3
Controlling Trace Organics with Passive Activated Carbon Filters
Carbon filters have been used on municipal waste combustors (MWCs) and hazardous waste incinerators in Europe since the early 1990s (see Appendix c). The NRC first recommended that the Army look into this technology in 1991, and in 1992 the Army sent a study team to Europe. Carbon filters in combination with other gas-scrubbing systems now represent a highly efficient emission-control technology. The following passage appeared in a 1994 technical paper on an MWC in Rotterdam, Holland:
This type of retrofit designed as a five-stage gas cleaning system has become state-of-the-art technology in central Europe. Especially in Holland, Germany, Austria, and Switzerland, virtually all new MWC plants as well as most existing facilities are being equipped with these systems.
The extremely high removal efficiencies obtained by these advanced flue gas cleaning systems, especially attributable to the activated char adsorbers, yield an increase in political and public acceptance of MWCs in central Europe (Hartenstein, 1994).
Activated carbon is commonly used for separating organic chemicals in process plants and recovering solvents in dry cleaning and printing press operations. Controlling emissions of flue gas with passive beds of activated carbon is a more recent application with about a decade of experience. Carbon bed filters were first used in the late 1980s in Germany as NOx-control catalysts for large stationary sources like coal-fired steam-electric plants. An activated coke filter was commissioned in the Herten Hazardous Waste Incineration Plant (Germany) in August 1991 to "polish" reheated flue gas from the scrubber towers. Since then, the design problems listed below have been addressed:
-
plugging of the bed by fine particulates that penetrated the particulate-control device preceding the filter
-
rapid saturation of the leading edge of the bed with acid gases
-
development of practical, zero-leakage isolation dampers
-
movement of the bed and generation of fine particulates within the bed
New standards for emissions of dioxins and mercury for incinerators in several European countries in 1989 and 1990 led to the widespread use of activated carbon filters as a final stage for polishing flue gas (Hartenstein, 1993). Standards for chlorinated dioxins/ furans were set at 0.1 ng ITEQ/dnm3 at 11 percent 02, but the reported emission levels have been substantially below this level (see Table 3-1).1Table 3-2 shows some reported "before" and "after" data on chlorinated dioxin/furan cleanup with a carbon filter. The gas temperatures for these operations were in the range of 100 to 150»C (212 to 302»F). Gas-flow rates and bed thicknesses were not disclosed but are believed to be close to the design conditions for the Anniston and Umatilla PFS (pollution abatement system carbon filter system) discussed in Chapter 4. These and other data demonstrate
TABLE 3-1 Emission Levels of Chlorinated Dioxins for Some European Incinerators
|
Incinerator Type |
Dioxins/Furans (ng ITEQ/dnm3 @ 11% O2) |
|
Medical waste incinerator (Germany) |
0.003 |
|
Medical waste incinerator (Netherlands) |
0.03 |
|
Hazardous waste incinerator (Netherlands) |
0.03 |
|
Source: Adapted from Brueggendick and Pohl, 1993. |
|
more than 95 percent removal of dioxins/furans from already low levels in the gas stream.
Early testing of these facilities showed that emissions of particulates, acid gases (HCl and SO2), mercury, and dioxins and furans were reduced to levels near or below the detection limits for available sampling and analysis methodologies. Typical concentrations of stack gas emissions included: particulates around 1 mg/dsm3 at 7 percent O2; HCl and SO2 below 3 ppmdv at 7 percent O2; ITEQ dioxins and furans below 0.04 ng/dsm3 at 7 percent 02; and mercury below 0.003 μg/dsm3 at 7 percent O2 (Brueggendick and Pohl, 1993). Tests of installations in Germany and Japan have had similar results.
The available data from field tests indicate that carbon filter systems control adsorbable chemical species and act as depth (gravel bed) filters to separate particulates. (The carbon can also become a source of particulates as it erodes with use.) The proposed Army design
TABLE 3-2 "Before" and "After" Data on Chlorinated Dioxin/Furan Cleanup with a Carbon Filter
|
|
Raw Gas (ng/dnm3 @ 11% O2) |
Clean Gas (ng/dnm3 @ 11% O2) |
||
|
Dioxin Species |
In |
Out |
In |
Out |
|
Tetra CDFa (total) |
44 |
0.72 |
4.2 |
0.17 |
|
Penta CDF (total) |
32 |
0.55 |
3.7 |
0.18 |
|
Hexa CDF (total) |
13 |
0.28 |
2.3 |
0.16 |
|
Hepta CDF (total) |
2.9 |
0.06 |
1.4 |
0.12 |
|
Octa CDF |
0.9 |
0.02 |
0.6 |
0.1 |
|
a CDF = chlorinated dioxins/furans Source: Schüller, 1994. |
||||
incorporates a high-efficiency particulate air (HEPA) filter at the outlet of the filter to control dust. Unfortunately, the available information does not indicate actual performance because the outlet concentrations are frequently, but not always, below detection limits. Industrial experience with activated-carbon adsorption systems has shown that the amount of each SOPC separated is a complex function of the adsorption characteristics of individual chemical species, species concentrations, and the depth, temperature, pressure, and face velocity of the adsorption bed.
The Stockpile Committee's major concern in 1991, when the recommendation that the Army study carbon filters was made, was the possibility of "puffs." Although the existing PAS effectively cleans the flue gas during "normal" operations, occasional pressure pulses in furnaces, which are common in industry, could result in increases in emissions. These puffs could potentially be controlled by a carbon-bed filter.
Puffs were most likely to occur in the DFS. Whereas the other furnaces showed only minor variations in recorded pressures and possible puffs only during transients at start-up or shutdown, puffs in the DFS could be caused by small explosions from the processing of explosives and propellants. The Army has reported that puffs have been relatively infrequent, (e.g., one per week), and in any case have not been of great concern. No agent has ever been measured in the stack as a consequence, and any release from the furnace into the room air is passed with the air through carbon beds before it is released to the atmosphere.
Although the major adsorbable species of the flue gas from chemical agent incinerators have been characterized, the performance of carbon filters on this specific flue gas with its particular SOPCs has not been experimentally determined. Consequently, engineering calculations have been used to predict the performance of carbon bed filter systems applied to this novel gas stream.
FUNDAMENTALS OF ADSORPTION
The selective separation of individual components of a gas mixture by adsorption on a solid is widely practiced and has been well documented (e.g., Ruthven, 1984; Tien, 1995). Many solids can selectively separate individual materials. One common requirement for the adsorbent is that it have a large surface area; up to severed hundred square meters per gram of solid is common. Pore size is also an important characteristic of the materials to be adsorbed (chlorinated dioxins and
furans in this case). Because activated carbon has already been used for capturing SOPCs and other organic gases in flue-gas streams, the Army selected it as the adsorbent of choice for the scrubbed flue gas from the incineration of chemical agents.
The materials of most interest, hexavalent chromium, chlorinated dioxins and furans, mustard agent, and arsenic (because most of the health risk from the flue gas is associated with them), are at levels below the threshold of regulatory concern (U.S. Army, 1998a). Most of the chromium (solid) and arsenic will be removed by the HEPA filter that precedes the carbon filter in the proposed design. Carbon beds can also act as filters for finely divided suspended solids, therefore, as a backup for the HEPA filter.
The chlorinated dioxins and furans measured during trial bums were all at very low concentration levels, and the mustard, if present at all, was below the detection level. The Army and its contractor attempted to characterize carbon-filter adsorption, with special emphasis on these materials experimentally and theoretically (Mitretek Systems, 1997).2
Carbon beds on incinerators are usually designed to operate for extended periods of time before they become saturated (in the case of the PFS, for more than a year). Ultimately, a portion of the bed will become saturated, and SOPCs will start to appear in the atmospheric exhaust gas, indicating that it is time to change the carbon in the bed.
Most of the process analysis for the carbon bed presented in this chapter is based on ''normal'' operation of the baseline system incinerators with SOPCs present at very low levels. However, some higher concentrations (e.g., 10-fold) could occur during short-term system upsets. Because most SOPCs cannot be continuously monitored, a sudden increase could go undetected. In these circumstances, a carbon bed would have a comparative advantage over other flue gas filtration systems because it could adsorb higher-than-normal concentrations of SOPCs for extended periods of time (e.g., days).
Three properties of the flue gas/carbon adsorbent combination are considered in this chapter:
-
the maximum amount of material that can be retained by the carbon adsorbent, which is important in determining the on-stream time for a given bed
-
the rate of transfer of material from gas to solid phase (rate of adsorption), which dictates how close to the maximum the adsorbent capacity of the carbon can be used
-
the chemical stability of the SOPCs adsorbed on the carbon, which could affect the amount of SOPCs adsorbed on the bed at any given time
Although other adsorbent properties, such as catalytic activity, hygroscopicity, and resistance to acidic attack, may also be significant, carbon was chosen because it has been used extensively in many incinerators in Europe, as well as for the adsorption and control of chemical agents in facility ventilation systems.
The performance objective is to reduce the gas-phase concentrations of SOPCs—such as dioxins and furans—by a large factor (e.g., a 90 percent or greater reduction). The performance lifetime objective of the carbon bed is one year or more. The Army has taken a twofold approach to determining whether these objectives can be met:
-
a theoretical prediction (based on experimental work with other materials) of the thermodynamic equilibrium for adsorption of dioxins and furans on carbon at the very low gas-phase concentrations of interest, as well as the adsorption characteristics of more polar materials (water in particular)
-
a kinetic analysis of the adsorption from the flowing gas, based on relations for mass-transfer rate and axial flow dispersion found in the literature
(Both theoretical treatments are outlined in some detail in Appendix D. For a full exposition, see Mitretek Systems, 1997.)
Although the predictions must be considered approximate, they indicate very long performance lifetimes for the suggested design—a good indication that the one-year lifetime objective will be met. The committee attempted to confirm this conclusion by comparing the predictions with results reported for
commercial incinerators with carbon filters. Commercial data suggest that "capacity" (the amount of dioxin adsorbed at equilibrium) should not limit the "breakthrough" time. That is, the carbon in the PFS has the capacity to adsorb dioxins from the flue gas for many years before becoming saturated. The committee did not attempt to validate the kinetic analysis through an independent analysis.
MAXIMUM ADSORPTION FROM THE GAS PHASE
For any separable component in the gas phase, there is a maximum amount that can be adsorbed on the solid. The relation between the adsorbed component and its concentration in the gas phase is called the adsorption isotherm. This equilibrium relation varies with temperature and with the other adsorbed components (see Appendices D and E for details on adsorption phenomena).
Operational Modes
Two methods are commonly used for affecting contact between the effluent gas and the solid carbon adsorbent: (1) continuous relatively long-term flow through a packed bed of adsorbent, and (2) injection of a small amount of finely divided solid adsorbent into the flowing gas stream, with separation of the two after a few seconds. These two approaches can result in very different amounts of adsorption:
-
In packed-bed filters, most of the solid comes to equilibrium with the gas at the inlet concentration of the material being adsorbed (i.e., at its maximum gas concentration). This equilibrium can be represented by an isotherm of a general shape referred to as "favorable." Adsorption of many materials on carbon yields equilibrium relations with this general shape.
-
In dilute-phase (flowing) contact systems (e.g., carbon injection), the solid approaches equilibrium with the gas at the outlet concentration of the material being adsorbed (i.e., at its minimum gas concentration).
Packed-bed filters are capable of adsorbing more material from the gas phase than dilute-phase contact systems. Depending on the shape of the adsorption isotherm and other operating factors, the difference could be as much as 100-fold. A drawback to packed-bed filters is that they increase the system pressure drop, which results in higher power requirements. In practice, a combination of the two approaches is often used. MWCs (municipal waste combustors) typically disperse powdered activated carbon into the flue gas and separate it with a fabric filter. Thus, some separation occurs in the dilute phase, and more is adsorbed in the thin packed bed formed by the filter cake. The packed-bed design (i.e., a fixed bed of carbon) was chosen by the Army because, in addition to its higher adsorption capacity, it has two other advantages: (1) a fixed bed minimizes the amount of spent carbon adsorbent requiting disposal, and (2) a fixed bed is always present to capture accidental releases.
Application To The Incineration Of Chemical Agents And Munitions
There are two basic problems in determining the adsorption isotherms for a fixed carbon bed (the PFS) with the Army's baseline incineration system:
-
The SOPCs in the flue gas—possibly unburned agent and chlorinated dioxins/furans—are present at extremely low concentrations, frequently lower than the detection levels of today's best sampling and analytic methods. Because equilibrium adsorption isotherms have not been measured to such low levels, they must be predicted from experimental measurements at much higher concentrations or from related industrial experience at higher concentrations.
-
Many materials in the flue gas (SOPCs, other organics, and some vapor-phase metals) compete for adsorption sites on the activated carbon. Predictions for these interactions are not well established in theory.
Adsorption Equilibrium At Low Concentrations
Several methods have been described in the literature for determining the performance of adsorption systems. Mitretek, an Army contractor, has chosen an approach developed by Dubinin and coworkers (the Dubinin-Radushkevich [D-R] relation), a summary of which appears in Ruthven (1984) (Mitretek Systems, 1997). This approach has a reasonable theoretical justification for nonpolar materials, for which the energy of adsorption is due primarily to Van der Waals forces,
which are independent of temperature. In addition, this theory has been found to be useful for determining adsorption equilibria over activated carbon. A brief summary of the theory and its value for generalizing from a very few measurements is given in Appendix D.
The D-R relation has been used frequently (see for example Prakash et al., 1994) and is a useful engineering approach and a good approximation for estimating adsorption data for many materials. However, its applicability to the baseline system (equipped with the PFS) raises a serious problem:
The expression for the characteristic curve does not reduce automatically to Henry's Law in the low concentration limit. This is a theoretical requirement for any thermodynamically consistent physical isotherm equation, although the practical consequences of this deficiency may not be important if the equation is applied only in the high concentration range (Ruthven, 1984).
Unfortunately, the concentrations of most interest in the PFS application (chlorinated dioxin/furans and chemical agents) are in a low range where the D-R relation is not accurate (it is known to overpredict the amounts that can be adsorbed, but the amount of overprediction is uncertain). This problem is discussed semiquantitatively in Appendix D.
An alternative approach, also based on the D-R relation (and described in Appendix D), underpredicts the amount that can be adsorbed. The two approaches yield a range that includes the actual adsorbed amount, although the range can be quite large. The problem at hand is limited to a few SOPCs present at very low concentrations. Most of the SOPCs reported are at concentrations that are well beyond the range of Henry' s Law.
An alternative to theoretical modeling based on a small number of measurements is to use actual plant data for substances at low concentrations. Activated carbon has been used in industry to control adsorbable SOPCs in flue gas from various sources, including incinerators. If sufficient data are available, the plant data can be used to estimate dioxin loading at low gas-phase concentration. This approach has been used by the committee as a check on the theoretical analysis.
Adsorption Data
The committee examined two types of adsorption data: (1) laboratory data on individual compounds that were then used by Mitretek as a basis for estimating the adsorption of materials of direct concern, and (2) full-scale plant data on chlorinated dioxins. Mitretek measured adsorption equilibrium data for about a dozen compounds representing typical SOPCs (from a list of trace organics identified in the atmospheric exhaust gas during the JACADS trial bums). These compounds varied in type and polarity: hydrocarbons (i.e., hexane and toluene); alcohols (i.e., 1-propanol and 2-hexanol); refrigerants (mainly chlorofluorocarbons)3 (i.e., R123, R113, R11, R318, R22, R143a); methylene chloride; acetone (a ketone); and dimethyl methylphosphonate (DMMP, a simulant for GB). The D-R relation can be checked using Mitretek's data,. Either all of the data can be matched with one "characteristic curve," or all of the adsorption data can be predicted by the measurement of the equilibrium for one compound.
Mitretek also used their data with some success to predict multicomponent adsorption. The analysis focused on water and carbon dioxide, which are present in flue gas in much higher concentrations than the other SOPCs. Mitretek determined that water had little effect on adsorption if the relative humidity of the gas was 60 percent or lower. The Mitretek analysis used ideal solution theory to predict multicomponent adsorption, including water—which obviously will not form an ideal solution with the other components. Based on experimental data, Mitretek determined that the effect of water was consistently overpredicted. Mitretek did not modify the theory but pointed out that the allowance for water led to a "conservative" result (i.e., the actual adsorption of SOPCs would be greater than the predicted adsorption). Mitretek concluded that carbon dioxide is adsorbed so weakly (despite its relatively high concentration) that it should not affect the adsorption of most SOPCs.
Plant data from the municipal waste incinerator in Camden County, New Jersey, and a small experimental medical waste incinerator were examined by the committee (NORIT Americas Inc., 1996). In these plants, powdered activated carbon was injected into the gas stream and then separated using either an electrostatic precipitator (municipal waste incinerator) or fabric filter (medical waste incinerator) prior to the cleaned gas being exhausted through the stack. Initial
and final gas concentrations for the two incinerators were measured, as well as flow rates for the gas and solid-carbon adsorbent. The committee then calculated the amounts adsorbed on the carbon by material balance. The concentration of chlorinated dioxin adsorbed on the solid and the final gas concentrations for four different runs (two in each incinerator) are given in Table 3-3. The numbers represent measured values that combine all dioxin constituents.
The NORIT data show how much of the key materials can be adsorbed at a very low gas-phase concentration. (No laboratory data at this low concentration were available.) These data may represent a reasonable approach to equilibrium for the following reasons:
-
The powdered carbon used for gas injection is very fine, typically passing through 325 mesh. Calculations by the committee indicated a rapid approach to equilibrium for such fine material (e.g., 90 percent in a few seconds).
-
In one test, a fabric filter was used to separate the gas and solid. The solid cake built up on the filter provides a contact time of about an hour, more than enough to reach equilibrium. In the other test, electrostatic separation was used, but the level of adsorption was about the same.
The data in Table 3-3 were collected at a much higher temperature (270ºF) than the Army's PFS (160ºF). The lower temperature will have a substantial effect on the adsorption equilibrium. Hence, the baseline system PFS should be more efficient in reducing dioxin emissions than the carbon adsorption systems for the two incinerators in Table 3-3.
The concentration of dioxins from trial burn data (see Appendix B) is lower than the concentrations in Table 3-3. The possible loadings on the solid adsorbent have been adjusted downward by the committee by assuming they are proportional to the gas concentration. This procedure, which treats the data as though Henry's Law applies, is conservative (i.e., the prediction of a loading on the solid may be low).
Adsorption Of Multicomponents
A major problem with using a theory-based approach for the PFS is the large number of materials that may be competing for adsorption sites. The "key" components in this case are the chlorinated dioxins and furans, which are present at relatively low concentrations (ng/dsm 3). Many other SOPCs are present in much larger concentrations (1,000-fold to 10,000-fold higher concentration). Industrial experience has shown that chlorinated dioxins and furans are tightly adsorbed with breakthrough times in deep beds (e.g., 6 feet) of many years. Most SOPCs, however, are much less strongly adsorbed, and breakthrough times of days or weeks should be anticipated. Determining the adsorption equilibrium of many different constituents is a daunting task because each new species affects the adsorption of the others. Attempts to predict multi-component equilibria from single component isotherms have been reported in the literature. This strategy has been moderately successful for systems of materials with generally similar properties (e.g., all nonpolar materials of similar vapor pressures). This strategy was also used by Mitretek (Mitretek Systems, 1997).
Several theoretical approaches for predicting multi-component equilibria have been suggested. Perhaps the simplest is the Ideal Adsorbed Solution Theory (Ruthyen, 1984). As the name implies, the adsorbed materials are assumed to form an "ideal solution" in the solid adsorbent; each component then exerts a vapor pressure proportional to its mole fraction and its single
TABLE 3-3 Chlorinated Dioxins Adsorbed on Powdered Activated Carbon
|
|
Concentration of Dioxin in Gas Phase (ng/dsm3) |
|||
|
Concentration of Dioxin on Solids (grams/kg carbon) |
Inlet |
Outlet |
Incinerator Type |
Adsorption Temperature |
|
0.34 × 10-3 |
128 |
4.66 |
municipal waste |
270ºF (132ºc) |
|
1.2 × 10-3 |
411 |
16 |
municipal waste |
349ºF (176ºc) |
|
3.5 × 10-3 |
416 |
6 |
medical waste |
unknown |
|
0.32 × 10-3 |
94 |
2 |
medical waste |
unknown |
|
Source: Derived from NORIT Americas Inc., 1996. |
||||
adsorption isotherm pressure. The Mitretek report uses a modification of this theory developed by Grant and Manes (1987) for computational convenience. The approach appears to be reasonable, although the predictions for equilibrium adsorption are necessarily approximate. This is particularly true for the effect of water; the Ideal Adsorbed Solution Theory consistently predicts that more material will be displaced by water than has actually been observed (see Appendix D).
Carbon Bed Breakthrough Times
Calculated equilibrium values are used to determine breakthrough times. Fortunately, approximate equilibrium values can be used for the chlorinated dioxins and furans, whose calculated breakthrough times turn out to be quite long. Consequently, even an error of a few-fold in the equilibrium saturation value can be tolerated in calculating an estimate of adsorbent lifetime.
Breakthrough times for 14 different materials calculated by Mitretek (as described above) for carbon beds using coconut-shell carbon are shown in Table 3-4. All 14 materials were detected in agent-incinerator flue gas during trial bums at JACADS, and all are considered carcinogenic. The calculations show a wide range of breakthrough times, long times for the chlorinated dioxins and furans, and much shorter times for most of the other (more volatile) materials.
Two breakthrough times for each material are shown in Table 3-4: (1) the breakthrough time if each material were present alone, based on the corresponding single adsorption isotherm; and (2) the breakthrough time based on modeling of a multicomponent mixture. The multicomponent mixture included 41 compounds detected during JACADS trial bums (i.e., the 14 compounds shown plus other trace compounds in the gas). The competition for space on the solid adsorbent has a surprisingly large effect on the breakthrough times for all materials.
For this report, the committee compared the plant data shown in Table 3-3 with the calculations in Table 3-4 as a check on whether the calculations were reasonable. The comparison was based on the following approximation and assumption:
-
The dioxin/furan loadings were estimated directly from the data in Table 3-3. No attempt was made to correct them for temperature, although a much
TABLE 3-4 Estimated Carbon Filter Breakthrough Times for Substances of Potential Concern in Stack Gases from the Chemical Agent Disposal Facility Liquid Incineratora
|
Substance |
Estimated Initial Concentration ng/m3 |
Estimated Breakthrough Time as Single Componentb |
Estimated Time for Multicomponent PFS Flue Gasc |
|
Tetra CDD |
0.13 |
1.5 × 107 years |
> 5 years |
|
Octa CDD |
0.24 |
1.7 × 107 years |
> 5 years |
|
Tetra CDF |
0.21 |
6.8 × 107 years |
> 5 years |
|
Penta CDF |
0.088 |
2.6 × 107 years |
> 5 years |
|
Hexa CDF |
0.11 |
2.2 × 107 years |
> 5 years |
|
Hepta CDF |
0.16 |
1.8 × 107 years |
> 5 years |
|
Bis (2-Ethylhexyl) phthalate |
19,000 |
650 years |
> 5 years |
|
Benzene |
90,000 |
2.4 years |
14.2 hours |
|
Carbon tetrachloride |
35,000 |
4.1 years |
7.1 minutes |
|
Chloroform |
22,000 |
2.5 years |
5.7 hours |
|
Chloromethane |
780,000 |
1.8 years |
7.4 minutes |
|
cis-l,3-dichloropropene |
250,000 |
1.7 years |
1.0 days |
|
Methylene chloride |
5,900,000 |
38 days |
2.1 hours |
|
Vinyl chloride |
4,500 |
1.7 days |
9.5 minutes |
|
a Bed dimension = 214 square feet, 1 foot deep, 3,030 kg of carbon b Calculated based on D-R equation assuming complete saturation of filter at 135ºF c Based on multicomponent computer model, 135ºF, 67 percent relative humidity Source: Adapted from Mitretek Systems, 1997. |
|||
-
larger loading on the carbon can be expected at a lower operating temperature.
-
A sharp concentration "front" was assumed, so that most of the solid would be used to adsorb material before breakthrough occurred. This is a reasonable assumption (although not certain) based on the very long breakthrough times shown in Table 3-4, which in turn were based on detailed kinetic analyses. Assuming a sharp concentration front is also reasonable based on the long breakthrough times of commercial units.
Based on the flow rates and dioxin concentrations at JACADS, the breakthrough time for the PFS was estimated to be approximately two years. This is a very conservative number because it is based on observed plant operation at a much higher temperature (270ºF) than is planned for the PFS (160ºF). Because more can certainly be adsorbed at the lower temperature, the predicted breakthrough time at 160ºF would be longer.4 The data in Table 3-3 represent multicomponent adsorption, so the estimated breakthrough time for chlorinated dioxins and furans should be compared with the right-hand column of Table 3-4, "Estimated Breakthrough Time for Multicomponent PFS Flue Gas." Because the estimates are in roughly the same range, they lend some confidence to the calculated results in Table 3-4. Based on these analyses, the committee concludes that the carbon will be effective for reducing the emissions of dioxins and furans—and will continue to be effective for well over a year before the carbon will have to be replaced.
Similar data are not available for the adsorption of chemical agents on carbon. If any agent is present in the flue gas, it is below the level of analytical detection, so experimentally determining the vapor-to-solid equilibrium relation is very difficult. However, the committee used the theoretical analysis developed by Mitretek, based on the D-R relation, to estimate the equilibria for the three agents. The purpose was to determine whether the carbon bed would be effective in retaining agent under these conditions. In addition, the analysis would predict how much agent might be accumulated on the filter in the event of an accident that drove off the accumulated material in a short time. The range of adsorption quantities was based on the uncertainty of applying the D-R relation at very low concentration (see Appendix D).
For the analysis presented here, the committee assumed that the agents were present in the gas at the detection limit quoted by Mitretek in their analysis of the JACADS trial-burn measurements (U.S. Army, 1998a). Of course, the agents are probably present at lower concentrations or not at all. Assuming that they are present, however, and because measurements always show some fluctuation around the mean value, this method gives a conservative result. The committee made two calculations for each agent: the D-R relation as done by Mitretek, and an approximate Henry's Law calculation (see Appendix D).
The first calculation yields equilibrium adsorption levels that are too high, the second that are too low, probably bracketing the correct level. The results differ significantly, as indicated by the ranges for each agent shown in Table 3-5. GB shows the largest "spread" because it is the farthest away from the Dubinin criterion for application of the D-R relation.
For comparison, the amount of each agent that might reach the carbon was also estimated, based on Mitretek's assumed agent concentrations and a 144-week life of the plant:
|
GB and VX: |
0.00156 g/kg carbon |
|
HD: |
0.23 g/kg carbon |
The calculations show that the capacity of the carbon to adsorb agent at equilibrium levels greatly exceeds the amount that might plausibly reach it—by some orders of magnitude for VX and HD. Although the
TABLE 3-5 Calculated Range of Carbon Adsorption of Agents
|
Agent |
Gas Concentrationa ng/dsm3 |
Range of Calculated Adsorption Capacity (g/kg carbon) |
|
GB |
60 |
0.478-0.0076 |
|
VX |
60 |
19.1-0.0156 |
|
HD |
8,700 |
27-11.25 |
|
a These are the "detection limit" concentrations quoted by Mitretek for trial-burn data at JACADS and are simply taken as 20 percent of the Army's acceptable stack concentrations (U.S. Army, 1998a). The trial-bum data from JACADS and the TOCDF discussed in Chapter 2 resulted in much lower values. |
||
committee did not evaluate breakthrough times, it appears unlikely that VX and HD (strongly adsorbed) would break through at all. GB might also be captured, but this is judged to be less certain. By comparison, the air from the plant, which is passed through carbon filters before being exhausted to the atmosphere, may contain measurable quantities of agent when it reaches the carbon filters, although no agent has ever been detected past the second four-inch bed of this system. These filters consist of six four-inch beds in series.
As indicated previously, chlorinated dioxins, furans, and mustard constitute a substantial fraction of the calculated risk associated with the flue gas. The other major risk contributors, chromium and arsenic, are solids and should be captured by the HEPA filters, as well as the carbon-bed filters. The analyses show that more volatile materials, such as benzene, carbon tetra-chloride, and others, are retained on the carbon bed for a relatively short time before they start to break through. These materials contribute to the 5 percent of the calculated risk not associated with the major risk drivers. A carbon-filter bed will have a negligible effect on their concentrations in the flue gas.
The adsorption capacities described above are very low, less than one gram of GB per kilogram of carbon, for example. This reflects the extremely low concentrations (below the detection limit) that might exist in the gas phase. If a large amount of agent were released during a major accident, the carbon could adsorb much more (e.g., as much as 2 or 3 percent of its own weight). Because each bed contains approximately 10,000 kilograms of carbon, there would be enough capacity to adsorb an extremely large accidental release. The carbon would have to be replaced immediately, but the design of the carbon bed provides for easy replacement.
Predicting adsorptive filter performance is complex and difficult (Mitretek Systems, 1997), and the predicted breakthrough times are probably accurate only to within a factor of a few-fold. The breakthrough times in Table 3-4 are long enough, however, that operation would be considered satisfactory even if the actual times were substantially shorter (e.g., 3-fold). In addition, the plant data support the laboratory findings and, thus, reinforce the conclusion derived from theoretical modeling that the PFS carbon bed would effectively remove the compounds of greatest public concern for an extended period of time. Two beds in series would offer a safety backup; if sampling data after the first bed show a breakthrough of SOPCs, the second bed would capture them until the beds could be replaced.
Filter Upsets
Trace organics accumulate on the carbon filters as they are removed from the flue gas. In the event of a major upset (e.g., a large temperature rise), these accumulated materials could be desorbed in a short period of time, raising a question of the potential risk of desorption, particularly to workers who might be exposed to the highest concentration. The PFS is designed to prevent a rise in temperature, but the possibility cannot be ruled out.
If all of the adsorbed material is assumed to be desorbed in a short period of time (e.g., one hour) the results show that no material could exceed the "acute threshold level" (ATV).5 Detailed results are presented in the Mitretek report, as well as in the PFS risk assessment reports for Anniston and Umatilla (Mitretek Systems, 1997; U.S. Army, 1998a, 1998b).
CHEMICAL STABILITY OF ADSORBED MATERIALS
Chemical agents can be decomposed at elevated temperatures. They are also destroyed by hydrolysis. The chemical stability of agents that might accumulate on the carbon, and the extent of decomposition during the lifetime of the PFS carbon filters, are examined below.
The thermal decomposition of agents has been tested to demonstrate that the 5X criterion for agent destruction (15 minutes at 1,000ºF) will ensure "complete safety" (PMCD, 1989). These tests showed that VX is the most stable, GB less stable, and HD the least stable. Only a few of the tests were done with temperature/ residence time relationships that were well enough defined to yield reaction-rate relationships. GB was the agent most extensively studied (PMCD, 1989; Baier and Weller, 1967). None of the tests involved decomposition on carbon.
More recent information has been reported on the off-gassing of agents from carbon filters (Karwacki et al., 1998), which are commonly used to treat the ventilation air flowing through agent destruction facilities, laboratories, and other facilities. Data on GB and HD equilibria (gas and solid adsorbent concentrations) and decomposition rates over a temperature range of 30ºC
to 90ºC (86ºF to 194ºF) have also been reported. The reaction rates reported were markedly faster than those of the earlier studies cited above, possibly because water was present on the carbon. (Chemical agents are susceptible to hydrolysis, so the presence of water would open another pathway for decomposition.)
Rate relations given in the 1989 PMCD (Program Manager for Chemical Demilitarization) report have been used by the committee to make the following rough estimates of the stability of the chemical agents at 71ºC (160ºF), the design temperature of the carbon filter. The experiments on which these rate relations are based were done in a dry atmosphere to minimize hydrolysis. The reaction data indicate first-order agent decomposition with respect to agent concentration:
The reaction-rate constants have been related to temperature in the usual way using the Arrhenius relation:
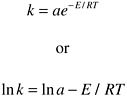
The rate relations chosen for the calculations presented in this report are shown below.
Rate Relations (units for k [1/sec]; T [K]):
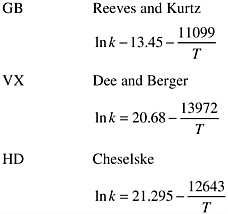
The half-lives (t1/2) calculated from these equations for a carbon bed temperature of 160ºF (71ºC), are:
|
GB: |
102 × 106 seconds |
(3.2 years) |
|
VX: |
315 × 106 seconds |
(10 years) |
|
HD: |
3.5 × 106 seconds |
(40 days) |
The higher reaction rates reported by Karwacki et al. (1998) would indicate much shorter half-lives (by a factor of 10 or more). There is strong evidence that for mustard (HD) in particular, and GB to a lesser extent, a great deal of any agent adsorbed on the carbon bed would be decomposed during the period of anticipated plant operation. For example, Karwacki et al. report that about 70 percent of the mustard on carbon was decomposed after 16 weeks at 90ºC (194ºF).
Very little information is available on the products of decomposition. The thermal decomposition of GB at 395ºC (743ºF) showed propylene and methylphosphonofluoridic acid as products with little else (Baier and Weller, 1967):
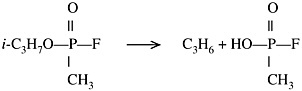
The data for GB on dry carbon at the much lower temperature of 50ºC to 100ºC (122ºF to 212ºF) showed isopropyl methylphosphonoic acid as the major product (Karwacki et al., 1998). Isopropanol was also present at the higher temperatures. The half-life of GB on dry carbon was reported to be about 63 days at 30ºC (86ºF). The products of GB decomposition noted above are not expected to show significant nerve agent activity.
For mustard, the data showed the principal volatile reaction products to be 1,4-thioxane, 1,4-dithiane, and 2-chloroethyl-ethylenesulfide. The latter would probably undergo further dechlorination with time (Ward, 1998). The 2-chloroethyl-ethylenesulfide product from HD was expected to show some vesicant properties, but at a lower level than HD; it is not known whether it retains the carcinogenic quality of HD (Ward, 1998).
Chlorinated dioxins are very stable materials, much more stable than the agents. Decomposition rates have been measured, but only at a very high temperature (i.e., 800ºC [1,470ºF]). Based on results from tests of oil emulsions in soils, their decomposition rate at 160ºF is assumed to be negligible.
SUMMARY
Carbon filters have been added to many combustion facilities in Europe (see Appendix C) and have proven to be very effective in reducing already low
concentrations of SOPCs and other troublesome components, such as mercury in flue gases. A theoretical analysis of adsorption on carbon developed by Mitretek for the Army leads to the same conclusion. Based on these studies, reasonable ranges can be predicted for the quantities of SOPCs adsorbed, and from them a prediction can be made of the useful life of the carbon beds. The calculations suggest that the carbon beds would last for the duration of the munitions destruction programs at both Anniston and Umatilla.
Chemical agents represent a special case because they are not very stable materials, and GB and mustard in particular react on carbon and decompose in a matter of days. The agents are routinely monitored in the stack gas, however, and none has been observed at JACADS or the TOCDF. The committee' s analysis indicates that any agent that reaches the carbon would be completely adsorbed and that most of it would decompose. Thus, little if any agent would remain on the carbon when it was removed for disposal.











