Appendix G
Inhibition of Cholinesterases and an Evaluation of the Methods Used to Measure Cholinesterase Activity
ESTERASES
CHOLINESTERASES (ChEs) are enzymes that hydrolyze esters of choline. One is acetylcholinesterase (AChE, acetylcholine hydrolase, EC 3.1.1.7). Another is butyryl cholinesterase (BuChE, acylcholine acylhydrolase, EC 3.1.1.8), sometimes known as nonspecific cholinesterase or pseudocholinesterase. The preferred substrates for AChE and BuChE are acetylcholine (ACh) and butyryl choline or propionylcholine, respectively. AChE and BuChE are inhibited by organophosphate (OP) esters that react at the catalytic site of the enzymes, forming enzyme-inhibitor complexes that block the action of the enzyme. Carboxyesterases (CaE, EC 3.1.1.1) are a third type of enzyme. One CaE, known as neuropathy target esterase (NTE), is an enzyme associated with organophosphate-induced delayed neuropathy (OPIDN) (Johnson 1977). The biochemistry, determination, pharmacology, toxicology, and biology of the ChEs and their inhibitors have been reviewed (Augustinsson 1948; Aldridge and Reiner 1972; Silver 1974; Whittaker 1986; Hoffmann et al. 1989; Gallo and Lawryk 1991; Ballantyne and Marrs 1992; Chambers and Levi 1992; EPA 1992; Ecobichon 1994; Taylor 1994, 1996).
AChEs, BuChEs, and CaEs are specialized carboxylic ester hydrolases classed among the B esterases, enzymes that are inhibited by OPs. Another class of enzymes are the A esterases (e.g., paraoxonase and DFPase) that actively hydrolyze OPs, destroying their toxic potential
(Aldridge and Reiner 1972). The A esterases have a serine catalytic site. The tertiary structure and amino acid sequences of several AChEs and BuChEs have been established (Taylor 1994). AChEs regulate excitation at cholinergic synapses, destroying the neurotransmitter ACh. AChE is one of the most active enzymes known, cycling within a few milliseconds (Taylor 1996). AChEs are found at synapses and neuromuscular junctions, and in central-nervous-system (CNS) neuron cell bodies, axons, muscles, red blood cells (RBCs), and platelets of ovine and rodent species (Silver 1974; Traina and Serpietri 1984; Hoffmann et al. 1989).
There is AChE-like activity in the plasma of some birds and mammals (Traina and Serpietri 1984; Smucker and Wilson 1990). Plasma ChE of rodents, such as the laboratory rat, is high in both AChE and BuChE activities (Traina and Serpietri 1984). AChE activity in human blood is found mainly in RBCs (Silver 1974). AChE activity also occurs in the serum of developing mammals and birds, decreasing to adult levels after birth (Smucker and Wilson 1990). Together with AChEs, BuChEs are also found at synapses, motor end plates, and muscle fibers. BuChE activity in blood is restricted to serum (Silver 1974)
Substrate preferences for AChEs and BuChEs vary with the species. Both mammal and bird AChEs rapidly hydrolyze ACh and its thiocholine analog acetylthiocholine (AcTh) (Silver 1974). Plasma-BuChE activity in the rat is reported to favor propionyl rather than butyryl substrates (Augustinsson 1948; Hoffmann et al. 1989). AChEs and BuChEs respond differently to increasing substrate concentration. AChEs are inhibited by excess substrate above 1–2 millimolars (mM) (Wilson et al. 1997; Silver 1974). BuChEs are less sensitive. BuChEs are preferentially inhibited by the selective inhibitor iso-OMPA and quinidine, and AChEs by the bisquaternary compound BW284c51.
BIOCHEMISTRY
The action of AChE on ACh is a multistep process, represented below by the formation of a reversible enzyme-substrate complex (EAX), acetylation of the catalytic site (EA), and hydrolysis of the enzyme-substrate complex yielding acetic acid, choline, and the regenerated enzyme (E + A). A similar reaction scheme is applicable to BuChEs.
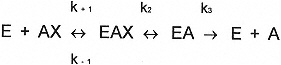
Scheme 1 Action of AChE on ACh. E, enzyme; AX, substrate (ACh) or inhibitor; EAX, reversible enzyme complex; k, reaction-rate constants.
Reaction of an OP with AChE, BuChE, or other B esterases is similar to the reaction of AChE with ACh, except that the hydrolysis step is much slower or, in some cases, might not occur at all. Its basis is a phosphorylation of the enzyme. A serine hydroxyl at the catalytic site reacts with the phosphorus atom of the inhibitor to form an OP-ChE complex. A side group on the phosphorus atom, known as the leaving group (X), is lost. With some OPs, the phosphorylated enzyme might, in time, reactivate by rehydrolysis (Wilson et al. 1992). Spontaneous reactivation might take hours to days. OP-ChE complexes also undergo a reaction known as aging, in which a second group is lost from the phosphate, stabilizing the OP-ChE complex and blocking its reactivation.
TOXICITIES
The toxicities of OPs and carbamates usually correlate with the extent of their inhibitions of brain AChE. For example, a plot of intraperitoneal LD50 versus pI50 (50% inhibition) in mice shows the relationship between the toxicity in vivo of 30 directly acting OPs and their inhibition of AChE in vitro (Gallo and Lawryk 1991).
Many of the physiological effects of anti-ChEs are attributable to excess neurotransmitter ACh (Taylor 1996). The precise symptoms and the time course depend on the chemicals and the localization of the receptors affected. Early symptoms of cholinergic poisoning represent stimulation of muscarinic neuro-effectors of the parasympathetic system. Effects include slowing of the heart (bradycardia), constriction of the pupil of the eye, diarrhea, urination, lacrimation, and salivation. Actions at nicotinic skeletal neuromuscular junctions (motor end plates) result in muscle fasciculation (disorganized twitching) and, at higher doses,
muscle paralysis. Anti-ChE actions at the cholinergic junctions of the sympathetic and parasympathetic autonomic ganglia affect the eye, bladder, heart, and salivary glands. Anti-ChEs also affect junctions of the CNS, producing symptoms that include hypothermia, tremors, headache, anxiety, convulsions, coma, and death. Whether exposure to low doses of OPs results in consistent behavioral effects, such as deficits in learning and memory, is a matter of current research. Behavioral effects (performance deficits) in the absence of miosis (usually taken as the most sensitive indicator of airborne exposure to nerve agents), salivation or fasciculation, are described in primates receiving oral GD under controlled conditions (Hartgraves and Murphy 1992).
Excess ACh produced at motor end plates also causes reversible subjunctional myopathy (Dettbarn 1984). ACh opens receptor channels, promoting influx of Ca+2 and other ions into the post-synaptic cell. In rats, that activity brings about regions of necrosis in 10–30% of muscle fibers around the motor end plates. Prolonged muscle weakness and muscle damage might last several weeks or longer after exposure to high concentrations of some OPs, including methyl parathion, fenthion, and dimethoate. These experimental observations might correspond to the proximal muscle weakness that sometimes develops in individuals after an organophosphate pesticide-induced cholinergic crisis. That phenomenon has been termed ''intermediate syndrome" because it follows the cholinergic crisis and precedes the appearance of peripheral neuropathy (in the event the organophosphate is able to induce neuropathy).
Although most of the effects of OPs are considered to be due to AChE inhibition, there is evidence that anti-ChEs directly affect ACh receptor channels (Rocha et al. 1996; Katz et al. 1997), that anti-AChE pesticides depress the immune system in experimental animals (Casale et al. 1993), and that choline itself might act as an allosteric regulator of nicotinic receptors in the CNS (Alkondon et al. 1997).
A few OPs have been shown to cause OPIDN, a retrograde degeneration of long and large nerve fibers in the spinal cord and peripheral nerves of humans and experimental animals. Some OPs, such as GB, chlorpyrifos, and isofenphos, require very high doses to be acutely neuropathic (WHO 1986; Lotti 1991). Inhibition of approximately 70% or more of the carboxylesterase NTE often is associated with the disorder (WHO 1986; Lotti 1991). Onset of OPIDN is usually 10 days to several weeks after exposure. It is not clear whether OPIDN can occur after long-term exposure to low concentrations of OPs.
Genetic variation between individuals plays a role in the toxicity of anti-ChEs. An example is humans with inherited low concentrations of plasma BuChEs. Although usually symptomless, such persons given succinylcholine (or a similar drug) during surgery to bring about muscle relaxation, cannot speedily destroy the drug, intensifying and prolonging its activity, sometimes with fatal consequences (Whittaker 1986). Studies on experimental animals indicate that depressing ChEs with anti-ChEs intensifies cocaine's effects. Artificially high blood ChEs protect against chemical-warfare agents, and there is some evidence that low blood ChEs enhance their toxicity (Shih et al. 1998).
EVALUATION OF METHODS USED TO MEASURE CHE
The methods used to determine ChE activity were reviewed by the subcommittee with several questions in mind: (1) Were the methods accurate enough to meet the objectives of the study? (2) Were the methods standardized enough to permit comparisons of one study to another? (3) Were the findings suitable for deriving RfDs?
GA, GB, AND GD STUDIES
In its evaluation of the critical studies of GA, GB, and GD, the subcommittee noted that ChE assay kits and instructions developed for humans appeared to be applied without question to studies of experimental animals. Validation of such applications has not been done, and, therefore, ChE measurements reported in the critical studies of GA (Bucci et al. 1992a), GB (Bucci and Parker 1992), and GD (Bucci et al. 1992b) are of questionable accuracy.
In the critical studies of GA, GB, and GD, the manual Boehringer-Mannheim kit or an Encore II centrifugal analyzer using Boehringer-Mannheim conditions were used to measure ChE activity. RBC-AChE activities were calculated by determining the difference between whole-blood and plasma-ChE concentrations using a formula from the reagent manufacturer (Boehringer-Mannheim Diagnostics 1981). Relatively high activity of plasma AChE (Table G-1) and RBC thiol oxidase (Table G-2), which are present in rats but not humans, was not taken into account; resulting in low estimates of blood-AChE (plasma and RBC combined)
TABLE G-1 The Ellman Assay of Cholinesterase Activities in Plasma from 18 Rats
|
Total ChE (mU/mL) |
BuChE (mU/mL) |
AChE (mU/mL) |
|
(Mean ± SE) |
(Mean ± SE) |
(Mean ± SE) |
|
452 ± 17 |
175 ± 12 |
210 ± 8 |
|
Substrates: acetylthiocholine for ChE, butyrylthiocholine for BuChE, and acetylthiocholine and 0.1 mM iso-OMPA. Abbreviations: AChE, acetylcholinesterase; BuChE, butyrylcholinesterase; ChE, cholinesterase; iso-OMPA, tetraisopropylpyrophosphoramide Source: Traina and Serpietri (1984). |
||
activity. Moreover, the studies were conducted with a substrate concentration of acetylthiocholine of 5.4 mM and a pH of 7.2, which have been shown to reduce human blood-AChE activity by approximately 40% (Wilson et al. 1997). Although the results of the individual studies might be consistent internally, the lack of optimal conditions and the lack of correction for the thiol oxidase activity make it difficult to compare the findings between studies.
The problems with the assay methods might have been minimized by Oak Ridge National Laboratory's (ORNL) use of relative rather than
TABLE G-2 Red-Blood-Cell Dithiobisnitrobenzoate (DTNB) Background Reaction in Various Species
|
Species |
Percent Total Activity |
|
Man |
<10 |
|
Monkey |
18–27 |
|
Dog |
30–60 |
|
Rabbit |
30–50 |
|
Rat |
50–70 |
|
Mouse |
60–75 |
|
Brain (All) |
<4 |
|
Plasma (All) |
Not applicable |
|
Transient activity during the first 5–10 min of DTNB by using the Ellman method. Source: Loof (1992). |
|
absolute values. It is not clear, however, whether the effects on the dose-response curves were sufficient to materially affect the levels at which changes in activity could be detected.
Table G-3 illustrates the effect of a constant high blank that would occur when the thiol oxidase activity of rodent RBCs is taken into consideration using arbitrary values. When the data are presented in absolute units, the slopes of the curves are the same but the intercepts differ by a value equal to the level of the blank. However, when the data are expressed as a percentage of the total activity, the intercepts are little affected, and the higher the blank, the lower the slopes. Regardless, because the dose values are unchanged, the relative value at which the effect of the inhibition appears seems little affected.
TABLE G-3 Effect of a Constant Blank on Acetylcholinesterase Activity
|
Dose |
0-ACT |
20-ACT |
30-ACT |
40-ACT |
%-20 |
%-30 |
%-40 |
|
1 |
100 |
120 |
130 |
140 |
100 |
100 |
100 |
|
2 |
90 |
110 |
120 |
130 |
92 |
92 |
93 |
|
3 |
70 |
90 |
100 |
110 |
75 |
77 |
79 |
|
4 |
40 |
60 |
70 |
80 |
50 |
54 |
57 |
|
5 |
25 |
45 |
55 |
65 |
38 |
42 |
46 |
|
6 |
8 |
28 |
38 |
48 |
23 |
29 |
34 |
|
7 |
5 |
25 |
35 |
45 |
21 |
27 |
32 |
|
8 |
3 |
23 |
33 |
43 |
19 |
26 |
31 |
|
SLOPE |
-15.5 |
-15.5 |
-15.5 |
-15.5 |
-13.0 |
-11.9 |
-11.1 |
|
INTCPT |
112.5 |
132.5 |
142.5 |
152.5 |
110.6 |
109.4 |
109.0 |
|
CORR |
-0.969 |
-0.969 |
-0.969 |
-0.969 |
-0.970 |
-0.968 |
-0.967 |
|
The effect of a constant high blank, such as that occuring with the thiol oxidase activity of rodent red blood cells, is illustrated above. Arbitrary units of blank activity 0, 20, 30, and 40 are added to a 100-unit enzyme activity. When the data are plotted in absolute numbers, the slopes of the curves are the same, but the intercepts differ by a value equal to the level of the blank. When the data are presented as a percent of the total activity, the intercepts are little affected, and the higher the blank, the lower the slopes. Because the dose values are unchanged, the relative value at which the effect of the inhibition appears should be little affected. Abbreviations: ACT, activity; INTCPT, intercept; CORR, correction. |
|||||||
VX STUDIES
As with its evaluation of the critical studies of GA, GB, and GD, the subcommittee noted that ChE assay kits and instructions developed for humans were applied to sheep (Rice et al. 1971) and rats (Goldman et al. 1988) in experimental studies of VX. Validation of such applications was not done; therefore, the accuracy of the ChE measurements reported in the studies are questionable.
In its evaluation of the methods used to measure AChE activity in the Rice et al. (1971) study of VX in sheep, the subcommittee noted that the study relied on whole-blood samples collected in heparinized tubes and used within 3 days of sampling. No mention was made of the conditions of sample storage (i.e., whether the samples were iced or refrigerated). A pH method was used to determine AChE activity with acetylcholine as a substrate. Substrate-activity curves showed a clear-cut inhibition of activity with excess substrate, providing good evidence that the measurements were mainly of AChE. Other investigators (e.g., Mohammad et al. 1997) reported that sheep blood has little if any pseudocholinesterase, suggesting that AChE was presumably all that was measured by Rice et al. (1971). The investigators reported that they used a substrate concentration that was greater than optimal, meaning that they started their measurements in the inhibitory range. The reason given was to avoid problems of substrate depletion. The data suggest, however, that the reaction time of the assay might have been too long. Because the values were obtained from end points, there is no way of knowing whether samples of high ChE activity yielded values that were too low.
The subcommittee also evaluated the method used to measure AChE in the Goldman et al. (1988) study of VX in rats. Using a Technicon Autoanalyzer II system, the investigators followed an automated procedure for the assay based on the Ellman method devised to measure exposure of individuals applying pesticides (Knaak et al. 1978). The AChE activity of the RBCs was obtained using acetylthiocholine as a substrate. The activity of the plasma from heparin-treated samples was subtracted from the activity of the whole blood to obtain an estimate of what was believed to be the RBC activity. Values were corrected with hematocrits. No corrections were made for the high AChE concentrations in plasma. At best, subtraction of the plasma values presumably yielded an estimated activity of the total AChE activity in the blood, whether it was from RBCs, serum, or platelets. Second, as was true of
the studies on GA, GB, and GD, no correction was made for an indeterminate amount of thiol oxidase activity in rat RBCs (Loof 1992; Wilson et al. 1996).
DETERMINING LOAELS
ORNL focused on establishing lowest-observed-adverse-effect levels (LOAELs) as the first step in deriving reference doses (RfDs) for the chemical-warfare agents. On balance, that choice might have been good. Errors made in the techniques used might not affect determination of the first detectable decrease in enzyme activity as much as determinations of the slope of the dose-response curve and the values derived from it. In this case, the ORNL experiments were not designed to establish a minimal detectable inhibition. Indeed, many of the dose studies started with inhibitions in the 40% range. It is accepted that decreases of approximately 15% can be reliably detected in carefully performed experiments designed for the purpose.
SUMMARY
The critical studies used by ORNL to derive RfDs for the nerve agents and many other studies in the literature have serious flaws in their ChE determinations and interpretations. Those flaws include
-
Application of commercial kits developed for humans to experimental animals without validating them for the species to be used.
-
Failure to consider RBC-thiol-oxidase activity. Rat RBCs contain high thiol oxidase activity and low AChE activity, which can result in large errors in calculating percentage of inhibition if uncorrected. There was no indication that such corrections were made in the assays used in the critical studies of GA, GB, and GD. For the critical studies on VX, consideration of whether similar thiol oxidase activity occurs in sheep RBCs was not relevant, because the sheep ChEs were assayed with choline and not thiocholine esters.
-
Failure to consider high plasma-AChE activity in the rat. Whether the activity is due to platelet AChE or to soluble AChE is not clear, because the distribution of platelet activity would depend on the specifics
-
of the collection of samples, their storage, and the method of isolation of blood particulates.
-
Failure to consider propionyl cholinesterases. Strictly speaking, rat plasma does not contain a BuChE; it is known to favor propionyl esters more than butyryl esters and both of those esters more than acetyl esters. Sheep plasma is said to have little if any ChE activity, although it is known to have platelet AChE.
CONCLUSIONS
A case can be made that the critical studies on GA, GB, GD, and VX were not optimal for the purpose of establishing LOAELs; they were not designed for that purpose and were not performed with assays validated for the species used and the conditions of the experiments. The subcommittee believes, however, that the critical studies are probably sufficiently reliable to permit their use in deriving RfDs. One reason is that the absolute values of the enzyme activities might not be as important as the relative inhibitions because it is the highest dose at which an inhibition can be detected that is important. Another reason is that methods that subtracted plasma activities from whole-blood activities avoided the problem of plasma-AChE activities, so long as the relative changes in total AChE activity were not affected. However, the problem of high thiol oxidase activity in the rat might represent an uncorrectable confounding factor, because its contribution to total enzyme activity becomes greater as more enzyme activity is inhibited.










