HEALTH RISK ASSESSMENT FOR SULFUR MUSTARD (HD)
DRAFT REPORT
September 1996
(editorial corrections made April 1997)
Prepared for
U.S. Department of the Army
Army Environmental Center
under Interagency Agreement No. 1769-1769-A1
Prepared by
Life Sciences Division
OAK RIDGE NATIONAL LABORATORY*
Oak Ridge, Tennessee 37831
Submitted to
Material/Chemical Risk Assessment Working Group
Advisory and Coordinating Committee
Environmental Risk Assessment Program
PREFACE
This report assesses the potential non-cancer and cancer effects of sulfur mustard (HD) (CAS No. 505-60-2).
This document supports the activities of the Material/Chemical Risk Assessment Working Group of the Environmental Risk Assessment Program, a cooperative endeavor of the Department of Defense, Department of Energy, and Environmental Protection Agency. This working group is developing toxicity values for selected chemicals of concern at federal facilities. Toxicity values will be submitted for consideration by the EPA's IRIS Consensus Process for inclusion on IRIS (EPA's Integrated Risk Information System). The Material/Chemical Risk Assessment Working Group consists of Drs. Jim Cogliano (chair) and Harlal Choudhury (U.S. EPA), Dr. Bruce Briggs (Geo-Centers); Lt. Cmdr. Warren Jederberg and Dr. Robert L. Carpenter (U.S. Naval Medical Research Institute); Dr. Elizabeth Maull and Mr. John Hinz (U.S. Air Force Occupational and Environmental Health Directorate); Drs. Glenn Leach and Winnie Palmer (U.S. Army Center for Health Promotion and Preventive Medicine); Drs. Robert Young and Po-Yung Lu (Oak Ridge National Laboratory).
This document was written by Drs. Dennis M. Opresko and Rosmarie Faust, Life Sciences Division, Oak Ridge National Laboratory (ORNL), Oak Ridge, TN. Internal peer review was provided by Dr. Robert Young, Dr. Annetta Watson, and Mr. Robert Ross. External review of the toxicity data was provided by Dr. Thomas J. Bucci, Integrated Services, White Hall, AR and Dr. I.K Ho of the U. of Mississippi Medical Center, Jackson MS. External review of the derivation of the RfDs was provided by Drs. Michael Dourson and Susan Velazquez of Toxicology Excellence for Risk Assessment, Cincinnati, OH, and Dr. William Hartley of Tulane Medical Center, New Orleans LA. Additional reviews were provided by Mr. Joe King, Dr. Jack Heller, Ms. Veronique Hauschild, Ms. Bonnie Gaborek, Mr. Maurice Weeks, Maj. Robert Gum, and Mr Kenneth Williams of the U.S Army.
LIST OF TABLES
|
Table 1. |
Ocular effects of sulfur mustard in dogs |
|||
|
Table 2. |
Toxicity of sulfur mustard to dogs |
|||
|
Table 3. |
Lowest doses of sulfur mustard causing maternal and fetal effects in rats and rabbits |
|||
|
Table 4. |
Incidences of skin tumors in McNamara et al. (1975) toxicity study |
|||
|
Table 5. |
Incidences of skin tumors in McNamara et al. (1975) cancer study |
|||
|
Table 6. |
Summary of toxicity data for sulfur mustard |
|||
|
Table 7. |
Skin tumor data (toxicity study) used in EPA quantitative assessment |
|||
|
Table 8. |
Skin tumor data (carcinogenicity study) used in EPA quantitative assessment |
1. INTRODUCTION
Sulfur mustard (HD) is a chemical vesicant capable of causing severe skin and eye damage at very low concentrations. The chemical name, synonyms, identification codes, molecular formula and structural formula for this agent are as follows:
Sulfur mustard
bis(2-chloroethyl)sulfide
1,1'-thiobis(2-chlorethane)
1-chloro-2-(2-chloroethylthio)ethane
Distilled mustard
Agent HD
CAS No. 505-60-2
C4H8Cl2S
1.1. PHYSICAL/CHEMICAL PROPERTIES
Pure sulfur mustard (HD) is a colorless, odorless, oily liquid with a molecular weight of 159.08 (MacNaughton and Brewer, 1994). Commercial products, however, have a yellow-brown color and sweet odor due to contaminants (MacNaughton and Brewer, 1994). Sulfur mustard has a vapor density of 5.5 (air = 1), a liquid density of 1.27 g/mL at 25°C, a vapor pressure of 0.11 mm Hg at 25°C, and a water solubility of 0.092 g per 100 g at 22°C (DA, 1974).
1.2. ENVIRONMENTAL FATE
1.2.1 Air
The vapor pressure of sulfur mustard is 0.11 mm Hg at 25°C, indicating moderate volatility. A vapor concentration of 920 mg/m3 has been reported for a temperature of 25°C (DA, 1974) (although not adequately described in the reference, this presumably is the saturation concentration above a pure liquid). Information on the half-life of HD in air under various environmental conditions was not found in the available literature.
1.2.2 Water
The water solubility of sulfur mustard has been reported as 0.092 g per 100 g water at 22°C (DA, 1974), and 5 × 10-3 M at room temperature (MacNaughton and Brewer, 1994). In dilute aqueous solutions sulfur mustard hydrolyzes almost completely to thiodiglycol and hydrochloric acid (Papirmeister et al., 1991). For dissolved HD, the hydrolysis half-life ranges from about 4 to 15 min for temperatures of 20–25°C; however, bulk HD may persist in water for up to several years (Small, 1984). Small (1984) reported that it would take 15 days for the mass of a 1 cm droplet of HD in quiescent water to decrease by one half.
The Henry's Law Constant for HD has been estimated to be 2.1 × 10-5 atm m3/mol (MacNaughton and Brewer, 1994), indicating a moderate potential for evaporation from water.
1.2.3 Soil
Sulfur mustard can be very persistent in soil (Rosenblatt et al., 1995). Persistence depends on the soil type, pH, moisture content, and whether the agent is at the soil surface or buried. Small (1984) reported that when HD was applied to the soil surface, volatilization would be the main route of HD loss (half-life about 30 min), but if the soil was wet, hydrolysis would be the main loss pathway. When sprayed onto soil, a vesicant action was still apparent after about 2 weeks; when the agent leaked into the soil, however, a vesicant action was still present after 3 years (DA, 1974). Rosenblatt et al. (1995) state that the persistence of sulfur mustard in soil is due to the formation of oligomeric degradation products that coat the surface of the mustard agent and that are resistant to hydrolysis.
Sulfur mustard has a log Kow of 1.37 and a Koc of 133, indicating that binding to soil organics would limit transport through soil to groundwater (MacNaughton and Brewer, 1994). MacNaughton and Brewer (1994) calculated a leaching index of 7.2 for HD, (i.e., the number of leachings required to reduce the HD soil concentration to one-tenth of the original amount, assuming that for each leaching one kilogram of soil is in equilibrium with one liter of water).
2 MECHANISM OF ACTION
The acute toxic effects of mustard vesicants are usually attributed to the consequences of alkylation reactions with organic compounds including nucleoproteins such as DNA. Alkylation reactions can result in physiological and metabolic disturbances as well as genotoxic effects. Several hypotheses have been advanced concerning the primary cause of cell death following acute exposures. As reviewed by Papirmeister et al. (1991), the three major hypotheses are:
-
Poly(ADP-ribose) polymerase (PADPRP) hypothesis. - In this theory DNA is the initial target of the mustard agent. Alkylated DNA purines undergo spontaneous and enzymatic depurination, leading to the production of apurinic sites which are cleaved by apurinic endonucleases to yield DNA breaks. Accumulation of DNA breaks leads to activation of the chromosomal enzyme PADPRP, which utilizes nicotinamide adenine dinucleotide (NAD+) as a substrate to ADP-ribosylate and a variety of nuclear
-
proteins, causing severe lowering of cellular NAD+. Depletion of NAD+ results in the inhibition of glycolysis, and stimulation of the nicotinamide adenine dinucleotide phosphate (NADP+)-dependent hexose monophosphate shunt (HMS) pathway follows as a result of the accumulation of glucose-6-phosphate, a common precursor for both glycolysis and the HMS. Induction and secretion of proteases is stimulated as a result of enhanced HMS activity, and this leads to pathological changes in the cell.
-
Thiol-Ca+2 peroxidation hypothesis. The first step in this process is thought to be the alkylation of glutathione (GSH) by the mustard agent. Depletion of GSH subjects protein sulfhydryl groups to damage from the agent or from reactive cellular oxidants. Proteins most susceptible to damage include Ca2+ translocases (Ca2+-stimulated, Mg2+-dependent ATPase) which are dependent on thiol groups to maintain cellular Ca2+ homeostasis, and microfilamentous proteins, where loss of sulfhydryl groups could result in disruptions of the cytoskeletal and structural integrity of the plasma membrane.
-
Lipid peroxidation hypothesis. According to this hypothesis the mustard agent causes depletion of GSH which, in turn leads to the buildup of highly toxic oxidants, usually through H2O2-dependent reaction sequences. The oxidizing agents react with membrane phospholipids to form lipid peroxides, initiating a chain reaction of lipid peroxidation which can lead to alterations in membrane fluidity, loss of membrane protein function, and loss of membrane integrity.
3. TOXICOLOGY
3.1 Introduction
Sulfur mustard vesicants are acutely toxic by direct contact. Edema, ulceration, and necrosis of the skin and respiratory tract epithelium can occur, as well as conjunctivitis and blindness. General symptoms of systemic toxicity include nausea, vomiting, fever, and malaise (ITII, 1975). Delayed effects which may occur following acute exposures include: eye lesions, chronic bronchitis, and cancers of the respiratory tract and skin. However, information on adverse effects following long-term exposures to less-than acutely toxic concentrations is very limited. Health effects of sulfur mustard agents have recently been reviewed by ATSDR (1992), Somani (1992), Sidell and Hurst (1992), Watson and Griffin (1992), and the Institute of Medicine (1993). The following is a brief summary of the most important toxicological data for sulfur mustard.
3.2 Acute Toxicity
Acute exposures to sulfur mustard can result in skin and eye damage, gastrointestinal irritation, and depressed myelopoiesis (resulting in leukopenia and anemia) (Vogt et al., 1984). Damage to the respiratory tract, which is the principal cause of mortality in the first few days to weeks after exposure to sulfur mustard, involves acute edema, inflammation, and destruction of the airway epithelial lining (Institute of Medicine, 1993). Infection of the respiratory tract resulting in bronchopneumonia is a common complication of exposure to sulfur mustard.
The skin and eyes are especially sensitive to the toxic effects of sulfur mustard. When applied to human skin, about 80% of the dose evaporates and 20% is absorbed (Vogt et al., 1984). About 12% of the amount absorbed remains at the site and the remainder is distributed systemically (Renshaw, 1946). Doses up to 50 µg/cm2 cause erythema, edema, and sometimes small vesicles. Doses of 50-150 µg/cm2 cause bullous-type vesicles, and larger doses cause necrosis and ulceration with peripheral vesication. Droplets of liquid sulfur mustard containing as little as 0.0025 mg may cause erythema (Ward et al., 1966). Eczematous sensitization reactions were reported in several early studies and may occur at concentrations below those causing direct primary irritation (Rosenblatt et al., 1975). In humans, the LCt50 (estimated concentration x exposure period lethal to 50% of exposed individuals) for skin exposures is 10,000 mg-min/m3 (DA, 1974) (for masked personnel; however, the amount of body surface area exposed was not reported). The ICt 50 (estimated concentration x exposure period incapacitating to 50% of exposed individuals) for skin exposures is 2000 mg-min/m3 at 70–80°F in a humid environment and 1000 mg-min/m3 at 90°F in a dry environment (DA, 1974, 1992). The ICt50 for contact with the eyes is 200 mg-min/m 3 (DA, 1974, 1992). The LDLo for skin exposure is 64 mg/kg and the LD50 is estimated to be about 100 mg/kg (DA, 1974, 1992).
Repeated exposure to 1.4 mg-min/m3 produced no eye irritation or injury to laboratory animals (Rosenblatt et al., 1975). In humans, a Ct of ≤12 mg-min/m3 is considered a no-effect dose for eye irritation (McNamara et al., 1975) at ambient temperatures. At higher temperatures (≥32°C), threshold and other biological effects occur at lower concentrations. Cts of 12–70 mg-min/m3 cause mild reddening of the eyes (McNamara et al., 1975); Cts of 40–90 can cause eye irritation and conjunctivitis after a latency period of 2 to 48 hr; and Cts of 90–100 mg-min/m3 produce moderately severe burns, ulcers, opacity, and perforation after a latency period of 2 to 10 hr (Doull et al., 1980). In some cases there may be a recurrent vascularization and ulceration many years after the initial exposure.
The LCt50 for inhalation exposures in humans has been estimated to be 1500 mg-min/m3 (DA, 1992). In animals, median lethal Ct values for sulfur mustard range from 600 to 1900 mg-min/m3 for 10-min exposures (see Rosenblatt et al., 1975 for review). An LCLo (lowest lethal concentration) of 189 mg/m3/10 min has been reported for mice (Lewis and Sweet, 1984), and a 5-min LCLo of 77 ppm has been reported for dogs (ITII, 1975).
Information on the acute oral toxicity of sulfur mustard is quite limited. The oral LDLo for humans has been estimated to be 0.7 mg/kg (DA, 1992). The oral LD50 for rats is 17 mg/kg (DA, 1974). Rats treated with 2.5 mg/kg/day for 14 days developed inflammation, petechial hemorrhage, thickening, and sloughing of the gastric mucosa (Hackett et al., 1987).
3.3 Subchronic Toxicity
In a subchronic study conducted by Sasser et al. (1989a), Sprague-Dawley rats (12/sex/group) were dosed by gavage with 0, 0.003, 0.01, 0.03, 0.1 or 0.3 mg sulfur mustard (in sesame oil)/kg body weight/day, 5 days/week, for 13 weeks. No mustard-related mortality occurred at any dose level. Body weights were significantly decreased in animals in the high-dose group. Epithelial hyperplasia of the forestomach occurred in 5/12 males and 5/12 females of the high-dose group and in 1/12 males receiving 0.1 mg/kg/day, but not in any other treatment group. Forestomach lesions were not seen in any of the control animals. No other treatment-related pathological lesions, clinical chemistry changes, or hematological abnormalities were reported.
3.4 Chronic Toxicity
3.4.1 Human Data
The U.S. Department of the Army (DA, 1992) states that chronic exposure to sulfur mustard can cause sensitization and chronic lung impairment (cough, shortness of breath, chest pain); however, specific information on dose-response functions for these effects was not found in the available literature. Limited information on the chronic toxicity of sulfur mustard comes from studies of workers at chemical agent manufacturing and weapons plants. Morgenstern et al. (1947) reported that many workers in a munitions plant handling sulfur mustard developed chronic bronchitis which in some cases developed into bronchiectasis. Wada et al. (1962a, b) reported that a large proportion of workers at a Japanese plant manufacturing mustard, as well as Lewisite and several other agents, exhibited productive cough, irregular fever, chronic bronchitis, emphysematous changes, and pleural adhesions. It is likely that in this case the reported effects were due to concentrations of sulfur mustard sufficiently high to cause acute toxic effects; exposure levels were estimated to reach as high as 50–70 mg/m3 at times (Inada et al., 1978).
3.4.2 Animal Studies
McNamara et al. (1975) exposed male and female SDW rats (140), A/J mice (140), rabbits (12), guinea pigs (30), and dogs (6 initially) to a sulfur mustard vapor concentration of 0.001 mg HD/m3 for 24 hr/day, 5 days/wk, for varying exposure durations up to one year (Note: the investigators reported that the experimental protocol involved a continuous exposure, implying that it was for 7 days/wk; however, in several places in the report it is specifically mentioned that the exposures were for only 5 days/wk). The same number of animals of each species were exposed to 0.1 mg HD/m3 for 6.5 hr followed by 0.0025 mg HD/m3 for 17.5 hr per day, 5 days/week for up to one year. The latter exposure is equivalent to a 5 day/wk time-weighted average concentration of 0.029 mg/m3. Unexposed controls consisted of 10 dogs, 7 rabbits, 20 guinea pigs, 100 rats and 120 mice. Exposed animals were sacrificed periodically during the study and were replaced with new animals. One hundred ICR mice were added to the test chambers about 6 months after the tests began, and 50 A/J mice were added to the chambers about 3 months later.
Signs of toxicity that could be attributed to the sulfur mustard exposure occurred only in rats and dogs. Of 39 rats exposed to 0.001 mg HD/m3 for 12 months, 5 exhibited chronic keratitis, a condition that McNamara et al. (1975) reported could possibly have been agent-related; however, this effect was not observed in any rats exposed to 0.1 mg HD/m3. No signs of toxicity were seen in any of the dogs exposed to 0.001 mg HD/m3; however, it should be noted that only 2 animals were exposed for the full 52-week period and only 4 animals were exposed for 32 weeks. The major signs of toxicity seen in the dogs exposed to 0.1 mg HD/m3 were ocular changes consisting of corneal opacity, pannus, vascularization, pigmentation, keratitis, and granulation. McNamara et al. (1975, p. 12) state that chronic keratitis and conjunctivitis occurred in 3 of 10 dogs exposed for 7.5 or 12 months. The tabulated data presented by McNamara et al. (see Tables 1 and 2) indicate that chronic keratitis was also seen in some animals as early as 16 weeks after exposure began, and may have occurred in as many as 5 of 10 animals exposed for 32, 40 or 52 weeks. McNamara et al. (1975) concluded that it was ''possible" that these effects were agent-related. Pneumonitis occurred in several of the dogs exposed to 0.1 mg HD/m3, but this condition was also seen in the control animals, and because no other respiratory tract lesions were found, McNamara et al. (1975) indicated that the observed pneumonitis was not agent-related. There were no changes in
Table 1. Ocular effects of sulfur mustard in dogsa
blood chemistry of the exposed dogs except for a possible increase in serum glutamic oxaloacetic transaminase after 12–28 weeks of exposure to 0.1 mg/m3. As shown in Table 2, two dogs exposed to 0.1 mg HD/m 3 for 12 months also exhibited anaphylactic syndrome, gastroenteritis, and petechia.
Table 2. Toxicity of sulfur mustard to dogsa
|
No. Animals |
Exposure (months) |
Post-exposure (wk) |
Gross findings |
Microscopic findings |
|
4 |
2 |
5 |
See micro. |
Splenic infarct, 1/4; Pneumonia, granulomatous, 1/4; Pneumonitis, chronic 1/4 |
|
1 |
4 |
4 |
NSLb |
NSL |
|
1 |
4 (control) |
4 |
NSL |
NSL |
|
1 |
4 |
4 |
NSL |
NSL |
|
1 |
7.5 |
4 |
NSL |
Keratitis, pigmentary; Pneumonitis, chronic |
|
1 |
7.5 |
4 |
NSL |
Keratitis, chronic |
|
1 |
7.5 (control) |
4 |
NSL |
Pneumonitis, chronic |
|
1 |
7.5 (control) |
4 |
NSL |
Pneumonitis, chronic, active |
|
1 |
12 |
10 |
Gastroenteritis; Multiple petechiae; Anaphylactic syndrome |
Congestion, liver, spleen, lung; Hemorrhage, pancreas; Ulcerative colitis; Keratitis, chronic; Conjunctivitis, lymphocytic |
|
1 |
12 |
10 |
Anaphylactic syndrome |
Gastroenteritis, hemorrhagic; Heart, petechia; Keratitis, acute |
|
SOURCE: McNamara et al., 1975, Table A-37, p. 53 a 0.1 mg HD/m3 for 6.5 hr followed by 0.0025 mg HD/m3 for 17.5 hr per day, 5 days/week b NSL= no significant lesions |
||||
Although these effects were considered by McNamara et al. (1975) to be unrelated to the exposure to sulfur mustard, they are consistent with the known vesicant and sensitization actions of the agent. It is possible that the HD condensed on the fur of the animals and was subsequently ingested as a result of grooming behavior. Gastroenteritis could then have resulted from direct contact of the vesicant with the gastrointestinal epithelium.
3.5 Delayed Toxicity
Acute exposures to sulfur mustard can also result in long-term respiratory damage manifested as asthma-like conditions, emphysematous bronchitis, and increases in incidence of secondary respiratory infections (bronchopneumonia and tuberculosis) (see review by Watson and Griffin, 1992). Beebe (1960) evaluated the occurrence of respiratory tract disease among a group of World War I soldiers. Soldiers who had been exposed to mustard gas exhibited greater mortality from tuberculosis and pneumonia than either of two reference groups. Manning et al. (1981) reported a significantly increased incidence of mortality from pneumonia among 428 former workers of a sulfur mustard manufacturing facility. The ratio of observed to expected cases was 2 (p <0.05). Some individuals exposed to sulfur mustard concentrations that are damaging to the eyes are susceptible to relapsing keratitis (delayed keratopathy) (see review by Watson and Griffin, 1992). The condition may reappear 8 to 40 years after recovery from the initial exposure (Dahl et al., 1985).
3.6 Developmental and Reproductive Effects
Azizi et al. (1995) investigated changes in serum concentrations of reproductive hormones and sperm counts in men who had been exposed to sulfur mustard during wartime. In 16 individuals, serum free and total testosterone and dehydroepiandrosterone were markedly decreased in the first five weeks after exposure; but levels returned to normal by 12 weeks. In 28 of 42 men evaluated one to three years after exposure, sperm counts were less than 30 million cells/mL and follicle-stimulating hormone was increased compared to controls having sperm counts above 60 million cells/mL. Testicular biopsy of the test subjects revealed partial or complete arrest of spermatogenesis.
In a study conducted by Hackett et al. (1987), sulfur mustard (dissolved in sesame oil) was administered by intragastric intubation to rats and rabbits on gestation days 6–15 (rats) or 6–19 (rabbits). Female rats were dosed with 0, 0.2, 0.4, 0.8, 1.6, 2.0 or 2.5 mg/kg/day in a range-finding study (3–9 animals per dose group of which 2–7 per dose group were pregnant) and with 0, 0.5, 1.0, or 2.0 mg/kg/day in a teratology study (25–27 animals per dose group of which 20–26 per dose group were pregnant). Maternal and fetal toxicity was observed at all dose levels (see Table 3). In the range-finding study significant (p <0.05) maternal effects included mortality (1/3) at the highest dose; severe gastric lesions (petechial hemorrhage and sloughing of gastric mucosa) at 2.0 and 2.5 mg/kg/day; and inflamed mesenteric lymph nodes at doses of 0.4 mg/kg/day and higher. Significant decreases in body weight and decreased extragestational weight occurred at 1.6 mg/kg/day and decreased hematocrit at 0.8 mg/kg/day. There were no adverse effects on fetal weight and no evidence of morphological abnormalities in the fetuses. In the rat teratology study, maternal toxicity was evidenced by gastric inflammation at 2.0 mg/kg/day, and inflamed mesenteric lymph nodes at doses of 0.5 mg/kg/day and higher. Decreased body weight and decreased extragestational weight occurred at 0.5 mg/kg/day; decreased hematocrit at 1.0 mg/kg/day; and decreased weight of the placenta and gravid uteri at 2.0 mg/kg/day. Fetal effects included decreased weight in females and hydroureter at 0.5 mg/kg/day; decreased weight of males at 1.0 mg/kg/day; increased incidences of supernumerary ribs, misaligned sternebrae, and reduced ossification of sternebrae at 2.0 mg/kg/day. The investigators reported that the study did not reveal any evidence for a sulfur mustard-induced teratogenic effect in rats because all of the observed fetal changes occurred at dose levels that also produced maternal toxicity. The NOAEL for maternal and fetal toxicity was reported to be <0.5 mg/kg/day.
Table 3. Lowest doses of sulfur mustard causing maternal and fetal effects in rats and rabbits
|
Effects |
Rat studies |
Rabbit studies |
|||
|
|
|
Range-finding (mg/kg/day) |
Teratology (mg/kg/day) |
Range-finding (mg/kg/day) |
Teratology (mg/kg/day) |
|
Maternal Effects: |
mortality |
2.5 |
— |
1.0 |
0.8 |
|
|
gross lesions: |
|
|
|
|
|
|
majora |
2.0 |
— |
1.0 |
0.4 |
|
|
minorb |
0.4 |
0.5 |
0.5 |
0.4 |
|
|
decreased weight: |
|
|
|
|
|
|
body |
1.6 |
0.5 |
2.0 |
0.8 |
|
|
extragestational |
1.6 |
0.5 |
— |
— |
|
|
extragestational gain |
0.4 |
0.5 |
— |
0.8c |
|
|
gravid uterus |
— |
2.0 |
— |
— |
|
|
decreased hematocrit |
0.8 |
1.0 |
— |
0.8 |
|
|
resorptions |
0.4d |
— |
— |
— |
|
Fetal Effects: |
decreased weights: |
|
|
|
— |
|
|
female fetuses |
— |
0.5 |
2.0 |
— |
|
|
male fetuses |
— |
1.0 |
2.0 |
— |
|
|
placenta |
— |
2.0 |
— |
— |
|
|
fetal morphology |
|
|
|
|
|
|
misaligned sternebrae |
— |
2.0e |
— |
— |
|
|
supernumerary ribs |
— |
2.0e |
— |
— |
|
|
reduced ossification |
|
|
|
|
|
|
vertebrae |
— |
— |
— |
|
|
|
sternebrae |
— |
2.0e |
— |
— |
|
|
hydroureter |
— |
— |
— |
|
|
Source: Hackett et al., 1987 aGastric lesions or infections bInflamed mesenteric lymph nodes in rats; enlarged Peyer's patch in rabbits cSignificantly different from lowest dose group, but not from controls dNot significant in the highest dose group eSignificance based on fetal unit fSignificance based on litter unit |
|||||
In the second part of the Hackett et al., (1987) study, rabbits were dosed with 0, 0.5, 1.0, 2.0, and 2.5 mg/kg/day in a range-finding study (7–8 per dose group), and with 0, 0.4, 0.6, or 0.8 mg/kg/day in the teratology study (7–8 per dose group). Dose levels of 0.8 mg/kg/day or higher were lethal to the dams. Damage to the gastric mucosa and enlarged Peyer's patches were observed in animals that received the lowest dose (0.4 mg/kg/day). Depressed body weight, depressed extragestational weight gain, and depressed hematocrit values occurred at 0.8 mg/kg/day. In the range-finding study a significant depression in fetal body weights occurred at a dose level of 2.0 mg/kg/day; however, in the teratology study no significant effects were observed on intrauterine survival, placental and fetal body weights, or incidence of fetal abnormalities. The investigators concluded that the study provided no evidence that sulfur mustard induced a teratogenic effect in rabbits. The NOAELs for maternal and fetal toxicity were reported to be <0.4 mg/kg/day and >0.8 mg/kg/day, respectively.
In a two-generation reproductive toxicity study conducted by Sasser et al. (1989b), groups of Sprague-Dawley rats (27 females and 20 males/group/generation) were gavaged with 0, 0.03, 0.1 or 0.4 mg/kg/day. The animals were treated according to the following exposure protocol: male and female rats were dosed 5 times/week for 13 weeks prior to mating and during a 2-week mating period; female rats were dosed daily throughout the 21-day gestation and parturition period; and females were dosed 4–5 times/week during the 21-day lactation period. Males who had mated with females were sacrificed at the birth of their pups; dams who had given birth were sacrificed when the pups were weaned. Male and female F1 pups received sulfur mustard until they were mated, the females became pregnant, and gave birth. At this point, F1 males were sacrificed and F1 dams continued on the dosage schedule until weaning, at which point the study was terminated. Thus, two generations of rats received subchronic exposure to sulfur mustard, with each generation going through a mating cycle. Similarly, two generations of pups were born to parents who had received sulfur mustard. Body weight gain was significantly (p <0.05) lower than control values in the F1 rats of both sexes born to parents who had received the highest dose of sulfur mustard. There were no significant adverse effects on reproductive parameters at any dose level. However, dose-related lesions of the squamous epithelium of the forestomach (acanthosis and hyperplasia) occurred in both sexes of each treatment group. The lesions were described as mild at the lowest dose level, 0.03 mg/kg, compared with the higher dose groups. The incidence and severity of acanthosis was 0/94 in the controls, 71/94 in the low-dose group, 89/94 in the mid-dose group, and 94/94 in the high-dose group. Benign neoplasms of the forestomach occurred in 8/94 animals in the 0.1 mg/kg group and in 10/94 animals of the 0.4 mg/kg group. The results of this study indicate that lowest dose tested (0.03 mg/kg/day) is a LOAEL for maternal toxicity.
McNamara et al. (1975) reported no increased fetal mortality rate when groups of 10 rat dams were exposed by inhalation to 0.001 mg HD/m3, 24 hr/day or to 0.1 mg HD/m3 for 6.5 hours followed by 0.0025 mg HD/m3 for 17.5 hours during the first, second, or third week, or for the entire period of gestation. In another study, groups of 10 unexposed female rats were bred to male rats which had been exposed to the same exposure concentrations of HD for 1, 2, 4, 8, 24, 36, or 52 weeks to gain information on dominant lethal mutagenesis. There was no evidence of mutagenesis and fetal mortality was considered within normal limits. Both studies had a number of short-comings; in particular, the authors stated that the fetuses were examined, but they did not indicate whether there were fetal abnormalities.
3.7 Carcinogenicity
Several studies on workers occupationally exposed to sulfur mustard have revealed elevated risks of respiratory tract and skin tumors after long-term exposure. In addition, animal studies, mutagenicity studies, genotoxicity data, and the fact that sulfur mustard is a potent DNA alkylating agent, all provide supporting evidence for the carcinogenicity of this chemical agent.
The International Agency for Research on Cancer (IARC) has classified "mustard gas" as a Group 1 carcinogen (IARC, 1987), and the National Toxicological Program (NTP) includes "mustard gas" in the category of Substances or groups of substances, occupational exposures associated with a technological process, and medical treatments that are known to be carcinogenic (NTP Annual Report on Carcinogens, 1994). The State of Maryland also considers "mustard gas" as a "known human carcinogen" (a Class I.A. Toxic Air Pollutant as defined by the Code of Maryland Regulations, CMR Title 26 Subtitle 11, as amended).
3.7.1 Human Data
IARC (1975), Waters et al. (1983), Watson et al. (1989), and the Institute of Medicine (1993) have summarized the epidemiological evidence concerning the potential carcinogenicity of sulfur mustard in humans. Much of this information has come from studies of soldiers exposed during World War I as well as from studies of workers at chemical agent manufacturing facilities.
Case and Lea (1955) reported 29 deaths from cancer of the lungs and pleura among a sample of 1267 World War I veterans who had been exposed to sulfur mustard, 80% of whom also suffered from chronic bronchitis. In comparison, 14 cases would have been expected in a population of that size based on the mortality rates for the male population of England and Wales. The mortality ratio (207) indicated a significantly elevated risk for respiratory tract neoplasms (p between 0.0001 and 0.01). A similar tumor incidence rate and mortality ratio were found in a population of veterans who had never been exposed to mustard but who were suffering from bronchitis. Case and Lea (1955) concluded that the evidence did not support the view that sulfur mustard was a direct carcinogen. IARC (1975), however, noted that the high tumor rate in the group not exposed to mustard may have been due, in part, to smoking habits (a significantly higher proportion of men injured by mustard gas had given up smoking by the age of 40).
Beebe (1960) evaluated the occurrence of respiratory tract cancers among a group of 2718 American soldiers exposed to sulfur mustard during World War I and found that the ratio of observed to expected cases was 1.47 (based on U.S. mortality rates) compared with 1.15 for wounded soldiers not exposed to sulfur mustard, and 0.81 for soldiers who had pneumonia, but who had not been exposed to mustard. Norman (1975) evaluated the same group of soldiers after a 10-year follow-up period (study completed in 1965) and found that the exposed men had a 40% excess of lung cancer mortality, with an estimated relative risk of 1.3 (95% confidence limits of 0.9–1.9) compared to a control group consisting of wounded soldiers without exposure to mustard. The latency period was estimated to be 22–37 years. Norman (1975) also reported that in a limited subgroup of veterans, the relative risk of lung cancer mortality among cigarette smokers who were exposed to mustard agents was approximately equal to that of veterans exposed to mustard who stated that they did not smoke (4.3 vs 4.4). Norman (1975) concluded that there was no evidence in this limited data set that mustard exposure and cigarette smoking had a synergistic effect on lung cancer mortality.
Retrospective studies of Japanese workers who had been employed at a chemical agent manufacturing plant from 1929 to 1945 have revealed that these individuals have an increased risk of developing respiratory tract cancers. Although sulfur mustard was the main product of the facility, lewisite, diphenylarsine, hydrocyanic acid, phosgene, and chloroacetophenone were also produced there (Inada et al., 1978), and it is not known to what degree these other chemicals contributed to the observed effects. The concentration of mustard in the workplace was estimated to be as high as 50–70 mg/m3 (Nakamura, 1956), and reportedly, the workers frequently exhibited signs of mustard toxicity including acute conjunctivitis, acute rhinitis, acute bronchitis, and acute dermatitis with blister formation. Studies completed in the 1950's documented individual cases of bronchial and laryngeal carcinoma in this population of workers (Yamada et al., 1953, 1957). Yamada (1963) reported that 16.3% of 172 deaths of former workers were due to cancers of the respiratory tract and oropharynx. The incidence rate among 5030 non-exposed inhabitants from the same geographic area was reported to be of 0.4% (Yamada, 1963). Mortality rates among the former factory workers during the years 1952–1967 were studied by Wada et al. (1968) who found that the incidence of mortality due to respiratory tract cancer was 33/495 (30 confirmed by histological evaluation) compared to an expected 0.9, based on national mortality rates for males with the same age distribution as the mustard workers. Of 930 former factory workers not directly involved in the mustard production process, three had died of respiratory tract cancer compared to 1.8 expected.
Neoplasms occurred in the tongue, pharynx, sphenoidal sinus, larynx, trachea, and bronchi; only one occurred peripherally in the lung. The median length of employment was 7.4 years, and the median interval between first employment and death from cancer of the respiratory tract was 24.4 years (Wada et al., 1968). Additional studies of this population of workers were conducted by Nishimoto et al. (1983, 1988) who incorporated histopathological and mortality data gathered between 1952 and 1986. For 1632 of these workers, the standardized mortality ratio (SMR) for respiratory tract tumors was 3.9 (70 observed vs. 17.8 expected, p<0.001, based on data for the Japanese male population) and the SMR for all malignant tumors was 1.2 (173 observed vs. 142 expected, p<0.01). These individuals were divided into three groups; (A) those directly involved in the manufacture of sulfur mustard or lewisite; (B) those not involved in mustard or lewisite manufacture, but who experienced some exposure; and (C) those engaged in the manufacture of other gases and those who were never exposed. The SMR for groups A and B (1.6 and 1.9) were also significantly elevated (p<0.001) whereas that for group C was not. Nishimoto et al. (1988) also showed that the SMR was about 2.7 for individuals who had worked at the factory 0.5 to 5 years, but 7.17 for individuals who had been employed for more than 5 years. The SMR was not significantly elevated for individuals who had worked at the factory for 7 months or less. SMRs were also calculated for each of six age groups. For individuals 30–39 years old the SMRs for respiratory tract cancer were not significantly elevated; however, the SMRs for the 40–49, 50–59, 60–69, and 70–79 yr olds were 10.3, 3.9, 4.4, and 2.5, respectively; all statistically significant at p<0.01 or p<0.001.
There is some evidence that these former factory workers may have also suffered elevated rates of digestive tract and skin tumors. Histopathological studies conducted by Yamada (1974, as reported by Inada et al., 1978) on 94 autopsy cases and 8 surgical cases revealed 17 cases of digestive tract cancers among these workers (no comparisons with control groups were reported). Of 488 former workers examined dermatologically, 115 had abnormal pigmentation and 22 had skin tumors of which 8 were cases of Bowen's disease (Inada et al., 1978). Pigmentation disorders were present in 57 cases out of 109 engaged only in the production of mustard and in only 1 of 16 cases engaged only in the production of lewisite. Hyperkeratotic skin lesions such as Bowen's disease, basal cell carcinomas, and hyperkeratotic papular eruptions, were present in 14 cases out of 109 engaged only in mustard production and in 1 case out of 16 engaged only in lewisite production. No abnormalities were observed in 77 former factory workers who had no exposure to chemical agents (Inada et al., 1978). It was also observed that the longer an individual had been exposed to mustard, the more marked the skin lesions tended to become (Inada et al., 1978).
The studies of Nishimoto et al. (1988), Yamada (1974) and Inada et al., (1978) provide strong evidence for a causal link between chemical agent exposure and cancer; however, because the workers were exposed to multiple chemicals, it is not possible to state conclusively that the cancers were due solely to sulfur mustard. Furthermore, it should be noted that several possible confounding factors, such as tobacco smoking habits, pre-existing health conditions, and post-exposure occupational histories of the workers, were not evaluated. In addition, SMRs themselves may not provide an accurate estimate of relative cancer risk if they do not correlate with tumor incidence rates in exposed and control groups (i.e., if social/economic or other differences between control and exposed groups result in differences in health care which affect survival rates).
Weiss and Weiss (1975) conducted studies evaluating the health of 271 workers employed for varying lengths of time between 1935–1945 at a munitions depot where the production, testing and destruction of sulfur and nitrogen mustard (as well as bromoacetone, phosgene, chloropicrin and organic arsenicals) had occurred. Ninety percent of the group had chronic health problems and 114 had died by the end of 1974. Thirty-five percent died from cancer of which 38% were bronchial cancers. The total number of deaths from cancer was significant (p<0.01) and the number of bronchial cancers was also
significant (11 observed vs. 5 expected for the population of the geographic region where the facility was located). The number of cancers of the gastrointestinal tract was 35% greater than expected. The average tumor induction time was 21.6 years. IARC (1975) notes that the study was limited to workers with available medical records, which "raises the possibility that the proportion with cancer may have been inflated, since medical records or autopsy records would more likely have been preserved for workers with cancer". Furthermore, IARC (1975) does not indicate whether smoking habits and other confounding factors were accounted for in the study of Weiss and Weiss (1975).
According to Klehr (1984), German workers involved in the dismantling of a sulfur mustard facility developed multiple skin lesions including basal cell carcinomas, Bowen's disease, Bowen's carcinomas, and carcinoma spinocellulare. The incidence rate for all tumors (including skin tumors) was 34% in 53 workers evaluated.
Manning et al. (1981) evaluated the incidence of cancer among former workers of a British mustard manufacturing facility (1939–1945). As of 1974, the number of deaths from all neoplasms combined (45) was slightly greater than that expected from national death rates, but the increase was not statistically significant. Two deaths were attributed to cancer of the larynx and one to carcinoma of the trachea, compared with an expected number of 0.40 (p<0.02; relative risk 7.5). Seven individuals were known to have developed cancer of the larynx, compared with 0.75 expected (p<0.001; relative risk 9.3). Lung cancer deaths were also elevated (21 observed vs. 13.43 expected) but not to significant levels (relative risk 1.6). In follow-up investigations of this group of workers, Easton et al. (1988) evaluated the mortality records of 3354 individuals and found greater numbers of cancer deaths when compared to national mortality rates. Significant increases were observed in deaths from cancer of the larynx (11 observed, 4.04 expected, p = 0.003), pharynx (15 observed, 2.73 expected, p<0.001), and all other buccal cavity and upper respiratory sites combined (12 observed, 4.29 expected, p = 0.002). There were also 200 deaths from lung cancer compared with 138.39 expected (p<0.001). It was also reported that the risks of developing cancer of the lung and pharynx were significantly related to the duration of employment. Significant excess mortality was also observed for cancers of the esophagus (20 observed vs. 10.72 expected) and stomach (70 observed vs. 49.57 expected) but there was no correlation with time since first exposure or duration of exposure.
Manning et al. (1981) concluded that it was very likely that the observed cancers of the pharynx, larynx and other upper respiratory sites were due to exposure to sulfur mustard because the excesses were too large to be accounted for by confounding factors (the effects of smoking, however, were not evaluated), increased with increasing duration of employment, and were limited to the period more than 10 years after first employment. Evidence for a causal relationship between sulfur mustard exposure and other cancers, including lung cancer, was not considered to be as strong.
Although a large number of American military personnel were exposed to sulfur mustard in chamber and field tests conducted during World War II, the morbidity and mortality records of this cohort have not been adequately evaluated to document long-term health risks (Institute of Medicine, 1993).
3.7.2 Animal Studies
Information on the potential carcinogenicity of sulfur mustard is available primarily from studies on rats and mice. McNamara et al. (1975) exposed SDW rats, ICR Swiss albino and A/J mice, rabbits, guinea pigs, and dogs to sulfur mustard vapors for varying exposure durations up to one year. The test animals were exposed to 0.001 mg HD/m3 continuously or to 0.1 mg HD/m3 for 6.5 hr followed by
0.0025 mg HD/m3 for 17.5 hr per day, 5 days/week. In the rat study, 70 males and 70 females were exposed at each of the two concentrations, and 50 of each sex were maintained as controls. No tumors were observed in rabbits, guinea pigs, dogs, or mice; however, skin tumors were seen in the rats and these were considered to be the result of exposure to sulfur mustard. The rats were tested in two separate studies; a "toxicity study" in which the animals were exposed for up to 52 weeks and then followed for 6 months at which time they were sacrificed, and a "carcinogenicity study" in which the animals were exposed for varying times and then observed for varying periods of time before being sacrificed. In both studies skin tumors occurred in animals exposed to the highest concentration, but not in those exposed to the lower concentration. Of the tumors observed in the exposed animals, McNamara et al. (1975) considered basal cell and squamous cell carcinomas, trichoepitheliomas, and keratoacanthomas of the skin to be related to the sulfur mustard exposure; the incidence of these tumors are shown in Tables 4 and 5.
Table 4. Incidences of skin tumors in McNamara et al. (1975) toxicity study1,2
|
|
|
Control |
0.001 mg/m3 |
0.1/0.0025 mg/m3 |
|||
|
Exposure duration (months) |
Post-exposure (days) |
M |
F |
M |
F |
M |
F |
|
2 |
|
|
0/5 |
0/5 |
0/5 |
0/5 |
|
|
3 |
|
0/5 |
0/5 |
0/5 |
0/5 |
0/5 |
0/5 |
|
4 |
|
|
|
|
0/5 |
0/5 |
|
|
6 |
|
0/5 |
0/5 |
0/5 |
0/5 |
|
|
|
8 |
|
0/5 |
0/5 |
0/5 |
0/5 |
0/5 |
0/5 |
|
12 |
|
0/5 |
0/5 |
0/5 |
0/5a |
0/5 |
|
|
12 |
70 |
|
|
|
|
4/44b |
|
|
12 |
90 |
0/4 |
0/4c |
0/4 |
0/5 |
0/1 |
0/5 |
|
12 |
180 |
0/7 |
0/6 |
0/14e |
0/6 |
||
|
SOURCE: McNamara et al., 1975; adapted by U.S. EPA, 1991 1 Superscripts indicate the number and types of tumors: a. subcutaneous fibroma; b. skin, squamous cell carcinoma; c. squamous cell carcinoma of uterus; d. pulmonary adenoma; e. papilloma of the skin; f. basal cell carcinoma of the skin; g. thyroid adenoma 2 Only tumor types b, and f, were considered by the authors to be related to the HD exposure and only these types are counted in the numerators of this table. |
|||||||
Table 5. Incidences of skin tumors in McNamara et al. (1975) cancer study (Data for both sexes pooled)1,2
|
Exposure duration (weeks) |
Post-exposure (months) |
Control |
0.001 mg/m3 |
0.1 mg/m3 |
|
1 |
13 |
|
0/1 |
|
|
1 |
15 |
|
|
0/1a |
|
1 |
21 |
|
0/4b |
0/4 |
|
2 |
20 |
|
0/5 |
0/5c |
|
4 |
16 |
|
0/1 |
0/1 |
|
4 |
20 |
|
0/4 |
0/5 |
|
8 |
15 |
0/4 |
0/2 |
0/4 |
|
8 |
17 |
|
0/1 |
|
|
8 |
18 |
|
0/1d |
|
|
12 |
12 |
|
0/2 |
|
|
12 |
17 |
|
0/3e |
|
|
26 |
14 |
|
0/4 |
3/43f |
|
26 |
18 |
|
1/1f |
|
|
393 |
11 |
|
0/3e |
|
|
52 |
2 |
|
|
1/1f |
|
52 |
4 |
|
|
1/1h |
|
52 |
6 |
|
|
1/1f |
|
52 |
7 |
|
|
0/1 |
|
52 |
10 |
0/22e |
0/17 |
|
|
52 |
17 |
0/1e |
|
0/1e |
|
52 |
18 |
|
|
4/4f |
|
SOURCE: McNamara et al., 1975; adapted by U.S. EPA, 1991 1 Superscripts indicate the number and types of tumors: a. subcutaneous lipoma b. axillary lipoma c. subcutaneous fibroma d. astrocytoma e. skin, fibroma f. skin, squamous cell carcinoma g. skin, basal cell carcinoma h. skin, trichoepithelioma i. skin, keratoacanthoma 2 Only types f, g, h, and i, were considered by the authors to be related to the HD exposure and only these types are counted in the numerators. At 0.1 mg/m3, one type h tumor co-occured in one animal with a squamous cell carcinoma. |
||||
Heston (1950) reported an increase in the occurrence of pulmonary tumors in strain A mice injected intravenously with 0.25 mL of a 1:10 dilution of a saturated solution of HD in water (0.06–0.07%) at 2-day intervals for a total of 4 doses. The tumor incidence was 93.3% with 2.6 tumors/mouse compared with 61% in the controls (0.9 tumors/mouse). In a second test in which a slightly lower dose was used, pulmonary tumors were found in 68% of the surviving treated animals (1.09 tumors/mouse) compared with 13% in the controls (0.13 tumors/mouse) (p<0.001). A significant increase in the incidence of pulmonary tumors in strain A mice was also seen in an inhalation study in which the test animals were exposed for 15 min to vapors released from 0.01 mL of HD applied to filter paper (Heston and Levillain, 1953; exposure levels were not otherwise quantified). Eleven months after exposure, lung tumor incidence was 49% (33/67) in the exposed animals and 27% (21/77) in the controls (p<0.01).
In another study, Heston (1953) found that subcutaneous injections of HD (0.05 cc of a 0.05% solution at weekly intervals for 6 weeks, or 0.1 cc of a 0.1% solution in olive oil at 2-day intervals for a total of 6 doses) into the mid-dorsal region of mice (strains A, C3H, and C3Hf) resulted in injection-site tumors, whereas injections of vehicle alone did not induce tumor formation. Tumors occurring at the injection site included sarcomas, sarcomas neurogenic in origin, a rhabdomyosarcoma, papillomas, a squamous cell carcinoma, a hemangioendothelioma, and a mammary carcinoma.
3.8 Genotoxicity
IARC (1975), Fox and Scott (1980) and ATSDR (1992) have summarized the available evidence concerning the genotoxicity of sulfur mustard. Because sulfur mustard is a strong DNA alkylating agent, genotoxic effects occur through cross-link formation, inhibition of DNA synthesis and repair, point mutations, and chromosome and chromatid aberrations (ATSDR, 1992). Some of these conditions have been observed in humans following exposure to sulfur mustard, others in various test systems including bacteria, yeast, insects, and mammalian cell cultures.
In studies conducted on a group of 28 former employees of a chemical agent manufacturing plant, Yanagida et al. (1988) found that the frequency of mutations to hypoxanthine-guanine-phosphoribosyl-transferase (HGPRT) deficiency was significantly elevated when compared to two control groups matched for age and smoking status. One control group consisted of healthy men and the other of individuals with bronchitis. The data also showed that the mutations were significantly more frequent in those workers who had longer exposures to sulfur mustard. A chromosome study of 16 former workers of this same factory indicated a significantly higher incidence of sister chromatid exchanges in peripheral lymphocytes when compared to a control group (p<0.03) (Shakil et al., 1993). Two individuals with chronic myelocytic leukemia had an almost three-fold higher SCE rate than controls and also a high (12.1%) incidence of chromosome abnormalities (Shakil et al., 1993). In an evaluation of the p53 mutations found in lung tumors of sulfur mustard workers, Takeshima et al. (1994) found that the mutations were similar to those in lung tumors of tobacco smokers (the factory workers were also tobacco smokers), however, the prominence of G:C to A:T transitions and the occurrence of double mutations in two of twelve cases suggested that the exposure to sulfur mustard did contribute to the development of the lung cancers.
Wulf et al. (1985) reported significant (p<0.001) increases in sister chromatid exchanges in lymphocytes of eleven fisherman who had accidently been exposed to sulfur mustard in sufficiently high concentrations to cause signs of acute toxicity.
Sulfur mustard has found to be genotoxic and mutagenic in several microbial assays. The agent caused alkylation of DNA in the yeast Saccharomyces cerevisiae (Kircher and Brendel, 1983), and
interstrand DNA cross-links (Venitt, 1968) and inhibition of DNA synthesis (Lawley and Brookes, 1965) in Escherchia coli. Using the histidine reversion assay, Stewart et al. (1989) found that sulfur mustard induced point mutations in Salmonella typhimurium strain TA102 and frameshift mutations in TA 97, but neither type of mutation in strains TA98 and TA100.
Sulfur mustard inhibited DNA synthesis in mouse lymphoma cells (Crathorn and Roberts, 1965), HeLa cells (Crathorn and Roberts, 1966), and L-strain mouse fibroblasts (Walker and Thatcher, 1968). It also induced chromosomal aberrations in cultured rat lymphosarcoma and mouse lymphoma cells (Scott et al., 1974), and chromosomal aberrations and reverse mutations in male BDF1 mice in a host-mediated assay using murine leukemia L5178Y/Asn cell line as an indicator (Capizzi et al., 1973).
Several studies have also demonstrated that sulfur mustard causes dominant lethal mutations. Rozmiarek et al. (1973) reported a dominant lethal mutation rate of 9.4% (±1.9%) in rats after adult males had been exposed to 0.1 mg HD/m3 for 12 weeks. Sasser et al. (1990) reported that a dominant lethal effect occurred after male Sprague-Dawley rats were dosed orally with 0.5 mg HD/kg/day 5 days/week for 10 weeks. The observed effects included increases in early fetal absorptions, preimplantation losses, and decreases in total live embryo implants. A significant increase in the percentage of abnormal sperm was also reported. Dominant lethal mutations, as well as chromosome rearrangements, have also been observed in Drosophila melanogaster exposed to sulfur mustard (Auerbach and Robson, 1946).
The cytotoxic, clastogenic and mutagenic effects of HD in Chinese hamster ovary cells have also been evaluated by Jostes et al. (1989). Chromosomal aberration frequency increased in a dose-dependent manner over the dose range of 0.0625 to 0.25 μM. Mutation induction at the HGPRT locus was sporadic, but the majority of the exposures resulted in mutation frequencies that were 1.2 to 4.0 fold higher than the spontaneous frequencies.
4. ORAL REFERENCE DOSE
4.1 Selection of the Principal Study
The available toxicity data for sulfur mustard are summarized in Table 6. The experimental study considered most suitable for the derivation of an oral RfD for sulfur mustard is the rat two-generation reproductive toxicity study conducted by Sasser et al. (1989b). This study, extending over 42 weeks, provides the lowest LOAEL. Acanthosis and hyperplasia of the epithelial tissue of the forestomach were the critical endpoints. Gastric lesions have also been observed in other oral toxicity studies on sulfur mustard (see Section 3.6). Some investigators have noted that the absence of a forestomach in humans suggests that the rat may not be a reliable human surrogate for evaluating the toxic effects of sulfur mustard. In addition, effects of gavage treatment in which the tissues are in immediate contact with sulfur mustard dissolved in sesame oil may not be comparable to the more dispersed contact expected when sulfur mustard is administered in drinking water.
Table 6. Summary of toxicity data for sulfur mustard
|
Study |
Type |
NOAEL (mg/kg/day) |
LOAEL (mg/kg/day) |
Comments |
|
Hackett et al., 1987 |
rabbit teratology |
> 0.8 |
No fetal toxicity |
|
|
Hackett et al., 1987 |
rabbit teratology |
0.4 |
maternal toxicity |
|
|
Hackett et al., 1987 |
rat teratology |
0.5 |
maternal and fetal toxicity |
|
|
Sasser et al., 1989a |
rat subchronic |
0.1 |
0.3 |
Epithelial hyperplasia of the forestomach |
|
Sasser et al., 1989b |
rat 2 generation |
0.03 |
maternal toxicity acanthosis and hyperplasia of the forestomach |
|
|
Sasser et al., 1989b |
rat 2 generation |
0.4 |
reproductive effects |
4.2 Derivation of the Oral Rfd
In the Sasser et al. (1989b) study, male and female Sprague-Dawley rats were gavaged with 0, 0.03, 0.1, or 0.4 mg/kg/day dissolved in sesame oil (see Section 3.6 for details of the experimental protocol). There was no evidence of adverse reproductive effects at the dose levels tested. There was a significantly (p <0.05) decreased body weight gain in the F1 rats of both sexes born to parents who had received the highest dose of sulfur mustard. In addition, dose-related lesions of the squamous epithelium of the forestomach (acanthosis and hyperplasia) occurred in both sexes of each treatment group. The lesions were described as mild at the lowest dose level, 0.03 mg/kg, compared with the higher dose groups. The incidence and severity of acanthosis was 0/94 in the controls, 71/94 in the low-dose group, 89/94 in the mid-dose group, and 94/94 in the high-dose group. Benign neoplasms of the forestomach occurred in 8/94 animals in the 0.1 mg/kg group and in 10/94 animals of the 0.4 mg/kg group. The investigators reported that the NOAEL for toxicity was <0.03 mg/kg and the NOAEL for reproductive effects >0.4 mg/kg.
The lowest dose tested, 0.03 mg/kg, can be considered a LOAEL for rats subchronically exposed to sulfur mustard, with epithelial acanthosis and hyperplasia of the forestomach as the critical effect. Using this LOAEL, a human chronic RfD can be derived by adjusting the dose to a 7 day/week exposure protocol and then applying the result to the RfD methodology. Dose adjustments for discontinuous exposure can be made as follows: female rats were gavaged 5 times/week for 15 weeks (75 days), total
dose = 2.25 mg/kg; daily for 3 weeks (21 days), total dose = 0.63 mg/kg; and 4 times/week for 3 weeks (12 days), total dose = 0.36 mg/kg. The combined total dose over the 21-week exposure period, therefore, is 3.24 mg/kg; dividing the combined total dose of 3.24 mg/kg by 147 days (21 weeks) results in an adjusted LOAEL of 0.022 mg/kg/day. The adjusted LOAEL can then be applied to the equation for the derivation of an RfD:
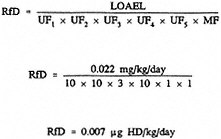
where:
|
LOAEL |
= |
0.022 mg/kg/day |
|
UF1 |
= |
10 (to protect sensitive individuals) |
|
UF2 |
= |
10 (for animal to human extrapolation) |
|
UF3 |
= |
3 (for estimating the NOAEL from the LOAEL) |
|
UF4 |
= |
10 (for subchronic to chronic extrapolation) |
|
UF5 |
= |
1 (data base adequate) |
|
MF |
= |
1 (no additional modifications needed). |
An uncertainty factor of 3000 was applied, accounting for protection of sensitive subpopulations (10), animal-to-human extrapolation (10), LOAEL-to-NOAEL extrapolation (3), and extrapolation from a subchronic to chronic exposure (10).
A LOAEL-to-NOAEL uncertainty factor of 10 is not considered to be necessary because the observed effect was reported to be ''mild"; the critical effect may have been enhanced due to the vehicle used (sesame oil in which sulfur mustard is fully soluble) and the route of administration (i.e., gavage is more likely to result in localized irritant effects). Although the target organ (rat forestomach) is of questionable relevance to humans, because sulfur mustard is a direct alkylating agent, tissue damage would be expected to occur at the point of contact, even if it were another part of the gastrointestinal tract.
The data base for sulfur mustard contains two developmental toxicity studies in different species, a reproductive bioassay and a standard subchronic toxicity study in one species. In addition, chronic inhalation studies have been conducted on sulfur mustard using rats, mice, guinea pigs and dogs. The principal study identifies a toxic effect that is consistent with the vesicant properties of sulfur mustard. There is no evidence that any other experimental species would be more sensitive to ingested sulfur mustard; therefore, additional oral toxicity studies in other species are not considered critical.
4.3 Confidence in the RfD
Study: High
Data Base: Medium
RfD: Medium
The principal study is a well-designed and well-conducted reproductive toxicity study in rats. The identified endpoint (gastric lesions), also identified in a subchronic study and in two developmental studies, supports the conclusion that direct contact with epithelial tissue is the primary mechanism of toxicity. However, the principal study did not identify a NOAEL for this effect and the route of administration (gavage) may have led to an enhanced response due to the bolus type of dosing. Consequently, the overall confidence in the RfD must be considered medium.
5. CARCINOGENICITY ASSESSMENT
Inhalation unit risks have been derived for sulfur mustard directly from experimental animal data as well as from an analysis of the relative carcinogenic potency of sulfur mustard in comparison with that of known carcinogens for which there are both long-and short-term data. Epidemiological and long-term animal data are not available to directly derive an oral slope factor for sulfur mustard. Estimates of the oral slope factor are made from the inhalation unit risk and from the relative potency method.
5.1 Inhalation Unit Risk
5.1.1 Inhalation Unit Risk Derived from Experimental Animal Data
U.S. EPA (1991) derived a cancer inhalation unit risk for sulfur mustard based on the results of inhalation animal studies conducted by McNamara et al. (1975, see Section 3.7.2); however, it was emphasized in the EPA report that the studies of McNamara et al. (1975) contained deficiencies which made a quantitative analysis difficult. Conducted in 1970, the studies do not conform to the modern norms of acceptable experimental protocol, and it is likely that there was bias in the assignment of the animals to the test categories (U.S. EPA, 1991). In addition, many of the exposures were very brief, included only a few animals, and many of the animals were sacrificed (and some were replaced) before their capacity to develop late-appearing tumors was fully developed (U.S. EPA, 1991). Despite these shortcomings, it was noted by EPA that the McNamara et al. data are the best available for estimating the carcinogenic potency of sulfur mustard. The authors of the EPA report analyzed two sets of McNamara's data; one from a toxicity study and one from a carcinogenicity study (see Section 3.7.2).
In the toxicity study only those animals tested and observed long enough to exceed the demonstrated minimum latency period for first tumor appearance (70 days post-exposure, see Table 4) were included in the data analysis. The resulting tumor incidence rates are shown in Table 7.
Table 7. Skin tumor dataa (toxicity study) used in EPA quantitative assessment
|
Group |
Control |
0.001b mg/m3 |
0.1/0.0025b mg/m3 |
|
Males |
0/11 |
0/10 |
4/11 |
|
Females |
0/8 |
0/19 |
5/18 |
|
Both sexes |
0/19 |
0/29 |
9/29 |
|
SOURCE: U.S. EPA, 1991, derived from data of McNamara et al., 1975 a Includes only data for rats surviving longer than the time of first tumor appearance b Low exposure was 0.001 mg/m3 continuously; high exposure was 0.1 mg/m3 for 6.5 hr daily and 0.0025 mg/m3 for the remaining 17.5 hr, d days/wk. |
|||
Because the rats were observed for 6 months after the exposures ended, the daily average exposure was estimated as being equal to 2/3 of the nominal concentration for 52 weeks (U.S. EPA, 1991). Therefore, for the group exposed to 0.1/0.0025 mg/m3, the average concentration for 18 months was 0.019 mg/m3, and for the group exposed to 0.001 mg/m3, the average concentration for 18 months was 0.00067 mg/m3. These data were applied to the GLOBAL 86 computer program for estimating a multistage model dose-response curve. The resulting unit risk was 2.9 × 10-2 per µg/m3 (U.S. EPA, 1991). The unit risk was then adjusted for a rat lifetime exposure of 24 months by multiplying by the ratio of the lifetime to experimental duration to the third power [i.e., (24 mo/18 mo)3]. This adjustment resulted in an estimated unit risk of 6.8 × 103 per µg/m3. The data (including the times that the animals were sacrificed) were also subjected to time-to-tumor analysis using the WEIBULL 82 computer program. Empirically, the latency time estimated from the program was about 2 months, and the lifetime upperbound unit risk was estimated to be 8.5 × 10-2 per µg/m3 (U.S. EPA, 1991).
The results of the McNamara et al. (1975) carcinogenicity study were also analyzed by EPA using the GLOBAL 86 program (U.S. EPA, 1991). The data were grouped into 17 lifetime tumor incidences (two dose levels times eight exposure durations plus a control group), and the exposures were converted to lifetime average concentrations, assuming a lifespan of 2 years (Table 8). The major assumptions used in EPA calculations were that cancer incidence would be linearly related to the product of HD concentration and exposure duration even at very low concentrations, and that the dose-response relationship in humans would be the same as that in animals, when doses are expressed as lifetime average air concentrations. The linearized upper bound on the low-dose slope (q1*) estimated from these data by GLOBAL 86 was 9.4 × 10-2 per µg/m3 (U.S. EPA, 1991).
Considering all the above data, the U.S. EPA (1991) selected the unit risk of 8.5 × 10-2 per µg/m3, derived from the Weibull time-to-tumor model, as the recommended upper bound estimate of the carcinogenic potency of sulfur mustard for a lifetime exposure to HD vapors. However, U.S. EPA (1991) stated that "depending on the unknown true shape of the dose-response curve at low doses, actual risks may be anywhere from this upper bound down to zero". The Weibull model was considered to be the most suitable because the exposures used were long-term, the effect of killing the test animals before a full lifetime was adjusted for, and the sample size was the largest obtainable from the McNamara et al. (1975) data.
Table 8. Skin tumor data (carcinogenicity study) used in EPA quantitative assessment
|
Exposure Duration (weeks) |
Exposure Concentrationa |
Lifetimeb average daily exposure (µg/m3) |
Incidence of skin carcinomas |
|
Control |
0.0 |
0/27 |
|
|
1 |
low |
0.0096 |
0/5 |
|
2 |
low |
0.0192 |
0/5 |
|
4 |
low |
0.0385 |
0/5 |
|
8 |
low |
0.0769 |
0/4 |
|
12 |
low |
0.115 |
0/5 |
|
26 |
low |
0.250 |
0/4 |
|
1 |
high |
0.279 |
0/5 |
|
39 |
low |
0.375 |
0/3 |
|
52 |
low |
0.500 |
0/17 |
|
2 |
high |
0.558 |
0/5 |
|
4 |
high |
1.12 |
0/6 |
|
8 |
high |
2.23 |
0/4 |
|
12 |
high |
3.35 |
4/5 |
|
26 |
high |
7.25 |
4/5 |
|
39 |
high |
10.9 |
4/4 |
|
52 |
high |
14.5 |
10/23 |
|
SOURCE: U.S. EPA, 1991, derived from data of McNamara et al., 1975 a Low exposure was 0.001 mg/m3 continuously; high exposure was 0.1 mg/m3 for 6.5 hr daily and 0.0025 mg/m3 for the remaining 17.5 hr, 5 days/wk. b A 2-yr lifetime was assumed. |
|||
5.1.2 Inhalation Unit Risk Derived from Relative Potency
The inhalation carcinogenicity of sulfur mustard has also been evaluated using relative potency methods. Using the results of studies by Heston (1950, see Section 3.7.2) and Shimkin and McClelland (1949), U.S. EPA (1991) determined that the potency of sulfur mustard to induce pulmonary tumors in strain A mice was equivalent to that of 20-methylcholanthrene (MC). EPA then used the results of studies conducted by Stoner et al. (1984) to determine that MC was 10–13 times more potent than benzo(a)pyrene (BaP) in inducing lung tumors in this same strain of mice. Since the potency of sulfur mustard was
considered to be the same as that for MC, it was estimated that the unit risk for sulfur mustard would be 10–13 times the inhalation cancer unit risk for BaP. The latter was derived from the oral slope factor of 11.5 (mg/kg/day)-1 using the standard defaults of 20 m3/day for ventilation rate and 70 kg for body weight. The resulting inhalation unit risk estimate for sulfur mustard, based on the relative potency method, was 0.033–0.043 (µg/m3)-1 (U.S. EPA, 1991).
5.2 Oral Slope Factor
Long-term oral carcinogenicity studies have not been conducted on sulfur mustard. In oral subchronic studies in which the agent was administered by gavage to rats (see section 3..3), epithelial hyperplasia of the forestomach occurred in 5/12 males and 5/12 females dosed with 0.3 mg HD/kg/day (in sesame oil), 5 days/week for 13 weeks. In light of the known carcinogenicity of sulfur mustard, the epithelial hyperplasia is suggestive of a pre-neoplastic condition.
To derive an oral slope factor in the absence of long-term experimental data, two non-standard approaches can be considered. One involves the direct conversion of the inhalation unit risk to an oral slope factor, and the other involves the use of relative potency methods. Both approaches are summarized in the following sections.
5.2.1 Oral Slope Factor Derived from Inhalation Unit Risk
The U.S. EPA (1991) identified an "inhalation" unit risk of 8.5 × 10-2 per µg/m3, derived from the Weibull time-to-tumor model, as the most appropriate estimate of the carcinogenic potency of sulfur mustard. This unit risk can be converted to a slope factor by normalizing the value for a 70 kg man inhaling 20 m3 of air per day. The resulting slope factor is 0.3 (µg/kg/day)-1.
Although it can be argued that conversion of an inhalation unit risk to an oral slope factor might be acceptable under certain conditions, including cases where 1) the cancer target organ is the same regardless of the route of exposure, 2) where differences in g.i. and pulmonary absorption can be taken into account, and 3) where metabolic activation is not critical or, if it is, the differences in first-pass metabolism in the liver and lung are accounted for. In the case of sulfur mustard, the inhalation unit risk is based on the occurrence of skin tumors in rats following exposure to sulfur mustard vapors. There is no evidence that the tumors occurred following systemic absorption and distribution to the skin. Considering the vesicant action of the agent, it is likely that the skin tumors resulted from the direct contact of the vapors or condensation droplets on the skin of the test animals. As mentioned above, for oral exposures the only evidence available suggests that if tumors did occur, they would be localized in the epithelial tissue of the g.i. tract. Therefore, it is unlikely that the target organs would be the same, although it could be argued that in both cases an epithelial surface is the target. Because a dose per unit surface area of skin can not be determined from the McNamara et al. (1975) study, quantitative extrapolation from a skin response to a g.i. tract response is not possible. Furthermore, because sulfur mustard is subject to rapid hydrolysis, the amount ingested that actually reaches the epithelial surface of the g.i. tract may be considerably limited, and, in fact, a significant dose may only be possible through gavage administration in an organic solvent vehicle, as was done in the animal toxicity studies.
As noted in section 3.7.1, there is only limited evidence that sulfur mustard causes digestive tract tumors in humans. Yamada (1974, as reported by Inada et al., 1978) found 17 cases of digestive tract cancers among 94 autopsy cases and 8 surgical cases of former workers at a chemical warfare
manufacturing facility. No comparisons were made with control groups; therefore, the significance of this finding cannot be determined. It is known that many of these workers were exposed to sulfur mustard concentrations thought to be sufficiently high (est. 50–70 mg/m3) to produce signs of acute toxicity (i.e., acute conjunctivitis, acute rhinitis, acute bronchitis, and acute dermatitis with blister formation) (Nakamura, 1956).
5.2.2 Oral Slope Factor Derived from Relative Potency Estimates
As described in section 5.1.2, U.S. EPA (1991) derived an inhalation unit risk for sulfur mustard using the relative potency method in which sulfur mustard was considered to be 10–13 times more potent than BaP. The oral slope factor for BaP, as currently listed on IRIS, is 7.3 (mg/kg/day)-1 (U.S. EPA, 1996). Multiplying this slope factor by the relative potency range of 10–13, results in an oral slope factor of 0.073–0.095 (µg/kg/day)-1 for sulfur mustard.
Watson et al. (1989) estimated the carcinogenic potency of HD by the "rapid screening of hazard" (RASH) method developed by Jones et al. (1988). This approach compares exposures that produce documented toxic effects of a chemical of interest to exposures of a reference chemical producing a similar effect. The RASH procedure was applied to Heston's intravenous (1950) and subcutaneous injection studies (1953). Comparing the carcinogenicity of HD and the well characterized industrial carcinogen benzo[a]pyrene (BaP), Watson et al. (1989) showed that the two compounds are of approximate equivalent carcinogenic potency in experimental animals with a best estimate relative potency of 1.3 for sulfur mustard relative to BaP and an interquartile range (middle 50% of distribution) of 0.6-2.9. Consistent results were obtained when the potency of sulfur mustard was compared to that of bis-chloromethyl ether (BCME, CAS No. 542-88-1) another alkylating agent that is a powerful lung and eye irritant, as well as an IARC Class 1 carcinogen (Watson et al., 1989; IARC, 1987)
The estimated comparative carcinogenic potency can be used to derive a slope factor (SF) or q1* for sulfur mustard. The slope factor converts the estimated daily intake averaged over a lifetime exposure to incremental risk of an individual developing cancer. Because the slope factor is an upper 95th percentile confidence limit on the probability of response based on experimental animal data, the carcinogenic risk will generally be an upper-bound estimate.
Applying the Watson et al. (1989) estimated relative potency of 1.3 and using the currently accepted oral slope factor of 7.3 per (mg/kg)/day for BaP, a SF for HD can be calculated as follows:
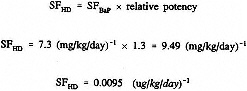
5.3 Confidence in the Quantitative Estimates of Carcinogenic Potency
5.3.1 Inhalation Unit Risk
U.S. EPA (1991) notes that the dose-response estimates derived from the McNamara et al. (1975) study are highly uncertain due to the fact that the study was not of a standard design and too few animals were exposed and followed for a lifetime to give adequate sensitivity for detecting long-term effects. In addition, the uncertainty concerning the experimental conditions was too great to allow confidence about the absolute carcinogenic potency value.
In view of the fact that in the McNamara et al. (1975) study, malignant tumors appeared only at the highest mustard concentration and only late in life, U.S. EPA (1991) observed:
"perhaps it may exert its carcinogenic activity secondarily through lifelong exposure to its cytotoxic or irritating effects. Under such circumstances, human exposures at low concentrations for limited times may entail much less risk than implied by the unit risk factor estimated from lifetime effects at higher doses. On the other hand, the lack of low-dose responses and early-appearing tumors in the McNamara data may be due simply to the inherent difficulty of detecting low-risk levels in experiments of reasonable size".
Because sulfur mustard is known to be a strong and direct DNA alkylating agent, the likelihood is very high that it functions as a non-threshold carcinogen. Consequently, the risks associated with exposures to low concentrations require evaluation, and the McNamara et al. (1975) study provides the only data set that allows for a quantification of carcinogenic potency following exposure to sulfur mustard vapors.
5.3.2 Oral Slope Factor
Although human and animal data are lacking, there is indirect evidence suggesting that sulfur mustard may be carcinogenic by the oral exposure route. The mechanism of action of sulfur mustard as a direct DNA alkylating agent, its known genotoxicity in exposed humans and in various animal bioassays, its induction of respiratory tract and skin tumors following inhalation exposures, and its induction of forestomach hyperplasia in rats following subchronic gavage dosing (see Section 3.3), all support the conclusion that this compound functions as a point of contact carcinogen on epithelial tissues. Furthermore, the mechanism of action of sulfur mustard would be expected to be similar to that of other known or suspected mustard carcinogens such as nitrogen mustard (sulfur mustard tumorigenicity was determined to be comparable to that of the nitrogen mustard agents HN2 and HN2-HCl and the therapeutic nitrogen mustard compounds melphalan and BCME; see Institute of Medicine, 1993, Appendix I), as well as bis-(chloro) ethyl ether (BCEE).
In the absence of human or animal oral dose-response data, the relative potency approaches developed by Watson et al. (1989) and U.S. EPA (1991) are considered to be appropriate methods for estimating the tumorigenic potency of sulfur mustard by the oral route of exposure. The oral slope factor derived by Watson et al. is approximately one order of magnitude less than the one derived from the relative potency estimated by U.S. EPA (1991). In the emerging area of relative potency analysis, a factor of 10 difference represents a good fit. There is no significant difference between the estimates of sulfur mustard carcinogenic potency relative to B(a)P published by Watson et al. (1989) and U.S. EPA (1991).
Although there are dose-response data from an animal inhalation exposure study (McNamara et al., 1975, see Section 5.1.1), route-to-route extrapolation (from inhalation to oral, as calculated in Section 5.2.1) is not considered appropriate because the exposure protocol of McNamara et al. (1975) resulted in rat skin tumors which might have occurred, not a result of systemic uptake, but as a result of dermal contact with sulfur mustard vapor (perhaps trapped by the rat pelt). Therefore, there is no method for estimating the dermal dose of sulfur mustard, or for converting this to an oral dose.
6.REFERENCES CITED
ATSDR (Agency for Toxic Substances and Disease Registry). 1992. Toxicological Profile for Mustard Gas. ATSDR, Atlanta, GA, TP-91/22.
Auerbach, C and J.M. Robson. 1946. Chemical production of mutagens. Nature (Lond.) 157:302.
Azizi, F., A. Keshavarz, F. Roshanzamir, et al. 1995. Reproductive function in men following exposure to chemical warfare with sulfur mustard. Med. War 11:34–44.
Beebe, G.W. 1960. Lung cancer in World War I veterans: possible relation to mustard gas injury and 1918 influénza epidemic. J. Natl. Cancer Inst. 25:1231–1252.
Capizzi, R.L., W.J. Smith, R. Field and B. Papirmeister. 1973. A host-mediated assay for chemical mutagens using L5178Y/Asn murine leukemia. Mutat. Res. 21:6.
Case, R.A.M. and A.J. Lea. 1955. Mustard gas poisoning, chronic bronchitis and lung cancer. An investigation into the possibility that poisoning by mustard gas in the 1914–1918 war might be a factor in the production of neoplasia. Brit. J. Prev. Med. 9:62–72. (as cited by IARC, 1975; Beebe, 1960)
Crathorn, A.R. and J.J. Roberts. 1965. Reactions of cultured mammalian cells of varying radiosensitivity with the radiomimetic alkylating agent mustard gas.. Prog. Biochem. Pharmacol. 1:320–326.
Crathorn, A.R. and J.J. Roberts. 1966. Mechanism of the cytotoxic action of alkylating agents in mammalian cells and evidence for the removal of alkylated groups from deoxyribonucleic acid. Nature (Lond) 211:150–153.
DA (U.S. Department of the Army). 1974. Chemical Agent Data Sheets, vol. 1. Edgewood Arsenal Special Report, EO-SR 74001. Defense Tech, Inform. Center, Alexandria, VA.
DA (U.S. Department of the Army). 1992. Material Safety Data Sheets: HD and THD. Edgewood Research, Development and Engineering Center, Aberdeen Proving Ground, MD.
Dahl, H., B. Glund, P. Vangstad and M. Norn. 1985. Eye lesions induced by mustard gas. Acta Ophthalmol. 63(suppl. 173):30–31. (as cited in Watson and Griffin, 1992)
Doull, J., C.D. Klaassen and M.O. Amdur (eds.). 1980. Casarett and Doull's Toxicology. The Basic Science of Poisons. 2nd ed., MacMillan, New York, NY.
Easton, D.F., J. Peto and R. Doll. 1988. Cancers of the respiratory tract in mustard gas workers. Br. J. Ind. Med. 45(10):652–659.
Fox, M. and D. Scott. 1980. The genetic toxicology of nitrogen and sulfur mustard. Mutat. Res. 75:131–168.
Hackett, P.L., R.L. Rommereim, F.G. Burton, R.L. Buschbom and L.B. Sasser. 1987. Teratology Studies on Lewisite and Sulfur Mustard Agents: Effects of Sulfur Mustard in Rats and Rabbits. Final Report. AD A 187495. Pacific Northwest Laboratory, Richland, WA. for the U.S. Army Medical Research and Development Command, Fort Detrick, MD.
Heston, W.E. 1950. Carcinogenic action of mustards. J. Natl. Cancer Inst. 11:415–423.
Heston, W.E. 1953. Occurrence of tumors in mice injected subcutaneously with sulfur mustard and nitrogen mustard. J. Natl. Cancer Inst. 14:131–140.
Heston, W.E. and W.D. Levillain. 1953. Pulmonary tumors in strain A mice exposed to mustard gas. Proc. Soc. Exp. Biol. 82:457–460.
IARC (International Agency for Research on Cancer). 1975. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Some Aziridines, N, S, & O-mustards and Selenium, vol. 9, pp. 181–207. International Agency for Research on Cancer, Lyons, France.
IARC (International Agency for Research on Cancer). 1987. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs Volumes 1–42, Suppl. 7, p. 67. International Agency for Research on Cancer, Lyons, France.
Inada, S., K. Hiragun, K. Seo and T. Yamura. 1978. Multiple Bowen's disease observed in former workers of a poison gas factory in Japan, with special reference to mustard gas exposure. J. Dermatol. 5:49–60.
Institute of Medicine, Committee to Survey the Health Effects of Mustard Gas and Lewisite, Division of Health Promotion and Disease Prevention. 1993. Veterans at Risk; The Health Effects of Mustard Gas and Lewisite, C.M. Pechura and D.P. Rall, eds. National Academy Press, Washington, DC.
ITII (International Technical Information Institute). 1975. Toxic and Hazardous Industrial Chemicals Safety Manual. p. 351. International Technical Information Institute, Tokyo, Japan.
Jones, TD, P.J Walsh, A.P. Watson, et al. 1988. Chemical scoring by a Rapid Screening Hazard (RASH) method. Risk Anal. 8:99–118.
Jostes, R.F., L.B. Sasser and R.J. Rausch. 1989. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Genetic Toxicity of Sulfur Mustard (HD) in the Chinese Hamster Ovary Cells. Final Report from Pacific Northwest Laboratories (PNL-6916) to U.S. Army Medical Research and Development Command, Fort Detrick, MD.
Kircher, M. and M. Brendel. 1983. DNA alkylation by mustard gas in yeast Saccharomyces cerevisiae strains of different repair capacity. Chem.-Biol. Interact. 44:27–39.
Klehr, N. 1984. Cutaneous late manifestation in former mustard gas workers. Z. Hautkrankh. 59:1161–1170. (In German with English abstract)
Lawley, P.D. and P. Brookes. 1965. Molecular mechanism of the cytotoxic action of difunctional alkylating agents and of resistance to this action. Nature (Lond.) 206:480–483.
Lewis, R.J. and D.V. Sweet, eds. 1984. Registry of Toxic Effects of Chemical Substances. 1983 supplement to the 1981–82 edition. pp. 1153, 1169. U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, Cincinnati, OH.
MacNaughton, M.G. and J.H. Brewer. 1994. Environmental Chemistry and Fate of Chemical Warfare Agents. Southwest Research Institute, San Antonio, TX
Manning, K.P., D.C.G. Skegg, P.M. Stell, et al. 1981. Cancer of the larynx and other occupational hazards of mustard gas workers. Clin. Otolaryngol. 6:165–170.
McNamara, B.P., E.J. Owens, M.K. Christensen, et al. 1975. Toxicological basis for controlling levels of mustard in the environment. EASP EBSP 74030. Biomedical Laboratory, Department of the Army, Headquarters, Edgewood Arsenal, Aberdeen Proving Ground, MD.
Morgenstern, P., F.R. Koss, and W.W. Alexander. 1947. Residual mustard gas bronchitis; effects of prolonged exposure to low concentrations. Ann. Internal Med. 26:27–40.
Nakamura, T. 1956. Studies on the warfare gas-injury in Japan. Report I. On the general condition of the poison gas island. Hiroshima Med. J. 4:1141–1149. (In Japanese, as cited in Inada et al., 1978)
NTP (National Toxicology Program). 1994. Annual Report on Carcinogens. National Toxicology Program. Research Triangle Park, NC.
Nishimoto, Y., M. Yamakido, T. Shinegobu, et al. 1983. Long term observations of poison gas workers with special reference to respiratory cancers. J. UOEH 5(Suppl.):89–94.
Nishimoto, Y., M. Yamakido, S. Ishioka, et al. 1988. Epidemiological studies of lung cancer in Japanese mustard gas workers. In: Unusual Occurrences as Clues to Cancer Etiology, R.W. Miller et al., (eds). Japan. Sci. Soc. Press., Tokyo. pp. 95–101.
Norman, J. 1975. Lung cancer mortality in World War I veterans with mustard gas injury: 1919–1945. J. Natl. Cancer Inst. 54:311–317.
Papirmeister, B., A.J. Feister, S.I. Robinson and R.D. Ford. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. CRC Press, Boca Raton, FL. 359 pp.
Renshaw, B. 1946. Mechanisms in production of cutaneous injuries by sulfur and nitrogen mustards. In: Chemical Warfare Agents and Related Chemical Problems, vol. 1, pp. 479–518. U.S. Office of Scientific Research and Development, National Defense Research Committee, Washington, D.C. (as cited in Vogt et al., 1984).
Rosenblatt, D.H., T.A. Miller, J.C. Dacre, I. Muul and D.R. Cogley. 1975. Problem Definition Studies on Potential Environmental Pollutants. II. Physical, Chemical, Toxicological, and Biological Properties of 16 Substances. Tech. Report 7509, AD AO30428. U.S. Army Medical Bioengineering Research and Development Laboratory , Fort Detrick, MD.
Rosenblatt, D.H., M.J. Small, T.A. Kimmell and A.W. Anderson. 1995. Agent Decontamination Chemistry Technical Report. U.S. Army Test and Evaluation Command (TECOM) Technical Report, Phase I. Draft Report, Argonne National Laboratory.
Rozmiarek, H., R.L. Capizzi, B. Papirmeister et al. 1973. Mutagenic activity in somatic and germ cells following chronic inhalation of sulfur mustard. Mutat. Res. 21:13–14.
Sasser L.B., R. A. Miller, D.R. Kalkwarf, et al. 1989a. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Subchronic Toxicity of Sulfur Mustard (HD) in Rats. Final Report from Pacific Northwest Laboratories (PNL-6870) to U.S. Army Medical Research and Development Command, Fort Detrick, MD, AD A2144555.
Sasser L.B., R. A. Miller, D.R. Kalkwarf, et al. 1989b. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Two-Generation Reproduction Study of Sulfur Mustard (HD) in Rats. Final Report from Pacific Northwest Laboratories (PNL-6944) to U.S. Army Medical Research and Development Command, Fort Detrick, MD, AD A216423.
Sasser L.B., R. A. Miller, J.A. Cushing and J.C. Dacre. 1990. Dominant lethal effect of sulfur mustard in rats. Toxicologist 10:225. (Abstract)
Scott, D., M. Fox and B.W. Fox. 1974. The relationship between chromosomal aberrations, survival and DNA repair in tumor cell lines of differential sensitivity to x-rays and sulphur mustard. Mutat. Res. 22:207–221.
Shakil, F.A., A. Kuramoto, M. Yamakido, et al. 1993. Cytogenetic abnormalities of hematopoietic tissue in retired workers of the Ohkunojima poison gas factory. Hiroshima J. Med. Sci. 42:159–165.
Shimkin, M.B. and J.N. McClelland. 1949. Induced pulmonary tumors in mice. IV. Analysis of dose response data with methylcholanthrene. J. Natl. Cancer Inst. 10:597–603.
Sidell, F.R. and C.G. Hurst. 1992. Clinical Considerations in Mustard Poisoning. In: Chemical Warfare Agents. A.M. Somani, ed., pp. 51–67, Academic Press, New York.
Small, M.J. 1984. Compounds Formed from the Chemical Decontamination of HD, GB, and VX and Their Environmental Fate. Technical Report 8304, AD A149515, US Army Medical Bioengineering Research and Development Laboratory, Fort Detrick, Frederick, MD.
Somani, S.M. 1992. Toxicokinetics and Toxicodynamics of Mustard. In: Chemical Warfare Agents. A.M. Somani, ed., pp. 13–50, Academic Press, New York.
Stewart, D.L., E.J. Sass, L.K. Fritz and L.B. Sasser. 1989. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Mutagenicity of Sulfur Mustard in the Salmonella Histidine Reversion Assay. Final Report from Pacific Northwest Laboratories (PNL-6873) to U.S. Army Medical Research and Development Command, Fort Detrick, MD, AD A213102.
Stoner, G.D., E.S. Greisiger, H.A. Schuf, et al. 1984. A comparison of the lung adenoma response in strain A/J mice after intraperitoneal and oral administration of carcinogens. Toxicol. Appl. Pharmacol. 72:313–323
Takeshima, Y, K. Inai, W.P. Bennet, et al. 1994. P53 mutations in lung cancers from Japanese mustard gas workers. Carcinogenesis 15:2075–2079.
U.S. EPA. 1991. Upper-bound Quantitative Cancer Risk Estimate for Populations Adjacent to Sulfur Mustard Incineration Facilities. Office of Research and Development, U.S. Environmental Protection Agency, EPA/600/8–91/053.
U.S. EPA. 1996. Carcinogenicity Assessment for Benzo[a]pyrene. Integrated Risk Information Retrieval System, U.S. Environmental Protection Agency, Washington, DC. Online file
Venitt, S. 1968. Interstrand cross-links in the DNA of Escherichia coli B/r and Bs-1 and their removal by the resistant strain. Biochem. Biophys. Res. Commun. 31:355–360.
Vogt, R.F., Jr., A.M. Dannenberg, Jr., B.H. Schofield, N. A. Hynes and B. Papirmeister. 1984. Pathogenesis of skin lesions caused by sulfur mustard. Fundam. Appl. Toxicol. 4:S71-S83.
Wada, S., Y. Nishimoto, M. Miyanashi, et al. 1962a. Review of Okuno-jima poison gas factory regarding occupational environment. Hiroshima J. Med. Sci. 11:75–80.
Wada, S., Y. Nishimoto, M. Miyanshi, S. Katsuta, M. Nishiki, A. Yamada, S. Tokuoka, H. Umisa, and M. Nagal, 1962b. Malignant respiratory tract neoplasms related to poison gas exposure. Hiroshima J. Med. Sci. 11:81–91.
Wada, S., Y. Nishimoto, M. Miyanishi, et al. 1968. Mustard gas as a cause of respiratory neoplasm in man. The Lancet, June 1, 1968, pp. 1161–1163.
Walker, I.G. and C.J. Thatcher. 1968. Lethal effects of sulfur mustard on dividing mammalian cells. Radiat. Res 34:110–127.
Ward, D.M., N.M. Anson, P.A. Parent, and E.H. Enquist. 1966. Sulfur Mustard and Analogous Compounds as Special Purpose Agents (U). 12–33 EASP 100-7R1. Aberdeen Proving Ground, MD. As reported in Rosenblatt et al. 1975.
Waters, M.D., N.E. Garrett, C.M. CovonedeSerres, B.E. Howard, and H.F. Stack. 1983. Genetic toxicology of some known or suspected human carcinogens. In: Chemical Mutagens, Principles and Methods for their Detection, F.J. de Serres, ed. vol. 8. pp. 261–341. Plenum Press, New York.
Watson, A.P., T.D. Jones, and G.D. Griffin. 1989. Sulfur mustard as a carcinogen: Application of relative potency analysis to the chemical warfare agents H, HD, and HT. Reg. Toxicol. Pharmacol. 10:1–25.
Watson, A.P. and G.D. Griffin. 1992. Toxicity of vesicant agents scheduled for destruction by the chemical stockpile disposal program. Environ. Health Perspect. 98:259–280.
Weiss, A. and B. Weiss. 1975. Karzinogenese durch Lost-Exposition beim Menschen, ein wichtiger Hinweis fur die alkylantien-Therapie. Dtsch. med. Wschr. 100:919–923 (as cited in IARC 1975)
Wulf, H.C., A. Aasted, E. Darre and E. Niebuhr. 1985. Sister chromatid exchanges in fishermen exposed to leaking mustard gas shells. The Lancet, March 25, 1985, pp. 690–691.
Yamada, A., F. Hirose and M. Miyanishi. 1953. An autopsy of bronchial carcinoma found in a patient succumbed to occupational mustard gas poisoning. Gann 44:216–219. (as cited in IARC, 1975)
Yamada, A., F. Hirose, M. Nagai and T. Nakamura. 1957. Five cases of cancer of the larynx found in persons who suffered from occupational mustard gas poisoning. Gann 48:366–368. (as cited in IARC, 1975)
Yamada, A. 1963. On the late injuries following occupational inhalation of mustard gas, with special references to carcinoma of the respiratory tract. Acta Pathologica Japonica 13:131–155.
Yamada, A. 1974. Patho-anatomical studies on occupational poisoning. Tr. Soc Path. Jap 63:17–61. (as cited in Inada et al., 1978)
Yanagida, J., S. Hozawa, S. Ishioka, et al. 1988. Somatic mutation in peripheral lymphocytes of former workers at the Okuno-jima poison gas factory. Jap. J. Cancer Res. 79:1276–1283.
Yoshimura, K. and N. Yamashita. 1982. Clinical statistical observation of 4931 cases with lung cancer by histological type. Results of field study in Japan. Lung Cancer 22:1–17. (In Japanese, as cited by Nishimoto et al., 1988)








































