HEALTH RISK ASSESSMENT FOR THE NERVE AGENT GB
DRAFT REPORT
September 1996
(editorial corrections made April 1997)
Prepared for
U.S. Department of the Army
Army Environmental Center
under Interagency Agreement No. 1769-1769-A1
Prepared by
Life Sciences Division
OAK RIDGE NATIONAL LABORATORY*
Oak Ridge, Tennessee 37831
Submitted to
Material/Chemical Risk Assessment Working Group
Advisory and Coordinating Committee Environmental Risk Assessment Program
PREFACE
This report assesses the potential non-cancer and cancer effects of chemical agent GB (CAS No. 107-44-8).
This document supports the activities of the Material/Chemical Risk Assessment Working Group of the Environmental Risk Assessment Program, a cooperative endeavor of the Department of Defense, Department of Energy, and Environmental Protection Agency. This working group is developing toxicity values for selected chemicals of concern at federal facilities. Toxicity values will be submitted for consideration by the EPA's IRIS Consensus Process for inclusion on IRIS (EPA's Integrated Risk Information System). The Material/Chemical Risk Assessment Working Group consists of Drs. Jim Cogliano (chair) and Harlal Choudhury (U.S. EPA), Dr. Bruce Briggs (Geo-Centers); Lt. Cmdr. Warren Jederberg and Dr. Robert L. Carpenter (U.S. Naval Medical Research Institute); Dr. Elizabeth Maull and Mr. John Hinz (U.S. Air Force Occupational and Environmental Health Directorate); Drs. Glenn Leach and Winnie Palmer (U.S. Army Center for Health Promotion and Preventive Medicine); Drs. Robert Young and Po-Yung Lu (Oak Ridge National Laboratory).
This document was written by Dr. Dennis M. Opresko, Life Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN. Internal peer review was provided by Dr. Robert Young, Dr. Annetta Watson, and Mr. Robert Ross. External review of the toxicity data was provided by Dr. Thomas J. Bucci, Integrated Services, White Hall, AR and Dr. I.K Ho of the U. of Mississippi Medical Center, Jackson MS. External review of the derivation of the RfDs was provided by Drs. Michael Dourson and Susan Velazquez of Toxicology Excellence for Risk Assessment, Cincinnati, OH, and Dr. William Hartley of Tulane Medical Center, New Orleans LA. Additional reviews were provided by Mr. Joe King, Dr. Jack Heller, Ms. Veronique Hauschild, Ms. Bonnie Gaborek, Mr. Maurice Weeks, Maj. Robert Gum, and Mr Kenneth Williams of the U.S Army.
LIST OF TABLES
|
Table 1. |
RBC-ChE activity in different species |
|||
|
Table 2. |
Lethality data for agent GB |
|||
|
Table 3. |
RBC-ChE levels in 90-day subchronic study of GB type I in CD rats |
|||
|
Table 4. |
RBC-ChE levels in 90-day subchronic study of GB type II in CD rats |
|||
|
Table 5. |
Plasma-ChE levels in 90-day subchronic study of GB type II in CD rats |
|||
|
Table 6. |
Sacrifice schedule for GB chronic study |
|||
|
Table 7. |
Incidence of tracheitis in colony rats in GB chronic study |
|||
|
Table 8. |
Comparison of RfD with human toxicity data for GB |
1. INTRODUCTION
Military nerve agents are organophosphate compounds containing either a fluorine, sulfur, or cyanide substituent group (Dacre, 1984). GB contains a fluorine substituent group (GA contains a cyanide substituent group and VX a sulfur group). The chemical synonyms, Chemical Abstract Service (CAS) and Army identification numbers (DA, 1974, 1992; Dacre, 1984), and chemical formula for GB are as follows:
Phosphonofluoridic acid, methyl-, 1-methylethyl ester
Phosphonofluoridic acid, methyl-, isopropyl ester
Isopropoxymethylphosphoryl fluoride;
Isopropyl methylfluorophosphate;
Isopropyl methanefluorophosphonate;
O-Isopropyl methylphosphonofluoridate;
O-Isopropyl methylisopropoxyfluorophosphine oxide;
Isopropyl-methyl-phosphoryl fluoride:
Isopropoxymethylphosphonyl fluoride;
Methylphosphonofluoridic acid isopropyl ester;
Methylfluorophosphonic acid, isopropyl ester;
Methylphosphonofluoridic acid 1-methylethyl ester;
Methylisopropoxyfluorophosphine oxide;
Sarin
CAS No. 107-44-8;
Edgewood Arsenal No. 1208
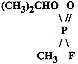
1.1 PHYSICAL/CHEMICAL PROPERTIES
Agent GB is a colorless liquid with a molecular weight of 140.1 (DA, 1974, MacNaughton and Brewer, 1994); it has a vapor density of 4.8 (air = 1) and a liquid density of 1.09 g/mL at 25°C (DA, 1974). The vapor pressure of GB is 2.9 mm Hg at 25°C. It is miscible with water and readily soluble in organic solvents (DA, 1974).
1.2. ENVIRONMENTAL FATE
1.2.1. Air
GB is very volatile with a vapor pressure of 2.9 mm Hg at 2519MMacNaughton and Brewer, 1994). A vapor concentration of 22 g/m3 has been reported for a temperature of 25°C (DA, 1974) (although not adequately described in the reference, this presumably is the saturation concentration above a pure liquid). No information was found on the atmospheric half-life of GB.
1.2.2 Water
GB is completely miscible with water (DA, 1974). Its rate of hydrolysis is dependent on temperature, pH, and other water quality parameters (Epstein, 1974; Morrill et al., 1985; Clark, 1989). At 20°C, the half-life ranges from 461 hr at pH 6.5 to 46 hr at pH 7.5. At 25°C, the half-life is 237 hr at pH 6.5 and 24 hr at pH 7.5. GA is much more persistent at low temperature; at 0°C, its half-life is 8,300 hours at pH of 6.5. The rate of hydrolysis under natural conditions is accelerated by the presence of ions (dissolved solids) in solution. Metal cations such as copper and manganese in seawater increase the rate of hydrolysis (Epstein, 1974).
Based on an estimated Henry's Law Constant of 5.4 × 10-7 atm m3/mol (MacNaughton and Brewer, 1994), evaporation of GB from water is expected to be slow.
1.2.3 Soil
According to Morrill et al. (1985), evaporation is the primary mechanism for the loss of GB from soil, and this is supported by the estimated volatility potential (slope of the vapor pressure vs. concentration in soil organics) of 4.9 × 10-8 mm Hg/mg/kg and by the air-soil partition coefficient of 135 × 10-5 mg/m3 (for a soil density of 1.4 g/cm3) as reported by MacNaughton and Brewer (1994). In a field test conducted in Finland detectable concentrations of GB (>1 pg/dm3) were found in the air for up to 9 days following application of 10 mg of GB over a 10 × 10 meter area of moss (temperature 2.5–8 °C, humidity 60–100%, wind speed 1–10 m/s) (Sanches et al., 1993).
Studies conducted with soil samples from Dugway Proving Ground and Edgewood Arsenal showed that 90% of GB added to soil and maintained in closed containers at room temperature (20–25°C) was lost in the first 5 days (Small, 1984).
Binding of GB to soil organics is likely to be limited considering the relatively low log Kow of 0.72 and low Koc value of 59 (MacNaughton and Brewer, 1994); therefore, there is a potential for leaching and groundwater contamination. MacNaughton and Brewer (1994) calculated a leaching index of 3.7 for GB, (i.e., the number of leachings required to reduce the GB soil concentration to one-tenth of the original amount, assuming that for each leaching one kilogram of soil is in equilibrium with one liter of water). However, the amount reaching ground water is likely to be limited by hydrolysis.
2. MECHANISM OF ACTION
Nerve agents are inhibitors of acetylcholinesterase (AChE), an enzyme responsible for deactivating the neurotransmitter acetylcholine at some neuronal synapses and myoneural junctions. By a mechanism of phosphorylation, nerve agents act as substrates for the enzyme, thereby preventing deactivation of acetylcholine. The organophosphate-inhibited enzyme can be reactivated by dephosphorylation, but this occurs at a rate that is slower than the rate of reactivation of acetylcholine. Consequently, there is a depletion of acetylcholinesterase and a buildup of acetylcholine. In addition, the nerve agent-enzyme complex can also undergo an ''aging" process (thought to be due to a loss of an alkyl or alkoxy group), whereby it becomes resistant to dephosphorylation (see review by Munro et al., 1994). Differences in rates of aging and reactivation may be important in evaluating toxicity data especially when extrapolating from animal studies to humans. In vitro tests conducted by Grob and Harvey (1958) indicate that both GA and GB combine with cholinesterase almost irreversibly during the first hour of their reaction. Sidell and Groff (1974) reported that the GB-ChE complex ages very rapidly in vivo, with 45–70% completion by 5 hours after infusion. In contrast, the complex formed between ChE and the nerve agent VX does not age significantly, and the rate of spontaneous reactivation can be as fast as 1%/hr in humans (Sidell and Groff, 1974).
2.1 Effects of Organophosphate Agents on the Nervous System
The anticholinesterase effects of the organophosphate nerve agents can be characterized as being muscarinic, nicotinic, or central nervous system (CNS)-related. Muscarinic effects occur in the parasympathetic system (bronchi, heart, pupils of the eyes; and salivary, lacrimal and sweat glands) and result in signs of pulmonary edema, bradycardia, miosis, tearing, and sweating. Nicotinic effects occur in somatic (skeletal/motor) and sympathetic systems, and result in muscle fasciculation, muscle weakness, tachycardia, and diarrhea. Effects on the CNS by organophosphates are manifested as giddiness, anxiety, emotional lability, ataxia, confusion, and depression (O'Brien, 1960).
Although the inhibition of cholinesterase within neuro-effector junctions or the effector itself is thought to be responsible for the major toxic effects of organophosphate agents, these compounds can apparently affect nerve-impulse transmission by more direct processes as well. Direct effects may occur on excitable tissues, receptors, and ionic channels. According to Somani et al. (1992), the direct action of nerve agents on nicotinic and muscarinic ACh receptors may occur when concentrations in the blood rise above micromolar levels, whereas at lower levels the action is mainly the result of inhibition of AChE. Albuquerque et al. (1985) have shown that agent GA, as well as agents GB and GD are capable of changing receptor sites in a manner similar to that exhibited by acetylcholine, which promotes the conductance of electrophysiological signals associated with stimulation of neuromuscular function. VX "may directly affect a small population of muscarinic ACh receptors that have a high affinity for [3H]-cis-methyldioxalane binding" (Somani et al., 1992). VX may also counteract the effects of ACh by acting as an open channel blocker at the neuromuscular junction, thereby interrupting neuromuscular function (Rickett et al., 1987).
Exposure to some organophosphate cholinesterase inhibitors results in a delayed neuropathy characterized by degeneration of axons and myelin. This effect is not associated with the inhibition of acetylcholinesterase, but rather with the inhibition of an enzyme described as neuropathy target esterase (NTE); however, the exact mechanism of toxicity is not yet fully understood (Munro et al., 1994). For some organophosphate compounds, delayed neuropathy can be induced in experimental animals at relatively low exposure levels, whereas for others the effect is only seen following exposure to supralethal
doses when the animal is protected by antidotes from the acute toxic effects caused by cholinesterase inhibition.
Although there is the potential for nerve agents to have direct toxic effects on the nervous system, there is no evidence that such effects occur in humans at doses lower than those causing cholinesterase inhibition. For the purpose of evaluating potential health effects, inhibition of blood cholinesterase is generally considered the most useful biological endpoint.
2.2 Effect on Blood Cholinesterases
In addition to being found in the nervous system, acetylcholinesterase also occurs in the blood where it is bound to the surface of red blood cells (termed RBC-ChE). RBC-ChE activity, as well as the activity of a second type of cholinesterase found in blood plasma (butyrylcholinesterase, or plasma cholinesterase) have been used to monitor exposure to organophosphate compounds (pesticides and nerve agents). Both RBC-AChE and plasma-ChE have been used as bioindicators of potential toxic effects. There is some evidence that RBC-AChE is as sensitive as brain ChE to the effects of nerve agents. Grob and Harvey (1958) reported that the in vitro concentrations producing 50% depression of brain-ChE and RBC-AChE activity were the same in the case of GA (1.5 × 10-8 mol/L), and only slightly different (3 × 10-9 mol/L and 3.3 × 10 mol/L) in the case of GB. However, in vivo animal studies indicate a poor correlation between brain and RBC-AChE in cases of acute exposures (Jimmerson et al., 1989), and this is reflected in the fact that blood cholinesterase activity may not always be correlated with exposure or with signs and symptoms of toxicity. Acute exposures to high concentrations may cause immediate toxic effects before significant changes occur in blood ChE activity, and repeated exposures over a period of several days may result in a sudden appearance of signs and symptoms due to cumulative effects (Grob and Harvey, 1958). Conversely, blood ChE activity can become very low without overt signs or symptoms during chronic exposures to low concentrations of organophosphates. This may be due to a slower rate of recovery of RBC-ChE compared to tissue ChE, or to a noncholinesterase-dependent recovery pathway for neural tissue (Grob and Harvey, 1958). Sumerford et al. (1953) reported that orchard workers exposed to organophosphate insecticides had RBC-AChE values as low as 13% of average preexposure levels without any other signs or symptoms of toxicity. Animal studies have demonstrated that chronic exposures to low concentrations of organophosphate insecticides can also result in increased tolerance levels (Barnes, 1954; Rider et al., 1952; Dulaney et al., 1985). Similarly, Sumerford et al. (1953) reported increased levels of tolerance to organophosphate insecticides in people living near orchards subject to insecticide applications. Such adaptation may result from increased rates of formation of blood ChE, or from increased rates of detoxification. Additional information on the development of tolerance to organophosphate cholinesterase inhibitors can be found in a review paper by Hoskins and Ho (1992).
The blood cholinesterases and other esterases may, to some degree, provide a protective effect by binding with some fraction of the anticholinesterase compound (Wills, 1972). However, not all nerve agents bind equally well with all cholinesterases. Agent GB inhibits both RBC-ChE (80–100%) as well as plasma-ChE (30–50%) (Grob and Harvey, 1958). In contrast, agent VX preferentially inhibits RBC-ChE (70% compared with about 20% inhibition of plasma ChE) (Sidell and Groff, 1974). Rodents (but not humans) have other enzymes in the blood, termed aliesterases, which can bind to organophosphates, thereby reducing the amount available for binding with acetylcholinesterase (Fonnum and Sterri, 1981). Agent GB binds with aliesterases; however, according to Fonnum and Sterri (1981), VX has a quaternary ammonium group which prevents it from being a substrate for aliesterases. The strong specificity of agent VX to AChE may account, in part, for the fact that it is much more acutely toxic than agents GA and GB (see Appendix A).
2.2.1 Intra- and Interspecies Variation in Blood Cholinesterase Activity
Although blood cholinesterase activity is used as a measure of exposure to organophosphate compounds, baseline activity levels can vary between individuals and between species. According to Wills (1972), both plasma- and RBC-ChE activity are generally lower in women than in men. Sidell and Kaminskis (1975) reported that, for a test population of 22 human subjects, the highest coefficient of variation of RBC-ChE was 4.1% per single subject; the average range of variation was ± 2.1% for men and ± 3.1% for women. In individuals studied for one year, the RBC-ChE activity varied by 11% in men and 16% in women. Yager et al. (1976) reported a 10.0% intra-individual coefficient of variation for RBC-ChE and 14.4% for plasma-ChE. Callaway et al. (1951) estimated that with only one pre-exposure measurement, the smallest measurable decrease was 15% of the baseline value for RBC-ChE activity and 20% of the baseline for plasma-ChE.
A small subpopulation of men and women have a genetic defect causing their blood cholinesterase activity to be abnormally low (Evans et al., 1952; Harris and Whittaker, 1962). For homozygous individuals, the activity can be as low as 8–21% of the normal mean (Bonderman and Bonderman, 1971). Morgan (1989) suggests that these individuals may be unusually sensitive to organophosphate anticholinesterase compounds.
Data compiled by Ellin (1981) reveal that the RBC-ChE activity for humans is slightly higher than that for monkeys and much higher than that for rats and other laboratory animals (Table 1).
Table 1. RBC-ChE activity in different species
|
Species |
RBC-ChE activity (µmol/mL/min) |
Optimum substratea concentration (M) |
|
Human |
12.6 |
2 × 10-3 |
|
Monkey |
7.1 |
2 × 10-3 |
|
Pig |
4.7 |
1 × 10-3 |
|
Goat |
4.0 |
2 × 10-3 |
|
Sheep |
2.9 |
2 × 10-3 |
|
Mouse |
2.4 |
2 × 10-3 |
|
Dog |
2.0 |
2 × 10-2 |
|
Guinea pig |
2.7 |
2 × 10-3 |
|
Rabbit |
1.7 |
5 × 10-3 |
|
Rat |
1.7 |
5 × 10-3 |
|
Cat |
1.5 |
5 × 10-3 |
|
Source: Ellin, 1981 a Acetylthiocholine iodide concentration for maximum RBC-ChE activity. |
||
These differences in RBC-ChE activity may affect a species' sensitivity to a particular organophosphate compound. At the same time, the relative amount of plasma cholinesterase and other compounds in the blood that can bind to the organophosphate agents must also be considered. For example, rodents, but
not humans, have high levels of aliesterases (AE) in the blood, and these compounds may provide rats and mice with a higher level of resistance to some anticholinesterase compounds (McNamara and Leitnaker, 1971).
2.2.2 Potency of Nerve Agents as Cholinesterase Inhibitors
The potency of the anticholinesterase activity of nerve agents and other organophosphates is expressed by the bimolecular rate constant (ki) for the reaction of the phosphate compound with the enzyme and by the molar concentration causing 50% inhibition of the enzyme (I 50). The relationship between I50 and ki as a function of time (t) is expressed by the following equation (Eto, 1974):
(1)
I50 data for several organophosphate nerve agents have been tabulated by Dacre (1984). The pI50 (negative log of the molar concentration causing 50% inhibition) for GB was reported to be 8.8 by Tammelin (1958) and 8.9 by Dacre (1984), and calculated as 8.5 from an I50 of 3.7 × 10-9 mol/L reported by Grob and Harvey (1958).
The potency of nerve agents can also be expressed in terms of the dose necessary to produce 50% inhibition of cholinesterase (ChE50). In humans, RBC-ChE50 values for GB are 0.003 mg/kg and 0.01 mg/kg, respectively, for i.v. and oral doses (Grob and Harvey, 1958). The relative effectiveness of nerve agents in inhibiting cholinesterase is closely correlated with their acute toxicity (see Appendix A).
3. TOXICOLOGY
3.1 Introduction
Health and environmental impacts of nerve agents and related compounds (i.e., organophosphate insecticides) have been reviewed by O'Brien (1960), Matsumura (1976), Dacre (1984), Carnes and Watson (1989), Watson et al. (1989), and Munro et al. (1994). A brief general discussion of the toxicology of nerve agents and related organophosphate pesticides is given below.
Nerve agents are acutely toxic by all routes of exposure. Initial symptoms of acute poisoning are fatigue, headache, mild vertigo, weakness, and loss of concentration. Moderate exposures result in miosis and excessive sweating, tearing, and salivation. Acidosis and hyperglycemia may also occur in addition to muscular weakness, muscular twitching, lacrimation, urination, and defecation. Acute poisoning can result in prostration, clonic convulsions (rapid repetitive movements) and tonic convulsions (limbs stretched and rigid) (Matsumura, 1976). Exposures sufficiently high to cause convulsions have resulted in brain lesions and cardiomyopathy in laboratory animals (Singer et al., 1987).
In addition to the immediate toxicity of the nerve agents, there is concern that exposures may lead to chronic neurological effects similar to those reported for some organophosphate insecticides. Included among these possible effects are organophosphate-induced delayed neuropathy (OPIDN), EEG changes, and long-term psychological disturbances (Munro et al., 1994). OPIDN, which appears 5–30 days after
exposure, manifests itself as muscle weakness, tingling, and twitching followed by paralysis (Munro et al., 1994). Histopathological changes, which consist of degeneration of axons and myelin of the nervous system, can be correlated, not with inhibition of AChE, but rather with inhibition of NTE. There is no evidence that agent GB causes OPIDN in humans. There are some data indicating that GB can induce this effect in chickens but only at supralethal dose levels when the animals are protected from immediate toxic effects by pretreatment with antidotes (see section 3.5). Neuropathological changes suggestive of OPIDN have also been observed in one study on mice exposed to GB; but the exposure levels were relatively high, and such effects were not reported in other studies on rodents (see section 3.5). The overall data indicate that GB is not likely to cause OPIDN in humans at exposure levels below those causing acute toxicity or cholinesterase inhibition.
Acute exposures to nerve agents are known to cause EEG changes (Grob and Harvey, 1958; Sidell, 1992) which may persist for long periods of time after exposure (Metcalf and Holmes, 1969; Burchfiel et al., 1976; Duffy et al., 1979; Duffy and Burchfiel, 1980); however, the reported changes have been considered to be clinically insignificant and not correlated with behavioral or physiological changes (DHHS, 1988). Acute exposures can also induce neuropsychological changes; however, there is no evidence of these effects persisting for months or years as has been reported for some organophosphate insecticides (Savage et al., 1988; Gershon and Shaw, 1961; Mick, 1974; Rodnitzky, 1974; Wagner, 1983; Tabershaw and Cooper, 1966). The available data for the organophosphate insecticides suggest that chronic neuropsychological effects (excluding OPIDN) do not occur in the absence of significant changes in blood cholinesterase. The same conclusion may apply to the organophosphate nerve agents.
3.2 Acute Toxicity
In tests on humans, Grob and Harvey (1958) found that a single oral dose of 0.022 mg GB/kg produced mild toxic effects including anorexia, nausea, heartburn, tightness in the stomach and chest, increased fatigue, nervous tension, anxiety, and other CNS responses including insomnia and excessive dreaming. An additional dose of 0.008 mg/kg within 8 hr resulted in moderate toxic effects including stomach cramps, vomiting, diarrhea, increased salivation and lacrimation, slightly decreased heart rate, and abnormal breathing. According to Thienes and Haley (1972), a single dose of 0.002 mg GB/kg caused excessive dreaming and talking during sleep and a dose of 0.020 mg/kg caused insomnia, excessive dreaming, withdrawal, and depression. At high exposures, brain damage may occur as a result of oxygen deprivation in brain tissue during GB-induced convulsions (Sidell, 1992).
Grob and Harvey (1958) reported that the first appearance of toxicity in humans occurred when RBC-ChE activity was depressed 88% (to 12% of the baseline value) following a single oral dose of GB. The single dose oral ChE50 value was reported to be 0.01 mg GB/kg, and the lethal oral dose was estimated to be 0.14 mg/kg. In comparison, the single dose intra-arterial ChE50 was reported to be 0.003 mg/kg, and the lethal intramuscular dose was estimated to be 0.03 mg/kg. Following i.v. administration, toxic effects occurred when RBC-ChE activity was depressed 40–50% (60-50% of baseline) indicating a more immediate effect on the nervous system than that caused by oral dosing (Grob and Harvey, 1958). LD50 data for selected species are shown in Table 2. Values for humans are estimates derived from animal data.
Table 2. Lethality data for agent GB
|
Exposure route |
Speciesa |
LD50 (µg/kg) |
References |
|
oral |
human human monkey rat rat rat |
71—285b; 140c — 550; 600; 870–1060 |
Somani et al., 1992; Grob and Harvey, 1958 — RTECS, 1995 Grob and Harvey, 1958 DA, 1974 |
|
dermal |
human human human monkey pig rat mouse |
28,000 24,000; 1,429–7,143b — 115,900 2500 1080 |
RTECS, 1995 DA, 1974 Somani et al., 1992 — DA, 1974 DA, 1974 RTECS, 1995 |
|
intravenous |
human monkey pig rat |
14 20 15 39 45 |
DA, 1974 DA, 1974 DA, 1974 RTECS, 1995 DA, 1974 |
|
subcutaneous |
human monkey rat |
— — 103–108 |
— — RTECS, 1995 |
|
intramuscular |
human monkey rat |
30c 22 170 108 112 |
Grob and Harvey, 1958 RTECS, 1995 Grob and Harvey, 1958 RTECS, 1995 DA, 1974 |
|
intraperitoneal |
human monkey rat |
— — 218 250 |
— — DA, 1974 RTECS, 1995 |
|
a Values for humans estimated from animal data b Based on 70 kg body weight c Lethal level |
|||
Grob and Harvey (1958) also administered to human volunteers multiple oral doses of GB over a period of 3 days (3–24 hr apart; average 7.5 hr). In two individuals, doses of 0.0005 or 0.005 mg/kg, totaling 0.007 mg/kg over the 3-day period, reduced RBC-ChE 33% and 27%, respectively, but neither produced toxic effects. Multiple doses of 0.008-0.016 mg/kg, totaling 0.088 mg/kg over the 3-day period, produced mild symptoms of toxicity. Similar incremental doses, totaling 0.102 mg/kg over 3 days, produced moderate symptoms of toxicity and >90% reduction in RBC-ChE activity. Grob and Harvey (1958) reported that exposure to GB had a cumulative effect that resulted in increased sensitivity to the chemical.
Bucci et al. (1991) and Bucci and Parker (1992) conducted range-finding studies with GB Type I (GB containing tributylamine as a stabilizer) and GB Type II (GB containing diisopropylcarbodiimide as a stabilizer). The chemicals were administered by gavage once per day, 5 days per week for 3 weeks. These studies indicated that for both GB mixtures, the maximum tolerated dose was 0.3 mg/kg/day and a dose of 0.5 mg/kg/day was lethal to the test animals.
3.3 Subchronic Toxicity
In a subchronic study conducted by the National Center for Toxicological Research (NCTR), male and female CD rats were administered GB Type I (GB containing tributylamine as a stabilizer) or GB Type II (GB containing diisopropylcarbodiimide as a stabilizer) by gavage at dose levels equivalent to 0, 0.075, 0.15, or 0.3 mg GB/kg/day (Bucci et al., 1991; Bucci and Parker, 1992). The doses were given once per day, 5 days per week for 13 weeks. All animals were observed daily for clinical signs of toxicity and weighed weekly. Necropsy examination was performed on all animals. Terminal body and organ weights were recorded. Microscopic evaluation was performed on all high-dose and control animals, and on those tissues of lower dose animals that were abnormal at necropsy Hematological analyses and clinical chemistry (including RBC and plasma cholinesterase) were evaluated in the same 6 male and 6 female rats in each dose group one week before the exposures began and also at weeks 1, 3, 7, and 13. In addition, at necropsy a hemisection of each brain was prepared and tested for NTE activity. In both studies there were several statistically significant changes in clinical chemistry (i.e., aspartate aminotransferse in mid-dose males exposed to GB Type II) and hematology (decrease at week 7 in white blood cells in high-dose males exposed to GB Type II and increase at week 13 in erythrocytes in mid-dose females exposed to GB Type II); however, these effects were not sufficiently consistent to suggest organ dysfunction. Brain NTE was not altered significantly in any rats dosed with GB Type II; however, it was significantly decreased (p <0.05) in female rats dosed with 0.3 mg/kg/day GB Type I. The latter, however, did not exhibit any histological signs of delayed neuropathy. GB Type II was not associated with any neoplastic or non-neoplastic lesions. Two high-dose females and one low-dose female dosed with GB Type I had brain lesions consisting of necrosis and vacuolization of individual hippocampal pyramidal cells. It was reported by Bucci et al. (1991) that this type of lesion is consistent with hippocampal hypoxia resulting from the respiratory convulsant effects of GB; however, Bucci et al. (1991) also noted that post-mortem autolysis could have mimicked cerebral necrosis in two of the animals which were found dead. The third animal exhibited signs consistent with nerve agent toxicity (e.g., rapid breathing, salivation, lacrimation, hemorrhage in the urinary wall, and possible right forelimb paralysis), and the observed neural lesions were attributed to GB. This animal was in the test group receiving 0.075 mg/kg/day. As noted above, none of the rats dosed with up to 0.3 mg/kg/day of GB Type II exhibited brain lesions. GB Type I contains the stabilizer tributylamine and GB Type II contains diisopropylcarbodiimide. Subchronic and chronic toxicity data for these stabilizers are lacking and in terms of acute toxicity, the stabilizers have only a fraction of the toxicity of the nerve agents (e.g., the oral LD50 for tributylamine is 114 mg/kg in rats; RTECS, 1995). Although there is one report indicating that tributylamine is a CNS stimulant (Windholz et al., 1983), there is no evidence to suggest that it contributed to the neurotoxic effect seen in the GB Type I study.
In the NCTR studies, RBC cholinesterase levels in the dosed animals were compared to control values for the same sampling times (Tables 3–5). In both studies there were significant decreases in plasma and RBC-ChE levels at certain time periods. The results for the GB Type II study were more internally consistent than those for GB Type I; i.e., the control values did not vary as greatly and the test groups in general exhibited more clearly defined dose-related changes in enzyme activity. For GB Type II, plasma ChE activity in high- and mid-dose males at week 1; in high- and mid-dose females at weeks 1 and 7; and in high-dose females at week 3 was significantly lower than the corresponding control values for the same time periods (Table 5).
Table 3. RBC-ChE levels in 90-day subchronic study of GB Type I in CD ratsa
|
|
|
Week of treatment |
||||||||
|
Dose (µg/kg/d) |
Sex |
-1 |
1 |
%b |
3 |
%b |
7 |
%b |
13 |
%b |
|
0 |
F |
2343(118) |
1885(196) |
80 |
1765(143) |
75 |
3177(118) |
136 |
1533(375) |
65 |
|
75 |
F |
2420(168) |
1964(121) |
81 |
1584(109) |
65 |
2101(94)c |
87 |
1265(383) |
52 |
|
150 |
F |
2656(94) |
1814(188) |
68 |
1867(143) |
70 |
2217(158)c |
83 |
1318(288) |
50 |
|
300 |
F |
2518(127) |
1564(99) |
62 |
1557(97) |
62 |
2061(126)c |
82 |
1673(441) |
66 |
|
0 |
M |
2085(267) |
1771(205) |
85 |
1954(97) |
94 |
2346(124) |
113 |
1547(285) |
74 |
|
75 |
M |
2219(114) |
1886(282) |
85 |
1834(53) |
83 |
1749(82) |
79 |
1405(166) |
63 |
|
150 |
M |
2444(177) |
1835(151) |
75 |
1907(104) |
78 |
1917(135) |
78 |
1522(173) |
62 |
|
300 |
M |
2632(218) |
1921(126) |
73 |
1990(96) |
76 |
2006(94) |
76 |
1478(98) |
56 |
|
Source: Bucci et al., 1991 a Values given as mean IU/L and (SEM) b Percent of baseline c p <0.05, different from control value (0 µg/kg) |
||||||||||
Table 4. RBC-ChE levels in 90-day subchronic study of GB Type II in CD ratsa
|
|
|
Week of treatment |
||||||||
|
Dose (µg/kg/d) |
Sex |
-1 |
1 |
%b |
3 |
%b |
7 |
%b |
13 |
%b |
|
0 |
F |
1728(174) |
1777(223) |
103 |
1497(182) |
87 |
1828(280) |
106 |
2313(285) |
130 |
|
75 |
F |
1603(149) |
1405(67) |
88 |
1545(221) |
96 |
1353(135) |
84 |
1753(249) |
109 |
|
150 |
F |
1678(132) |
1060(54)c |
63 |
1085(62) |
65 |
985(83)c |
59 |
1553(161)c |
93 |
|
300 |
F |
1653(75) |
968(41)c |
59 |
895(41) |
54 |
865(87)c |
52 |
1510(79)c |
91 |
|
0 |
M |
1118(20) |
1032(40) |
92 |
1270(42) |
114 |
1002(76) |
90 |
1097(54) |
98 |
|
75 |
M |
1180(78) |
728(33) |
62 |
910(47)c |
77 |
748(96)c |
63 |
992(72) |
84 |
|
150 |
M |
1097(46) |
665(51) |
61 |
782(73)c |
71 |
602(36)c |
55 |
835(78)c |
76 |
|
300 |
M |
1156(59) |
612(25) |
53 |
764(91)c |
66 |
606(30)c |
52 |
910(97)c |
79 |
|
Source: Bucci and Parker, 1992 a Mean IU/L and (SEM) b Percent of baseline (week -1). c p <0.05, different from control value (0 µg/kg) |
||||||||||
Inhibition of RBC-AChE was dose-related for females in the two highest dose groups and for males in all dose groups. Maximum RBC-ChE depression (48%) occurred in week 7 in both high-dose males and females. Male rats exposed to the lowest dose of GB Type II exhibited a 38% decrease in RBC-ChE activity in week 1; females exhibited a 12% decrease. By week 13, RBC-ChE activity levels
in females returned to near pre-exposure levels (>90%); however, levels in males were still depressed 16–24%.
The AChE data were re-analyzed by ORNL (using standard deviations) with ANOVA and Dunnett's Comparison (see Appendix B). This analysis indicated that RBC-AChE levels in males were significantly lower (p <0.05) than baseline values in all dose groups at weeks 1, 3, and 7, and for the two highest dose groups at week 13. The values for all dose groups were also significantly lower than controls at week 1, 3, and 7. Similar results were seen in females except that the RBC-AChE levels were not significantly different from controls or baseline values in the low-dose group (Appendix B).
Table 5. Plasma ChE levels in 90-day subchronic study of GB Type II in CD ratsa
|
|
|
Week of treatment |
||||||||
|
Dose (µg/kg/d) |
Sex |
-1 |
1 |
%b |
3 |
%b |
7 |
%b |
13 |
%b |
|
0 |
F |
1267(189) |
1935(304) |
153 |
1461(246) |
115 |
1761(353) |
139 |
2100(350) |
166 |
|
75 |
F |
1381(288) |
1460(206) |
106 |
1483(350) |
107 |
1475(188) |
107 |
1574(280) |
114 |
|
150 |
F |
1136(105) |
705(104)c |
62 |
736(53) |
65 |
790(136)c |
70 |
1280(149) |
113 |
|
300 |
F |
1316(55) |
611(73)c |
46 |
475(58)c |
36 |
481(95)c |
36 |
1311(146) |
100 |
|
0 |
M |
437(33) |
578(59) |
132 |
353(29) |
81 |
254(15) |
58 |
313(18) |
72 |
|
75 |
M |
461(25) |
407(53) |
88 |
296(22) |
64 |
174(12) |
38 |
312(25) |
68 |
|
150 |
M |
443(40) |
239(26)c |
54 |
229(9) |
52 |
122(7) |
28 |
246(13) |
56 |
|
300 |
M |
375(19) |
249(52)c |
66 |
187(15) |
50 |
109(9) |
29 |
257(4) |
69 |
|
Source: Bucci and Parker, 1992 a Mean IU/L and (SEM) b Percent of baseline (week -1). c p <0.05, different from control value (0 µg/kg) |
||||||||||
In a subchronic inhalation study conducted on Fischer 344 rats, no signs of toxicity were observed in animals exposed to 0.0001 or 0.001 mg GB/m3, 6 hr/day, 5 days/week, for up to 24 weeks (Weimer et al., 1979). Compared to the dose levels used in the subchronic oral studies described above, the exposures used in the Weimer et al. (1979) study were relatively low. For example, assuming an inhalation rate of 0.29 m3/day and an average body weight of 0.35 kg for rats and 100% pulmonary absorption, the highest concentration in the Weimer et al. study would be equivalent to an internal dose of only 0.15 µg/kg/day.
3.4 Chronic Toxicity
There is limited information concerning the effects of GB following prolonged exposure to low concentrations. In a retrospective study of workers occupationally exposed to GB for one year or longer prior to the testing, increased brain beta activity, increased delta and theta slowing, decreased alpha activity, and increased amounts of REM (rapid-eye-movement) sleep were observed (Duffy et al., 1979;
Burchfiel and Duffy, 1982). DHHS (1988) considered these changes to be of questionable importance due to the absence of clinically significant neuropsychological effects.
There are no animal studies involving chronic oral exposures to GB. In chronic inhalation studies conducted by Weimer et al. (1979), I.C.R. Swiss and strain A mice, Sprague-Dawley/Wistar and Fischer 344 rats, and purebred beagle dogs were exposed to 0, 0.0001, or 0.001 mg GB/m3, 6 hr/day, 5 days/week, for up to 52 weeks. Four male and 8 female beagles were exposed to each test concentration. In the rodent studies, 50 animals of each sex of each strain were exposed to each test concentration. The control groups were identical to the test groups except that an additional 100 F344 rats and A strain mice were used. Animals were sacrificed according to the schedule listed in Table 6. RBC-AChE activity levels were monitored throughout the study for all the test species. No dose-related, statistically significant changes in RBC-AChE occurred in any species at any sampling time. Using an inhalation rate of 0.29 m3/day for rats, and assuming 100% pulmonary absorption, the
Table 6. Sacrifice schedule for GB chronic study
|
Species |
Number of animals sacrificed |
||||||||
|
Months of exposure |
6-month post exposure |
||||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
9 |
12 |
||
|
Colony rats |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
20 |
|
Fischer 344 rats |
— |
— |
20 |
— |
— |
20 |
20 |
20 |
20 |
|
Colony mice |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
20 |
|
A strain mice |
— |
— |
20 |
— |
— |
20 |
20 |
20 |
20 |
|
Beagle dogs |
2 |
2 |
2 |
— |
— |
2 |
2 |
2 |
— |
|
Source: Weimer et al., 1979 |
|||||||||
6 hr/day, 5 day/week exposure would correspond to an average daily dose of 0.00015 mg/kg. This dose is considerably below the gavage doses of 0.075 mg/kg/day that produced cholinesterase depression in the subchronic studies described in Section 3.3.
Five of 20 dogs exhibited abnormal EKGs at the time of sacrifice; elevated P waves were suggestive of right atrial hypertrophy; however, there was no evidence of enlargement or physical abnormalities of the heart. The absence of pre-exposure data precludes identifying this effect as due to GB exposure. A higher incidence of tracheitis occurred in colony rats (a Sprague Dawley/Wistar population) and in Fischer rats exposed to GB in comparison to control animals (Table 7). The most severe cases occurred in the high-exposure group. The investigators could not determine whether the occurrence of tracheitis was agent-related. No other overt signs of GB-related toxicity were observed at either exposure level. Atrophy of the seminiferous tubules, starting at 12 weeks of exposure, was also seen in the Fischer rats. The investigators noted that this inbred strain of rat is susceptible to numerous genetically based defects which may appear under experimental conditions of stress. The tests were repeated using the same experimental protocol for 12 and 24 weeks. None of the rats in this second assay exhibited testicular atrophy.
Table 7. Incidence of tracheitis in colony rats
|
|
Dose group |
||
|
Exposure period (wk) |
Control |
0.0001 mg/m3 |
0.001 mg/m3 |
|
4 |
0/10 |
5/10 |
0/10 |
|
8 |
0/10 |
4/10 |
9/10 |
|
12 |
0/10 |
5/8 |
5/7 |
|
16 |
0/9 |
0/10 |
1/10 |
|
20 |
0/10 |
0/5 |
2/6 |
|
24 |
1/10 |
1/5 |
1/10 |
|
36 |
0/9 |
2/5 |
2/7 |
|
52 |
2/20 |
1/20 |
6/20 |
|
6-month post-exposure |
0/20 |
7/19 |
9/28 |
|
Totals |
3/108 |
25/98 |
34/108 |
|
Source: Weimer et al., 1979 |
|||
3.5 Nervous System Toxicity
As noted in Section 3.1, the neurotoxic effects following acute exposures to nerve agent such as GB can range from minor symptoms such as fatigue, headache, mild vertigo, weakness, and loss of concentration to convulsions, respiratory arrest, and death. In laboratory animals exposed to GB, single subcutaneous injections sufficiently high to cause convulsions resulted in brain lesions (Singer et al., 1987; see also McLeod, 1985). Brain lesions in the absence of convulsions have also been reported in rats dosed by gavage with 0.3 mg GB Type I/kg/day for 90 days (Bucci et al., 1991).
Exposures to nerve agents have also been associated with subtle neurological effects manifested as altered EEGs (Metcalf and Holmes, 1969; Burchfiel et al., 1976; Duffy et al., 1979; Duffy and Burchfiel, 1980) and psychological changes (Sidell, 1992). The reported EEG abnormalities have not been correlated with behavioral or physiological parameters.
Organophosphate-induced delayed neuropathy (OPIDN) has not been observed in humans exposed to acutely toxic levels of GB (Munro et al., 1994), nor in cats receiving single supralethal doses or multiple low doses of GB for up to 10 days (Goldstein et al., 1987; Goldstein, 1989). In subchronic rat studies, Bucci et al. (1991) found that male and female rats receiving 0.3 mg GB Type I/kg/day for 90 days had reduced brain NTE activity levels (significant at the p <0.05 level in females) but no histopathological signs of OPIDN. Decreases in brain NTE were not seen in a related study in which rats were dosed with the same amount of GB Type II (Bucci and Parker, 1992)). However, signs suggestive of OPIDN have been observed in female Swiss albino mice exposed to 5 mg GB/m3 for 20 min daily for 10 days (Husain et al., 1993). Muscular weakness of the limbs and slight ataxia occurred on the 14th day after the start of the exposures. These changes were accompanied by significant (p <0.001) inhibition of NTE activity in the brain (59.2%), spinal cord (47.4%), and platelets (55.4%). Histological examination
of the spinal cord revealed focal axonal degeneration which was reported to be moderate in two animals and light in four. The same exposure inhibited AChE in blood by 27.3% and in brain by 19.2% but was not associated with any anti-AChE symptoms. Some studies have also demonstrated that GB can induce OPIDN in chickens, but only at supralethal doses. Davies et al. (1960), Davies and Holland (1972) and Gordon et al. (1983) reported that signs of OPIDN appeared in chickens dosed with 20 and 30 times the LD50 if the animals were protected from acute toxic effects by the use of antidotes such as atropine and oxime compounds. However, Bucci et al. (1992a) reported that White Leghorn chickens (atropine-protected) given single oral doses of 70.2, 140.4, or 280.7 µg/kg GB Type II exhibited no clinical signs of OPIDN such as ataxia when evaluated 8 to 43 days after the treatment. At sacrifice, samples of nervous tissue were examined microscopically, but showed no evidence of OPIDN pathology. In a related study conducted by Bucci et al. (1992a), hens were administered the same total doses of GB but in one-third increments given one week apart. There were no signs of neuropathology when the animals were sacrificed 43 days later. In a third study, Bucci et al. (1992a) evaluated the effects of GB on NTE in hens given single doses of GB Type II ranging from 70.2 to 750 µg/kg. Bucci et al. (1992a) reported that under the conditions of the test GB Type II did not cause a significant dose-related change in NTE in the brain or spinal cord.
3.6 Developmental and Reproductive Effects
No data are available to evaluate the potential reproductive and developmental effects of GB in humans; however, studies in laboratory animals indicate that such effects are not likely even at dose levels that are maternally toxic. LaBorde and Bates (1986) conducted developmental toxicity studies on agent GB Type I and GB Type II using CD rats and New Zealand rabbits. In the rat studies, the test animals were dosed with 0, 100, 240, or 380 µg/kg of GB orally on days 6–15 of gestation. Females were weighed on gestational days 0, 6–16 and prior to sacrifice on gestational day 20. The test animals were observed for clinical signs of toxicity. At sacrifice, gravid uteri were weighed and examined for number and status of implants (alive, resorbed or dead). Individual fetal body weight and internal or external malformations were recorded. Maternal toxicity (evidenced by excessive salivation, ataxia, lacrimation) and mortality (8/29 for GB Type I and 13/29 for GB Type II) occurred in the high-dose group. There were no significant differences among treatment groups in the incidence of resorptions or in the average body weight of live fetuses per litter. The only fetal morphological anomaly was fetal hydroureter which occurred at a rate of 5.2, 1.9, 5.3 and 2.1% with GB Type I and 4, 5, 3.2, and 0.5% with GB Type II in the 0, 100, 240, and 300 µg/kg dose groups, respectively. The observed effect was not dose-related and was, therefore, considered to be a spontaneous variant. Skeletal and cartilage variants occurred between dose group but these were not statistically significant. In similar studies conducted on New Zealand rabbits using the same experimental protocol, oral doses of 0, 5, 10, or 15 µg/kg/day on gestational days 6–19, resulted in no fetal toxicity or teratogenicity (Laborde and Bates, 1986). The only observed fetal anomaly was retinal folding which occurred at a rate of 6.8, 3.9, 4.3, and 7.4% for GB Type I and 17, 18, 25, and 19% for GB Type II in the 0, 5, 10, and 15 µg/kg dose groups, respectively. The frequency of the anomaly was not dose-related and the variant was, therefore, considered to be a spontaneously occurring malformation. Maternal toxicity, evidenced by excessive salivation, ataxia, and lacrimation, occurred at the highest dose.
The developmental toxicity of GB was also evaluated by Denk (1975). In this study Sprague-Dawley rats were exposed to GB vapors (0.1 and 1 µg/m3) for 6 hr/day, 5 days/wk, for varying time periods. In one series of tests, male rats were exposed for 1 week to 1 yr, and then mated to unexposed females. Nineteen days after mating, the females were sacrificed and examined for number of corporalutea, deciduomata, number of fetal deaths, and number of live fetuses. In a second series of tests mated pairs of rats were exposed to GB for 1, 2, or 3 weeks or until the pups were whelped. The incidence of
intrauterine deaths was recorded and all fetuses were examined for abnormalities. In a third series of tests, males and females were exposed to GB for 10 months and then mated. The F1 generation was mated, as was the F2 generation. The number and sex of offspring, number of preweaning deaths, number weaned, and pup weights at various ages were recorded. Denk (1975) reported that GB, at the doses and by the route used, had no adverse effects with respect to dominant lethal mutations, reproductive performance, fetal toxicity, and teratogenesis.
3.7 Carcinogenicity
There are no human data to suggest that GB is carcinogenic. As part of chronic inhalation studies conducted by Weimer et al. (1979) (see section 3.4), the tissues of animals exposed to GB for up to one year were examined for microscopic lesions including tumors. The test species included I.C.R. Swiss mice, strain A mice, Sprague-Dawley/Wistar rats, Fischer 344 rats, and purebred beagle dogs. The exposures were to 0.0001 or 0.001 mg GB/m3, 6 hr/day, 5 days/week. Weimer et al. (1979) reported that agent-related tumors did not occur in any of the exposed species. Pulmonary tumors did occur in strain A mice; after 52 weeks of exposure, pulmonary adenomas were present in 3/19 animals exposed to 0.0001 mg GB/m3' in 3/20 animals exposed to 0.001 mg GB/m3, and in 0/20 controls; and for animals maintained for 6 months post-exposure, the incidence rates for pulmonary adenocarcinomas were 5/19, 6/18, and 9/29, respectively. However, these lesions were not considered to be agent-related. Strain A mice have a high natural propensity to form pulmonary tumors; the incidence of spontaneous pulmonary tumors being about 53% in animals 12 months of age and 90% in animals 18 months of age (Heston, 1942). Overall, the studies of Weimer et al. (1979) indicate that agent GB is not carcinogenic.
3.8 Genotoxicity
No information is available regarding the genotoxicity of GB in humans. In bioassays using bacteria and mammalian cell cultures, GB was not genotoxic or mutagenic when tested with or without metabolic activation (Goldman et al., 1987). GB did not induce biologically significant increases in mutations when tested in the Ames Salmonella assay using five revertant strains (TA135, TA100, TA98, TA1537, and TA1538) (Goldman et al., 1987). GB Type I and GB Type II did not induce a significant increase in forward mutations when tested on mouse L5178Y lymphoma cells at concentrations of 50, 100, or 200 µg/mL (Goldman et al., 1987). An increase in sister chromatid exchanges (SCE) was not observed in Chinese hamster ovary cells exposed in vitro to 200 µg/mL of GB (Goldman et al., 1987). Mice treated in vivo with a maximally tolerated intraperitoneal dose of 360 µg GB/kg did not exhibit a significant increase in SCE in splenic lymphocytes (Goldman et al., 1987). Exposure of rat hepatocytes to GB concentrations as high as 2.4 × 10-3 M resulted in a decrease in DNA repair synthesis, leading Goldman et al. (1987) to conclude that GB probably did not damage DNA directly but that it might inhibit DNA synthesis after non-agent-induced DNA damage had occurred.
4. ORAL REFERENCE DOSE FOR GB
4.1 Cholinesterase Inhibition as an RfD Endpoint
The endpoint for identifying a no-observed-adverse-effect level (NOAEL) for nerve agents such as GB is the level at which there is no significant depression in blood cholinesterase activity. In humans, 15% inhibition of RBC-AChE is generally considered to be the minimal change that can be observed with any statistical reliability (Callaway et al., 1951). Existing response data for other organophosphates indicate that RBC-ChE inhibition of as much as 20% is not associated with adverse clinical signs or symptoms in humans and should be considered only as evidence of exposure (Marquis, 1988). This contention is supported by the U.S. EPA (1995a) which reports scientific agreement that statistically significant inhibition of cholinesterase in multiple organs and tissues accompanied by clinical effects constitutes a hazard; however, in the absence of clinical effects, such inhibition may not be of biological significance. It is generally agreed that inhibition of RBC and/or plasma cholinesterase contributes to the overall hazard identification of cholinesterase inhibiting agents by serving as biomarkers (U.S. EPA, 1995a). Animal data have shown that exposure to low doses of nerve agents for extended periods of time can result in low blood ChE activity levels without signs of toxicity. Bucci et al. (1992b) found no evidence of toxicity in rats dosed i.p. with GA (up to 112 µg/kg), even though RBC-AChE activity was reduced about 37% in females (relative to controls). In oral toxicity studies conducted on GB, Bucci and Parker (1992) found that gavage doses of 0.3 mg/kg/day to rats caused nearly a 50% reduction in RBC-AChE activity without signs of toxicity. Goldman et al. (1988) reported no signs of toxicity, but 78–80% reduction in RBC-AChE activity, in Sprague-Dawley rats dosed subcutaneously with 1.0 µg VX/kg/day over 30 days. Rice et al. (1971) reported that whole blood cholinesterase of sheep dosed orally with 15 µg VX/day was reduced to 5% of the normalized baseline values (during the last 3 weeks of the dosing period) without any signs of toxicity. Rice et al. (1971) also found that sheep showing signs of toxicity at higher dose levels recovered fully after the exposures ended. Further complicating the evaluation is the extreme variability in ChE levels of individual animals and different sexes and ages of the same species (Halbrook et al., 1992). Possible changes in blood ChE that may occur with increasing age of the animals requires comparisons with concurrent controls, because the absence of a significant difference from preexposure value may be due to age-related increases in ChE in the dosed animals.
Blood ChE activity has been used by EPA as the critical endpoint in the establishment of oral RfDs for organophosphate insecticides (U.S. EPA 1995b-d). In the case of malathion (U.S. EPA, 1995a), the no-observed-effect level (NOEL) was identified as the highest oral dose level at which no significant change in RBC-AChE or plasma-ChE activity was recorded in 5 human volunteers who received the compound orally for 47 days (Moeller and Rider, 1962). The next highest dose was associated with a depression of about 25% in both RBC-AChE and plasma ChE, but no clinical signs of toxicity. The EPA approach, also used for other organophosphate pesticides (U.S. EPA, 1995b), is, therefore, to identify the lowest-effect level (LEL) as the dose at which statistically significant decreases in ChE levels (RBC-AChE, plasma-ChE, or brain-ChE) occur, and then to base an RfD on the dose level where the change in ChE is not statistically significant. This approach is also used in this report so that the RfDs developed for the nerve agents will not be disproportionally different from those for organophosphate insecticides; however, it should be emphasized that these values may be overly conservative. Furthermore, in evaluating the experimental data for the nerve agents, added weight was given to those cases where significant changes in ChE occurred relative to both control and pre-exposure values and where there was evidence of a dose-response relationship.
4.2 Derivation of the Oral RfD
In the derivation of an oral RfD, human oral exposure data are preferred (as in the case of malathion); however, the only available human data for GB pertain to acute exposures. Although such data can be used to establish short-term exposure limits; acute toxicity endpoints are generally not used for developing subchronic or chronic reference values.
The only subchronic or chronic exposure studies for GB that were found in the available literature consist of a 90-day study in which rats were given GB Type I (Bucci et al., 1991) or GB Type II (Bucci and Parker, 1992) by gavage, and a 1-year study in which rats, mice, and dogs were exposed to GB by inhalation (Weimer et al., 1979). For the development of an oral RfD, a study involving the same exposure pathway is preferred even though the exposure period may be less than chronic.
The use of the subchronic rat study for developing an oral RfD for GB is complicated by the fact that rodents have a much lower RBC-AChE activity level compared to humans (Ellin, 1981, see Table 1). By itself, this could cause rats to be relatively more sensitive than humans to anticholinesterase compounds; however, the lower RBC-ChE activity may be offset by the presence of aliesterases in rat blood. Aliesterases, which are not present in humans (Cohen et al., 1971), are known to bind to and thereby reduce the toxicity of GB (Fonnum and Sterri, 1981). Other species differences, such as the rates of aging of the GB-ChE complex, the rates of synthesis of plasma cholinesterase in the liver, and the levels of AChE in various parts of the nervous system (see Ivanov et al., 1993) may also result in differences in species' sensitivities. There is insufficient data to determine the relative susceptibilities of humans and rodents to GB; therefore, for the purpose of this assessment, the EPA method will be followed which assumes that humans may be as much as ten times more sensitive to a chemical than laboratory animals.
The subchronic rat study conducted by Bucci and Parker (1992) with GB Type II is used here to derive an oral RfD for GB. This study is described in detail in Section 3.3. Briefly summarized, the results of this study showed statistically significant (p <0.05) decreases in plasma and RBC-ChE activity levels in male and female CD rats dosed by gavage once per day, 5 days per week for 13 weeks. The RBC-ChE levels are shown in Table 4. Significant reductions in RBC-AChE relative to controls and to baseline values were seen in male rats in all dose groups (Appendix B). Although no other toxic effects were observed in the rats dosed with GB Type II, brain lesions (hippocampal necrosis) occurred in rats dosed with GB Type I (in 1 of 12 females dosed with 0.075 mg/kg/day, and in 2 of 12 females dosed with 0.3 mg/kg/day, but in none of the females dosed with 0.150 mg/kg/day, and in the of the males dosed with 0.075, 0.15, or 0.3 mg/kg/day) (Bucci et al., 1991). The absence of effects at the mid-dose, and the possibility that post-mortem autolysis contributed to the findings (see section 3.3) makes it difficult to select a LOAEL or NOAEL for this endpoint.
The lowest tested dose (0.075 mg/kg/day) is considered a lowest-observed-adverse-effect level (LOAEL) because of the statistically significant reduction in RBC-AChE seen in male rats dosed with GB Type II. This dose is adjusted to a 7 days/week exposure period by using a factor of 5/7; i.e., 5/7 × 0.075 mg/kg/day = 0.054 mg/kg/day. The RfD can then be calculated according to the following formula:
(2)
where:
|
UF1 |
= |
10 (sensitive subpopulations) |
|
UF2 |
= |
10 (animal to human extrapolation) |
|
UF3 |
= |
3 (subchronic-to-chronic extrapolation) |
|
UF4 |
= |
3 (LOAEL-to-NOAEL extrapolation) |
|
UF5 |
= |
3 (data base incomplete) |
|
MF |
= |
1 (modifying factor - not needed) |
An uncertainty factor of 10 for sensitive subpopulations is considered necessary because some individuals have a genetic defect causing their blood cholinesterase activity to be abnormally low (Evans et al., 1952; Harris and Whitaker, 1962). For homozygous individuals, the activity can be as low as 8–21% of the normal mean (Bonderman, and Bonderman, 1971). These individuals may be unusually sensitive to organophosphate anticholinesterase compounds (Morgan, 1989).
An uncertainty factor of 10 is used for animal-to-human extrapolation because there is ample evidence that humans are more sensitive to GB than laboratory rodents. In humans, the single dose oral RBC-AChE 50 (dose required to lower red blood cell cholinesterase by 50%) is 0.01 mg/kg (Grob and Harvey, 1958), and an average daily dose of 0.034 mg/kg for three days resulted in moderate signs of toxicity. In comparison, rats receiving 0.3 mg GB Type II/kg/day for 90 days exhibited decreases in blood cholinesterase levels but no signs of toxicity (Bucci and Parker, 1992).
An uncertainty factor of 3 is used to extrapolate from a subchronic to chronic exposure. In the derivation of oral RfDs for other organophosphate compounds, EPA has used NOAELs for cholinesterase inhibition following short-term exposures without adjustment for a more prolonged exposure period because of the unlikelihood that the endpoint would change over time (i.e., a subchronic-to-chronic UF of 1 was used). In addition, animal data indicate that maximum ChE inhibition may occur 30–60 days or more after exposure begins after which it levels off or even shows signs of recovery. In the Bucci and Parker study plasma and RBC-ChE activity levels at week 13 were no longer significantly different from both baseline and control values, particularly for the lowest dose level (see Appendix B); therefore, increased ChE inhibition is not expected to occur at longer exposure periods. However, an uncertainty factor of 3 is used here because studies are not available to verify that adverse effects would not occur following chronic exposures.
A LOAEL-to-NOAEL uncertainty factor of 3 is used instead of 10 because the endpoint, cholinesterase inhibition, was not associated with signs of toxicity.
The data base for GB consists of two well designed and well conducted subchronic toxicity studies in rats, developmental studies in two species (rats and rabbits), delayed neuropathy studies in chickens and rats, and chronic inhalation studies in mice, rats and dogs. In addition, there is substantial human data for acute and short-term exposures. These studies support the use of cholinesterase inhibition as the critical endpoint for deriving an oral RfD. The data base for GB is, however, lacking a multi-generational reproductive toxicity study. Because studies on other organophosphate cholinesterase inhibitors, including a multi-generational study on agent VX, indicate that reproductive effects are unlikely, a full Uncertainty Factor of 10 is not considered necessary.
Therefore,
(3)
(4)
(5)
4.3 Overall Confidence in the Oral RfD
Study: High
Data Base: Medium
RfD: Medium
The principal study was well-designed and well-conducted, used a relevant exposure pathway, and examined the appropriate toxicological endpoints. The data base for GB also contains a second oral subchronic study in rats, chronic inhalation studies in rats, mice and dogs, teratology studies in rats and rabbits, and delayed neuropathy studies in mice and chickens. Deficiencies in the data base consist primarily of a lack of a multi-generational reproductive toxicity study, and a standard toxicity study in a second species. Consequently, the overall confidence in the RfD is medium.
4.4 Comparison of the RfD with Human Toxicity Data
The RfD is compared to the available human toxicity data in Table 8. One study in humans indicated that an oral dose of 2.3 µg/kg/day for three days resulted in 27 and 33% RBC-AChE inhibition but no toxic effects. This dose is about 115 times greater than the derived RfD. For an adverse effect level (i.e., mild toxic effect at 29 µg/kg/day; Grob and Harvey, 1958), the ''margin of safety" would be about 10 times greater than that for the 27–33% RBC-AChE inhibition.
5. CARCINOGENICITY ASSESSMENT
The potential carcinogenicity of GB cannot be determined; however, limited data from animal inhalation studies suggest that agent GB is not carcinogenic (see Section 3.7). The results of mutagenicity assays on bacteria, in vitro tests on mammalian cell cultures, and in vivo studies on mice (see Section 3.8) indicate that GB is not genotoxic or mutagenic. These data provide supporting evidence that GB is not likely to be carcinogenic.
Table 8. Comparison of RfD with Human Toxicity Data for GB
|
Dose (µg/kg) |
Exposure Route |
Endpoint |
References |
|
0.02 |
oral |
RfD - no inhibition of RBC-AChE |
This report |
|
2 |
oral |
excessive dreaming |
Thienes and Haley, 1972 |
|
2.3 |
oral; average daily dose for three days |
RBC-AChE reduced 27 and 33%, but no toxic effects |
Grob and Harvey, 1958 |
|
10 |
oral |
50% inhibition of AChE |
Grob and Harvey, 1958 |
|
20 |
oral |
insomnia, withdrawal, depression |
Thienes and Haley, 1972 |
|
22 |
oral |
mild toxic effects, anorexia, nausea, hearburn |
Grob and Harvey, 1958 |
|
29 |
oral; average daily dose for three days |
mild toxic effects |
Grob and Harvey, 1958 |
|
30 |
oral |
moderate toxic effects |
Grob and Harvey, 1958 |
|
34 |
oral; average daily dose for three days |
moderate toxic effects; > 90% reduction in RBC-AChE activity |
Grob and Harvey, 1958 |
|
140 |
oral |
Estimated lethal oral dose |
Grob and Harvey, 1958 |
6.
REFERENCES CITED
Albuquerque, E.X., S.S. Deshpande, M. Kawabuchi, Y. Aracava, M. Idriss, D.L. Rickett, and A.F. Boyne. 1985. Multiple actions of anticholinesterase agents on chemosensitive synapses: Molecular basis for prophylaxis and treatment of organophosphate poisoning. Fund. Appl. Toxicol. 5:S182–S203.
Barnes, J.M. 1954. Organo-phosphorus insecticides. The toxic action of organo-phosphorus insecticides in mammals. Chem. and Ind. January 2, 1954, pp. 478–480.
Bonderman, R.P. and D.P. Bonderman. 1971. A titrimetric method for differentiating between atypical and inhibited human serum pseudocholinesterase. Arch. Environ. Health 22:578–581. (Cited in Hayes, 1982)
Bucci, T.J., R.M. Parker, J.A. Crowell, et al. 1991. Toxicity Studies on Agents GB and GD (Phase II), 90 Day Subchronic Study of GB (Sarin Type I) in CD-Rats. Final Report. FDA 224-865-0007. Prepared for U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, MD. DTIC AD-A248617.
Bucci, T.J. and R.M. Parker. 1992. Toxicity Studies on Agents GB and GD (Phase II), 90 Day Subchronic Study of GB (Sarin Type II) in CD-Rats. Final Report. Prepared for the U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, MD. DTIC AD-A248618.
Bucci, T.J., R.M. Parker and P.A. Gosnell. 1992a. Delayed Neuropathy Study of Sarin, Type II, in SPF White Leghorn Chickens. Technical Report. Prepared by the National Center for Toxicological Research, Jefferson, AK, for U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, MD. NTCR Rept Nos. 478, 479.
Bucci, T.J., R.M. Parker, J.A. Crowell, J.D. Thurman and P.A. Gosnell. 1992b. Toxicity Studies on Agent GA (Phase II): 90 Day Subchronic Study of GA (Tabun) in CD Rats. Final Report. Prepared for the U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, MD. DTIC AD-A258020.
Burchfiel, J.L., F.H. Duffy and V.M. Sim. 1976. Persistent effects of sarin and dieldrin upon the primate electroencephalogram. Toxicol. Appl. Pharmacol. 35: 365–369.
Burchfiel, J.L. and F.H. Duffy. 1982. Organophosphate neurotoxicity: chronic effects of sarin on the electroencephalogram of monkey and man. Neurobehav. Toxicol. Teratol. 4: 767–778.
Callaway, S., D.R. Davies and J.P. Rutland. 1951. Blood cholinesterase levels and range of personal variation in a healthy adult population. Br. Med. J. 2:812-816. (Cited in Hayes, 1982)
Carnes, S.A. and A.P. Watson. 1989. Disposing of the U.S. chemical weapons stockpile: An approaching reality. JAMA 262:653-659.
Clark, D.N. 1989. Review of Reactions of Chemical Agents in Water. AD-A213 287, Defense Technical Information Center.
Cohen, E.M., P.J. Christen and E. Mobach. 1971. The inactivation by oximes of Sarin and Soman in plasma from various species. I. The influence of diacetylmonoxime on the hydrolysis of Sarin. J.A. Cohen memorial issue. North-Holland Publishing Company, Amsterdam.
DA (U.S. Department of the Army). 1974. Chemical Agent Data Sheets, vol. 1. Edgewood Arsenal Special Report, EO-SR 74001. Defense Tech. Inform. Center, Alexandria, VA.
DA (U.S. Department of the Army). 1992. Material Safety Data Sheets: GB. Edgewood Research, Development and Engineering Center, Aberdeen Proving Ground, MD.
Dacre, J.C. 1984. Toxicology of some anticholinesterases used as chemical warfare agents - a review. In: Cholinesterases: Fundamental and Applied Aspects, M. Brzin, E.A. Barnard and D. Sket, eds., Walter de Gruyter, New York. pp. 415–426.
Davies, D.R., P. Holland and M.J. Rumens. 1960. The relationship between the chemical structure and neurotoxicity of alkyl organophosphorus compounds. Brit. J. Pharmacol. 15:271–278.
Davies, D.R. and P. Holland. 1972. Effect of oximes and atropine upon the development of delayed neurotoxic signs in chickens following poisoning by DFP and sarin. Biochem. Pharmacol. 21:3145–3151.
Denk, J.R. 1975. Effects of GB on Mammalian Germ Cells and Reproductive Parameters. EB-TR-74087. (Cited in Weimar et al., 1979)
DHHS (U.S. Department of Health and Human Services, Centers for Disease Control). 1988. Final recommendations for protecting the health and safety against potential adverse effects of long-term exposure to low doses of agents: GA, GB, VX, Mustard Agent (H, HD, T), and Lewisite (L). Federal Register 53(50):8504–8507.
Duffy, F.H., J.L. Burchfiel. P.H. Bartels, et al. 1979. Long-term effects of an organophosphate upon the human electroencephalogram. Toxicol. Appl. Pharmacol. 47:161–176.
Duffy, F.H. and J.L. Burchfiel. 1980. Long-term effects of the organophosphate sarin on EEGs in monkeys and humans. Neurotoxicol. 1:667–689.
Dulaney, M.D., B. Hoskins and I.K. Ho. 1985. Studies on low dose sub-acute administration of soman, sarin, and tabun in the rat. Acta Pharmacol. Toxicol. 57:234–241.
Ellin, R.I. 1981. Anomalies in Theories and Therapy of Intoxication by Potent Organophosphorus Anticholinesterase Compounds. Special Publication USABML-SP-81-003, AD A101364. U.S. Army Medical Research and Development Command, Biomedical Laboratory, Aberdeen Proving Ground, MD.
Epstein, J. 1974. Properties of GB in water. J. Am. Water Works Assoc. 66:31–37.
Eto, M. 1974. Organophosphorus Pesticides: Organic and Biological Chemistry. CRC Press, Cleveland, OH. pp. 123–231.
Evans, F.T., P.W.S. Gray, H. Lehmann and E. Silk. 1952. Sensitivity to succinylcholine in relation to serum cholinesterase. Lancet 1:1129–1230. (Cited in Hayes, 1982).
Fonnum, F. and S.H. Sterri. 1981. Factors modifying the toxicity of organophosphorus compounds including soman and sarin. Fund. Appl. Toxicol. 1:143–147.
Gershon, J.L. and F.H. Shaw. 1961. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet (June 24, 1961):1371–1374.
Goldman, M., A.K. Klein, T.G. Kawakami and L.S. Rosenblatt. 1987. Taxicity Studies on Agents GB and GD. Final Report from the Laboratory for Energy-Related Health Research to U.S. Army Medical Research and Development Command, Fort Detrick, MD. AD A187841 .
Goldman, M., B.W. Wilson, T.G. Kawakami, L.S. Rosenblatt, M.R. Culbertson, J.P. Schreider, J.F. Remsen, and M. Shifrine. 1988. Toxicity Studies on Agent VX. Final Report from the Laboratory for Energy-Related Health Research to U.S. Army Medical Research and Development Command, Fort Detrick, MD. AD A201397.
Goldstein, B.D. D. R. Fincher and J.R. Searle. 1987. Electrophysiological changes in the primary sensory neuron following subchronic soman or sarin: alterations in sensory receptor function. Toxicol. Appl. Pharmacol. 9:55–64.
Goldstein, B.D. 1989. Changes in spinal cord reflexes following subchronic exposure to soman and sarin. Toxicol. Lett. 47:1–8.
Gordon, J.J., R.H. Inns, M.K. Johnson, et al. 1983. The delayed neuropathic effects of nerve agents and some other organophosphorus compounds. Arch. Toxicol. 52:71–82. (Cited in Munro et al., 1994)
Grob, D. and J.C. Harvey. 1958. Effects in man of the anticholinesterase compound Sarin (isopropyl methyl phosphonofluoridate). J. Clin. Invest. 37(1):350–368.
Halbrook, R.S., L.R. Shugart, A.P. Watson, N.B. Munro and R.D. Linnabary. 1992. Characterizing biological variability in livestock blood cholinesterase activity for biomonitoring organophosphate nerve agent exposure. J. Amer. Vet. Med. Assoc. 201:714–725.
Harris, H. and M. Whittaker. 1962. The serum cholinesterase variants. Study of twenty-two families selected via the "intermediate" phenotype. Ann. Hum. Genet. 26:59–72. (Cited in Hayes, 1982)
Heston, W.E. 1942. Inheritance of susceptibility to spontaneous pulmonary tumors in mice. J. Natl. Cancer Inst. 3:79–82.
Heston, W.E. and W.D. Levillain. 1953. Pulmonary tumors in strain A mice exposed to mustard gas. Proc. Soc. Exp. Biol. 82:457–460.
Hoskins, B. and I.K. Ho. 1992. Tolerance to organophosphate cholinesterase inhibitors. In: Organophosphates: Chemistry, Fate and Effects, J.E. Chambers and P.E. Levi, eds. Academic Press, New York, pp. 285–297.
Husain, K., R. Vijayaraghavan, S.C. Pant, et al. 1993. Delayed neurotoxic effect of Sarin in mice after repeated inhalation exposure. J. Appl. Toxicol. 13:143–145.
Ivanov, P., B. Georgiev, K. Kirov, L. Venkov. 1993. Correlation between concentration of cholinesterase and the resistance of animals to organophosphorus compounds. Drug Chem. Toxicol. 16:81–99
Jimmerson, V.R. T-M. Shih and R.B. Mailman. 1989. Variability in soman toxicity in the rat: Correlation with biochemical and behavioral measures. Toxicology 57:241–254.
LaBorde, J.B. and H.K. Bates. 1986. Developmental Toxicity Study of Agent GB-DCSM Types I and II in CD Rats and NZW Rabbits. Final Report. National Center for Toxicological Research, FDA, Jefferson, AR. Prepared for U.S. Army Medical Research and Development Command, Fort Detrick, MD
Marquis, J.K. (ed.). 1988. Cholinesterase inhibition as an indication of adverse toxicologic effects. Review draft (June, 1988). Prepared for the Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC.
MacNaughton, M.G. and J.H. Brewer. 1994. Environmental Chemistry and Fate of Chemical Warfare Agents. Southwest Research Institute, San Antonio, TX
Matsumura, F. 1976. Toxicology of Insecticides. Plenum Press, New York, NY, pp. 17–46, 64–78, 142–152, 403–444, 462–464.
McLeod, C.G. 1985. Pathology of nerve agents: perspectives on medical management. Fund. Appl. Toxicol. 5:S10–S16.
McNamara, B.P. and F. Leitnaker. 1971. Toxicological Basis For Controlling Emission of GB Into the Environment. EASP 100-98, AD 914271L. U.S. Army, Medical Research Laboratory, Edgewood Arsenal, Aberdeen Proving Ground, MD.
Metcalf, D.R. and J.H. Holmes. 1969. EEG, psychological, and neurological alterations in humans with organophosphorus exposure. Ann. N. Y. Acad. Sci. 357–365.
Mick, D.L. 1974. Collaborative study of neurobehavioral and neurophysiological parameters in relation to occupational exposure to organophosphate pesticides. In: Behavioral Toxicology: Early Detection of Occupational Hazards. C. Xintaras, B.L. Johnson and I. de Groot, eds. Center for Disease Control, National Institute for Occupational Safety and Health, Washington, DC. pp. 152–153.
Moeller, H.C. and J.A. Rider. 1962. Plasma and red blood cell cholinesterase activity as indications of the threshold of incipient toxicity of ethyl-p-nitrophenyl thiobenzenephosphorate (EPN) and malathion in human beings. Toxicol. Appl. Pharmacol. 4:123–130. (Cited in U.S. EPA, 1995a)
Morgan, D.P. 1989. Recognition and Management of Pesticide Poisonings, 4th ed., EPA-540/9-88-001, U.S. Environmental Protection Agency, Washington, DC.
Morrill, L.G., L.W. Reed and K.S.K. Chinn. 1985. Toxic Chemicals in the Soil Environment. Volume 2. Interaction of Some Toxic Chemicals/Chemical Warfare Agents and Soils. Oklahoma State University TECOM Project 2-CO-210-049, Stillwater, OK. Available from DTIC, AD-A158 215.
Munro, N.B., K.R. Ambrose and A.P. Watson. 1994. Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: Implications for public protection. Envir. Health Perspect. 102:18–38.
O'Brien, R.D. 1960. Toxic Phosphorus Esters: Chemistry. Metabolism, and Biological Effects. Academic Press, New York NY, pp. 175–239.
Rice, G.B., T.W. Lambert, B. Haas and V. Wallace. 1971. Effect of Chronic Ingestion of VX on Ovine Blood Cholinesterase. Technical Report DTC 71–512, Deseret Test Center, Dugway Proving Ground, Dugway UT.
Rickett, D.J., J.F. Glenn and W.E. Houston. 1987. Medical defense against nerve agents: New directions. Mil. Med. 152:35–41.
Rider, J.A., L.E. Ellinwood and J.M. Coon. 1952. Production of tolerance in the rat to octamethylpyrophosphoramide (OMPA). Proc. Soc. Exptl. Biol. Med. 81:455–459.
Rodnitzky, R.L. 1974. Neurological and behavioral aspects of occupational exposure to organophosphate pesticides. In: Behavioral Toxicology: Early Detection of Occupational Hazards. C. Xintaras, B.L. Johnson and I. de Groot, eds. Center for Disease Control, National Institute for Occupational Safety and Health, Washington, DC. pp. 165–174.
Rosenblatt, D.H., M.J. Small, T.A. Kimmell and A.W. Anderson. 1995. Agent Decontamination Chemistry Technical Report. U.S. Army Test and Evaluation Command (TECOM) Technical Report, Phase I. Draft Report, Argonne National Laboratory.
RTECS (Registry of Toxic Effects of Chemical Substances). 1995. MEDLARS Online Information Retrieval System, National Library of Medicine, Computer printout.
Sanches, M.L., C.R. Russell, and C.L. Randolf. 1993. Chemical Weapons Convention (CWC) Signature Analysis. DNA-TR-92-73, AD B171788, Defense Technical Information Center.
Savage, E.P., T.J. Keefe, L.M. Mounce, et al. 1988. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch. Environ. Health 43:38–45.
Sidell, F.R. 1992. Clinical considerations in nerve agent intoxication. In: Chemical Warfare Agents, S. Somani, ed., Academic Press, N.Y., pp 155–194.
Sidell, F.R. and W.A. Groff. 1974. The reactivatibility of cholinesterase inhibited by VX and Sarin in man. Toxicol. Appl. Pharmacol. 27:241–252.
Sidell, F.R. and A. Kaminskis. 1975. Temporal intrapersonal physiological variability of cholinesterase activity in human plasma and erythrocytes. Clin. Chem. 21:1961–1963.
Singer, A.W., N.K. Jaax, J.S. Graham and C.G. McLeod, Jr. 1987. Cardiomyopathy in Soman and Sarin intoxicated rats. Toxicol. Letters 36:243–249.
Small, M.J. 1984. Compounds Formed from the Chemical Decontamination of HD, GB, and VX and Their Environmental Fate. Technical Report 8304, AD A149515, US Army Medical Bioengineering Research and Development Laboratory, Fort Detrick, Frederick, MD.
Somani, S.M., R.P. Solana and S.N. Dube. 1992. Toxicodynamics of nerve agents. In: Chemical Warfare Agents, S.M. Somani, ed., Academic Press, Inc. New York. pp. 67–123.
Sumerford, W.T., W.J. Hayes, J.M. Johnston, K. Walker and J. Spillane. 1953. Cholinesterase response and symptomatology from exposure to organic phosphorus insecticides. AMA Arch. Ind Hyg. Occup. Med. 7:383–398.
Tabershaw, I.R. and W.C. Cooper. 1966. Sequelae of acute organic phosphate poisoning. J. Occup. Med. 8:5–20.
Tammelin, L.E. 1958. Organophosphorylcholines and cholinesterases. Arkiv. Kemi. 12(31):287–298.
Thienes, C.H. and T.J. Haley. 1972. Clinical Toxicology. Lea and Febiger, Philadelphia, PA. pp. 95–115.
U.S. EPA (U.S. Environmental Protection Agency). 1995a. Proposed Guidelines for Neurotoxicity Risk Assessment: Notice. Federal Register 60(192):52032–52056.
U.S. EPA (U.S. Environmental Protection Agency). 1995b. Oral RfD Assessment for Malathion. Integrated Risk Information System (IRIS). Online file. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Cincinnati, OH.
U.S. EPA (U.S. Environmental Protection Agency). 1995c. Oral RfD Assessment for Ethion. Integrated Risk Information System (IRIS). Online file. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Cincinnati, OH.
U.S. EPA (U.S. Environmental Protection Agency). 1995d. Oral RfD Assessment for Aldicarb Sulfone. Integrated Risk Information System (IRIS). Online file. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Cincinnati, OH.
Wagner, S.L. 1983. Organophosphates. In: Clinical Toxicology of Agricultural Chemicals. Noyes Data Corporation, Park Ridge, NJ, pp. 205–246.
Watson, A.P., K.R. Ambrose, G.D. Griffin, et al. 1989. Health effects of warfare agent exposure: implications for stockpile disposal. Environ. Prof. 11:335–353.
Weimer, J.T., B.P. McNamara, E.J. Owens, et al. 1979. Proposed Revision of Limits for Human Exposure to GB Vapor in Nonmilitary Operations Based on One-Year Exposures of Laboratory Animals to Low Airborne Concentrations. ARCSL-TR-78056. U.S. Army Armament Research and Development Command, Chemical Systems Laboratory, Aberdeen Proving Ground MD.
Wills, J.H. 1972. The measurement and significance of changes in the cholinesterase activities of erythrocytes and plasma in man and animals. CRC Crit. Rev. Toxicol. 1:153–202.
Windholz, M. S. Budavari, R.F. Blumetti and E.S. Otterbein, eds. 1983. The Merck Index. An Encyclopedia of Chemicals and Drugs. 10th ed. Merck and Co. Rahway, NJ.
Yager, J., H. McLean, M. Hudes and R.C. Spear. 1976. Components of variability in blood cholinesterase assay results. J. Occup. Med. 18:242–244.
APPENDIX A Comparison of RfDs, ChE Inhibition and Toxicity Data for GA, GB, GD, and VX
|
Endpoint |
GA (µg/kg/day) |
GB (µg/kg/day) |
GD (µg/kg/day) |
VX (µg/kg/day) |
Ref. |
|
RfD |
0.04 |
0.02 |
0.004 |
0.0006 |
This report |
|
Estimated no-effect level for RBC-AChE inhibition |
- |
1.0 |
- |
0.24 |
GBd VXa |
|
27–33% inhibition of RBC-AChE in humans/oral dose |
- |
2.3 (3 days) |
- |
0.2-2.0 |
GB - Grob and Harvey, 1958; VX-this report |
|
RBC-AChE inhibition in humans/i.v. dose |
- |
- |
1.5-2.0 |
1.0 (50%) |
DA, 1974; Sidell and Groff, 1974 |
|
50–60% RBC-AChE inhibition in humans/oral dose |
- |
10 |
- |
2.4 |
GB - Grob and Harvey, 1958; VX-Sidell and Groff, 1974 |
|
50% brain ChE inhibition in vitro |
1.5 × 10-8 (c) |
0.3 × 10-8 (c) |
- |
- |
Grob and Harvey, 1958 |
|
Acute toxic effects in humans/oral dose |
- |
20–30 |
- |
2–4.5 |
GB - Thienes and Haley 1972; Grob and Harvey, 1958; VX-Sidell and Groff, 1974 |
|
human oral LD50 (estimated) |
25–50b |
5–20b |
5–20 |
3–10b |
Somani et al., 1992 |
|
rat oral LD50 |
3700 |
870–1060 600 |
400 |
77–128 |
DA, 1974 Grob & Harvey, 1958 |
|
monkey i.v. LD50 |
50 |
20 |
- |
6–11 |
DA, 1974 |
|
rat i.v. LD50 |
70 |
45–63 |
50 |
6.9–10.1 |
Dacre, 1984 |
|
rat i.p. LD50 |
490, 800 |
250 218 |
- |
37–55 |
DA, 1974 RTECS, 1995 |
|
a Based on ration of oral to i.v. doses (2.4 and 1.0 µg/kg, respectively) required for 50% RbC-ChE inhibition and the estimated i.v. no effect dose of 0.1 µg/kg. b Values were estimated from animal data. c Molar concentration d Estimated from RBC-ChE50 values for GB and VX. |
|||||
Appendix B Statistical Analysis of GB-Induced ChE Inhibition in Rats
GB Type II - RBC Cholinesterase Inhibition in Female Ratsa (ANOVA and Dunnett's Comparison)
GB Type II - RBC Cholinesterase Inhibition in Male Ratsa (ANOVA and Dunnett's Comparison)
GB Type II - RBC Cholinesterase Inhibition in Female Ratsa (ANOVA and Dunnett's Comparison)
GB Type II - RBC Cholinesterase Inhibition in Male Ratsa (ANOVA and Dunnett's Comparison)




































