1
Introduction And Background
For more than half a century, the United States has maintained a stockpile of extremely hazardous chemical agents and munitions at eight sites in the continental United States and Johnston Atoll in the Pacific Ocean. The United States has decided to destroy this stockpile because of its age, its lack of utility as weapons or as a deterrent, the continuing costs of maintenance, and the potential for accidental release with resultant harm to human health and the environment.
In 1985, Congress passed Public Law 99-145, which directed the U.S. Department of Defense to destroy at least 90 percent of the stockpile of unitary1 chemical agents and munitions, especially M55 rockets, which were deteriorating and becoming increasingly hazardous. After setting several intermediate goals and dates, on October 23, 1992, Congress passed the National Defense Authorization Act for fiscal year 1993 (Public Law 102-484), which directed the Army to dispose of the entire stockpile of unitary chemical warfare agents and munitions by December 31, 2004. Congress also directed that the Army's Chemical Stockpile Disposal Program (CSDP) ensure the maximum protection of workers, the public, and the environment.
The CSDP has evolved in parallel with worldwide attempts to control and eliminate chemical agents and munitions. Over the course of several decades, a broad, complex agreement known as the Chemical Weapons Convention (CWC) has been negotiated. This agreement, which went into effect on April 29, 1997, requires that all chemical agents and munitions be destroyed in ten years (i.e., by April 29, 2007); the congressional mandate has been modified to reflect the CWC deadline. The agreement allows each country to determine the method of destruction, as long as it ensures public safety and protects the environment.
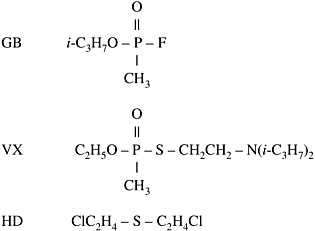
The chemical formulas for the three principal agents in the U.S. stockpile to be destroyed are given below. GB and VX are nerve agents; HD, commonly called "mustard," is a blister agent.
SELECTION AND DEVELOPMENT OF THE BASELINE INCINERATION SYSTEM
In the 1970s, the Army commissioned studies of many different disposal technologies and tested several
of them. In 1982, disassembly followed by component incineration was selected as the method for disposing of agents and associated propellants and explosives and for thermally decontaminating metal parts. In 1984, the National Research Council (NRC) Committee on Demilitarizing Chemical Munitions and Agents reviewed a range of disposal technologies and endorsed the Army's selection of incineration as an adequate technology for the safe disposal of chemical warfare agents and munitions (NRC, 1984).
Pursuant to the enactment of Public Law 99-145, the Army developed and tested prototype components of the baseline incineration system at the Chemical Agent Munitions Disposal System (CAMDS) facility at Deseret Chemical Depot (DCD), formerly Tooele Army Depot South, Utah. To date, two baseline incineration systems are in operation: the Johnston Atoll Chemical Agent Disposal System (JACADS), located on Johnston Island about 700 miles southwest of Hawaii, and the Tooele Chemical Agent Disposal Facility (TOCDF) at DCD in Utah, about 50 miles west of Salt Lake City. JACADS, the first full-scale version of the baseline system, commenced operational verification testing in July 1990. Construction on the TOCDF, the first baseline incineration system disposal facility in the continental United States, began in 1989. The destruction of agent and munitions from the DCD stockpile at the TOCDF began in August 1996. JACADS is scheduled to complete the destruction of the stockpile at Johnston Island in 2000; the TOCDF is scheduled to operate until 2005. In 1997, the construction of baseline incineration system disposal facilities was begun at two other storage sites: Anniston Chemical Activity, Anniston, Alabama, and Umatilla Chemical Depot, Hermiston, Oregon. As of early March 1999, construction of these facilities was 36 percent and 40 percent complete, respectively. Construction of a baseline incineration system at Pine Bluff Chemical Activity, Pine Bluff, Arkansas, began in January 1999.
Incineration processes have raised concerns about potentially harmful emissions. People who fear that contaminants in the exhaust gas could have adverse effects on their health and the environment have remained resolutely opposed to the baseline incineration system. Properly operated, the incineration system produces mostly relatively harmless products: carbon dioxide, water, and other completely oxidized products in their most stable state. However, incinerator emissions also contain small quantities of products of incomplete combustion (PICs) and other trace contaminants, collectively known as substances of potential concern (SOPCs). The presence of SOPCs and trace quantities of agent at levels below the monitoring detection limits have become matters of concern to the public. The chemical agent is sometimes called the principal organic hazardous constituent (POHC) being incinerated. In keeping with normal practice for hazardous-waste incineration operations, stack emissions are continuously monitored for the presence of chemical agent. However, SOPCs (other than carbon monoxide), which include particulates, heavy metals, and acid gases, are not routinely monitored; and current monitoring techniques cannot provide real-time analysis. These substances are usually measured only during tests required to obtain or maintain federal and state operating permits.
There appear to be two levels of public concern. Some people are convinced that incinerator operations will not keep SOPCs at relatively benign levels. Others believe that the presence of any SOPCs—no matter how small—is unacceptable because any discharge adds to the pollution burden to which people are exposed. These well documented concerns are not limited to the incineration of chemical agents and munitions but have also been expressed about waste incineration, the burning of fossil fuels, other combustion processes, and industrial chemical processes in general.
ROLE OF THE NATIONAL RESEARCH COUNCIL
In 1987, at the request of the Undersecretary of the Army, the NRC Committee on Review and Evaluation of the Army Chemical Stockpile Disposal Program (Stockpile Committee) was established under the aegis of the Board on Army Science and Technology to provide the Army with technical advice and counsel on specific aspects of the disposal program. Under this charter, the Stockpile Committee has completed 17 reports evaluating various stages of progress and aspects of the program. See Appendix A for a list of these reports.
After a workshop sponsored by the Stockpile Committee in 1991, the Army was urged to evaluate a number of improvements in the pollution control systems for cleaning the incinerator off-gases at stockpile disposal sites in the continental United States (NRC, 1991). One of the technologies to be evaluated was the use of activated carbon to adsorb SOPCs:
Use of an activated carbon filter downstream of the scrubbers would remove pulses of agent [puffs]2 and low-level organics...The ability to reduce mercury vapor and dioxin emissions is an additional feature of carbon. While such a system is currently considered redundant in municipal and industrial waste incineration, the use of redundant air pollution control could substantially enhance public confidence, particularly if the redundant system is independent of system operating conditions.
At the time, carbon filtration was routinely used for the separation and recovery of trace organic compounds from various sources (e.g., storage tanks, chemical process plants, automobiles, etc.), but it had not been used as an air pollution control system for incinerators in the United States. Hence, activated carbon filters were not included in the pollution abatement system (PAS) at JACADS, the first full-scale chemical agent disposal facility.
The workshop was followed by a letter report in 1992 by the Stockpile Committee that included several recommendations based on workshop discussions for improving the performance of the baseline system PAS (NRC, 1992). One of these mentioned carbon filters:
The Army should consider incorporating passive controls, such as activated carbon beds, to ensure the lowest emissions even under temporary upsets (e.g., "puffs") that might not be controlled by the existing afterburner. (Recommendation 2, NRC, 1992).
The Army divided its consideration of the NRC recommendations into three subtasks:
-
Subtask 1: a review of literature and existing data on PASs
-
Subtask 2: a PAS plant survey and applicability study
-
Subtask 3: a comparison of new and existing PAS technologies
The results of the Army' s study were presented in three reports (U.S. Army, 1994a, 1994b, and 1994c), but no decision for change was made at that time.
Meanwhile, in March 1991, as a result of growing public concerns about and opposition to the baseline incineration system and the rising cost of the CSDP, the Stockpile Committee suggested, and the Army agreed, that a study be undertaken of alternatives to incineration for the destruction of the stockpile.
In January 1992, the NRC, at the request of the Office of the Assistant Secretary of the Army for Installations, Logistics and Environment, established the Committee on Alternative Chemical Demilitarization Technologies (Alternatives Committee) to develop a comprehensive list of alternative technologies, review their capabilities, and evaluate their potential for the disposal of agents and munitions. In June 1993, this committee published its report, Alternative Technologies for the Destruction of Chemical Agents and Munitions (NRC, 1993). Continuing public concerns about emissions from the baseline incineration system were expressed during a public forum on June 30, 1993, in Washington, D.C., sponsored jointly by the Alternatives Committee and the Stockpile Committee.
Based on the report of the Alternatives Committee and the Stockpile Committee's knowledge of the baseline system and disposal requirements, the Stock-pile Committee recommended that alternatives to incineration be investigated. The recommendations were published in a report in February 1994, Recommendations for the Disposal of Chemical Agents and Munitions (NRC, 1994). Although the committee still considered the baseline system to be adequate for disposal of the stockpile, the committee included the following finding and recommendation for enhancing the performance of the baseline incineration system.
Finding 13. The Stockpile Committee finds the baseline system to be adequate for disposal of the stockpile. Addition of activated carbon filter beds to treat all exhaust gases would add further protection against agent and trace organic emissions, even in the unlikely event of a substantial system upset. If the beds are designed with sufficient capacity to absorb the largest amount of agent that might be released during processing, addition of these beds could provide further protection against inadvertent release of agent.
Recommendation 13. The application of activated charcoal filter beds to the discharge from baseline system incinerators should be evaluated in detail, including estimations of the magnitude and consequences of upsets, and site-specific estimates of benefits and risks. If warranted, in terms of site-specific advantages, such equipment should be installed.
The Army responded to the NRC finding and recommendation in two ways. First, the trial burns at JACADS and the TOCDF (required as part of the permitting process) showed, as expected, that numerous SOPCs were present at very low levels. Chlorinated dioxin concentrations measured at JACADS and the TOCDF were much lower than the levels reported for sources using activated carbon filters in Europe (Clarke, 1991). Because adsorption efficiency is a function of many parameters, including inlet concentrations, a question arose as to how effectively carbon beds would reduce SOPCs (e.g., chemical agent, dioxins, etc.) even further. The Army performed an experimental and theoretical study to determine what might be achieved by carbon adsorption with very dilute inlet concentrations (Mitretek Systems, 1997). The study also considered the risk of a sudden release of sorbed materials from the carbon in the event of an operational failure. Second, anticipating that the Mitretek study would show potential benefits, the Army included a carbon bed filter in the pollution abatement designs submitted for permitting of the baseline incineration systems at Umatilla, Oregon; Anniston, Alabama; and Pine Bluff, Arkansas.
Carbon filters have been shown to reduce emissions of some SOPCs (see for example, Hartenstein, 1994). The health benefits of reducing residual SOPC emissions may be inconsequential, however, because the emissions are already very low. However, further reductions may increase public confidence.
Carbon filters can introduce other types of risk. First, there is a possibility of a carbon fire, although to the committee's knowledge none has been reported in an incinerator application. Second, SOPCs from incinerator exhaust gas will accumulate and concentrate on the filters, and there is a possibility that the accumulated SOPCs could be driven off of the filters by a thermal transient or a sudden increase in the gas humidity. Third, the SOPC-loaded activated carbon could be considered contaminated and would, therefore, have to be treated as hazardous waste. Thus, carbon filters should be thoroughly evaluated in quantitative risk assessments (QRAs), health risk assessments (HRAs), and hazard evaluations (HEs) for their overall beneficial and harmful effects.
Carbon filters are already in the designs and permits for the Anniston and Umatilla plants, and construction of both of these facilities is well under way. A carbon filter is also included in the design submitted as part of the RCRA Part B permit application for the baseline facility at Pine Bluff, Arkansas, the fifth baseline facility to be built. Construction at Pine Bluff began in January 1999. If the decision to install or remove a carbon filter system is made after a permit has been issued, a permit modification would be required, which could delay the completion of the disposal program and increase the overall risk.
In response to NRC recommendations concerning the use of risk assessments and the need for public involvement, the Army established a formal procedure called the change management process (CMP) for making significant changes to a facility's configuration or operations (U.S. Army, 1997a). The CMP evaluates changes to the risk estimates in the QRA and HRA from proposed modifications to equipment or operations. The CMP also includes public input into decisions to make changes in equipment, operational procedures, or any aspect of the operation of concern to the public. The decision about carbon filters was intended to be an early application of the CMP.
PURPOSE OF THE REPORT
This report reviews the Army's evaluation of carbon filters, as well as its process for reaching decisions on their utilization. Members of the Stockpile Committee have been actively following the testing of stack gas emissions at JACADS and the TOCDF, the development of the carbon filter simulation model, the conceptual design of a PAS modified with an activated carbon filter system (the PFS), and the two major risk assessments conducted for each continental disposal site, namely, the QRA (a quantitative risk assessment of the likelihood and consequences of accidental agent releases) and the HRA (a health risk assessment of potential effects of facility emissions during mild and severe upset conditions on human health and the environment).
In the Statement of Task for this report, the committee was asked to do the following tasks:
-
Gather and assess trial bum data from JACADS) and the TOCDF.
-
Acquire from appropriate Army and contractor sources data and information regarding the design concept for a PFS for the Anniston and Umatilla Chemical Agent Disposal Facilities.
-
Assess the PAS carbon filter simulation model.
-
Review the changes in previous QRAs and HRAs after the addition of carbon filters to the PAS of the baseline incineration system.
-
Travel to Umatilla Chemical Agent Disposal Facility to observe the change management process as applied to any design change that might affect the carbon filter system.
-
Gather relevant data, information, and literature on carbon filters and carbon filter performance based on other (e.g., municipal and hazardous waste) incinerators.
-
Produce a report that reflects the background of the Army' s incineration program and previous relevant NRC recommendations; provides data on flue gas emissions and public concerns; details filter performance principles; and reviews the PAS carbon filter design, operation, maintenance, and disposal requirements.
-
Assess the Army's QRAs and HRAs evaluating the addition of carbon filters and provide appropriate findings and recommendations.
The committee could only base its evaluation on the Anniston and Umatilla HRAs and Phase 1 QRAs because the Phase 2 QRAs3 were not yet complete. Therefore, no formal characterization of worker risk was available. The committee has used the available information to suggest additional steps the Army could take to implement its decisions.
The Stockpile Committee had already initiated its review of carbon filters in a recent NRC report, Risk Assessment and Management at Deseret Chemical Depot and the Tooele Chemical Agent Disposal Facility (NRC, 1997a). That report included the following findings and recommendation concerning the addition of carbon filters to the PAS.
The proposed methodology [using an HRA/QRA risk-based evaluation], if well implemented, is appropriate for evaluating whether or not to install a PFS on a site-specific basis.
Carbon filters appear to be effective in reducing the level of dioxins/furans to below the limits of detection and to have a useful life of at least one year. Because these levels cannot be measured, however, credit only for reducing them to the detection limit appears in the HRA.
Recommendation 10. The Army should proceed with the application of its proposed methodology for evaluating the use of PAS carbon filters on a site-specific basis. For consistency with the HRA assumptions, the QRA should take into account the possible sudden release of agent that may have accumulated on the filter at a gas concentration equal to the lower detection limit.
In preparing the present report, the Stockpile Committee evaluated developments pertaining to the findings and recommendation cited above. Chapters 2, 3, and 4 are focused on science and engineering aspects of carbon filters, particularly their application to flue gas emissions from chemical agent disposal by incineration. Detailed discussions in these chapters address the composition of trace gaseous emissions, the control of trace gas emissions with carbon filters, and the impact of carbon filters on facility design. Chapter 5 is focused on the risk assessments and public involvement in the Army's decisions about using PAS carbon filters. Chapter 6 contains the committee' s findings and recommendations.





