potential risks. Results of one study suggested that 3 g of vitamin C per day for 6 days reduced high-altitude resistance in normal adults (Schrauzer et al., 1975). Other studies have indicated evidence of systemic conditioning (the accelerated metabolism of ascorbic acid) following abrupt discontinuation of prolonged, high dose vitamin C supplementation (Omaye et al., 1986, Schrauzer and Rhead, 1973; Tsao and Leung, 1988).
Recent research conducted at the National Institutes of Health (NIH) indicated that daily intakes of 100 mg of vitamin C saturated circulating immune cells and that this is reflective of other tissues in the body. Bioavailability of oral doses of vitamin C up to 200 mg was found to be 80%. Above that level, bioavailability declined rapidly. Consumption of vitamin C above 500 mg results in steady state conditions, the entire absorbed dose being excreted (Levine et al., 1996).
Although supplementation of up to 500 mg of vitamin C per day appears to pose little risk, data also indicate there is little benefit in providing supplements in excess of the 100 mg per day required for tissue and enzyme system saturation.
Vitamin E
Widely varying doses of vitamin E have been evaluated in numerous studies. In the α-Tocopherol, β-Carotene Cancer Prevention studies (ATBC Study Group, 1994) where subjects were given 50 mg tocopherol equivalents daily for 5 to 8 years, there was an increased risk of hemorrhagic stroke in the groups receiving vitamin E. The age of these subjects ranged from 50 to 69 yr. which is, on average, older than that of the military population. In a smaller study reported by Steiner et al. (1995), patients at high risk of stroke (as shown by transient ischemic attacks (TIA) and other transient neurologic deficits which often predate the occurrence of major strokes) were given 400 mg vitamin E plus aspirin for 2 years. Hemorrhagic stroke occurred in 2 of the 50 patients receiving vitamin E plus aspirin, while none were reported for those receiving aspirin only, although according to the investigator, this was not of statistical significance. Nevertheless it should be pointed out that the study population was selected on the basis of a tendency to form small blood clots in that they all had TIA and were therefore not likely to be classified as bleeders.
Individuals being treated with coumarin or Warfarin drugs have been reported to be at increased risk of hemorrhage if taking high doses of vitamin E (Corrigan and Ulfers, 1981). Jandak et al (1988) reported a significant reduction in platelet adhesion in healthy adults given 400 mg of d-α-tocopherol per day for 2 wk. Similar adverse effects have not been reported in other studies in healthy subjects given supplements of vitamin E as high as 800 mg to 1200 mg per day (Stampfer et al., 1988; Kitagawa and Mino, 1989; Kim and White, 1996).
Thus, supplements of vitamin E may pose a minor risk for the military population. Individuals routinely ingesting non-steroidal anti-inflammatory drugs such as aspirin, and anticoagulants may be at risk if supplemented with more than 50 mg of vitamin E.
Data supplied to the committee from Fort Hood, Texas, which houses 41,500 active duty soldiers, indicated that in 1997 44 % of soldiers were taking anti-inflammatory drugs, primarily ibuprofen, naprosyn, piroxicam, and aspirin, and that this estimate was considered to be a very conservative one (MAJ Vicky Thomas, Office of the Surgeon General, personal communication, September 22, 1998). Additional research is needed to define better any potential risk of vitamin E supplementation for those individuals who may be routinely consuming non-steroidal inflammatory drugs or anticoagulants. Concerns with respect to prolonged clotting times would be magnified in battlefield situations.
Beta Carotene
Carotenoids are widely distributed in fruits and vegetables with β-carotene being the most prevalent. For many years nutritionists have regarded high intakes of β-carotene as being innocuous and having no adverse effects other
than causing skin discoloration. More recently, there have been a number of adverse effects of high levels of β-carotene reported in the scientific literature that would be of concern to military personnel.
Several reports have addressed the occurrence of amenorrhea associated with high intakes of β-carotene from fruits and vegetables (Duester et al., 1986; Frumar et al., 1979; Kemmann et al., 1983). Another study reported an interaction of ethanol with β-carotene causing delayed blood clearance of ethanol and enhanced hepatotoxicity (Leo et al., 1992).
Two recent prospective intervention trials designed to test the hypothesis that β-carotene might have a favorable chemopreventive effect against lung cancer in smokers revealed unexpected results (ATBC Study Group, 1994; Omenn et al., 1996a, b). In the ATBC trial, with a total of 29,133 male smokers, participants receiving β-carotene (20 mg per day alone, or in combination with 50mg of α-tocopherol) had an 18% increase in lung cancer and an 8% increase in mortality. In the Carotene and Retinol Efficacy Trial (CARET) (Omenn et al., 1996a, b) involving 18, 314 smokers and asbestos workers, 30 mg of β-carotene plus 25000 IU of retinol per day increased lung cancer incidence by 28% and mortality by 17%. However, in the Physicians Health Study, which included both smokers and non-smokers, no adverse effects of β-carotene were observed, nor were any benefits observed (Hennekens et al., 1996). In one long term study, Chinese subjects who were deficient in vitamin A received approximately 15 mg β-carotene daily, risk of stomach cancer decreased with no evidence of adverse effects (Blot et al., 1993). Several shorter studies with 25-90 mg of β-carotene per day showed positive effects in treating oral pre-malignant lesions, with no adverse side effects (Stich et al., 1988; Garewal et al., 1995).
The evidence suggests there may be an adverse effect of high doses of supplemental β-carotene in current heavy smokers and asbestos-exposed populations. Very recent data obtained using ferrets has demonstrated a plausible mechanism of β-carotene interaction with tobacco smoke that enhances lung tumor development (Wang et. al., 1999).
In view of these data, the committee does not recommend supplementation of β-carotene, even in high stress situations, especially since self-report data from the 1995 DoD World-wide Health Survey indicates that across all services (Army, Navy, Air Force, and Marines) 34% of males and 28% of females are smokers. Broken down by age group, 41% of males and 31% of females in the 18-25 age group are smokers. In addition, in the 18-25 age group (again by self-report which probably under-reports true incidence), 28.1% of males and 7.7% of females are heavy alcohol drinkers (defined as consuming 5 or more drinks in one day, at least one day per week).
It would appear that significant improvements in health status of military personnel could best be achieved through the promotion of healthy life-style changes.
References
Alessio, H.M. and Goldfarb, A.H. 1988. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J. Appl. Physiol. 64:1333–1336.
Askew, E.W. 1995. Environmental and physical stress and nutrient requirements. Am. J. Clin. Nutr. 61(suppl):631S–637S.
ATBC (α-Tocopherol, β-carotene Cancer Prevention Study Group). 1994. The effect of vitamin E and β-carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330:1029–1035.
Bendich, A. 1997. Vitamin C safety in humans. Pp. 367–379 in Vitamin C in Health and Disease. L. Packer and J. Fuchs (eds.). New York: Marcel Dekker, Inc.
Bendich, A. and Cohen, M. 1990. Ascorbic acid: Analysis of factors affecting iron absorption. Toxicology Lett.51:189–201
Bernton, E., D. Hoover, R. Galloway, and K. Popp. 1995, Adaptation to chronic stress in military trainees. Ann. NY. Acad. Sci. 774:217–231.
Blot, W.J., J-Y. Li, P. R. Taylor, W. Guo, S. Dawsey, G.-Q. Wang, C.S. Yang, S.-F. Zheng, M. Gail, G.-Y. Li, Y. Yu, B.-Q. Lin, J. Tangrea, Y.-H. Sun, F. Liu, J.F. Fraumani, Jr., Y.-H. Zhang, and B. Li. 1993. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 85:1483–1491.
Briggs, M. 1976. Letter: Vitamin C-induced hyperoxaluria. Lancet I:154.
Calabrese, E.J., J. Victor, and M.A. Stoddard. 1985. Influence of dietary vitamin E on susceptibility to ozone exposure. Bull. Environ. Contam. Toxicol. 34:417–422.
Chalmers, A.H., D.M. Crowley and J.M. Brown. 1986. A possible etiological role for ascorbate in calculi formation. Clin. Chem. 32:333.
Clarkson, P.M. 1993. The effect of exercise and heat on vitamin requirements. In Nutritional Needs in Hot Environments pp.137–171. B. Marriott (ed.). Washington, D.C.: National Academy Press.
Collins, A.R., M. Dusinska, C.M. Gedik, and R. Stetina. 1996. Oxidative Damage to DNA: do we have a reliable biomarker. Environ. Health. Perspect. 104:465–469.
Corrigan, J.J., Jr., and L.L. Ulfers. 1981. Effect of vitamin E on prothrombin levels in warfarin-induced vitamin K deficiency. Am. J. Clin. Nutr. 34:1701–1705.
Curhan, G.C., W.C. Willett, E.B. Rimm and M.J. Stampfer. 1996. A prospective study of the intakes of vitamins C and B6 , and the risk of kidney stones in men. J. Urol. 155:1847–1851.
Davanipour, Z., E. Sobel, J.D. Bowman, Z. Qian, A.D. Will. 1997. Amyotrophic lateral sclerosis and occupational exposure to electromagnetic fields. Bioelectromagnetics 18:28–35.
Deuster, P.A., S.B. Kyle, P.B. Moser, R.A. Vigersky, A. Singh, E.B. Schoomaker. 1986. Nutritional intakes and status of highly trained amenorrheic and eumenorrheic women runners. Fertil. Steril. 46: 636–643.
DHRS (Department of Health and Human Services). 1991. Healthy People 2000: National Health Promotion and Disease Prevention Objectives. DHHS Publication No. (PHS) 91-50213. Washington, D.C.: U.S. Government Printing Office.
Elsayed, N.M., N.V. Gorbunov, V.E. Kagan. 1997. A proposed biochemical mechanism involving hemoglobin for blast overpressure-induced injury. Toxicol. 121:81–90.
Elsayed, N.M. 1997. Toxicology of blast overpressure. Toxicol. 121:1–15.
Fituri, N., N. Allawi, M. Bentley, and J. Costello. 1983. Urinary plasma oxalate during ingestion of pure ascorbic acid: A re-evaluation. Eur. Urol. 9:312–315.
Francis, K.T. and T. Hoobler. 1986. Failure of vitamin E and delayed muscle soreness. Alabama Medicine, J. of MASA. March, 1986 15–18.
Frumar, A.M., D.R. Meldrum, and H.L. Judd. 1979. Hypercarotenemia in hypothalamic amenorrhea. Fert. Steril. 32:261–264.
Garewal, H., F. Meyskens, R.V. Katz, 1995. β-carotene produces sustained remissions in oral leukoplakia: results of a 1 year randomized, controlled trial. Proc. Am. Soc. Clin. Oncol. (Abstract) 14:496.
Grayson, J.K. 1996. Radiation exposure, socioeconomic status and brain tumor risk in the U.S. Air Force: A nested case control study. Am. J. Epidemiol 143:480–486.
Gunnarsson, L.G., L. Bodin, B. Sφderfeldt, O. Axelson. 1992. A case-control study of motor neuron disease: Its relation to heritability, and occupational exposures, particularly solvents. Br. J. Ind. Med. 49:791–798.
Hackney, J.D. W.S. Linn, R.D. Buckley, M.P. Jones, L.H. Wightman, S.K. Karuza, R.L.
Blesseg, and H.J. Hislop. 1981. Vitamin E supplementation and respiratory effects of ozone in humans. J. Toxicol. Environ. Health. 7:383–390
Hatch, M., S. Mulgrew, E. Bourke, B. Keogh, and J. Costello. 1980. Effect of megadoses of ascorbic acid on serum and urinary oxalate. Eur. Urol. 6:166–169.
Hammeren, J., S. Powers, J. Lawler, D. Criswell, D. Lowenthal, and M. Pollack. 1993. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity. Internat. J. Sports Med. 13: 412–416.
Hartmann, A., A.M. Nieb, M. Grunert-Fuchs, B. Poch, and G.Speit. 1995. Vitamin E prevents exercise-induced DNA damage. Mutation Res. 346:195–202.
Hennekens, C.H. 1998. Antioxidant Vitamins: Current and Future Directions. Presented at IOM Workshop: Antioxidants and the Effects of Oxidative Stress in Military Personnel. July 30, 1998. Washington, D.C.
Hennekens, C.H., J.E. Buring, J.E. Manson, M. Stampfer, B. Rosner, N.R. Cook, C. Belanger, F. LaMotte, J.M. Gaziano, P.M. Ridker, W. Willett, and R. Peto. 1996. Lack of effect of long-term supplementation of beta carotene on the incidence of malignant neoplasms and cardiovascular disease. New Engl. J. Med. 334:1145–1149.
Hornig, D.H., U. Moser, B.E. Glatthaar. 1988. Ascorbic acid. Pp. 417–435 in Modern Nutrition in Health and Disease. 7th Edition. M.E. Shils and V.R. Young (eds.). Philadelphia: Lea & Febiger.
Hughes, C. S. Dutton, A.S. Truswell. 1981. High intakes of ascorbic acid and urinary oxalate. J. Hum. Nutr. 35:274.
IOM (Institute of Medicine). 1993. Nutritional Needs in Hot Environments. B. Marriott (ed.). Washington, D.C.: National Academy Press.
IOM. 1995. Not Eating Enough. B. Marriott (ed.). Washington D.C.: National Academy Press
IOM. 1998. Military Strategies for Sustainment of Nutrition and Immune Function in the Field. Washington, D.C: National Academy Press
IOM. 1996. Nutritional Needs in Cold and High Altitude Environments. B. Marriott and S.J. Carlson (eds.). Washington, D.C. National Academy Press.
Jacob, R.A. 1994. Vitamin C. Pp. 432–448 in Modern Nutrition in Health and Disease. 8th Edition. M.E. Shils, J. A. Olson, and M. Shike (eds.) Philadelphia, PA: Lea & Febiger.
Jakeman, P. and S. Maxwell. 1993. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur. J. Appl. Physiol. 67:426–430
Jandak, J., M. Steiner, and P.D. Richardson. 1988. Reduction of platelet adhesiveness by vitamin E supplementation in humans. Thromb. Res. 49:393–404.
Janero, D.R. 1990. Malondialdehyde and thiobarturic acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue damage. Free Radic. Biol. Med. 9:515–540.
Kantner, M. 1998. Free radicals, exercise and antioxidant supplementation. Proc. Nutr. Soc. 57:9–13.
Kemmann, E., S.A. Pasquale, and R.Skaf. 1983. Amenorrhea associated with carotenemia. J. Am. Med. Assoc. 249:926–929.
Kim, J.M., and R.H. White. 1996. Effect of vitamin E on the anticoagulant response to warfarin. Am. J. Cardiol. 77:545–546.
Kitagawa, M. and M. Mino. 1989. Effects of elevated d-alpha-tocopherol dosages in man. J. Nutr. Sci. Vitaminol. (Tokyo) 35:133–142.
Lawrence, J.D., R.C. Bower, W.P.Riehl, and J.L. Smith. 1975. Effects of alpha-tocopherol acetate on the swimming endurance of trained swimmers. Am. J. Clin. Nutr. 28:205–208.
Leo, M.A., C. Kim, N. Lowe, and C.S. Lieber. 1992. Interaction of ethanol with beta-carotene: delayed blood clearance and enhanced hepatotoxicity. Hepatology 15:883–891.
Levine, M., C. Conry-Cantilena, Y. Wang, R.W. Welch, P.W. Washko, K.R. Dhariwal, J.B. Park, A. Lazarev, J.F. Graumlich, J. King, and L.R. Cantilena. 1996. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 93:3704–3709.
Meydani, M., W.J. Evans, G. Handelman, L. Biddle, R.A. Fielding, S.N. Meydani, J. Burrill, M.A. Fiatarone, J.B. Blumburg, and J.G. Cannon. 1993. Protective effect of vitamin E on exercise-induced oxidative damage in young and older adults. Am. J. Physiol. 264:R992–R998.
Moore, R.J., K.E. Friedl, T.R. Kramer, L.E. Martinez-Lopez, R.W. Hoyt, R.T. Tulley, J.P. DeLany, E.W. Askew, and J.A. Vogel. 1992. Changes in soldier nutritional status and immune function during the Ranger training course . Technical Report T13–92. Natick, MA: U.S. Army Research Institute of Environmental Medicine.
NRC (National Research Council). 1989. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, D.C.: National Academy Press.
Omaye, S.T., J.H. Skala, R.A. Jacob. 1986. Plasma ascorbic acid in adult males: Effects of depletion and supplementation. Am. J. Clin. Nutr. 44:257–264.
Omenn, G.S., G.E. Goodman, M.D. Thornquist, J. Balmes, M.R. Cullen, A. Glass, J.P. Keogh, F.L. Meyskens, B. Valanis, J.H. Williams, S. Barnhart, and S. Hammar. 1996(a). Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New Engl. J. Med. 334:1150–1155.
Omenn, G.S., G.E. Goodman, M.D. Thorriquist, J. Balmes, M.R. Cullen, A. Glass, J.P. Keogh, F.L. Meyskens, B. Valanis, J.H. Williams, S. Barnhart, and S. Hammar. 1996(b). Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial . J. Natl. Cancer Inst. 88:1550–1559.
Patrono, C., and G.A. Fitzgerald. 1997. Isoprostanes: potential markers of oxidative stress in artherothrombotic disease. Arterioscler. Thromb. Vasc. Biol. 17: 2309–2315.
Posin, C.I., K.W. Clark, M/P/ Jones, R.D. Buckley, and J.D. Hackney. 1979. Human biochemical response to ozone and vitamin E. J. Toxicol. Environ. Health. V: 1049–1058.
Reynolds, R.D. 1996. Effects of cold and altitude on vitamin and mineral requirements. Pp. 215–244 in Nutritional Needs in Cold and High Altitude Environments B.M. Marriott and S.J. Carlson, eds.. IOM. Washington D.C.: National Academy Press.
Reynolds, K.L., H. A. Heckel, C.E. Witt, J.N. Martin, J.A. Pollard, J.J. Knapick, and B.H. Jones. 1994. Cigarette smoking, physical fitness, and injuries in infantry soldiers. Am. J. Prev. Med. 10:145–150.
Rokitzki, L., E. Logemann, G. Huber, E. Keck, and J. Keul. 1994. Alpha-tocopherol supplementation in racing cyclists during extreme endurance training. Int. J. Sports Nutr. 4:253–264.
Schrauzer, G.N., D. Ishmael, and G.W. Kiefer. 1975. Some aspects of current vitamin C usage: Diminished high-altitude resistance following overdosage. Ann. NY Acad. Sci. 258:377–381.
Schrauzer, G.N. and W.J. Rhead. 1973. Ascorbic acid abuse: Effects of long-term ingestion of excessive amounts on blood levels and urinary excretion. Int. J. Vit. Nutr. Res. 43: 201–211.
Seis, H. 1986. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 25:1058–1071.
Sen, C.K., 1995. Oxidants and antioxidants in exercise. J. Appl. Physiol. 79:675–686.
Sen, C.K., M. Atalay, and O. Hanninen. 1994. Exercise induced oxidative stress: Glutathione supplementation and deficiency J. Applied Physiol. 77: 2177–2187.
Sharman, I.M., M.G. Down, and N.G. Norgan. 1976. The effects of vitamin E on physiological function and athletic performance of trained swimmers. J. Sports Med. 16:215–225.
Shippee, R., K. Friedl, T. Kramer, M. Mays, K. Popp, E.W. Askew, B. Fairbrother, R. , J. Vogel, L. Marchitelli, P. Frykman, L. Martinez-Lopez, E. Bernton, M. Kramer, R. Tulley, J. Rood, J. Delany, D. Jezior, and J. Arsenault. 1994. Nutritional and immunological assessment of Ranger students with increased caloric intake. Technical Report T95-5. Natick, MA: U.S. Army Research Institute of Environmental Medicine.
Simon-Schnass, I. 1996. Oxidative stress at high altitudes and effects of vitamin E. Pp. 393–418 in Nutritional Needs in Cold and High Altitude Environments. B. Marriott and S.J. Carlson, eds.. IOM. Washington D.C.: National Academy Press
Simon-Schnass, I. and H. Pabst. 1988. Influence of vitamin E on physical performance. Internat. J. Vit. Nutr. Res. 58:49–54.
Sobel, E., Z. Davanipour, R. Suldava, T. Erkinjuntti, J. Wikstrom, V.W. Henderson, G. Buckwalter, J.D. Bowman, P.S. Lee. 1995. Occupations with exposure to electromagnetic fields: A possible risk factor for Alzheimer's disease. Am. J. Epidemiol. 142:515–524.
Sobel, E., M. Dunn, Z. Davanipour, Z. Qian, H.C. Chui. 1996. Elevated risk of Alzheimer's disease among workers with likely electromagnetic field exposure. Neurology 47:1477–1481.
Stampfer, M.J., J.A. Jakubowski, D. Faigel, R. Vaillancourt, and D. Deykin. 1988. Vitamin E supplementation effect on human platelet function, arachidonic acid metabolism, and plasma prostacyclin levels. Am. J. Clin. Nutr. 47:700–706.
Steiner, M. 1991. Influence of vitamin E on platelet function in humans. J. Am. Coll. Nutr. 10:466–473.
Stich, H.F., M.P. Rosin, P. Hornby, B. Matthew, R. Sankaranarayanan, M.K. Nair. 1988. Remission of oral leukoplakias and micromiclei in tobacco/betel quid chewers treated with β-carotene and with β-carotene plus vitamin A. Int. J. Cancer 42:195
Tsao, C.S. and P.Y. Leung. 1988. Urinary ascorbic acid levels following the withdrawal of large doses of ascorbic acid in guinea pigs. J. Nutr. 118:895–900.
Tsao, C.S., and S.L. Salimi. 1984. Effect of large intake of ascorbic acid on urinary and plasma oxalic acid levels. Int. J. Vit. Nutr. Res. 54:245.
USDA (U.S. Department of Agriculture) and DHHS. 1995. Nutrition and Your Health: Dietary Guidelines for Americans, Fourth Edition. Home and Garden Bulletin No. 232. Washington, D.C.: U.S. Government Printing Office.
Wang, X-D., C. Liu, R.T. Broderick, D.E. Smith, N.I. Krinsky, and R.M. Russell. 1999. Retinoid signaling and activator protein-1 expression in ferrets given β-carotene supplements and exposed to tobacco smoke. J. Nat. Cancer Inst. 91:60–66.
Antioxidants and Oxidative Stress in Military Personnel
July 29–31, 1998
National Academy of Sciences
2101 Constitution Avenue, N.W.
Washington, D.C. 20418
Workshop Participants
CMNR
Robert Nesheim
Salinas, CA
Lawrence E. Armstrong Ph.D.
Professor, Departments of Physiology and Neurobiology, and Exercise Science
Human Performance Laboratory
University of Connecticut
Storrs, CT
William R. Beisel, M.D.
Adjunct Professor, Department of Molecular
Microbiology and Immunology
The Johns Hopkins University School of Hygiene and Public Health
Baltimore, MD
Wanda Chenoweth, Ph.D., R.D.
Professor, Department of Food Science and Human Nutrition
Michigan State University
East Lansing, MI
Robin B. Kanarek, Ph.D.
Professor and Chair of Psychology
Professor of Nutrition
Tufts University
Medford, MA
Orville A. Levander, Ph.D.
Research Leader, Nutrient Requirements and Functions Laboratory
USDA-ARS Beltsville Human Nutrition Research Center
Beltsville, MD
Esther M. Sternberg, M.D.
Chief, Neuroendocrine Immunology and Behavior
National Institute of Mental Health-NIH
Bethesda, MD
John E. Vanderveen, Ph.D.
Rockville, MD
Douglas W. Wilmore, M.D.
Frank Sawyer Professor
Department of Surgery
Brigham and Women's Hospital
Boston, MA
Food and Nutrition Board Liaison
Johanna T. Dwyer, D.Sc., R.D.
Professor, Departments of Medicine and of Community Health
Tufts Medical School and School of Nutrition Science and Policy
Director, Frances Stern Nutrition Ctr., New England Medical Ctr.
Boston, MA
U.S. Army Grant Officer Representative
LTC Karl Friedl, Ph.D.
Program Director
Army Operational Medicine Research
USAMRMC
Fort Detrick, MD
Speakers
Eldon Wayne Askew, Ph.D.
Professor and Director
Division of Foods and Nutrition
University of Utah
Salt Lake City, UT
Lt. Gen. Ronald Blanck
Surgeon General, U.S. Army
Patrick Dunne, Ph.D.
U.S. Army Natick Research, Development, and Engineering Center
U.S. Army Soldier System Command
Natick, MA
Nabil M. Elsayed, Ph. D.
Chief, Pulmonary Biochemistry Section
Department of Respiratory Research
Division of Medicine
Walter Reed Army Institute of Research
Washington, D.C.
Hank Gardner, Ph.D.
Director
USARIEM
Fort Detrick, MD
Charles Hennekens, M.D., Ph.D.
Eugene Braunwald Professor of Medicine
Harvard Medical School
Chief, Div. of Preventive Medicine
Brigham and Womens Hospital
Boston, MA
MAJ William H. Karge, Ph.D.
Nutritional Biochemist
USARIEM
Natick, MA
Susan Taylor Mayne, Ph.D.
Associate Professor in Chronic Disease Epidemiology
Department of Epidemiology and Public Health
Yale University School of Medicine
New Haven, CT
Harold Schmitz, Ph.D.
Group Manager
M&M Mars Company
Hackettstown, NJ
Ronald L. Seaman, Ph.D.
Research Scientist
Microwave Bioeffects Branch,
USARMD
Brooks AFB, TX
Chandan K. Sen, Ph.D.
Staff Scientist
Biological Technologies Section
Lawrence Berkeley National
Laboratory/EETD
University of California
Berkeley, CA
Maret G. Traber, Ph.D.
Principal Investigator,
Linus Pauling Institute
Associate Professor, Dept. of Nutrition and Food Management
Oregon State University
Corvallis, OR
Steven Wood, Ph.D., R.D.
Senior Clinical Project Leader
Ross Products Division
Columbus, OH
FNB Staff
Mary Poos, Ph.D.
Sydne Newberry, Ph.D.
Melissa Van Doren
Allison Yates, Ph.D.




























