ATTACHMENT C
BACKGROUND ON THE HIGH-LEVEL WASTE PROGRAM AT SAVANNAH RIVER
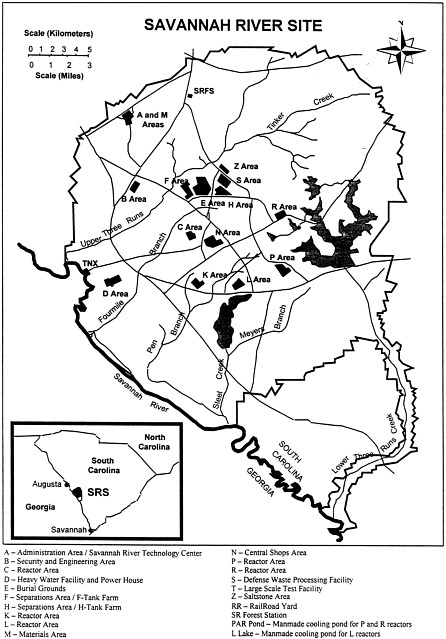
During and immediately following the Second World War, the U.S. Government established large industrial complexes at several sites across the United States to develop, manufacture, and test nuclear weapons. One of these complexes was established in 1950 at the Savannah River Site to produce strategic isotopes, mainly plutonium and tritium, for defense purposes. The site is located adjacent to the Savannah River near the Georgia-South Carolina border ( Figure C.1 ) and comprises an area of about 800 square kilometers (~300 square miles).
The Savannah River Site is host to an extensive complex of facilities that includes fuel and target fabrication plants, nuclear reactors, chemical processing plants, underground storage tanks, and waste processing and immobilization facilities. Plutonium and tritium were produced by irradiating specially prepared metal targets in the nuclear reactors at the site. After irradiation, the targets were transferred to the F Canyon or H Canyon, where they were processed chemically to recover these isotopes. This processing resulted in the production of large amounts of highly radioactive liquid waste, known as high-level waste (HLW), that is being stored in two underground tank farms at the site (in the F Tank Farm and H Tank Farm). The U.S. Department of Energy has the responsibility for waste management at the Savannah River Site and has implemented a program to stabilize this high-level waste and close the tank farms.
The information used in this attachment is taken from the documents cited in Attachment E and from copies of the presentations provided at the committee’s first information-gathering meeting.
HIGH-LEVEL WASTE SYSTEM AT SAVANNAH RIVER SITE
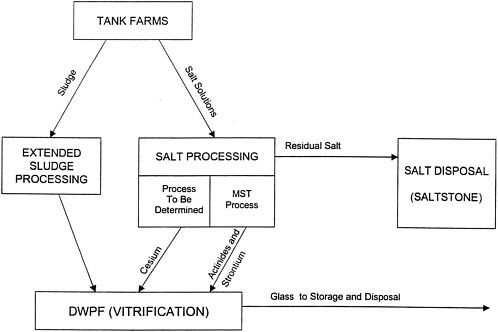
A simplified schematic representation of the HLW system at the Savannah River Site is shown in Figure C.2 . This system comprises the following major components:
Waste Concentration and Storage. The high-level waste resulting from operations in the F and H Canyons is currently being stored in 48 underground carbon-steel tanks 17 in the F and H Tank Farms. The tanks range in size from about three million to five million liters (750,000 to 1.3 million gallons). The high-level waste was made alkaline with NaOH before being transferred to the tanks to reduce corrosion of the carbon steel primary containment. Consequently, the waste has a high pH (>14) and a high salt content.
Approximately 400 million liters (100 million gallons) of high-level waste have been produced at Savannah River since operations began in the 1950s, but this volume has been reduced to about 130 million liters (34 million gallons) by removal of excess water through evaporator processing operations. About 10% of the waste by volume is in the form of a metal precipitate, or sludge, that contains most of the actinides and fission products. This sludge was formed by natural settling and by precipitation when NaOH was added to the waste. The remaining waste consists of salt in a solid, or saltcake, form that contains cesium and minor amounts of actinides and other fission products. Salt was produced by processing the alkaline
Radionuclide Immobilization. The Defense Waste Processing Facility (DWPF) was constructed to immobilize radioactive waste in borosilicate glass for eventual shipment to and disposal in a geological repository. The glass-making process is referred to as vitrification. This glass is produced by combining the processed high-level waste (the processing operations are discussed below) with specially formulated glass frit and melting the mixture at about 1150 ºC. The molten glass is then poured into cylindrical stainless steel canisters, allowed to cool, and sealed. The DWPF canisters are about 60 centimeters (2 feet) in diameter and about 300 centimeters (10 feet) in length and contain about 1,800 kilograms (4,000 pounds) of glass. About 700 canisters have been produced to date, and Savannah River estimates that a total of about 6,000 canisters will be produced by 2026, when the tank waste processing program is planned to be completed. These canisters will be stored at the site until a repository is opened and ready to receive them.
Extended Sludge Processing is used to prepare the sludge portion of the tank waste for processing into glass. The sludge is removed from the tanks by hydraulic slurrying, and it is then washed to remove aluminum and soluble salts, both of which can interfere with the glass-making process. The washed sludge is transferred to the DWPF for further processing before being incorporated into glass.
Salt Processing will be used to remove radionuclides from the HLW salt for eventual processing into glass. This processing step is not yet operational and is the focus of the present study. The salt will be redissolved and transferred out of the tanks. It will then be mixed with a sorbent to remove any remaining actinides (mainly uranium and plutonium) and strontium. The currently planned sorbent is monosodium titanate (MST). The solution will then be subjected to another (and as-yet undetermined) process to remove cesium. The separated actinides and cesium will be washed to remove soluble salts and sent to the DWPF for immobilization.
Salt Disposal. A variety of secondary waste streams are formed during the processing operations described above. Some of these waste streams are recycled back to the tanks, other wastes are recycled within the various processing operations, and yet other wastes are treated and released to the environment. Most notably, the residual salt solutions (i.e., the solutions remaining after actinide and cesium removal) will be disposed of onsite in a waste form known as saltstone. This solution is classified as “incidental waste” from the processing of high-level waste. Saltstone is created by mixing the residual salt solutions with fly ash, slag, and Portland cement to create a grout slurry. This slurry is then poured into concrete vaults, where it cures and is eventually covered with soil. The saltstone also contains some radionuclides, for example technetium-99 and tin-126. The saltstone production facility is permitted by the South Carolina Department of Health and Environmental Control as waste water treatment facility. The saltstone vaults are designed as a controlled release landfill disposal site. The operating permit limits the average concentrations of radioactive contaminants to the limits specified by the U.S. Nuclear Regulatory Commission for Class A Waste 18 . In the direct grout processing option, which is discussed below, cesium also would be immobilized in the grout.
At present, Savannah River is processing sludge from the tanks to make glass at the DWPF, and it has a wastewater permit from South Carolina to produce saltstone. The current high-level waste processing schedule calls for the saltcake to be processed to recover the
actinides, strontium, and cesium beginning about 2008. The 2008 schedule has been proposed to maintain operations at the DWPF and to ensure that there is sufficient space in the tank farms to continue operations at the site 19 . To meet this schedule, however, Savannah River must develop, test, and implement a process for removing actinides, strontium, and cesium from the salt in the tanks. A brief review of Savannah River’s efforts to develop this process is provided in the next section.
SALT PROCESSING OPTIONS
The objective of the salt processing step is to reduce the volume of salt waste to be immobilized in glass and, consequently, to reduce the time and cost of the immobilization operations. There are approximately 120 million liters (31 million gallons) of HLW salt in the F and H Tank Farms, but Savannah River estimates that this salt could be processed to yield about 11 million liters (3 million gallons) of actinide and cesium-bearing solutions or precipitates for vitrification, roughly a ten-fold reduction in volume.
At present, Savannah River plans to remove actinides, strontium, and cesium from the salt solutions in two processing steps. As noted previously, actinides and strontium will be removed by mixing the salt solutions with MST. The resulting reaction leads to the sorption of actinides and strontium. The product of this reaction could be removed from the salt solutions by filtration for subsequent processing and immobilization. This process has been demonstrated at the Savannah River Site’s In-Tank Precipitation Facility.
The removal of cesium from the salt solutions is potentially feasible through a number of processes, for example, precipitation reactions, ion exchange, or solvent extraction. In the 1980’s, Savannah River developed a process for removing cesium from salt solutions through a precipitation reaction involving sodium tetraphenylborate (TPB):
The Savannah River Site refers to this process as in-tank precipitation (ITP). The TPB was to be added directly to a large waste tank to produce a cesium-bearing precipitate, which could then be processed like tank sludge. Savannah River undertook an ITP pilot project in 1983 to demonstrate proof of principle. The process removed cesium from the salt solution, but it also resulted in the generation of benzene from radiolytic reactions and possibly from catalytic reactions with trace metals in the waste, in particular, palladium and copper.
In September 1995, Savannah River initiated ITP processing operations in a tank that contained about 1.7 million liters (450,000 gallons) of salt solutions. The operations were halted after about three months because of higher-than-expected rates of benzene generation. Savannah River staff then initiated a research program to understand the mechanisms of benzene generation and release, and staff also considered possible design changes so that the benzene, which is highly flammable, could be handled safely during processing operations. In 1996, the Defense Nuclear Facility Safety Board (DFNSB) issued Recommendation 96-1, which urged DOE to halt all further testing and to begin an investigative effort to understand the mechanisms of benzene formation and release (DNFSB, 1996):
The additional investigative effort should include further work to (a) uncover the reason for the apparent decomposition of precipitated TPB in the anomalous experiment, (b) identify the important catalysts that will be encountered in the course of ITP, and develop quantitative understanding of the action of these catalysts, (c) establish, convincingly, the chemical and physical mechanisms that determine how and to what extent benzene is retained in the waste slurry, why it is released during mixing pump operation, and any additional mechanisms that might lead to rapid release of benzene, and (d) affirm the adequacy of existing safety measures or devise such additions as may be needed.
Investigations by Savannah River in 1997 uncovered the possible role of metal catalysts in the benzene formation process. However, Savannah River concluded that safety and simultaneous production requirements could not be met, which led to the suspension of operations altogether in early 1998. At the time of suspension, Savannah River had spent $489 million to develop and implement the ITP process.
In March 1998, the Savannah River contractor, Westinghouse Savannah River Company (WSRC), formed a systems engineering team to identify alternatives to the ITP process for separating cesium. This team was comprised of 10 members with expertise in science and engineering, operations, waste processing, and safety and regulations. The team interacted with experts throughout the DOE complex and undertook a historical review and literature survey to identify about 140 processes that could potentially be used to separate cesium from the salt solutions. These processes were grouped into an “initial list” of 18 alternative processing options, which were subsequently screened using a multi-attribute analysis to obtain a “short list” of four alternative processing options: (1) small tank tetraphenylborate (TPB) precipitation, (2) crystalline silicotitanate (CST) non-elutable ion exchange, (3) caustic side solvent extraction, and (4) direct disposal in grout.
Small tank TPB precipitation is carried out in specially designed processing vessels to control benzene generation. TPB is the same precipitating agent used in the ITP process. The process allows for closer temperature control and faster cycling times to reduce the generation of benzene and improved agitation of the liquid to facilitate benzene removal. The process is also designed with secondary containment and positive pressure control so that the processing vessels could be blanketed with nitrogen to reduce explosion hazards and facilitate benzene removal. The process generates a precipitate slurry that will be transferred to the DWPF.
CST non-elutable ion exchange is based on conventional ion exchange concepts but utilizes a non-elutable inorganic solid that has a high selectivity for cesium over other alkali metals. The waste would be processed by pumping it through columns packed with this material. As the salt solutions pass through the CST, cesium is trapped. Once loaded with cesium, the CST would then be sent directly to the DWPF, where it would be treated with formic acid treated sludge before immobilization. Although ion exchange for cesium removal has been used in the nuclear industry, CST ion exchange has never been used in a large-scale waste application, and CST has never been manufactured in commercial-scale quantities.
Caustic side solvent extraction also is based on conventional solvent extraction concepts, such as those used in the widely known PUREX process to separate U and Pu from dissolved irradiated fuel. The process involves the mixing and subsequent separation of two immiscible feed streams: an aqueous solution containing the radionuclide to be extracted and an organic solvent containing a chelating agent (also known as the extractant) for that
radionuclide. Other chemicals may be added to improve the extraction efficiency or to inhibit the formation of undesirable reaction products.
The two feed streams are pumped through a series of centrifugal contactors, where they are mixed and subsequently separated on the basis of density. During the mixing process, the radionuclide is chelated by the extractant, which results in its transfer from the aqueous feed stream to the organic feed stream. The radionuclide is then recovered through a series of stripping steps, and the organic solvent is recycled back into the front end of the extraction process.
For caustic side solvent extraction of cesium, the organic solvent consists of a diluent (Isopar®-L, a mixture of branched alkanes), modifier (Cs-3, a fluorinated alcohol that prevents the formation of additional chemical phases), and extractant (BOB Calix 20 , a calixarene crown ether). When the salt solution is mixed with the organic solvent, cesium ions are complexed by the extractant (“L” in the following reaction) to form a cesium nitrate ion pair:
This ion pair is subsequently extracted into the organic solvent and then is recovered by separation of the aqueous stream from the solvent stream followed by a series of acid washing steps. The Isopar®-L and BOB Calix are recycled, and the cesium nitrate liquid can be sent directly to the DWPF without further processing.
Although solvent extraction is a mature technology for separating radionuclides from acid solutions (acid side solvent extraction), solvent extraction of cesium from highly alkaline solutions has never been demonstrated on an industrial scale, and the chelator (BOB Calix) has never been produced in commercial quantities. It is currently being manufactured in small quantities and is priced at about $500 per gram. The price could presumably be reduced significantly once production was scaled up.
Direct grouting is very similar to the saltstone process that was to have been used to immobilize the residual salt solutions from ITP operations. After removal of the actinides and strontium with MST, the remaining liquid would be mixed with fly ash, slag, and Portland Cement and poured into concrete vaults on the site. About 520,000 cubic meters (18 million cubic feet) of grout would be produced. This waste would meet the limits of Class C low-level waste 21 .
Savannah River performed a flowsheet analysis, risk analysis, and lifecycle cost analysis of the four alternatives in late 1998. Based on this analysis, WSRC recommended small tank TPB as the preferred alternative and CST non-elutable ion exchange as the backup alternative. However, the Department of Energy’s (DOE) analysis of the information concluded that additional research was needed before a preferred alternative could be selected. Additional R&D needs were identified for each of these alternatives, and work to address these needs was underway as the committee began this study.