8
Evaluation of the Army's Interim Reference Dose for Lewisite
THE CHEMICAL-WARFARE agent lewisite is an organic, trivalent arsenic compound. It is classified as a vesicating agent because of its ability to cause blisters on exposed skin. Lewisite is present at several stockpile and nonstockpile munitions sites in the United States. At the request of the U.S. Army, Oak Ridge National Laboratory (ORNL) conducted a health risk assessment of lewisite. The assessment included a detailed analysis of lewisite's physical and chemical properties, environmental fate, toxicokinetics, mechanism of action, and animal and human toxicity data (see Appendix F, Health Risk Assessment of Lewisite, ORNL 1996). On the basis of that assessment, ORNL proposed a reference dose (RfD) of 1 × 10-4 mg/kg of body weight per day for noncancer health effects of lewisite. Because there was no evidence that lewisite is carcinogenic, a slope factor was not derived. The Army's Surgeon General accepted ORNL's proposed RfD as an interim exposure value until an independent evaluation of the proposed RfD was conducted by the National Research Council (NRC). This chapter contains the NRC's independent assessment of the scientific validity of the Army's interim RfD for lewisite.
DERIVATION OF THE ARMY'S INTERIM RfD
The Army's interim RfD for lewisite is 1 × 10-4 mg/kg per day. ORNL (1996) calculated that value on the basis of a two-generation reproduc-
tive study (Sasser et al. 1989a) in which the highest oral dose of lewisite did not produce forestomach lesions (necrosis and hyperplasia) in rats. In that study, male and female rats were intragastrically intubated with lewisite for 5 days per week for 13 weeks. Female rats were also dosed for 7 days per week during the 3-week gestation period and 4 days per week during the 3-week lactation period. The highest no-observed-adverse-effect level (NOAEL) for lewisite was 0.6 mg/kg per day. Because of the discontinuous exposure regimen, ORNL adjusted the NOAEL (NOAELadj) to a time-weighted average. That adjustment was done by calculating the total dose administered during the different exposure protocols:
0.6 mg/kg per day × (5/7) = 0.43 mg/kg per day for 13 weeks.
0.6 mg/kg per day × (7/7) = 0.6 mg/kg per day for 3 weeks.
0.6 mg/kg per day × (4/7) = 0.34 mg/kg per day for 3 weeks.
Using those values, ORNL calculated the NOAELadj to be 0.44 mg/kg per day as follows:
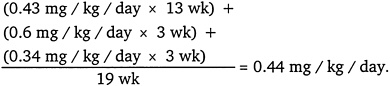
The RfD for lewisite was calculated to be 1 × 10-4 mg/kg per day by dividing the NOAELadj by 3,000, the product of the uncertainty factors and the modifying factor selected by ORNL.
The subcommittee is aware that the interim RfD for lewisite was reviewed by the Material/Chemical Risk Assessment (MCRA) Working Group of the Environmental Risk Assessment Program, which represents multiagency (U.S. Environmental Protection Agency (EPA), Department of Defense, and Department of Energy) expertise in deriving and validating toxicity values. The MCRA Working Group concluded that the forestomach lesions appeared to be an artifact of administering doses of lewisite directly to the forestomach in a short period of time and that the overall data base for lewisite was poor. Its consensus was that the RfD for lewisite be considered unverifiable due to data deficiencies. For those reasons and because it also considered lewisite to degrade to inor-
ganic arsenic in environmental media, the MCRA Working Group decided that the existing RfD for inorganic arsenic (3 × 10-4 mg/kg per day) would be an applicable surrogate, although the group recognized that the chemical structure of lewisite might imply toxic activity different from that of inorganic arsenic.
APPROPRIATENESS OF THE CRITICAL STUDY
ORNL estimated the NOAEL for lewisite by considering two studies—a two-generation reproductive study (Sasser et al. 1989a) and a 90-day toxicity study (Sasser et al. 1989b, 1996). In the two-generation reproductive study (Sasser et al. 1989a), Sprague-Dawley rats (20 males and 25 females per group) were intragastrically intubated with lewisite dissolved in sesame oil at doses of 0.10, 0.25, and 0.60 mg/kg per day. Males and females were dosed for 5 days per week for 13 weeks before mating. Female rats were also dosed for 7 days per week during the 3-week gestation period and 4 days per week during the 3-week lactation period. No significant adverse effects on reproductive performance or fertility were found at any dose through two consecutive generations, nor were any other toxic effects observed.
In the 90-day toxicity study (Sasser et al. 1989b, 1996), Sprague-Dawley rats (10 male and 10 females per group) were intragastrically intubated with lewisite dissolved in sesame oil at doses of 0.01, 0.1, 0.5, 1.0, and 2.0 mg/kg per day for 5 days per week for 13 weeks. The most significant adverse effects observed were necrosis and hyperplasia of the forestomach. Those forestomach lesions were found only in rats treated with lewisite at 1.0 or 2.0 mg/kg per day; the incidence was 1 of 20 and 12 of 20, respectively. Thus, the highest NOAEL for this study was 0.5 mg/kg per day. ORNL noted that no forestomach lesions were found at a slightly higher dose of 0.6 mg/kg per day in the two-generation reproductive study (Sasser et al. 1989a) and concluded that this higher value would be an appropriate estimate of the NOAEL. As described earlier, that NOAEL was adjusted to a time-weighted average of 0.44 mg/kg per day, and ORNL used the adjusted value to calculate the RfD.
ORNL also considered a teratogenicity study (Hackett et al. 1987) in which rabbits were administered lewisite by intragastric intubation on days 6–19 of gestation at doses of 0.07, 0.2, and 0.6 mg/kg per day. Increased mortality of 13% (in fact, 15% but reported incorrectly by
Hackett et al. (1987) and by ORNL), 46%, and 69%, respectively, were reported. In addition, gastric lesions (mucosal inflammation, edema, necrosis, and mucosal sloughing) were found at all doses, and teratogenic effects (fetal stunting and supernumerary ribs) were observed at the highest dose. On the basis of gastric lesions and mortality, the lowest-observed-adverse-effect level (LOAEL) for this study was 0.07 mg/kg per day.
ORNL considered this study to be compromised statistically primarily because of the small number of survivors in each treatment group. The subcommittee disagrees with that conclusion because the statistical weakness identified appears to compromise only the detection of teratogenic effects and not necessarily the effects of mortality or marked gastric lesions that were observed in the adult female rabbits at all administered doses. Furthermore, comparisons between the results of this study and a similar teratogenicity study in rats conducted by the same investigators (Hackett et al. 1987) suggest that rabbits might be more susceptible to lewisite than rats. On the basis of these considerations, the subcommittee concludes that even though the teratogenicity study in rabbits has shortcomings, the data indicating a LOAEL of 0.07 mg/kg per day for mortality and gastric lesions in the maternal rabbits should not be dismissed.
The subcommittee also considered other possible critical studies. In a study by Leitch et al. (1941), groups of 10 rats each were fed drinking water containing lewisite at 10 or 16 ppm for 98 or 133 days, respectively. No adverse effects were observed. However, the subcommittee noted that the study had a number of deficiencies, including an undefined effect concentration and lack of data on the actual concentration of lewisite consumed in drinking water. Earlier, Daniels (1990) came to the same conclusion. In addition, the test animals' water consumption, which is critical for determining an actual or estimated dose of lewisite, was not reported. Furthermore, the consumed concentration of lewisite might have varied from the target concentration because of degradation. Lewisite is hydrolyzed to 2-chlorovinylarsine oxide in the presence of water (IOM 1993); therefore, the actual exposure in the Leitch et al. (1941) study was not to lewisite but to its hydrolysis product. The subcommittee also reviewed the Institute of Medicine's (IOM 1993) evaluation of health effects of lewisite and found no other relevant studies with respect to derivation of the RfD.
On the basis of its evaluation of the available studies on lewisite, the
subcommittee recommends that the teratogenic study in rabbits (Hackett et al. 1987) be used as the critical study from which to derive the RfD for lewisite instead of the two-generation reproductive study in rats by Sasser et al. (1989a). Although the study was not ideal and the administration period was only 14 days, the rabbit appears to be more susceptible than the rat to lewisite, and the range of doses and number of animals in each dose group are considered credible. This recommendation contradicts the consensus judgment of the MCRA Working Group, who concluded that the available data on lewisite were inadequate for deriving an RfD for lewisite, and the RfD for inorganic arsenic should be used as a surrogate RfD for lewisite. The working group's recommendation was based on the assumption that lewisite degrades to inorganic arsenic in environmental media, an assumption the subcommittee regards as not necessarily certain under all circumstances. The subcommittee believes that use of the Hackett et al. (1987) rabbit study allows for the possibility that the chemical structure of lewisite represents a toxic activity that exceeds that of inorganic arsenic; the study is reasonable despite the limitations of the data; and the study does not presume lewisite will degrade to inorganic arsenic.
APPROPRIATENESS OF CRITICAL END POINT
The NOAELadj of 0.44 mg/kg per day used by ORNL for derivation of the interim RfD for lewisite was based on the highest dose at which necrosis and hyperplasia of the forestomach were not observed in rats (Sasser et al. 1989a). The subcommittee recommends, however, the use of the reproductive rabbit study (Hackett et al. 1987) in which a LOAEL of 0.07 mg/kg per day was identified on the basis of maternal mortality and gastric lesions. Although the possibility exists that those effects resulted from administration of lewisite directly to the stomach over a short period, resulting in an RfD that could be overprotective of noncancer health effects, there are two reasons for applying such a conservative value at this time. First, the available dose-response data are too sparse to establish conclusively that the dose-administration process primarily is responsible for the observed effects. Second, the available data on lewisite are inadequate for determining its carcinogenic potential. Because the chemical structure of lewisite includes arsenic and vinyl groups, the possibility exists for lewisite to be degraded in the environ
ment or to be metabolized into inorganic arsenic and for vinyl chloride to be formed. Inorganic arsenic and vinyl chloride are considered human carcinogens (EPA 1997a,b; NRC 1996).
APPROPRIATENESS OF UNCERTAINTY FACTORS
Because the subcommittee recommends the use of the teratogenicity study in rabbits (Hackett et al. 1987) as the basis for deriving the RfD for lewisite, it assigned values to the uncertainty factors and the modifying factor with respect to that study below.
EXTRAPOLATION FROM ANIMAL TO HUMAN
The subcommittee believes that lewisite is a highly corrosive agent, and when it is introduced directly into the stomach in a short period, its behavior is likely to be similar across species. For that reason, the subcommittee considers the typical default value of 10 for the uncertainty factor for extrapolation of data from animals to humans (UFA) to be too high and recommends a lower value of 3. The lower value is meant to indicate some similarity in action across species without excluding the possibility of some greater human susceptibility.
PROTECTING SUSCEPTIBLE SUBPOPULATIONS
The subcommittee considers the appropriate value for the uncertainty factor for protecting susceptible subpopulations (UFH) to be 3 because the rabbits were pregnant females and can be considered more susceptible than healthy nonpregnant animals but not the most susceptible subpopulation.
EXTRAPOLATION FROM LOAEL TO NOAEL
Because a LOAEL was used to derive the RfD, the subcommittee assigns a value of 10 to the uncertainty factor for extrapolation from a LOAEL to a NOAEL (UFL).
EXTRAPOLATION FROM SUBCHRONIC TO CHRONIC EXPOSURES
The subcommittee considers it appropriate to use a value of 10 for the uncertainty factor for extrapolation from subchronic to chronic exposures (UFS) because the LOAEL was estimated from a 14-day exposure study in rabbits.
DATA-BASE ADEQUACY
The subcommittee believes the uncertainty factor for data-base adequacy (UFD) should be assigned a value of 10 because no long-term exposure studies involving lewisite are available, only a few studies that address the acute or subchronic toxicity of lewisite are available, and little or no information about the metabolism of lewisite or its degradation products is available.
MODIFYING FACTOR FOR ADDITIONAL UNCERTAINTY
The subcommittee believes that the values assigned to the uncertainty factors above are supported by the data on lewisite and, therefore, supports a modifying factor (MF) of 1.
SUMMARY
Table 8-1 presents the uncertainty factors recommended by the subcommittee for deriving an RfD based on the rabbit teratogenic study (Hackett et al. 1987). The product of those factors is 9,000. On the basis of that value, the RfD for lewisite is 8 × 10-6 mg/kg per day (0.07 mg/kg per day ÷ 9,000), which reasonably can be rounded to 1 × 10 -5 mg/kg per day.
WEIGHT AND STRENGTH OF EVIDENCE
Because of a poor data base on lewisite, the strength of evidence for deriving the RfD for lewisite is weak.
TABLE 8-1 Uncertainty Factors Used to Calculate the RfD for Lewisite on the Basis of the Hackett et al. (1987) Study
|
Uncertainty Factor |
Description |
NRC |
|
UFA |
For animal-to-human extrapolation |
3 |
|
UFH |
To protect susceptible subpopulations |
3 |
|
UFL |
For LOAEL-to-NOAEL extrapolation |
10 |
|
UFS |
For Subchronic-to-chronic extrapolation |
10 |
|
UFD |
For data-base adequacy |
10 |
|
MF |
Modifying factor for additional uncertainty |
1 |
|
TOTAL UF |
|
9,000 |
|
Abbreviations: LOAEL, lowest-observed-adverse-effect level; MF, modifying factor; NOAEL, no-observed-adverse-effect level; NRC, National Research Council; RfD, reference dose; UF, uncertainty factor |
||
CONCLUSIONS
The approach used by ORNL to calculate the RfD for lewisite is consistent with the guidelines of the EPA. The subcommittee does not agree, however, with ORNL's proposed RfD of 1 × 10-4 mg/kg per day, which is based on studies in the rat, and recommends deriving the RfD on the basis of a rabbit study. The RfD for lewisite recommended by the subcommittee is 1 × 10-5 mg/kg per day, which is an order of magnitude more conservative than the Army's interim RfD.
DATA GAPS AND RESEARCH RECOMMENDATIONS
The major gaps in the available information on lewisite are the lack of information on the implications of administering lewisite directly to the stomach over a short time and the absence of chronic oral toxicity data from which to derive an RfD. Because of those deficiencies, the RfD for lewisite is estimated by extrapolating from a less-than-ideal animal study to humans. Confidence in the RfD can be increased if subchronic studies in rabbits and rats are conducted that compare the effects of chronic oral exposure to low concentrations of lewisite with the effects of short-term intragastric administration of small volumes. These crucial studies will provide not only the data needed to better understand the implications
of dosing techniques but also more pertinent information on whether the rabbit is a more appropriate animal model than the rat for deriving an RfD for lewisite.
The subcommittee believes the potential environmental and metabolic breakdown products of lewisite are not well identified. There is a possibility that inorganic arsenic and perhaps even vinyl chloride, two known carcinogens, may be break down products. Accordingly, the subcommittee recommends that the environmental degradation and metabolic products of lewisite be determined, and, if those breakdown products are found to be produced, that the carcinogenic potential of those substances, as well as lewisite, be considered in future assessments.
REFERENCES
Daniels, J.I. 1990. Lewisite. Pp. 6-1 to 6–22 in Evaluation of Military Field Water Quality, Vol. 4. Health Criteria and Recommendations for Standards, Part 2. Interim Standards for Selected Threat Agents and Risks from Exceeding these Standards. UCRL-21008 DTIC AD-A241523. Prepared by Lawrence Livermore Laboratory, University of California, Livermore, Calif., for the U.S. Army Medical Research and Development Comand, Fort Detrick, Frederick, Md.
EPA (U.S. Environmental Protection Agency). 1997a. Report on the Expert Panel on Arsenic Carcinogenicity: Review and Workshop. Prepared by the Eastern Research Group, Inc., Lexington, Mass., for the U.S. Environmental Protection Agency, National Center for Environmental Assessment, Washington, D.C.
EPA (U.S. Environmental Protection Agency). 1997b. Integrated Risk Information System (IRIS). Online file. http://www.epa.gov/ngispgm3/iris/irisdat/0278.DAT (Accessed March 1, 1997).
Gaylor, D.W. and J.J. Chen. 1996. A simple upper limit for the sum of the risks of the components in a mixture. Risk Anal. 16: 395–398.
Hackett, P.L., L.B. Sasser, R.L. Rommereim, J.A. Cushing, R.L. Buschbom, and D.R. Kalkwarf. 1987. Teratology studies of lewisite and sulfur mustard agents: Effects of lewisite in rats and rabbits. PNL-6408. DTIC AD-A198423. Prepared by Pacific Northwest Laboratory, Richland, Wash., for the U.S. Army Medical Research and Development Command, Fort Detrick, Frederick, Md.
Israeli, M., and C.B. Nelson. 1992. Distribution and expected time of residence for U.S. households. Risk Anal. 12:65–72.
IOM (Institute of Medicine). 1993. Veterans at Risk: The Health Effects of Mustard Gas and Lewisite. Washington, D.C.: National Academy Press.
Leitch, J.L., T.H. Ginsburg, and M.E. Price. 1941. MD(EA) Memorandum Report 18. Purification of Water Contaminated with Lewisite. A Toxicological Study of Water Containing 10 ppm and 16 ppm of Lewisite. Medical Research Division, Edgewood Arsenal, Aberdeen Proving Ground, Edgewood, Md.
NRC (National Research Council). 1996. Table B-1, pp. 381–391 in Carcinogens and Anticarcinogens in the Human Diet: A Comparison of Naturally Occurring and Synthetic Substances. Washington, D.C.: National Academy Press.
ORNL (Oak Ridge National Laboratory). 1996. Health Risk Assessment for Lewisite. Draft Report. Interagency Agreement No. 1769–1769-A1. Prepared by Oak Ridge National Laboratory, Life Sciences Division, Oak Ridge, Tenn., for U.S. Department of the Army, Army Environmental Center, Aberdeen Proving Ground, Edgewood, Md.
Rodricks, J.V., S.M. Brett, and G.C. Wrenn. 1987. Significant risk decisions in federal regulatory agencies. Regul. Toxicol. Pharmacol. 7:307–320.
Sasser, L.B., R.A. Miller, D.R. Kalkwarf, R.L. Buschbom, and J.A. Cushing. 1989a. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Two-Generation Reproduction Study of Sulfur Mustard (HD) in Rats. Final Report. PNL-6944. DTIC AD-A216423. Prepared by Pacific Northwest Laboratory, Richland, Wash., for the U.S. Army Medical Research and Development Command, Fort Detrick, Frederick, Md.
Sasser, L.B., R.A. Miller, D.R. Kalkwarf, P.W. Mellick, and R.L. Buschbom. 1989b. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Subchronic Toxicity of Sulfur Mustard (HD) in Rats. Final Report. PNL-6860. DTIC AD-A217886. Prepared by Pacific Northwest Laboratory, Richland, Wash., for the U.S. Army Medical Research and Development Command, Fort Detrick, Frederick, Md.
Sasser, L.B., J.A. Cushing, P.W. Mellick, D.R. Kalkwarf, and J.C. Dacre. 1996. Subchronic toxicity evaluation of lewisite in rats. J. Toxicol. Environ. Health 47:321–334.










