PANEL 4
Does our current understanding of the processes that led from chemical to biological evolution place constraints on the size of early organisms?
If size is not constrained, are there chemical signatures that might record the transition to living systems?
Discussion
Summarized by Leslie Orgel, Panel Moderator, and Laura Ost, Consultant
Did Life Originate in an RNA World?
Free-living organisms today require two biopolymers—DNA and RNA, which store and transmit genetic information—as well as proteins, which catalyze chemical reactions. A primitive organism might have relied on a single biopolymer, RNA, which might or might not have catalyzed its own reactions. Such an organism would not have required proteins, ribosomes, and other modem cellular machinery and conceivably could have been the smallest self-sustaining chemical system capable of Darwinian evolution.
If life began with RNA, then it must have started with nucleotides made accidentally in a prebiotic process, but it must also have been "clever enough" to invent the materials needed for the next phase of evolution, Dr. Orgel observed. Panel members offered varying perspectives on how this may have occurred, reflecting not outright disagreements but rather different areas of expertise and interest and perhaps different phases of evolution.
Dr. Ferris suggested that, instead of trying to "downsize a Mercedes into a Yugo," it might be Useful to think in terms of the comic strip B.C.'s round stone with a stick through the center. He proposed that RNA-based life-forms originated from monomers present on the primitive Earth. RNA may have catalyzed its own reactions, and other necessary compounds might have been formed in a variety of ways. Bases formed from hydrogen cyanide in aqueous solution could produce adenine and guanine, and purines could be generated from these two compounds or, alternatively, brought in by meteorites. Formaldehyde can be converted to ribose and many other sugars. Montmorillonite clay acts as a catalyst in making RNA oligomers, which, once they are long enough (perhaps 50 mers), may have catalytic properties.
In Dr. Ferris's concept, the RNA, vesicles, and proteins—not enclosed by a cell membrane—would bind to mineral surfaces. Their shape and dimensions would be determined by the features of the substrate and rates of formation of RNA. These organisms could be as small as some purported nanobacteria, or about twice the size of the Q-beta virus, which contains three genes consisting of about 1,500 bases each. Dr. Ferris concluded that the fossil signature of such RNA-based life-forms would be difficult to identify.
Dr. Szostak proposed that a simple ancestral cell with the capability to evolve into a more complex cell may have started with polynucleotides, which can have catalytic activity, and vesicles, which are spontaneously assembling systems. Once a replicase and vesicle are brought together, a synergistic evolution could build up to produce a megabase of information that leads to a free-living organism. This process sets the stage for peptide synthesis and large-scale structural and regulatory components.
Dr. Szostak observed that evolution is inhibited by the free interaction of replicases in solution. The only way to achieve interesting Darwinian evolution is to have a compartmentalized system that can grow and divide, thus providing a selective advantage for mutations. But how can there be a cell cycle without any internal encoded machinery? Small vesicles, 30 to 100 nm in size, could interact and fuse to generate larger ones that combined different internal molecules. In the laboratory an artificial system can be created in which cells divide, fuse, divide, and fuse. Much larger vesicles can be fragmented with mild shear forces.
Dr. Benner proposed that the minimum cell size would be determined by the robustness of a single-biopolymer system in making the chemical compromise between genetics and catalysis, which pose competing and contradictory demands (e.g., in terms of the biopolymer's complexity, ease of folding, and capability to change physical properties). The problem is that nucleic acids are generally not good catalysts: one must sort through 2 × 1013 random RNA sequences to find one that modestly increases the rate of a templated ligation. Adding functional groups does improve catalytic power and versatility, but it is not clear whether functionalized RNA can sustain Darwinian evolution.
Dr. Benner said that chemical studies attempting to resolve these contradictions will help define life's origins on Earth and how best to find life elsewhere. In the meantime, short of historical context, information content is the only reliable signature of a Darwinian chemical system. A single-biopolymer system must be able not only to replicate but also to evolve. There is evidence that life before proteins had functionalized RNA, so this chemistry should be sought in samples from Mars. He also proposed that a genetic molecule needs a polycharged backbone to exhibit the behavior needed to support Darwinian evolution. Such a chemical structure would be fairly easy to detect on Mars, perhaps robotically.
The First Biopolymer System
A question was raised concerning how the first biopolymer was formed, given that even modern cells must work hard to make highly activated molecules. In fact, as Dr. Orgel noted, this is a matter of dispute within the prebiotic research community. Dr. Ferris said that a primitive process for forming such molecules is plausible, because polymeric phosphates can be made by heating phosphates.
Dr. Fraenkel asked whether prebiotic evolution would have been assisted by high temperatures. Dr. Benner noted that high temperature speeds all reactions, whether desirable or not, and it destroys the secondary structure of nucleic acids. Dr. de Duve noted that some scientists believe that life originated at cold temperatures—below zero degrees centigrade.
Dr. Ferris said that scientists have been looking for a polymer system other than RNA that could have driven early life-forms, but they have failed so far, so RNA remains the best model. Dr. Orgel
noted that some other systems behave much like RNA and DNA but have modified backbones. Peptide nucleic acid, for example, which lacks the components that form the deoxyribosyl-phosphate backbone, may be marginally simpler than RNA. However, it has been difficult to synthesize.
The First Cell Membrane
The issue of encapsulation was revisited by Dr. Orgel, who asked when, on the evolutionary scale, an impermeable cell membrane stopped being a disadvantage and became a necessity. A cell that depends on external resources produced by abiotic processes clearly cannot obtain them if it is surrounded by such a membrane. Similarly, a cell that makes its own metabolites cannot allow them to escape. Dr. de Duve suggested that when encapsulation evolved, it enabled competition between cells instead of molecules.
Dr. Szostak said that permeable membranes might have been formed from short-chain fatty acids or alcohols. A cell with an impermeable membrane would need to be complex enough to both evolve the barter and encode a transport system, perhaps with nucleic acids or peptides serving transport functions. Dr. Benner said that membranes likely to emerge in a primitive environment would contain multiple organic molecules, would be defective, and would be permeable. He suggested phosphorylation as a way of holding resources inside leaky membranes. Dr. Ferris said that nutrient flow might be restricted and noted that no peptides Would have been available in the RNA world. Dr. Osborn postulated that inorganic phosphates inside and outside the cell might reach an equilibrium through a leaky membrane. But she asked whether any intrinsically leaky membranes are known; membranes are not made of fatty acids, but rather from phospholipids.
If a membrane-like structure is observed in a sample, then how big must it be before it can realistically be considered a cell membrane, and does the cell need multiple genes, Dr. Orgel asked. Dr. Szostak suggested that tens or hundreds of genes would be needed; a one-gene cell could not encode transport and would have a leaky membrane. Dr. Fraenkel asked what types of molecules would need to be transported—nucleotide triphosphates? Resources such as carbon dioxide can pass through modem membranes without a transport system, but phosphates cannot. Dr. Benner said that his comments refer to life-forms that are just sophisticated enough to achieve a metastable state—probably the type most likely to be found on Mars. By contrast, Dr. Szostak said that there is no reason to think that the evolution of protein synthesis is difficult.
Time Frame for Evolution of Life on Mars
Earth was formed 4.5 billion years ago, and approximately 1 billion years later bacteria resembling modem cyanobacteria had evolved. When, and for how long, did Mars offer a suitable environment (i.e., water) for evolution? Just as scientists are uncertain about what it takes to create a fully competent organism, so also is the time frame for the aqueous history of Mars subject to debate, although the surface had probably dried out about 3 billion years ago.
Dr. Ferris said that life is unlikely to have survived on the surface of Mars because of the inhospitable environment. Presumably life originated and was shut down quickly. John Rummel said that life may have evolved over a long time period because the massive outflow features on Mars suggest that there may be large quantities of liquid water beneath the permafrost. There may also be subsurface volcanic or hydrothermal activity. Furthermore, as part of the natural cross-contamination between celestial bodies, terrestrial materials bearing viable organisms may have been transported to Mars at a time when water flowed on the surface.
Dr. Benner said that the search for life on the martian surface is a surrogate for the search for life elsewhere. He assumed that life would have emerged on Mars and on Earth at about the same time. One billion years is 10 percent of the life of a star; of the billions and billions of planets, scientists are examining the one planet most readily available. If it took a long time for protein translation to emerge, then martian life-forms might exhibit the primitive character of a single-biopolymer system, but they might have made the transition to a two-polymer system.
Dr. de Duve said it is unlikely that life took a long time to emerge because it involved chemical reactions. He proposed that life arose rapidly, perhaps many times, until it finally was sustained. He disputed the notion of tiny protocells harboring RNA molecules that exhibited both genetic and catalytic activity swimming in a sea of activated nucleotides. The nucleotides would not feed through the cell membrane to enable adenosine triphosphate or guanosine triphosphate to pass through. Although the basic premise of the RNA world may be correct, he said that a complex proto-metabolism was needed that was catalyzed by clays, metals, or peptides instead of RNA molecules, because a catalyst was needed to make the first RNA molecule.
Dr. Szostak agreed that primitive organisms might have evolved quickly, adding that it might have taken just a few years to evolve from a one-gene cell to a free-living organism with perhaps 100 genes.
Minimum Cell Size
Dr. Orgel said that the discussion suggested that a replicating, single-biopolymer system could be compressed into a very small volume just slightly larger than the genome, in contrast to two-biopolymer systems, which must be at least 5 to 10 times the volume of the genome. Dr. Benner suggested that a single-biopolymer system could be packed into a 50-nm sphere. Thus, although a sphere of 50 nm in a terrestrial sample would not represent a life-form, a similar structure in a martian sample would warrant study of the organic chemistry to determine whether it had a genome.
Summary and Consensus
As yet, there is no consensus view of how life originated. There is, however, broad agreement that the first living systems were far simpler than the simplest free-living organisms known today. The concept that life passed through a stage in which RNA, or a polymer much like it, provided both genetic information and catalysis suggests what such a simple organism might have been like. Organisms characterized by such single-bioploymer chemistry could have been minute, perhaps as small as 50 nm in diameter. This means that the minimum size observable in living cells may not be applicable in setting limits for biological detection on Mars or Europa. The earliest organisms on Earth (or elsewhere) would probably be extremely difficult to recognize as fossils.
Primitive Life: Origin, Size, and Signature
James P. Ferris
Department of Chemistry Rensselaer Polytechnic Institute
Abstract
The question of the size of the fast life was brought to the forefront by the proposal that the martian meteorite ALH84001 contains nanometer size fossils of martian life. In this paper estimates of the size of the fast life were made on the basis of the essential requirements for life and research progress toward the understanding of the origin of life. One model for the fast life is based on RNA bound to the mineral that catalyzed the formation of the RNA. The essential life processes, with the exception of the synthesis of monomers, were carried out by the RNA bound to the mineral surface. The size of this life was determined by the size of the mineral surface and the rates of formation and decomposition of the RNA. The second model for the fast life assumes that the RNA and other essential biomolecules were protected from dispersal by a membrane. Here it is assumed that synthesis of monomers took place within the membrane. The size of this life was estimated from the sizes of RNA viruses, and it was concluded that the first life could have been as small as the proposed “nannobacteria."
Introduction
The proposal that the Mars meteorite ALH84001 contained fossils of "nannobacteria" (McKay et al., 1996) prompted, among other discussions, one on the minimal size for life. A point in favor of such small life-forms is that the first life on Earth and Mars would have been much smaller and simpler than the present life on Earth, so comparisons to contemporary cellular life are probably not valid. I will examine the possible size and shape of the first life on Earth and/or Mars. This will be done by reviewing some of the experimental data concerning the pathway to the first life. I will then extrapolate from that data to two different possibilities for the first life and then use these models for life together with the known sizes of the genomes of viruses to estimate the sizes of the fast life. Finally, the possibilities of finding fossil signatures of this life on Earth and Mars will be discussed.
What Is Life?
It will be necessary to provide a definition of the basic requirements of life before it is possible to suggest what constitutes a minimal form of life. What is life? is a controversial scientific question because it is intimately associated with the particular scenario that the scientist is investigating for the origin of life. He/she does not want a definition that would invalidate their paradigm of the origin of life. The definition of life was the topic of a recent paper by Luisi (1998). He provided a brief review of the definitions put forward over the past 100 years and then focused his discussion on recent definitions. The simplest is, "Life is a self-sustaining chemical system undergoing Darwinian evolution." He proposed a modification of this definition for "adherents of the RNA world" that life is "a population of RNA molecules (a quasi-species) which is able to self-replicate and evolve in the process." I will use the modified definition not only because I am one of those "adherents" but also because it provides a useful metric (RNA) for the size of primitive life. As Luisi noted, this definition implies the presence of
an external source of energy and/or reactive nutrients to maintain the life. It also specifies the need for RNA but no other molecular species, but it is likely that some other organics were required.
Many scientists feel that this definition of life is inadequate because it does not require that this first life was protected from the vagaries of the environment on the primitive Earth by a surrounding compartment. This more complicated model of life was defined by Luisi as "a system which is spatially defined by a semipermeable component of its own making and which is self-sustaining by transforming external energy/nutrients by its own process of component production." Here I will also make the assumption that genetic information was also stored in RNA in this model of life. This more elaborate life-form may require additional biomolecules such as proteins for the synthesis of the monomers required for the biopolymers and the membrane.
It should be noted here that other biopolymers are also under consideration as either precursors to the RNA world or alternatives to it. For example, peptides have been synthesized in the laboratory (not by "prebiotic reactions") that are self-replicating by template-directed ligation (Lee et al., 1996; Severin et al., 1997).
A Summary of the Current State of Prebiotic Synthesis of RNA
The basic ingredients required for proposed models of primitive life are RNA, peptides, or proteins and membrane constituents. I recognize that the first life may not have utilized the types of biomolecules present in contemporary life, but at the present time there is very little information as to the possible structures of alternative life so the focus here will be on those molecules where information on possible prebiotic syntheses exists.
RNA
There has been progress in the understanding of the prebiotic synthesis of RNA monomers, but it is generally agreed there is much to be done to establish that these monomers were formed in sufficient amounts on the primitive Earth to lead to the formation of RNA oligomers. The research that has been done and those things that need to be accomplished were summarized (Ferris, 1987), and recent progress is reported by Zubay and coworkers (Zubay, 1994, 1998; Reimann and Zubay, 1999). Studies on a related ribopyranose-based RNA have been reported by Eschenmoser and coworkers (Pitsch et al., 1995).
It has been possible to catalyze the synthesis of RNA oligomers that contain up to 10 monomer units by the montmorillonite-catalyzed condensation of the 5'-phosphorimidazolides of 5'-nucleotides (ImpN; Figure 1; Ertem and Ferris, 1997; Kawamura and Ferris, 1994; Prabahar and Ferris, 1997). The RNAs are linked by 2', 5'- and 3',5'-phosphodiester bonds, pyrophosphate bonds and contain cyclic and linear oligonucleotides. Oligo(A)s as long as 50 mers have been prepared by the stepwise elongation of a decameric primer bound to montmorillonite by the daily addition of ImpA over a period of fourteen days (Figure 2; Ferris et al., 1996). This finding suggests that it may have been possible to form RNAs on mineral surfaces that were long enough to have served as templates for template-directed synthesis (Joyce and Orgel, 1993) and as catalysts for RNA ligation (Szostak and Ellington, 1993).
The replication of RNA, or any other genetic material, was a key process in the first life. It has not been possible to attain the non-enzymatic replication of RNA, but the template-directed synthesis of a complementary RNA has been demonstrated. Oligo(G)s over 40 mers in length are obtained in the template-directed reaction of 2-MeImpG on a poly(C) template (Figure 3; Inoue and Orgel, 1982). A less efficient template-directed synthesis is the formation of >6 mers of oligo(A)s by the reaction of

Figure 1.
The montmorillonite-catalyzed oligomerization of activated mononucleotides.
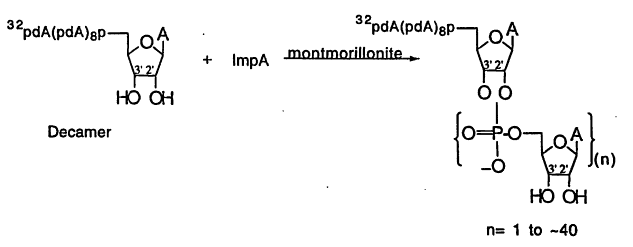
Figure 2.
The montmorillonite-catalyzed elongation of a nucleic acid primer.
ImpA on a poly(U) template in the presence of Pb+2 (Sleeper et al., 1979). It has not been possible to demonstrate the non-enzymatic template-directed synthesis of pyrimidine oligomers on a polypurine nucleotide template or the template-directed synthesis on a heterogeneous template that contains more purine than pyrimidine nucleotides (Haertle and Orgel, 1986; Joyce and Orgel, 1986). It was observed that the heterogeneous RNAs formed in clay-catalyzed reactions, which contain 2'5'- and 3',5'-phosphodiester bonds, pyrophosphate links, and both cyclic and linear oligomers, do serve as templates for the synthesis of the complementary RNAs (Ertem and Ferris, 1997).
Prebiotic Syntheses of Polypeptides
There have been many reports of the prebiotic synthesis of short peptides in aqueous solution (for a brief summary see Liu and Orgel, 1998a), and it has been possible to make those that contain more than
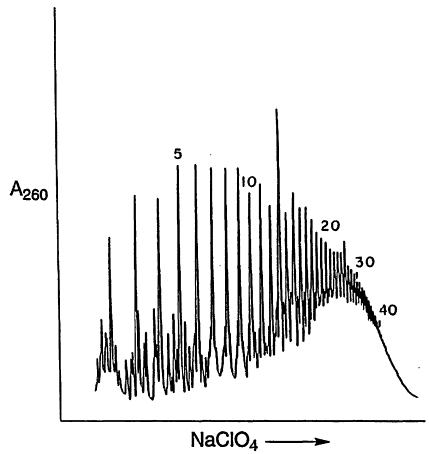
Figure 3.
The template-directed synthesis of oligo(G)s from 2-MeImpG on poly(C) template. Reprinted, by permission, from T. Inoue and L.E. Orgel, The Journal of Molecular Biology (1982). Copyright © 1982 by Academic Press.
10 amino acids on mineral surfaces. Polymerization of the aminoacyladenylates of α-amino acids on montmorillonite yields polypeptides (Katchalsky, 1973; Paecht-Horowitz and Eirich, 1988; and previous papers in this series). Positively and negatively charged α-amino acids and β-amino acids form long chain polypeptides on mineral surfaces when carboxyl activating groups are added to the reaction mixture 20 to 50 times (Figure 4; Hill, Jr., et al., 1998; Liu and Orgel, 1998b). Hydroxyapatite, FeS2, and the clay mineral illite were used in these studies. These findings suggest the possibility of the presence of catalytic polypeptides in the first life on Earth.
Prebiotic Membrane Formation
The formation of bilayer membranes requires the formation of a linear hydrocarbon chain containing greater than 10 to 12 carbon atoms with a charged or polar group on one end of the chain. No reports
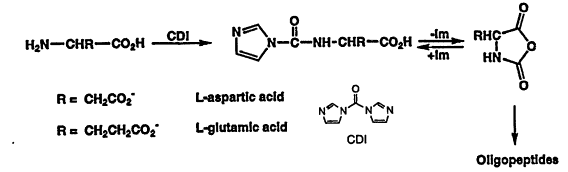
Figure 4.
The elongation of a decameric glutamate and aspartate on hydroxyapatite and illite using carbonyl-diimidazole as the condensing agent. Reprinted, by permission, from Origins of Life and Evolution of the Biosphere by Aubrey Hill, Jr. et al. (1998). Copyright © 1998 by Kluwer Academic Publishers.
of the prebiotic synthesis of these long chains have been issued; however, their synthesis from formic and oxalic acids via the Fisher-Tropsch process in hydrothermal systems may be possible (Ferris, 1992; McCollum et al., 1999). The hydrolysis of esters and anhydrides of fatty acids results in their conversion to fatty acids, which associate into vesicles with diameters that range from 10 to 45 microns (Figure 5) (Walde et al., 1994). The presence of vesicles catalyzes the formation of additional vesicles as the hydrolysis proceeds.
An alternative source of vesicles may have been material brought to the primitive Earth by meteorites. A fraction isolated from the neutral extract of the Murchison meteorite forms vesicles capable of encapsulating a soluble dye during their formation in basic solution (Deamer and Pashley, 1989). The structure of this vesicle-forming material is not known, but it may be a carboxylic acid because these are one of the major constituents of the Murchison meteorite (Cronin et al., 1988)
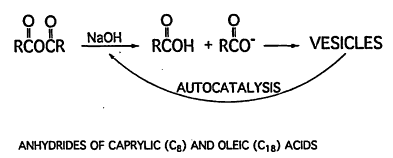
Figure 5.
The formation of vesicles by hydrolysis of carboxylic acid anhydrides with base. The hydrolysis is catalyzed by the vesicles formed.
Size Estimates of the First Life
Life on the Rocks: A Minimal Form of Life
“Life on the rocks," a designation coined by Leslie Orgel, describes a system in which key processes for the formation of the biopolymers of life occur on mineral surfaces. The concept can be extended to a simple living system if the integrity of the life depends on the binding of molecules undergoing synthesis, replication, and mutation to the surface of a mineral assemblage. Such a system does not need to be bounded by a compartment to maintain its integrity.
In an RNA world on the rocks, the mineral-catalyzed synthesis of RNAs would generate RNAs capable of replication and evolution. The RNAs formed initially would have components capable of catalyzing these requisite functions. This would result in the preferential buildup of RNAs that carried out these essential tasks. Those RNAs that became detached from the mineral surface would initiate new centers of life when they became bound to other minerals. As the monomers for life are not synthesized on the mineral, this scenario requires the presence of a proximate source of activated monomers.
In the life on the rocks model there is no compartment surrounding the living system, so its size is determined by the size of the mineral assemblage that catalyzes the formation of the RNAs and the rates of synthesis and decomposition of the RNAs that are key to life processes.
Life Bounded by a Semipermeable Membrane
Life within a semipermeable membrane may be more resistant to changes in the environment, but it will also require the presence of a larger array of biomolecules than life on the rocks (Luisi, 1998). I assume that in the simplest case such life will require RNAs for the larger genome as well as RNAs to catalyze the synthesis of the RNA monomers and RNAs for synthesis and assembly of the semiperme-able membrane (assumed to be constructed from fatty acids). Proteins may also have a role in this minimal life, but I will assume that randomly formed peptides and other biomolecules were adequate for the first simple life because this avoids the need for the translation machinery of protein synthesis.
The level of complexity of primitive life within a semipermeable membrane is comparable to the complexity of contemporary viruses. This is not to claim that the first life was a virus that evolved to a cell but rather that both are simple devices. The virus is able to replicate only with the aid of the biomolecules of a living cell. It has been proposed that viruses preserve a record of macromolecular evolution and may be molecular fossils of the RNA world (Maizels and Weiner, 1993). For an alternative view of both the RNA world and the thesis that RNA viruses contain vestigial RNA see Benner and Ellington (1987). Primitive life probably required the presence of preformed biomolecules that could be appropriated for its own purposes. Consequently, I have chosen virus as a metric for the measurement of the size of the first life. In Table 1 are listed size data on some RNA viruses with simple shapes so that their volume can be calculated assuming they are spheres. The Qβ virus, which has the 3 genes, is able to efficiently pack RNA within its membrane wall while the L-A virus has double-stranded RNA and presumably more protein and other biomolecules than does Qβ.
If it is assumed using Qβ as the model that a single-stand RNA gene has about 1,500 bases, then it is possible to estimate the volume required for a simple life-form with a variety of genes assuming the close packing present in the Qβ virus. One can use the same approach for calculating the radius of a primitive cell with double-stranded RNA and a larger proportion of other molecules by using the L-A virus as the model (Table 2). The radius of the particles based on the RNA content of the Qβ virus is less
Table 1 Dimensions and RNA Content of Some RNA Virusesa
|
|
Base Pairs or Bases |
Radius Inside Capsid (nm)b |
Base Pairs or Bases per nm3 |
|
Double-stranded |
|
|
|
|
Reoviruses |
˜22,000 |
25 |
0.34 |
|
L-A Virus |
4,600 |
19 |
0.16 |
|
Single-stranded |
|
|
|
|
Polio Virus |
6,100 |
10 |
1.5 |
|
Qβ Virus |
4,600 |
8 |
2.1 |
|
a Data from Casjens (1997), Fraenkel-Conrat et al. (1988), and Metzler (1977). b Not counting the phospholipid membrane, which is assumed to be 4 nm thick. |
|||
Table 2 Volume and Radii of Spherical Primitive Life Determined on the Basis of the RNA Packing in Qβ and L-A Virusesa
than one-half that of the L-A virus, and the available volume for RNA and other molecules is larger by the cube of the differences in the radii.
If it was possible to have membrane-bounded life based with a minimum of five genes (ligase, replicase, monomer synthase, fatty acid synthase, and membrane synthase ribozymes) and it had that RNA packing density of Qβ, then one would need a 3,580-nm3 volume in addition to the volume of the surrounding membrane. As noted previously, proteins and other biomolecules were probably present as well. This volume would be 50,000 nm3 if the contents of this simple life were more like the L-A virus.
How do these values correspond to the "nannobacteria" postulated for ALH84001? The tubular structures are said to be 20 to 100 nm in length (McKay et al., 1996). A crude approximation of the dimensions of these "nannobacteria" was made by measuring one of them (shown in Figure 6B of McKay et al., 1996). It is estimated to be 120 nm long and 10 nm in diameter. Correcting for a 4-nm phospholipid membrane layer, the internal dimensions are 112 nm long and 2 nm in diameter. Its internal volume is 350 nm3, assuming it is a cylinder. Since the volume is a function of the square of the radius and the least reliable measure is the diameter of the "nannobacteria" in this photograph, the volume was calculated on the basis of a diameter of 14 nm and therefore an internal diameter of 6 nm. Here the volume is estimated to be 3,170 nm3, a value close to that of 4 to 5 genes packed as they would be in the Qβ virus but only one-third of a gene if it were packed as it is in the L-A virus. The conclusion from this exercise is that primitive life may have been as small as large “nannobacteria” if there was an efficient mechanism for packing its RNA.
There are many concerns that can be raised with this approximation. First, a better estimate of the dimensions of these "nannobacteria" is needed. A small error in the diameter results in a major change in the calculated volume Consequently the compartment size for the first life may have been larger. Third, the genes for primitive life were probably shorter than 1,500 bases so would have required a smaller container. It is assumed that these factors more or less cancel out in these approximations.
The Signature of the First Life
It is unlikely that there will be a direct fossil record of the RNA world on the rocks because its structure is determined by the mineral assemblage to which it is attached. In addition, it is unlikely that this life would be recognized in ancient rock formations on Earth or Mars because it would have left behind few unique signatures. Circumstantial evidence for such life may be found if it has been established that the mineral is an efficient catalyst for the formation of an essential biopolymer. An exhaustive survey of potential mineral catalysts is required before undertaking such a search for these minerals in ancient rock formations. Such a search would be facilitated if there was data that suggested that primitive life provided conditions resulting in the deposition of a mineral that does not form under the usual environmental conditions. This was observed by the formation of apatite containing occluded organics in a banded iron formation in Greenland (Mojizsis et al., 1996). Unfortunately, even if such minerals were discovered they would not be a marker unique for establishing the former presence of life on the rocks since any type of living system may have initiated the formation of the marker crystals (Schopf, this volume, pp. 88-93).
The possibility of detecting primitive compartmentalized life on Mars is much higher than it is on Earth. If life did exist on Mars, the probability of its detection is higher there because it has no history of plate tectonics which would have destroyed most of these fossils by recycling the lithosphere. The principal challenge will be to distinguish these spherical microfossils from other small spherical objects of the same size.
Acknowledgments
Drs. Michael Gaffey, William Hagan, Jr., and Sandra Nierzwicki-Bauer provided helpful comment on an initial draft of this manuscript. The study was supported by NSF grant CHE-9619149, NASA grant NAGS-4557, and NASA NSCORT grant NAGS-7598.
References
Benner, S.A., and A.D. Ellington (1987). The last ribo-organism. Nature 329: 295-296.
Casjens, S. (1997). Principles of virion structure, function and assembly . Pp 3-37 in Structural Biology of Viruses. W. Chiu, R.M. Burnett, and R.L. Garcea (eds.). New York: Oxford University Press.
Cronin, J.R., S. Pizzarello, and D.P. Cruickshank (1988). Organic matter in carbonaceaous chondrites, planetary satellites, asteroids and comets. Pp. 819-857 in Meteorites and the Early Solar System. J.F. Kerridge and M.S. Matthews (eds.). Tuscon, Ariz.: University of Arizona Press.
Deamer, D.W., and R.M. Pashley (1989). Amphiphilic components of the Murchison carbonaceous chondrite: Surface properties and membrane formation. Origins Life Evol. Biosphere 19: 21-38.
Ertem, G., and J.P. Ferris (1997). Template-directed synthesis using the heterogeneous templates produced by montmorillonite catalysis. A possible bridge between the prebiotic and RNA worlds. J. Am. Chem. Soc. 119: 7197-7201.
Ferris, J.P. (1987). Prebiotic synthesis—problems and challenges. Cold Spring Harbor Symposia on Quantitative Biology 52: 29-35.
Ferris, J.P. (1992). Marine hydrothermal systems and the origin of life: Chemical markers of prebiotic chemistry in hydrothermal systems. Origins Life Evol. Biosphere 22: 109-134.
Ferris, J.P., A.R. Hill, Jr., R. Liu, and L.E. Orgel (1996). Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381: 59-61.
Fraenkel-Conrat, H., P.C. Kimball, and J.A. Levy (1988). Virology 2. Englewood Cliffs, N.J.: Prentice-Hall, p. 440.
Haertle, T., and L.E. Orgel (1986). The template properties of some oligodeoxynucleotides containing cytidine and guanosine. J. Mol. Evol. 23: 108-112.
Hill, Jr., A.R., C. Böhler, and L.E. Orgel (1998). Polymerization on the rocks: Negatively-charged a-amino acids. Origins Life Evol. Biosphere 28: 235-243, scheme 1 on p. 236.
Inoue, T., and L.E. Orgel (1982). Oligomerization of (guanosine 5'-phosphor)-2-methylimidazolide on poly(C): An RNA polymerase model. J. Mol. Biol. 162: 201-217, fig. 3(c) on p. 207.
Joyce, G.F., and L.E. Orgel (1986). Non-enzymic template-directed synthesis on RNA random copolymers: poly(C,G) templates. J. Mol. Biol. 188: 433-441.
Joyce, G.F., and L.E. Orgel (1993). Prospects for understanding the origin of the RNA World. Pp. 1-25 in The RNA World. R.F. Gesteland and J.F. Atkins (eds.). Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
Katchalsky, A. (1973). Prebiotic synthesis of biopolymers on inorganic templates. Naturwiss. 60: 215-220.
Kawamura, K., and J.P. Ferris (1994). Kinetic and mechanistic analysis of dinucleotide and oligonucleotide formation from the 5'-phosphorimidazolide of adenosine on Na+-montmorillonite. J. Am. Chem. Soc. 116: 7564-7572.
Lee, D.H., J.R. Granja, J.A. Martinez, K. Severin, and M.R. Ghadiri (1996). A self-replicating peptide. Nature 382: 525-528.
Liu, R., and L.E. Orgel (1998a). Polymerization of b-amino acids in aqueous solution. Origins Life Evol. Biosphere 28: 47-60.
Liu, R., and L.E. Orgel (1998b). Polymerization on the rocks: b-amino acids and arginine. Origins Life Evol. Biosphere 28: 245-257.
Luisi, P.L. (1998). About various definitions of life. Origins Life Evol. Biosphere 28: 613-622.
Maizels, N., and A.M. Weiner (eds.) (1993). The genomic tag hypothesis: Modem viruses as molecular fossils of ancient strategies for genome replication. Pp. 577-602 in The RNA World. Cold Spring Harbor, New York:Cold Spring Harbor Laboratory Press.
McCollum, T.M., G. Ritter, and B.R.T. Simoneit (1999). Lipid synthesis under hydrothermal conditions. Origins Life Evol. Biosphere 29: in press.
McKay, D.S., E.K. Gibson, Jr., K.L. Thomas-Kepra, C.S. Romanek, S.J. Clemett, X.D.F. Chillier, C.R. Maechling, and R.N. Zare (1996). Search for past life on Mars: Possible relic biogenic activity in martian meteorite ALH84001. Science 273: 924-930.
Metzler, D.E. (1977). Biochemistry. New York: Academic Press, p. 1129
Mojizsis, S.J., G. Arrhenius, K.D. McKeegan, T.M. Harrison, A.P. Nutman, and C.R.L. Friend (1996). Evidence for life on Earth before 3,800 million years ago. Nature 384: 55-59.
Paecht-Horowitz, M., and F.R. Eirich (1988). The polymerization of amino acid adenylates on sodium-montmorillonite with preadsorbed peptides. Origins Life Evol. Biosphere 18: 359-387.
Pitsch, S., R. Krishnamurthy, M. Bolli, S. Wendeborn, A. Holzer, M. Minton, C. Lesuer, I. Schloenvogt, B. Jaun, and A. Eschenmoser (1995). Pyranose-RNA('p-RNA'): Base-pairing selectivity and potential to replicate. Helv. Chim. Acta 78: 1621-1635.
Prabahar, K.J., and J.P. Ferris (1997). Adenine derivatives as phosphate-activating groups for the regioselective formation of 3',5'-linked oligoadenylates on montmorillonite: Possible phosphate-activating groups for the prebiotic synthesis of RNA. J. Am. Chem. Soc. 119: 4330-4337.
Reimann, R., and G. Zubay (1999). Nucleoside phosphorylation: A feasible step in the prebiotic pathway to RNA. Origins Life Evol. Biosphere 29: in press.
Severin, K., D.H. Lee, J.A. Martinez, and M.R. Ghadiri (1997). Peptide self-replication via template-directed ligation. Chemistry-A European J. 3: 1017-1024.
Sleeper, H.L., R. Lohrmann, and L.E. Orgel (1979). Temple-directed synthesis of oligoadenylates catalyzed by Pb2+ ions. J. Mol. Evol. 13: 203-214.
Szostak, J.W., and A.D. Ellington (1993). In vitro selection of functional RNA sequences. Pp. 511-533 in The RNA World. R.F. Gesteland and J.F. Atkins (eds.). Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
Walde, P., R. Wick, M. Fresto, A. Mangone, and P.L. Luisi (1994). Autopoietic self-replication of fatty acid vesicles. J. Am. Chem. Soc. 116: 11649-11654.
Zubay, G. (1994). A feasible prebiotic pathway to the purines. Chemtracts-Biochem. Mol. Biol. 5: 179-189.
Zubay, G. (1998). Studies on the lead-catalyzed synthesis of aldopentoses. Origins Life Evol. Biosphere 28: 13-26.
Constraints on the Sizes of the Earliest Cells
Jack W. Szostak
Howard Hughes Medical Institute and Department of Molecular Biology Massachusetts General Hospital
Abstract
Any discussion of constraints on the minimum size of simple, early cells must be based on speculative deductions about the structure of long-extinct ancestral life-forms. I first discuss reasons for thinking that early cells were surrounded by lipid membranes, and then explore the implications of such a structure. The physical properties of membranes are strongly influenced by their degree of curvature, which is related to vesicle size. Small vesicles of 50- to 100-nm diameter have properties which might, under suitable conditions, result in the establishment of a spontaneous cell cycle. Even such small cells could encapsulate a simple genome and cellular metabolism. I conclude that small early cells are a viable possibility. Given present uncertainties, it seems wise to be prepared to detect life-forms of a wide range of sizes.
Introduction: Early Cellular Life
It is important to distinguish between truly early cellular life and the last common ancestor of existing life. The structural and biochemical similarities of all existing branches of life point to a complex cellular structure for the last common ancestor, characterized by a DNA genome encoding at least several hundred and possibly several thousand genes, ribosome-catalyzed protein synthesis using the standard genetic code, membrane-surrounded cells with a wide range of protein transporters, and a complex metabolism supporting sugar, amino acid, nucleotide, cofactor, and lipid biosynthesis based on ATP synthesis from an electrochemical proton gradient (1). Such a cell would clearly have internal mechanisms for the control of basic processes such as cell growth and division. The last common ancestor is not, in its fundamentals, much simpler or even much different from current eubacterial or archaebacterial cells. We must look back much further in time to find simpler evolutionary precursors of such cells, and further still to find structures simple enough to have formed spontaneously by molecular self-assembly, yet complex enough to have evolved into life as we know it.
What might such ancestral forms have looked like? The arguments for early cells with an RNA genome arid ribozymes as catalysts have been made many times (2) and will not be repeated here. However, the results of numerous recent experiments have confirmed the ability of ribozymes to catalyze a wide range of chemical transformations, including peptide and nucleotide synthesis (3-5). Given these results, it does not seem too unreasonable to postulate an intermediate between the earliest cells and the last common ancestor in which coded protein synthesis had not yet evolved, but which had evolved to a level of moderate complexity. Such a cell would be a membrane-bounded compartment containing a nucleic acid (RNA or DNA) genome that Was transcribed to yield several hundred ribozymes that maintained a complex metabolism similar to the primary metabolism of modem cells (6). Perhaps the greatest uncertainty in regard to the plausibility of such a cell is a mechanism of membrane transport in the absence of complex coded proteins. Peptides or polyketides synthesized by sequential enzymatic steps (as cells still do today) may have played a key role in membrane-related processes.
Such a cell is still far too complex to be anything but the result of a long process of Darwinian evolution, starting from a much simpler ancestor. It is the ability of such an ancestral cell to evolve into
more complex structures by Darwinian evolution that places the most severe constraints on its structure. I shall argue that, in addition to a genome that can be replicated with reasonable but not perfect accuracy, some form of compartmentation is required to enable Darwinian evolution. The ability to evolve is what distinguishes systems that are alive in a biologically relevant sense from prebiotic chemical systems and from other types of growth and propagation. Consideration of the simplest possible structures capable of evolution provides a framework for discussion of the question of the minimal size of such a structure.
Role of the Membrane Compartment
The function of the RNA (or RNA precursor) in our hypothetical progenitor cell is to provide a mechanism for the storage and replication of information in a form that is both heritable and mutable. The capacity for mutation allows the organism to explore new ways of adapting to its environment, while the heritability of such changes means that a selective advantage can be passed on to future generations. What then is the role of the membrane, or more generally of any form of compartmentation that places a boundary between the inside and the outside of a cell? For complex cells, a membrane-bound compartment is required for the co-localization of genes with gene products and metabolites. But for very simple cells, the idea of a membrane-bound compartment raises problems such as how nutrients can be imported, and how growth and cell division occur. Nevertheless, the membrane performs a subtle but critical function, which is to keep RNA molecules that are related by descent together, thus allowing natural selection to work. Because this function is so important, and so little appreciated, I will discuss it first to provide a rationale for the subsequent discussion of the properties of simple membrane-bound cells.
Perhaps the easiest Way to understand this function is to consider what would happen in the absence of compartmentation. Imagine an initial population of RNA replicase molecules in free solution with activated monomers, but without any other RNA molecules present to complicate matters. Each replicase could copy any other RNA replicase that it happened to use as a template. If a mutant RNA replicase arose, with superior efficiency or accuracy, it would be better at replicating other RNA replicases, but would have no selective advantage for itself. Even worse, when it chanced to be replicated, its daughter molecules would diffuse away from each other, and thus could not even help each other preferentially.
In contrast, replicases that are replicating inside a growing and dividing membrane-bound compartment or vesicle would be capable of evolving. In this simple cellular system, each vesicle would contain some finite number of replicase molecules, which would use each other as templates for replication. Division would result in smaller vesicles, each containing a random subset of the replicase molecules from the parental cell. Through successive generations of such growth and division, the replicase molecules present in any one vesicle would tend, on average, to be more closely related by descent than replicase molecules in different vesicles. A mutant replicase that arose within such a system would have a selective advantage because it would be replicating its close relatives. During successive divisions, random segregation into daughter dells would eventually result in the formation of cells containing only the mutant replicase. Such cells would replicate their (mutant) genome more efficiently than cells containing only the parental replicase, and would eventually predominate in the population. Although selection for being a good template can occur in solution, selection for being a good replicase requires compartmentalization. Other new functions that favor propagation of the whole system could also evolve only in a compartmentalized system. The key to rapid and sustained evolution lies in the synergistic interaction between the molecules of inheritance and the molecules of compartmentation.
An independent reason for favoring a compartment-based cellular system is that this would allow for replicases to self-assemble from separate molecular segments. Many ribozymes can be assembled from a set of smaller RNA fragments; this attractive idea has the advantage that only relatively short segments need to be copied (7). However, to keep the segments together, some boundary, such as a membrane, would be needed.
Is a compartment absolutely required? In principle, selection for replicase activity could occur with dimers or higher multimers of a replicase, in which the various units would take turns acting as replicase and template; however, these ideas imply very long RNAs and require rather complex and unlikely dynamics such as partial strand separation so that newly copied material can re-fold into a replicase, while remaining attached to its template. For these reasons I do not favor the idea of a "living molecule," i.e., a replicating evolving molecule that exists in free solution. If the requirement for compartmentation is valid, the search for life should focus on cellular structures. On the other hand, evidence for life, or even critically important pre-biotic structures, might be found at either the molecular or cellular size scale.
If RNA without a compartment can't evolve (other than to be a better template), what about compartments without RNA? The beauty of membrane vesicles is that they are self-assembling structures that form spontaneously once a critical concentration of amphiphilic molecules exists. A variety of proposals have been made for the origin of life in self-sustaining metabolizing structures. Some such structures invoke networks of catalytic peptides or other molecules, while others postulate surface catalysis on colloidal particles of clay, FeS, or other materials. Autocatalytic networks are attractive from a theoretical perspective because they encode information in a distributed form, and can evolve by incorporating new catalytic processes. However, I suspect that the rarity of efficient catalysts in sequence space makes such models physically unrealistic. Vesicles containing catalytically active colloidal particles might develop quite complex chemistries, and may have been significant in the generation of monomers for the synthesis of RNA or its progenitors. Such particles could even be pre-biotic precursors of the first living cells. However, without a mechanism for heritable variation, they could not evolve in a Darwinian sense.
What about forms of compartmentation other than membranes? In principle, any medium that limits macromolecular diffusion more than small molecule diffusion, such as the interior of a gel matrix, or a micro or nano-porous rock, might suffice to keep molecules related by descent preferentially together. Fascinating experiments with Qβ replicase at least raise the possibility of such a mechanism (8). In such a scenario, it is hard to even say what the relevant size domain is. Another interesting possibility that has been suggested is an emulsion, in which small aqueous compartments in a non-aqueous matrix house replicating molecules (9). The variety of such possible systems emphasizes the importance of looking for life or its precursors in a wide range of environments. Nevertheless, since all present-day life consists of membrane-bound cells, any such precursor of life must at some point have made a transition to a membrane-bound compartment housing a replicating informational molecule. It is therefore worth considering if there are any significant size constraints on such a possible ancestral form of cellular life.
Size and the Cell Cycle
Membrane vesicles can be made from a wide range of phospholipids and other components, in a wide range of sizes. The vesicles that bud spontaneously from the surface of dried phospholipid films suspended in buffer tend to be large (1-10 µm) and multilamellar. However, when subjected to strong
shear forces, either by sonication or by being forced through small pores under pressure, unilamellar vesicles as small as 50 nm in diameter are readily generated.
Arguments can be made in favor of either large or small vesicles as the most likely basis for early cellular life. The key challenge here is to come up with a plausible mechanism for cell growth and division, given that early primordial cells lacked all of the sophisticated internal machinery evolved by modem cells to control their growth and to mediate the physical process of division. Very small vesicles have an important property that may be relevant in this regard: because of their small size and strong curvature, the membrane is highly strained. The growth of such strained vesicles is thermodynamically favored by the relaxation that occurs as size increases and curvature decreases. Growth can occur spontaneously, either slowly by incorporation of additional lipid molecules, or rapidly by fusion with other vesicles. Incorporation of additional lipid can occur by transfer through solution from micelles or other small vesicles, and transfer is faster into smaller vesicles (10). Further studies of vesicle growth by this mechanism would be very useful in assessing this model for spontaneous growth. Vesicle fusion processes have been studied in much more detail (both as models of biological membrane fusion events and because of the potential importance of vesicles in drug delivery). Depending on the nature of the lipid, vesicle fusion can be mediated by Ca++, by dehydration or by certain "fusogenic" peptides. Smaller vesicles tend to fuse much more readily than larger vesicles, although they are also less stable to phase changes in some conditions (11). Once larger vesicles have been generated, whether by growth or by fusion, they can divide into smaller vesicles with essentially no contents leakage, by shear-force-induced fission. In the laboratory, the simplest way of accomplishing this is by pressure-driven passage through small pores. The fact that vesicle growth and division can occur entirely under the influence of external environmental conditions raises the possibility of a primitive cell cycle, driven entirely by external physical forces, which is quite satisfying when considering a very simple cell that would lack all internal machinery for the control of growth and division. On the other hand, it is not clear what, if any, natural setting could provide the physical basis for the vesicle fission part of the cycle (passage of water in a hydrothermal vent system through microporous rock? wave action at the surface of a lake?). An interesting alternative possibility involves small vesicles formed spontaneously from short-chain lipids with a high intrinsic membrane curvature; synthesis of additional lipid by internal metabolic processes leads to vesicle growth and spontaneous (thermodynamically favored) fission (12).
Can we conceive of an analogous spontaneous cell cycle for larger vesicles? Large vesicles form spontaneously and fragment under mild shear forces. Here, however, it is the growth part of the cycle that is problematic, because of the absence of a thermodynamic driving force. Growth by incorporation of lipid molecules from solution seems unlikely (unless they are internally generated), and the fusion reactions of larger vesicles are less well studied. In general, concentrations of divalent cations that lead to the fusion of unstrained vesicles are very close to the concentrations that cause lipid phase changes and complete vesicle disruption (11). However, it must be emphasized that there have been very few studies of vesicle-based model systems for cell growth and division. One recent study suggests that hydrocarbons and single chain lipids may facilitate the fusion of larger vesicles (13). This observation raises the fundamental problem that, because the pre-biotic synthesis of amphiphilic molecules is so poorly studied, we have little idea of what kinds of molecules we should be looking at when studying model vesicle systems. Shorter lipid chains are known to generate less stable vesicles that are more permeable to small molecules such as nucleotides (14). Clearly, further studies of the physical properties of vesicles generated from pre-biotically plausible amphiphilic molecules would go a long way toward constraining the possible sizes of early cellular vesicles.
Very Small Cells Probably Transient
Although reasonable arguments can be made that the first cells might have been very small, it seems likely that such life-forms would have been quite transient, and soon superseded by the evolution of larger and more complex cells. Simple calculations based on volume suggest that a small vesicle could hold a maximum of about 1,000 medium-sized ribozymes (4- to 5-nm diameter, ˜70 nucleotides), which, allowing for some redundancy, means that up to perhaps 100 distinct functions could be encoded. Thus, a very small (50-nm diameter) organism might in principle evolve to a level of moderate complexity without having to enlarge to the point of changing the basic physical phenomena involved in the cell cycle. However, complexity beyond this level would almost certainly require an increase in size. Since a vesicle of 50-nm internal diameter has a volume of ˜60,000 nm3 vs. 1 nm3 for one base-pair in a duplex, the absolute upper limit on packing a double-stranded genome is about 60 kb, or 40 kb at a reasonable packing density. Even to hold a genome of 1 Mb (all free-living bacteria have genomes larger than this) would require a 150-nm diameter vesicle, and to keep the genome to less than 10% of total cell volume would require a minimum of a 300-nm diameter vesicle, or alternatively a 100-nm diameter cylinder of length 1,500 nm. Such a level of genomic complexity could easily accommodate considerable metabolic and structural complexity, including protein synthesis and internal regulation of cell growth, shape, and division. Although small relative to the size of most present-day microorganisms, this size may represent the lower limit necessary for organisms to maintain the complexity required to be competitive as a free-living life-form. Such life-forms would presumably have out-competed and driven to extinction their smaller and simpler relatives, unless there were physiological factors or specialized ecological niches that favored the survival of small cells.
One obviously relevant physiological factor is that the high surface to volume ratio of small cells could help to compensate for the difficulties involved in transport of nutrients across membranes before the advent of protein transporters. Of course this is a two-edged sword, and the loss of essential metabolic intermediates would become a serious problem. This problem is exacerbated for small vesicles, since a single molecule in a 50-nm vesicle has a concentration of ˜30 µM. A high-radiation environment might also initially favor small, simple cells, with a restricted genomic and cellular target size. However, the example of M. radiodurans shows that the evolution of repair functions can more than compensate for such environmental factors. As mentioned above, cell division of very small early cells would, at least initially, require an external source of energy in the form of an environment that provided very high shear forces. Although selection for the ability to grow outside such a restricted environment would be very strong, growth within that niche could be difficult or impossible for larger cells, at least until the evolution of rigid cell walls. Another niche that might be limited to very small cells could be nanoporous media such as, perhaps, compressed sediments. Such considerations may suggest that very small cells, if they ever existed, would be severely restricted in their distribution, both temporally and spatially.
Conclusions
Our present degree of knowledge is inadequate to strongly constrain the possible sizes of early cells. Vesicles as small as 50-nm diameter can be generated, could encapsulate small replicating informational polymers, and have at least some attractive properties in terms of the potential for a spontaneous cell cycle. Although such structures may not be the most likely form of primordial life, it would not be wise to ignore this possibility.
References
1. Bonner, S.A., Ellington, A.D., and Tauer, A. (1989). Proc. Natl. Acad. Sci. USA 86, 7054-7058.
2. Gilbert, W. (1986). Nature 319, 618.
3. Zhang, B. and Cech, T.R. (1997). Nature 390, 96-100.
4. Unrau, P.J. and Bartel, D.P. (1998). Nature 395, 260-263.
5. Wilson, D. and Szostak, J.W. (1998). Ann. Rev. Biochem. in press.
6. Benner, S.A. and Ellington, A.D. (1987). Nature 329, 295-296.
7. Doudna, J.A., Couture, S., and Szostak, J.W. (1991). Science 251 , 1605-1608.
8. Chetverina, H.V. and Chetverin, A.B. (1993). Nucleic Acids Res. 21, 2349-2353.
9. Tawfik, D.S. and Griffiths, A.D. (1998). Nat. Biotechnol. 16, 652-656.
10. Brown, R.E. and Hyland, K.J. (1992). Biochemistry 31, 10602-10609.
11. Wilschut, J., Duzgunes, N., Hoekstra, D., and Papahadjopoulos, D. (1985). Biochemistry 24, 8-14.
12. Schmidli, P.K., Schurtenberger, P., and Luisi, P.L. (1991). J. Am. Chem. Soc. 113, 8127-8130.
13. Basanez, G., Goni, F.M., and Alonso, A. (1998). Biochemistry 37, 3901-3908.
14. Chakrabarti, A.C., Breaker, R.R., Joyce, G.F., and Deamer, D.W. (1994). J. Mol. Evol. 39, 555-559.
How Small Can a Microorganism be?
Steven A. Benner
Departments of Chemistry and Anatomy and Cell Biology University of Florida
Abstract
Much of the volume of a bacterial cell is filled with machinery (ribosomes) that converts information in the genetic biopolymer (DNA) into information in the catalytic biopolymer (protein). This places a limit on the size of a two-biopolymer living system that all but certainly excludes cells as small as (for example) the structures observed in the Allan Hills meteorite derived from Mars. Life that uses a single biopolymer to play both genetic and catalytic roles could conceivably fit within a smaller cell, however. No biopolymer has yet been found that can play both roles, and the chemical demands for genetics and catalysis are frequently contradictory. A catalytic biopolymer should have many building blocks; a genetic biopolymer should have few. A catalytic biopolymer should fold easily; a genetic biopolymer should not. A catalytic biopolymer must change its physical properties rapidly with few changes in its sequence; a genetic biopolymer must be COSMIC-LOPER (Capable Of Searching Mutation-space Independent of Concern over Loss of Properties Essential for Replication), with physical properties largely unchanged by changes in sequence. This article reviews the chemical plausibility of a single biopolymer that might make an effective compromise between these competing demands, and therefore permit life within very small cells.
Two-biopolymer Life-forms and One-biopolymer Life-forms
In terms of its macromolecular chemistry, life on Earth is a "two-biopolymer" system. Nucleic acid is the genetic biopolymer, storing information within an organism, passing it to its descendants, and suffering the mutation that makes evolution possible. Nucleic acids also direct the biosynthesis of the second biopolymer, proteins. Proteins generate most of the selectable traits, from structure to motion to catalysis. The two-biopolymer strategy evidently works well. It has lasted on Earth for billions of years, adapting to a remarkable range of environments, surviving formidable efforts by the cosmos to extinguish it, and generating intelligence capable of exploring beyond Earth.
The terrestrial version of two-biopolymer life contains a well-recognized paradox, however, one relating to its origins. It is difficult enough to envision a non-biological mechanism that would allow either proteins or nucleic acids to emerge spontaneously from non-living precursors. But it seems astronomically improbable that both biopolymers arose simultaneously and spontaneously, and even more improbable that both arose spontaneously, simultaneously, and as an encoder-encoded pair.
Accordingly, "single-biopolymer" models have been proposed for life that may have preceded the two-biopolymer system that we know on contemporary Earth (Joyce et al., 1987). Such models postulate that a single biopolymer can perform both the catalytic and genetic roles and undergo the Darwinian evolution that defines life (Joyce, 1994). RNA was proposed some time ago as an example of such a biopolymer (Rich, 1962; Woese, 1967; Orgel, 1968; Crick, 1968). This proposal became more credible after Cech, Altman, and their coworkers (Cech et al., 1981; Zaug and Cech, 1986; Guerrier-Takada et al., 1983) showed that RNA performs catalytic functions in contemporary organisms. The notion of an "RNA world," an episode in natural history when RNA served both genetic and catalytic roles, is now part of the culture of molecular biology (Watson et al., 1987).
Single-biopolymer Systems and Extraterrestrial Life
“Single-biopolymer” models for Darwinian chemistry have relevance to the search for extraterrestrial life. For example, some biologists have argued that the microstructures identified by McKay et al. (1996) in the Allan Hills meteorite, which are 20 to 100 nanometers across, are too small to be the remnants of living cells (Kerr, 1997). The argument is that the ribosome is 25 nm across, ribosomes are a requirement for life, and placing ribosomes (ca. four ribosomes across the short dimension of the "cell") in the cell would exclude virtually every other biomolecule.
This view is narrowly formulated. Ribosomes are a requirement for a two-biopolymer life-form, such as those known on contemporary Earth. If a single-biopolymer (such as RNA) can serve both genetic and catalytic functions, ribosomes are not required. A smaller cell may be sufficient to hold a single-biopolymer life-form.
How much smaller might the cells of a single-biopolymer life-form be (excluding parasitic cells)? Translation places demands upon the volume of a typical two-biopolymer cell. If we do not consider water, approximately half of the material inside an E. coli cell is ribosomes, tRNA, and mRNA (Lewin, 1985). Thus, a single-biopolymer cell can be half the size of a two-biopolymer cell simply by discarding the translation material. Of the remaining half of the dry weight of the intracellular contents of E. coli, aminoacyl tRNA synthetases, proteins that form transcription complexes, and proteins catalyzing amino acid biosynthesis are a major contributor. Together, biomolecules required to support translation comprise more than half of the soluble proteins that form the "core metabolism" encoded by the protogenome (Benner et al., 1993), the organism at the hypothetical threefold point joining Archaea, Eucarya, and Eukaryota in the universal tree of life.
Models can be built for a minimal metabolism that might be used by a single-biopolymer life-form. If that biopolymer is RNA, Figure 1 offers an autotrophic metabolism that involves fixation of carbon dioxide (4 catalysts, by analogy with the reductive tricarboxylic acid cycle), carbohydrate biosynthesis (5 catalysts exploiting cyanide-based couplings and aldol reactions), triphosphate generation (6 catalysts), nitrogen metabolism (3 catalysts), and nucleotide biosynthesis (28 catalysts, adopted directly from contemporary pathways). Ignoring the thermodynamics of this pathway (which are expected to be favorable under reducing conditions; see McCollom and Shock, 1997), this model sustains a single-biopolymer life-form with ca. 50 biocatalysts. Although additional macromolecules would undoubtedly be useful to biosynthesize membrane components, transport metal ions and cofactors, and participate in gene regulation, a plausible model for minimal single-biopolymer autotrophic life could almost certainly be limited to fewer than 100 macromolecules, less than 10% of the number found in a typical autotrophic two-biopolymer genome. If different types of catalysts can have the same size, a single-biopolymer life-form might fit within a cell having 5% the volume of a contemporary terrestrial bacterium. This implies that the microstructures in the martian meteorite might not be too small to be fossils of a single-biopolymer form of life. Conversely, if the meteorite structures are indeed fossils, then they almost certainly are fossils of an organism that used only a single biopolymer.
Does a Single Biopolymer Exist That Is Capable of Genetics and Catalysis?
This discussion suggests that the answer to the title question depends on the answer to the question: Does a single biopolymer exist that can robustly do both genetics and catalysis. In this discussion, we focus on RNA as the most highly regarded candidate for the single biopolymer.
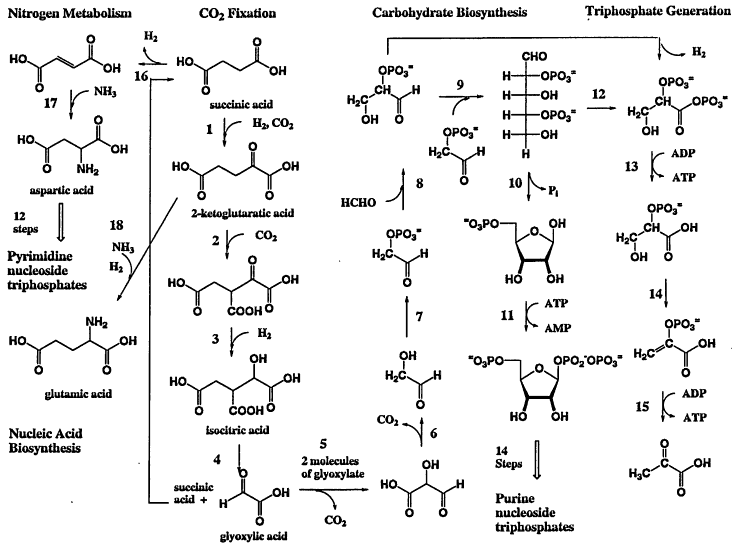
Figure 1.
A hypothetical metabolism for a single-biopolymer autotrophic life-form based on RNA. Each reaction has a precedent in known chemistry, biological or non-biological. The driving force for the overall synthesis is not defined. The overall process would be exergonic under reducing conditions (see McCollum and Shock, 1997).
The Requirements for Genetics
A NASA workshop defined life as "a self-sustaining chemical system capable of undergoing Darwinian evolution" (Joyce, 1994). The genetic component of this definition is contained within the concept of Darwinian evolution. It includes not only the ability to be reproduced, but also the ability to survive mutation in a way that can create a change in phenotype that is selectable.
As discussed elsewhere (Benner and Switzer, 1998), many molecular systems can be reproduced and can form structures, catalysis, or other lifelike phenotypes. The most substantial challenge facing those attempting to develop a system that models life is to identify a biopolymer that can undergo mutation in a non-destructive way. Specifically, to support Darwinian evolution, a biopolymer must be able to search "mutation-space" independent of concern that it will lose properties essential for replication. If a substantial fraction of the mutations possible within a genetic information system cause a biopolymer to precipitate, unfold, or otherwise no longer be recognizable by the catalyst responsible for replication, then the biopolymer cannot evolve. We designate polymers that have this property as COSMIC-LOPER biopolymers (Capable of Searching Mutation-space Independent of Concern over Loss Of Properties Essential for Replication).
DNA and RNA are COSMIC-LOPER biopolymers. A mutant of a DNA sequence is as likely to dissolve in water, pair via Watson-Crick rules, template complementary strands, and be a substrate for DNA polymerases as its parent. The COSMIC-LOPER behavior is not absolute. If an RNA sequence wanders into a G-rich region of sequence space, it may become insoluble, or otherwise incapable of acting as a template. But these regions are exceptions.
Because of the familiarity of the "rule-based" molecular recognition properties displayed by DNA and RNA, the uniqueness of nucleic acids with respect to their COSMIC-LOPER behavior is often overlooked. In fact, very few classes of organic molecules can suffer changes in structures without significant changes in their physical properties. Perhaps the best example is proteins. The physical properties of proteins (including their solubility) can change dramatically upon point mutation within the mutation space allowed by the 20 standard amino acids. Again, there are many examples of this phenomenon in Nature (for example, hemoglobin in sickled cells). Designed peptides provide other examples. For example, altering their structure of a peptide designed to catalyze the decarboxylation of oxaloacetate by a single acetyl group changed substantially their level of aggregation, while altering their internal sequence at a single residue changes substantially their helicity (Johnsson et al., 1990, 1993). If solubility and/or helicity are essential to the replicatability of a peptide template, a large range of plausible mutation would destroy it. Protein is not COSMIC-LOPER, and is not expected to serve well as a genetic biopolymer, despite its acknowledged virtues as a catalytic biopolymer.
Starting in the 1980s, various groups altered the structure of nucleic acids to learn what structural features enable the rule-based molecular recognition properties (for a review, see Benner et al., 1998). The polyanionic nature of the oligonucleotide backbone appeared to be an important component of the COSMIC-LOPER behavior of nucleic acids; modifications of that backbone to remove the repeating charges created a biopolymer that no longer displayed rule-based molecular recognition (Richert et al., 1996).
Further, work expanding the number of letters in the genetic alphabet uncovered an intriguing relationship between the number of building blocks in a biopolymer and the fidelity of its synthesis. A genetic polymer should be replicated with a high (if not perfect) degree of fidelity. From both theory and experiment (Szathmary, 1992; Lutz et al., 1996), one expects higher fidelity with smaller genetic alphabets than large genetic alphabets.
The Requirements for Catalysis
Binding and catalysis (which may be viewed as binding to a transition state) require that the biopolymer present a series of specific interacting groups to the substrate. Here, diversity is advantageous. A case can be made that the 20 amino acid side chains found in natural proteinogenic amino acids provide a good sampling of the diversity that is available, in that it includes cationic groups, anionic groups, hydrophilic neutral groups, hydrophobic aliphatic groups, aromatic groups, and heterocycles, general acids and general bases, and nucleophilic groups. It has deficiencies. The standard 20 amino acids underrepresent heterocycles (compared, for example, with the U.S. Pharmacopoeia), it lacks a range of redox active side chains, and it is missing an electrophilic reactivity. But much of the diversity required for catalysis is present in standard proteins.
Catalysts must also surround a transition state, delivering contacting interactions from all sides. This, in turn, requires folding. Via a backbone with an equal number of hydrogen bond donors and acceptors, peptides fold well. Indeed, the feature most characteristic of proteins is that they precipitate (Benner, 1988b). Precipitation is folding, arising when the peptide prefers to interact with other pep-tides than with solvent. DNA and RNA in contrast, have a backbone of repeating negative charges. In the absence of cofactor (most commonly, divalent metal ion), there is no backbone-backbone interaction that supports the folding of an oligonucleotide (Richert et al., 1996).
The Contradicting Chemical Features Required for a Biopolymer That Does Both
This discussion makes evident that catalysis on one hand and genetics on the other place competing and contradictory demands on molecular structure. This implies in turn that it is difficult to find a single biopolymer that does both, suggesting that single-biopolymer life-forms might be less robust than two-biopolymer life-forms and that the small cells that single-biopolymer life enables might be scarcer in the universe than large cells. Let us review three specific contradictions:
|
1. |
A biopolymer specialized to be a catalyst must have many building blocks, so that it can display a rich versatility of chemical functionality required for catalysis. A biopolymer specialized for genetics must have few building blocks, as a way of ensuring faithful replication. |
|
2. |
A biopolymer specialized to be a catalyst must fold easily so that it can form an active site. A biopolymer specialized for genetics should not fold easily, so that it can serve as a template (Richert et al., 1996). |
|
3. |
A biopolymer specialized for catalysis must be able to change its physical properties rapidly with few changes in its sequence, enabling it to explore "function space" during divergent evolution. A biopolymer specialized for genetics must have physical properties largely unchanged even after substantial change in sequence (the COSMIC-LOPER property). |
At the very least, a single-biopolymer attempting to support Darwinian evolution must reflect some sort of structural compromise between these goals. No fundamental principle guarantees that a polymeric system will make this compromise in a satisfactory way, however. The demands for functional diversity, folding, and rapid search of function space might be so stringent, and the demands for few building blocks, templating ability, and COSMIC-LOPER ability so stringent, that no biopolymer structure achieves a suitable compromise. Even if one exists, it may perform genetics and/or catalysis with poor robustness. Single-biopolymer life would then be fragile and easily extinguished. Life would be scarce in the universe because most of the initial forms would be driven to extinction before they
could leap to a two-biopolymer structure. Conversely, if many-polymeric systems exist that make an acceptable compromise between the demands of catalysis and the demands of information storage, life would have emerged rapidly via single-biopolymer forms and be abundant in the universe in diverse forms.
Theoretical Evidence for a Robust Single-biopolymer System
Well before experiments were brought to bear on this problem, a theoretical argument was available that suggested that a single-biopolymer life-form might be possible. It began with three stipulations: (1) that life on Earth did not arise via divine intervention, (2) that spontaneous generation of a two-biopolymer system is not possible, but (3) that spontaneous generation of one biopolymer is possible. From the (obvious) fact that life exists on Earth, it can be concluded that a single biopolymer must have existed that performs both genetics and catalysis; this is the only way to explain the origin of life on Earth.
This proposal in one of various forms was made in the 1960s (Rich, 1962; Woese, 1967; Orgel, 1968; Crick, 1968). The extent to which the proposal begs questions was ameliorated by a rational analysis of contemporary biochemistry that began in the 1970s, when Usher and McHale (1976), White (1976), and Visser and Kellogg (1978) suggested that elements of contemporary metabolism (in particular, the structure of cofactors) might be viewed as vestiges of an "RNA world" (Gilbert, 1986). The emerging field of genomics was then used to generate internally consistent reconstructions for the ancient single-biopolymer life-forms. These reconstructions concluded from the abundance of its vestiges in modem metabolism that the RNA world was metabolically complex (Benner, 1988a; Benner et al., 1989; Benner et al., 1993). In modem metabolism, RNA fragments play roles for which they are not intrinsically suited. This suggests that these fragments originated during a time in natural history where RNA was the only available biopolymer, rather than by convergent evolution or recruitment in an environment where chemically better-suited biomolecules could be encoded. If the RNA world developed the RNA cofactors, ATP, coenzyme A, S-adenosylmethionine, and NADH, it follows that the RNA world needed these, presumably for phosphorylations, Claisen condensations, methyl transfers, and oxidation-reduction reactions (respectively).
These models imply that the RNA-based single-biopolymer life upon which all terrestrial life is founded had a complicated metabolism. This, in turn, implies that RNA can catalyze a wide variety of chemical reactions. This may be taken as indirect support for the existence of single-biopolymer life-forms, and from there, the possibility of very small cells.
The Experimental Evidence
These types of arguments, together with the discovery of RNA catalysis, made hopes high when Szostak (1988), Joyce (1989a,b), Gold (Irvine et al., 1991), and their coworkers introduced "in vitro selection" as a combinatorial tool to identify RNA molecules that catalyze specific reactions. If RNA was indeed as effective a catalyst as the reconstruction of the RNA world would imply, in vitro selection should rapidly generate the ultimate goal, an RNA (or DNA) molecule that catalyzes the template-directed polymerization of RNA (or DNA), a molecular system able to undergo Darwinian evolution. If selection procedures were appropriately designed, they should also produce RNA catalysts for almost any other reaction as well.
In contrast with these hopes (and only by this contrast), in vitro selection has been disappointing. RNA has proven to be an intrinsically poor matrix for obtaining catalysis, especially when compared
with proteins. For example, to have a 50% chance of obtaining a single RNA molecule capable of catalyzing a template-directed ligation reaction by a modest (by protein standards) factor of 10,000, Bartel and Szostak (1993) estimated that one must sift through 2 × 1013 random RNA sequences 220 nucleotides in length. To obtain a catalyst with a factor of 10 greater catalytic power, one must increase the size of the library being searched by a factor of 1,000. This is poor catalysis, at least by comparison with proteins.
Although many laboratories have tried, only a few have managed to extend the scope of RNA catalysis beyond the phosphate transesterification reactions in which it was originally observed. For example, attempts to obtain an RNA catalyst for a Diels-Alder reaction using in vitro selection failed (Morris et al., 1994); the same reaction is readily catalyzed by protein antibodies (Gouverneur et al., 1993). Attempts to obtain RNA that catalyzes amide synthesis have succeeded, but with difficulty (Zhang and Cech, 1997; Wiegand et al., 1997). The fact that such successes came only after many attempts is indicative of a relatively poor catalytic potential in oligonucleotides.
The comparison with peptides is instructive. For example, short (14 amino acids) peptides accelerate the rate-determining step for the amine-catalyzed decarboxylation of oxaloacetate by more than three orders of magnitude (Johnsson et al., 1993), not far below the acceleration observed for the first-generation ligases observed in the Bartel-Szostak selection beginning with 1013 random RNA sequences. Further, the peptide is less than 10% the size of the RNA motif. Combinatorial experiments starting from this design (Perezpaya et al., 1996; Baltzer, 1998) suggested that perhaps only 107 random sequences must be searched to get a similar catalytic effectiveness as is observed in a library of 1013 RNA molecules. This suggests that peptides are intrinsically a millionfold fitter as catalysts than RNA.
The comparison is imperfect, of course, as it involves different reactions and different design strategies. This imperfection characterizes most of the comparisons that can be made at present. Not surprisingly, ribozymes are most frequently sought for reactions where oligonucleotides are most likely to be effective catalysts (for example, where oligonucleotides themselves are substrates), while peptide catalysts are most frequently sought for reactions suited for peptide catalysts (for example, those that make use of functional groups found on amino acid side chains). This makes the comparison non-quantitative, but useful nevertheless as an estimate of how well oligonucleotides and oligopeptides respectively perform when challenged by their favorite target reactions.
Biopolymers That Are Not (Exactly) RNA Or DNA
The failure of in vitro selection experiments with RNA to rapidly generate self-replicating systems challenges the notion that life emerged in a fashion directly analogous to the way in which in vitro selections are presently being done in the laboratory. This, in turn, means that these experiments failed to provide positive evidence that a single-biopolymer system exists, which in turn implies that we cannot confidently invoke a single-biopolymer life-form when we wish to argue that a very small structure (for example, on Mars) is a vestige of a primitive cell.
These experiments provided a direction, however. The apparent superiority of proteins as catalysts compared with RNA reflects (at the very least) the availability to proteins of a wider range of building blocks and catalytic functionality than in RNA. RNA lacks the imidazole, thiol, amino, carboxylate, and hydrophobic aromatic and aliphatic groups that feature so prominently in protein-based enzymes. RNA has only hydroxyl groups, polar aromatic groups, and phosphate groups. An uncounted number of studies with natural enzymes and their models has illustrated the use of this functionality by protein catalysts (Dugas, 1989).
Several groups are now seeking to add functionality to RNA and DNA. RNA might gain functionality
using cofactors, much as contemporary proteins gain the functionality that they lack through vitamins. In a sense, this was already done in in vitro selection experiments, which nearly universally use the divalent magnesium cation, essentially as a cofactor. More recently, Breaker and his coworkers have expanded the approach to include organic molecules as second ligands in riboenzymes (Tang and Breaker, 1997).
A second solution was to append functionality to the standard nucleotides (Tarasow et al., 1997). Prompting this suggestion was the observation that contemporary tRNA and rRNA contain much of the functionality found in proteins but lacking in contemporary encoded RNA, including amino, carboxylate, and aliphatic hydrophobic groups (Limbach et al., 1994). These functional groups are introduced by post-transcriptional modification of encoded RNA. Some of these might even be placed by parsimony in the protogenome (Benner et al., 1989).
A third way to expand the functional diversity of nucleic acids is to increase the number of nucleotides in the nucleic acid alphabet. This can be done by using the non-standard hydrogen-bonding patterns permitted by the geometry of the Watson-Crick base pair (Switzer et al., 1989; Piccirilli et al., 1990). Additional letters in the genetic alphabet could carry a richer diversity of functionality. Indeed, one might imagine a new type of biopolymer, one carrying functionalization like proteins but able to be copied like nucleic acids (Kodra and Benner, 1997).
Conclusions
Each approach outlined above to increase the catalytic power of RNA as a single-biopolymer is only beginning to be explored. The title question will be answered only as this work proceeds. We believe that some of the most exciting results in chemistry in the next decade will come from efforts attempting to resolve the contradictions between catalysis and genetics in single-biopolymer systems in a way that will generate a biopolymer capable of both genetics and catalysis.
This question has implications for planetary exploration. The experiments with nucleic acid analogs has suggested as a hypothesis that a universal chemical characteristic of genetic biopolymers in water is a repeating charge, either an anion or a cation. This repeating charge may be both necessary and sufficient for COSMIC-LOPER behavior (Richert et al., 1996; Benner and Switzer, 1998). A repeating charge is a convenient biomarker for non-terrean genetic molecules. Future planetary probes might well search for such molecules.
Further, a single-biopolymer system should sustain work on Earth to learn how metabolic pathways might have emerged. In vitro selection permits would permit sequential selection for catalysts for individual metabolic steps (as shown in Figure 1). This would provide an experimental approach to identify the minimal cell, may generate new biomarkers, and could assist in the search for life on other planets.
Acknowledgments
We are indebted to NASA and the Office of Naval Research for supporting some of the work described here, and to the collaborators whose published work is cited.
References
Baltzer, L., 1998. Functionalization of designed folded peptides. Opinions in Structural Biology 8, 466-470.
Bartel, D.P., and Szostak, J.W., 1993. Isolation of new ribozymes from a large pool of random sequences. Science 261, 1411-1418.
Benner, S.A., 1988a. Reconstructing the evolution of proteins. Pps. 115-175 in Redesigning the Molecules of Life, Benner, S.A., ed. Heidelberg: Springer-Verlag.
Benner, S.A., 1988b. Evolution, physical organic chemistry, and understanding enzymes. Adv. Clin. Enzymol. 6, 14-23.
Benner, S.A., 1989. Enzyme kinetics, and molecular evolution. Chem. Rev. 89, 789-806.
Benner, S.A., and Switzer, C.Y., 1998. Chance, and necessity in biomolecular chemistry. Is life as we know it universal? In Simplicity and Complexity in Proteins and Nucleic Acids, Frauenhofer, H., Deisenhofer, J., and Wolynes, P.G., eds. Dahlem Workshop Report. Berlin: Dahlem University Press, in press.
Benner, S.A., Battersby, T.R., Kodra, J., Switzer, C.Y., Moroney, S.E., Vögel, J., MacPherson, L., yon Krosigk, U., Hammer, C., Richert, C., Huang, Z., Horlacher, J., Schneider, K.C., König, M., Blättler, M., Arslan, T., Hyrup, B., Egli, M., Bäschlin, D., Müller, E., Schmidt, J., Piccirilli, J., Roughton, A., Held, H., Lutz, M., Eschgfäller, B., Jurczyk, S., Lutz, S., Hutter, D., Nambiar, K.P., and Stackhouse, J. , 1998. Redesigning nucleic acids. Pure Appl. Chem. 70, 263-266.
Benner, S.A., Cohen, M.A., Gonnet, G.H., Berkowitz, D.B., and Johnsson, K., 1993. Reading the palimpsest: Contemporary biochemical data and the RNA World. Pp. 27-70 in The RNA World, Gesteland, R., and Atkins, J., eds. New York: Cold Spring Harbor Laboratory Press.
Benner, S.A., Ellington, A.D., and Tauer, A., 1989. Modem metabolism as a palimpsest of the RNA World. Proc. Natl. Acad. Sci. USA 86, 7054-7058.
Cech, T.R., Zaug, A.J., and Grabowski, P.J., 1981. In vitro splicing of the ribosomal RNA precursor of Tetrahymena. Involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 27, 487-496.
Crick, F.H.C., 1968. The origin of the genetic code. J. Mol. Biol. 38, 367-379.
Dugas, H., 1989. Bioorganic Chemistry, 2nd Ed. New York: Springer-Verlag.
Gilbert, W., 1986. The RNA world. Nature 319, 618.
Gouverneur, V.E., Houk, K.N., Depascualteresa, B., Beno, B., Janda, K.D., and Lerner, R.A., 1993. Control of the exo-pathway, and endo-pathway of the Diels-Alder reaction by antibody catalysis. Science 262, 204-208.
Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N., and Altman, S., 1983. The RNA moiety of RNase P is the catalytic subunit of the enzyme. Cell 35, 849-857.
Irvine, D., Tuerk, C., and Gold, L., 1991. Selexion. Systematic evolution of ligands by exponential enrichment with integrated optimization by non-linear analysis. J. Mol. Biol. 222, 739-761.
Johnsson, K., Allemann, R.K., and Benner, S.A., 1990. Designed enzymes. New peptides that fold in aqueous solution, and catalyze reactions. Pp. 166-187 in Molecular Mechanisms in Bioorganic Processes, Bleasdale, C., and Golding, B.T., eds. Cambridge: Royal Society of Chemistry.
Johnsson, K., Allemann, R.K., Widmer, H., and Benner, S.A., 1993. Synthesis, structure, and activity of artificial, rationally designed catalytic polypeptides. Nature 365, 530-532.
Joyce, G.F., 1989a. Amplification, mutation, and selection of catalytic RNA. Gene 82, 83-87.
Joyce, G.F., 1989b. Building the RNA world. Evolution of catalytic RNA in the laboratory. UCLA Symp. Mol. Cell Biol. New Ser. 94, 361-371.
Joyce, G.F., 1994. Forward in Origins of Life: The Central Concepts , Deamer, D.W., and Fleischaker, G.R., eds. Boston: Jones and Bartlett.
Joyce, G.F., Schwartz, A.W., Miller, S.L., and Orgel, L.E., 1987. The case for an ancestral genetic system involving simple analogs of the nucleotides. Proc. Natl. Acad. Sci. USA 84, 4398-4402.
Kerr, R.A., 1997. Ancient Life on Mars? Putative Martian microbes called microscopy artifacts. Science 278, 1706-1707.
Kodra, J., and Benner, S.A., 1997. Synthesis of an N-alkyl derivative of 2'-deoxyisoguanosine . Syn. Lett., 939-940.
Lewin, B., 1985. Genes II. New York: John Wiley & Sons, p. 95.
Limbach, P.A., Crain, P.F., and McCloskey, J.A., 1994. Summary. The modified nucleosides of RNA. Nucl. Acids Res. 22, 2183-2196.
Lutz, M.J., Held, H.A., Hottiger, M., Hübscher, U., and Benner, S.A., 1996. Differential discrimination of DNA polymerases for variants of the non-standard nucleobase pair between xanthosine, and 2,4-diaminopyrimidine, two components of an expanded genetic alphabet. Nucl. Acids Res. 24, 1308-1313.
McCollum, T.M., and Shock, E.L. 1997. Geochim. Cosmochim. Acta. 61, 4375-4391.
McKay, D.S., et al., 1996. Science 273, 924-930.
Morris, K.N., Tarasow, T.M., Julin; C.M., Simons, S.L., Hilvert, D., and Gold, L., 1994. Enrichment for RNA molecules that bind a Diels-Alder transition-state analog. Proc. Natl. Acad. Sci. USA 91, 13028-13032.
Orgel, L.E., 1968. Evolution of the genetic apparatus. J. Mol. Biol . 38, 381-393.
Perezpaya, E., Houghten, R.A., and Blondelle, S.E., 1996. Functionalized protein-like structures from conformationally de-fined synthetic combinatorial libraries. J. Biol. Chem. 271, 4120-4126.
Piccirilli, J.A., Krauch, T., Moroney, S.E., and Benner, S.A., 1990. Extending the genetic alphabet: Enzymatic incorporation of a new base pair into DNA, and RNA. Nature 343, 33-37.
Rich, A., 1962. On the problems of evolution, and biochemical information transfer. Pp. 103-126 in Horizons in Biochemistry, Kasha, M., and Pullman, B., eds. New York: Academic Press.
Richert, C., Roughton, A.L., and Benner, S.A., 1996. Nonionic analogs of RNA with dimethylene sulfone bridges. J. Am. Chem. Soc. 118, 4518-4531.
Switzer, C.Y., Moroney, S.E., and Benner, S.A., 1989. Enzymatic incorporation of a new base pair into DNA, and RNA . J. Am. Chem. Soc. 111, 8322-8323.
Szathmary, E., 1992. What is the optimum size for the genetic alphabet? Proc. Natl. Acad. Sci. USA 89, 2614-2618.
Szostak, J.W., 1988. Structure, and activity of ribozymes. Pp. 87-114 in Redesigning the Molecules of Life, Benner, S.A., ed. Heidelberg: Springer-Verlag.
Tang, J., and Breaker, R.R., 1997. Rational design of allosteric ribozymes. Chem. Bio. 4, 453-459.
Tarasow, T.M., Tarasow, S.L., and Eaton, B.E., 1997. RNA-catalyzed carbon-carbon bond formation. Nature 389, 54-57.
Usher, D.A., and McHale, A.H., 1976. Hydrolytic stability of helical RNA: A selective advantage for the natural 3',5'-bond. Proc. Natl. Acad. Sci. USA 73, 1149-1153.
Visser, C.M., and Kellogg, R.M., 1978. Biotin. Its place in evolution. J. Mol. Evol. 11, 171-178.
Watson, J.D., Hopkins, N.H., Roberts, J.W., Steitz, J.A., and Weiner, A.M., 1987. Molecular Biology of the Gene, 4th Ed., p. 1115. Menlo Park, Calif.: Benjamin Cummings.
White III, H.B., 1976. Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 7, 101-104.
Wiegand, T.W., Janssen, R.C., and Eaton, B.E., 1997. Selection of RNA amide synthases. Chem. Biol. 4, 625-683.
Woese, C.R., 1967. The Genetic Code. The Molecular Basis for Genetic Expression. New York: Harper & Row.
Zaug, A.J., and Cech, T.R., 1986. The intervening sequence RNA of Tetrahymena is an enzyme. Science 231, 470-475.
Zhang B.L., and Cech T.R., 1997. Peptide bond formation by in vitro selected ribozymes. Nature 390, 96-100.






























